Abstract
It has become increasingly apparent that gene expression is regulated by the functional interplay between spatial genome organization and nuclear architecture. Within the nuclear environment a variety of distinct nuclear bodies exist. They are dynamic, self-organizing structures that do not assemble as pre-formed entities but rather emerge as a direct reflection of specific activities associated with gene expression and genome maintenance. Here I summarize recent findings on functions of some of the most prominent nuclear bodies, including the nucleolus, Cajal body, PML nuclear body, Polycomb group body and the 53BP1 nuclear body. The emerging view is that their organization is orchestrated by similar principles, and they function in fundamental cellular processes involved in homeostasis, differentiation, development and disease.
Introduction
The dynamic spatial organization of the cell nucleus is thought to play a primary role in genome function and maintenance [1]. Within the nuclear space, which is characterized by a lack of defining membranes, chromosomes occupy specific nonrandom territories which overlap at their edges. These chromosome territories harbor a variety of functionally distinct nuclear bodies (NBs) [2,3]. NBs are highly dynamic structures, the components of which can rapidly exchange with the surrounding nucleoplasm, and their structural integrity is mediated by transient protein-protein and likely protein-RNA interactions. Some NBs form as a direct reflection of specific activities involved in gene expression and genome maintenance and they assemble by self-organization [3]. NBs are frequently associated with specific gene loci whose activities contribute to their formation. Both coding and noncoding RNAs can initiate the formation of some NBs with which they are physiologically associated [4**,5**,6].The primary role of NBs is to concentrate substrates, enzymes and assembly intermediates within their confined space to accelerate these enzymatic and assembly reactions (Figure 1). Moreover, NBs can positively or negatively regulate concentrations of specific essential factors by sequestering and releasing them as needed [2,7].
Figure 1. Nuclear bodies (NBs) facilitate more efficient interactions between substrates and enzymes.

NBs act as a depot to concentrate a variety of reactive molecules or enzymes – each of which is represented as a Pac-Man in the schematic - and their cognate substrates (triangles) relative to the larger nucleoplasmic space. As a result, production of the relevant products (squares) is accelerated. The variety of enzymes, substrates and products are represented by different colors.
In the following sections I focus on recent findings regarding the functional properties of five prominent NBs and how they relate to the organization of the cell nucleus, regulation of gene expression and genome stability. I will also discuss the roles they play in regulatory pathways controlling fundamental processes such as the cell cycle, stress responses, DNA damage/repair, pluripotency and cell death.
Nucleolus
The primary function of the nucleolus, the most prominent NB, is the production of ribosomal RNA and subunits. Nucleolar function is reflected in the structural organization of the organelle into three distinct regions: the fibrillar center surrounded by the dense fibrillar component, both of which are embedded in the granular component [8,9]. However, it is still a matter of debate how transcriptionally active tandemly repeated arrays of ribosomal genes are topologically organized inside the nucleolus and how their arrangement fits in the tripartite nucleolar structure [8,10]. Recent work [11**] proposed a model in which the key transcriptional initiation complex SL1 binds and holds together the promoter, upstream regulatory region and terminator. In this scenario, there is DNA looping between the sites of transcription initiation and termination. The SL1-core functions as the anchoring hub of the rDNA structure, with the transcribed rDNA region wrapped around it in a pattern of consecutive rows of non-intersecting DNA rings in a helix-like cylindrical manner. The pulling forces of elongating RNA polymerases I, which after initiation in the core move to the outer rim of the helix, rotate the entire anchoring core around its axis, with nascent transcripts radiating away from the rDNA helix, preventing them from intertwining (Figure 2).
Figure 2. The core-helix model depicting the topology of transcribing ribosomal RNA genes within the mammalian nucleolus.
(A) The key transcriptional initiation complex SL1 binds and holds together the promoter, upstream regulatory region and terminator, which establishes DNA looping between the sites of transcription initiation and termination. The SL1-core functions as the anchoring hub of the rDNA structure, with the transcribed ribosomal DNA region wrapped around it in a pattern of consecutive rows of non-intersecting DNA rings in a helix-like cylindrical manner. The pulling forces of elongating RNA polymerases I, which after initiation at the promoter in the core move to the outer rim of the helix, rotate the entire anchoring core around its axis, with nascent pre-rRNA transcripts radiating away from the ribosomal DNA helix, preventing them from intertwining. Only a subset of polymerases at the top and bottom of the coiled rDNA are shown for clarity (dashed lines). (B) Scheme of three subnucleolar compartments in frontal projection of the core-helix structure model. SL1 initiation complex is located in the centrally positioned Fibrillar center (FC). The active ribosomal genes with engaged RNA polymerases I are located at the periphery of the FC. Growing nascent pre-rRNA transcripts are cotranscriptionally assembled into processing ribonucleoprotein particles which form dense fibrillar component (DFC). Large (60S) and small (40S) pre-ribosomal subunits are assembled in the granular component (GC). The images were adapted from [11**].
The classical view of the nucleolus is that it serves primarily as a factory for building preribosome subunits. But the nucleolus has functions far beyond ribosome biogenesis, acting as the site of key regulatory pathways controlling fundamental processes such as nuclear organization, the cell cycle, proliferation, the stress response and aging [8]. In addition, several lines of evidence suggest that the nucleolus may act as a genome organization center [12*,13*]. Large genome regions with low gene density are nonrandomly associated with the periphery of the nucleolus. These regions are prominently enriched in transcriptionally repressed genes and specific satellite repeats [10,12*,13*]. In addition, centromeres nonrandomly associate with nucleoli in mammalian cells and transcriptionally active tRNA gene clusters and 5S rRNA genes are preferentially localized at the nucleolar periphery in multiple organisms [10]. The association dynamics of these genomic regions and the identity of the elements responsible for recruiting and/or tethering these regions to the nucleolus remain to be elucidated.
A key non-ribosome synthesis role for the nucleolus is in regulating protein turnover. It appears that the nucleolus plays an essential role in the regulation of the tumor suppressor p53 under stress conditions [9]. Under normal physiological conditions p53 levels are very low due to ubiquitination by the Mdm2 E3 ubiquitin ligase, which leads to p53 nuclear export and degradation by the proteosome [9,14]. Recent evidence indicates that this ubiqutination is a nucleolar event as the entire nuclear pool of p53 transits through nucleoli where p53 is polyubiquitinylated by Mdm2 [15**]. p53 ubiquitination is blocked by the stress-elevated expression of the tumor suppressor p14ARF, a predominantly nucleolar protein, whose stabilization in the nucleolus is achieved via interaction with the major nucleolar protein B23/nucleophosmin (NPM) [16]. When p14ARF is upregulated through oncogenic stress, it is delocalized from the nucleolus to the nucleoplasm where it binds and inactivates Mdm2, which in turn reduces Mdm2's ability to interact with and target p53 for degradation. Additionally, during nucleolar stress, which is induced by disruption of ribosome biogenesis, Mdm2 directly associates with a set of ribosomal proteins (RPL5, RPL11, RPL23, RPS7) released from the nucleolus, again preventing the ubiquitin-mediated degradation of p53 [17]. Recent work [18**] identified the nucleolar protein PICT1 as responsible for retaining RPL11 in the nucleolus, thereby blocking the induction of p53 stabilization by nucleolar stress. These findings support a model where stress-induced nucleolar disruption causes extensive redistribution of nucleolar components that bind and sequester Mdm2 to the nucleoplasm, which in turn disrupts the p53-Mdm2 interaction, thereby stabilizing and activating p53 [9]. Once stabilized, p53 enhances expression of its target genes, followed by either cell cycle arrest or apoptosis. Collectively these data reveal an intimate link between p53 regulation and the nucleolus, and suggest that the nucleolus acts as a stress sensor responsible for the suppression of p53 levels.
Increasing evidence suggests that non-coding RNAs play a functional role in the nucleolus [8,19]. Small nucleolar RNAs (snoRNAs) carry out a key role as guides in modification and processing of pre-rRNA [8]. However, a large proportion of snoRNAs can be further processed into smaller molecules that act as micro RNAs (miRNAs), regulating gene expression post-transcriptionally [20,21]. The mature miRNAs derived from snoRNA-like precursors are predominantly localized in the nucleus [22] and the major nucleolar protein B23/NMP has been implicated in miRNA export [23]. Interestingly, the miRNA processing endonuclease Drosha has also been detected in the nucleolus [24] and the miRNA processor Dicer has been found associated with ribosomal genes [25]. Depletion of Dicer and Drosha leads to defects in prerRNA processing and alteration in the nucleolar structure [26]. Moreover, intergenic transcripts from a spacer RNA polymerase I promoter about 2kb upstream of the pre-rRNA start site are processed into a mixture of 150–250 nucleotide RNAs, termed pRNAs, which overlap the rDNA promoter. The conserved hairpin structure within pRNA is stabilized by the nucleolar remodeling complex (NoRC) and recruits it to the rDNA promoter to induce long-term transcriptional silencing of a fraction of rDNA genes [27]. Interestingly, pRNA is transcribed from hypomethylated rRNA genes during mid to late S phase and acts in trans to inherit transcriptional repression of silent rDNA clusters during cell division [27].
Recent efforts to understand the regulation of embryonic stem (ES) cell pluripotency demonstrated that three key transcription factors, Oct4, Sox2 and Nanog, all essential for maintenance of ES cells and creation of induced pluripotent stem (iPS) cells physically interact with the nucleolar protein B23/NPM [28]. Furthermore, a genome-wide functional screen in ES cells revealed that knockdown of the nucleolar protein Mki67ip induces the differentiation of ES cells. Importantly, Mki67ip forms a complex with B23/NPM in ES cells and both are highly expressed in undifferentiated ES cells and downregulated upon ES differentiation. Their knockdown leads to downregulation of Oct4, Sox2 and Nanog and loss of pluripotency [29]. Thus, B23/NPM seems to play multiple key roles in ribosome biogenesis, cell proliferation, cancer transformation, miRNA export [16,23], but is also involved in the regulation of ES cell functions. Future studies will likely highlight the significance of the nucleolus in the regulation of pluripotency.
Cajal body
Cajal bodies (CBs) are prominent NBs, which contain numerous factors involved in pre-mRNA splicing, pre-rRNA processing, histone pre-mRNA 3' end processing, and telomere maintenance implicating them in the biogenesis of RNA-processing complexes.
It is now well established that CBs play a role in the assembly of spliceosomal snRNPs [30]. After transcription, U1, U2, U4 and U5 snRNAs are exported to the cytoplasm, where each is assembled with the core Sm proteins into the SMN protein complex. Consequently, snRNPs are reimported into nuclei and targeted to CBs to finalize their maturation before associating with transcription sites or nuclear speckles [31]. Two key CB components, coilin and SMN, are essential for CB formation and structural integrity [3]. Importantly, the WRAP53/TCAB1 protein, recently identified as a component of CB-specific scaRNPs and telomerase [32], recruits the SMN complex from the cytoplasm to CBs by facilitating interactions between SMN, importin β and coilin. Depletion of WRAP53/TCAB1 leads to complete CB disassembly and relocalization of coilin and SMN to nucleoli, indicating its importance for coilin-SMN complex formation and CB maintenance [33**].
It appears that the phosphorylation status of coilin and SMN functionally impacts CB formation and the recruitment of components to CB. In primary cells, which typically lack CBs, coilin is hyperphosphorylated on C-terminal phosphoserine residues reducing its self-interaction ability. In contrast, in transformed cells, which typically have a large number of CBs, coilin is hypophosphorylated and able to self-interact [34]. SMN is hyperphosphorylated in the cytoplasm and able to recruit proteins to the SMN complex [31]. A recent model proposes how snRNPs might be exchanged between the SMN complex and coilin in CBs [35*]. In this view, snRNPs are imported into the nucleus as components of the SMN complex which is recruited to CBs by hypophosphorylated coilin. Coilin is then hyperphosphorylated on its C-terminus, which disrupts its interaction with SMN, which in turn may promote SMN release from the CB and enhance coilin-snRNP interaction. Thus, snRNPs are transferred from SMN to coilin and become available for further modifications in CBs. As a consequence, coilin is dephosphorylated possibly by phosphatase PPM1G, which facilitates the release of snRNPs [35*].
CBs are also directly involved in spliceosome recycling by aiding the stepwise assembly of U4/U6 di-snRNP followed by U4/U6-U5 tri-snRNP formation after each round of splicing [30]. Based on in vivo measurements, the U4/U6 di-snRNP assembly rate is increased 11-fold in cells with at least one CB in contrast to cells without them [36] and U4/U6-U5 tri-snRNP assembly is 10-fold faster in the CB than in the surrounding nucleoplasm [37*]. These findings strongly support the view that the CB functions as a depot for snRNP biogenesis, significantly accelerating the assembly of macromolecular complexes by increasing the speed of reactions by concentrating the components required for their association and decreasing inappropriate interactions. In support of this, knockdown of coilin leads to the loss of CBs and developmental arrest in zebrafish embryos by reduced mRNA production. Importantly, developmental defects can be rescued by injection of mature pre-assembled human snRNPs, but not the snRNAs or snRNP proteins alone, indicating involvement of CBs in maturation of snRNPs [38**]. Conversely, cellular functions attributed to CBs, such as modification of spliceosomal snRNAs and assembly of snRNPs and snoRNPs, occur in many cell types lacking CBs albeit with lower efficacy [30].
The impairment in assembly of spliceosomal snRNPs associated with CB function is also reflected in Spinal muscular atrophy (SMA), an autosomal recessive disease characterized by the loss of lower motor neurons and muscular atrophy. The disease is caused by loss or mutation of the survival motor neuron 1 gene (SMN1) [31]. Thus, patient cells have lowered levels of SMN, leading to CB disruption and significantly reduced snRNP assembly activity, yet the total amount of major snRNPs is almost unchanged. However, defective snRNP assembly causes a decrease of U4/U6-U5 tri-snRNP formation and severely reduces levels of the minor U4atac/U6atac/U5 tri-snRNP plus U11 and U12 snRNPs. These data demonstrate a correlation between SMN deficiency associated with CB disruption in SMA leading to defects in minor trisnRNP assembly and inefficient splicing of particular minor introns [39**,40]. Moreover, the WRAP53/TCAB1 protein, which is responsible for targeting the SMN complex to CBs, shows reduced binding to SMN in cells derived from SMA patients indicating its role in SMN dysfunction [33**].
CBs have also been shown to play an important role in regulation of telomeres. Telomere shortening is counteracted by extending the 3' ends of linear chromosomes by telomerase, which consists of telomerase RNA (TR) and telomerase reverse transcriptase (TERT) [41]. Telomerase biogenesis requires an assembly pathway common to a class of H/ACA snoRNAs and scaRNAs, involved in modification of pre-rRNA and splicing snRNAs [30]. The human telomerase RNA (hTR) contains a short sequence motif called the CAB box also present in H/ACA scaRNAs, but not in snoRNAs, which is recognized by a newly identified telomerase and scaRNP component WRAP53/TCAB1 [32,33**]. This CAB box-binding protein targets hTR and scaRNAs to CBs. Depletion of WRAP53/TCAB1 or mutations in the CAB box prevents hTR association with CBs by retargeting it to nucleoli, but also fully eliminates telomerase association at telomeres and disrupts telomere synthesis [42,43*]. It seems that hTR accumulation in CBs is not needed for assembly of the catalytic core of telomerase but telomerase is likely modified in CBs to promote its processivity and competency for telomere association [44**]. hTR, and presumably telomerase holoenzyme, localizes to CBs throughout G1 and G2 phase but appears to transiently leave the CBs for telomere association specifically during mid-S phase when human telomeres are replicated and synthesized. Importantly, during S phase CBs associate only with a small number (8–12) of telomeres which is in agreement with the recent observation that during one cell cycle only a small subset of telomeres are elongated by telomerase in human cells [44**]. Taken together, these findings suggest that CBs might deliver telomerase to the subset of elongating telomeres. Interestingly, in mouse mTR does not localize to CBs but is present in nuclear foci distinct from CBs which also likely deliver telomerase to telomeres [45].
PML nuclear body
Promyelocytic leukemia protein (PML) NBs have long been thought of as sites of inactivity functioning as nuclear storage sites for the accumulation or sequestration of proteins which are released when required [2]. Recent findings challenge that view suggesting that PML NBs may play a more active regulatory role.
PML NBs have extensive contacts with chromatin and have been functionally implicated in DNA repair, transcriptional regulation, cell senescence, and apoptosis. The major structural component of PML NBs is the PML protein and the formation of PML NBs depends on the SUMOylation status of PML and key components such as Sp100 and Daxx [46**].
Daxx functions as a transcription co-repressor. It is SUMOylated on its C-terminus which is critical for its localization in PML NBs, as well as for its SUMO-1-dependent association with gene promoters. Remarkably, as a response to osmotic stress the activity of CK2 kinase is upregulated, which phosphorylates the SUMO-1-interacting motif of Daxx in turn enhancing its SUMOylation. Thus, the phosphorylation stabilizes interactions between Daxx and SUMO-1-modified PML protein and targets Daxx to PML NBs. In addition, it also recruits Daxx to promoters of genes encoding anti-apoptotic factors. Altogether, these results suggest that PML NBs play an active role in enhancing gene repression by working as an assembly factory for Daxx-containing SUMO-1-dependent co-repressor complexes [46**].
PML NBs may represent important cellular nodes for DNA repair or recombination since many forms of genotoxic stress increase their number. Therefore, changes in their number may have a functional impact on fidelity or efficiency of DNA damage signaling. Recent work [47*] has shed some light on how chromatin regulators control PML NB number upon DNA damage. DNA damage increases PML NB number concomitantly with the activation of ATM kinase which phosphorylates the KAP1 protein, a regulator of chromatin structure, and thus induces rapid decondensation of chromatin [47*]. In contrast, dephosphorylation of KAP1 inhibits the number of PML NBs. Interestingly, depletion of KAP1 by RNAi results in a similar increase in PML NB numbers without DNA damage suggesting that ATM prevents the KAP1-dependent NB number decrease. Moreover, KAP1 knockdown leads to reduction of chromatin density [47*]. Collectively, these data suggest that KAP1 is a downstream mediator by which ATM can regulate PML NB number by altering the structural organization of chromatin in response to DNA damage. Future studies are required to determine how changes in PML NB number affect DNA damage signaling in the cell.
Polycomb Group body
Increasing evidence suggests that long-range chromatin interactions are not limited to events linked with activation of gene expression such as targeting gene loci to transcription factories, but also to those associated with gene repression, by positioning their target genes into Polycomb group (PcG) bodies. PcG proteins are epigenetic regulators which form two main repressive complexes, Polycomb repressive complex 1 (PRC1) and 2 (PRC2). They silence many important genes for development and cell fate specification by compacting the chromatin structure via post-translational modification of histones. Mechanistically this appears to be achieved through the methylation of histone H3 at lysine 27 by PRC2, which recruits PRC1 to promote chromatin condensation [48]. Moreover, PcG target genes interact extensively with each other. PcG repressive complexes bind to PcG responsive elements (PREs) present on PcG target genes and modulate their activity by the formation of a multi-looped chromatin structure in which PREs are clustered by long-range chromosomal interactions [49*,50**] (Figure 3). This clustered chromatin architecture is detectable as nuclear foci termed PcG bodies visible by immunofluoresce microscopy of PcG proteins [50**]. However, at the electron microscopy level, PcG bodies are not recognizable as canonical NBs distinct from the surrounding nucleoplasm in human cells, but as a translucent local accumulation of large-scale heterochromatin fibers [51*].
Figure 3. Polycomb group (PcG) bodies act as a hub to recruit and silence multiple specific gene loci.
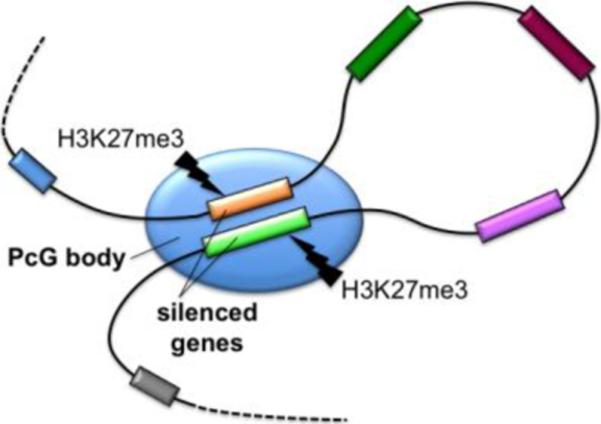
PcG bodies contribute to gene silencing by harboring long-range interactions of PcG-target genes within these bodies by the PcG repressive complexes. Methylation of histone H3 at lysine 27 (H3K27me3) by PcG repressive complex PRC2 recruits the silencing complex PRC1 to enact chromatin condensation.
53BP1 nuclear body
A clear example of the concept that many NBs are formed as a reflection of specific activity are 53BP1 NBs. Formerly described as OPT domains, these large NBs form around DNA lesions generated during mitosis at loci that failed to complete DNA replication. When these “under-replicated” loci enter mitosis they are converted to gaps or breaks by chromosome condensation. Some of these DNA lesions are transmitted to daughter cells, where they are sequestered into large chromatin domains enriched in 53BP1 (p53 Binding protein 1) and other markers associated with DNA-damage-modified chromatin such as phosphorylated histone H2AX [52**,53]. It has been proposed that 53BP1 NBs shield unrepaired lesions against erosion by nucleases and thus protect such loci until repair mechanisms become available in G1 to early S phase when these bodies gradually disassemble [54**].
Conclusions
This review has focused on several of the many recent findings of NBs function and how their activities are correlated in the dynamic functional network of genome function and nuclear organization. These insights illustrate remarkable recent progress, but also highlight that many conceptually important questions in NB biology are just begining to be understood. Recent advances in genome-wide screening, super-resolution imaging, and computational modeling will certainly uncover new, unexpected insights in this rapidly evolving field.
Acknowledgements
I sincerely apologize to all colleagues whose recent works on nuclear bodies could not be cited due to space limitations. I thank Dr. Serguei Denissov for original Figure 2. MD is funded by NIH R01GM090156 from NIGMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
**of outstanding interest
- 1.Rajapakse I, Groudine M. On emerging nuclear order. J Cell Biol. 2011;192:711–721. doi: 10.1083/jcb.201010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao YS, Zhang B, Spector DL. Biogenesis and function of nuclear bodies. Trends Genet. 2011;27:295–306. doi: 10.1016/j.tig.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dundr M, Misteli T. Biogenesis of nuclear bodies. Cold Spring Harb Perspect Biol. 2010;2:a000711. doi: 10.1101/cshperspect.a000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4**.Mao YS, Sunwoo H, Zhang B, Spector DL. Direct visualization of the co-transcriptional assembly of a nuclear body by noncoding RNAs. Nat Cell Biol. 2011;13:95–101. doi: 10.1038/ncb2140. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using an inducible live-cell imaging system the authors visualized the de novo formation of paraspeckle, a nuclear domain involved in nuclear retention of edited RNAs. Paraspeckle is assembled around nascent paraspeckle-specific non-coding RNA NEAT1 acting as a seed to recruit additional building components.
- 5**.Shevtsov SP, Dundr M. Nucleation of nuclear bodies by RNA. Nat Cell Biol. 2011;13:167–173. doi: 10.1038/ncb2157. [DOI] [PubMed] [Google Scholar]; Using a novel RNA-tethering assay this study demonstrates that both coding and non-coding RNAs act as a seeding scaffold for recruitment and retention of RNA-binding components to build specific nuclear bodies.
- 6.Dundr M. Seed and grow: a two-step model for nuclear body biogenesis. J Cell Biol. 2011;193:605–606. doi: 10.1083/jcb.201104087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matera AG, Izaguire-Sierra M, Praveen K, Rajendra TK. Nuclear bodies: random aggregates of sticky proteins or crucibles of macromolecular assembly? Dev Cell. 2009;17:639–647. doi: 10.1016/j.devcel.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pederson T. The nucleolus. Cold Spring Harb Perspect Biol. 2011;3:a000638. doi: 10.1101/cshperspect.a000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulon S, Westman BJ, Hutten S, Boisvert FM, Lamond AI. The nucleolus under stress. Mol Cell. 2010;40:216–227. doi: 10.1016/j.molcel.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Németh A, Längst G. Genome organization in and around the nucleolus. Trends Genet. 2011;27:149–156. doi: 10.1016/j.tig.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 11**.Denissov S, Lessard F, Mayer C, Stefanovsky V, van Driel M, Grummt I, Moss T, Stunnenberg HG. A model for the topology of active ribosomal RNA genes. EMBO Rep. 2011;12:231–237. doi: 10.1038/embor.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]; By using a combination of chromatin immunoprecipitation and 3C assays the authors propose a new model for topological organization of active ribosomal genes which is reflected in the tripartite nucleolar structure.
- 12*.Németh A, Conesa A, Santoyo-Lopez J, Medina I, Montaner D, Péterfia B, Solovei I, Cremer T, Dopazo J, Längst G. Initial genomics of the human nucleolus. PLoS Genet. 2010;6:e1000889. doi: 10.1371/journal.pgen.1000889. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides the high-resolution, genome-wide map of nucleolus-associated chromatin domains with one thousand thirty-seven genes identified within these domains.
- 13*.van Koningsbruggen S, Gierlinski M, Schofield P, Martin D, Barton GJ, Ariyurek Y, den Dunnen JT, Lamond AI. High-resolution whole-genome sequencing reveals that specific chromatin domains from most human chromosomes associate with nucleoli. Mol Biol Cell. 2010;21:3735–3748. doi: 10.1091/mbc.E10-06-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work provides genome-wide analysis of chromatin naturally associated with nucleoli. These nucleolar-associated domains are characterized by low gene density and transcriptionally repressed genes.
- 14.Miliani de Marval PL, Zhang Y. The RP-Mdm2-p53 pathway and tumorigenesis. Oncotarget. 2011;2:234–238. doi: 10.18632/oncotarget.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Boyd MT, Vlatkovic N, Rubbi CP. The nucleolus directly regulates p53 export and degradation. J Cell Biol. 2011;194:689–703. doi: 10.1083/jcb.201105143. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that p53 polyubiquitinylation by Mdm2 E3 ligase, thus marking it for degradation by proteosome, occurs in the nucleolus and it depends on an intact nucleolar structure and function.
- 16.Lindström MS, Zhang Y. B23 and ARF: friends or foes? Cell Biochem Biophys. 2006;46:79–90. doi: 10.1385/CBB:46:1:79. [DOI] [PubMed] [Google Scholar]
- 17.Chakraborty A, Uechi T, Kenmochi N. Guarding the `translation apparatus': defective ribosome biogenesis and the p53 signaling pathway. Wiley Interdiscip Rev RNA. 2011;2:507–522. doi: 10.1002/wrna.73. [DOI] [PubMed] [Google Scholar]
- 18**.Sasaki M, Kawahara K, Nishio M, Mimori K, Kogo R, Hamada K, Itoh B, Wang J, Komatsu Y, Yang YR, Hikasa H, Horie Y, Yamashita T, Kamijo T, Zhang Y, Zhu Y, Prives C, Nakano T, Mak TW, Sasaki T, Maehama T, Mori M, Suzuki A. Regulation of the MDM2-P53 pathway and tumor growth by PICT1 via nucleolar RPL11. Nat Med. 2011;17:944–951. doi: 10.1038/nm.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that nucleolar protein PITC1, whose expression is altered in cancers, inhibits p53 responses to stress via Mdm2-mediated ubiquitination of p53 by sequestering the Mdm2-binding ribosomal protein RPL11 in the nucleolus, thus promoting tumor growth. This indicates that PICT1 is a potent regulator of p53 activity and promotes tumor progression.
- 19.Politz JC, Hogan EM, Pederson T. MicroRNAs with a nucleolar location. RNA. 2009;15:1705–1715. doi: 10.1261/rna.1470409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kishore S, Khanna A, Zhang Z, Hui J, Balwierz PJ, Stefan M, Beach C, Nicholls RD, Zavolan M, Stamm S. The snoRNA MBII-52 (SNORD 115) is processed into smaller RNAs and regulates alternative splicing. Hum Mol Genet. 2010;19:1153–1164. doi: 10.1093/hmg/ddp585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ono M, Scott MS, Yamada K, Avolio F, Barton GJ, Lamond AI. Identification of human miRNA precursors that resemble box C/D snoRNAs. Nucleic Acids Res. 2011;39:3879–3891. doi: 10.1093/nar/gkq1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taft RJ, Simons C, Nahkuri S, Oey H, Korbie DJ, Mercer TR, Holst J, Ritchie W, Wong JJ, Rasko JE, Rokhsar DS, Degnan BM, Mattick JS. Nuclear-localized tiny RNAs are associated with transcription initiation and splice sites in metazoans. Nat Struct Mol Biol. 2010;17:1030–1034. doi: 10.1038/nsmb.1841. [DOI] [PubMed] [Google Scholar]
- 23.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38:7248–59. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiohama A, Sasaki T, Noda S, Minoshima S, Shimizu N. Nucleolar localization of DGCR8 and identification of eleven DGCR8-associated proteins. Exp Cell Res. 2007;313:4196–4207. doi: 10.1016/j.yexcr.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 25.Sinkkonen L, Hugenschmidt T, Filipowicz W, Svoboda P. Dicer is associated with ribosomal DNA chromatin in mammalian cells. PLoS One. 2010;5:e12175. doi: 10.1371/journal.pone.0012175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang XH, Crooke ST. Depletion of key protein components of the RISC pathway impairs pre-ribosomal RNA processing. Nucleic Acids Res. 2011;39:4875–4889. doi: 10.1093/nar/gkr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santoro R, Schmitz KM, Sandoval J, Grummt I. Intergenic transcripts originating from a subclass of ribosomal DNA repeats silence ribosomal RNA genes in trans. EMBO Rep. 2010;11:52–58. doi: 10.1038/embor.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansson H, Simonsson S. Core transcription factors, Oct4, Sox2 and Nanog, individually form complexes with nucleophosmin (Npm1) to control embryonic stem (ES) cell fate determination. Aging. 2010;2:815–822. doi: 10.18632/aging.100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abujarour R, Efe J, Ding S. Genome-wide gain-of-function screen identifies novel regulators of pluripotency. Stem Cells. 2010;28:1487–1497. doi: 10.1002/stem.472. [DOI] [PubMed] [Google Scholar]
- 30.Nizami Z, Deryusheva S, Gall JG. The Cajal body and histone locus body. Cold Spring Harb Perspect Biol. 2010;2:a000653. doi: 10.1101/cshperspect.a000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cauchi RJ. SMN and Gemins: `we are family' … or are we?: insights into the partnership between Gemins and the spinal muscular atrophy disease protein SMN. Bioessays. 2010;32:1077–1089. doi: 10.1002/bies.201000088. [DOI] [PubMed] [Google Scholar]
- 32.Venteicher AS, Abreu EB, Meng Z, McCann KE, Terns RM, Veenstra TD, Terns MP, Artandi SE. A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science. 2009;323:644–648. doi: 10.1126/science.1165357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Mahmoudi S, Henriksson S, Weibrecht I, Smith S, Söderberg O, Strömblad S, Wiman KG, Farnebo M. WRAP53 is essential for Cajal body formation and for targeting the survival of motor neuron complex to Cajal bodies. PLoS Biol. 2011;8:e1000521. doi: 10.1371/journal.pbio.1000521. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study characterizes WRAP53/TCAB1 protein as a new critical self-interacting factor required for the CB formation and maintenance. WRAP/TCAB1 recruits the SMN protein complex from the cytoplasm to CB by mediating interactions between SMN, importinβ and coilin. In patients with spinal muscular atrophy the interaction between WRAP53/TCAB1 and SMN is disrupted which reflects the failure of SMN accumulation in CBs.
- 34.Hearst SM, Gilder AS, Negi SS, Davis MD, George EM, Whittom AA, Toyota CG, Husedzinovic A, Gruss OJ, Hebert MD. Cajal-body formation correlates with differential coilin phosphorylation in primary and transformed cell lines. J Cell Sci. 2009;122:1872–1881. doi: 10.1242/jcs.044040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Toyota CG, Davis MD, Cosman AM, Hebert MD. Coilin phosphorylation mediates interaction with SMN and SmB'. Chromosoma. 2010;119:205–215. doi: 10.1007/s00412-009-0249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work suggests that the phosphorylation status of coilin's C-terminus regulates targeting of spliceosomal snRNPs with the SMN protein complex to CBs and their release for further steps of their maturation in CBs.
- 36.Klingauf M, Stanek D, Neugebauer KM. Enhancement of U4/U6 small nuclear ribonucleoprotein particle association in Cajal bodies predicted by mathematical modeling. Mol Biol Cell. 2006;17:4972–4981. doi: 10.1091/mbc.E06-06-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Novotný I, Blažíková M, Staněk D, Herman P, Malinsky J. In vivo kinetics of U4/U6·U5 tri-snRNP formation in Cajal bodies. Mol Biol Cell. 2011;22:513–523. doi: 10.1091/mbc.E10-07-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work predicts by in vivo kinetic modeling that U4/U6-U5 tri-snRNP assembly is ~10-fold faster in CBs than in the surrounding nucleoplasm implicating the importance of CBs for snRNP formation and recycling after splicing.
- 38**.Strzelecka M, Trowitzsch S, Weber G, Lührmann R, Oates AC, Neugebauer KM. Coilin-dependent snRNP assembly is essential for zebrafish embryogenesis. Nat Struct Mol Biol. 2010;17:403–409. doi: 10.1038/nsmb.1783. [DOI] [PubMed] [Google Scholar]; This study demonstrates that coilin is required for CB formation and embryogenesis in zebrafish by playing the role in the biogenesis and function of snRNPs. Injection of mature preassembled human snRNP, but not snRNAs or snRNP proteins alone, can rescue developmental defects in coilin-deficient embryos.
- 39**.Boulisfane N, Choleza M, Rage F, Neel H, Soret J, Bordonné R. Impaired minor tri-snRNP assembly generates differential splicing defects of U12-type introns in lymphoblasts derived from a type I SMA patient. Hum Mol Genet. 2011;20:641–648. doi: 10.1093/hmg/ddq508. [DOI] [PubMed] [Google Scholar]; This study demonstrates that SMN deficiency in spinal muscular atrophy does not dramatically affect the biogenesis of major spliceosomal snRNPs. However, it significantly decreases the level of the minor U4atac/U6atac/U5 tri-snRNPs and affects some splicing events carried out the minor spliceosome.
- 40.Coady TH, Lorson CL. SMN in spinal muscular atrophy and snRNP biogenesis. Wiley Interdiscip Rev RNA. 2011;2:546–564. doi: 10.1002/wrna.76. [DOI] [PubMed] [Google Scholar]
- 41.Egan ED, Collins K. Specificity and stoichiometry of subunit interactions in the human telomerase holoenzyme assembled in vivo. Mol Cell Biol. 2010;30:2775–2786. doi: 10.1128/MCB.00151-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Batista LF, Pech MF, Zhong FL, Nguyen HN, Xie KT, Zaug AJ, Crary SM, Choi J, Sebastiano V, Cherry A, Giri N, Wernig M, Alter BP, Cech TR, Savage SA, Reijo Pera RA, Artandi SE. Telomere shortening and loss of self-renewal in dyskeratosis congenita induced pluripotent stem cells. Nature. 2011;474:399–402. doi: 10.1038/nature10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Zhong F, Savage SA, Shkreli M, Giri N, Jessop L, Myers T, Chen R, Alter BP, Artandi SE. Disruption of telomerase trafficking by TCAB1 mutation causes dyskeratosis congenita. Genes Dev. 2011;25:11–16. doi: 10.1101/gad.2006411. [DOI] [PMC free article] [PubMed] [Google Scholar]; Telomere shortening plays an important role in dyskeratosis congenita (DS). This study identifies DC patients with missense mutations in WRAP53/TCAB1, a telomerase protein, which facilitates targeting of telomerase to CBs. Mutations in WRAP53/TCAB1 disrupt telomerase localization in CBs which prevent telomerase trafficking to telomeres and lead to DC.
- 44**.Zhao Y, Abreu E, Kim J, Stadler G, Eskiocak U, Terns MP, Terns RM, Shay JW, Wright WE. Processive and distributive extension of human telomeres by telomerase under homeostatic and nonequilibrium conditions. Mol Cell. 2011;42:297–307. doi: 10.1016/j.molcel.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that the association of telomerase with CBs positively affects its processivity and CBs might deliver a limited number of telomerases to telomeres when they elongate.
- 45.Tomlinson RL, Li J, Culp BR, Terns RM, Terns MP. A Cajal body-independent pathway for telomerase trafficking in mice. Exp Cell Res. 2010;316:2797–2809. doi: 10.1016/j.yexcr.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46**.Chang CC, Naik MT, Huang YS, Jeng JC, Liao PH, Kuo HY, Ho CC, Hsieh YL, Lin CH, Huang NJ, Naik NM, Kung CC, Lin SY, Chen RH, Chang KS, Huang TH, Shih HM. Structural and functional roles of Daxx SIM phosphorylation in SUMO paralog-selective binding and apoptosis modulation. Mol Cell. 2011;42:62–74. doi: 10.1016/j.molcel.2011.02.022. [DOI] [PubMed] [Google Scholar]; This study study shows that phosphorylation of transcriptional co-repressor Daxx increases both its sequestration in PML NBs and its recruitment to specific gene promoters. These observations suggest that PML NBs play more active role in gene repression.
- 47*.Kepkay R, Attwood KM, Ziv Y, Shiloh Y, Dellaire G. KAP1 depletion depletion increases PML nuclear body number in concert with ultrastructural changes in chromatin. Cell Cycle. 2011;10:308–22. doi: 10.4161/cc.10.2.14551. [DOI] [PubMed] [Google Scholar]; This study demonstrates that KAP1 protein regulates the number of PML NBs upon DNA damage acting as inhibitor of PML NB formation.
- 48.Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–349. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49*.Tolhuis B, Blom M, Kerkhoven RM, Pagie L, Teunissen H, Nieuwland M, Simonis M, de Laat W, van Lohuizen M, van Steensel B. Interactions among Polycomb domains are guided by chromosome architecture. PLoS Genet. 2011;7:e1001343. doi: 10.1371/journal.pgen.1001343. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using 4C (circular chromosome conformation capture) assays the authors demonstrated that long-range chromatin interactions contribute to the regulation of polycomb group target genes.
- 50**.Bantignies F, Roure V, Comet I, Leblanc B, Schuettengruber B, Bonnet J, Tixier V, Mas A, Cavalli G. Polycomb-dependent regulatory contacts between distant Hox loci in Drosophila. Cell. 2011;144:214–226. doi: 10.1016/j.cell.2010.12.026. [DOI] [PubMed] [Google Scholar]; This study demonstrates that two separated Hox genes clusters in Drosophila are repressed by Polycomb group (PcG) proteins. Using 4C method authors show that both Hox gene clusters interact by long-range interactions within PcG bodies where they are epigenetically silenced by PcG proteins.
- 51*.Smigová J, Juda P, Cmarko D, Raška I. Fine structure of the “PcG body” in human U-2 OS cells established by correlative light-electron microscopy. Nucleus. 2011;2:219–228. doi: 10.4161/nucl.2.3.15737. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using light-electron microscopy this study demonstrates that PcG bodies are more likely local accumulation of large-scale heterochromatin fibers than a canonical protein-enriched nuclear body.
- 52**.Lukas C, Savic V, Bekker-Jensen S, Doil C, Neumann B, Pedersen RS, Grøfte M, Chan KL, Hickson ID, Bartek J, Lukas J. 53BP1 nuclear bodies form around DNA lesions generated by mitotic transmission of chromosomes under replication stress. Nat Cell Biol. 2011;13:243–253. doi: 10.1038/ncb2201. [DOI] [PubMed] [Google Scholar]; This study characterizes 53BP1 nuclear bodies which form around DNA lesions generated during mitosis at loci that failed to complete DNA replication. 53BP1 nuclear bodies shield these fragile chromatin sites against erosion.
- 53.Noon AT, Goodarzi AA. 53BP1-mediated DNA double strand break repair: Insert bad pun here. DNA repair. 2011;10:1071–1076. doi: 10.1016/j.dnarep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 54**.Harrigan JA, Belotserkovskaya R, Coates J, Dimitrova DS, Polo SE, Bradshaw CR, Fraser P, Jackson SP. Replication stress induces 53BP1-containing OPT domains in G1 cells. J Cell Biol. 2011;193:97–108. doi: 10.1083/jcb.201011083. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrate that incomplete DNA synthesis during DNA replication in S phase leads to DNA damage response and formation of 53BP1 nuclear bodies after mitosis in subsequent G1 phase.



