Abstract
The use of dendritic cell (DC) vaccines as treatment for malignancy is complicated by immune evasion tactics often employed by carcinomas such as head and neck squamous cell carcinoma (HNSCC). The present study aims to determine if an immune response can be elicited by administering a DC vaccine during the premalignant stages of HNSCC, prior to development of immune escape. Mice treated with the carcinogen 4-nitroquinoline 1-oxide (4NQO) in drinking water develop premalignant oral lesions that progress to HNSCC. As previous studies demonstrated that premalignant lesions and HNSCC overexpress common tumor antigens, bone marrow-derived DCs were pulsed with premalignant lesion lysate (DCpm) and administered to 4NQO-treated mice exhibiting premalignant lesions. Lesion progression was tracked through endoscopy, which revealed that DCpm vaccination and control vaccination with dendritic cells pulsed with normal tongue epithelium lysate (DCnt) significantly decreased lesion burden at 8 weeks. Analysis of lymph node cells revealed that while DCnt vaccination resulted in a rapid increase in total lymphocyte count, levels of activated conventional CD4+ T cells and Th1, Tc1, Th17, Tc17, and Th2 cells, DCpm vaccination results in a delayed, yet substantial, increase in these immune effector mechanisms. This suggests that dendritic cell vaccination may have a beneficial effect on clinical outcome regardless of type of antigenic stimulation. Also, pulsing DCs with premalignant lysate rather than normal tongue epithelium lysate affects the dendritic cells in a way that delays the immune effector response upon vaccination of premalignant lesion-bearing mice.
Keywords: HNSCC, premalignant, immunotherapy, dendritic cell vaccination
Introduction
Head and neck squamous cell carcinoma (HNSCC) is an aggressive malignancy that has had a 5-year survival rate of around 50% for over 30 years [1, 2]. Developing from oral keratinizing epithelial cells in a progressive fashion, both premalignant oral lesions and HNSCC have a high incidence of recurrence. This malignancy has also been consistently associated with extensive immune manipulation, including upregulation of both immunosuppressive regulatory T cells and inflammatory cell types [3–8]. The high incidence of recurrence and systemic immune manipulation are large contributors to the low survival rate of HNSCC [1, 2, 9].
Dendritic cell vaccination involves the exposure of dendritic cells to tumor-associated antigens (TAAs) through one of a variety of mechanisms. Primed dendritic cells are then matured and injected into the recipient with the intention of stimulating the recipient’s own T cells to specifically target tumor tissue [10]. In the case of HNSCC, however, the patient’s immune system at the point of initiation of immunotherapy may have been compromised to the point that it is not capable of mounting a sufficient immune response to vaccination. While established HNSCC has been shown to be associated with extensive immune manipulation, during the premalignant stages of progression, levels of activated T cells with a memory phenotype, including inflammatory Th1, Tc1 and Th17 cells increase [3]. As such, this time may be optimal for initiation of immunotherapeutic techniques, since the immune system is already stimulated.
The utilization of this technique during the premalignant stage of HNSCC depends on the presence of common antigens that are upregulated at both the premalignant and malignant stages compared to normal tissue. Our lab identified several common TAAs, including EGFR, RAGE, and MUC1, in mice with 4NQO carcinogen-induced premalignant oral lesions and HNSCC, as well as in patients [11, 12]. Previous work also revealed that in vitro sensitization of human PBML with autologous premalignant lesion lysate resulted in increased IFN-γ release from sensitized PBML upon subsequent challenge with autologous premalignant lesion or HNSCC lysate and increased cytolytic activity of sensitized PBML against challenge with premalignant lesion or HNSCC cells [11]. This provides the rationale for the use of premalignant tissue as the source of antigen to stimulate a protective immune response against the further development of premalignant lesions and HNSCC.
In the current study, mice treated with 4NQO until the development of premalignant oral lesions were vaccinated with dendritic cells pulsed with either premalignant lesion lysate or normal tongue epithelium lysate. Three separate vaccinations were administered: the first was given at the onset of premalignant lesions, the second was given 1 week after the first, and the final booster was given 7 weeks after the first (Fig S1). To determine immune response in vaccinated mice over time, mice were sacrificed at two timepoints: an early timepoint at 4 weeks after the first vaccination and a late timepoint at 8 weeks after the first vaccination (1 week after the final booster) (Fig S1). Lesion progression as well as immune response to vaccination was monitored. Somewhat surprisingly, both mice treated with the premalignant lesion-pulsed dendritic cell vaccine (DCpm) and, to a lesser extent, mice treated with the normal tongue epithelium lysate-pulsed dendritic cells (DCnt) had an improved clinical course compared to 4NQO-treated controls. DCnt mice had an early increase in cervical lymph node cellularity and levels of multiple immune effectors, while DCpm treated mice had a delayed increase in these same effectors.
Materials and Methods
Oral HNSCC carcinogenesis
Five mg/ml 4NQO was administered in the drinking water (diluted to 50 μg/ml) of 2 month old (at start) female C57BL/6 mice (Charles Rivers Laboratory) until development of premalignant oral lesions (6–8 weeks) or HNSCC (12–16 weeks). Control mice received propylene glycol diluent control. To monitor development of premalignant oral lesions and HNSCC, oral cavities of 4NQO-treated mice were endoscoped weekly using a Stryker 1.9mm × 30° scope and a Stryker 1088 HD camera. Mice were sedated with inhaled isoflurane (Piramal Healthcare) during the procedure.
Dendritic cell generation, pulsing, and maturation
Cells were collected from C57BL/6 mouse femoral bone marrow and cultured with 1000 U/ml GM-CSF (R&D Systems) to stimulate the development of dendritic cells. Dendritic cells were pulsed by 12 hours of incubation with 25 μg/ml lysate of premalignant lesion-bearing tissue from 4NQO-treated mice or normal tongue epithelial tissue from control mice. Tongue epithelium was obtained after incubation of tongue fragments with Dispase II (Roche) and lysed through sonication, after which protein concentration was determined by BCA protein assay (Pierce) per manufacturer’s instructions. Dendritic cells were then matured by 48 hrs of culture with 10 ng/ml GM-CSF and 0.1 μg/ml LPS (R&D Systems).
Vaccine regimen
Vaccination was by injection of 1×106 bone marrow-derived premalignant lesion lysate- or normal tongue epithelium lysate-pulsed dendritic cells in 50 μl serum-free medium into the ventral tongue of 4NQO-treated mice under inhaled isoflurane sedation (Piramal Healthcare). 4NQO-treated controls were injected with 50 μl of saline into the ventral tongue of 4NQO-treated mice. The first vaccination was given to mice once all 4NQO-treated mice were determined through oral cavity endoscopy to exhibit premalignant oral lesions. A second vaccination was administered 7 days after the first. Some mouse groups were sacrificed four weeks following the first vaccination. A third vaccination was administered 7 weeks after the first vaccination for the remaining mice, and these mice were sacrificed 7 days later. Tongues were harvested, sectioned, and stained with hematoxylin and eosin for histologic analysis.
Cervical lymph node processing
Cervical lymph nodes were harvested from mice and homogenized via a Stomacher 80 homogenizer (Seward) set on high for 90 sec. Cells were passed through a 40-μm cell strainer (BD Falcon, San Jose, CA) and rinsed with Hank’s Buffered Saline Solution (HBSS, Invitrogen) to remove debris. Cell number was determined by counting cells excluding trypan blue using a hemocytometer.
Flow cytometric analysis of surface markers and Foxp3 expression
All reagents in this section were from BD Bioscience unless otherwise stated. In order to detect levels of CD8 and CD4 positive cells in relation to Foxp3, ex vivo cervical lymph node cells in single-cell suspension were washed once in Stain Buffer and resuspended at 1×107 cells/ml. In order to detect expression of MHCII (I-Ab for C57/bl6 mice), CD80, and CD86 on CD11c positive dendritic cells, cultured matured dendritic cells in single-cell suspension were washed once in Stain Buffer and resuspended at 1×107 cells/ml. Nonspecific staining of total 1×106 cells was blocked with FBS and anti-CD16/32 monoclonal antibody prior to cell surface staining with the following antibodies: PerCP-Cy5.5 CD4, FITC CD8a, FITC CD11c, PE CD25, PE I-Ab, APC CD69, APC CD80, and PE-Cy7 CD86. Intracellular staining for PE-Cy7 Foxp3 was performed after fixation with Foxp3 Fixation Buffer and permeabilization with Foxp3 Permeabilization Buffer. Extent and frequency of positively stained cells was visualized using flow cytometry (FACSCanto).
Flow cytometric analysis of cytokine expression
All reagents in this section were from BD Bioscience unless otherwise stated. In order to detect intracellular cytokines, single-cell suspensions of cervical lymph node cells were restimulated for 4 hours at 37°C with 50 ng/ml phorbol 12-myristate 12-acetate (PMA), 1 μg/ml ionomycin, and brefelden A solution. Nonspecific staining of total of 1×106 cells was blocked with FBS and anti-CD16/32 monoclonal antibody prior to cell surface staining with the following antibodies: PerCP-Cy5.5 CD4 and PE-Cy7 CD8a. Intracellular staining for PE IL-17A, FITC IFN-γ, APC IL-4, APC IL-10 and Alexa Fluor 488 IL-13 (eBioscience) was performed after fixation/permeabilization with Cytofix/Cytoperm. Extent and frequency of positively stained cells was visualized using flow cytometry (FACSCanto).
Cytokine bead array
All reagents in this section were from BD Bioscience unless otherwise stated. In order to detect cytokines released into supernatant, single-cell suspensions of cervical lymph node cells or dendritic cells were restimulated for 4 hours at 37°C with 50 ng/ml PMA and 1 μg/ml ionomycin without addition of brefeldin A or cervical lymph node cells were activated for 3 days in the presence of anti-CD3/anti-CD28 beads. To determine degree of cytokine release in response to challenge, some lymph node cells were incubated for three days with 25μg/ml of normal tongue epithelium lysate, premalignant epithelium lysate or HNSCC epithelium lysate in the presence of anti-CD3/anti-CD28 beads. Presence/level of IFN-γ, IL-17A, IL-4, IL-10, and IL-13 in lymph node cell supernatant and IL-12, IL-6, TNF, IL-10, IL-1α and IL-1β in dendritic cell supernatant was determined using a mouse cytometric bead array Th1/Th2/Th17 cytokine kit and cytometric bead array flex sets for individual cytokines according to the manufacturer’s instructions. Relative amount of each cytokine was analyzed using FCAP Array software.
Statistical analysis
Data were reported using the mean as a measure of central tendency ± standard error of the mean. To compare one variable condition between groups, the 2-tailed Student’s t-test was used. To evaluate significant differences between lesion numbers for each mouse group, the Mann-Whitney U Test was used. To evaluate if any significant differences exist between histologic scores, a chi squared test was used. Significance was reported in the 95% confidence interval.
Results
DCpm vaccination and, to a lesser extent, DCnt vaccination result in an improved clinical response compared to 4NQO-treated controls
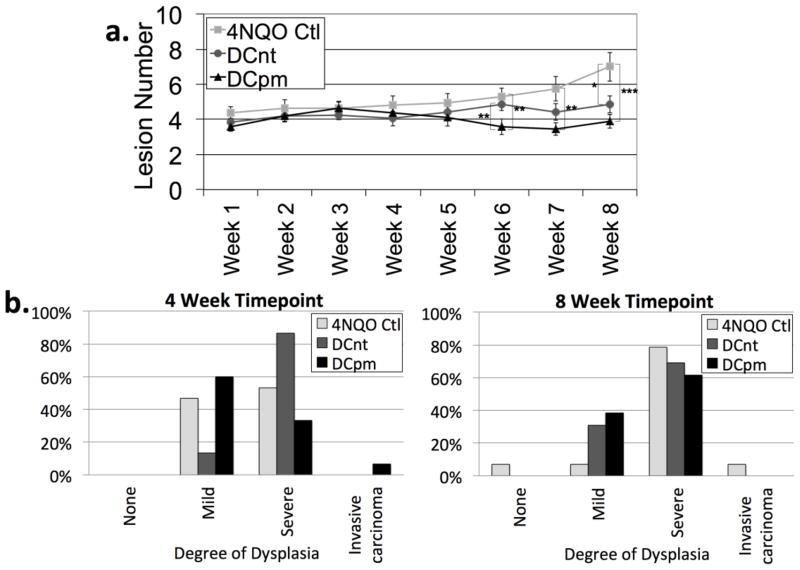
The ultimate goal of any interventional immunotherapeutic approach is an improvement in clinical status. To monitor development of lesions in 4NQO-treated mice, oral cavities were examined through weekly endoscopy and number of visible lesions was counted. Analysis of lesion number revealed that DCpm vaccination resulted in a significant decrease in the number of lesions compared to 4NQO control at 6, 7, and 8 weeks (Fig 1a). DCnt vaccination resulted in a significant decrease in number of lesions compared to 4NQO control at 8 weeks only (Fig 1a). Change in lesion size over time was also observed (data not shown), but data was highly inconsistent, as is somewhat expected in this model, making proper analysis problematic.
Fig 1. DCpm vaccination and DCnt vaccination result in an improved clinical response compared to 4NQO-treated controls.
Number of visible lesions (a) as determined by weekly endoscopy of oral cavities of 4NQO-treated mice administered dendritic cells pulsed with normal tongue epithelium lysate (DCnt), dendritic cells pulsed with premalignant lesion lysate (DCpm), or saline (4NQO Ctl) with at least 15 mice per groups. Data represent mean ± SEM. *, p < 0.05. **, p < 0.01. ***, p < 0.001 (Mann-Whitney U test). At 4 weeks and 8 weeks post-first vaccination, 4NQO Ctl, DCnt and DCpm mice were sacrificed and tongues were harvested, sectioned, and random sections were stained with hematoxylin and eosin for histopathologic analysis (b) with tongues from at least 11 mice per group. Data represent percentage, and statistical significance was determined by chi squared test. Four weeks post-vaccination: n = 15 mice per group; df = 6; χ2 = 10.12; p = 0.11. Eight weeks post-vaccination: n = at least 13 mice per group; df = 6; χ2 = 6.90; p = 0.33.
Mice were sacrificed at 4 and 8 weeks post-vaccination and tongues were harvested, processed, and random paraffin-embedded sections were analyzed for histologic score (normal, mild dysplasia, moderate-severe dysplasia, invasive carcinoma). Histologic analysis revealed a trend (p = 0.11) towards an increase in the overall degree of dysplasia at 4 weeks in mice administered the DCnt vaccine compared to 4NQO-treated controls. Similar to the results from gross analysis, histologic analysis also revealed a trend (p = 0.33) towards a decrease in the overall degree of dysplasia at 8 weeks in mice administered either the DCnt or DCpm vaccine compared to 4NQO-treated controls (Fig 1c). This indicates that DCpm vaccination may decrease the clinical lesion burden in mice treated with 4NQO, and, surprisingly, DCnt vaccination also appears to decrease clinical lesion burden, though at a later timepoint.
DCnt vaccination results in an early increase in lymph node cell count, while DCpm vaccination results in a delayed increase in lymph node cell count
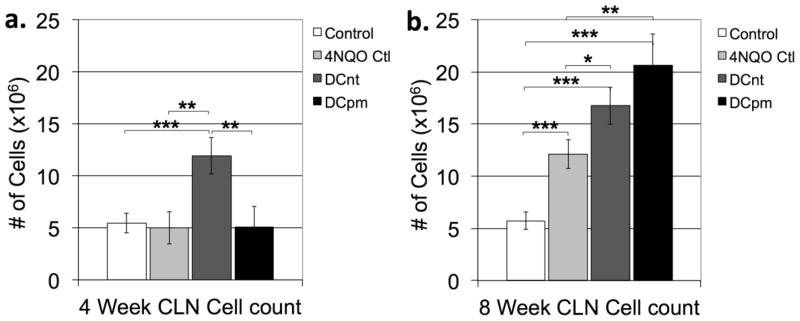
The development of malignancies is often associated with hyperplasia of draining lymph nodes, whether due to reactive hyperplasia, metastasis, or both [13]. To determine the degree of lymph node hyperplasia among the various mouse groups, cervical lymph nodes from control mice, 4NQO-treated control mice, 4NQO-treated DCnt mice, and 4NQO-treated DCpm mice were harvested at 4 and 8 weeks post-first vaccination, and the number of trypan blue-excluding cells were counted. At 4 weeks post-first vaccination (Fig 2a), DCnt vaccinated mouse lymph nodes contained significantly more cells than all other groups. At 8 weeks post-first vaccination (Fig 2b), however, DCpm- and DCnt-vaccinated mouse lymph nodes contained greater numbers of cells compared to control and 4NQO control mouse lymph nodes. Also at 8 weeks, 4NQO control mouse cervical lymph nodes contained significantly more cells than control mice (Fig 2b). This indicates that there is a more rapid response in lymph node cell proliferation after DCnt vaccination compared to DCpm vaccination. However, the proliferative response to the DCpm vaccine occurs to a great extent by the later timepoint.
Fig 2. DCnt vaccination results in an early increase in lymph node cellularity, while DCpm vaccination results in a delayed increase in lymph node cellularity.
At 4 weeks and 8 weeks post-first vaccination, control mice, 4NQO-treated control mice (4NQO Ctl), DCnt-vaccinated mice (DCnt) and DCpm-vaccinated mice (DCpm) were sacrificed and cervical lymph nodes were harvested and processed to single-cell suspensions, and trypan-blue excluding cells were counted with at least 5 mice per group. Data represent mean ± SEM of cell number at 4 weeks post-first vaccination (a) and 8 weeks post-first vaccination (b). *, p < 0.05. **, p < 0.01. ***, p < 0.001 (2-tailed Student’s t-test).
DCnt vaccination results in an early increase in levels of Tconv cells and CD4 Tregs, while both DCnt and DCpm vaccination result in a decreased percentage of CD4 Tconv cells and increased absolute number of CD8 Tconv cells and Tregs at 8 weeks compared to 4NQO-treated controls
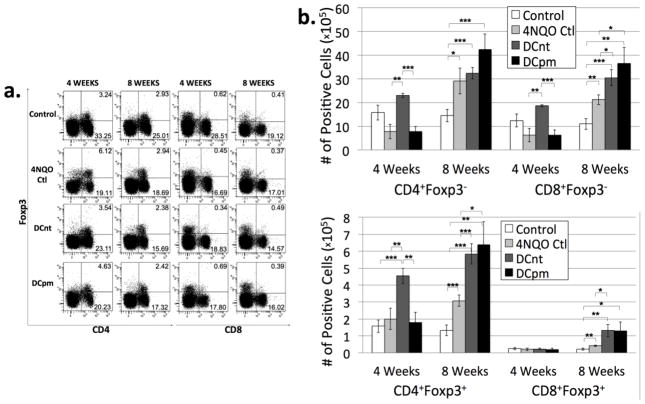
Many solid carcinomas have been shown to be associated with local and systemic immunosuppression [4–6]. This includes downregulation of stimulatory immune cells, such as CD4+ and CD8+ conventional T cells, and upregulation of suppressive immune cells. One suppressive cell population that is often upregulated as a part of this phenomenon is the regulatory T cell population, identified by expression of the transcription factor Foxp3 [4–6]. Our lab had previously shown that locoregional regulatory and conventional T cell numbers are both increased in mice with HNSCC [3]. To determine the relative composition of conventional and regulatory T cells in 4NQO-treated mice administered DC vaccination, lymph node cells collected at 4 and 8 weeks post-first vaccination were stained for surface expression of CD4 and CD8 and intracellular expression of Foxp3. Proportions of cells expressing each marker were determined through flow cytometric analysis (Fig 3a), and numbers of cells expressing each markers were determined by multiplying the percent positive by the number of lymph node cells for each individual mouse (Fig 3b). At 4 weeks post-first vaccination, DCnt mice exhibited an increase in absolute number of Tconv cells and CD4 Tregs compared to 4NQO-treated controls (Fig 3a and b). At 8 weeks post-first vaccination, analysis of cell numbers revealed an increase in the absolute number of CD8 Tconv cells and Treg cells in both DCnt and DCpm mouse lymph nodes compared to 4NQO controls (Fig 3b). This data indicates that DCnt vaccination may work early on to stimulate an increase in Tconv and Treg cells. While DCpm mice show no differences from 4NQO controls early on, DCpm vaccination appeared to affect a delayed increase in Tconv and Treg cells at 8 weeks after the initial vaccination.
Fig 3. DCnt vaccination results in an increase in levels of Tconv cells and CD4 Tregs at 4 weeks, while both DCnt and DCpm vaccination result in a decreased percentage of CD4 Tconv cells and increased absolute number of CD8 Tconv cells and Tregs at 8 weeks compared to 4NQO-treated controls.
Representative results (a) of flow cytometric staining of cervical lymph node cells from control, 4NQO-treated control (4NQO Ctl), DCnt-vaccinated (DCnt) and DCpm-vaccinated (DCpm) mice at 4 and 8 weeks post-first vaccination with at least 5 mice per group. Total numbers of populations (b) were determined by multiplying the percent positive by the number of cervical lymph node cells for each individual mouse. Data represent mean ± SEM. *, p < 0.05. **, p < 0.01. ***, p < 0.001 (2-tailed Student’s t-test). Results for absolute numbers of CD8+Foxp3+ cells at 4 weeks (Ctl: 2.55 × 104 ± 6.65 × 103; 4NQO Ctl: 2.06 × 104 ± 7.83 × 103; DCnt: 2.34 × 104 ± 3.08 × 103; DCpm: 2.00 × 104 ± 7.21 × 103).
DCnt vaccinated mice exhibit a decrease in percentage but increase in absolute number of activated CD4 Tconv cells 4 weeks, while both DCnt and DCpm vaccinated mice exhibit an increase in absolute number of activated CD8 Tconv cells at 8 weeks compared to 4NQO-treated controls
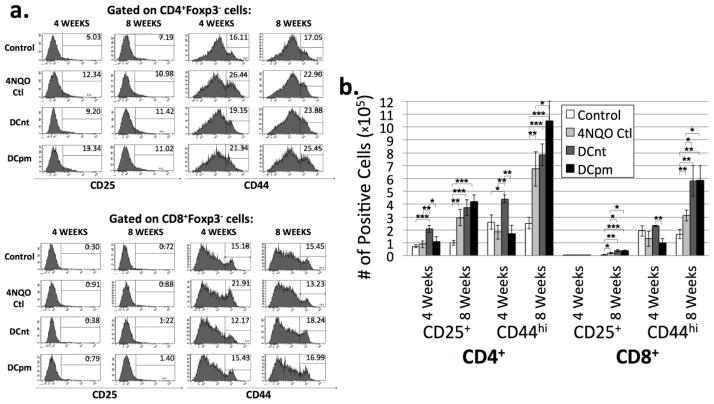
An optimal immune response to antigenic stimulation involves the activation of conventional T cells [14]. To determine the proportions of conventional T cells bearing markers for activation, CD4+Foxp3− and CD8+Foxp3− T lymph node cells were analyzed for expression of CD25 and CD44. While often used as a marker for regulatory T cells, CD25 expression on foxp3− conventional T cells is expressed within 2–24 hours after stimulation and persists for a few days after withdrawal of the stimulus. CD44 is a glycoprotein involved in cell adhesion and migration, and its increased expression is indicative of T cell activation and memory [15, 16]. Percentages of cells bearing CD25 and CD44 were determined by flow cytometric analysis (Fig 4a), and total numbers of cells bearing these markers were determined by multiplying percent positive by the number of lymph node cells for each individual mouse (Fig 4b). At 4 weeks post-first vaccination, DCnt vaccinated mouse lymph nodes were found to have an increased absolute number of CD25+ and CD44hi CD4 Tconv cells compared to 4NQO controls (Fig 4b). At 8 weeks post-first vaccination, both vaccinated groups see a significant increase in number of CD25+ and CD44hi CD8 Tconv cells compared to 4NQO controls, and DCpm-vaccinated mice also exhibit an increase in CD44hi CD4 Tconv cells compared to 4NQO controls (Fig 4b). This data indicates that both DCnt and DCpm vaccination tend to result in an increase in activated T cells compared to 4NQO treatment alone by 8 weeks post-first vaccination.
Fig 4. DCnt vaccinated mice exhibit a decrease in percentage but increase in absolute number of activated CD4 Tconv cells 4 weeks, while both DCnt and DCpm vaccinated mice exhibit an increase in absolute number of activated CD8 Tconv cells at 8 weeks compared to 4NQO-treated controls.
Representative results (a) of flow cytometric staining of cervical lymph node cells from control, 4NQO-treated control (4NQO Ctl), DCnt-vaccinated (DCnt) and DCpm-vaccinated (DCpm) mice at 4 and 8 weeks post-first vaccination with at least 5 mice per group. Total numbers of populations (b) were determined by multiplying the percent positive by the number of cervical lymph node cells for each individual mouse. Data represent mean ± SEM. *, p < 0.05. **, p < 0.01. ***, p < 0.001 (2-tailed Student’s t-test). Results for absolute number of CD8+Foxp3− CD25+ cells at 4 weeks (Ctl: 3.95 × 103 ± 1.15 × 103; 4NQO Ctl: 5.21 × 103 ± 1.97 × 103; DCnt: 6.01 × 103 ± 4.34 × 102; DCpm: 5.01 × 103 ± 1.93 × 103). Results for absolute number of CD8+Foxp3− CD25+ cells at 8 weeks (Ctl: 6.81 × 103 ± 1.74 × 103; 4NQO Ctl: 1.78 × 104 ± 4.54 × 103; DCnt: 3.78 × 104 ± 8.92 × 103; DCpm: 3.66 × 104 ± 6.05 × 103).
DCnt vaccination results in an early increase in Th1 and Tc1 levels, while both DCnt and DCpm vaccination result in an increase in Tc1 levels at 8 weeks compared to 4NQO-treated controls
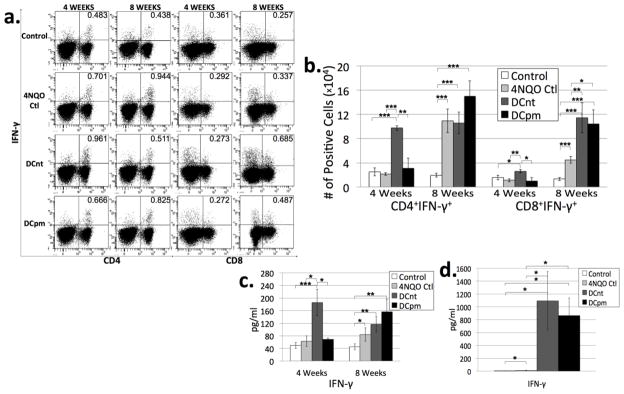
T cells can be further subdivided based on their expression of certain cytokines that dictate differing effector functions. Th1 cells (and a smaller subset of CD8+ cells known as Tc1 cells) secrete IFN-γ and are generally considered as inflammatory. They are traditionally thought of as beneficial for anti-tumor immunity [17]. However, recent research in our laboratory has shown that Th1 and Tc1 cells are upregulated in both the premalignant and malignant stages of HNSCC [3]. To evaluate the populations of Th1 and Tc1 cells in draining lymph nodes of control, 4NQO control, DCnt, and DCpm mice, lymph node cells were stained for surface expression of CD4 and CD8 and intracellular expression of the Th1 and Tc1 cytokine IFN-γ after 4 hours incubation with PMA, Ionomycin and brefeldin A. In addition, supernatants of lymph node cells incubated for 4 hours in PMA and Ionomycin were analyzed by cytometric bead array for levels of IFN-γ. At 4 weeks post-first vaccination, lymph nodes from DCnt vaccinated mice contain a greater absolute number of Th1 cells and Tc1 cells compared to all other groups (Fig 5b). This is supported by CBA data showing an increase in IFN-γ released into the supernatant of DCnt mouse lymph node cells compared to all other groups (Fig 5c).
Fig 5. DCnt vaccination results in an increase in Th1 and Tc1 levels at 4 weeks, while both DCnt and DCpm vaccination result in an increase in Tc1 levels at 8 weeks compared to 4NQO-treated controls.
Representative results (a) of flow cytometric staining of cervical lymph node cells from control, 4NQO-treated control (4NQO Ctl), DCnt-vaccinated (DCnt) and DCpm-vaccinated (DCpm) mice at 4 and 8 weeks post-first vaccination with at least 5 mice per group. Total numbers of populations (b) were determined by multiplying the percent positive by the number of cervical lymph node cells for each individual mouse. Data represent mean ± SEM. *, p < 0.05. **, p < 0.01. ***, p < 0.001 (2-tailed Student’s t-test). Cytometric bead array analysis (c) of supernatant of 4 week and 8 week control, 4NQO Ctl, DCnt, and DCpm mouse cervical lymph node cells after stimulation for four hours with PMA and ionomycin, with at least 5 mice per group. Data represent mean ± SEM. *, p < 0.05. **, p < 0.01. ***, p < 0.001 (2-tailed Student’s t-test). Cytometric bead array analysis (d) of supernatant of 8 week control, 4NQO Ctl, DCnt, and DCpm mouse cervical lymph node cells after 3 day stimulation with anti-CD3/anti-CD28 beads with at least 4 mice per group. Data represent mean ± SEM. *, p < 0.05 (2-tailed Student’s t-test). Results for pg/ml of IFN-γ released after 3 day stimulation (Ctl: 4.73 ± 1.31; 4NQO Ctl: 11.85 ± 2.53; DCnt: 1095.05 ± 452.47; DCpm: 864.98 ± 274.21).
At 8 weeks post-first vaccination, both DCnt and DCpm mouse lymph nodes were found to contain a greater absolute number of Tc1 cells compared to 4NQO controls (Fig 5b). Analysis of cytokines secreted in response to 4 hour stimulation with PMA and Ionomycin revealed no significant changes in the levels of IFN-γ in the supernatant of ex vivo lymph node cells from vaccinated mice compared to 4NQO controls (Fig 5c). To determine cytokine release in response to more extended stimulation, lymph node cells from 8 week post-first vaccination mice were cultured for 3 days in the presence of anti-CD3/anti-CD28. After the three day stimulation, cytometric bead array was used to measure levels of IFN-γ in the supernatant. Cytokine analysis revealed a huge increase in IFN-γ levels in the supernatant of DCpm and DCnt mouse lymph node cells compared to control and 4NQO control (Fig 5d). This data indicates that DCnt vaccination induces an early increase in Th1 and Tc1 cells, but by the later timepoint, both DCnt and DCpm vaccination induce a Tc1-type reaction.
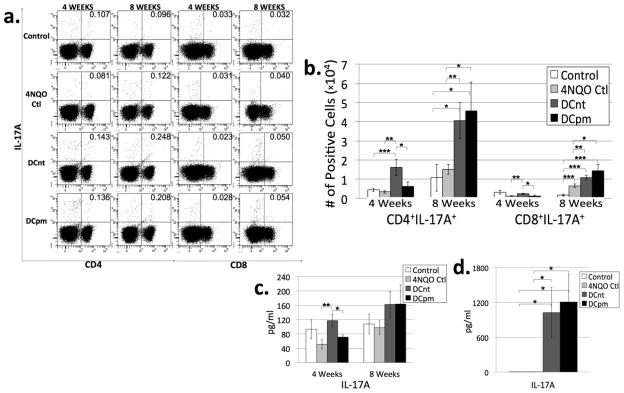
DCnt vaccination results in an early increase in absolute number of Th17 and Tc17 cells, while both DCnt and DCpm vaccination result in an increase in absolute number of Th17 and Tc17 cells at 8 weeks compared to 4NQO-treated controls
Th17 cells (and a smaller subset of CD8+ cells known as Tc17 cells) secrete IL-17A, among other cytokines, and induce a greater degree of inflammation compared to Th1 cells. Their role in antitumor immunity is less clear. They are highly proinflammatory and proangiogenic, qualities that generally promote tumorigenesis. They are also upregulated in many different tumor types; however, in some tumor types, like lung cancer and ovarian cancer, an increased presence of IL-17A and Th17 cells is actually associated with improved prognosis [18–21]. Recent data from our lab showed that Th17 cells are highly upregulated in mice bearing premalignant oral lesions, only to decrease in the draining lymph nodes of HNSCC-bearing mice [3]. To evaluate the populations of Th17 and Tc17 cells in draining lymph nodes of control, 4NQO control, DCnt, and DCpm mice, lymph node cells were stained for surface expression of CD4 and CD8 and intracellular expression of the Th17 and Tc17 cytokine IL-17a after 4 hours incubation with PMA, Ionomycin and brefeldin A. In addition, supernatants of lymph node cells incubated for 4 hours in PMA and Ionomycin were analyzed by cytometric bead array for levels of IL-17A. At 4 weeks post-first vaccination, DCnt mouse lymph nodes have an increased absolute number of Th17 and Tc17 cells compared to 4NQO controls, corresponding to an increased secretion of IL-17A from DCnt lymph node cells compared to 4NQO lymph node cells in response to 4 hour stimulation (Fig 6b and c).
Fig 6. DCnt vaccination results in an increase in absolute number of Th17 and Tc17 cells at 4 weeks, while both DCnt and DCpm vaccination result in an increase in absolute number of Th17 and Tc17 cells at 8 weeks compared to 4NQO-treated controls.
Representative results (a) of flow cytometric staining of cervical lymph node cells from control, 4NQO-treated control (4NQO Ctl), DCnt-vaccinated (DCnt) and DCpm-vaccinated (DCpm) mice at 4 and 8 weeks post-first vaccination with at least 5 mice per group. Total numbers of populations (b) were determined by multiplying the percent positive by the number of cervical lymph node cells for each individual mouse. Data represent mean ± SEM. *, p < 0.05. **, p < 0.01. ***, p < 0.001 (2-tailed Student’s t-test). Results for absolute number of CD8+IL-17A+ cells at 4 weeks (Ctl: 3.20 × 103 ± 9.96 × 102; 4NQO Ctl: 1.21 × 103 ± 1.75 × 102; DCnt: 2.38 × 103 ± 2.82 × 102; DCpm: 1.29 × 103 ± 4.90 × 102). Cytometric bead array analysis (c) of supernatant of 4 week and 8 week control, 4NQO Ctl, DCnt, and DCpm mouse cervical lymph node cells after stimulation for four hours with PMA and ionomycin, with at least 5 mice per group. Data represent mean ± SEM. *, p < 0.05. **, p < 0.01. ***, p < 0.001 (2-tailed Student’s t-test). Cytometric bead array analysis (d) of supernatant of 8 week control, 4NQO Ctl, DCnt, and DCpm mouse cervical lymph node cells after 3 day stimulation with anti-CD3/anti-CD28 beads with at least 4 mice per group. Data represent mean ± SEM. *, p < 0.05 (2-tailed Student’s t-test). Results for pg/ml of IL-17A released after 3 day stimulation (Ctl: 3.06 ± 1.06; 4NQO Ctl: 4.07 ± 1.38; DCnt: 1026.99 ± 430.89; DCpm: 1210.87 ± 471.49).
At 8 weeks post-first vaccination, both DCnt and DCpm mice have an increased absolute number of Th17 and Tc17 cells compared to control and 4NQO control (Fig 6b). While the supernatant of ex vivo lymph node cells shows no significant change in IL-17A levels in vaccinated mice compared to controls (Fig 6c), supernatant of DCnt and DCpm mouse lymph node cells stimulated for 3 days with anti-CD3/anti-CD28 contained much higher levels of IL-17A compared to controls (Fig 6d). This indicates that vaccination with DCnt results in an increase in Th17 and Tc17 cells early on, while vaccination with either DCnt or DCpm results in an increase in the Th17 and Tc17 response at 8 weeks post-vaccination.
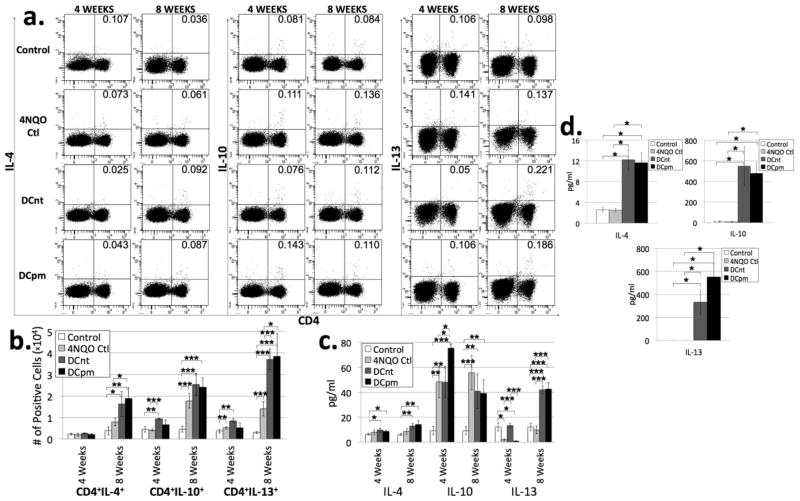
DCpm vaccination results in increased levels of IL-10 at 4 weeks, while both DCnt and DCpm vaccination results in increased levels of IL-13 at 8 weeks compared to 4NQO-treated controls
Th2 cells can have inflammatory or anti-inflammatory effects, and they can release multiple different cytokines, including IL-4, IL-10, and IL-13. These cells have been traditionally associated with a pro-tumorigenic phenotype. They are often upregulated in cancers, antagonizing a beneficial Th1 response [22–25]. However, our lab has shown that levels of Th2 cytokines remain largely unchanged in the draining lymph nodes of premalignant lesion-bearing and HNSCC-bearing mice compared to controls [3]. To evaluate the populations of Th2 cells in draining lymph nodes of control, 4NQO control, DCnt, and DCpm mice, lymph node cells were stained for surface expression of CD4 and intracellular expression of the Th2 cytokines IL-4, IL-10, and IL-13 after 4 hours incubation with PMA, Ionomycin and brefeldin A. In addition, supernatants of lymph node cells incubated for 4 hours in PMA and Ionomycin were analyzed by cytometric bead array for levels of IL-4, IL-10, and IL-13. At 4 weeks post-first vaccination, DCnt mouse lymph nodes were found to have an increased absolute number of IL-10 expressing CD4 T cells as well as an increased level of IL-13 in supernatant of ex vivo lymph node cells compared to 4NQO control (Fig 7b and c). Also at this timepoint, data shows an increase in IL-10 released into the supernatant of ex vivo DCpm mouse lymph node cells incubated for 4 hours with PMA and Ionomycin compared to all other groups (Fig 7c).
Fig 7. DCpm vaccination results in increased levels of IL-10 at 4 weeks, while both DCnt and DCpm vaccination results in increased levels of IL-13 at 8 weeks compared to 4NQO-treated controls.
Representative results (a) of flow cytometric staining of cervical lymph node cells from control, 4NQO-treated control (4NQO Ctl), DCnt-vaccinated (DCnt) and DCpm-vaccinated (DCpm) mice at 4 and 8 weeks post-first vaccination with at least 5 mice per group. Total numbers of populations (b) were determined by multiplying the percent positive by the number of cervical lymph node cells for each individual mouse. Data represent mean ± SEM. *, p < 0.05. **, p < 0.01. ***, p < 0.001 (2-tailed Student’s t-test). Cytometric bead array analysis (c) of supernatant of 4 week and 8 week control, 4NQO Ctl, DCnt, and DCpm mouse cervical lymph node cells after stimulation for four hours with PMA and ionomycin, with at least 5 mice per group. Data represent mean ± SEM. *, p < 0.05. **, p < 0.01. ***, p < 0.001 (2-tailed Student’s t-test). Cytometric bead array analysis (d) of supernatant of 8 week control, 4NQO Ctl, DCnt, and DCpm mouse cervical lymph node cells after 3 day stimulation with anti-CD3/anti-CD28 beads with at least 4 mice per group. Data represent mean ± SEM. *, p < 0.05 (2-tailed Student’s t-test). Results for pg/ml of IL-4 released after 3 day stimulation (Ctl: 2.59 ± 0.45; 4NQO Ctl: 2.50 ± 0.36; DCnt: 12.22 ± 2.04; DCpm: 11.67 ± 1.90). Results for pg/ml of IL-10 released after 3 day stimulation (Ctl: 11.52 ± 5.60; 4NQO Ctl: 10.13 ± 2.99; DCnt: 550.06 ± 191.82; DCpm: 479.50 ± 107.11). Results for pg/ml of IL-13 released after 3 day stimulation (Ctl: 0.00 ± 0.00; 4NQO Ctl: 0.00 ± 0.00; DCnt: 333.04 ± 106.89; DCpm: 550.70 ± 171.80).
At 8 weeks post-first vaccination, DCpm mouse lymph nodes were found to contain an increased number of IL-4-expressing CD4 T cells compared to 4NQO controls (Fig 7b). Both DCnt and DCpm mouse lymph nodes have an increased absolute number of IL-13-expressing CD4 T cells compared to 4NQO control (Fig 7b). Analysis of supernatant of ex vivo cells stimulated for 4 hours with PMA and Ionomycin revealed a significant increase in IL-13 in both vaccinated groups compared to 4NQO control and control (Fig 7c). Analysis of supernatant of cells stimulated for 3 days with anti-CD3/anti-CD28 revealed a large increase in IL-10, IL-13 and IL-4 in supernatant from DCnt and DCpm mouse lymph node cells compared to control and 4NQO control (Fig 7d). This data indicates that both DCnt and DCpm vaccination result in an increase in multiple Th2 cytokines, especially IL-13.
Discussion
The immune reaction to tumor development is a complicated and multifaceted phenomenon. While the immune system is capable of recognizing and attacking most dysplastic tissues, some dysplastic tissue is bound to avoid elimination and, given time and the selective pressure of the immune system, variants arise which have the capacity to escape recognition and/or manipulate the immune system in ways which further the progression of the malignancy [26]. This is the case with HNSCC, a cancer that is known to upregulate immunosuppressive cell populations such as Treg cells and promote smoldering inflammation through chronic upregulation of inflammatory cell types [3–8]. In this study, we sought to halt the progression of HNSCC at the premalignant stage through dendritic cell vaccination, with the rationale that premalignant and HNSCC tissue share common TAAs and the immune system at the premalignant stage has not yet been compromised by the advanced immune-manipulative tactics of an established tumor [3, 11, 12]. Unexpectedly, both DCpm vaccination and DCnt vaccination, though to a lesser extent, resulted in a decrease in lesion burden compared to 4NQO treatment alone. Also, evaluation of lymph node cells from DCpm vaccinated mice for response to a 3 day challenge with premalignant or HNSCC lysate showed that a specific response was not being generated (Fig S3). Analysis of the ex vivo immune response in vaccinated mice, as summarized in Table 1, revealed an early increase in draining lymph node cell number and levels of stimulatory immune effectors, including activated CD4 Tconv cells, CD8 Tconv cells, Th1, Tc1, Th17, and Tc17 cells, for DCnt mice, while the immune response in DCpm mice at 4 weeks mirrored that of 4NQO control mice. At the later timepoint, when both vaccinated groups appear to provide beneficial clinical effects, both DCnt and DCpm mouse lymph nodes have a greater number of cells, consisting of immunostimulatory cells such as activated CTLs, Tc1, Th17 and Tc17 cells, and, to a lesser extent, immunosuppressive Tregs and Th2 cells. These data indicate that DC vaccination, regardless of whether the DCs were pulsed with normal tongue or premalignant antigens, provides a non-specific but substantial immune stimulatory response and may be clinically beneficial.
Table I.
Summary of significant differences in absolute numbers of cell populations between the 4NQO control group and the DCnt and DCpm-vaccinated groups.
| 4 Weeks | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Absolute # (compared to 4NQO Control) | Tconv | Treg | Activated Tconv | Th1/Tc1 | Th17/Tc17 | Th2 | ||||
| CD4+ Foxp3− | CD8+ Foxp3− | CD4+ Foxp3+ | CD25+ CD4 Tconv | CD44hi CD4 Tconv | CD4+ IFN-γ+ | CD8+ IFN-γ+ | CD4+ IL-17A+ | CD8+ IL-17A+ | CD4+ IL-10+ | |
| DCnt | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| DCpm | ||||||||||
| 8 Weeks | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Absolute # (compared to 4NQO Control) | Tconv | Treg | Activated Tconv | Tc1 | Th17/Tc17 | Th2 | |||||
| CD8+ Foxp3− | CD4+ Foxp3+ | CD8+ Foxp3+ | CD25+ CD8 Tconv | CD44hi CD4 Tconv | CD44hi CD8 Tconv | CD8+ IFN-γ+ | CD4+ IL-17A+ | CD8+ IL-17A+ | CD4+ IL-4+ | CD4+ IL-13+ | |
| DCnt | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ||
| DCpm | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
This study presents the evaluation of immune response in the draining cervical lymph nodes of vaccinated and unvaccinated 4NQO-treated mice. This method of evaluation was used because the immune response to tumor is likely to be orchestrated in draining lymph nodes and because tongue epithelium was determined to contain very low numbers of T cells. However, immune response to treatment and to tumor development involves not only loco-regional responses, but also a myriad of changes in cellular localization of immune populations following activation. As such, it will be important to investigate immune populations present in tongue epithelium and in the periphery to fully determine the response to vaccination and tumor development.
Evaluation of dendritic cell maturation markers and cytokines revealed no differences between the dendritic cells pulsed with premalignant lesion lysate and the dendritic cells pulsed with normal tongue epithelium lysate (Fig S2). Investigation into response to premalignant lysate or HNSCC lysate challenge showed no indications of specificity of reaction of DCpm mouse lymph node cells or DCnt mouse lymph node cells (Fig S3). However, DCnt vaccination clearly elicited a more immediate response compared to DCpm vaccination, though this did not correlate with a stronger clinical response compared to DCpm mice. DCpm vaccination, on the other hand, resulted in very little in the way of an immediate reaction. At the later timepoint, however, both DCnt and DCpm vaccination resulted in increased immune stimulation and improved clinical response. Additional research into what delineates the different responses to these two vaccinations is necessary to determine why the DCpm vaccination promotes a delayed response compared to the immediate response to the DCnt vaccine.
Part of the rationale for using premalignant lesion lysate as the source of antigen to stimulate a specific in vivo response to premalignant and malignant tissue involved the over-expression of multiple common TAAs in both premalignant and HNSCC tissue [11, 12]. However, as determined in the current study, pulsing DCs with premalignant antigen did not result in the development of a specific response of lymph node cells from vaccinated mice to premalignant or HNSCC antigens, and both DCnt vaccination and DCpm vaccination resulted in a similar clinical response. To evaluate if the clinical response seen with DCnt and DCpm vaccination is a result of administering activated dendritic cells regardless of pulsing with lysate, another control group was vaccinated with unpulsed, activated DCs. Like both the DCnt and DCpm-vaccinated groups, there was no specific response of DC-vaccinated mouse lymph node cells to either premalignant or HNSCC challenge (data not shown). Also, while DC-vaccinated mice did have a clinically significant decrease in lesion burden, there was no difference in lesion burden between the DC-vaccinated and DCnt- or DCpm-vaccinated groups (data not shown). Therefore, it is likely that the clinical benefit seen is solely due to an increase in numbers of activated DCs. It is possible that in using tissue lysate as an antigen source, the extraneous components of the lysate prevented sufficient stimulation of cells with the intended premalignant and HNSCC tissue-associated TAAs. Future experimentation into vaccination of mice with specific TAA-pulsed DCs may lead to the development of an immunotherapy providing a specific immune response with improved clinical benefit.
HNSCC is an aggressive malignancy that has a complicated relationship with the immune system. The development of immunotherapeutic strategies to combat this cancer is highly important to increasing the poor survival rate of patients with HNSCC. While the dendritic cell vaccination described in this study reduced lesion burden by only a small amount, the immune system interactions described help to advance our understanding of the functions of various immune cell subtypes during the development of HNSCC. Further investigation into the intricacies of the tumor-immune system relationship will allow for more efficient development of treatments that have a better chance of being effective in the future.
Supplementary Material
Immature bone marrow-derived dendritic cells were pulsed with lysates of premalignant epithelium (DCpm vaccine) or normal tongue epithelium (DCnt vaccine), then matured with LPS. Phenotyping of dendritic cells by flow cytometric staining (a) and cytometric bead array (b) of at least 4 separate experiments reveal no significant differences between dendritic cell maturation markers or cytokines between the two groups (2-tailed Student’s t-test).
Cytometric bead array analysis of supernatant of 4 week control, 4NQO-treated control (4NQO Ctl), DCnt-vaccinated (DCnt) and DCpm-vaccinated (DCpm) mouse cervical lymph node cells after 3 day challenge with 25 μg/ml of normal tongue epithelium lysate (NT), premalignant epithelium lysate (PM), or HNSCC lysate (HNSCC) in the presence of anti-CD3/anti-CD28 beads with at least 4 mice per group. No significant differences in IL-2 release (a), IFN-γ release (b), IL-17A release (c), or IL-4 release (d) were found for the different challenges within each mouse group (2-tailed Student’s t-test).
Highlights.
A DC vaccine was administered during the premalignant stage of HNSCC.
DCpm and DCnt vaccination significantly decreased lesion burden at 8 weeks.
DCnt vaccination resulted in a rapid increase in stimulatory immune effectors.
DCpm vaccination resulted in a delayed increase in stimulatory immune effectors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345(26):1890–900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 2.Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52(4):195–215. doi: 10.3322/canjclin.52.4.195. [DOI] [PubMed] [Google Scholar]
- 3.De Costa AM, Schuyler CA, Walker DD, Young MR. Characterization of the evolution of immune phenotype during the development and progression of squamous cell carcinoma of the head and neck. Cancer Immunol Immunother. 2011 doi: 10.1007/s00262-011-1154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bose A, Chakraborty T, Chakraborty K, Pal S, Baral R. Dysregulation in immune functions is reflected in tumor cell cytotoxicity by peripheral blood mononuclear cells from head and neck squamous cell carcinoma patients. Cancer Immun. 2008;8:10. [PMC free article] [PubMed] [Google Scholar]
- 5.Bergmann C, Strauss L, Wang Y, Szczepanski MJ, Lang S, Johnson JT, et al. T regulatory type 1 cells in squamous cell carcinoma of the head and neck: mechanisms of suppression and expansion in advanced disease. Clin Cancer Res. 2008;14(12):3706–15. doi: 10.1158/1078-0432.CCR-07-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12(2):465–72. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 7.Young MR, Wright MA, Lozano Y, Prechel MM, Benefield J, Leonetti JP, et al. Increased recurrence and metastasis in patients whose primary head and neck squamous cell carcinomas secreted granulocyte-macrophage colony-stimulating factor and contained CD34+ natural suppressor cells. Int J Cancer. 1997;74(1):69–74. doi: 10.1002/(sici)1097-0215(19970220)74:1<69::aid-ijc12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 8.Schaefer C, Kim GG, Albers A, Hoermann K, Myers EN, Whiteside TL. Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer. 2005;92(5):913–20. doi: 10.1038/sj.bjc.6602407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauswald H, Simon C, Hecht S, Debus J, Lindel K. Long-term outcome and patterns of failure in patients with advanced head and neck cancer. Radiat Oncol. 2011;6(1):70. doi: 10.1186/1748-717X-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilett EJ, Prestwich RJ, Melcher AA. The evolving role of dendritic cells in cancer therapy. Expert Opin Biol Ther. 2010;10(3):369–79. doi: 10.1517/14712590903559830. [DOI] [PubMed] [Google Scholar]
- 11.Young MR, Neville BW, Chi AC, Lathers DM, Boyd Gillespie M, Day TA. Oral premalignant lesions induce immune reactivity to both premalignant oral lesions and head and neck squamous cell carcinoma. Cancer Immunol Immunother. 2007;56(7):1077–86. doi: 10.1007/s00262-006-0242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young MR. Use of carcinogen-induced premalignant oral lesions in a dendritic cell-based vaccine to stimulate immune reactivity against both premalignant oral lesions and oral cancer. J Immunother. 2008;31(2):148–56. doi: 10.1097/CJI.0b013e31815bdbf5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verastegui E, Morales R, Barrera JL, Mueller A, Guzman B, Meneses A, et al. Immunological approach in the evaluation of regional lymph nodes of patients with squamous cell carcinoma of the head and neck. Clin Immunol. 2002;102(1):37–47. doi: 10.1006/clim.2001.5130. [DOI] [PubMed] [Google Scholar]
- 14.Berinstein NL. Strategies to enhance the therapeutic activity of cancer vaccines: using melanoma as a model. Ann N Y Acad Sci. 2009;1174:107–17. doi: 10.1111/j.1749-6632.2009.04935.x. [DOI] [PubMed] [Google Scholar]
- 15.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–23. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 16.Pure E, Cuff CA. A crucial role for CD44 in inflammation. Trends Mol Med. 2001;7(5):213–21. doi: 10.1016/s1471-4914(01)01963-3. [DOI] [PubMed] [Google Scholar]
- 17.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 18.Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, et al. Th17 cells in cancer: help or hindrance? Carcinogenesis. 2011;32(5):643–9. doi: 10.1093/carcin/bgr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kesselring R, Thiel A, Pries R, Trenkle T, Wollenberg B. Human Th17 cells can be induced through head and neck cancer and have a functional impact on HNSCC development. Br J Cancer. 2010;103(8):1245–54. doi: 10.1038/sj.bjc.6605891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye ZJ, Zhou Q, Gu YY, Qin SM, Ma WL, Xin JB, et al. Generation and differentiation of IL-17-producing CD4+ T cells in malignant pleural effusion. J Immunol. 2010;185(10):6348–54. doi: 10.4049/jimmunol.1001728. [DOI] [PubMed] [Google Scholar]
- 21.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114(6):1141–9. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang M, Wang J, Lee P, Sharma S, Mao JT, Meissner H, et al. Human non-small cell lung cancer cells express a type 2 cytokine pattern. Cancer Res. 1995;55(17):3847–53. [PubMed] [Google Scholar]
- 23.Sato M, Goto S, Kaneko R, Ito M, Sato S, Takeuchi S. Impaired production of Th1 cytokines and increased frequency of Th2 subsets in PBMC from advanced cancer patients. Anticancer Res. 1998;18(5D):3951–5. [PubMed] [Google Scholar]
- 24.Shurin MR, Lu L, Kalinski P, Stewart-Akers AM, Lotze MT. Th1/Th2 balance in cancer, transplantation and pregnancy. Springer Semin Immunopathol. 1999;21(3):339–59. doi: 10.1007/BF00812261. [DOI] [PubMed] [Google Scholar]
- 25.Filella X, Alcover J, Zarco MA, Beardo P, Molina R, Ballesta AM. Analysis of type T1 and T2 cytokines in patients with prostate cancer. Prostate. 2000;44(4):271–4. doi: 10.1002/1097-0045(20000901)44:4<271::aid-pros2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immature bone marrow-derived dendritic cells were pulsed with lysates of premalignant epithelium (DCpm vaccine) or normal tongue epithelium (DCnt vaccine), then matured with LPS. Phenotyping of dendritic cells by flow cytometric staining (a) and cytometric bead array (b) of at least 4 separate experiments reveal no significant differences between dendritic cell maturation markers or cytokines between the two groups (2-tailed Student’s t-test).
Cytometric bead array analysis of supernatant of 4 week control, 4NQO-treated control (4NQO Ctl), DCnt-vaccinated (DCnt) and DCpm-vaccinated (DCpm) mouse cervical lymph node cells after 3 day challenge with 25 μg/ml of normal tongue epithelium lysate (NT), premalignant epithelium lysate (PM), or HNSCC lysate (HNSCC) in the presence of anti-CD3/anti-CD28 beads with at least 4 mice per group. No significant differences in IL-2 release (a), IFN-γ release (b), IL-17A release (c), or IL-4 release (d) were found for the different challenges within each mouse group (2-tailed Student’s t-test).