Abstract
Opioid conjugate vaccines have shown promise in animal models as a potential treatment for opioid addiction. Individual vaccines are quite specific and each targets only a limited number of structurally similar opioids. Since opioid users can switch or transition between opioids, we studied a bivalent immunization strategy of combining 2 vaccines that could target several of the most commonly abused opioids; heroin, oxycodone and their active metabolites. Morphine (M) and oxycodone (OXY) haptens were conjugated to keyhole limpet hemocyanin (KLH) through tetraglycine (Gly)4 linkers at the C6 position. Immunization of rats with M-KLH alone produced high titers of antibodies directed against heroin, 6-monoacetylmorphine (6-MAM) and morphine. Immunization with OXY-KLH produced high titers of antibodies against oxycodone and oxymorphone. Immunization with the bivalent vaccine produced consistently high antibody titers against both immunogens. Bivalent vaccine antibody titers against the individual immunogens were higher than with the monovalent vaccines alone owing, at least in part, to cross-reactivity of the antibodies. Administration of a single concurrent intravenous dose of 6-MAM and oxycodone to rats immunized with the bivalent vaccine increased 6-MAM, morphine and oxycodone retention in serum and reduced the distribution of 6-MAM and oxycodone to brain. Vaccine efficacy correlated with serum antibody titers for both monovalent vaccines, alone or in combination. Efficacy of the individual vaccines was not compromised by their combined use. Consistent with the enhanced titers in the bivalent group, a trend toward enhanced pharmacokinetic efficacy with the bivalent vaccine was observed. These data support the possibility of co-administering two or more opioid vaccines concurrently to target multiple abusable opioids without compromising the immunogenicity or efficacy of the individual components.
Keywords: addiction, antibodies, vaccine, prescription opioids, heroin, oxycodone
INTRODUCTION
Opioid abuse and addiction in the USA encompasses a wide variety of opioids [1, 2]. Prior to the 1990s, heroin abuse was predominant and was the focus of treatment strategies. Over the past 15 years prescription opioid abuse has increased dramatically and is now substantially more common than that of heroin [3, 4]. Oxycodone and to a lesser extent hydrocodone or oxymorphone abuse have been increasingly reported in various populations, including teens and USA military personnel [1, 5–7]. Patterns of opioid abuse are also diverse. Daily use by intravenous injection or smoke inhalation is common with heroin, while more occasional oral or intravenous use is more common with prescription opioids [8].
Existing medications for the treatment of opioid addiction are effective and helpful, yet are taken advantage of by only a small fraction of opioid abusers [9]. Agonist therapies including methadone and buprenorphine are themselves addictive. Their use requires substantial oversight, and is legally restricted to established daily opioid users. Despite the potential benefits, some opioid addicts object to taking an addictive treatment medication. Because abuse of prescription opioids is often episodic, continuous agonist therapy is a less attractive option to treat this pattern of abuse. The opioid antagonist naltrexone is effective for heroin addiction, but compliance is generally poor. Additional treatment options are needed which address the diversity of both the opioids abused and their different routes of administration and patterns of use [10].
Vaccines targeting drugs of abuse are being developed as an alternative or supplemental approach to addressing addictions [11]. These vaccines stimulate production of antibodies, which bind the target drug, alter its distribution to brain and reduce drug-related behaviors in animals. Vaccines against cocaine and nicotine have reached clinical trials [12, 13]. A number of vaccines have been developed, which elicit antibodies that bind heroin and its sequentially produced active metabolites 6-monoacetylmorphine (6-MAM), morphine, and morphine-6-glucuronide. Some of these vaccines have been shown to reduce heroin- or morphine-induced behaviors, including self-administration in animals [14–19]. These vaccines show structural specificity and little binding of other opioids such as methadone, buprenorphine, or naltrexone. This structural specificity is advantageous in that use of such vaccines should not preclude the concurrent use of agonist therapies, but high specificity also means that these vaccines do not appreciably bind other abusable opioids such as oxycodone. An oxycodone vaccine was recently described, which binds oxycodone and its active metabolite oxymorphone but has a much lower affinity for heroin and its metabolites, reduces oxycodone distribution to brain and reduces oxycodone-induced hot plate analgesia in rats [20]. The availability of this vaccine presents the possibility of combining heroin and oxycodone vaccines in order to achieve broader opioid binding activity.
In the current study rats received an oxycodone-KLH conjugate vaccine (OXY-KLH) targeting oxycodone and its active metabolite oxymorphone, or a morphine-KLH conjugate vaccine (M-KLH) targeting heroin, 6-MAM and morphine. The two vaccines were administered alone or in combination to determine whether their combined use would preserve their individual efficacies. Because heroin is rapidly converted in vivo to 6-MAM which is considered largely responsible for its acute effects, and resulting heroin levels are quite low [21], 6-MAM was used as a model opioid in this study rather than heroin. Rats vaccinated with M-KLH and OXY-KLH vaccines concurrently developed high titers of antibodies to all of the targeted drugs and showed substantial reductions in the distribution of 6-MAM and oxycodone to brain.
MATERIAL AND METHODS
Drugs and reagents
6-monoacetylmorphine (6-MAM), morphine, oxycodone and opioids used for competitive binding studies were obtained through the NIDA Drug Supply Program and Sigma (St. Louis, MO). [Leu5]-enkephalin was purchased from Tocris Biosciences (Ellisville, MO). All drug doses and concentrations are expressed as the weight of the base.
Synthesis of haptens
OXY(Gly)4 hapten consisting of oxycodone with a tetraglycine (Gly)4 linker at the C6 position was synthesized as previously described (Figure 1) [20]. The analogous morphine M(Gly)4 hapten with C6 linker position was synthesized as detailed below (see Supplemental Figure 1). This linker position was selected because of its efficacy in previously reported immunogens for eliciting antibodies against heroin, 6-MAM, morphine and morphine-6-glucoronide [14, 18], and its efficacy for the OXY-KLH vaccine [20]. The (Gly)4 linker length was found to be more effective than shorter linkers for the OXY-KLH vaccine and is similar to the linker length of an heroin vaccine shown to be highly immunogenic and effective in blocking heroin-induced behaviors [14].
Figure 1.
Morphine, oxycodone, their immunogens and linker structures.
For characterization of synthetic intermediates and haptens, 1H and 13C nuclear magnetic resonance (NMR) spectra were taken on a Bruker Avance 400 MHz instrument (Brucker, Billerica, MA) and calibrated using an internal reference. Elemental analyses were performed by M-H-W Laboratories (Phoenix, AZ). Analytical thin-layer chromatography (TLC) was performed on EM Science (Gibbstown, NJ) silica gel 60 F254 (0.25 mm) and plates visualized by UV light, iodine vapor, or ninhydrine solution. 3-Boc-M (1): Morphine sulfate (5 mmol) was dissolved in a dimethylformamide (DMF)/H2O mixture (9/1: v/v) and catalytic 4-dimethylaminopyridine (DMAP) (0.025 mmol) was added. Tert-Butyloxycarbonyl (Boc) anhydride (1.1 equivalent) dissolved in DMF was added over 30 min and the mixture stirred at room temperature overnight. Solvents were evaporated under reduced pressure and the residue was purified by flash chromatography (3% MeOH/DCM/1%NH4OH) on silica gel (230 – 400 mesh, Fisher Scientific, Pittsburgh, PA) to afford 1 (84% yield) (See Supplemental Figure 1 for synthesis scheme).
3-BOC-M-6-O-tertButylglycolate (2): A suspension of sodium hydride (60% dispersion in mineral oil) (2.9 mmol) in dry tetrahydrofuran (5 ml) at 0°C under nitrogen was gradually added to 1 (1.9 mmol) and the reaction mixture was allowed to stir at room temperature for 30 min, followed by the addition of tert-butylbromoacetate (2.9 mmol). The reaction mixture was stirred at room temperature for 8 hr. The reaction was concentrated under vacuum, diluted with water and then extracted with dichloromethane (DCM). The organic layers were combined, dried over sodium sulfate, filtered and concentrated under vacuum to furnish a final crude residue. This residue was subjected to flash column chromatography using a DCM/MeOH/NH4OH mixture (97/2/1) to afford 2 as an oil (67% yield).
M-6-O-glycolic acid (3). Trifluoroacetic acid (TFA) (20% v/v) was added to a solution of the ester 2 (2 mmol) in DCM (20 ml). The resultant solution was stirred at room temperature. Upon complete disappearance of starting material, the solvent was removed under vacuum. The crude reaction mixture was subjected to azeotropic drying using toluene. The residue was taken up in a methanol/diethyl ether (Et2O) mixture and the precipitate was filtered and washed with Et2O to afford 3 as a white solid (85% yield). M(Gly)4. The M-6-O-glycolic acid (3) was coupled to tetraglycine tertbutyl esters (Gly4tBu) using Dicyclohexylcarbodiimide (DCC)/hydroxybenzotriazole (HOBt) followed by acid hydrolysis to complete the synthesis of M(Gly)4 as described before for OXY(Gly)4 [20]. M-6-O-glycolic acid (3) (0.15–0.4 mmoles), DCC (1.3 equivalent) and HOBt (1.2 equivalent) were dissolved in 5 ml of anhydrous DMF. The solution was cooled to 0°C, placed under a nitrogen atmosphere, and after 15 minutes Gly4tBu (0.15–0.4 mmoles) was added. The solution was sealed under a nitrogen atmosphere and was allowed to reach room temperature and then stir overnight. The reaction mixture was filtered to remove dicyclohexylurea into water (10x initial volume of DMF) and extracted with ethylacetate. The combined organic layers were dried on magnesium sulfate, and concentrated under reduced pressure. The residue was purified by flash column chromatography (DCM/MeOH/NH4OH: 94/5/1) and to a solution of this protected intermediate (0.15–0.4 mmol scale) in DCM (5 ml) was added TFA (20% v/v). The resultant solution was stirred at room temperature. Upon complete disappearance of starting material, the solvent was removed under vacuum. The crude reaction mixture was subjected to azeotropic drying using toluene. The residue was taken up again in a methanol/Et2O mixture and the precipitate was filtered and washed with Et2O. The final crude was purified by reverse phase high pressure liquid chromatography using an acetonitrile/H20/TFA (80/20/0.1%) mixture to provide M(Gly)4 as a slightly yellow solid (43% over 2 steps) (see Supplemental Figure 1 for synthesis scheme). M(Gly)4 was converted to its TFA salt for conjugation to carrier proteins.
Conjugation and purification of conjugates
Haptens for use in the vaccines were conjugated to keyhole limpet hemocyanin (KLH) because this protein is highly immunogenic and acceptable for use in humans. For use as coating antigen in ELISA assays, haptens were conjugated to bovine serum albumin (BSA) or chicken albumin (OVA). Hapten 5 mM and ethyl-N′-(3 dimethylaminopropyl)carbodiimide hydrochloride 50 mM were dissolved in 0.1M MES buffer at pH 4.5.. BSA, OVA or KLH (Thermo Fisher Rockford, IL) were added and reactions were stirred for 3 hr at room temperature followed by dialysis and storage at +4° C as previously described [20]. Molar hapten:protein conjugation ratios (moles of hapten conjugated per mole of protein) for the BSA and OVA conjugates were quantitated by MALDI-TOF (Reflex III, Bruker).
Vaccination
All animal studies have been carried out in accordance with EU Directive 2010/63/EU and approved by the Minneapolis Medical Research Foundation Animal Care and Use Committee. Male Holtzman rats weighing 350 grams (Harlan Laboratories, Madison, WI) were housed with a 12/12 hrs standard light/dark cycle. Conjugates were injected i.p. in a final volume of 0.4 ml in complete Freund’s adjuvant for the first injection and incomplete Freund’s adjuvant for 2 subsequent booster injections at 3 and 6 weeks. Experiments were conducted 7–10 days after the 3rd immunization. Rats (n=12 group) were immunized as follows, with unconjugated KLH added as needed so that all groups received a total KLH protein dose of 50 μg: Group (1) KLH 50 μg; Group (2) Monovalent M-KLH 25 μg + KLH 25 μg; Group (3) Monovalent OXY-KLH 25 μg + 25 μg KLH; Group (4) Bivalent M-KLH 25 μg + OXY-KLH 25 μg.
To compare vaccine route and adjuvant male BALB/c mice (n=5 per group) (Harlan Laboratories, Madison, WI) were vaccinated on days 0, 14 and 28 with 25 μg of monovalent OXY-KLH. Vaccine was administered either i.p. in a final volume of 0.2 ml in complete Freund’s adjuvant for the first dose and incomplete Freund’s for the remaining doses, or s.c. in a final volume of 0.2 ml containing alum adjuvant (Thermo Fisher) at a final concentration of 5 mg/ml. Blood was obtained on day 35 for serum antibody titer measurement.
Antibody cross-reactivity and specificity
ELISA plates were coated with 5 ng/well of M-BSA or OXY-OVA conjugate or unconjugated protein control in carbonate buffer at pH 9.6 and blocked with 1% gelatin. Rat primary antibodies were incubated with goat anti-IgG antibodies conjugated to horseradish peroxidase, while rabbit anti-mouse IgG antibodies were used to measure mouse immunized serum. The extent of cross- reactivity to immunogen between antibodies generated by each monovalent vaccine was assessed by measuring titers against both the M-BSA and OXY-OVA coating immunogens. Cross-reactivity for the M-KLH vaccine was calculated as the ratio of its ELISA titer to OXY-OVA divided by its ELISA titer to M-BSA and vice versa for the OXY-KLH vaccine. Antibody specificity was characterized by competitive ELISA and IC50 values for each inhibitor were obtained as described before [20].
Morphine-specific antibody concentration
Morphine-specific IgG concentrations from immunized rats were measured by ELISA using a standard curve constructed using commercially available murine monoclonal anti-morphine IgG. The morphine-conjugate used to generate the monoclonal anti-morphine IgG utilized the same C6 linker position for conjugation to carrier protein (BSA) as the M(Gly)4 immunogen used in the current study (Qed Biosciences, San Diego, CA). A corresponding oxycodone monoclonal antibody with C6 linker position was not available so this approach could not be used to measure anti-oxycodone IgG concentrations.
Because the serum morphine-specific antibody being measured was from rats while the monoclonal IgG used for the standard curve was from mice, preliminary experiments were performed to determine dilutions of anti-mouse IgG-horseradish peroxidase (HRP) or anti-rat IgG-HRP, which produced the same optical density reading when added to wells containing 5 ng of commercially obtained purified mouse or rat IgG, respectively.
These secondary antibody dilutions were then used for the standard curve or serum ELISAs to quantitate morphine-specific IgG levels in vaccinated rats. For analysis of rat serum, wells were coated with 5 ng of M-BSA and serum dilutions were added followed by goat anti-rat IgG-HRP at a 1:50,000 dilution. The corresponding standard curve was obtained by coating ELISA wells similarly and adding various concentrations of anti-morphine monoclonal antibody followed by rabbit anti-mouse IgG-HRP at a 1:10,000 dilution. Controls consisting of wells coated with BSA alone showed no binding of either the monoclonal antibody or immune serum to this protein.
Effects of vaccination on 6-MAM, morphine and oxycodone distribution
The effects of immunization with the bivalent M-KLH + OXY-KLH vaccine on drug distribution were measured in rats receiving 0.1 mg/kg (0.32 μmol/kg) 6-MAM and the same dose of oxycodone administered concurrently i.v.. 6-MAM was dissolved in 2% (v/v) DMSO in physiological saline while oxycodone was dissolved directly in saline on the day of the experiment. 6-MAM and oxycodone were mixed 1:1 (vol: vol) in the same syringe prior to intravenous infusion. One week after the final vaccine dose rats were anesthetized with ketamine/xylazine (respectively 100 mg/kg and 10mg/kg) and an indwelling catheter was placed in their right external jugular vein. Blood was withdrawn for ELISA assays, and 6-MAM + oxycodone were administered as a 60 sec infusion. Rats were decapitated 4 minutes later and trunk blood and brain collected. Oxycodone and 6-MAM doses were chosen as the largest doses that, when combined, would avoid respiratory depression or overdose.
Oxycodone assay
Serum and brain oxycodone or oxymorphone concentrations were measured by gas chromatography coupled to mass spectrometry as previously described [20]. The reported oxycodone concentrations represent the total drug (protein or antibody-bound as well as free) in each sample.
6-MAM and morphine assay
All solutions and samples were kept on ice. Whole blood samples were collected into a syringe containing ice-cold sodium fluoride (4 mg/ml) and heparin (100 IU/ml). Plasma was separated and 0.5 ml of plasma was diluted 1:1 with formate buffer (pH 3.0, 10mM). Brain halves were rinsed with 10 mM formate (pH 3.0), placed into pre-weighed vials, then weighed and 10 mM formate pH 3.0 was added (4 parts by weight). Samples were homogenized 30–40 sec and stored at −20°C until extraction.
Samples underwent solid-phase extraction using Strata-X-RP 1 ml extraction cartridges and were quantified by liquid-chromatography coupled to mass spectrometry (LC-MS). All samples were extracted immediately after sample collection to avoid degradation of heroin and 6-MAM. Standard curves were prepared using bovine serum and were linear in the range of 5 ng/ml to 200 ng/ml for 6-MAM and 10 ng/ml to 200 ng/ml for morphine. The internal standards were the deuterated analogues of 6-MAM and morphine (Cerilliant Analytical Reference Standards, Round Rock, TX). Samples were analyzed on a 2010A Shimadzu (Tokyo, Japan) single-quadrupole LC-MS with reversed phase Agilent (Santa Clara, CA) Zorbax XDB-C18 (2.1 mm × 50 mm i.d., 3.5 μm) column with a reversed phase 4 mm × 2 mm C18 guard column (Phenomenex, Torrance, CA) and a gradient mobile phase mixture of 10 mM formate buffer pH 3.0 and acetonitrile. Total run time was 5 minutes. Validation was based on a six point standard curve prepared in triplicate (within-run) for each day on five different days (between run, N=15). The within day variability was between 2.2% and 7.9%, the between day variability 1.9% and 6.9%, and the limit of quantitation 5 ng/ml.
Stoichiometric relationships
The total number of moles/kg of anti-morphine IgG elicited in rats vaccinated with M-KLH was calculated as the product of the mean serum anti-morphine IgG concentration and its reported volume of distribution of 131 ml/kg in rats [22]. The corresponding number of drug-binding sites of IgG was twice that number since there are 2 binding sites per IgG. The % saturation of anti-morphine IgG in serum was calculated as the molar ratio of the antibody-bound serum ligand concentration (6-MAM + morphine) to the serum anti-morphine IgG binding site concentration. Antibody-bound drug concentration in serum was calculated as (total 6-MAM + morphine concentration in the M-KLH group) minus (mean 6-MAM + morphine concentration in the control KLH group). The drug assay used measured total (bound and unbound) drug in each sample so that the total concentration in the M-KLH group represented both bound and unbound drug, while the concentrations in the control group represented only unbound drug since there are not antibodies present. The difference between these therefore provided an estimate of the bound drug concentration.
Statistical analysis and calculations
Group differences were analyzed by one-way analysis of variance followed by Bonferroni post hoc test. Relationships between serum antibody titers or concentrations and drug concentrations in serum and brain were analyzed by linear regression within groups and analysis of covariance was used to compare their linear regression slopes across groups.
RESULTS
Synthesis and conjugation of M-KLH and OXY-KLH vaccines
Haptenation ratios for M(Gly)4 or OXY(Gly)4 conjugated to BSA or OVA ranged from 17 to 21 moles of hapten per mole of carrier protein. Haptenation ratios for the M-KLH and OXY-KLH conjugates could not be measured by mass spectrometry due to the larger size of KLH.
Antibody response in rats
Monovalent M-KLH
Immunization with M-KLH alone elicited serum antibodies that were highly specific for heroin, 6-MAM and morphine. Serum from rats vaccinated with M-KLH showed 20% cross-reactivity with the OXY-OVA coating antigen on ELISA, and negligible cross-reactivity with unconjugated OVA alone (Table 1). Competition ELISA showed high relative affinities of antiserum (low IC50 values) for heroin, morphine and 6-MAM, and a low relative affinity for oxycodone (See Supplemental Table 1). Cross-reactivity with the (Gly)4 linker alone was negligible. Serum anti-morphine IgG concentrations were consistently high, with a mean ± SD of 558 ± 62 μg/ml and no values below 452 μg/ml.
Table 1.
Serum antibody titers (× 103) and cross-reactivity (%)#
| Vaccine | |||
|---|---|---|---|
| M-KLH | OXY-KLH | M-KLH + OXY-KLH | |
| Coating immunogen | |||
| M-BSA | 140 ± 47 | 18 ± 14 (18%) | 325 ± 220* |
| OXY-OVA | 29 ± 17 (21%) | 102 ± 78 | 239 ± 172* |
Cross-reactivity for each monovalent vaccine was calculated as the ratio of titer against the non-target immunogen/target immunogen.
p< 0.05 v. monovalent control
Monovalent OXY-KLH
Immunization with OXY-KLH alone elicited serum antibodies that were highly specific for oxycodone and oxymorphone. Serum from rats vaccinated with OXY-KLH showed 18% cross-reactivity with the M-BSA coating antigen on ELISA and negligible cross-reactivity with unconjugated BSA alone (Table 1). Competition ELISA values showed high relative affinities for oxycodone and oxymorphone, low relative affinity for heroin, 6-MAM or morphine, and negligible cross-reactivity with the (Gly)4 linker alone or the endogenous opioid Leu-enkephalin similarly to our previous report [20].
Bivalent M-KLH and OXY-KLH
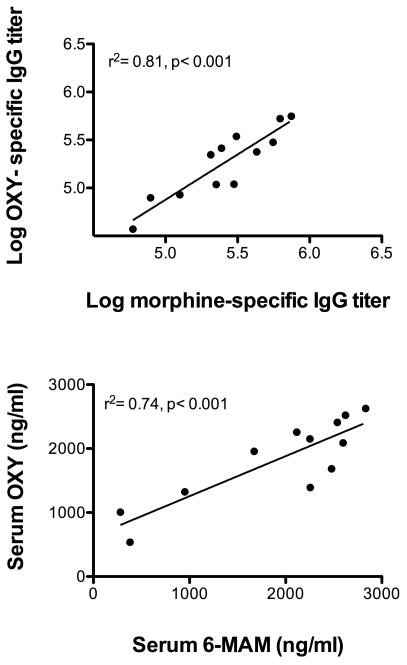
Rats immunized with the bivalent vaccine developed serum antibody titers against each immunogen that were higher than in rats vaccinated with monovalent vaccines alone (Table 1). These titers were greater than could be accounted for simply by adding the increase in titers attributable to cross-reactivity of the antibodies as determined by ELISA. For example, the anti-morphine titer in the bivalent group of 325 × 103 was greater than the anti-morphine titer of 140 × 103 in the group vaccinated with monovalent M-KLH even allowing for an additional titer of 29 × 103, which would be expected in the bivalent group due to cross-reactivity from immunization with OXY-KLH. Antibody specificities in the bivalent group, as determined by IC50 values, were similar to those obtained with the monovalent vaccines. While there was considerable individual variability in serum antibody titers in the bivalent group, there was a high correlation between anti-morphine and anti-oxycodone antibody titers measured in individual animals (Figure 2).
Figure 2.
(Upper panel) Relationship of log serum morphine-specific and oxycodone-specific antibody titers in the bivalent vaccine group showing a significant positive correlation. (Lower panel) Serum 6-MAM and oxycodone concentrations in the bivalent group were also correlated.
Antibody response in mice
Monovalent OXY-KLH vaccine produced comparable antibody titers in BALB/c mice whether injected i.p in Freund’s adjuvant (43±14 × 103, mean±SE) or s.c. in alum adjuvant (44±16 × 103) (p> 0.05).
Effects of vaccination on drug distribution
Monovalent M-KLH
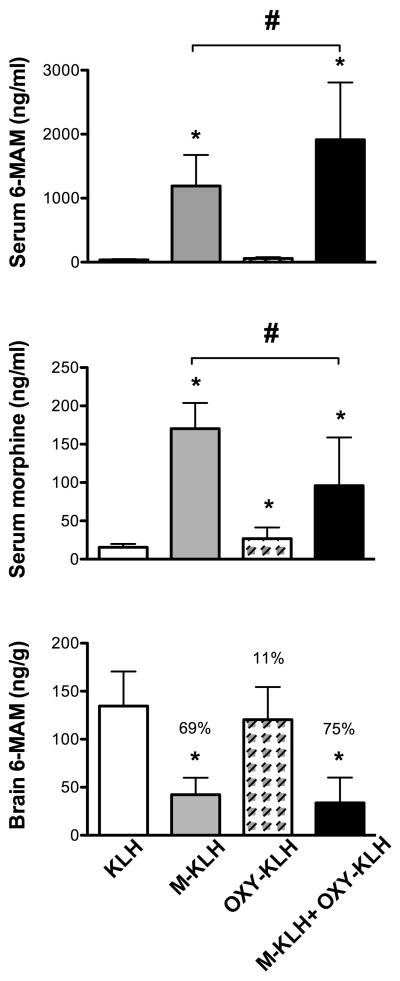
Vaccination with M-KLH substantially increased the retention of 6-MAM and morphine in serum (Figure 3). Distribution of 6-MAM to brain was reduced by 69% compared to controls. Morphine concentrations in brain in all groups were below assay sensitivity (<50 ng/ml). Serum anti-morphine antibody concentration was inversely correlated with the brain 6-MAM concentration (See Supplemental Figure 2). Vaccination with M-KLH also produced a small (33%) but significant decrease in distribution of oxycodone to brain (Figure 4), consistent with ELISA titers showing some cross-reactivity of anti-morphine antibodies with the oxycodone immunogen. The mean total number of IgG drug-binding sites in rats vaccinated with M-KLH was 0.98 μmol/kg, nearly 3-fold greater than the administered 6-MAM dose of 0.32 μmol/kg. The calculated saturation of serum anti-morphine IgG with its ligands (6-MAM + morphine) in the monovalent M-KLH group was 54 ± 15% (mean±SD).
Figure 3.
Vaccine effects on (upper panel) 6-MAM retention in serum, (middle panel) morphine retention in serum, and (lower panel) 6-MAM distribution to brain. Note 10-fold different vertical axis scales for 6-MAM and morphine serum concentrations. Numbers in parentheses are the % decrease compared to the KLH control group brain concentrations. Morphine distribution to brain is not shown because concentrations were below the assay detection limit. Data are the mean±SD. * p < 0.05 compared to KLH control, # p< 0.05 bivalent compared to monovalent vaccine.
Figure 4.
Vaccine effects on oxycodone concentrations in serum (upper panel) and brain (lower panel). Numbers in parentheses are the % decrease compared to the KLH control group. Data are the mean±SD, * p < 0.05 compared to KLH control.
Monovalent OXY-KLH
Vaccination with OXY-KLH increased retention of oxycodone in serum and reduced oxycodone distribution to brain by 66% (Figure 4). OXYM concentrations in all groups were too low to quantitate (<5 ng/ml). Vaccination with OXY-KLH produced a small increase in serum retention of morphine (Figure 3) but had no significant effect on serum or brain 6-MAM concentrations.
Bivalent M-KLH and OXY-KLH
Vaccination with the bivalent vaccine preserved the effects of the monovalent vaccines, significantly increasing 6-MAM and oxycodone retention in serum and decreasing their distribution to brain (Fig 3 and 4). The effects of the bivalent vaccine were generally similar to those of the monovalent vaccines alone. The bivalent vaccine increased serum retention of 6-MAM significantly more than the monovalent M-KLH vaccine. However the bivalent vaccine produced less retention of morphine in serum than the monovalent M-KLH vaccine, possibly due to increased binding of the precursor 6-MAM by the bivalent vaccine resulting in less conversion to morphine. Brain (p = 0.12) or serum (p = 0.06) oxycodone concentrations did not significantly differ between the bivalent and monovalent groups, although the difference in serum concentrations approached significance.
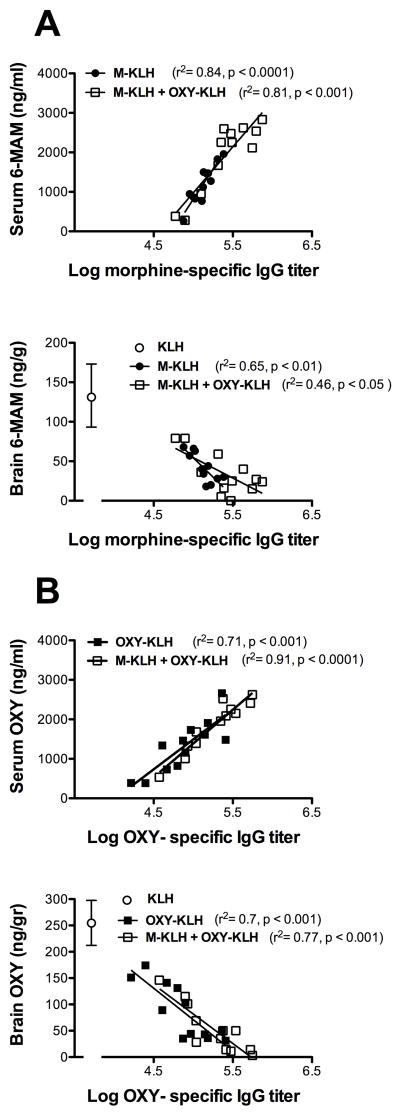
Vaccine effects on serum and brain 6-MAM or oxycodone concentrations were highly correlated with the corresponding serum antibody titers (Figure 5). The relationships between drug-specific titers and drug concentrations regression slopes were not significantly different between the monovalent and bivalent groups (all p >0.2).
Figure 5.
Relationships between log serum antibody titers and serum or brain 6-MAM (A) or oxycodone (B) concentrations. Brain concentrations of drug for the KLH control group are also shown (white open circle). Separate regression lines are shown for monovalent and bivalent vaccines in each panel, and their slopes did not differ significantly between groups.
DISCUSSION
Opioid vaccines are of interest as a potential adjunct to the treatment of opioid abuse or addiction but the wide variety of commonly abused opioids makes this challenging. Individual opioid vaccines reflect the high specificity of humoral antibody responses and each targets only a limited range of opioids. Vaccines based on morphine conjugates produce antibodies, which bind heroin and its active metabolites 6-MAM and morphine but have substantially lower affinity for oxycodone [14, 19]. A vaccine based on an oxycodone conjugate produces antibodies, which bind oxycodone and its active metabolite oxymorphone but have substantially lower affinity for heroin and its metabolites [20]. In the current study combining M-KLH and OXY-KLH vaccines allowed their concurrent use while fully retaining their individual immunogenicity and effect on opioid pharmacokinetics. These observations provide proof of concept that a broad range of opioid coverage can be obtained through the use of multivalent vaccines.
The use of multivalent vaccines is well established for infectious diseases. Unrelated vaccines (e.g. DPT = diphtheria, pertussis, tetanus; MMR = measles, mumps, rubella) can be combined for convenience, or administered as separate injections at the same time, with little or no loss of efficacy. In principle the same should be true of conjugate vaccines constructed from small molecule haptens. This has been shown to be feasible for nicotine vaccines through the use of different linker positions to create immunologically distinct haptens [23]. Each of these haptens activated different populations of B cells to produce distinct populations of antibodies to nicotine. The current study extends this approach to opioids with the goal of targeting a wider range of opioids.
The efficacy of the M-KLH vaccine was studied using 6-MAM, which has a higher affinity for the mu opioid receptor than heroin and is present in brain at higher concentrations. 6-MAM is considered the principal mediator of the early reinforcing and rewarding effects of heroin [21]. A number of morphine-conjugate vaccines similar to M-KLH, which also utilize the C6 linker position, have been shown to block heroin self-administration in animals [14, 15, 18]. While the influence of M-KLH on heroin pharmacokinetics needs to be specifically studied, the high serum concentration of antibodies produced is comparable to the highest reported with other morphine-conjugate vaccines [15].
The OXY-KLH vaccine targeted oxycodone but the resulting antibodies also had a high affinity for oxymorphone. Oxymorphone is only a minor metabolite of oxycodone in humans but it is marketed separately as an analgesic medication. Tighter regulation of oxycodone prescribing and reformulation of oxycodone into tablets that are more difficult to dissolve for intravenous injection has led to increased abuse of alternative opioids including oxymorphone [6]. The potential use of the OXY-KLH vaccine to block oxymorphone distribution and effects is therefore of interest. Similarly, the relatively low IC50 values of both the M-KLH and OXY-KLH vaccines for hydrocodone suggest their efficacy might extend to this drug as well.
Antibodies generated by both vaccines showed some cross-reactivity as measured by binding to immunogen conjugates on ELISA plates. Cross-reactivity in the setting of a bivalent vaccine is beneficial, as it allows both immunogen to contribute to binding either 6-MAM or oxycodone. Antibody titers to each immunogen in the bivalent group were however higher than could be accounted for by ELISA cross-reactivity alone. Since antibodies may bind differentially to the drug hapten alone compared to the corresponding hapten-linker immunogen [24], it is possible that there was additional cross reactivity of antibodies that was not detected by ELISA. The ELISA used immunogens with linkers attached, which may cover some epitopes that free drug in serum could nevertheless bind to. It is also possible that antibody affinity was enhanced in the bivalent group, but the generally similar IC50 values for bivalent and monovalent vaccines suggest this was not the case.
Consistent with enhanced titers in the bivalent group, 6-MAM retention in serum was greater and distribution to brain lower compared to the monovalent M-KLH group. Morphine retention in serum was less in the bivalent group, perhaps because increased binding of 6-MAM led to less conversion to morphine. Oxycodone concentrations showed a similar but non-significant trend toward greater efficacy in the bivalent group. Nevertheless, the most important result from the drug level measurements is confirmation that the two vaccines maintained their efficacy and did not interfere with each other when combined.
A limitation with available nicotine and cocaine vaccines has been the high variability in antibody titers and concentrations they produce in both animals and humans, with some subjects having levels too low to produce their desired effect [12, 13, 25–27]. The absence of such non-responders to M-KLH or OXY-KLH vaccines, and the very high serum antibody concentrations produced, suggests that their efficacy may be more uniform than that of nicotine or cocaine vaccines. Morphine-conjugate vaccines studied by others have also reported uniformly high antibody titers or concentrations and it may be that opioids in general are particularly effective haptens compared to nicotine or cocaine [15, 19].
A cautionary note regarding the high serum antibody concentrations produced by opioid vaccines is that it is often more difficult to achieve very high serum antibody concentration in humans than it is in experimental animals. The reasons for this are not entirely clear. The current study used the i.p. route and Freund’s adjuvant in rats, which are not appropriate for use in humans. However vaccination of mice by the s.c. route with alum adjuvant, which is acceptable in humans, generated anti-oxycodone antibody titers as high as vaccination i.p. with Freund’s. Other heroin or morphine vaccine studies have also successfully used the s.c. route and alum adjuvant. Whatever the reason, it may be challenging to produce serum antibody concentrations in humans as high as those measured in the current study. Because antibody concentrations were uniformly high, our data do not directly comment on whether such high concentrations were necessary to achieve substantial efficacy. However magnitude of effect did correlate with antibody titer or concentration within the range of values measured, and this will be an important question to address.
A limitation of this study is that animals received only a single dose of opioid rather than repeated or chronic administration, and only one dose size. Because serum antibody concentrations were quite high, the calculated number of antibody drug-binding sites provided by M-KLH vaccination (0.98 μmol/kg) exceeded the dose of 6-MAM administered (0.32 μmol/kg). It may be more challenging to block the effects of higher or repeated 6-MAM doses. Also, effects of vaccination on opioid pharmacokinetics were evaluated but vaccine effects on opioid-induced behaviors were not. However the OXY-KLH vaccine, used alone, has already been shown to reduce oxycodone distribution to brain at a 5-fold higher i.v. oxycodone dose and to block hot plate analgesia induced by a 20-fold higher s.c. oxycodone dose [20]. In addition, several morphine-conjugate vaccines have been shown to block heroin or morphine self-administration in rats, which involves repeated drug dosing [14, 19]. These questions need to be addressed for the bivalent vaccine, but similar efficacy seems likely.
The possible role of vaccines in addiction treatment is unclear because these vaccines are in early stages of development and the vaccines that have reached clinical trials to date have not had satisfactory immunogenicity [12]. It is unlikely that addiction vaccines will replace current therapies but they could serve as useful adjuncts. An opioid vaccine could be useful for opioid abusers who refuse agonist or antagonist treatment or who do not qualify for it because their use is occasional. Opioid vaccines may also be usable in combination with agonist therapy to provide efficacy during periods of agonist medication noncompliance or for those who persistently abuse opioids even while on agonist therapy. Opioid vaccines like M-KLH and OXY-KLH have been designed so that they target the desired opioids but not opioids, which may be needed for therapeutic use such as methadone, buprenorphine or naltrexone. The utility of this approach is not known, but highly immunogenic vaccines provide a tool for studying its potential. A bivalent vaccine which blocks a wide range of abusable opioids may have distinct advantages in areas of the world, like the USA, in which many different opioids are available and abused.
Supplementary Material
Highlights.
Two morphine and oxycodone vaccines were combined in a bivalent vaccine
Bivalent vaccine antibody titers were higher than monovalent vaccines alone.
Bivalent vaccine reduced distribution of both 6-MAM and oxycodone to brain
Combining two or more opioid vaccines to target multiple opioids is possible
Acknowledgments
This work was supported by NIH NIDA grants DA026300, DA030715, T32-DA07097 and a Minneapolis Medical Research Foundation Career Development Award (MP).
Abbreviations
- 6-MAM
6-monoacetylmorphine
- M
morphine
- OXY
oxycodone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Survey on Drug Use and Health: National Findings. U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Substance Abuse and Mental Health Services Administration Office of Applied Studies 2010; http://www.oas.samhsa.gov/nsduh/2k8nsduh/2k8Results.cfm
- 2.Maxwell JC. The prescription drug epidemic in the United States: a perfect storm. Drug Alcohol Rev. 2011;30(3):264–70. doi: 10.1111/j.1465-3362.2011.00291.x. [DOI] [PubMed] [Google Scholar]
- 3.Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83(Suppl 1):S4–7. doi: 10.1016/j.drugalcdep.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103–7. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 5.2008 Department of Defense Survey of Health Related Behaviors Among Active Duty Military Personnel. Department of Defence. 2009 http://www.tricare.mil/2008HealthBehaviors.pdf.
- 6.Oxymorphone Abuse: A growing Threat Nationwide. Department of Justice 2011; EWS Report 000011; http://www.justice.gov/ndic/pubs44/44817/sw0011p.pdf
- 7.EPIDEMIC: RESPONDING TO AMERICA’S PRESCRIPTION DRUG ABUSE CRISIS. Executive office of the president of the United States 2011; http://www.whitehouse.gov/sites/default/files/ondcp/policy-and-research/rx_abuse_plan.pdf
- 8.Butler SF, Black RA, Cassidy TA, Dailey TM, Budman SH. Abuse risks and routes of administration of different prescription opioid compounds and formulations. Harm Reduct J. 2011;8:29. doi: 10.1186/1477-7517-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodrill CL, Helmer DA, Kosten TR. Prescription pain medication dependence. Am J Psychiatry. 2011;168(5):466–71. doi: 10.1176/appi.ajp.2010.10020260. [DOI] [PubMed] [Google Scholar]
- 10.Wu LT, Woody GE, Yang C, Blazer DG. How do prescription opioid users differ from users of heroin or other drugs in psychopathology: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Addict Med. 2011;5(1):28–35. doi: 10.1097/ADM.0b013e3181e0364e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi KR. Vaccines move forward against a range of addictions. Nat Med. 2011;17(2):146. doi: 10.1038/nm0211-146. [DOI] [PubMed] [Google Scholar]
- 12.Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, Tashkin DP, Reus VI, Akhavain RC, Fahim RE, Kessler PD, Niknian M, Kalnik MW, Rennard SI. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther. 2011;89(3):392–9. doi: 10.1038/clpt.2010.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66(10):1116–23. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anton B, Leff P. A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents. Vaccine. 2006;24(16):3232–40. doi: 10.1016/j.vaccine.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 15.Anton B, Salazar A, Flores A, Matus M, Marin R, Hernandez JA, Leff P. Vaccines against morphine/heroin and its use as effective medication for preventing relapse to opiate addictive behaviors. Hum Vaccin. 2009;5(4):214–29. doi: 10.4161/hv.5.4.7556. [DOI] [PubMed] [Google Scholar]
- 16.Berkowitz B, Spector S. Evidence for active immunity to morphine in mice. Science. 1972;178(67):1290–2. doi: 10.1126/science.178.4067.1290. [DOI] [PubMed] [Google Scholar]
- 17.Berkowitz BA, Ceretta KV, Spector S. Influence of active and passive immunity on the disposition of dihydromorphine-H3. Life Sci. 1974;15(5):1017–28. doi: 10.1016/0024-3205(74)90016-2. [DOI] [PubMed] [Google Scholar]
- 18.Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature. 1974;252(5485):708–10. doi: 10.1038/252708a0. [DOI] [PubMed] [Google Scholar]
- 19.Stowe GN, Vendruscolo LF, Edwards S, Schlosburg JE, Misra KK, Schulteis G, Mayorov AV, Zakhari JS, Koob GF, Janda KD. A vaccine strategy that induces protective immunity against heroin. J Med Chem. 2011;54(14):5195–204. doi: 10.1021/jm200461m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pravetoni M, Le Naour M, Harmon T, Tucker A, Portoghese PS, Pentel PR. An oxycodone conjugate vaccine elicits oxycodone-specific antibodies that reduce oxycodone distribution to brain and hot-plate analgesia. J Pharmacol Exp Ther. 2012 doi: 10.1124/jpet.111.189506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersen JM, Ripel A, Boix F, Normann PT, Morland J. Increased locomotor activity induced by heroin in mice: pharmacokinetic demonstration of heroin acting as a prodrug for the mediator 6-monoacetylmorphine in vivo. J Pharmacol Exp Ther. 2009;331(1):153–61. doi: 10.1124/jpet.109.152462. [DOI] [PubMed] [Google Scholar]
- 22.Bazin-Redureau MI, Renard CB, Scherrmann JM. Pharmacokinetics of heterologous and homologous immunoglobulin G, F(ab′)2 and Fab after intravenous administration in the rat. J Pharm Pharmacol. 1997;49(3):277–81. doi: 10.1111/j.2042-7158.1997.tb06795.x. [DOI] [PubMed] [Google Scholar]
- 23.Pravetoni M, Keyler DE, Pidaparthi RR, Carroll FI, Runyon SP, Murtaugh MP, Earley CA, Pentel PR. Structurally distinct nicotine immunogens elicit antibodies with non-overlapping specificities. Biochem Pharmacol. 2011 doi: 10.1016/j.bcp.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tars K, Kotelovica S, Lipowsky G, Bauer M, Beerli RR, Bachmann MF, Maurer P. Different binding modes of free and carrier-protein-coupled nicotine in a human monoclonal antibody. J Mol Biol. 2012;415(1):118–27. doi: 10.1016/j.jmb.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 25.Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol Psychiatry. 2010;67(1):59–65. doi: 10.1016/j.biopsych.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kantak KM, Collins SL, Bond J, Fox BS. Time course of changes in cocaine self-administration behavior in rats during immunization with the cocaine vaccine IPC-1010. Psychopharmacology (Berl) 2001;153(3):334–40. doi: 10.1007/s002130000555. [DOI] [PubMed] [Google Scholar]
- 27.Pravetoni M, Keyler DE, Raleigh MD, Harris AC, Lesage MG, Mattson CK, Pettersson S, Pentel PR. Vaccination against nicotine alters the distribution of nicotine delivered via cigarette smoke inhalation to rats. Biochem Pharmacol. 2011;81(9):1164–70. doi: 10.1016/j.bcp.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.