Abstract
Background
While olfactory deficits have been reported in schizophrenia and youths at-risk for psychosis, few studies have linked these deficits to current pathophysiological models of the illness. There is evidence that disrupted cyclic adenosine 3’,5’-monophosphate (cAMP) signaling may contribute to schizophrenia pathology. As cAMP mediates olfactory signal transduction, the degree to which this disruption could manifest in olfactory impairment was ascertained. Odor-detection thresholds to two odorants that differ in the degree to which they activate intracellular cAMP were assessed in clinical risk and low-risk participants.
Method
Birhinal assessments of odor-detection threshold sensitivity to lyral and citralva were acquired in youths experiencing prodromal symptoms (n = 17) and controls at low risk for developing psychosis (n = 15). Citralva and lyral are odorants that differ in cAMP activation; citralva is a strong cAMP activator and lyral is a weak cAMP activator.
Results
The overall group-by-odor interaction was statistically significant. At-risk youths showed significantly reduced odor detection thresholds for lyral, but showed intact detection thresholds for citralva. This odor-specific threshold deficit was uncorrelated with deficits in odor identification or discrimination, which were also present. ROC curve analysis revealed that olfactory performance correctly classified at-risk and low-risk youths with greater than 97% accuracy.
Conclusions
This study extends prior findings of an odor-specific hyposmia implicating cAMP-mediated signal transduction in schizophrenia and unaffected first-degree relatives to include youths at clinical risk for developing the disorder. These results suggest that dysregulation of cAMP signaling may be present during the psychosis prodrome.
Keywords: schizophrenia prodrome, ultra high risk, cAMP, adenosine cyclase, DISC1
1. Introduction
There is compelling evidence that schizophrenia patients exhibit structural and functional abnormalities of the olfactory system (for a review, see: Turetsky et al., 2009a). While this olfactory dysfunction has been well-characterized in schizophrenia, relatively little is known about olfactory performance in youths at risk for psychosis. A nascent, but growing, literature suggests that at-risk youths have similar olfactory performance deficits (Brewer et al., 2003; Kamath et al., in press; Keshavan et al., 2009; Woodberry et al., 2010), and these may have utility in predicting conversion to schizophrenia (Brewer et al., 2003; Kamath et al., in press; Keshavan et al., 2009; Woodberry et al., 2010).
There are few studies, however, that have attempted to link behavioral olfactory impairments in schizophrenia to disturbed cellular and molecular processes. Recent evidence suggests that disrupted cyclic adenosine 3’,5’-monophosphate (cAMP) signaling may contribute to schizophrenia pathology (Harrison & Weinberger, 2005; Thomson et al., 2005; Vacic et al., 2011). cAMP is an intracellular secondary messenger involved in key biological and cognitive processes, which is regulated by genetic and molecular mechanisms that have also been implicated in schizophrenia (Carlyle et al., 2011; Millar et al., 2005; Murdoch et al., 2007). It is notable that cAMP mediated signal transduction is the primary signaling pathway in peripheral olfactory receptor neurons (ORNs) (Lowe et al., 1989). When odor molecules bind to ORNs in the nasal epithelium, a G protein-signaling cascade activates adenylyl cyclase, leading to the formation of cAMP (Chesler et al., 2007; Doty et al., 1990; Sklar et al., 1986). Importantly, odorants vary in the degree to which they activate adenylyl cyclase and increase intracellular cAMP (Doty et al., 1990). Given that schizophrenia patients have marked olfactory deficits and cAMP is central to olfactory intracellular signal transduction, we recently investigated whether behavioral olfactory impairments in schizophrenia could denote cAMP abnormalities.
Odor detection threshold sensitivity for odorants that induced high vs. low intracellular cAMP responses (citralva and lyral) was assessed in schizophrenia patients, demographically matched unaffected family members and healthy controls (Turetsky & Moberg, 2009b). Though lyral and citralva are odorants with qualitatively similar floral scents, citralva induces high levels of adenylyl cyclase activity, while lyral has only a weak effect on adenylyl cyclase (Gomez et al., 2000a; Gomez et al., 2000b). We found that patients and unaffected relatives showed a selective deficit for lyral, but intact thresholds for citralva. These data suggest that odor detection deficits in schizophrenia are odor-specific rather than universal. A differential impairment based on relative activation of adenylyl cyclase supports the hypothesis that cAMP signaling is implicated in the pathophysiology of schizophrenia and, specifically, that perturbations in this intracellular signaling mechanism influence olfactory sensitivity deficits in patients.
In the current study, we extended this investigation to individuals at risk for psychosis, to determine if at-risk youths exhibit impairments consistent with cAMP signal transduction abnormalities prior to the onset of psychosis. We hypothesized that at-risk youths would exhibit threshold deficits for lyral but not citralva, similar to the impairment profile observed in schizophrenia patients and unaffected family members.
2. Method
2.1 Participants
Young adults and adolescents were recruited into one of two groups as follows: 1) Clinical Risk (CR) individuals who exhibited prodromal symptoms (n=17), but did not meet criteria for any DSM-IV axis I psychotic disorder, and 2) Low Risk (LR) comparison subjects who were symptom free (n=15). Participants were recruited from flyers, follow-up contact with previous research participants who consented to be contacted for additional studies, referrals from local schools and community mental health centers, the Behavioral Health Center at Children’s Hospital of Philadelphia (CHOP) as well as primary care physicians and inpatient units in the University of Pennsylvania Health System and pediatric practices of the Children’s Hospital of Philadelphia. Low-risk participants were recruited from local schools and colleges. Respondents were screened by telephone; for minor participants, screens were be conducted with a parent or guardian. Written informed consent was obtained from all participants prior to study participation. For subjects under the age of 18, both parental consent and child assent were obtained.
2.2 Exclusion Criteria
Individuals were excluded based on: 1) lack of proficiency in English, 2) history of a neurological disorder that could affect brain function, 3) history of loss of consciousness or head trauma, 4) current substance abuse, 5) positive urine drug screen, 6) presence of a medical condition affecting olfactory functioning (e.g., upper respiratory infection, obvious craniofacial trauma/abnormality), 7) history of mental retardation or pervasive development disorder, 8) standard score below 70 on the (Reading Subtest of the Wide Range Achievement Test – 3rd edition; Wilkinson, 1993), an estimate of verbal intellectual functioning. LR subjects were also excluded if they had a first-degree relative with psychotic illness.
2.3 Clinical Assessment
Participants were administered the Diagnostic Interview for Genetic Studies (DIGS; Nurnberger et al., 1994), the Structured Clinical Interview for DSM-IV-F (Anxiety) Module (SCID; First et al., 1996), and the Family Interview for Genetic Studies (FIGS; NIMH Genetics Initiative, 1992). Information was also gathered from medical records and collateral informants (family members and caregivers for participants under age 18). In addition, the Structured Interview for Prodromal Syndromes (SIPS; McGlashan et al., 2003) that includes the Scale of Prodromal Symptoms (SOPS; McGlashan et al., 2001; McGlashan et al., 2003; Miller et al., 1999) was administered to characterize current prodromal symptomatology. The SIPS is now the established method for assessing prodromal symptomatology and its psychometric properties are well-documented (Miller et al., 2003). Individuals in the CR cohort were considered clinical risk if they exhibited at least one positive symptom rated 3–5 in severity or at least two negative and/or disorganized symptoms (rated 3–6 in severity) on the SOPS. Participants were additionally assessed for a DSM-IV axis I or axis II cluster A disorder (First et al., 1997). Prodromal symptomatology in the CR cohort was as follows: 11 CR subjects had a mixture of positive, negative and disorganized symptoms, 4 exhibited solely positive symptoms, and 2 had solely disorganized symptoms. Three CR subjects were also first-degree relatives of schizophrenia patients (1 sibling and 2 offspring from 3 families). Consensus best estimate diagnoses were assigned based on comprehensive case review by at least two doctoral level clinicians (MEC, KBW, CGK, REG).
2.4 Odor Detection Threshold Assessment
Each subject was administered two odor detection threshold tasks, with lyral or citralva as the active odorant. Presentation order was counterbalanced across subjects. Trained technicians administered the tasks birhinally in a single reversing staircase, forced-choice format. Two vials, one containing mineral oil and one containing the active odorant diluted in mineral oil, were presented sequentially and the subject was asked to identify the vial that “smells stronger.” Concentrations of both odorants ranged from 10−1 M (strongest) to 10−9 M (weakest) in 0.5 log step dilution increments. The test began at the 10−5 M step, and odor concentration was increased in full-molar steps until correct detection occurred on five consecutive trials at a given concentration. Odor concentration was then increased or decreased in half-molar increments, depending upon performance on two trials at each concentration step (i.e., odor concentration was decreased after two correct trials and increased after an incorrect trial). The geometric mean of the last four staircase reversal points (out of seven total reversals) was taken as the estimate of odor detection threshold sensitivity (i.e., the weakest odor concentration that could be reliably identified).
2.5 Odor Identification and Discrimination Assessment
Participants were also administered the Sniffin’ Sticks Odor Identification and Discrimination Test (Hummel et al., 1997; Kobal et al., 1996). In the odor identification task, subjects were asked to identify 16 different odors in a four-alternative multiple choice format. In the 16-trial odor discrimination test subjects were asked to indicate, on each trial, which one of three odors differed from the other two. The score for each task was the number correct out of a possible 16. Scores were unavailable for one CR subject. Results from these tests have been presented previously for a subset of the subjects (Kamath et al., in press), and are included here for the ROC analysis.
2.6 Data analysis
Statistical analyses were conducted using STATISTICA™ 9.0 (StatSoft® Inc., Tulsa Oklahoma). Group differences in age, smoking, education, and parental education were assessed using analysis of variance (ANOVA). Pearson chi-square was used to examine group sex differences. Multivariate analysis of variance (MANOVA) was conducted to assess group differences in odor detection threshold sensitivity, with odorant (lyral vs. citralva) as the repeated measure factors. Post-hoc analyses of significant effects examined pair-wise group contrasts on individual measures. A similar MANOVA was conducted for odor identification and discrimination test scores. Relationships among olfactory measures were examined via Pearson correlation coefficients; relationships between olfactory scores and prodromal symptom indices were examined with Spearman correlations.
A ROC curve analysis was conducted to determine the ability of these olfactory measures to distinguish the CR and LR groups. In an ROC curve, the true positive rate (sensitivity) is plotted against the false positive rate (1 – specificity) for each possible sensitivity/specificity pair (Zweig & Campbell, 1993). Prediction accuracy of a parameter increases as the area under the curve (AUC) approaches 1.0 and decreases as the AUC approaches 0.5. A parameter is considered statistically significant when the AUC confidence interval exceeds 0.5, indicating better-than-chance prediction of diagnostic category. Analyses were performed using MedCalc® 11.6.10 (Mariakerke, Belgium). Separate ROC analyses were conducted for each of the four olfactory tests (lyral threshold, citralva threshold, odor discrimination, odor identification), as well as an omnibus measure computed as a weighted average of the individual olfactory test scores. The weighting for each test was the parameter estimate derived from a logistic regression with the four test scores as predictors and group (LR vs. CR) as the dependent variable (Pepe et al., 2006).
3. Results
3.1 Demographic Analysis
CR and LR subjects differed with respect to age (F1,30=5.48, p=.03) and educational attainment (F1,29=8.44, p=.01), but not parental education (Wilks’ F2,24=1.44, p=.26) or sex (χ2=3.14, p=.08). Both groups had similar smoking habits as quantified by packs per day (F1,28=0.84, p=.37). Demographic and clinical information are presented in Table 1.
Table 1.
Demographic/Clinical Characteristics and Raw Odor Detection Thresholds
| Clinical Risk | Low Risk | |
|---|---|---|
| (n = 17) | (n = 15) | |
| Mean ± SD | Mean ± SD | |
| Age (years) | 18.59 ± 3.47 | 21.27 ± 2.94 |
| Sex (Male:Female) | 11:6 | 5:10 |
| Education level (years) | 11.38 ± 2.83 | 14.40 ± 2.97 |
| Pack-days | 0.11 ± 0.20 | 0.05 ± 0.14 |
| SOPS1 Total Score | 30.94 ± 12.81 | 2.87 ± 3.56 |
| Odor Detection Thresholds2 | ||
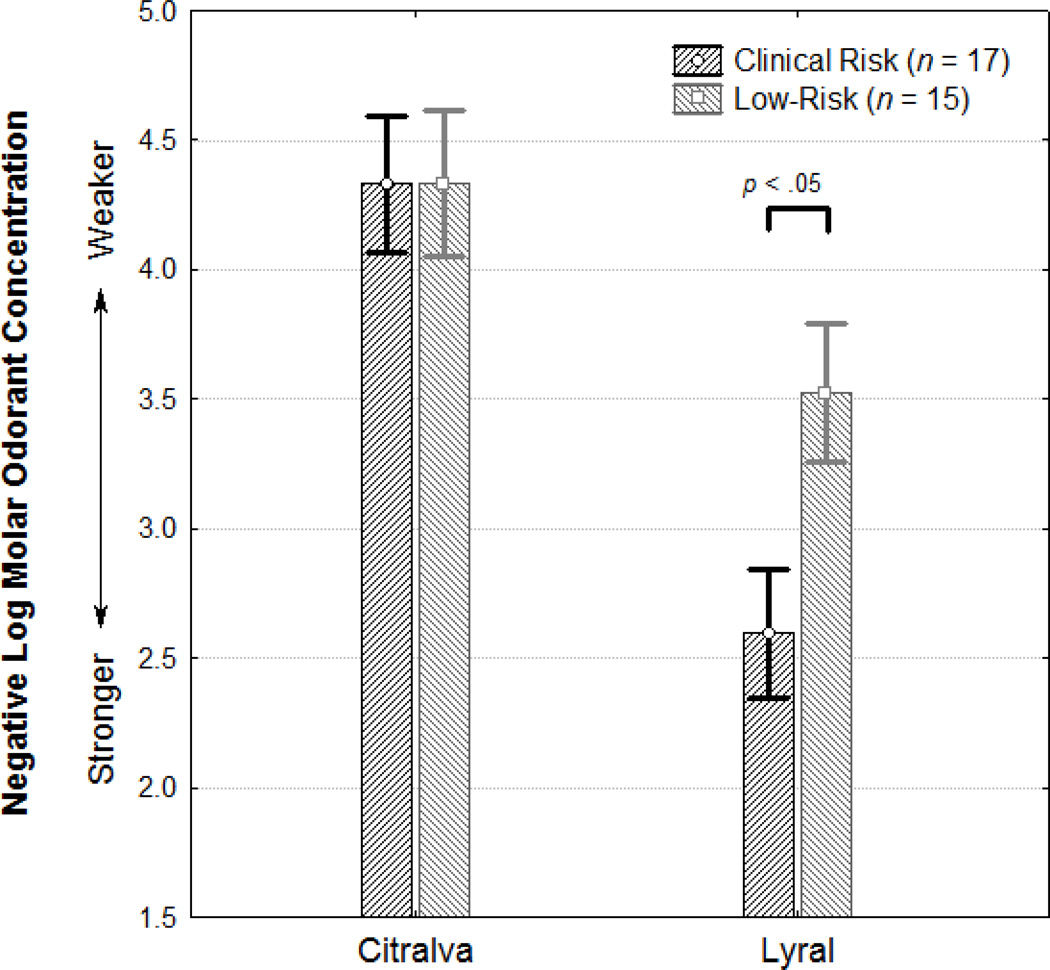
| Lyral | 2.60 ± 1.13 | 3.53 ± 0.91 |
| Citralva | 4.33 ± 1.40 | 4.33 ± 0.83 |
| Odor Identification3 | 11.06 ± 1.53 | 13.40 ± 1.24 |
| Odor Discrimination3 | 10.19 ± 2.32 | 12.07 ± 1.39 |
SOPS: Scale of Prodromal Symptoms (McGlashan et al., 2001; McGlashan et al., 2003; Miller et al., 1999)
Negative log molar concentration
Data available for n = 16 Clinical Risk subjects
3.2 Odor Detection Threshold Performance
There was a statistically significant interaction of group × odorant (F1,30=4.05, p=.05) (Figure 1). In paired contrasts, CR subjects were significantly worse than LR subjects at detecting lyral (F1,30=6.42, p=.02, Cohen’s d=0.90), but not citralva (F1,30>0.01, p>.99, Cohen’s d<0.01). Inclusion of age and sex as covariates did not alter these observed effects, although age emerged as a significant independent predictor of performance (age: F1,29=4.71, p=.04, sex: F1,29=0.04, p=.84). Exclusion of the 3 CR subjects who had a schizophrenia family member did not alter the results (group × odorant: F1,27=7.99, p<.01).
Figure 1. Mean odor threshold detection sensitivities to citralva and lyral in youth at clinical and low risk for psychosis.
Higher y-axis values indicate better odor detection threshold sensitivity.
3.3 Odor Identification and Discrimination Performance
There was a significant group difference (F1,29=20.09, p<.001), with CR subjects performing worse than LR subjects (see Table 1). There was also a significant test effect (F1,29=8.48, p<.01); scores on odor discrimination were lower, overall, than odor identification scores. However, there was no group × test interaction (F1,29=0.37, p=.55) and the groups differed significantly on each of the individual tests (identification: F1,29=21.70, p<.001; discrimination: F1,29=7.38, p=.01). Covarying for age and sex did not alter these results; this replicates our previous findings in a smaller sample (Kamath et al., in press).
3.4 Relationships Among Olfactory Performance Measures
We considered whether the measures on which CR subjects were impaired (lyral detection threshold, odor discrimination, odor identification) were correlated, suggesting disruption of a common underlying olfactory substrate - or whether they were relatively independent, suggesting multiple discrete abnormalities. There was a significant correlation, across groups, between odor identification and odor discrimination (r =.41, p=.02). However, these measures were not significantly correlated with either lyral detection threshold (identification: r = −.30, p=.11; discrimination: r = −.01; p=.98) or citralva detection threshold (identification: r = −.06, p=.73; discrimination: r = .18; p=.33). There was also no relationship between the detection thresholds for lyral and citralva (r = .27; p=.14). Examination of just the CR group revealed a similar pattern, though with lower significance reflecting the smaller sample size (identification vs. discrimination: r =.47, p=.07; lyral threshold vs. identification: r =.15, p=.57; lyral threshold vs. discrimination: r =.22, p=.42; lyral threshold vs. citralva threshold: r =.31, p=.25; citralva threshold vs. identification: r =−.26, p=.33; citralva threshold vs. discrimination: r =.35, p=.18). This suggests that odor identification and discrimination abnormalities may share a common underlying olfactory neuropathology, but abnormal lyral threshold detection may denote a separate pathological substrate. Moreover, the absence of any association between the threshold detections for lyral and citralva indicates that this is not just a generic dissociation of odor detection sensitivity from other higher-order olfactory domains.
3.5 Sensitivity and Specificity of Olfactory Measures
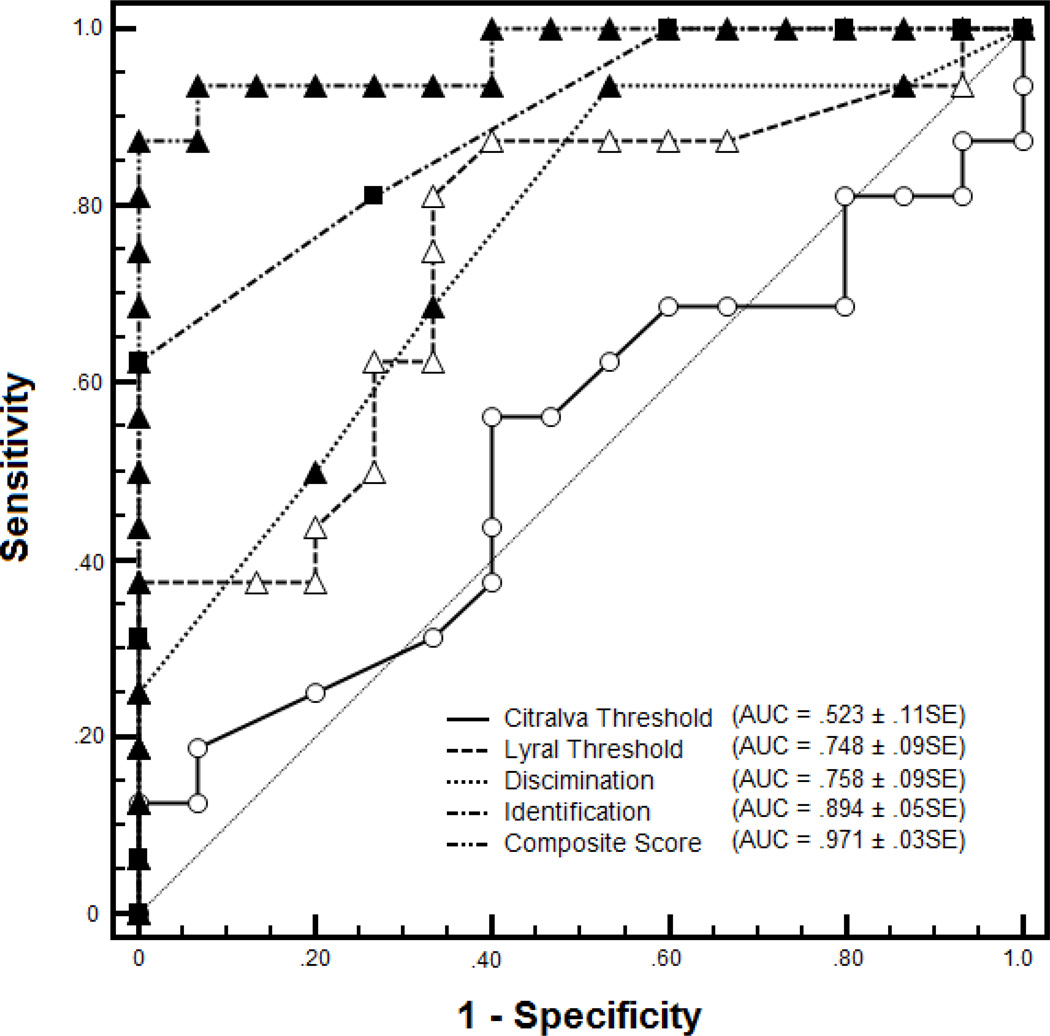
The ROC curves for each of the individual test scores and the omnibus measure are presented in Figure 2. Consistent with the findings above, AUC exceeded 0.5 (indicating better-than-chance predictive capacity) for each individual test except citralva threshold detection. AUC was 0.748±0.092 SE for lyral threshold, 0.894±0.052 SE for odor identification, and 0.758±.087 SE for odor discrimination. These values indicate the proportion of subjects correctly classified as CR or LR, based solely on performance on each individual test. Consistent with the idea that these are not redundant tests, but rather reflect relatively independent areas of impairment, the AUC for the omnibus score was substantially higher, 0.971±0.027 SE, indicating near-perfect classification accuracy when these olfactory measures are used in combination.
Figure 2. ROC Curve Analysis of Olfactory Tasks in Clinical Risk and Low Risk Subjects.
The composite score represents a weighted average based on the parameter estimates of a logistic regression analysis with all four olfactory variables included as predictors.
3.5 Relationships Between Olfactory Performance and Clinical Symptoms
None of the individual olfactory measures or the composite score was associated with prodromal symptom severity, as measured by the SOPS total score (all p>0.20).
4. Discussion
In this study, odor detection thresholds for odorants that induce high vs. low intracellular cAMP responses were examined in youths at clinical risk for psychosis. We found that individuals with prodromal symptoms showed the same odor-specific threshold deficit – i.e., an inability to detect lyral despite normal perception of citralva – previously observed in schizophrenia patients and adult first-degree relatives (Turetsky et al., 2009b). This observation provides further support for an odor-specific threshold decrement related to the cAMP dimension-of-interest that precedes the onset of acute psychosis and is unrelated to the effects of illness chronicity or antipsychotic medications. This abnormality is also relatively independent of other dimensions of olfactory processing (i.e., odor discrimination and odor identification), which are also impaired in this at-risk population (Kamath et al., in press) and may be harbingers of impending conversion to psychosis (Brewer et al., 2003). The ability of these latter measures to identify at-risk youths, as demonstrated by the ROC analysis, is quite high – i.e., 89% correct classification based on odor identification deficits alone. However, inclusion of lyral threshold as an additional predictor improved classification accuracy to greater than 97%. This is consistent with the idea that this threshold deficit reflects a fundamental dysregulation of cAMP-mediated signal transduction in peripheral ORNs, while identification and discrimination require additional “higher-order” elements of perceptual integration and memory, beyond mere odor detection.
The importance of cAMP for both the development and function of the olfactory system is clear. As noted above, cAMP is the secondary messenger through which olfactory receptor neurons respond to odor stimulation, and this is presumably the mechanism underlying this odor-specific threshold deficit. However, it has also been implicated in the axonal targeting of ORNs and glomerular development in the olfactory bulb (Chesler et al., 2007; Col et al., 2007; Imai et al., 2006; Zou et al., 2007). So, cAMP dysregulation may also contribute to the developmental anomalies observed in peripheral olfactory structures in schizophrenia (Turetsky et al., 2007; 2003; 2000). The implications of this, of course, extend beyond the domain of olfaction. The molecular mechanisms that underlie these olfactory impairments may also contribute to the broader pathophysiology of the illness. Among the candidate genes implicated as schizophrenia vulnerability markers, two at least - DISC1 and PDE4B (Andreasen et al., 2011) – are important regulators of intracellular cAMP activity (Andreasen et al., 2011). While it is not established that the heightened vulnerability conveyed by these genes is mediated through cAMP mechanisms, it is plausible that one manifestation of these vulnerability factors would be a functional disruption of cAMP activity in the olfactory system. In this case, the odor-specific threshold deficit that we have observed could be an indicator of this broader illness vulnerability in at-risk youths.
A critical question, of course, is whether the putative mechanism underlying this behavioral deficit can be confirmed through molecular analysis. The relatively non-invasive procedure of olfactory epithelial biopsy allows an examination of the molecular composition and reactivity of olfactory receptor neurons ex vivo (Borgmann-Winter et al., 2009; Gomez et al., 2000b; Hahn et al., 2005a; Hahn et al., 2005b). Studies currently underway in our program are examining cAMP signal transduction in ORNs obtained from both schizophrenia patients and at-risk youths to directly test this hypothesis. A second critical question, which requires longitudinal follow-up of a larger at-risk sample, is whether performance on this test, along with other structural and functional measures of olfaction, is predictive of subsequent conversion to overt illness. Finally, the question of specificity needs to be considered. It remains to be determined whether this odor-specific threshold abnormality is limited to schizophrenia or is also seen in other psychotic disorders.
Acknowledgements
We thank Dana Gatto and Jared Hammond for assistance with subject recruitment, task administration and data entry.
This study was funded in part by National Institutes of Health Grants MH63381 (PJM), K08MH79364 (MEC), and K23MH079498 (KBW). The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
VK, PJM, MEC, KBW, CGC and CGK, report no competing interests. REG and BIT report unrelated investigator-initiated research support from AstraZeneca Pharmaceuticals and Pfizer Inc.
Contributors
Dr. Kamath conducted literature review, statistical analyses, and wrote the first draft of the manuscript. Dr. Moberg contributed to the study design and protocol. Dr. Calkins, Dr. Borgmann-Winter, Ms. Conroy, Dr. Kohler, and Dr. Gur oversaw all aspects of participant recruitment, screening, diagnostic assessment, and case conference of the participants. Dr. Turetsky contributed to the study design, protocol, literature review, statistical analyses, and manuscript writing. All authors contributed to and have approved the final manuscript.
References
- Andreasen NC, Wilcox MA, Ho BC, Epping E, Ziebell S, Zeien E, et al. Statistical epistasis and progressive brain change in schizophrenia: An approach for examining the relationships between multiple genes. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgmann-Winter KE, Rawson NE, Wang HY, Wang H, Macdonald ML, Ozdener MH, et al. Human olfactory epithelial cells generated in vitro express diverse neuronal characteristics. Neuroscience. 2009;158(2):642–653. doi: 10.1016/j.neuroscience.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer WJ, Wood SJ, McGorry PD, Francey SM, Phillips LJ, Yung AR, et al. Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am J Psychiatry. 2003;160(10):1790–1794. doi: 10.1176/appi.ajp.160.10.1790. [DOI] [PubMed] [Google Scholar]
- Carlyle BC, Mackie S, Christie S, Millar JK, Porteous DJ. Co-ordinated action of disc1, pde4b and gsk3beta in modulation of cAMP signalling. Mol Psychiatry. 2011;16(7):693–694. doi: 10.1038/mp.2011.17. [DOI] [PubMed] [Google Scholar]
- Chesler AT, Zou DJ, Le Pichon CE, Peterlin ZA, Matthews GA, Pei X, et al. A g protein/cAMP signal cascade is required for axonal convergence into olfactory glomeruli. Proc Natl Acad Sci U S A. 2007;104(3):1039–1044. doi: 10.1073/pnas.0609215104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Col JA, Matsuo T, Storm DR, Rodriguez I. Adenylyl cyclase-dependent axonal targeting in the olfactory system. Development. 2007;134(13):2481–2489. doi: 10.1242/dev.006346. [DOI] [PubMed] [Google Scholar]
- Doty RL, Kreiss DS, Frye RE. Human odor intensity perception: Correlation with frog epithelial adenylate cyclase activity and transepithelial voltage response. Brain Res. 1990;527(1):130–134. doi: 10.1016/0006-8993(90)91070-w. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured clinical interview for dsm-iv axis ii personality disorders. Washington, D.C.: American Psychiatry Press; 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for dsm-iv -patient edition (scid-p, version 2.0) New York: New York State Psychiatric Institute; 1996. [Google Scholar]
- Gomez G, Rawson NE, Cowart B, Lowry LD, Pribitkin EA, Restrepo D. Modulation of odor-induced increases in [ca2+]i by inhibitors of protein kinases a and c in rat and human olfactory receptor neurons. Neuroscience. 2000a;98(1):181–189. doi: 10.1016/s0306-4522(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Gomez G, Rawson NE, Hahn C-G, Michaels R, Restrepo D. Characteristics of odorant elicited calcium changes in cultured human olfactory neurons. J Neurosci Res. 2000b;62:737–749. doi: 10.1002/1097-4547(20001201)62:5<737::AID-JNR14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Hahn CG, Gomez G, Restrepo D, Friedman E, Josiassen R, Pribitkin EA, et al. Aberrant intracellular calcium signaling in olfactory neurons from patients with bipolar disorder. Am J Psychiatry. 2005a;162(3):616–618. doi: 10.1176/appi.ajp.162.3.616. [DOI] [PubMed] [Google Scholar]
- Hahn CG, Han LY, Rawson NE, Mirza N, Borgmann-Winter K, Lenox RH, et al. In vivo and in vitro neurogenesis in human olfactory epithelium. J Comp Neurol. 2005b;483(2):154–163. doi: 10.1002/cne.20424. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. 'Sniffin' sticks': Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22(1):39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- Imai T, Suzuki M, Sakano H. Odorant receptor-derived cAMP signals direct axonal targeting. Science. 2006;314(5799):657–661. doi: 10.1126/science.1131794. [DOI] [PubMed] [Google Scholar]
- Kamath V, Turetsky BI, Calkins ME, Kohler CG, Conroy CG, Borgmann-Winter K, et al. Olfactory processing in schizophrenia, non-ill first-degree family members, and young people at-risk for psychosis. World J of Biol Psychiatry. doi: 10.3109/15622975.2011.615862. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Vora A, Montrose D, Diwadkar VA, Sweeney J. Olfactory identification in young relatives at risk for schizophrenia. Acta Neuropsychiatr. 2009;3:121–124. doi: 10.1111/j.1601-5215.2009.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, Wolf S. "Sniffin' sticks": Screening of olfactory performance. Rhinology. 1996;34(4):222–226. [PubMed] [Google Scholar]
- Lowe G, Nakamura T, Gold GH. Adenylate cyclase mediates olfactory transduction for a wide variety of odorants. Proc Natl Acad Sci U S A. 1989;86(14):5641–5645. doi: 10.1073/pnas.86.14.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan TH, Miller TJ, Woods SW, Hoffman RE, Davidson L. Instrument for the assessment of prodromal symptoms and states. In: Miller TJ, editor. Early intervention in psychotic disorders. Dordrecht: Kluwer Academic Publishers; 2001. pp. 135–150. [Google Scholar]
- McGlashan TH, Miller TJ, Woods SW, Rosen JL, Hoffman RE, Davidson L. Structured interview for prodromal syndromes, version 4.0. New Haven, CT: Prime Clinic Yale School of Medicine; 2003. [Google Scholar]
- Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, et al. Disc1 and pde4b are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310(5751):1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: Predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, et al. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70(4):273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Murdoch H, Mackie S, Collins DM, Hill EV, Bolger GB, Klussmann E, et al. Isoform-selective susceptibility of disc1/phosphodiesterase-4 complexes to dissociation by elevated intracellular cAMP levels. J Neurosci. 2007;27(35):9513–9524. doi: 10.1523/JNEUROSCI.1493-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIMH Genetics Initiative. Family interview for genetic studies (figs) Rockville, MD: National Institute of Mental Health; 1992. [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. Arch Gen Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863–844. [DOI] [PubMed] [Google Scholar]
- Pepe MS, Cai T, Longton G. Combining predictors for classification using the area under the receiver operating characteristic curve. Biometrics. 2006;62(1):221–229. doi: 10.1111/j.1541-0420.2005.00420.x. [DOI] [PubMed] [Google Scholar]
- Sklar PB, Anholt RR, Snyder SH. The odorant-sensitive adenylate cyclase of olfactory receptor cells: Differential stimulation by distinct classes of odorants. J of Biol Chem. 1986;261(33):15538–15543. [PubMed] [Google Scholar]
- Thomson PA, Wray NR, Millar JK, Evans KL, Le Hellard S, Condie A, et al. Association between the trax/disc locus and both bipolar disorder and schizophrenia in the scottish population. Mol Psychiatry. 2005;10:657–668. doi: 10.1038/sj.mp.4001669. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Glass CA, Abbazia J, Kohler CG, Gur RE, Moberg PJ. Reduced posterior nasal cavity volume: A gender-specific neurodevelopmental abnormality in schizophrenia. Schizophr Res. 2007 doi: 10.1016/j.schres.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Hahn CG, Borgmann-Winter K, Moberg PJ. Scents and nonsense: Olfactory dysfunction in schizophrenia. Schizophr Bull. 2009a;35(6):1117–1131. doi: 10.1093/schbul/sbp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ. An odor-specific threshold deficit implicates abnormal intracellular cyclic amp signaling in schizophrenia. Am J Psychiatry. 2009b;166(2):226–233. doi: 10.1176/appi.ajp.2008.07071210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Roalf DR, Arnold SE, Gur RE. Decrements in volume of anterior ventromedial temporal lobe and olfactory dysfunction in schizophrenia. Arch Gen Psychiatry. 2003;60(12):1193–1200. doi: 10.1001/archpsyc.60.12.1193. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Yousem DM, Doty RL, Arnold SE, Gur RE. Reduced olfactory bulb volume in patients with schizophrenia. Am J Psychiatry. 2000;157(5):828–830. doi: 10.1176/appi.ajp.157.5.828. [DOI] [PubMed] [Google Scholar]
- Vacic V, McCarthy S, Malhotra D, Murray F, Chou HH, Peoples A, et al. Duplications of the neuropeptide receptor gene vipr2 confer significant risk for schizophrenia. Nature. 2011;471(7339):499–503. doi: 10.1038/nature09884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS. Wide range achievement test (wrat3) administration manual. Wilmington, DE: Wide Range, Inc; 1993. [Google Scholar]
- Woodberry KA, Seidman LJ, Giuliano AJ, Verdi MB, Cook WL, McFarlane WR. Neuropsychological profiles in individuals at clinical high risk for psychosis: Relationship to psychosis and intelligence. Schizophr Res. 2010;123(2–3):188–198. doi: 10.1016/j.schres.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou DJ, Chesler AT, Le Pichon CE, Kuznetsov A, Pei X, Hwang EL, et al. Absence of adenylyl cyclase 3 perturbs peripheral olfactory projections in mice. J Neurosci. 2007;27(25):6675–6683. doi: 10.1523/JNEUROSCI.0699-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig MH, Campbell G. Receiver-operating characteristic (roc) plots: A fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561–577. [PubMed] [Google Scholar]