Abstract
Coccidioides is a fungal pathogen and causative agent of a human respiratory disease against which no clinical vaccine exists. In this study we evaluated a novel vaccine adjuvant referred to as EP67, which is a peptide agonist of the biologically active C-terminal region of human complement component C5a. The EP67 peptide was conjugated to live spores of an attenuated vaccine strain (ΔT) of C. posadasii. The non-conjugated ΔT vaccine provided partial protection to BALB/c mice against coccidioidomycosis. In this report we compared the protective efficacy of the ΔT-EP67 conjugate to the ΔT vaccine in BALB/c mice. Animals immunized subcutaneously with the ΔT-EP67 vaccine showed significant increase in survival and decrease in fungal burden over 75 days postchallenge. Increased pulmonary infiltration of dendritic cells and macrophages was observed on day 7 postchallenge but marked decrease in neutrophil numbers had occurred by 11 days. The reduced influx of neutrophils may have contributed to the observed reduction of inflammatory pathology. Mice immunized with the ΔT-EP67 vaccine also revealed enhanced expression of MHC II molecules on the surface of antigen presenting cells, and in vitro recall assays of immune splenocytes showed elevated Th1- and Th17-type cytokine production. The latter correlated with a marked increase in lung infiltration of IFN-γ- and IL-17-producing CD4+ T cells. Elevated expression of T-bet and RORc transcription factors in ΔT-EP67-vaccinated mice indicated the promotion of Th1 and Th17 cell differentiation. Higher titers of Coccidioides antigen-specific IgG1 and IgG2a were detected in mice immunized with the EP67- conjugated versus the non-conjugated vaccine. These combined results suggest that the EP67 adjuvant enhances protective efficacy of the live vaccine by augmentation of T-cell immunity, especially through Th1- and Th17-mediated responses to Coccidioides infection.
Keywords: Adjuvant, Vaccine, Coccidioides, C5a, EP67
1. Introduction
Coccidioides posadasii and C. immitis are primary fungal pathogens of humans which can cause a mild to potentially life-threatening respiratory disease known as coccidioidomycosis or San Joaquin Valley fever [1]. Although the two species show some degree of genetic diversity revealed by comparative genomic sequence analyses, they demonstrate no significant difference in virulence in mice [2]. Human infection typically occurs by inhalation of Coccidioides spores released into the air from the saprobic phase of the soil borne fungus. Coccidioidomycosis is a reemerging infectious disease indicated by a major increase in the number of cases reported in the United States during the past decade (3). The health impact and cost of long-term antifungal therapy of patients who contract this disease support the need for a vaccine against coccidioidomycosis [3–5]. A compelling argument for the feasibility of generating such a vaccine is based on retrospective clinical observations that symptomatic human infection with Coccidioides results in lifelong immunity against recurrent coccidioidal disease [6].
Both clinical and animal studies have confirmed that T cell-mediated immune responses to Coccidioides infection, especially those associated with T helper (Th)1 and Th17 signal pathways, are pivotal for protection against this respiratory pathogen [7,8]. The mouse has been the most extensively used animal model for studies of immunity to coccidioidal infection [9]. Results of these investigations have revealed that the effectiveness of vaccination against this respiratory mycosis can be reliably predicted by the nature of host immune responses within the first two weeks after intranasal challenge (8). Differences in susceptibility to disease have been observed between murine strains [10]. BALB/c mice are particularly susceptible to pulmonary and disseminated forms of coccidioidomycosis following intranasal challenge with a suspension of ≤ 50 viable spores. We have recently generated a genetically-engineered, live, attenuated vaccine (ΔT) that partially protects BALB/c mice against lung infection with a virulent isolate of Coccidioides. However, in spite of its stimulation of a robust immune response this vaccine does not achieve sterile immunity [11]. In addition, an increase in the intranasal challenge dose from 50 to 100 spores results in a marked reduction in the protective efficacy of the ΔT vaccine. We have used this animal model of coccidioidomycosis to test whether the protective response of BALB/c mice immunized with the live vaccine and challenged with the higher spore number could be enhanced by the addition of an adjuvant. The experimental adjuvant investigated in this study is EP67, a decapeptide characterized as a conformationally-biased, response-selective agonist of the biologically active C-terminal region of human complement component C5a [12, 13]. EP67 is the product of specific-residue substitutions in the C5a65-74 fragment. Its amino acid sequence is YSFKDMP(MeL)aR. The purpose of the substitutions is to restrict backbone flexibility in order to bias certain topographical features that allow a conformational distinction between beneficial C5a-like immune stimulatory responses versus undesirable inflammatory activity. The unique conformational features of the adjuvant are accommodated by the C5a receptor (C5aR/CD88) expressed on antigen presenting cells (APCs), particularly dendritic cells (DC) and macrophages, but not by C5a receptors on neutrophils [14, 15]. EP67 has been shown to induce Th1-type cytokine release from APCs and enhance antigen processing and presentation by human DC [12, 13, 16]. We have tested the adjuvant using vaccines in which target antigens were covalently linked to the N-terminus of EP67 and have reported that robust antigen-specific humoral and/or cell-mediated immune responses occurred [13, 16–18]. The functional concept is that the EP67 moiety directs the conjugated antigens to the C5aR-bearing DC and simultaneously activates the antigen processing and presentation capacity of the APCs. Our objective in this study was to compare the protective efficacy of a ΔT-EP67-conjugated, live attenuated Coccidioides vaccine versus the previously reported ΔT vaccine (11) against coccidioidomycosis in BALB/c mice challenged intranasally with a lethal inoculum of viable Coccidioides spores.
2. Materials and methods
2.1. Mice
Female, 8-week old BALB/c mice (20–25 gm) were obtained from the National Cancer Institute/Charles River Laboratory. The mice were housed in a pathogen-free animal facility at the University of Texas at San Antonio (UTSA) and handled according to guidelines approved by the university Institutional Animal Care and Use Committee. Mice were relocated prior to vaccination and infection to an animal biosafety level 3 (ABSL3) laboratory, which is located on the UTSA campus and has been certified by the Centers for Disease Control and Prevention (CDC).
2.2. Preparation of the ΔT and ΔT-EP67 vaccines
A previously-described genetically-engineered, live, attenuated mutant strain (Δcts2/ard1/cts3) derived from a virulent parental isolate (C735) of C. posadasii [11] was employed as a vaccine and designated “ΔT” [8, 11]. The mutant strain was grown on GYE medium (1% glucose, 0.5% yeast extract, 1.5 % agar) at 30 °C for 3 to 4 weeks to generate a confluent layer of spores on the agar surface. Spores were harvested from the saprobic culture as previously reported [11], washed twice, and resuspended in endotoxin-free PBS in preparation for immediate subcutaneous immunization of BALB/c mice as previously described [11], or for conjugation of the cells with EP67 prior to immunization as outlined below. The number of viable spores was determined by hemocytometer counts and culture analysis as reported [11].
Conjugation of EP67 to live spores isolated from the ΔT vaccine strain was performed by a single-step process as previously described [19]. Briefly, 107 viable spores were incubated with 1000 μg/ml of succinimidyl-4-benzoylhydrazino-nicotinamide-EP67 (S4BHyNic-EP67) in 1 ml of 0.1 M phosphate buffer, pH 8.0 for 2 h at room temperature. The S4BHyNic-modified N-terminus of EP67 in the presence of live spores of Coccidioides forms stable amide linkages with ε-amino groups of lysine residues of proteins exposed on the cell surface. The conjugated spores were washed three times with ice-cold PBS, resuspended in 1 ml of PBS, and stored at 4°C until used for vaccination of mice. The EP67 covalent linkage to the ΔT spores has a strong molar absorption at 354 nm. Aliquots of the conjugated spore suspension (106 spores in 1 ml of PBS) were tested for viability in dilution plate cultures, and subjected to UV spectrophotometric analysis (240–400 nm, path length of 1 cm) for evaluation of the amount of EP67 bound to the spore surface as previously reported [19]. The OD354 of the ΔT-EP67-conjugated spores ranged from 0.2 to 0.4. The amount of EP67conjugated to the cell surface was determined by the Beer/Lambert law (A = εlc) in which A = absorption at 354 nm, ε = molar absorptivity (L mol−1cm−1), l = cuvette path length, and c = concentration of EP67 in mol/L [19].
2.3. Immunization and challenge protocols
Primary immunization of BALB/c mice with either the ΔT vaccine or ΔT-EP67 conjugate vaccine was performed in the abdominal region by the subcutaneous route with 5.0 × 104 viable spores in 100 μl PBS. This initial immunization step was followed 14 days later with a boost of 2.5 × 104 spores of the same ΔT or conjugated vaccine. Control mice were immunized with 0.5 μg of EP67 alone (for both primary immunization and boost) dissolved in 100 μl PBS following the same vaccination schedule as above. The total amount of EP67 in the conjugate vaccine injected per mouse was estimated on the basis of the amount of EP67 bound to 7.5 × 104 Coccidioides spores, which ranged between 0.64 and 1.2 μg. In all cases, mice were challenged four weeks after completion of the vaccination protocol by intranasal instillation with ≥ 100 viable spores of the virulent isolate of C. posadasii (C735) suspended in 35 μl PBS as previously reported [11]. The fungal burden in lungs and spleen were determined at 14 and 75 days postchallenge by plating serial dilutions of separate lung and spleen homogenates on GYE agar containing 50 μg/ml chloramphenicol as reported [11]. The number of colony-forming units (CFU) of Coccidioides was expressed on a log scale and reported as box plots for each group of 20 animals as previously described [8].
2.4. Isolation of pulmonary leukocytes
Vaccinated and non-vaccinated mice infected with Coccidioides were sacrificed at 7 or 11 days postchallenge (4 mice per time point) and their excised lungs were used as sources of pulmonary leukocytes. The procedure employed for isolation of total pulmonary leukocytes was the same as previously reported [8]. The number of viable leukocytes obtained from each lung was visualized by light microscopy using a trypan blue exclusion test and quantified by hemocytometer counts as previously described [8].
2.5. Quantification of selected innate immune cell types and activated CD4+ and CD8+ T cells in Coccidioides-infected lungs
Isolated total pulmonary leukocytes (0.5–2.0 × 106 viable cells/ml) from vaccinated or non-vaccinated mice infected with Coccidioides spores were prepared for quantitative and qualitative analyses, which were conducted by fluorescence-activated cell sorting (FACS) with a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ) as previously reported [8, 20]. The absolute numbers of immune cells in lung homogenates were determined from data acquired by flow cytometry with CellQuest Pro software (BD Biosciences) and analyzed using a FlowJo software package (Tree Star, Inc., Ashland, OR). Selected fluorochrome-labeled monoclonal antibodies (MAb) were used for flow cytometry to identify and quantify neutrophils, dendritic cells (DC), tissue macrophages (MΦ), CD4+ T cells (both total and activated cells) in lung homogenates obtained from vaccinated and non-vaccinated, Coccidioides-infected mice at 7 and 11 days postchallenge as previously described [8]. Standard methodology was employed for direct MAb labeling and enumeration of selected pulmonary immune cells by FACS analysis as reported [8]. The expression level of CD44, an adhesion molecule that binds to hyaluronic acid, is elevated in activated T cells and was used as an activation marker [8]. A cocktail of fluorochrome-labeled anti-CD45, anti-CD4, anti-CD8, and anti-CD44 (BD Biosciences) was used to determine absolute numbers of activated CD4+ T cells as reported [8]. Although we did not examine CD8+ T cells in this study, it was necessary to include ant-CD8 MAb to specifically gate for CD4+ T cells. The total number of pulmonary leukocytes in each lung sample was quantified by multiplying the total number of hemocytometer-determined viable cell counts by the percentage of CD45+ cells determined by flow cytometry using fluorescent anti-CD45 MAb (clone 30-F11) obtained from BD Biosciences. The absolute numbers of each subpopulation of selected cell types in lung homogenates of vaccinated and non-vaccinated mice at 7 and 11 days postchallenge were calculated by multiplying the percentage of each gated subpopulation by the total number of viable pulmonary leukocytes derived from hemocytometer counts as described above.
2.6. Histopathology
Comparative histopathology was conducted with excised lungs of non-vaccinated, ΔT- vaccinated, and ΔT-EP67-vaccinated BALB/c mice (three animals per group) which were challenged intranasally with Coccidioides spores as described above. The non-vaccinated mice were injected subcutaneously with adjuvant (EP67) alone as above. Tissue fixation and embedding procedures were performed as described previously (8), and sections were stained with hematoxylin and eosin (H&E) by standard procedure. Lung sections were examined using a Leica DMI6000 microscope equipped with an automated turboscan stage (Objective Imaging, Ltd. Cambridge, UK). Microscope images of the infected tissue sections were acquired and analyzed using Surveyor software (Objective Imaging, Cambridge, UK). The mice were sacrificed at 14 days postchallenge.
2.7. Intracellular cytokine staining (ICS)
Permeabilized pulmonary leukocytes were stained with selected fluorochrome- conjugated monoclonal antibodies (anti-IFNγ, anti-IL5, or anti-IL-17A) to determine absolute numbers of the specific cytokine-producing CD4+ T cells in the lungs of vaccinated and non- vaccinated mice as previously described [8]. The absolute numbers of the specific cytokine- producing CD4+ T cells relative to the total lung-infiltrated leukocytes per lung homogenate at 7 and 11 days postchallenge were calculated as above. The percentages of specific cytokine- producing CD4+ T cells per lung at 11 days postchallenge relative to the total gated CD45+ CD4+ CD8− CD44+ T cells which had infiltrated the lungs of vaccinated and non-vaccinated mice were determined from data acquired by flow cytometry and analyzed using a FlowJo software package as above.
2.8. Quantification of APC surface expressed MHC II
Standard fluorocytometric methods were employed for quantification of MΦ (macrophages), dendritic cells (DC), and B cells (not shown) in lung homogenates as above, combined with determination of their expression levels of CD80/86 and CD40 costimulatory and MHC II molecules at 7 and 11 days postchallenge as previously described [7]. The median fluorescence intensity (MFI) of the MHC II-labeled antigen-presenting cells was determined for each gated population using FlowJo software.
2.9. In vitro recall assays of immune splenocytes isolated from vaccinated and non-vaccinated mice
At 7 days postchallenge spleens of vaccinated and non-vaccinated mice were separately harvested, pooled, and macerated as previously described [8, 20]. Non-vaccinated mice were immunized with EP67 alone. The T cell composition of splenocyte preparations was typically 22–26% CD4+ and 12–16% CD8+ T lymphocytes. Immune splenocytes derived from ΔT- and ΔT- EP67-vaccinated mice were cultured either for 48 h in media alone (negative control), or with Coccidioides T27K antigen (40 μg/ml). The latter is an immunogenic preparation derived from endosporulating spherule homogenates of C. posadasii [21]. The T27K antigen has been shown to stimulate peripheral blood monocytic cells of patients with confirmed Coccidioides infection, and is routinely used in ex vivo assays for evaluation of Coccidioides-specific immune cell responses [21]. The splenocytes were cultured in 24-well plates (4 × 106 cells/well) in 2 ml of RPMI 1640 medium containing 10% (v/v) heat-inactivated FBS, 100 I.U./ml penicillin and 100 μg/ml streptomycin [11]. Immune cells incubated with ConA (10 ng/ml) served as a non- specific, positive control. Supernatants of the cell cultures were collected and assays of IL-2, IFN-γ, IL-4, IL-5, IL-17A, IL-23, IL-1β, IL-6, and TGF-β concentrations were determined using a Bio-Plex suspension array kits (Bio-Rad Laboratories, Hercules, CA) as previously reported [20]. Assays of lung homogenates of individual mice were performed in triplicate.
2.10. Quantitative RT-PCR assays of expression of selected transcription factors
Total RNA was extracted from isolated pulmonary cells of Coccidioides-infected lungs of non-vaccinated, ΔT-vaccinated or ΔT-EP67-vaccinated BALB/c mice (three animals per group) using a RNeasy kit (Qiagen, Valencia, CA). The samples were treated with DNAase (Promega, San Luis Obispo, CA) to remove traces of contaminating DNA. Reverse transcription was performed using 5 μg total RNA in a 50 μl reaction mixture containing oligo-dT and SuperScript III (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The genes encoding the T-box transcription factor (T-bet) [22], mouse GATA binding protein 3 (GATA-3) [23], retinoid-related orphan receptor γ (RORγt, encoded by the RORc gene) [24], and forkhead box P3 protein (Foxp3) [25] are specific transcription factors which function in the differentiation of Th1, Th2, Th17 and regulatory T (Treg) cells, respectively. The relative expression levels of T-bet, GATA-3, RORc, and Foxp3 in the lungs of non-vaccinated and vaccinated mice were examined by QRT-PCR with a 7900HT Fast Real-Time PCR System (ABI Applied Biosystems; Foster City, CA) using a Power SYBR Green Master Mix reagent (ABI Applied Biosystems) as recommended by the manufacturer. The quantitative RT-PCR data were derived using the comparative CT as previously reported [26]. The primer pairs used in these assays were as follows: forward 5′-CAACAACC CCTTTGCCAAAG-3′, and reverse 5′-TCCCCCAA GCAGTTGACAGT-3′ for T-bet (GenBank no. AAF61242.1); forward 5′-GAAGGCATCCAG ACCC GAAC-3′ and reverse 5′-ACCCATGGCGGTGACCATGC-3′ for GATA-3 (GenBank no. NM_008091); forward 5′-TGTCCTGGGCTACCCTACTGA-3′ and reverse 5′-CAAGGGATC ACTTCAATTTGTG-3′ for RORc (GenBank no. NM_011281); and forward 5′-CAGCTGCCT ACAGTGCCCCTAG-3′ and reverse 5- CATTTGCCAGCAGTGGG TAG-3′ for Foxp3 (GenBank no. NM_054039). Each reaction was run in triplicate and initially normalized to expression of the constitutive GAPDH gene. The following primer pair was used to determine the expression level of the mouse GAPDH (GenBank no. NM_008084): forward 5′-CATGGCCTTCCGTGTT CCTA-3′ and reverse 5′-GCGGCACGTCAGATCCA-3′. The following formula was then used to quantify the fold differential expression of each specific gene in pulmonary cells of mice vaccinated with ΔT or ΔT-EP67 compared to gene expression in non-vaccinated mice as previously described [27]: 2−ΔΔCT, where ΔΔCT = (CT of a test gene − CT of GAPDH of the vaccinated mice) − (CT of a test gene − CT of GAPDH of the non-vaccinated mice). The data are represented as means of fold change ± SEM.
2.11. Measurement of anti-Coccidioides antibody titers
An indirect enzyme-linked immunsorbent assay (ELISA) was conducted to measure titers of Coccidioides-specific antibodies in mouse sera as previously reported [28]. Briefly, blood samples were collected from 4 mice per group of vaccinated or non-vaccinated mice by heart puncture exsanguination at 7 days postchallenge. The T27K antigen (100 ng in 100 μl PBS) described above was added to high-binding 96-well plates (Corning Life Sciences, Lowell, MA) and allowed to bind overnight at 4°C. The wells of the plates were blocked with PBS containing 1% BSA and washed four times with sterile PBS containing 0.05% Tween 20 (Fisher Scientific, Pittsburgh, PA). Mouse sera were applied to the T27K antigen-coated wells in 100 μl serial dilutions and incubated for 1 h. The plates were washed with buffer followed by the addition of horseradish peroxidase-conjugated anti-mouse antibodies (specific for total IgG, IgG1, IgG2a, IgG2b, or IgG3; SouthernBiotech, Birmingham, AL) diluted to 1:4000 in 100 μl PBS. The plates containing the secondary antibodies were incubated for 1 h, and then washed with buffer followed by the addition of 100 μl/well of SureBlue™ TMB I peroxidase substrate (KPL Inc., Gaithersburg, MD) and incubated for an additional 20 min. Sulfuric acid (2 M, 50 μl/well) was added to stop the reaction. The optical density (OD450) was determined using a microplate reader (SpectraMax M5/M5e, Molecular Devices, Sunnyvale, CA). The absorbance of wells incubated with secondary antibody alone was designated as background and these OD values were subtracted from the absorbance of the test samples. The titers were interpolated as the dilution of each serum sample equal to the geometric mean of the serum absorbance. If the absorbance of a serum sample was too low to interpolate its geometric mean the titer was designated as 50, which is the lowest dilution used in these assays.
2.12. Statistical analyses
The Student’s t-test was used to compare absolute cell numbers, levels of MHC II expression, cytokine concentrations, and percentages of specific cytokine-producing CD4+ T cells in lung homogenates obtained from vaccinated and non-vaccinated mice at the indicated time points postchallenge as previously reported [11]. The Mann-Whitney U test was used to compare differences in the median CFU values for statistical significance as previously described [11]. All data except the box plots of CFU are presented as mean values ± standard error of the mean (SEM). A P value of < 0.05 was considered statistically significant.
3. Results
3.1. Conjugation of EP67 with spores of the ΔT vaccine significantly enhanced survival of BALB/c mice and clearance of Coccidioides from their lungs and spleen
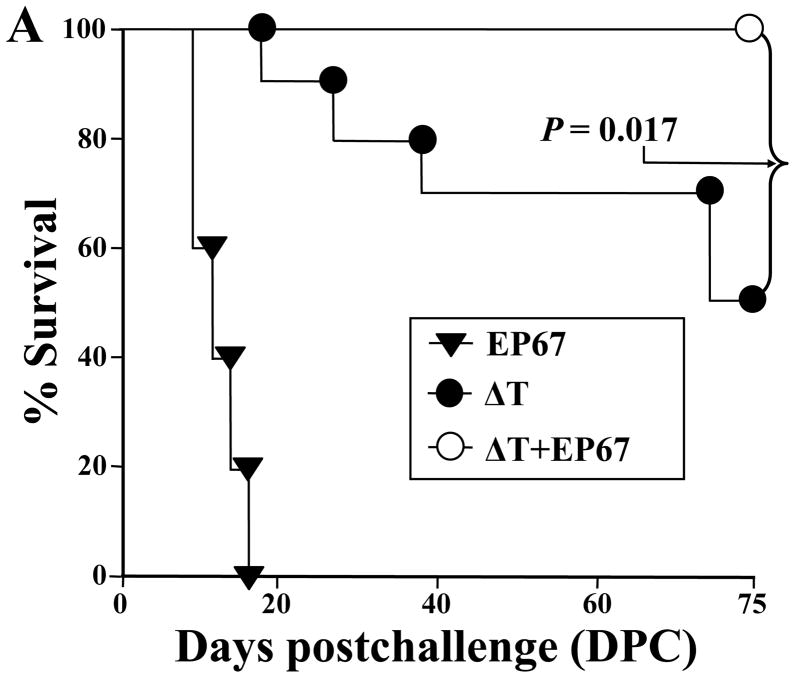
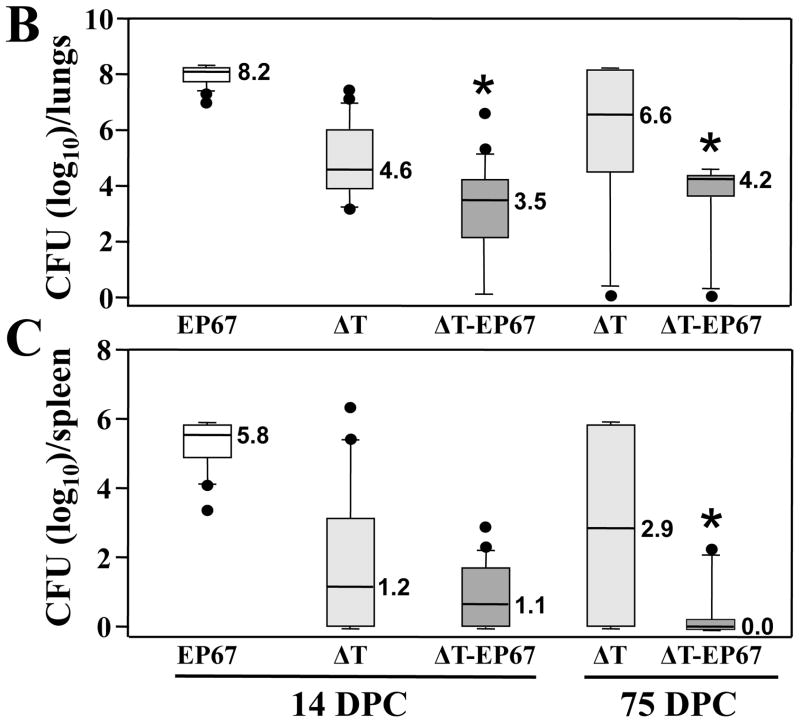
We speculated on the basis of our earlier vaccine studies with EP67 (13, 16–18) that addition of the adjuvant to the ΔT vaccine would significantly increase the protective immune response to Coccidioides infection in BALB/c mice. Protective immunity was evaluated by determining percent survival after intranasal challenge with a potentially lethal spore inoculum (Fig. 1A), and by measuring the fungal burden (CFU) in the lungs and spleen at 14 and 75 days postchallenge in mice that had been immunized with either the ΔT or ΔT-EP67 conjugate vaccine (Fig. 1B,C). Non-vaccinated mice immunized with EP67 alone showed signs of morbidity as early as 10 days postchallenge and all of these infected animals died by 18 days (Fig. 1A). At 14 days pcostchallenge, these control mice showed high CFUs in their lungs (8.2 log10) and spleen (5.8 log10) (Fig. 1B and C, respectively). The latter is indicative of a high level of dissemination of the pathogen from original sites of infection in the lungs. Mice immunized with the ΔT vaccine first showed signs of distress (e.g., ruffled fur) at 20 days after challenge, and by 75 days approximately 50% of these vaccinated animals had died. All mice immunized with the ΔT-EP67 conjugate vaccine, on the other hand, survived and appeared healthy throughout the course of examination. Both vaccinated groups of mice revealed significantly lower CFU in their lungs and spleen compared to non-vaccinated, infected control mice at 14 days postchallenge. Comparison of the fungal burden of vaccinated mice at 14 days revealed that the median CFUs in the lungs of ΔT-EP67-vaccinated mice (3.5 log10) was significantly lower than that of the ΔT-vaccinated mice (4.6 log10) (P = 0.02). At 75 days postchallenge the median CFUs in the lungs and spleen of mice immunized with the ΔT-EP67 conjugate vaccine were significantly lower than the median CFU values for the ΔT-vaccinated mice (P = 0.007 and 0.009, respectively).
Fig. 1.
BALB/c mice immunized with the ΔT-EP67-conjugate vaccine showed significantly increased survival (A) and reduced fungal burden in the lungs (B) and spleen (C) at 75 days postchallenge (DPC) compared to mice immunized with the non-conjugated, live, attenuated (ΔT) vaccine. Asterisks in (B) and (C) indicate statistically significant differences between CFUs in the lungs and spleen of the two groups of vaccinated mice. Although not significantly different, a trend was evident at 14 days postchallenge toward reduced fungal burden between the spleen of the ΔT-EP67-vaccinated versus ΔT-vaccinated mice. Control mice were immunized with EP67 alone. The boxes indicate the 25th and 75th percentiles. The horizontal lines within the boxes represent the medians. The bars above and below the boxes indicate the 90th and 10th percentiles, respectively. Outliers are indicated by solid circles and represent individual mice. The results are representative of two separate experiments.
3.2. BALB/c mice immunized with the ΔT-EP67 conjugate vaccine showed reduced inflammatory cell infiltration and less damage of host lung tissue compared to ΔT-vaccinated mice
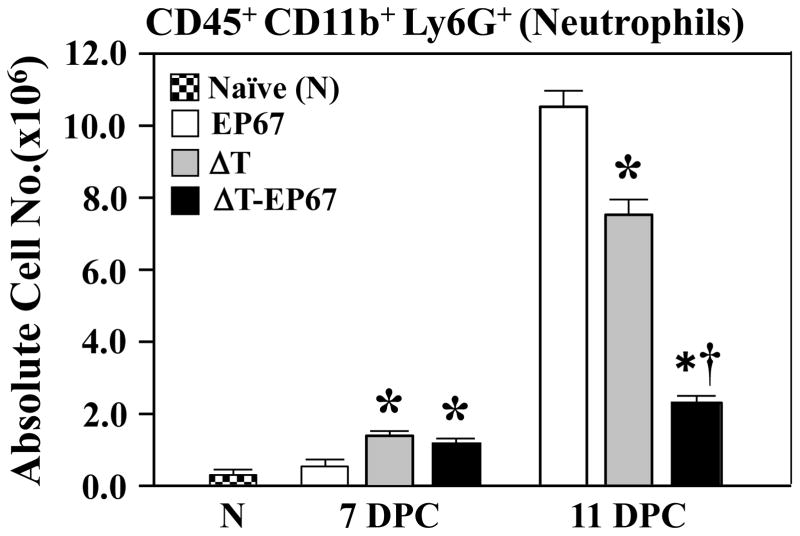
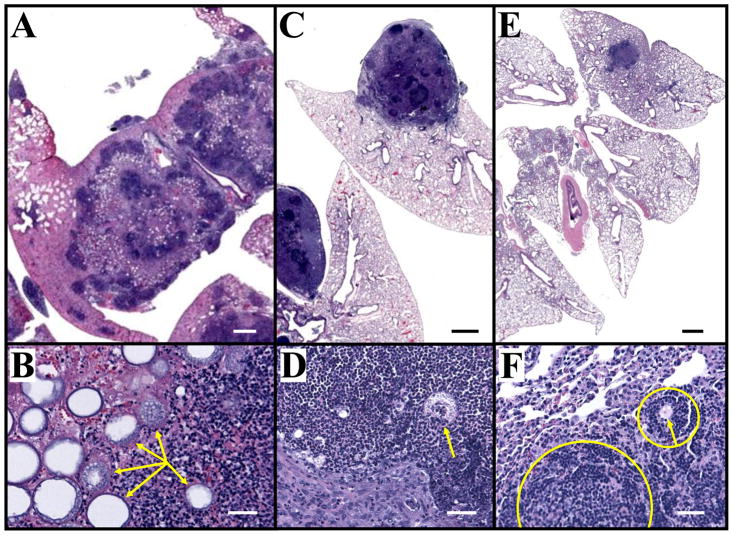
Recruitment of neutrophils to sites of Coccidioides infection in the lungs of mice and humans is a hallmark of pathogenic inflammation during early stages of coccidioidomycosis [8, 11, 29]. Flow cytometry was first employed to compare the absolute numbers of fluorescently- labeled CD45+CD11b+Ly6G+ cells which had infiltrated the infected lungs of ΔT- versus ΔT- EP67-vaccinated BALB/c mice at 7 and 11 days postchallenge (DPC; Fig. 2). Neutrophil numbers were equally and significantly elevated in the two groups of vaccinated mice compared to non-vaccinated controls at 7 days. However, at 11 days a statistically significant reduction of neutrophil recruitment was evident in lungs of mice vaccinated with the ΔT-EP67 conjugate vaccine compared to animals immunized with the non-conjugated ΔT vaccine. Control animals immunized with EP67 alone showed a major increase in infiltration of neutrophils into the lungs at 11 days postchallenge compared to the two groups of vaccinated mice. The high number of neutrophils detected in the lungs of non-vaccinated mice at 11 days correlated with microscopic observations in histological preparations at 14 days postchallenge of intense inflammatory response composed primarily of neutrophils, and indications of necrosis of lung tissue at multiple sites of pulmonary infection, (Fig. 3A,B). Paraffin sections of the lungs of mice immunized with the ΔT vaccine showed multiple large abscesses containing parasitic cells also surrounded by high numbers of neutrophils, monocytes, and lymphocytes without apparent organization (Fig. 3C,D). Some tissue damage was visible in these regions of inflammation. In contrast, the histopathology of infected lungs of mice immunized with the ΔT-EP67 conjugate vaccine revealed fewer and smaller abscesses that contained organized inflammatory cells surrounding the organisms which suggests an early stage of granuloma formation (Fig. 3E,F).
Fig. 2.
BALB/c mice immunized with the ΔT-EP67-conjugate vaccine revealed significantly reduced infiltration of neutrophils during early stages of Coccidioides infection compared to mice immunized with the non-conjugated ΔT vaccine. Bar graphs show absolute numbers of total neutrophils in lungs of ΔT- and ΔT-EP67-vaccinated mice at 7 and 11 postchallenge. Infected control mice were immunized with EP67 alone. Non-infected, non-vaccinated naïve mice were included to provide baseline values. Asterisks indicate statistically significant differences between mice vaccinated with the ΔT or ΔT-EP67 vaccine versus control mice immunized with EP67 alone (P < 0.05). The dagger represents statistically significant difference between the ΔT-EP67- and ΔT-vaccinated mice (P < 0.05). These results are representative of three separate experiments.
Fig. 3.
Histopathology of Coccidioides infected lungs of non-vaccinated (A, B), ΔT-vaccinated (C, D), and ΔT-EP67-vaccinated BALB/c mice (E, F) sacrificed at 14 days postchallenge. Arrows in (B), (D) and (F) locate parasitic cells of C. posadasii. Note the high density of infection sites in the lung of the non-vaccinated mouse (A). Most of the parasitic cells in the lungs of this representative mouse have a large, central vacuole and surrounding endospores, characteristic of the proliferation stage of the parasitic cycle. Much of the lung tissue in the region of the parasitic and inflammatory cells in (B) has undergone necrosis. The large abscesses of the ΔT-vaccinated mouse lung shown in (C) contain sites of concentrated inflammatory cells without apparent organization visible at higher magnification in (D). In contrast, the single, small abscess in the infected lung of the ΔT-EP67-vaccinated mouse (E) contains organized host inflammatory cells at infection sites (F), which is indicative of an early stage of granuloma formation (circled and partly circled host tissue). Bars in (A, C, E) represent 1.0 mm. Bars in (B, D, F) represent 100 μm.
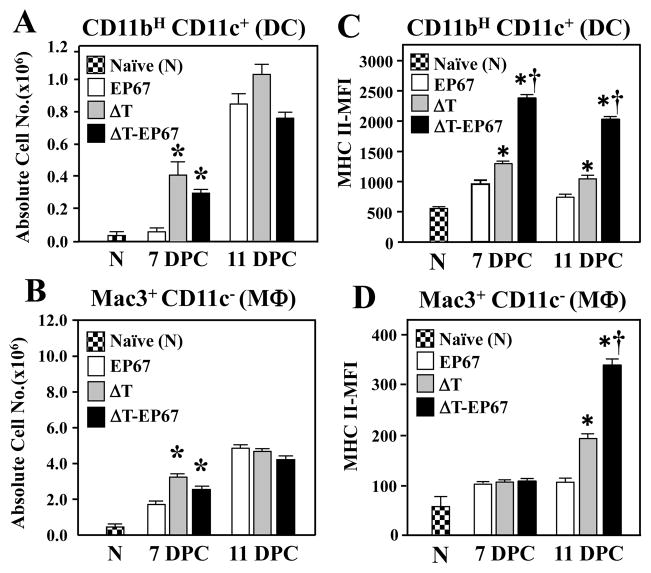
3.3. The ΔT-EP67 conjugate vaccine promoted increased expression of MHC II on the surface of APCs in infected lungs compared to ΔT-vaccinated and infected mice
A significant increase in lung infiltration of DC, MΦ, and B cells (not shown) was evident in vaccinated compared to non-vaccinated mice at 7 days postchallenge (Fig. 4A,B). We also observed that expression levels of costimulatory molecules CD80/86 and CD40 on the surface of these APCs were upregulated in the lungs of both groups of vaccinated animals at 7 and 11 days postchallenge compared to non-vaccinated mice (data not shown). Detection of CD80/86 and CD40 costimulatory molecules on the surface of APCs is indicative of their activation and ability to present antigens in the context of MHC II molecules. On this basis, we questioned whether EP67 conjugated to the ΔT vaccine could also increase the surface expression of MHC II (I-Ad haplotype) on professional APCs present in Coccidioides-infected lungs. Figure 4C shows that surface expression of MHC II molecules on DC of the ΔT-EP67-vaccinated mice was significantly increased compared to the DC of both ΔT-vaccinated and non-vaccinated mice at 7 and 11 days postchallenge. Surface expression of MHC II on macrophages of mice immunized with the conjugate vaccine was also significantly increased at 11 days (Fig. 4D), albeit to a lesser degree than dendritic cells. The median fluorescence intensity (MFI) of MHC II-labeled pulmonary DC from mice vaccinated with ΔT-EP67 compared to DC from ΔT-vaccinated mice at day 7 and 11 postchallenge had increased by 1.8-fold (2378 ± 32 vs 1300 ± 30 units) at 7 days (P < 0.01) and 1.9-fold (2029 ± 32 vs 1045 ± 57 units) by 11 days (P < 0.01) (Fig. 4C). The MFI of macrophages isolated from ΔT-EP67-vaccinated mice also increased by 1.8-fold on day 11 postchallenge compared to Mac3+-labelled cells isolated from lungs of ΔT-vaccinated mice (343 ± 13 vs 196 ± 9 units, respectively; P < 0.01) (Fig. 4D).
Fig. 4.
BALB/c mice immunized with the ΔT-EP67 conjugate vaccine showed enhanced expression of MHC II molecules on the surface of dendritic cells (DC) and tissue mcrophages (MΦ) which had infiltrated Coccidioides-infected lungs. (A, B) Absolute numbers of lung-infiltrated DC and MΦ, respectively, recorded at 7 and 11 days postchallenge. (C, D) Median fluorescence intensity (MFI) of MHC II molecules expressed by flow cytometry-gated DC and MΦ cells, respectively, at 7 and 11 days postchallenge. Asterisks indicate statistically significant differences between absolute numbers and MFI of innate cells isolated from vaccinated versus non-vaccinated control mice (P < 0.05). Daggers indicate statistically significant differences between MFI of MHC II on DC and MΦ isolated from ΔT-EP67- versus ΔT- vaccinated mice (P < 0.05). The results are representative of two separate experiments with 4 mice per group.
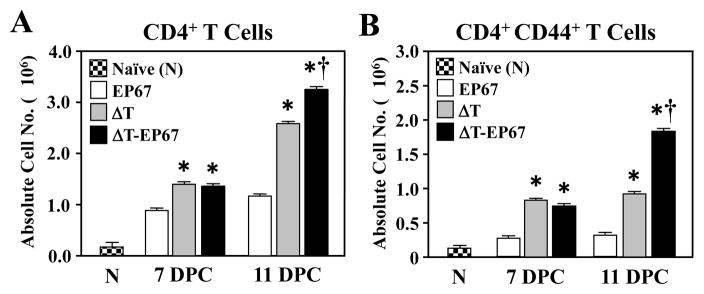
3.4. The EP67 conjugate vaccine enhanced lung infiltration and activation of CD4+ effector T cells
We used FACS analysis of lung homogenates to evaluate the recruitment of CD4+ effector T cells to the lungs of mice immunized with either the ΔT or ΔT-EP67 vaccine. Absolute numbers of total CD4+ T cells were significantly higher in infected lungs of vaccinated compared to non-vaccinated mice at 7 and 11 days postchallenge (Fig. 5A). In addition, the numbers of total and activated CD4+ T lymphocytes that had infiltrated the lungs of mice immunized with the EP67-conjugate vaccine were significantly higher at 11 days after challenge compared to absolute numbers of lung-infiltrated CD4+ cells of the ΔT-vaccinated mice (Fig 5A,B).
Fig. 5.
BALB/c mice immunized with the ΔT-EP67 conjugate vaccine showed increased infiltration of activated CD4+ T cells into their lungs during early stages of Coccidioides infection. (A)Absolute numbers of total CD4+ T cells versus (B) activated T cells which had infiltrated Coccidioides-infected lungs at 7 and 11 days postchallenge. Asterisks indicate statistically significant differences between absolute cell numbers of lung-infiltrated T cells (total and activated) of vaccinated versus non-vaccinated mice (P < 0.05). Daggers indicate statistically significant difference between absolute cell numbers of ΔT-EP67- versus ΔT-vaccinated mice (P < 0.05). The results are representative of two separate experiments with 4 mice per group.
3.5. ΔT-EP67 conjugate vaccine-primed splenocytes responded in vitro to Coccidioides antigen by production of significantly higher Th1- and Th17-type cytokines compared to ΔT-vaccinated mice
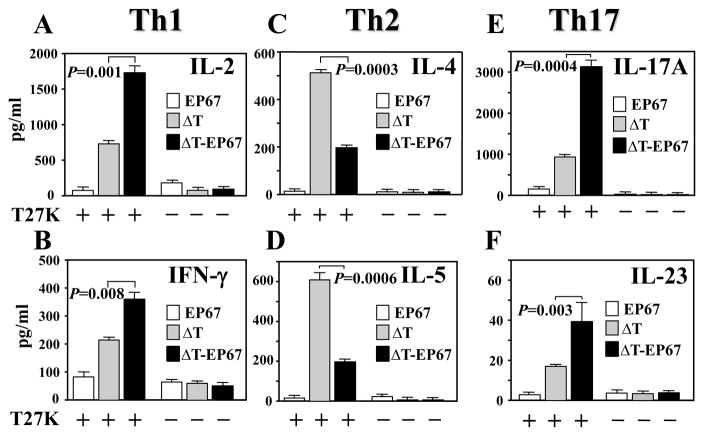
A recall response assay was conducted to determine whether immune splenocytes isolated from ΔT- or ΔT-EP67-vaccinated mice at 7 days postchallenge secrete a profile of cytokines which are indicative of Th1, Th2 or Th17 cell activation (Fig. 6A-F). As previously discussed, the Coccidioides-derived T27K antigen was employed to evaluate this in vitro response. We had previously determined that at 7 days postchallenge the pathogen had not yet disseminated from the lungs to the spleen of BALB/c mice [11]. The isolated immune splenocytes were cultured for 2 days in the presence (+) or absence (-) of the T27K antigen. Assays of the concentrations of representative Th1- (IL-2 and IFN-γ), Th2- (IL-4 and IL-5), and Th17-type cytokines (IL-17A, IL-23, IL6, TGF-β, IL-1β,) in the culture supernatants were measured by the Bio-Plex array system. The concentrations of IL-2 and IFN-γ (Fig. 6A,B) as well as IL-17A and IL-23 (Fig. 6E,F) were significantly higher in mice immunized with the EP67 conjugate vaccine compared to the ΔT vaccine. On the other hand, no significant difference was observed in the amount of T27K-stimulated immune splenocyte secretion of IL-6, TGF-β and IL-1β between the two groups of vaccinated mice (data not shown). The concentrations of cytokines representing activation of the Th2 signal pathway were significantly lower in culture supernatants of splenocytes isolated from ΔT-EP67- compared ΔT-vaccinated mice (Fig. 6C,D). Immune splenocytes isolated from mice immunized with EP67 alone and challenged with Coccidioides showed minimal in vitro production of the selected cytokines when co-cultured with the T27K antigen. Likewise, isolated immune splenocytes cultured in the absence of T27K consistently showed low levels of production of the selected cytokines.
Fig. 6.
BALB/c mice immunized with the ΔT-EP67-conjugated vaccine reveal elevated secretion of Th1- and Th17- type cytokines but suppressed Th2-type cytokine secretion by isolated immune splenocytes upon in vitro recall stimulation with Coccidioides T27K antigen. Splenocytes were isolated from mice immunized with the ΔT vaccine, ΔT-EP67 vaccine, or EP67 adjuvant alone and sacrificed at 7 days postchallenge. Splenocyte suspensions were cultured in the presence (+) or absence (-) of Coccidioides T27K antigen. Concentrations of representative Th1 cytokines (IL-2, IFN-γ) (A, B), Th2 cytokines (IL-4, IL-5) (C, D) and Th17 cytokines (IL-17A, IL-23) (E, F) in supernatants of antigen-stimulated immune cells were compared. Statistically significant differences in cytokine production between the ΔT-EP67- and ΔT-vaccinated mice are indicated by P values. The results are representative of three separate experiments with 4 mice per group.
3.6. Expression of transcription factors associated with promotion of T helper and regulatory T cell differentiation suggest dominance of Th17 cell response in ΔT-EP67-vaccinated mice
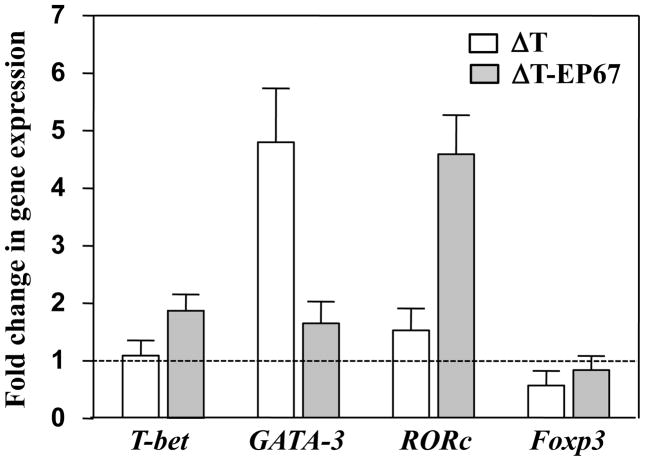
Quantitative RT-PCR analysis of RORc at 7 days postchallenge revealed that the level of expression of this transcription factor, indicative of the promotion of Th17 cell differentiation, was 3-fold higher in pulmonary cells obtained from mice immunized with the ΔT-EP67 vaccine compared to ΔT-vaccinated mice (Fig. 7). The opposite relationship between the vaccinated mice was revealed in the case of GATA-3 expression, which helps to drive the differentiation of Th2 cells. The expression level of T-bet which promotes Th1 cell differentiation was higher in mice vaccinated with the conjugate vaccine, while the difference in Foxp3 expression that helps to drive Treg cell differentiation was not statistically significant between the two vaccinated groups of mice.
Fig. 7.
Quantitative real-time PCR analysis of expression levels of selected transcription factors indicative of the differentiation of T helper cells (T-bet, GATA-3, RORc) and Treg cells (Foxp3). Total RNA of pulmonary cells isolated from non-vaccinated, ΔT-vaccinated, or ΔT-EP67-vaccinated mice at 7 days postchallenge was used in this assay. The horizontal dotted line represents the expression level of the transcription factors in pulmonary cells isolated from non-vaccinated mice and was arbitrarily indicated as 1. The assays were conducted in triplicate and the data are presented as mean values ± SEM.
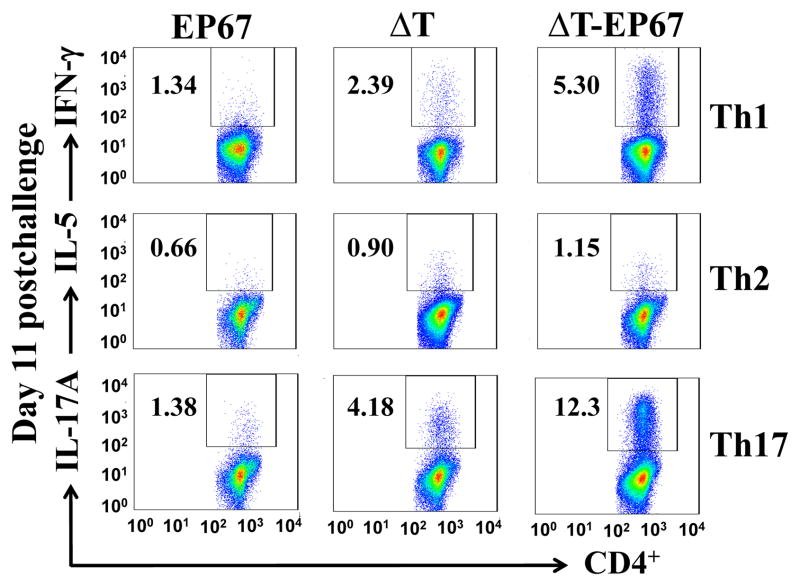
3.7. ΔT-EP67-vaccinated mice showed significantly greater expansion of Th1 and Th17 cells in infected lungs at 11 days postchallenge compared to ΔT-vaccinated mice
To further analyze the phenotype of T cells that had infiltrated the infected lungs we conducted an intracellular cytokine staining (ICS) assay to determine the percentages of activated CD4+ T cells present in lung homogenates which produce IFN-γ, IL-5, or IL-17A. The lung homogenates of ΔT-EP67 vaccinated mice revealed not only higher numbers of activated CD4+T cells at 11 days postchallenge (c.f. Fig. 5B), but also significantly higher percentages of IFN-γ- and IL-17A-producing CD4+ T cells compared to mice immunized with the ΔT vaccine (Fig. 8). On the other hand, the percentages of IL-5-producing CD4+ T cells were not significantly different between the two groups of vaccinated mice. Lung homogenates of both groups of vaccinated mice revealed infiltration of significantly higher percentages of Th1, Th2 and Th17 cells compared to control mice vaccinated with EP67 alone.
Fig. 8.
BALB/c mice immunized with the ΔT-EP67 conjugate vaccine show polarized activation of Th1 and Th17 effector cells during early stages of Coccidioides infection. FACS analyses of IFN-γ-, IL-5- and IL-17A-producing Th1, Th2, and Th17 cells, respectively, were conducted using lung homogenates of ΔT- or ΔT-EP67-vaccinated BALB/c mice versus control mice vaccinated with EP67 alone. The mice were sacrificed at 11 days postchallenge. The percentages of gated, specific cytokine-producing T helper cells are indicated as insets in each panel. The results are representative of two separate experiments with 4 mice per group.
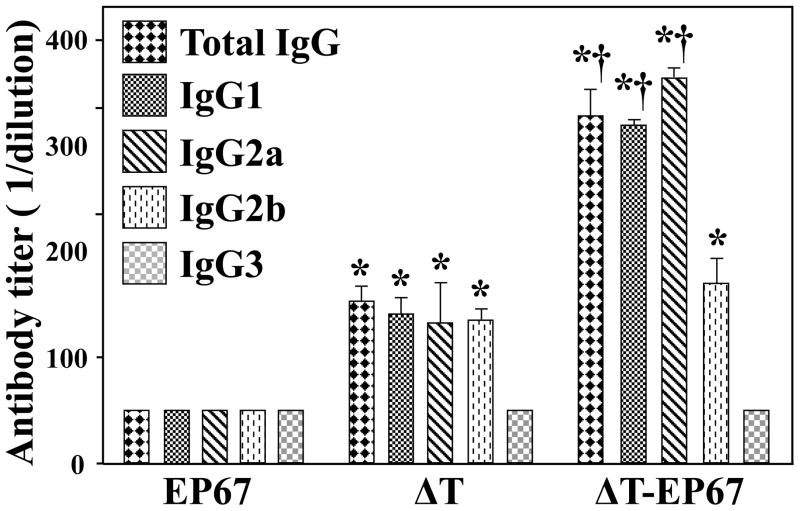
3.8. Mice immunized with the EP67-conjugate vaccine revealed significantly higher titers of antibody to Coccidioides antigen at 7 days postchallenge compared to ΔT-vaccinated mice
We determined that the serum titers of total IgG, as well as the IgG1 and IgG2a isotypes which recognized the Coccidioides T27K antigen were significantly higher in ΔT-EP67-vaccinated compared to ΔT-vaccinated mice at 7 days postchallenge. The titers of Coccidioides-specific IgG2b were comparable between the two groups of vaccinated mice. The IgG3 titers in vaccinated mice, like all the antibody titers examined in non-vaccinated mice were at the margin of detection and their titers were arbitrarily designated as 50.
4. Discussion
Evaluation of experimental adjuvants for their ability to augment appropriate pathways of protective immunity is an important component of vaccine research [4]. Coffman and colleagues [30] have proposed four compelling reasons to incorporate an adjuvant into a vaccine formulation: to enhance vaccine immunity in the heterogeneous human population, to improve the vaccine immune response of infants and the elderly, to facilitate use of smaller doses of antigen, and to achieve protection with fewer administrations of a vaccine. The functions of adjuvants used in vaccination protocols are now recognized to include more than just enhancement of the magnitude of the adaptive immune response. Evidence exists that adjuvants can contribute to both the specificity and clonotypic diversity of the CD4+ T cell response to an infectious agent [31]. Recent interest in adjuvants employed to augment vaccines against fungal infections has largely focused on their ability to polarize the activation of specific T helper cell populations (e.g., Th1, Th2, and Th17) [7, 32]. Only alum (aluminum hydroxide), monophosphoryl lipid A-oil emulsion (MPL), and synthetic oligodeoxynucleotides with CpG motifs (CpG ODN) have been extensively tested so far with experimental vaccines against coccidioidomycosis [4]. Alum has been shown to promote humoral immunity to Coccidioides infection [28, 33], but results of clinical and laboratory animal studies have indicated that dominance of a Th2 signal pathway compromises host protection against this mycosis and exacerbates the course of disease [34]. MPL interacts with Toll-like receptor 4 (TLR4), a pattern recognition receptor on antigen-presenting cells, and results in the release of proinflammatory cytokines as well as the stimulation of both humoral and cell-mediated immune responses [35]. The MPL adjuvant has been used in combination with a protein vaccine (Ag2/Pra) against coccidioidomycosis in BALB/c mice and was shown to promote the Th1 signal pathway through increased IFN-γ production resulting in enhanced protection against a potentially lethal intranasal infection of Coccidioides spores [36]. CpG ODN is a TLR9 agonist and has been used as an adjuvant with numerous experimental recombinant protein vaccines against coccidioidomycosis in mice [4, 28]. Prophylactic treatment of BALB/c mice with the synthetic oligodeoxynucleotide has been shown to induce a Th1-dominant environment while suppressing antigen-induced antibody production and infiltration of inflammatory cells into the lungs, a feature that may be advantageous for therapeutic application of CpG ODN to patients with severe allergy and asthma [37]. Non-vaccinated BALB/c mice tend to mount a Th2-biased response to Coccidioides infection characterized by production of IL-4 and IL-5 in infected lung tissue, which tends to hamper the activity of Th1 immunity. Mice immunized with experimental recombinant protein vaccines in the presence the CpG adjuvant have revealed an initial robust Th1 cell response to infection and a comparatively low level of Th2 cell stimulation [38].
In this study we examined both the protective efficacy and immunological effects of a novel C5a agonist (EP67) conjugated to a live, attenuated vaccine (ΔT) against coccidioidomycosis. We showed that this novel conjugate vaccine significantly enhanced protection against Coccidioides lung infection in BALB/c mice. This peptide adjuvant has been reported to promote the activation of Th1 cells when tested in vitro and in vivo as a conjugate with ovalbumin [13]. The amino acid sequence of the synthetic EP67 decapeptide is a modification of the biologically active C-terminal region of the complement-derived fragment C5a (C5a65-74; ISHKDMQLGR). As pointed out earlier in this paper specific residue substitutions have been introduced that enhance C5a-like immune stimulatory activities but not neutrophil-associated inflammatory response [12]. In fact, EP67 has been shown to be devoid of neutrophil activity due to its inability to bind to the C5a receptors of these innate immune cells while retaining the capacity of engagement with C5a receptor-bearing APCs [15]. Although infiltration of neutrophils into infected lung tissue during early stages of coccidioidomycosis is assumed to play a key role in host defense, perhaps in part by their production of IL-17A and promotion of the Th17 signal pathway [39], a persistent inflammatory response induced by the presence of these innate cells can also lead to acute lung injury which could exacerbate the course of disease [8]. Lung homogenates obtained from mice vaccinated with the ΔT-EP67 conjugate vaccine at 11 days postchallenge showed a significant decrease in the infiltration of neutrophils compared to animals vaccinated with the ΔT vaccine alone. These results correlated with a marked reduction in inflammatory pathology in the lungs of ΔT-EP67-vaccinated mice based on histopathological examinations. Few, small lung abscesses were visible in these mice and the response of primarily neutrophils, monocytes, and lymphocytes at infection sites appeared to be organized, indicative of early stages of granuloma formation. The lungs of infected, ΔT-vaccinated mice, on the other hand, revealed multiple large abscesses, a persistent and diffuse neutrophil response and associated host tissue damage.
Absolute numbers of dendritic cells and tissue macrophages recruited to infected lungs showed no significant difference between the two groups of vaccinated BALB/c mice. On the other hand, conjugation of EP67 with the ΔT vaccine induced a significant increase in expression of MHC II molecules on the surface of these APCs, especially dendritic cells, compared to mice immunized with the ΔT vaccine alone. Optimal antigen presentation and subsequent CD4+ T-cell responses require DC maturation, a process that includes upregulation of expression of costimulatory molecules CD80/86 and CD40 which we have observed in this study, as well as enhanced production of major histocompatibility complex class II molecules [40, 41]. It is possible that interaction between the C5a agonist and its receptor (C5aR) on the surface of DC contribute to activation of these antigen-presenting cells by upregulation of phosphatidylinositol 3-kinase activity and NF-κB signaling, which would promote the production of NF-κB-dependent MHC II expression and proinflammatory cytokine production [42]. Earlier studies have reported that EP67 engaged the C5aR on the surface of human DC and facilitated presentation of T and B cell epitopes to which EP67 was conjugated [12,16]. Denditric cells have been suggested to be major contributors to the host protective response to Coccidioides infection by bridging between the innate and adaptive immune systems [43].
BALB/c mice immunized with either the EP67-conjugate or ΔT vaccine stimulated increased infiltration and activation of CD4+ T cells at 7 and 11 days postchallenge compared to non-vaccinated mice. A large body of evidence supports the requirement of CD4+ T cells for host defense against Coccidioides infection, and the development of Th1 cells has been proposed to be crucial for protective immunity [4, 8]. Recent studies have also shown that Th17 cells are activated during early stages of Coccidioides infection in ΔT-vaccinated mice which is also essential for protective immunity [7, 8]. Previously tested adjuvants in combination with experimental vaccines designed to boost host defenses against Coccidioides infection have been shown to promote either a Th1 or combined Th1/Th2 cell response. However, no information is available on the ability of these adjuvants or EP67 to stimulate Th17 cell response to Coccidioides infection. Results of in vitro assays of immune splenocyte recall response to Coccidioides antigen revealed that immune cells isolated from ΔT-EP67-vaccinated mice produced significantly higher concentrations of Th1- and Th17-type cytokines than splenocytes derived from ΔT-vaccinated mice. Both IL-2 and IFN-γ produced by the primed splenocytes showed an approximate 2-fold increase in their concentration in supernatants of the in vitro-stimulated immune cells obtained from mice immunized with ΔT-EP67 compared to ΔT-vaccinated mice, which argues for a more potent activation of the Th1 signal pathway in the former. Elevated expression of T-bet in mice immunized with the conjugate vaccine, which correlates with the promotion of Th1 cell differentiation, supports this argument. Our observations of the production of higher concentrations of IL-17A and IL-23 by immune splenocytes isolated from ΔT-EP67-vaccinated mice, combined with increased expression of RORc expression in their pulmonary cells indicate that these mice also mounted a polarized Th17 response (7). In fact, the level of expression of the T-bet transcription factor was significantly lower than RORc expression in mice which received the conjugate vaccine, suggesting that a biased Th17 response to infection is stimulated in these animals. Evidence that vaccination with the ΔT-EP6 conjugate vaccine promoted a strong Th17 response to Coccidioides infection was further supported by FACS analysis of specific cytokine-producing CD4+ T cells in lung homogenates. These data revealed that CD4+IL-17A+ T cells were the dominant phenotype in infected lung preparations obtained from ΔT-EP67-vaccinated mice, and were present at significantly higher numbers than T cells with the same phenotype present in lung homogenates of the ΔT-immunized mice. Expression of Foxp3, which promotes the differentiation of Treg cells was apparently down-regulated at 7 days postchallenge in both groups of vaccinated mice compared to nonvaccinated controls. High IL-6 production by both ΔT- and ΔT-EP6-vaccinated mice revealed by the immune splenocyte recall assay may have an inhibitory effect on Foxp3 expression while enhancing Th17 cell activation [44]. IL-6 is a pleiotropic, proinflammatory cytokine produced by numerous cell types, including macrophages [45]. Recent studies have suggested that this cytokine participates in a positive feedback loop to increase the differentiation of Th17 cells [46]. These combined observations correlate with recent evidence that the Th17 signal pathway plays a pivotal role in early stages of protective immunity to pulmonary infection with Coccidioides in mice [7, 8]. In fact, it has been proposed that a vaccine against this mycosis should induce a major response of Th17 cells during the early course of infection if it is to be effective [7]. In a previous study we showed that IFN-γ−/− knock-out mice immunized with the ΔT vaccine alone could still be protected (100% survival at 50 days postchallenge), while only 40% of ΔT-vaccinated IL17 receptor A-deficient mice survived [8]. We proposed that both Th1 and Th17 signal pathways likely contribute to protection and share tasks associated with the control of Coccidioides infection in the immunocompetent host [47]. Whether the enhanced protection of BALB/c mice immunized with the ΔT-EP67 vaccine reported here is the associated with the independent or synergistic functions of activated Th1 and Th17 cells requires further investigation. Recent studies of vaccine immunity to Coccidioides infection have also suggested a role for production antibodies as components of the protective response through their collaboration with phagocytic cells and T lymphocytes to kill the pathogen, their ability to modulate inflammation to reduce host tissue damage [8], and possibly their requirement for Th17 cell differentiation [7]. Although our cytokine data and results of analysis of GATA-3 expression in ΔT-EP67-vaccinated mice indicated a down-regulation of the Th2 cell response to infection compared to ΔT-vaccinated mice, a significant difference in antibody titers was nevertheless observed between these two groups of immunized animals. We showed that the titers of total anti-Coccidioides IgG, IgG1 and IgG2a, but not IgG2b or IgG3 antibody, were significantly higher in mice immunized with the EP67 conjugate vaccine compared to the ΔT-vaccinated animals at 7 days postchallenge. Production of IgG2a is an indicator of activation of the Th1 signal pathway, and has been correlated with protective immunity to Coccidioides infection [4, 48]. Induction of an elevated humoral response to infection with preferential isotype class switching to IgG1 and IgG2a has also been correlated with the activation of Th17 cells [49]. However, the contributions of Th2 cells to protection against coccidioidomycosis are still undefined [50].
In summary, we have presented evidence that conjugation of EP67 with a live, attenuated vaccine contributes significantly to protection against a formidable pathogen in a mouse model that is highly susceptible to the induced life-threatening respiratory disease. The data presented demonstrate that the addition of EP67 to the live vaccine results in a significant increase in survival and reduction of the fungal burden, decrease in inflammatory pathology, polarization of Th1 and Th17 cell responses to infection, and promotion of antibody production. This is the first report which shows that conjugation of EP67 with surface proteins of a live, attenuated microbial vaccine strain can be used to enhance protective immunity against an infectious disease.
Fig. 9.
BALB/c mice immunized with the ΔT-EP67-conjugated vaccine revealed elevated production of specific antibody isotypes in response to Coccidioides infection. Sera were isolated from three groups of BALB/c mice immunized with the ΔT or ΔT-EP67 vaccine, or immunized with EP67 alone and sacrificed at 7 days postchallenge. Titers of Coccidioides T27K antigen-specific IgG and selected IgG subclasses (IgG1, IgG2a, IgG2b, IgG3) were determined by ELISA. The results are expressed as reciprocal titers (1/dilution) and presented as means ± SEM for sera from 4 mice in each group. The results are representative of two separate experiments.
Highlights.
The EP67 is an agonist of C5a, enhances protective immunity of a live, attenuated vaccine.
Ep67 was successfully conjugated to the surface of the live, attenuated vaccine strain.
EP67 enhanced the activation of Th1 and Th17 cells.
EP67 dampened inflammatory pathology associated with innate immune response to infection.
EP67 administration increased lung infiltration of IFN-γ and IL-17A producing CD4+ T cells.
Acknowledgments
Support for this work was provided by Public Health Service grants from the National Institute of Allergy and Infectious Disease (AI071118 and AI070891) awarded to GTC. Additional support was provided by the Margaret Batts Tobin Foundation, San Antonio, TX. We thank Ms. Natalia Castro-Lopez for her technical assistance in the determinations of fungal burden in Coccidioides-infected mice, and Drs. Karen Wozniak and Jieh-Juen Yu for their suggestions during the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Johnson RH, Stevens DA, et al. Coccidioidomycosis. Clin Infect Dis. 2005;41:1217–1223. doi: 10.1086/496991. [DOI] [PubMed] [Google Scholar]
- 2.Sharpton TJ, Stajich JE, Rounsley SD, Gardner MJ, Wortman JR, Jordar VS, et al. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 2009;19:1722–1731. doi: 10.1101/gr.087551.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hector RF, Rutherford GW, Tsang CA, Erhart LM, McCotter O, et al. The public health impact of coccidioidomycosis in Arizona and California. Int J Environ Public Health. 2011;8:1150–1173. doi: 10.3390/ijerph8041150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole GT, Xue JM, Okeke CN, Tarcha EJ, Basrur V, Schaller RA, et al. A vaccine against coccidioidomycosis is justified and attainable. Med Mycol. 2004;42:189–216. doi: 10.1080/13693780410001687349. [DOI] [PubMed] [Google Scholar]
- 5.Tsang CA, Anderson SM, Imholte SB, Erhart LM, Chen S, Park BJ, et al. Enhanced surveillance of coccidioidomycosis, Arizona, USA, 2007–2008. Emerg Infect Dis. 2010;16:1738–1744. doi: 10.3201/eid1611.100475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pappagianis D. Seeking a vaccine against Coccidioides immitis and serologic studies: Expectations and realities. Fungal Genet Biol. 2001;32:1–9. doi: 10.1006/fgbi.2000.1243. [DOI] [PubMed] [Google Scholar]
- 7.Wüthrich M, Gern B, Hung C-Y, Ersland K, Rocco N, Pick-Jacobs J, et al. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J Clin Invest. 2011;121:554–568. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung CY, Gonzalez A, Wüthrich M, Klein BS, Cole GT. Vaccine immunity to coccidioidomycosis occurs by early activation of three signal pathways of T helper cell response (Th1, Th2 and Th17) Infect Immun. 2011;79:4511–4522. doi: 10.1128/IAI.05726-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorensen KN, Clemons KV, Stevens DA. Murine models of blastomycosis, coccidioidomycosis, and histoplasmosis. Mycopathol. 1999;146:53–65. doi: 10.1023/a:1007081707287. [DOI] [PubMed] [Google Scholar]
- 10.Kirkland TN, Fierer J. Inbred mouse strains differ in resistance to lethal Coccidioides immitis infection. Infect Immun. 1983;40:912–916. doi: 10.1128/iai.40.3.912-916.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue J, Chen X, Selby D, Hung CY, Yu JJ, Cole GT. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect Immun. 2009;77:3196–3208. doi: 10.1128/IAI.00459-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan EL, Morgan BN, Stein EA, Vitrs EL, Thoman ML, Sanderson SD, et al. Enhancement of in vivo and in vitro immune functions by a conformationally biased, response-selective agonist of human C5a: Implications for a novel adjuvant in vaccine design. Vaccine. 2009;28:463–469. doi: 10.1016/j.vaccine.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan EL, Thoman ML, Sanderson SD, Phillips JA. A novel adjuvant for vaccine development in the aged. Vaccine. 2010;28:8275–8279. doi: 10.1016/j.vaccine.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor SM, Sherman SA, Kirnarsky L, Sanderson SD. Development of response-selective agonists of human C5a anaphylatoxin: Conformational, biological, and therapeutic considerations. Curr Med Chem. 2001;8:675–684. doi: 10.2174/0929867013373156. [DOI] [PubMed] [Google Scholar]
- 15.Vogen SM, Paczkowski NJ, Kirnarsky L, Short A, Whitmore JB, Sherman SA, et al. Differential activities of decapeptide agonists of human C5a: The conformational effects of backbone N-methylation. Int Immunopharmacol. 2001;1:2151–2162. doi: 10.1016/s1567-5769(01)00141-2. [DOI] [PubMed] [Google Scholar]
- 16.Hegde GV, Meyers-Clark E, Joshi SS, Sanderson SD. A conformationally-biased, response-selective agonist of C5a acts as a molecular adjuvant by modulating antigen processing and presentation activities of human dendritic cells. Int Immunopharmacol. 2008;8:819–827. doi: 10.1016/j.intimp.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 17.Duryee MJ, Bevins RA, Reichel CM, Murray JE, Dong Y, Thiele GM, et al. Immune responses to methamphetamine by active immunization with peptide-based, molecular adjuvant-containing vaccines. Vaccine. 2009;27:2981–2988. doi: 10.1016/j.vaccine.2009.02.105. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson SD, Cheruku SR, Padmanilayam MP, Vennerstrom JL, Thiele GM, Palmatier MI, et al. Immunization to nicotine with a peptide-based vaccine composed of a conformationally biased agonist of C5a as a molecular adjuvant. Int Immunopharmacol. 2003;3:137–146. doi: 10.1016/s1567-5769(02)00260-6. [DOI] [PubMed] [Google Scholar]
- 19.Phillips JA, Morgan EL, Dong Y, Cole GT, McMahan C, Hung C-Y, et al. Single-step conjugation of bioactive peptides to proteins via a self-contained succinimidyl bis-arylhydrazone. Bioconjug Chem. 2009;20:1950–1957. doi: 10.1021/bc9002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez A, Hung C-Y, Cole GT. Nitric oxide synthase activity has limited influence on the control of Coccidioides infection in mice. Microb Pathog. 2011;51:161–168. doi: 10.1016/j.micpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ampel NM, Kramer LA, Li L, Carroll DS, Kerekes KM, Johnson SM, et al. In vitro whole-blood analysis of cellular immunity in patients with active coccidioidomycosis by using the antigen preparation T27k. Clin Diagn Lab Immunol. 2002;9:1039–1043. doi: 10.1128/CDLI.9.5.1039-1043.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szabo SJ, Kim ST, Costa GL, Zhang XC, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 23.Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor Gata-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the Interleukin-5 gene. J Biol Chem. 1997;272:21597–21603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- 24.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: Differentiation, specification, subphenotypes. Nat Immunol. 2009;10:689–695. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 26.Xue J, Hung C-Y, Yu J-J, Cole GT. Immune response of vaccinated and non-vaccinated mice to Coccidioides posadasii infection. Vaccine. 2005;23:3535–3544. doi: 10.1016/j.vaccine.2005.01.147. [DOI] [PubMed] [Google Scholar]
- 27.Hardison SE, Wozniak KL, Kolls JK, Wormley FL. Interleukin-17 is not required for classical macrophage activation in a pulmonary mouse model of Cryptococcus neoformans infection. Infect Immun. 2011;78:5341–5351. doi: 10.1128/IAI.00845-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li K, Yu J-J, Hung C-Y, Lehmann PF, Cole GT. Recombinant urease and urease DNA of Coccidioides immitis elicit an immunoprotective response against coccidioidomycosis in mice. Infect Immun. 2001;69:2878–2887. doi: 10.1128/IAI.69.5.2878-2887.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hung C-Y, Seshan KR, Yu J-J, Schaller R, Xue J, Basrur V, Gardner MJ, Cole GT. A metalloproteinase of Coccidioides posadasii contributes to evasion of host detection. 2005;73:6689–6703. doi: 10.1128/IAI.73.10.6689-6703.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: Putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baumgartner CK, Malherbe LP. Antigen-driven T-cell repertoire selection during adaptive immune responses. Immunol Cell Biol. 2011;89:54–59. doi: 10.1038/icb.2010.117. [DOI] [PubMed] [Google Scholar]
- 32.Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5:e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmermann CR, Johnson SM, Martens GW, White AG, Zimmer BL, Pappagianis D. Protection against lethal murine coccidioidomycosis by a soluble vaccine from spherules. Infect Immun. 1998;66:2342–2345. doi: 10.1128/iai.66.5.2342-2345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirkland TN, Cole GT. Coccidioidomycosis: Pathogenesis, immune response and vaccine development. In: Calderone RA, Cihlar LC, editors. Fungal pathogenesis: Principles and applications. New York: Marcel Dekker; 2002. pp. 365–399. [Google Scholar]
- 35.Singh M, Srivastava I. Advances in vaccine adjuvants for infectious diseases. Cur HIV Res. 2003;1:309–320. doi: 10.2174/1570162033485195. [DOI] [PubMed] [Google Scholar]
- 36.Abuodeh RO, Shubitz LF, Siegel E, Snyder S, Peng T, Orsborn KI, et al. Resistance to Coccidioides immitis in mice after immunization with recombinant protein or a DNA vaccine of a proline-rich antigen. Infect Immun. 1999;67:2935–2940. doi: 10.1128/iai.67.6.2935-2940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu B, Zhang L, Liu J, Shan F, Wang E, Kimura Y. A prophylactic effect of an oligodeoxynucleotide containing a cytidine-guanosine motif against Japanese cedar pollen-induced T-helper type 2 allergic response. 2011;48:973–978. doi: 10.3109/02770903.2011.619288. [DOI] [PubMed] [Google Scholar]
- 38.Cox RA, Magee DM. Coccidioidomycosis: Host response and vaccine development. Clin Microbiol Rev. 2004;17:804–839. doi: 10.1128/CMR.17.4.804-839.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werner JL, Gessner MA, Lilly LM, Nelson MP, Metz AE, Horn D, et al. Neutrophils produce interleukin 17a (IL-17a) in a dectin-1- and IL-23-dependent manner during invasive fungal infection. Infect Immun. 2011;79:3966–3977. doi: 10.1128/IAI.05493-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macagno A, Neopolitani G, Lanzavecchia A, Sallusto F. Duration, combination and timing: the signal integration model of dendritic cell activation. Trends Immunol. 2007;28:227–233. doi: 10.1016/j.it.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Huang H, Ostroff GR, Lee CK, Specht CA, Levitz SM. Robust stimulation of humoral and cellular immune responses following vaccination with antigen-loaded β-glucan particles. mBio. 2010;1:e00164–10. doi: 10.1128/mBio.00164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng Q, Li K, Wang N, Li Q, Asgari E, Lu B, et al. Dendritic cell function in allostimulation is modulated by C5aR signaling. J Immunol. 2009;183:6058–6068. doi: 10.4049/jimmunol.0804186. [DOI] [PubMed] [Google Scholar]
- 43.Vilekar P, Awasthi V, Lagisetty P, King C, Shankar N, Awasthi S. In vivo trafficking and immunostimulatory potential of an intranasally-administered primary dendritic cell-based vaccine. BMC Immunology. 2010;11:60–77. doi: 10.1186/1471-2172-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, Lin J, Zhou Z, Huo R, Shen B, Li N. Up-regulation of Th 17 cells may underlie inhibition of Treg development caused by immunization with activated syngeneic T cells. PLoS One. 2011;6:e27289. doi: 10.1371/journal.pone.0027289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barton BE. IL-6: Insights into novel biological activities. Clin Immunol Immunopathol. 1997;85:16–20. doi: 10.1006/clin.1997.4420. [DOI] [PubMed] [Google Scholar]
- 46.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: Mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lexberg MH, Taubner A, Albrecht I, Lepenies I, Richter A, Kamradt T, et al. IFN-γ and IL-12 synergize to convert in vivo generated Th17 into Th1/Th17 cells. Eur J Immunol. 2010;40:3017–3027. doi: 10.1002/eji.201040539. [DOI] [PubMed] [Google Scholar]
- 48.Jiang C, Magee MD, Cox RA. Coadministration of interleukin 12 expression vector with antigen 2 cDNA enhances induction of protective immunity against Coccidioides immitis. Infect Immun. 1999;67:5848–5853. doi: 10.1128/iai.67.11.5848-5853.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitsdoerffer M, Lee Y, Jager A, Kim H-J, Korn T, Kolls JK, et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. PNAS. 2010;107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wuthrich M, Deepe G, Klein B. Adaptive immunity to fungi. Annu Rev Immunol. 2012;30:115–148. doi: 10.1146/annurev-immunol-020711-074958. [DOI] [PMC free article] [PubMed] [Google Scholar]