Abstract
Zebrafish (Danio rerio) have been used to study multiple effects of nicotine, for example on cognition, locomotion, and stress responses, relying on the assumption that pharmacological tools will operate similarly upon molecular substrates in the fish and mammalian systems. We have cloned the zebrafish nicotinic acetylcholine receptor (nAChR) subunits and expressed key nAChR subtypes in Xenopus oocytes including neuronal (α4β2, α2β2, α3β4, and α7) and muscle (α1β1bεδ) nAChR. Consistent with studies of mammalian nAChR, nicotine was relatively inactive on muscle-type receptors, having both low potency and efficacy. It had high efficacy but low potency for α7 receptors, and the best potency and good efficacy for α4β2 receptors. Cytisine, a key lead compound for the development of smoking cessation agents, is a full agonist for both mammalian α7 and α3β4 receptors, but a full agonist only for the fish α7, with surprisingly low efficacy for α3β4. The efficacy of cytisine for α4β2 was somewhat greater than typically reported for mammalian α4β2. The ganglionic blocker mecamylamine was most potent for blocking α3β4 receptors, least potent for α7, and roughly equipotent for the muscle receptors and the β2-containing nAChR. However, the block of β2-containing receptors was slowly reversible, consistent with effective targeting of these CNS-type receptors in vivo. Three prototypical α7-selective agonists, choline, tropane, and 4OH-GTS-21, were tested, and these agents were observed to activate both fish α7 and α4β2 nAChR. Our data therefore indicate that while some pharmacological tools used in zebrafish may function as expected, others will not.
Keywords: drug development, nicotine dependence, animal models
1.0 Introduction
The nicotinic acetylcholine receptor (nAChR) of the neuromuscular junction was the first molecular mediator of electro-chemical synaptic transmission to be studied in detail and ultimately isolated and cloned. Muscle-type nAChR are pentameric assemblies of subunits identified as α1, β1, δ, and ε or γ, with each receptor containing two α1 subunits. The mediators of nicotine's effects in the brain and autonomic nervous system were subsequently identified [1]. These neuronal nAChRs are also pentameric ligand-gated cation channels. They mediate synaptic transmission in autonomic ganglia and modulate synaptic and cell function in the central nervous system. Individual genes code for subunits of two general classes, based on the presence or absence of a key structural element in the α1 subunit of muscle-type receptors, a pair of adjacent cysteines in the ligand-binding subdomain. Neuronal nAChR beta subunits lack this feature. Functional neuronal nAChR subunits can be further classified into two major subfamilies: homomeric receptors with α7 and α9 subunits that may function without beta subunits, and heteromeric receptors which are assemblies of α (α2–α6) and β(β2–β4) subunits [2]. Heteromeric receptors constitute the high-affinity binding sites for nicotine in the nervous system.
Neuronal nAChRs are often located presynaptically in the CNS and modulate release of important neurotransmitters such as norepinephrine, serotonin, gamma aminobutyric acid, glutamate, and dopamine [3]. Signaling through nAChRs regulates and influences neural functions including several aspects of cognition and is involved in pathways mediating drug dependence and addictive behaviors [2]. nAChRs are also involved in schizophrenia, attention deficit hyperactivity disorder, Alzheimer’s, Tourette’s, Parkinson’s, autism, and epilepsy [2].
Neuronal nAChRs have been studied in animal models including non-human primates, rats, and mice. Although the specific nAChR subtypes involved in many of the various functions and diseases described above are not known for certain, studies of nAChR knockout mice have provided valuable leads, especially in regard to nicotine addiction. In many cases the in vivo pharmacology described in animal studies has been validated through the in vitro study of cloned receptor subunits in expressions systems such as the Xenopus oocyte [1]. As an outcome of these many research studies, numerous clinical studies have also been made or are presently underway for the indications noted.
Recently zebrafish (Danio rerio) have also been used to study the role of nAChRs in several behaviors including locomotor and stress responses, and cognitive and exploratory behaviors [4]. Zebrafish can be used for the study of nAChRs' role in normal development and the effects of nicotine on developing embryos [5]. This new information about zebrafish nAChRs, and the advantages of the zebrafish system, provide an opportunity to develop and test therapeutic agents targeted to neuronal nAChRs. Zebrafish have been used in some behavioral assays similar to those used with other vertebrates. Five-day old zebrafish possess locomotor and simple sensory capabilities, while older zebrafish exhibit additional behaviors, such as feeding and escape [5]. Zebrafish are amenable to relatively high throughput screening approaches to test compounds for effects on learning, memory, and anxiety. Zebrafish have been used to examine the anxiolytic effects of nicotine [6], spatial discrimination learning [7], and delayed spatial alternation [8]. Zebrafish are being used to screen for potential neuroprotective compounds in a model of Parkinson's [9]. The advantages of zebrafish for pharmaceutical screening [10, 11] can be exploited to complement existing cell culture and mouse studies to test and develop new cholinergic therapeutic compounds.
We have cloned eight zebrafish neuronal nAChR cDNAs (α2, α3, α4, α6, α7,β2, β3,β4) [12, 13] and zebrafish muscle nAChR subunit cDNAs [14]. These are largely expressed in regions analogous to structures in mammals. In order to interpret the behavioral studies and to lay the groundwork for possible use of zebrafish for high throughput screening, and as a model to study nAChRs, the basic pharmacological properties of the major nAChR subtypes present in zebrafish should be determined.
In this study we have expressed zebrafish neuronal α4β2, α2β2, α7, and α3β4 nAChRs and a muscle nAChR, α1β1bεδ, in Xenopus oocytes and determined the EC50s for acetylcholine, nicotine, and cytisine and the IC50 of mecamylamine for each subtype. We have also conducted preliminary tests of additional α7-selective compounds on each subtype (alone or with ACh) to determine whether these compounds may act similarly in zebrafish compared to other animal models.
2.0 Materials and Methods
2.01 Zebrafish maintenance
Zebrafish colonies were maintained at 28°C in stand-alone self-circulating systems following the guidelines of IACUC at the Ohio State University and the NIH / NIAAA.
2.02 Cloning of zebrafish nAChR cDNAs
RNA was isolated from zebrafish embryos using Trizol (Invitrogen, Eugene, OR). We first used polymerase chain reaction (PCR) with degenerate PCR primers in combination with 5' and 3' RACE (First Choice RLM RACE (Ambion, Austin, TX) to isolate partial 5' and 3' cDNAs encoding zebrafish neuronal nAChR α4, α2, β2, α7, α3, and β4 subunit cDNAs. The primers were based on conserved TM3 and TM 4 sequences present in human, rat, mouse, bovine, and chick nAChRs. Alignments were made and PCR primers designed to the most conserved regions. Information in the Sanger Centre Zebrafish Genome database (http://www.sanger.ac.uk) was also used to design gene-specific 5' and 3' RACE primers used in the cloning of some of the cDNAs. Reverse transcription using the Superscript III First Strand Synthesis System for RT-PCR (Invitrogen) followed by PCR with Platinum Pfx polymerase (Invitrogen) was used to isolate full-length nAChR clones using primers designed based on information obtained from the 5' and 3 ' RACE clones. The cDNAs were then cloned into PCRII TOPO vectors and sequenced. The muscle type nAChR subunits α1, β1b, ε, and δ were cloned as previously described [14].
After linearization and purification of cloned cDNAs, RNA transcripts were prepared in vitro using the appropriate mMessage mMachine kit from Ambion Inc. (Austin, TX).
2.03 In situ hybridization
In situ hybridization was performed as described in Ikenaga et al., [15]. The probes for β1a and β1b were 430 bps and 491 bps long, respectively. Regions for hybridization including 3'UTR were selected based on the low homology. Specifically, probe sequences were amplified by performing PCR with GGGTTGTTTGGAAAATAGCCTCAGA and TAGCGTCCGTCCACAGAGAGTACAG for β1a, and GGACTGGCAGTATGTTGCTATGGTG and GGGTAATTAGGCAAACCATAGTATAATGA for β1b.
2.04 Quantitative PCR
Total RNA was purified from pools of 20 embryos/larvae for each time point, 1, 2, 3, 8, and 21 days post fertilization (dpf), using RNeasy Micro (Qiagen, Valencia, CA). Total RNA was DNAse treated with TURBO DNAse (Ambion, Austin, TX). Following treatment, first-strand cDNA was synthesized using iScript cDNA Sythensis Kit (BioRad, Hercules, CA). Primers and probes, containing a 5’-6-FAM and a 3’-Black Hole Quencher-1, were synthesized by Integrated DNA Technologies (Coralville, IA). Primers corresponding to sequences in neighboring exons and a probe encompassing the exon junction were selected in order to eliminate amplification from the genomic DNA. Primers and probes were designed as follows: β1a: forward 5’-aacttactgcctcgctacttgggt-3, probe 5’-aggaaccagtggaggaagagccaaa-3’, reverse 5’-acagtgctctcgttatggcttcct-3’; β1b: forward 5’-ttcgtgcggagtgaaggtgacata-3’, probe 5’-agaagtggatcttcaacatgccctgg-3’, reverse 5’-actctcgctaaagcctgtgtccaa-3’; Elongation factor1-α-(elf1-α): forward 5’-ttgatgcccttgatgccattctgc-3’, probe 5’-attggaactgtacctgtgggtcgtgt-3’, reverse 5’-acaaccataccaggcttgaggaca-3’.
Elf1-α was used as an endogeneous control for all runs. TaqMan Fast Polymerase (Applied Biosystems, Carlsbad, California) was used in all experiments. Cycling conditions were as follows: an initial hold at 95°C for 20 seconds, followed by 40 cycles at 95°C for one second and at 60°C for 20 seconds. Results are an accumulation of 3–4 biological repeats with 3 internal repeats per run. Negative controls were run with each sample set. All qPCR runs were carried out using the StepOnePlus Real-Time PCR system (ABI, Foster City, CA).
Absolute transcript copy numbers were determined using the standard curve method. Quantification of known amounts of target DNA was used to establish a comparative for unknown transcript levels of specific subunits. From the standard curve, quantification of the transcript number was determined. Numbers were normalized to the endogenous control (elf1-α) to account for variations in concentration of starting templates. Normalized absolute numbers, from multiple runs, were plotted in Figure 2C.
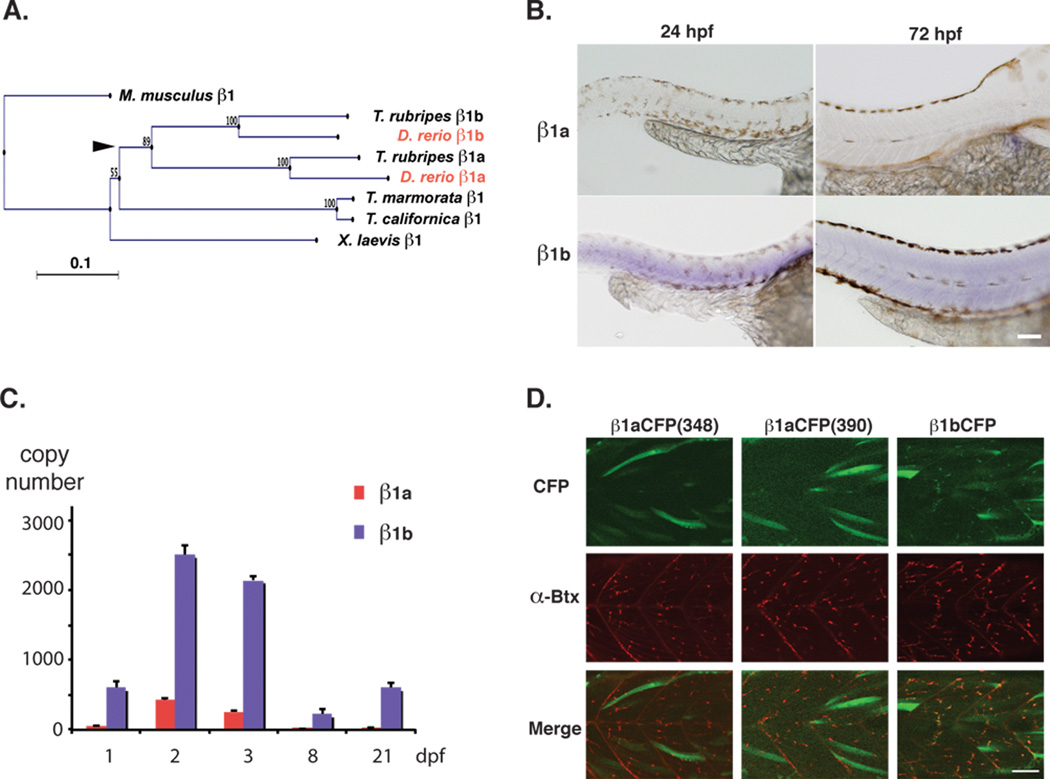
Figure 2.
Muscle β1 homologs in zebrafish. A) A phylogenetic tree constructed from β1 subunit sequences of Mus musculus, Xenopus laevis, Torpedo californica, Torpedo marmorata, Takifugu rubripes, and Danio rerio. Zebrafish sequences are shown in red. The presumed location of the fish specific genome duplication is indicated with an arrowhead. B) In situ hybridization of zebrafish β1a and β1b at 24 and 72 hpf. Scale 100 µm. C) Absolute copy number of β1a and β1b transcripts determined by real time PCR of whole larvae at 1, 2, 3, 8, and 21 dpf. Averages and standard deviations are shown. D) β1 clones conjugated to CFP in the III-IV intracellular loop were expressed in zebrafish muscle cells at 2 dpf. CFP signals (top), α-Btx stainings (middle), and merged images (bottom) are shown. Two β1a clones with different CFP insertion sites did not exhibit synaptic localization (left and middle), while the β1b CFP clone showed the signal of CFP overlapping with the α-Btx staining. Scale 50 µm.
2.05 Expression of CFP-conjugated β1s in zebrafish myocytes
β1a and β1b clones were modified so that they had in-frame insertions of Cyan Fluorescent Protein (CFP) in the III-IV intracellular loop. The modified clones were placed downstream of the α-actin promoter, as described in Epley et al. [16] for the δ subunit. The insertion site was after P348 and W390 for β1a, and S415 for β1b. The finished DNA construct was injected into fertilized embryos at the 1 cell stage. Staining of larvae with α-Btx was performed as previously described [16]. Two days post-fertilization (dpf) larvae were observed on an inverted 510 Meta confocal laser scanning microscope (Carl Zeiss Microimaging, Thornwood, NY). CFP was excited at 458 nm and the emission was filtered 465–490 nm. α-Btx conjugated with Alexa 555 (Invitrogen) was excited at 561 nm and the emission was filtered >570 nm. 25X water immersion lens (NA 0.8) was used for imaging. Images were taken using the Zen software (Carl Zeiss Microimaging) and later modified in Photoshop (Adobe Systems, San Jose, CA).
2.06 Expression of zebrafish nAChRs in oocytes
Mature (>9 cm) female Xenopus laevis African frogs (Nasco, Ft. Atkinson, WI) were used as a source of oocytes. Prior to surgery, the frogs were anesthetized by placing the animal in a 0.7g/l solution of MS222 (3-aminobenzoic acid ethyl ester) buffered with sodium bicarbonate for 15–30 min. Oocytes were removed from an incision made in the abdomen. To remove the follicular cell layer, harvested oocytes were treated with 1.25 mg/ml Type 1 collagenase (Worthington Biochemical Corporation, Freehold, NJ) for 3–4 hours at room temperature in calcium-free Barth's solution (88 mM NaCl, 1 mM KCl, 0.33 mM MgSO4, 2.4 mM NaHCO3, 10 mM HEPES (pH 7.6), 12 mg/l tetracycline chloride). Subsequently, stage 5 oocytes were isolated and injected with 50 nl (5–20 ng) each of the appropriate subunit cRNAs. The RNAs injected for alpha-beta pairs were injected at 1:1 ratios. Recordings were made 1 to 10 days after injection.
2.07 Chemicals
4OH-GTS-21 (3-(4-hydroxy, 2-methoxybenzylidene)anabaseine) was provided by Taiho Pharmaceutical (Tokyo, Japan). All other chemicals for electrophysiology were obtained from Sigma Chemical Co. (St. Louis, MO). Fresh ACh stock solutions were made daily in Ringer's solution and diluted.
2.08 Electrophysiology
Experiments were conducted using OpusXpress 6000A (Molecular Devices, Union City CA). OpusXpress is an integrated system that provides automated impalement and voltage clamp of up to eight oocytes in parallel. Cells were automatically perfused with bath solution, and agonist solutions were delivered from a 96-well plate. Both the voltage and current electrodes were filled with 3 M KCl. The agonist solutions were applied via disposable tips, which eliminated any possibility of cross-contamination. Drug applications alternated between ACh controls and experimental applications. Flow rates were set at 2 ml/min for experiments with α7 receptors and 4 ml/min for other subtypes. Cells were voltage-clamped at a holding potential of −60mV. Data were collected at 50 Hz and filtered at 20 Hz. ACh applications were 12 seconds in duration followed by 181-second washout periods with α7 receptors and 8 seconds with 241-second wash periods for other subtypes.
2.09 Measurement of functional responses
Pharmacological characterizations of ion channel responses often rely solely on measurement of peak currents. However, with desensitizing receptors like nAChR, the peak amplitudes of agonist-evoked responses cannot be interpreted in any straightforward manner, due to the nonstationary nature of channel activation. For heteromeric nAChR many factors determine peak current amplitudes, including agonist application rate, channel activation rates, desensitization, and even potentially channel block by agonist [17]. For α7 receptors, the peak currents are associated with synchronization of channel activation that occurs well in advance of the full agonist application [18]. Therefore, we have additionally measured the net charge of α7 agonist-evoked responses and mecamylamine inhibited currents, to add insight into an important aspect of the functional responses.
2.10 Experimental protocols and data analysis
Each oocyte received two initial control applications of ACh, then experimental drug applications, and additional follow-up control applications of ACh. The specific control concentrations were chosen because they gave robust, reproducible responses that did not show significant rundown or cumulative desensitization with repeated applications. The control ACh concentrations for fish α1β1bεδ, α4β2, α2β2, α3β4, and α7 receptors were 30 µM, 30 µM, 30 µM, 100 µM, and 300 µM, respectively. These concentrations represented the EC44, EC70, EC50, EC60, and EC100 values for each of the receptors, respectively (measured as net charge for α7 and peak current for all others).
Responses to each drug application were calculated relative to the preceding ACh control responses to normalize the data, compensating for the varying levels of channel expression among the oocytes. Drug responses were initially normalized to the ACh control response values and then adjusted to reflect the experimental drug responses relative to the ACh maximums. Responses were calculated as both the peak current amplitudes and as net charge [18]. Means and standard errors (SEM) were calculated from the normalized responses of at least four oocytes for each experimental condition. Since the application of some experimental drugs can cause subsequent ACh control responses to be reduced due to residual inhibition (or prolonged desensitization), subsequent control responses were compared to the pre-application control ACh responses. When cells failed to recover to at least 75% of the previous control, they were discarded and new cells were used for determination of effects at single concentrations. This approach was applied to the study of mecamylamine when the applications of the antagonist at high concentration produced inhibition that was not readily reversible during the normal washout period.
For concentration-response relations, data were plotted using Kaleidagraph 3.0.2 (Abelbeck Software; Reading, PA), and curves were generated from the Hill equation:
where Imax denotes the maximal response for a particular agonist/subunit combination, and n represents the Hill coefficient. Imax, n, and the EC50. Imax was constrained to equal 1 for the ACh responses, since we used the maximal ACh responses to define full agonist activity. Negative Hill slopes were applied for the calculation of IC50 values associated with inhibition.
3.0 Results
3.01 Neuronal nicotinic receptor subunits cloned from zebrafish
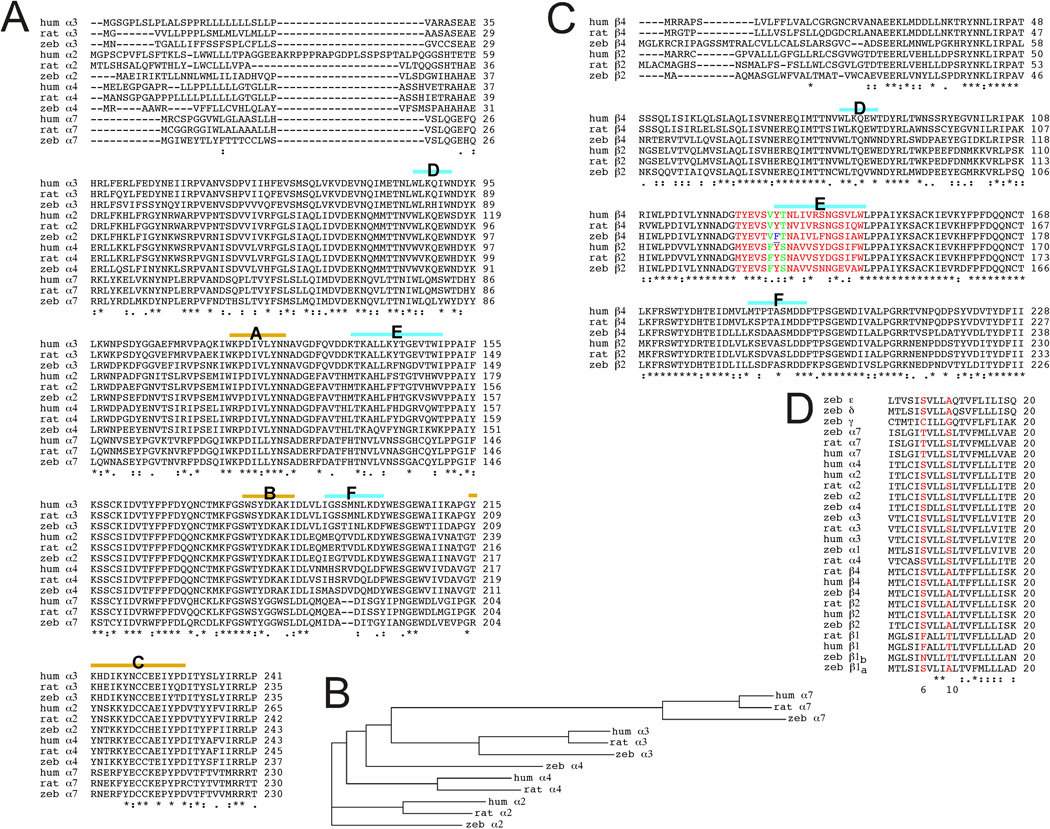
We have cloned eight zebrafish neuronal nAChR subunit cDNAs (α2, α3, α4, α6, α7, β2, β3, and β4) and focused on α2β2, α4β2, α3β4, and α7 neuronal nAChR subtypes for pharmacological characterization when expressed in Xenopus oocytes. In Figure 1 we compare the sequences of the expressed zebrafish nAChR subunits to those of rat and human nAChRs to determine how similar the zebrafish subunits are to those of other species in which the pharmacology of nAChRs is well characterized. Sequence identities/similarities were determined for pairwise comparisons between orthologous subunits using the programs EMBOSS Stretcher [19] and ClustalW2.1 [20] on the EMBL-EBI websites (http://www.ebi.ac.uk/Tools/psa/emboss_stretcher/ and http://www.ebi.ac.uk/Tools/msa/clustalw2/). We have focused on the extracellular domains (ECD) and the pore-forming second transmembrane (TM2) domain sequences as these may be most important for understanding contributions of specific amino acid differences to any differences in pharmacology. A comparison of the α subunit ECDs reveals a high degree of protein sequence identity and similarity across all the regions except the signal peptide sequences (Figure 1A). There is also a large amount of conserved sequence in the beta subunit ECDs (Figure 1C). A comprehensive comparison of the zebrafish neuronal ECD to the rat and human counterparts is given in Table 1.
Figure 1.
ClustalW2 Alignments A) Extracellular domain (ECD) regions of zebrafish (zeb), rat (rat), and human (hum) nAChR alpha subunits. The key subdomains of the ligand binding site, identified as loops A–F [45] are indicated above the sequence. The loops A–C are key elements associated with the primary surface of the bonding site restricted to alpha subunits (except α5). The loops D–F are key elements associated with the complementary surface of the ligand binding domain and are restricted to α7–α10 subunits and non-alpha subunits (except β3). B) A cladogram of the rat, human, and zebrafish neuronal alpha subunits, generated by ClustalW2 on whole sequences. C) Alignments of ECD regions of zebrafish (zeb), rat (rat), and human (hum) nAChR beta subunits. The key elements of the complementary portions of the agonist binding site are indicated above the sequences, and the extended E-loop sequence implicated as important for the efficacy of cytisine [31] is in red. The F residue conserved in beta 2 subunits is in green and the nonconservative Y to F residue change is blue and underlined. D) Alignments of TM2 regions (20 amino acids) of zebrafish (zeb), rat (rat), and human (hum) nAChRs. The two residues (6' and 10' in the internal TM2 numbering sequence), identified as most important for determining the selectivity of mecamylamine for neuronal compared to muscle-type nAChR, are red. The ClustalW2 alignment program was used on the EMBL-EBI site (http://www.ebi.ac.uk/Tools/clustalw2/) [20]. "*" denotes identical residues in all sequences, " : " conserved substitutions, and ". " semiconserved substitutions.
Table 1.
ECD protein sequence identities/similarities (%), Comparison of zebrafish to human and rat
| Zebrafish | Human | Rat |
|---|---|---|
| α2 | 76.7/83.1 | 86.0/92.1 |
| α3 | 84.8/92.9 | 83.8/91.9 |
| α4 | 82.3/96.6 | 83.7/91.6 |
| α7 | 82.2/91.8 | 81.2/92.3 |
| β2 | 76.1/86.8 | 75.6/86.8 |
| β4 | 72.9/87.0 | 72.0/87.0 |
Sequence identities for all the subunits are particularly high in TM2 (Fig. 1D), especially when comparing zebrafish, rat, and human orthologues. The high degree of sequence identity and similarity across the ECDs and TM2s support the use of zebrafish as an animal model in which to test the effects of nicotinic compounds.
3.02 Muscle-type β1 variants in zebrafish
Zebrafish have two genes corresponding to the mammalian β1, designated β1a and β1b [14]. A neighbor-joining analysis of β1 genes from vertebrate genomes (Figure 2A) suggests that the two zebrafish clones arose presumably from the fish-specific genome duplication (FSDG), which occurred between 335 and 404 million years ago after the divergence of cartilaginous fish [21]. Therefore both β1a and β1b are potentially functional. On the other hand, heterologous expression with other muscle-type subunit clones, α1, δ and ε, showed that the ACh current can be recorded with β1b, but not with β1a [14]. In order to determine whether β1a plays any physiological role in zebrafish in vivo, we performed in situ hybridization with probes specific for β1a and β1b (Figure 2B). Larvae at 24 hours post fertilization (hpf) and 72 hpf were used for the reaction. At both stages, the signal intensity for β1a was minimal, while β1b showed a robust signal in the skeletal muscle. This suggests that the amount of β1a transcript was smaller than that of β1b. Next we performed real time PCR (RT-PCR) with probes specific for β1a and β1b. We extracted mRNA from embryos/larvae at 1, 2, 3, 8, and 21 dpf. After reverse transcription, PCR was performed on normalized quantities of cDNA. The absolute copy number was calculated from each run, based on the standard curve. Both β1a and β1b showed peaks at 2–3 dpf. In agreement with the in situ hybridization data, the β1a copy number was much smaller than that of β1b at all examined stages (Figure 2C). Finally, to examine if β1a contributes to the synaptic function in zebrafish muscles in vivo, β1 clones with fused CFP were constructed and expressed in muscle cells of larvae (Figure 2D). β1b-CFP led to the surface expression of the produced protein and formed clusters of CFP signal that overlapped with the α-Btx staining. In contrast, β1a conjugated to CFP failed to show membrane clusters. Two insertion sites in the intracellular loop were tested, and similar results were obtained. Thus we did not obtain any evidence that β1a plays a significant role in the muscle physiology of zebrafish larvae.
Although our expression studies only followed animals to 21 dpf, due to the rapid development of sensory and motor systems, the behavior of zebrafish at 21 dpf are in most respects comparable to those of sexually mature adults [22]. Moreover, since the β1a subunit is not able to form functional receptors in heterologous expression systems [14], it is likely that the pattern of β1 subunit expression observed at 21 dpf is retained in the adult. Therefore, we focused on β1b in the following pharmacological analyses.
3.03 ACh evoked responses of fish nAChR expressed in Xenopus oocytes
Although nAChR can be formed with widely varying subunit combinations, studies of mammalian receptors have identified a few key subtypes, and these same subtypes are likely to perform similar functions in fish. These functional designations of receptor subtypes were based on pharmacological tools long before receptors could be categorized based on subunit composition. The first major distinction is between muscle and neuronal receptors. In our studies we reconstituted a representative fish muscle-type receptor by the co-expression of α1β1bεδ subunits. As noted above, a major distinction among neuronal receptors is between homomeric α7 receptors and the various heteromeric neuronal receptor subtypes. Among the heteromeric subtypes we distinguish α3β4-containing receptors as those most likely associated with synaptic transmission in autonomic ganglia [23]. Studies of rodents have clearly pointed at α4β2-containing receptors to be probably the most important heteromeric receptors in brain, constituting most of the nicotine binding sites and critically important for nicotine addiction and dependence [24]. Recent data, however, have suggested that an alternative alpha subunit, α2, which is not highly expressed in rat or mouse brain but is expressed in zebrafish nervous system (Boyd, R.T., unpublished), may also co-assemble with β2 and form another important receptor subtype in primates [25]. Therefore we have focused our studies on these five important nAChR subtypes: muscle (α1β1bεδ), α7, α3β4, α4β2, and α2β2.
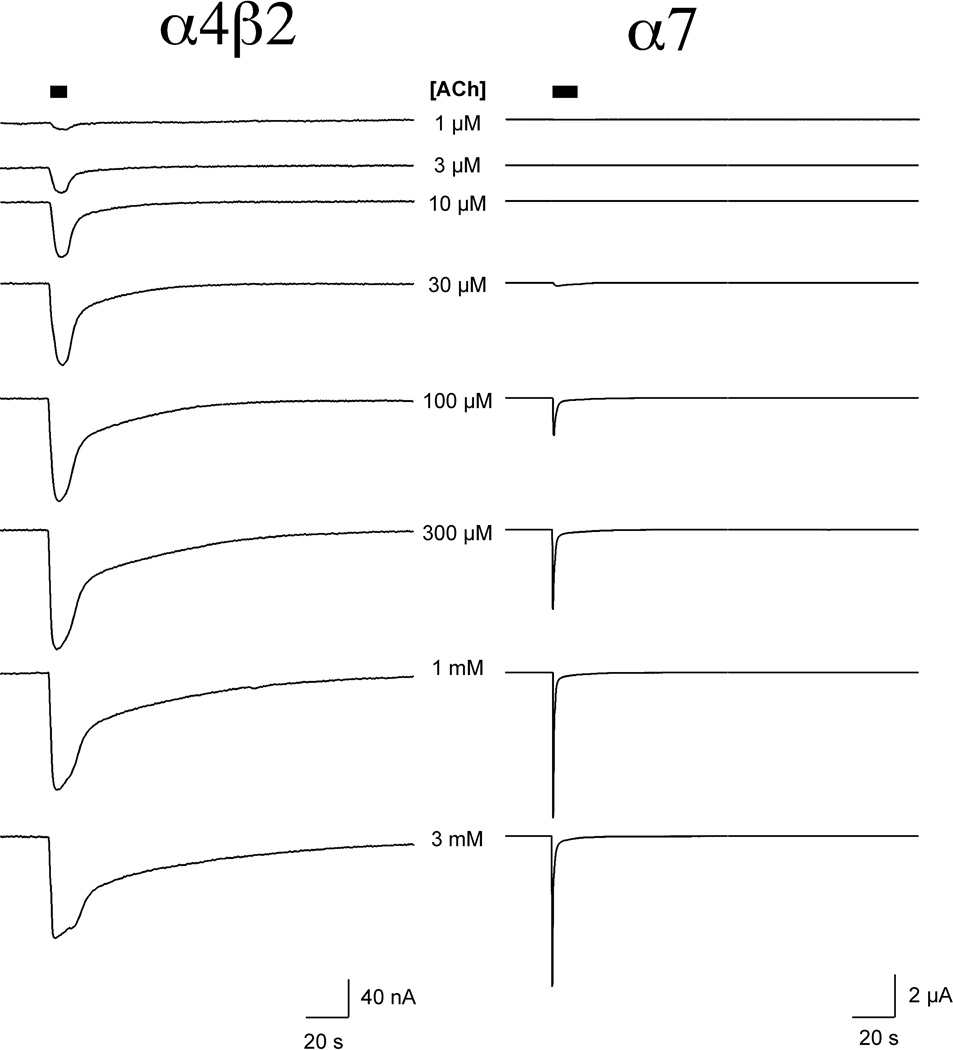
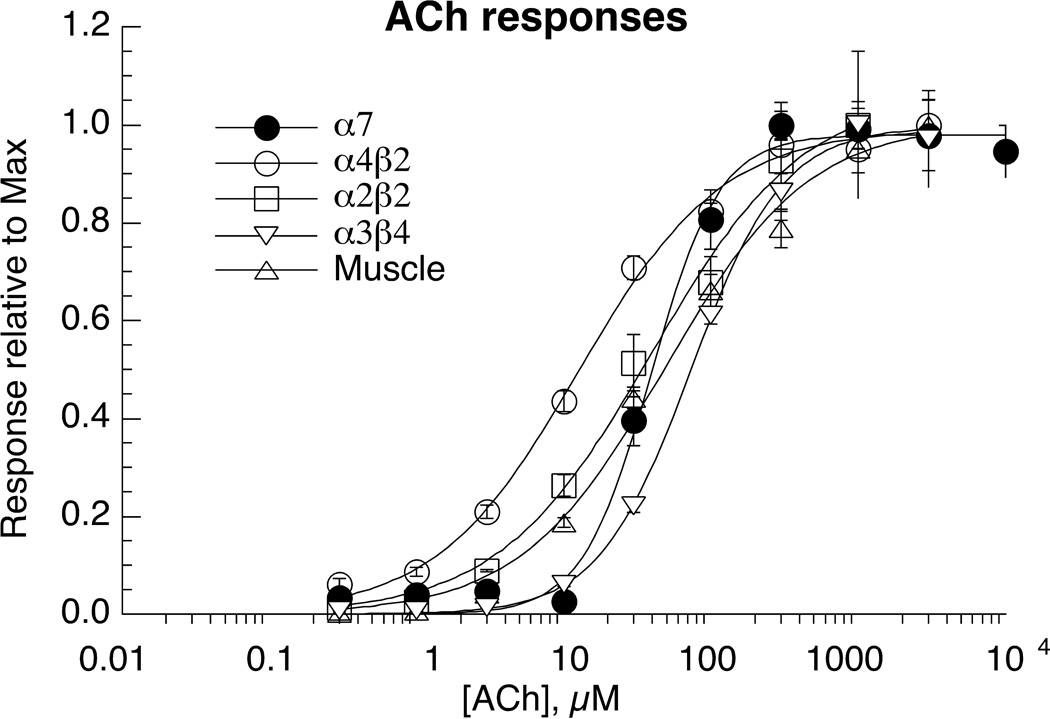
All of the receptors tested responded well to the application of ACh at concentrations of 3 µM or higher. Representative traces of α4β2 and α7 are shown in Figure 3, and concentration-response curves for all subtypes are shown in Figure 4. ACh was most potent for α4β2 receptors and least potent for α3β4 receptors (Table 2). However, while mammalian α4β2 nAChR typically generate large currents in the oocyte expression system, the fish α4β2 receptors consistently gave relatively small currents, due to either inefficient expression or an open probability intrinsically lower that associated with mammalian α4β2 receptors [26].
Figure 3.
ACh-evoked responses of zebrafish α4β2 and α7 nAChR. Displayed on the left are two-electrode voltage clamp recordings from a Xenopus oocyte expressing zebrafish α4β2 nAChR to the application of a range of ACh concentrations. Displayed on the right are recordings from a Xenopus oocyte expressing zebrafish α7 nAChR to the application the same range of ACh concentrations.
Figure 4.
ACh concentration-response studies of zebrafish nAChR expressed in Xenopus oocytes. Data were obtained by alternating applications of ACh at fixed control concentrations (see Methods) and ACh at increasing concentrations. The control responses were relatively consistent throughout the entire range of test concentrations. Test responses were initially normalized relative to the ACh controls, and the data are expressed relative to the observed ACh maximum responses. Data plotted are for the peak currents of the heteromeric receptors and for α7 net charge. Each point is the average ± SEM of responses from at least four oocytes. The solid curves represent fits of the Hill equation to the data, and that parameters for the fits are in Table 2.
Table 2.
Potency and efficacy of nicotinic agonists for the zebrafish nAChR
| ACh | nicotine | cytisine | |||
|---|---|---|---|---|---|
| Receptor | EC50 (µM) |
EC50 (µM) |
aImax | EC50 (µM) |
aImax |
| b α7 | 39 ± 3 | 27 ± 6 | 0.75 ± 0.04 | 15 ± 3 | 1.16 ± 0.06 |
| α4β2 | 13 ± 1 | 6 ± 2 | 0.7 ± 0.1 | 0.47 ± 0.09 | .26 ± 0.01 |
| α2β2 | 40 ± 8 | 6 ± 1 | 0.3 ± 0.05 | 4.1 ± 0.3 | 0.09 ±0.01 |
| α1β1bεδ | 48 ± 7 | 140 ± 50 | 0.13 ± 0.03 | 460 ± 20 | 0.13 ± 0.003 |
| α3β4 | 73 ± 3 | 13 ± 6 | 0.17 ± 0.02 | 11 ± 1 | 0.12 ± 0.002 |
Relative to ACh
Responses of α7 receptors are measured as net charge. All other data are based on peak current amplitude.
The data indicate that the ACh-evoked responses of the fish receptors are kinetically similar to what has been reported for mammalian receptors in the same expression system [27]. Likewise, as previously reported for mammalian heteromeric receptors, the ACh-evoked responses of all of the heteromeric zebrafish subtypes showed concentration-dependent increases in peak currents that were sustained for the duration of the agonist application. (See α4β2 responses as an example in Figure 3.) It is likely that, on the time scale of oocyte recordings, the receptors achieve some sort of equilibrium between activation and desensitization prior to the peak of the evoked responses, and the data indicate the concentration dependence of the pseudo steady-state phase of the evoked response.
The ACh-evoked responses of chick and mammalian α7 receptors have been reported to be qualitatively different from the responses of heteromeric receptors. In response to the applications of higher concentration of agonist, α7 receptor-mediated currents show progressively greater synchronization and diminished sustained responses. Although increased synchronization achieved with the application of high agonist concentrations has the effect of increasing the size of the peak currents, increasing agonist concentrations do not necessarily have the effect of increasing total channel activation. Moreover, when ACh is applied at concentrations ≥100 µM, peak currents occur on the leading edge of the drug application, so there is no correspondence between the height of the peak currents and the effects of the maximal drug concentration that will be seen by the cell. Therefore we use net charge as a more reliable and meaningful reporter of agonist potencies on α7-type receptors [18]. As shown in Figure 3, the α7 receptors of zebrafish showed the same pattern of concentration-dependent desensitization reported for α7 receptors of other species [18, 27].
The most important functional constraint on heteromeric neuronal nAChR is that they form two agonist binding sites at the interfaces between the alpha and non alpha subunits. It is known for mammalian receptors, especially β2-containing subtypes, that either an alpha or a beta subunit can take the position of the fifth subunit, with significant differences in the receptor function depending on which subunit is in that position. Receptors with three beta subunits and two alpha subunits often appear to respond more readily to low concentrations of agonists than do receptors with the reverse ratio [28]. In many studies, the oocyte expression system has been shown to be sufficiently permissive to allow both high-sensitivity (HS) and low-sensitivity (LS) populations of receptors to contribute to agonist-evoked responses. Since agonist potency for these subpopulations of receptors may differ by a factor of 10 or more, such data can clearly be better fit by a two-site model than by the single-site Hill model used to generate the curves in Figure 4. We investigated whether the α4β2 and α2β2 curves would be better fit by two-site models, supporting the likely presence of a mixed population of HS and LS forms of these receptors. However, in both cases the fits were not significantly improved by using a two-site model, and for α4β2 the fit was not even visibly changed (data not shown).
3.04 Response of zebrafish nAChR to nicotine and cytisine
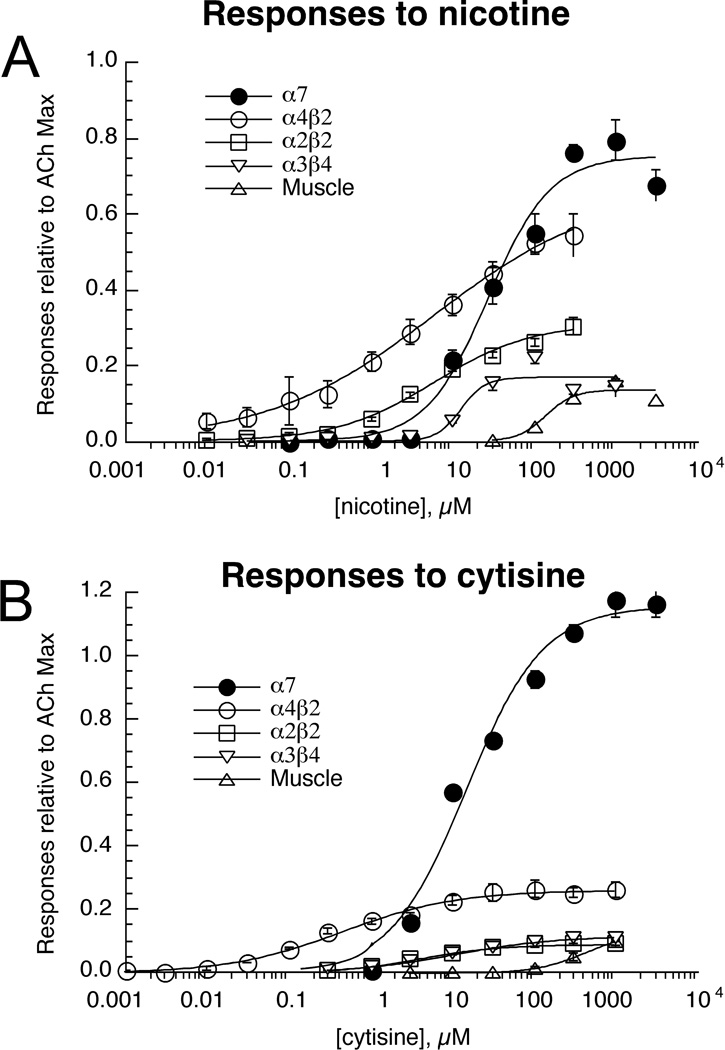
Since the zebrafish has been proposed as a screening system for nicotinic drugs and their behavioral effects, we determined the nicotine concentration-response relations for our battery of zebrafish nAChR subtypes. Additionally, we tested cytisine, a molecule which has become a lead compound for the development of smoking cessation agents and potentially also for the treatment of depression [29]. Compared to ACh, nicotine was a partial agonist for all of the heteromeric receptor subtypes, but a full agonist for α7 (Figure 5A). Among the heteromeric receptors, nicotine was most potent and efficacious for α4β2 and least potent and efficacious for muscle-type receptors.
Figure 5.
Nicotine (A) and cytisine (B) concentration-response studies of zebrafish nAChR expressed in Xenopus oocytes. Data were obtained by alternating applications of ACh at fixed control concentrations (see Methods) and the test compounds at increasing concentrations. The control responses were relatively consistent throughout the entire range of test concentrations. Test responses were initially normalized relative to the ACh controls, and the data are expressed relative to the observed ACh maximum responses as determined in the experiments illustrated in Figure 3. Data plotted are for peak currents of the heteromeric receptors and net charge for α7. Each point is the average ± SEM of responses from at least four oocytes.
The results obtained with cytisine were somewhat surprising. Cytisine is a full agonist for mammalian α7 and α3β4 receptors and a weak partial agonist for β2-containing receptors. We determined (Figure 5B) that while cytisine was also a full agonist for zebrafish α7 receptors, it was a relatively weak partial agonist for α3β4 receptors. The potency of cytisine was greatest for α4β2 receptors (Table 2), and the efficacy for this zebrafish subtype was greater than what is commonly reported for mammalian α4β2-type receptors [30].
3.05 Factors regulating the efficacy of Cytisine
Cytisine has previously been reported to be a very efficacious agonist of rat [30] and mouse α3β4 nAChR [27], giving rise to the hypothesis that off-target activity at ganglionic receptors might present a liability for autonomic side effects with the therapeutic use of cytisine for smoking cessation. It has previously been reported that sequence differences between β4 and β2 in the putative E-loop of the complementary face of the ligand binding domain regulate the efficacy of cytisine for β2 and β4-containing receptors [31]. The E-loop sequences of rat, human and zebrafish β2 and β4 E-loops are shown in Figure 1C highlighted in red.
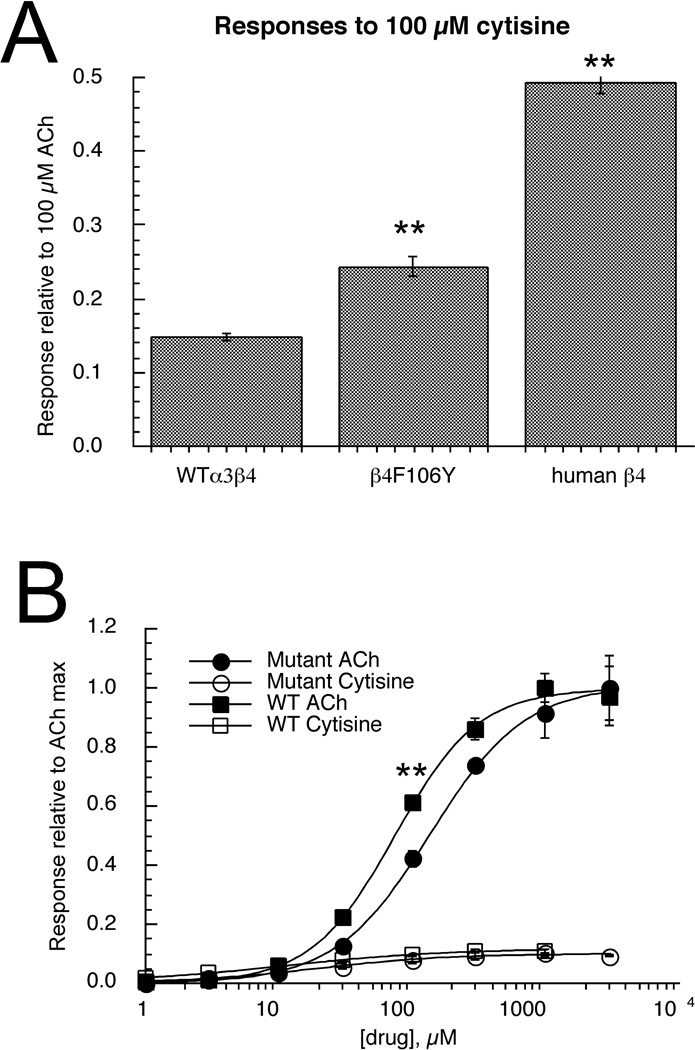
The residues in green are two previously implicated to be most important for regulating the efficacy of cytisine for mammalian receptors [31]. The zebrafish β4, which does not promote very effective activation by cytisine, contains the mammalian epitopes at these two sites but has a non-conservative Y to F difference between the two sites (in blue and underlined). We hypothesized that this difference might emulate the function of the conserved F residue found in β2 subunits, and so we made an F106Y mutation in the fish β4 subunit. When cells expressing the fish α3 subunit in combination with β4F106Y were stimulated with 100 µM cytisine, the relative amplitude of responses were significantly larger (p <.001) compared to 100 µM ACh control responses of wild-type fish α3β4 receptors (Figure 6A). However, this effect was not as large as the effect obtained when fish α3 subunits were co-expressed with human β4 subunits (Figure 6A). Although residues in the E-loop of the complementary side of the agonist binding site have been specifically implicated in regulating the efficacy of cytisine, this domain will likely affect activation by other agonists as well. Therefore, we conducted a full ACh concentration-response study of the α3β4F106Y receptors. As shown in Figure 6B, the β4 mutation had the effect of shifting the EC50 for ACh from 73 ±3 µM (wild-type, Table 2) to 134 ± 5 µM. This had a significant effect (P <.001) of decreasing the amplitude of the 100 µM ACh controls relative to the ACh maximum responses. When the cytisine data were expressed relative to the respective ACh maximums rather than the 100 µM controls, there was no significant effect of the F106Y mutation on the efficacy of cytisine (Figure 6B).
Figure 6.
The effects of the β4F106Y mutation on agonist-evoked responses of α3-containing zebrafish nAChR. A) Responses of wild-type fish α3β4 receptors to 100 µM cytisine compared to responses from oocytes expressing α3 and the β4F106Y mutant and to hybrid receptors expressing fish α3 subunits and human β4 subunits. In each case responses were measured relative to 100 µM ACh-evoked control responses. The wild-type cytisine responses were significantly less than those of either the mutant or hybrid receptors measured with this normalization procedure. B) In order to validate the normalization procedure, complete concentration-response studies were conducted for α3β4F106Y receptors for both ACh and cytisine. The data are shown compared to the wild-type data from Figures 4 (ACh) and 5B (cytisine). All of the data are normalized to the empirically determined ACh maximum responses. The main effect of the mutation appeared to be a shift in the potency of ACh, so that when expressed relative to ACh maximum, the 100 µM ACh responses of the mutants were significantly smaller (P <.001) than those of the wild-type receptors. Each bar or point is the average ± SEM of responses from at least four oocytes.
3.06 Evaluation of putative α7-selective agonists
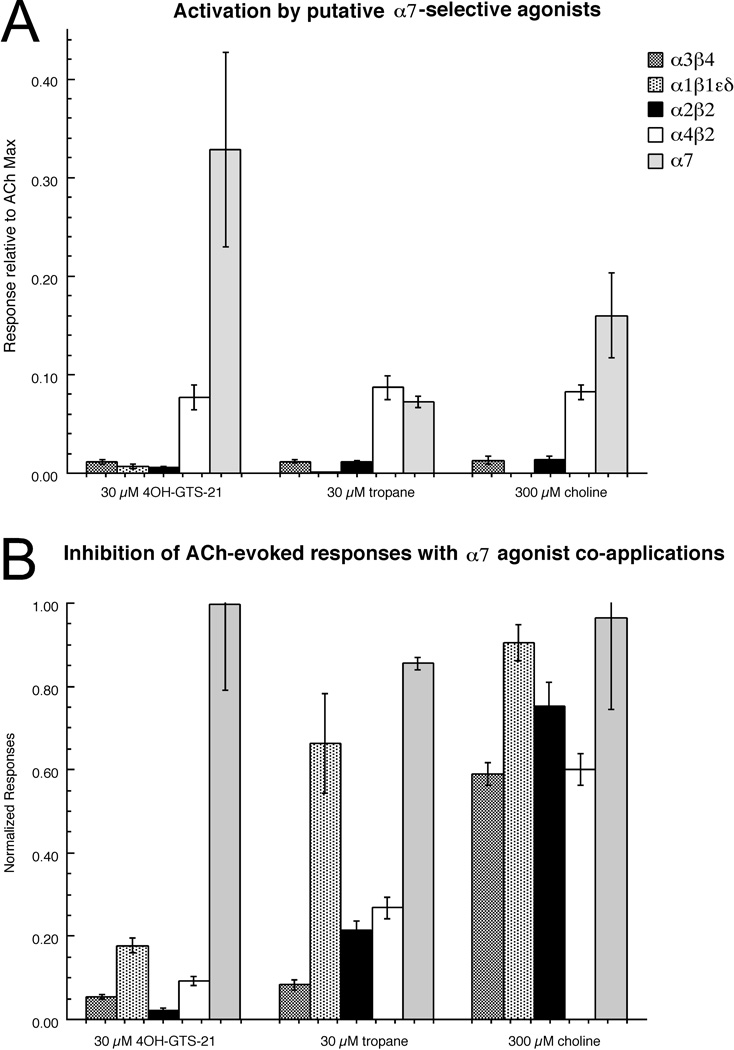
The therapeutic indications most commonly associated with α7 receptors as targets, e.g. Alzheimer's disease and schizophrenia, may not be easily addressable with zebrafish models. However, to test the underlying principles for α7-associated drug development, we investigated whether concepts developed for selectively targeting mammalian α7 could be applied to the fish receptors. Specifically, we tested three previously identified structural motifs which may be adapted to non-selective nicotinic agonists in order to create α7-selective agonists or partial agonists [32]. The eponymous prototypes defining these motifs are choline, tropane, and benzylidene anabaseine [32]. We choose 3-(4-hydroxy, 2-methoxybenzylidene)anabaseine (4OH-GTS-21) as a representative benzylidene anabaseine since it is among the best characterized drugs in this class and is a relatively efficacious partial agonist for both human and rat α7 receptors [18].
We used probe concentrations of 30 µM for 4OH-GTS-21 and tropane, and 300 µM for choline (the EC50 for human α7). As shown in Figure 7A, these agents failed to meet the criteria for truly α7-selective partial agonists since all three evoked significant responses from α4β2 receptors. We also tested whether probe compounds could inhibit the ACh-evoked responses of the heteromeric nAChR subtypes. While choline was relatively ineffective at decreasing ACh-evoked responses, 4OH-GTS-21 co-application inhibited all of the heteromeric receptors, and tropane was effective at inhibiting the neuronal heteromeric receptor subtypes (Figure 7B).
Figure 7.
The effects of α7-selective agonists on zebrafish nAChR. Three agents previously characterized as being selective activators of human α7 nAChR [32] were tested at the concentrations indicated for their ability to either activate zebrafish nAChR (A) or inhibit the ACh-evoked responses of the zebrafish nAChR (B). The ACh control concentrations used for the inhibition studies were 100 µM, 30 µM, 30 µM, 30 µM, and 60 µM for the α3β4, α1β1bεδ, α2β2, α4β2, and α7 receptors, respectively. Each column represents the average ± SEM of responses from at least four oocytes.
3.07 Mecamylamine inhibition of zebrafish nAChR subtypes
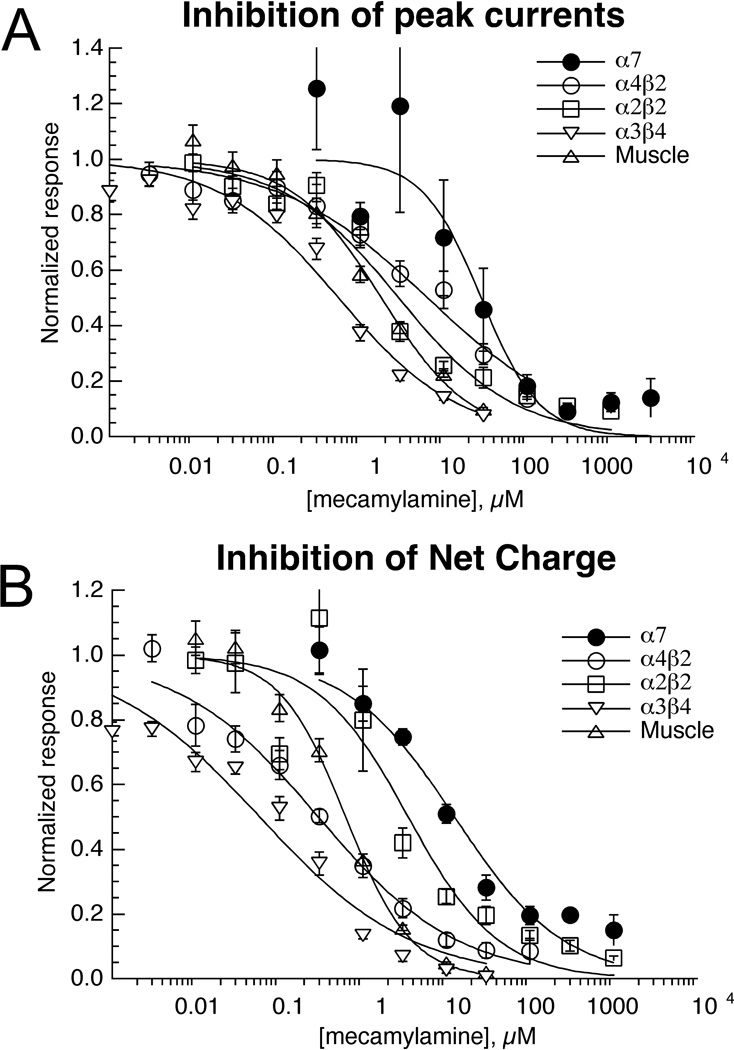
Many behavioral studies of nicotinic receptor function in mammals have relied on mecamylamine as a preferential antagonist of neuronal versus muscle-type nAChR. Since most drug development programs targeting nAChR either focus on heteromeric receptors of the CNS or on α7, mecamylamine effects on either muscle or ganglionic receptors might be considered "off-target". In acute co-application experiments we found that the rank order potency of mecamylamine for the inhibition of zebrafish nAChR was; α3β4 > α1β1bεδ > α2β2 > α4β2 > α7 for the inhibition of peak currents and α3β4 > α1β1bεδ > α4β2 > α2β2 > α7 for the inhibition of net charge (Table 3).
Table 3.
Inhibition of ACh-evoked peak currents and net charge responses by mecamylamine for zebrafish nAChR
| Peak current | Net charge | ||
|---|---|---|---|
| Receptor | IC50, µM | IC50, µM | Ratio |
| α3β4 | 0.6 ± 0.1 | .05 ± .01 | 12 |
| α1β1bεδ | 1.8 ± 0.21 | 0.6 ± .07 | 3.0 |
| α4β2 | 6.4 ± 1.5 | 0.29 ± 0.05 | 22 |
| α2β2 | 3.0 ± 0.7 | 3.5 ± 1.3 | 0.9 |
| α7 | 28 ± 11 | 13 ± 3 | 2.1 |
Based on the results obtained with single co-applications, our data (Figure 8, Table 3) might suggest that mecamylamine is not a selective antagonist of zebrafish CNS neuronal nAChR. Although it is more potent for the ganglion analog α3β4 compared to muscle-type receptors, the CNS subtypes tested appeared to be no more sensitive to mecamylamine than muscle receptors in these experiments. However, close inspection of the data revealed some important, albeit subtle, differences in mecamylamine's effects on the several receptor subtypes tested.
Figure 8.
Inhibition of zebrafish nAChR ACh-evoked responses by mecamylamine. Data were obtained by alternating applications of ACh at fixed control concentrations (see Methods) and ACh with increasing concentrations of mecamylamine. The data were normalized relative to the control responses to ACh applied alone. Data were calculated for both peak currents (A) and net charge (B). Each point is the average ± SEM of responses from at least four oocytes. As noted in the text, mecamylamine applications produced persistent inhibition of the ACh-evoked responses of the β2-containing receptors (see Figure 9), so separate sets of cells were used to evaluate the effects of mecamylamine at each of the higher concentrations for these subtypes.
Mecamylamine is a non-competitive antagonist and may produce use-dependent channel block, which may be either long-lived or rapidly reversible. Therefore, depending on the experimental conditions and the kinetics of both channel activation and drug inhibition, the net charge of responses evoked by co-applications of ACh and mecamylamine may be more strongly inhibited than the peak currents. With a simple co-application protocol, antagonist concentrations may still be rising while the peak synchronous channel activation is occurring. This is especially true with α7 receptors, since if ACh is co-applied at a high concentration, the peaks occur well before solution exchange is complete. Therefore, for measuring the mecamylamine antagonism of α7 we used 60 µM ACh as a control, a concentration that was approximately the EC70 for the net charge responses. Inhibition of peak currents may also be a poor measure of inhibitory action for heteromeric receptors since use-dependent inhibition requires that channels first activate before they can be inhibited [33]. The data presented in Figure 8 illustrate the concentration dependence of mecamylamine's effects on both the peak currents (8A) and net charge (8B) of the fish nAChR responses. As expected, in most cases the IC50 values for the inhibition of net charge were much lower than those for inhibition of peak currents (Table 3).
Figure 9 illustrates the qualitative differences in the effects of 3 µM mecamylamine co-application with ACh on all of the heteromeric receptor subtypes tested. As with mammalian receptors, we see that mecamylamine was most potent at inhibiting our ganglionic model, α3β4, which may account for undesirable but unavoidable side effects if the intended targets are α4-containing receptors in the brain. The α3β4 net charge measurements were 12-fold more sensitive to mecamylamine concentration than were the peak currents. Although α3β4 receptors were very effectively inhibited by 3 µM mecamylamine, it should be noted that inhibition was rapidly reversible, as illustrated in Figure 9.
Figure 9.
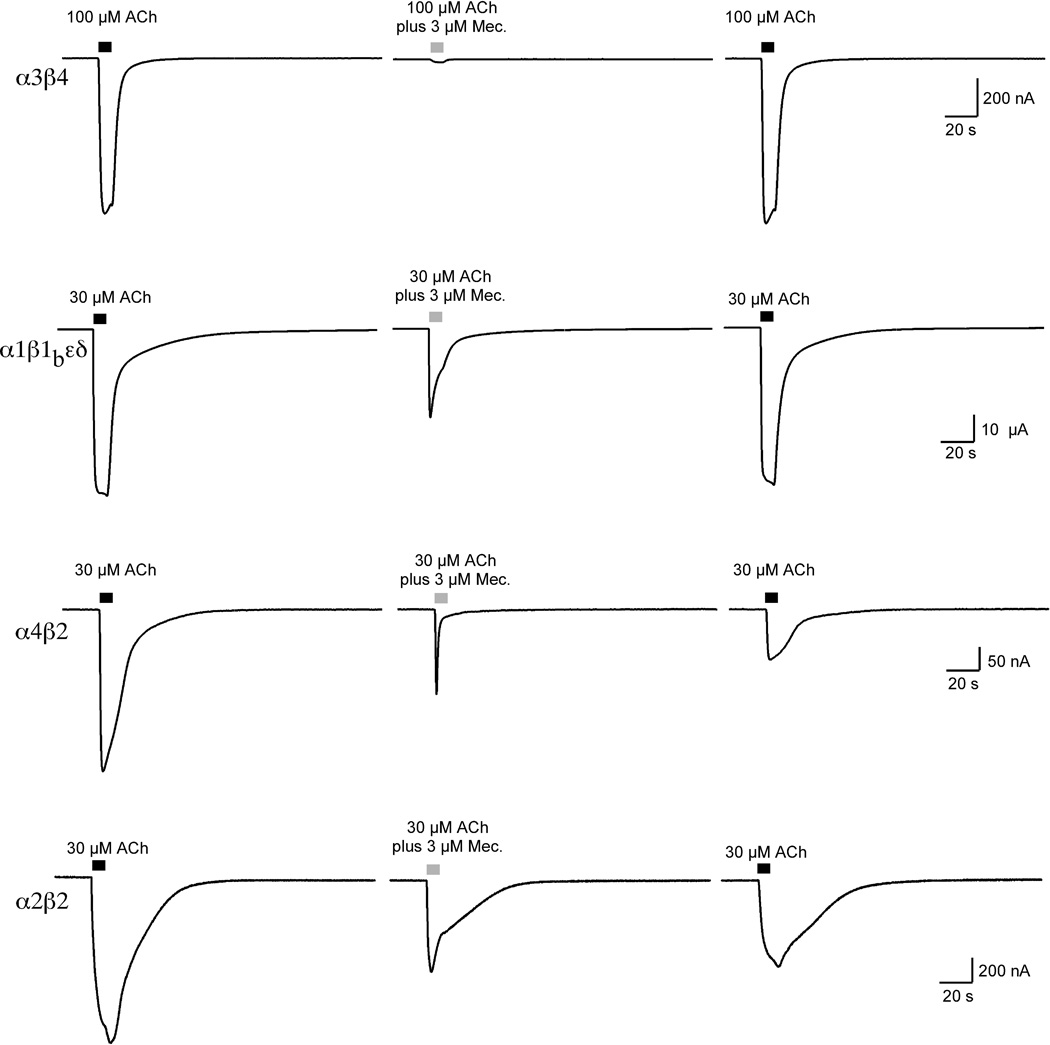
Representative traces illustrating qualitative differences in the mecamylamine inhibition of the zebrafish heteromeric nAChR and effects on subsequent ACh applications. The traces illustrate the effect of 3 µM mecamylamine co-application with ACh at the concentrations indicated. Note that for the α3β4 and muscle-type receptors there was good recovery of ACh control responses, but for α4β2 and α2β2 receptors there was only partial recovery of the control peak currents.
Mecamylamine also appeared to be a reasonably potent inhibitor of the fish muscle-type receptor, although the EC50 of approximately 2 µM is not too far different from what has been previously reported for mouse muscle receptors [27]. The IC50 for the inhibition of muscle receptor net charge was 3-fold lower than for peak current. As with α3β4 and α7 receptors (α7 data not shown), inhibition of the muscle-type receptors by mecamylamine was also readily reversible (Figure 7).
A remarkable exception to the pattern of increased potency for the inhibition of net charge was seen with α2β2 receptors, which showed no difference in potency for peak current and net charge inhibition (Table 3). These data were in most remarkable contrast to those for α4β2 receptors, for which mecamylamine was at least 10-fold more potent at inhibiting net charge than peak currents. On the basis of the net charge inhibition data alone, it might have been hypothesized that mecamylamine's block of α2β2 receptors is more rapidly reversible that the block of α4β2 receptors, so that the equilibrium inhibition of α2β2 receptors is achieved during the early phase of the co-application. However, an additional observation makes this mechanism unlikely. As shown in Figure 9, the ACh control responses of both α4β2 and α2β2 receptors failed to recover during the normal washout period. For both of these receptor subtypes, the mecamylamine inhibition studies had to be completed with separate sets of cells for each of the higher concentrations tested. Significant (>25%) residual inhibition was seen with α4β2 receptors at mecamylamine concentrations as low as 30 nM, and with α2β2 at concentrations ≥ 300 nM. Therefore it appears that mecamylamine can produce a persistent block of both of these β2-containing receptors, so that potency estimated with acute co-applications may underestimate the potency of block that would be obtained with a sustained presence of the drug in vivo. The difference between α4β2 and α2β2 in net charge inhibition may therefore relate to either a fundamental difference in the mechanism of inhibition or the activation kinetics of the receptors. Note that while mecamylamine inhibition of zebrafish α3β4 was readily reversible, it was previously reported for rat α3β4 that the co-application of 100 µM ACh with 3 µM mecamylamine produced 60% residual inhibition of ACh control responses following washout from the chamber [34].
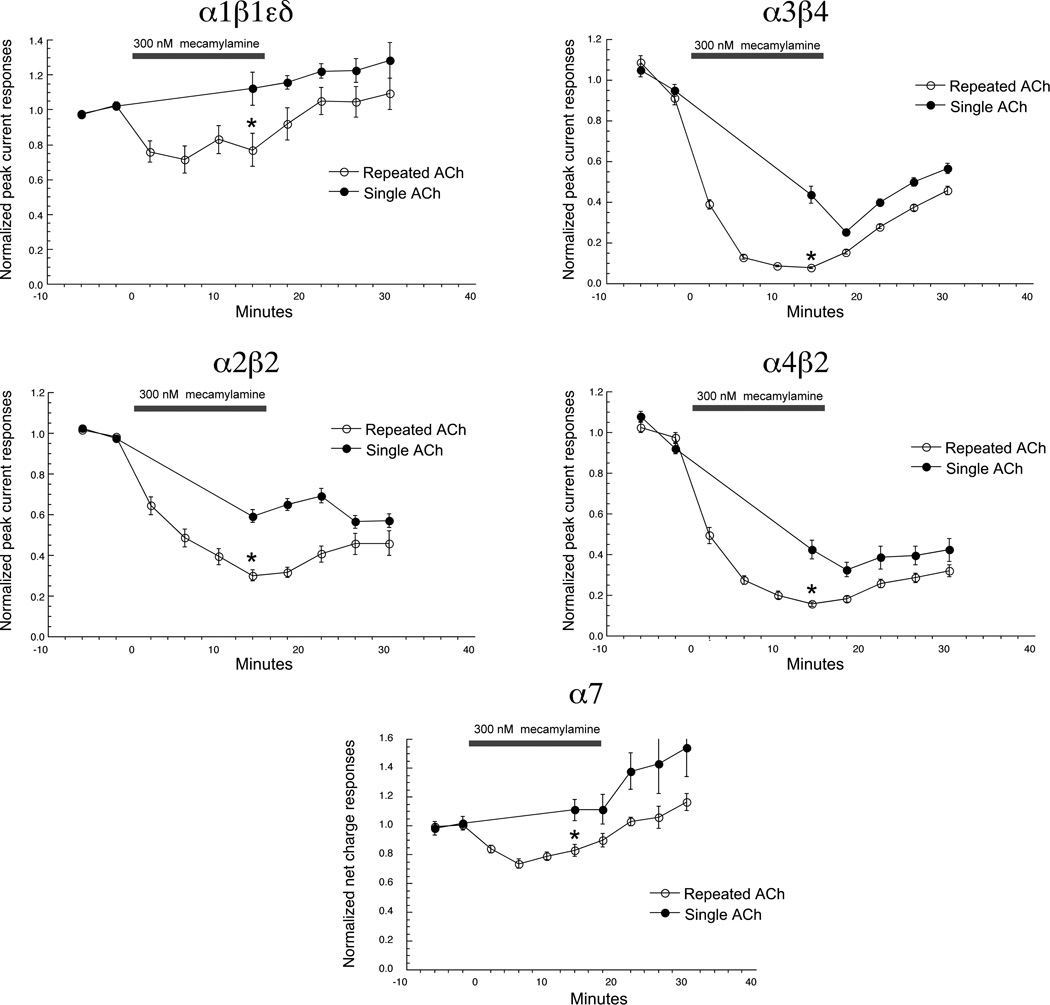
Although it is quite typical to characterize antagonists with simple co-application protocols, such an approach may underestimate the activity of the compound in an in vivo context, especially for compounds like mecamylamine, which may show significant use dependence. When used therapeutically or in whole animal experiments, compounds are likely to be present at low concentration for prolonged periods of time. Therefore, we used an alternative protocol to evaluate the selectivity and potential use-dependence of mecamylamine. After acquiring two control ACh-evoked responses from muscle-type and neuronal nAChR, the bath solution was switched to one containing 30 nM mecamylamine. To evaluate use-dependence, cells were either un-stimulated in the bath for 15 minutes and then given a single co-application of ACh and 300 nM mecamylamine, or given a series of four co-applications of ACh and 300 nM mecamylamine over the same period of time.
Using this protocol, the five receptor subtypes tested showed the same rank order sensitivity as in the acute co-application experiments (Table 3), α3β4 receptors being the most sensitive and α7 receptors the least (Figure 10). In all cases, the inhibition measured after 15 minutes was greater (p< 0.001) for the cells which received repeated stimulation, compared to cells receiving a single stimulation. Note that with this protocol the inhibition of the heteromeric receptor peak currents after repeated stimulations was relatively consistent with the inhibition of net charge measured in the acute co-application experiments (Table 3). Note also that for the α7-currents, the inhibitory effects of the low mecamylamine concentration were opposed by a tendency of the α7-mediated currents stimulated by repeated applications of the relatively low (60 µM) ACh concentration to run up over time, as previously reported for human α7 nAChR [35].
Figure 10.
Time and use dependence of mecamylamine inhibitory effects on zebrafish nAChR. Following the measurement of two control ACh control responses, cells expressing muscle-type or neuronal nAChR were switched to a bath solution containing 300 nM mecamylamine. Cells were then either stimulated with ACh and mecamylamine at 220s intervals (repeated ACh) or perfused with the mecamylamine buffer and given a single application of ACh and mecamylamine after 15 minutes. Cells were then switched back to control buffer and given 4 more stimulations with ACh alone. The data shown are peak current amplitudes for the heteromeric receptors and net charge for α7 nAChR. Each point is the average ± SEM of responses from at least four oocytes.
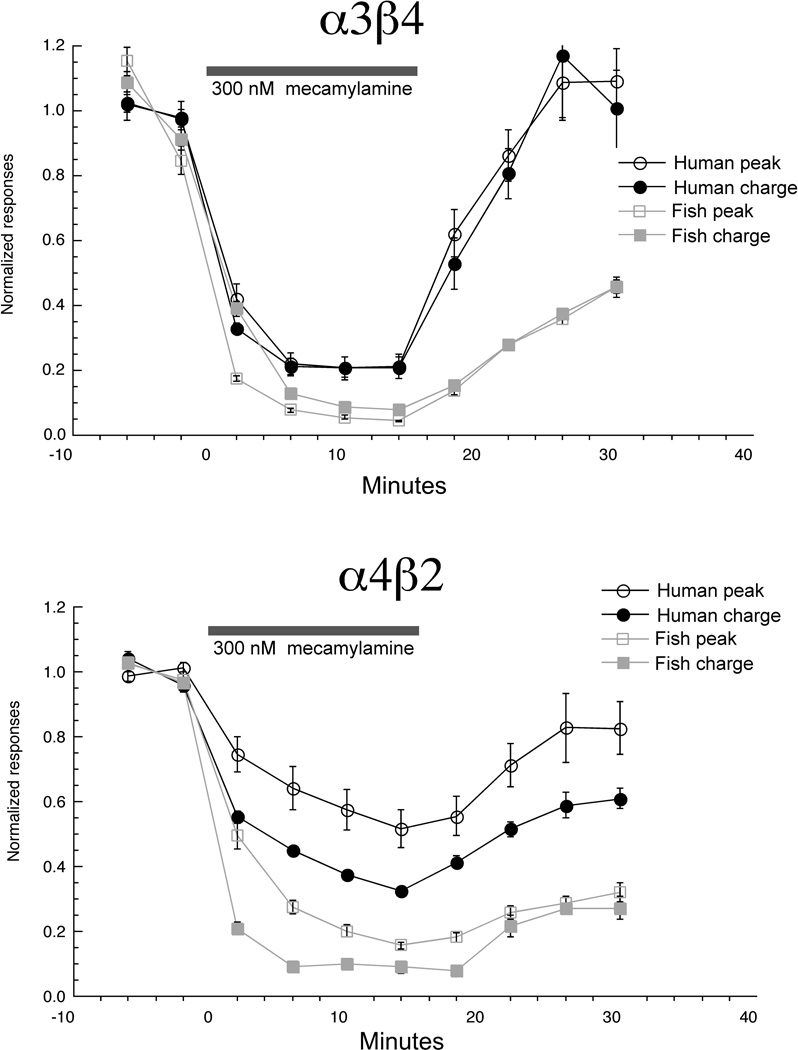
Seeking to further validate use of mecamylamine as a pharmacological tool in the zebrafish system, we made a direct comparison of mecamylamine's effects for human and fish neuronal nAChR, using the repeated application protocol. As shown in Figure 11, at all points during and after the series of ACh-mecamylamine co-applications, the inhibition of the fish receptor subtypes tested was significantly greater (p < 0.01) than for the human receptor subtypes.
Figure 11.
Mecamylamine inhibition of human and zebrafish neuronal receptors. The repeated stimulation with the concomitant bath application protocol shown in Figure 10 was used to measure the relative sensitivity of human and zebrafish α3β4 and α4β2 to a low concentration of mecamylamine. Data are plotted for both peak currents and net charge (the data for zebrafish peak currents are reproduced from Figure 10). Each point is the average ± SEM of responses from at least four oocytes.
4.0 Discussion
Our data indicate that key pharmacological tools used for characterization of nAChR in mammals will also be useful in the zebrafish model. Crucially, ACh, nicotine, cytisine and mecamylamine will be likely to work on the molecular level in zebrafish as they do in other animal model systems. However, our data also suggest that ligands shown to be selective for mammalian α7 nAChR may have broader ranges of activity in the zebrafish CNS than in mammals. Further consideration of zebrafish as model for nAChR targeting drugs will rely commonalities on the cellular and systems level for nAChR function.
Cholinergic cells are distributed in zebrafish expression in a pattern similar to that seen in other vertebrate animals, present in sites including autonomic ganglia, the telencephalon, cranial motor nuclei, spinal cord, olfactory bulb, retina, tegmentum, and cerebellum [36]. In addition, zebrafish express the same set of nAChR genes as occurs in other vertebrates. We have cloned nine zebrafish neuronal nAChR cDNAs (α2, α3, α4, α6, α7, α8, β2, β3, and β4) and shown that their sequences are very similar to those in other vertebrates [12, 13], this paper). We have also shown that numerous subunits (α2, α3, α4, α6, α7, β2, β3, and β4) are expressed early in zebrafish development, some within a few hours of fertilization. Assembled nAChRs are also present in zebrafish at least as early as 2 days post fertilization (dpf). Two high-affinity 3H-epibatidine binding sites have been detected in both 2 dpf and 5 dpf zebrafish, consistent with the presence of two nAChR subtypes [12], including a potential high affinity α4*-nAChR. RNA for α2 is expressed in spinal interneurons, forebrain, mid brain, and hindbrain regions in a transient pattern.β3 is expressed in retinal ganglion cells and α7 in hindbrain, although the characterization of α7 expression is limited. RNA for α6 is expressed in retina, forebrain, hindbrain, and in many catecholaminergic neurons analogous to those in mammals [13]. Interestingly, α4 is expressed in a pattern distinct from α6 and which also differs from that seen in other vertebrates, including limited forebrain expression and no expression in spinal cord or retina. It is possible that multiple α4 genes exist and that the expression pattern of only one has been examined [13].
Zebrafish have been used to determine the effects of nicotine and other cholinergic drugs on behaviors, and zebrafish are a promising new model and potential screening tool. Although zebrafish possess a limited telencephalon and no hippocampus, nicotine effects can be observed in several behavioral paradigms testing cognitive function, stress responses, and locomotor function. Low doses of nicotine (100 mg/l in the water) improved performance in a delayed spatial alternation task, while high doses impaired [8]. This inverted U pattern of the response is seen when nicotine is used in these paradigms in mammals as well. Mecamylamine blocked this nicotine-induced improvement in another spatial learning task when given 5 minutes before the task, but not if given 40 minutes prior to testing [7].
Nicotine was also anxiolytic at 100 mg/l in a fish model of anxiety, and this effect of nicotine was also blocked by 200 mg/l mecamylamine [6]. However, as in the spatial task, mecamylamine just before testing blocked the improvement produced by nicotine, but not when given 20 minutes prior. Nicotine also increases dihydroxyphenylacetic acid levels, and this increase is blocked by mecamylamine [37]. Thus, the effects of nicotine on learning and anxiety can be examined in zebrafish. Our current work on the pharmacology could possibly explain this timing of the effect of mecamylamine as being due to the actions on α2β2 versus α4β2, since our work indicates possible differences in activation kinetics or mechanisms of inhibition. Zebrafish express α4, α2, and β2 subunits and likely express both α2β2 and α4β2 nAChRs.
Our data largely support the use of both nicotine and mecamylamine as valid tools to target the nAChR subtypes in zebrafish that are homologous to their presumed targets in mammals. One caveat to this conclusion, however, is the uncertainty regarding the functional expression of the β1a subunit in zebrafish muscle. While our data suggest that most muscle-type receptors in the fish will contain β1b subunits, which we show are relatively resistant to inhibition by mecamylamine, the selectivity of mecamylamine for neuronal nAChR may not extend to neuromuscular junctions where β1a-containing receptors might be found. Two key residues, at the 6' and 10' positions of the pore-forming second transmembrane domain have been implicated in regulating the effectiveness of mecamylamine as a selective antagonist of mammalian neuronal nAChR [34]. Specifically, reciprocal mutations of these residues between neuronal beta subunits (β22 or β4) and the muscle β1 subunit reversed the pattern of mecamylamine selectivity [34]. As shown in Figure 1C, the fish β1a variant has sequence more similar to neuronal beta subunits at these sites and therefore would be predicted to be mecamylamine sensitive.
Another important area of nicotinic drug development is the refinement of partial agonist therapies for the treatment of nicotine dependence and more recently for depression [29, 38]. Cytisine and varenicline are lead compounds in these areas. They are believed to be potent but very weak partial agonists for α4β2 nAChR and to work primarily as time-averaged antagonists [35, 39]. People working on drug development accept that such agents will have side effect liabilities associated with their potential activation of α7 and ganglionic α3β4 receptors. Our data suggest that in zebrafish, while α7 may be associated with a similar spectrum of off-target effects, for cytisine, at least, there may be reduced off-target activity at ganglionic receptors.
The subunit selectivity of cytisine is of significant therapeutic importance since cytisine itself is being used for smoking cessation and it is the starting point for numerous drug development programs, including the one that brought varenicline to market. Selectivity between β2 and β4-containing receptors is key for cytisine structure-activity studies, and the data in the Figl et al. paper (1992) was thought to have identified the key epitope for cytisine selectivity. The purpose of the β4F106Y mutation was to confirm a hypothesis based on the Figl et al. (1992) paper. The fact that the hypothesis was not validated is therefore important since it calls into question the simple conclusion of the previous work and informs drug developers and modelers to look further into the receptor.
There is currently a great deal of interest in targeting α7 receptors for a variety of indications [40–42]. Our data suggest that drugs identified as selective agonists of mammalian α7 receptors may lack a certain amount of cross-validation in the zebrafish system. It is also interesting to note that pharmacological profiling of the putative α7-selective drugs indicated that cross-over effects might be more likely with α4β2 receptors than with α3β4 receptors, since for human receptors the activation profiles of α3β4 receptors were closer to α7 than were those of α4β2 receptors [32].
As the use of the zebrafish system is further developed for nicotinic drug design and testing, it will be necessary to validate additional pharmacological tools such as the antagonists methyllycaconitine (MLA) and dihydro-beta-erythroidine (DHβE). Previous work also showed that nicotine increases swimming activity in zebrafish, and this increase in activity can be blocked by either DHβE or MLA. DHβE and MLA also blocked the previously described anxiolytic effects of nicotine [43]. While DHβE and MLA are considered α4β2 and α7 nAChR antagonists, respectively, in other species, it is not clear that DHβE and MLA affect the same nAChRs subtypes in zebrafish as in other vertebrates, and further studies will be needed to clarify this point. Likewise, selective positive allosteric modulators of nAChR have been identified as another potentially important area for nicotinic drug development, and such drugs can now be evaluated with the cloned zebrafish receptors, potentially further expanding the usefulness of the system.
Ideally, in an experimental system the data obtained on the level of the whole organism will be congruent with data obtained on the molecular level. We show that in large measure, recent behavioral data with zebrafish can be considered consistent with the molecular pharmacology of zebrafish nAChRs. We also describe the tools and approaches that can be used to extend this work further into new areas of drug development.
5. Acknowledgements
We thank Shehd Abdullah Abbas Al Rubaiy, Sara Copeland, and Matthew Kimbrell for conducting OpusXpress experiments and Dr. Lynn Wecker (University of South Florida) for the use of an additional OpusXpress 6000. Zebrafish illustration in the graphic abstract is used by kind permission from Dries Knapen (http://www.egosumdaniel.se/illustrations/).
Supported by: James and Esther King Biomedical Research award 1KG12 and NIH GM57481 (RLP), and Intramural Research Program of the National Institutes of Health and National Institute on Alcohol Abuse and Alcoholism (FO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heinemann S, Boulter J, Deneris E, Conolly J, Duvoisin R, Papke R, et al. The brain nicotinic acetylcholine receptor gene family. Prog Brain Res. 1990;86:195–203. doi: 10.1016/s0079-6123(08)63177-5. [DOI] [PubMed] [Google Scholar]
- 2.Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Role L, Berg D. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- 4.Levin ED. Zebrafish assessment of cognitive improvement and anxiolysis: filling the gap between in vitro and rodent models for drug development. Rev Neurosci. 2011;22:75–84. doi: 10.1515/RNS.2011.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- 6.Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiol Behav. 2007;90:54–58. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Levin ED, Limpuangthip J, Rachakonda T, Peterson M. Timing of nicotine effects on learning in zebrafish. Psychopharmacology (Berl) 2006;184:547–552. doi: 10.1007/s00213-005-0162-9. [DOI] [PubMed] [Google Scholar]
- 8.Levin ED, Chen E. Nicotinic involvement in memory function in zebrafish. Neurotoxicol Teratol. 2004;26:731–735. doi: 10.1016/j.ntt.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 9.McKinley ET, Baranowski TC, Blavo DO, Cato C, Doan TN, Rubinstein AL. Neuroprotection of MPTP-induced toxicity in zebrafish dopaminergic neurons. Brain Res Mol Brain Res. 2005;141:128–137. doi: 10.1016/j.molbrainres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Langheinrich U. Zebrafish: a new model on the pharmaceutical catwalk. Bioessays. 2003;25:904–912. doi: 10.1002/bies.10326. [DOI] [PubMed] [Google Scholar]
- 11.Delvecchio C, Tiefenbach J, Krause HM. The zebrafish: a powerful platform for in vivo, HTS drug discovery. Assay Drug Dev Technol. 2011;9:354–361. doi: 10.1089/adt.2010.0346. [DOI] [PubMed] [Google Scholar]
- 12.Zirger JM, Beattie CE, McKay DB, Boyd RT. Cloning and expression of zebrafish neuronal nicotinic acetylcholine receptors. Gene Expr Patterns. 2003;3:747–754. doi: 10.1016/s1567-133x(03)00126-1. [DOI] [PubMed] [Google Scholar]
- 13.Ackerman KM, Nakkula R, Zirger JM, Beattie CE, Boyd RT. Cloning and spatiotemporal expression of zebrafish neuronal nicotinic acetylcholine receptor alpha 6 and alpha 4 subunit RNAs. Dev Dyn. 2009;238:980–992. doi: 10.1002/dvdy.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mongeon R, Walogorsky M, Urban J, Mandel G, Ono F, Brehm P. An acetylcholine receptor lacking both gamma and epsilon subunits mediates transmission in zebrafish slow muscle synapses. J Gen Physiol. 2011;138:353–366. doi: 10.1085/jgp.201110649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikenaga T, Urban JM, Gebhart N, Hatta K, Kawakami K, Ono F. Formation of the spinal network in zebrafish determined by domain-specific pax genes. J Comp Neurol. 2011;519:1562–1579. doi: 10.1002/cne.22585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epley KE, Urban JM, Ikenaga T, Ono F. A modified acetylcholine receptor delta-subunit enables a null mutant to survive beyond sexual maturation. J Neurosci. 2008;28:13223–13231. doi: 10.1523/JNEUROSCI.2814-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papke RL. Tricks of Perspective: Insights and limitations to the study of macroscopic currents for the analysis of nAChR activation and desensitization. Journal of Molecular Neuroscience. 2009;40:77–86. doi: 10.1007/s12031-009-9261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papke RL, Papke JKP. Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br J of Pharm. 2002;137:49–61. doi: 10.1038/sj.bjp.0704833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, Paern J, et al. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 2010;38:W695–W699. doi: 10.1093/nar/gkq313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 21.Meyer A, Van de Peer Y. From 2R to 3R: evidence for a fish-specific genome duplication (FSGD) Bioessays. 2005;27:937–945. doi: 10.1002/bies.20293. [DOI] [PubMed] [Google Scholar]
- 22.Colwill RM, Creton R. Imaging escape and avoidance behavior in zebrafish larvae. Rev Neurosci. 2011;22:63–73. doi: 10.1515/RNS.2011.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.David R, Ciuraszkiewicz A, Simeone X, Orr-Urtreger A, Papke RL, McIntosh JM, et al. Biochemical and functional properties of distinct nicotinic acetylcholine receptors in the superior cervical ganglion of mice with targeted deletions of nAChR subunit genes. Eur J Neurosci. 2010;31:978–993. doi: 10.1111/j.1460-9568.2010.07133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picciotto MR, Caldarone BJ, Brunzell DH, Zachariou V, Stevens TR, King SL. Neuronal nicotinic acetylcholine receptor subunit knockout mice: physiological and behavioral phenotypes and possible clinical implications. Pharmacol Ther. 2001;92:89–108. doi: 10.1016/s0163-7258(01)00161-9. [DOI] [PubMed] [Google Scholar]
- 25.Hoda JC, Wanischeck M, Bertrand D, Steinlein OK. Pleiotropic functional effects of the first epilepsy-associated mutation in the human CHRNA2 gene. FEBS Lett. 2009;583:1599–1604. doi: 10.1016/j.febslet.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Li P, Steinbach JH. The neuronal nicotinic alpha4beta2 receptor has a high maximal probability of being open. Br J Pharm. 2010;160:1906–1915. doi: 10.1111/j.1476-5381.2010.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papke RL, Wecker L, Stitzel JA. Activation and inhibition of mouse muscle and neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 2010;333:501–518. doi: 10.1124/jpet.109.164566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuryatov A, Onksen J, Lindstrom J. Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol Pharmacol. 2008;74:132–143. doi: 10.1124/mol.108.046789. [DOI] [PubMed] [Google Scholar]
- 29.Mineur YS, Eibl C, Young G, Kochevar C, Papke RL, Gundisch D, et al. Cytisine-based nicotinic partial agonists as novel antidepressant compounds. J Pharmacol Exp Ther. 2009;329:377–386. doi: 10.1124/jpet.108.149609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papke RL, Heinemann SF. The partial agonist properties of cytisine on neuronal nicotinic receptors containing the beta2 subunit. Mol Pharm. 1994;45:142–149. [PubMed] [Google Scholar]
- 31.Figl A, Cohen BN, Quick MW, Davidson N, Lester HA. Regions of beta4/beta2 subunit chimeras that contribute to the agonist selectivity of neuronal nicotinic receptors. FEBS Lett. 1992;308:245–248. doi: 10.1016/0014-5793(92)81284-s. [DOI] [PubMed] [Google Scholar]
- 32.Horenstein NA, Leonik FM, Papke RL. Multiple pharmacophores for the selective activation of nicotinic alpha7-type acetylcholine receptors. Mol Pharmacol. 2008;74:1496–1511. doi: 10.1124/mol.108.048892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francis MM, Choi KI, Horenstein BA, Papke RL. Sensitivity to voltage-independent inhibition determined by pore-lining region of ACh receptor. Biophys J. 1998;74:2306–2317. doi: 10.1016/S0006-3495(98)77940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webster JC, Francis MM, Porter JK, Robinson G, Stokes C, Horenstein B, et al. Antagonist activities of mecamylamine and nicotine show reciprocal dependence on beta subunit sequence in the second transmembrane domain. Br J Pharmacol. 1999;127:1337–1348. doi: 10.1038/sj.bjp.0702686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papke RL, Trocme-Thibierge C, Guendisch D, Abbas Al Rubaiy SA, Bloom SA. Electrophysiological perspectives on the therapeutic use of nicotinic acetylcholine receptor partial agonists. J Pharmacol Exp Ther. 2011;337:367–379. doi: 10.1124/jpet.110.177485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clemente D, Porteros A, Weruaga E, Alonso JR, Arenzana FJ, Aijon J, et al. Cholinergic elements in the zebrafish central nervous system: Histochemical and immunohistochemical analysis. J Comp Neurol. 2004;474:75–107. doi: 10.1002/cne.20111. [DOI] [PubMed] [Google Scholar]
- 37.Eddins D, Petro A, Williams P, Cerutti DT, Levin ED. Nicotine effects on learning in zebrafish: the role of dopaminergic systems. Psychopharmacology (Berl) 2009;202:103–109. doi: 10.1007/s00213-008-1287-4. [DOI] [PubMed] [Google Scholar]
- 38.Andreasen JT, Olsen GM, Wiborg O, Redrobe JP. Antidepressant-like effects of nicotinic acetylcholine receptor antagonists, but not agonists, in the mouse forced swim and mouse tail suspension tests. J Psychopharmacol. 2008 doi: 10.1177/0269881108091587. [DOI] [PubMed] [Google Scholar]
- 39.Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not "either/or": activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roncarati R, Scali C, Comery TA, Grauer SM, Aschmi S, Bothmann H, et al. Procognitive and neuroprotective activity of a novel alpha7 nicotinic acetylcholine receptor agonist for treatment of neurodegenerative and cognitive disorders. J Pharmacol Exp Ther. 2009;329:459–468. doi: 10.1124/jpet.108.150094. [DOI] [PubMed] [Google Scholar]
- 41.Leiser SC, Bowlby MR, Comery TA, Dunlop J. A cog in cognition: how the alpha7 nicotinic acetylcholine receptor is geared towards improving cognitive deficits. Pharmacol Ther. 2009;122:302–311. doi: 10.1016/j.pharmthera.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, Lin X, et al. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med. 2007;35:1139–1144. doi: 10.1097/01.CCM.0000259381.56526.96. [DOI] [PubMed] [Google Scholar]
- 43.Bencan Z, Levin ED. The role of alpha7 and alpha4beta2 nicotinic receptors in the nicotine-induced anxiolytic effect in zebrafish. Physiol Behav. 2008;95:408–412. doi: 10.1016/j.physbeh.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 45.Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, et al. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]