Abstract
The human organic anion and cation transporters are classified within two SLC superfamilies. Superfamily SLCO (formerly SLC21A) consists of organic anion transporting polypeptides (OATPs), while the organic anion transporters (OATs) and the organic cation transporters (OCTs) are classified in the SLC22A superfamily. Individual members of each superfamily are expressed in essentially every epithelium throughout the body, where they play a significant role in drug absorption, distribution and elimination. Substrates of OATPs are mainly large hydrophobic organic anions, while OATs transport smaller and more hydrophilic organic anions and OCTs transport organic cations. In addition to endogenous substrates, such as steroids, hormones and neurotransmitters, numerous drugs and other xenobiotics are transported by these proteins, including statins, antivirals, antibiotics and anticancer drugs. Expression of OATPs, OATs and OCTs can be regulated at the protein or transcriptional level and appears to vary within each family by both protein and tissue type. All three superfamilies consist of 12 transmembrane domain proteins that have intracellular termini. Although no crystal structures have yet been determined, combinations of homology modelling and mutation experiments have been used to explore the mechanism of substrate recognition and transport. Several polymorphisms identified in members of these superfamilies have been shown to affect pharmacokinetics of their drug substrates, confirming the importance of these drug transporters for efficient pharmacological therapy. This review, unlike other reviews that focus on a single transporter family, briefly summarizes the current knowledge of all the functionally characterized human organic anion and cation drug uptake transporters of the SLCO and the SLC22A superfamilies.
LINKED ARTICLES
BJP recently published a themed section on Transporters. To view the papers in this section visit http://dx.doi.org/10.1111/bph.2011.164.issue-7
Keywords: SLCO, SLC22A, OATP, OAT, OCT, drug transport, membrane transport
General introduction
Numerous endo- and xenobiotics including many drugs are organic anions or cations. Their disposition and elimination depend on the proper function of multispecific drug transporters that belong to two major superfamilies: solute carrier (SLC) transporters and ATP-binding cassette (ABC) transporters. Although most are capable of bidirectional transport, in general, ABC transporters are considered to be responsible for efflux of substrates, while SLC transporters mediate uptake of substrates into cells. Within the SLC transporters, there are two gene superfamilies that contain the major organic anion and cation transporters. These are the SLCO superfamily, made up of the organic anion transporting polypeptides (OATPs), and the SLC22A superfamily, which contains the organic cation transporters (OCTs) and the organic anion transporters (OATs). Individual members of these superfamilies are expressed in essentially every epithelium throughout the body. The members of both superfamilies mediate transport of a broad range of structurally diverse compounds with overlapping substrate specificities within the superfamilies. In general, OCTs transport cations, OATPs transport large and fairly hydrophobic organic anions, and OATs transport the smaller and more hydrophilic organic anions. This brief review will summarize our current knowledge about the human members of these three transporter families, with an emphasis on tissue distribution, substrate specificity, regulation of expression, transporter structure and pathology.
Nomenclature
OATPs are encoded by genes in the SLCO/Slco superfamily. This superfamily was originally named SLC21A; however, the nomenclature of its members was updated and standardized in 2004 based on phylogenetic relationships, and the superfamily was renamed to SLCO, the solute carrier family of the OATPs (Hagenbuch and Meier, 2004). Eleven human OATPs have been identified and are classified into six families based on their amino acid identity. The different proteins are named OATP (Oatp for the rodent proteins) followed by the family number (e.g. OATP1, OATP2), the subfamily letter (e.g. OATP1A, OATP1B) and then a consecutive number identifying the individual members within the family based on the historical order in which they have been identified (e.g. Oatp1a1, OATP1A2 and Oatp1a3). The corresponding gene symbols are SLCO followed by the same number–letter–number combination (e.g. Slco1a1, SLCO1A2 and Slco1a3). The best characterized OATPs belong to family 1, which in humans contains OATP1A2, OATP1B1, OATP1B3 and OATP1C1. A significant amount of gene duplication and divergence has occurred in this family, especially in rodents, complicating direct comparisons between human (OATP) and rodent (Oatp) studies. OATP1A2 has five rodent orthologues: Oatp1a1, Oatp1a3 (in rats only), Oatp1a4, Oatp1a5 and Oatp1a6. OATP1B1 and OATP1B3 have a single rodent orthologue, Oatp1b2. The other OATPs and their rodent orthologues are OATP1C1 (Oatp1c1), OATP2A1 (Oatp2a1), OATP2B1 (Oatp2b1), OATP3A1 (Oatp3a1), OATP4A1 (Oatp4a1), OATP4C1 (Oatp4c1), OATP5A1 and OATP6A1 (Oatp6b1, Oatp6c1 and Oatp6d1).
The SLC22A family includes OCT1-3 (SLC22A1-3), OCTN1 and OCTN2 (SLC22A4-5), OCT6 (SLC22A16, also known as CT2), OAT1-4 (SLC22A6-8, 11), OAT7 (SLC22A9), URAT1 (SLC22A12) and several additional not well characterized putative transporters. Most of these proteins have a single rodent orthologue, but OAT4 is specific to humans. OAT5 (SLC22A10) was cloned in 2001 but has not been functionally characterized (Sun et al., 2001); thus, it is considered an orphan OAT. It is believed that human OAT5 is not the orthologue of rodent Oat5 (Youngblood and Sweet, 2004). Additionally, in rodents, there is an Octn3 protein (Slc22a21) – although no human homologue has been conclusively identified, an antibody against mouse Octn3 cross-reacts in certain human tissues, which led the authors to suggest that a human OCTN3 does exist (Lamhonwah et al., 2005). The human and rodent SLCO and SLC genes and their corresponding proteins are listed in Table 1. Unless otherwise stated, all information included in this review refers to the human transporters.
Table 1.
Gene and protein names of human and rodent organic anion and cation transporters
| Human gene | Human protein | Rodent gene | Rodent protein |
|---|---|---|---|
| Slco1a1 | Oatp1a1 | ||
| SLCO1A2 | OATP1A2 | ||
| Slco1a3 (rat only) | Oatp1a3 | ||
| Slco1a4 | Oatp1a4 | ||
| Slco1a5 | Oatp1a5 | ||
| Slco1a6 | Oatp1a6 | ||
| SLCO1B1 | OATP1B1 | ||
| Slco1b2 | Oatp1b2 | ||
| SLCO1B3 | OATP1B3 | ||
| SLCO1C1 | OATP1C1 | Slco1c1 | Oatp1c1 |
| SLCO2A1 | OATP2A1 | Slco2a1 | Oatp2a1 |
| SLCO2B1 | OATP2B1 | Slco2b1 | Oatp2b1 |
| SLCO3A1 | OATP3A1 | Slco3a1 | Oatp3a1 |
| SLCO4A1 | OATP4A1 | Slco4a1 | Oatp4a1 |
| SLCO4C1 | OATP4C1 | Slco4c1 | Oatp4c1 |
| SLCO5A1 | OATP5A1 | ||
| SLCO6A1 | OATP6A1 | ||
| Slco6b1 | Oatp6b1 | ||
| Slco6c1 | Oatp6c1 | ||
| Slco6d1 | Oatp6d1 | ||
| SLC22A1 | OCT1 | Slc22a1 | Oct1 |
| SLC22A2 | OCT2 | Slc22a2 | Oct2 |
| SLC22A3 | OCT3 | Slc22a3 | Oct3 |
| SLC22A4 | OCTN1 | Slc22a4 | Octn1 |
| SLC22A5 | OCTN2 | Slc22a5 | Octn2 |
| SLC22A6 | OAT1 | Slc22a6 | Oat1 |
| SLC22A7 | OAT2 | Slc22a7 | Oat2 |
| SLC22A8 | OAT3 | Slc22a8 | Oat3 |
| SLC22A9 | OAT7 | ||
| SLC22A10 | OAT5 | ||
| SLC22A11 | OAT4 | ||
| SLC22A12 | URAT1 | Slc22a12 | Urat1 |
| SLC22A13 | OAT10 | Slc22a13 | Oat10 |
| SLC22A16 | OCT6 (CT2) | ||
| Slc22a19 | Oat5 | ||
| SLC22A20 | OAT6 | Slc22a20 | Oat6 |
| Slc22a21 | Octn3 |
OATPs
Organic anion transporting polypeptides (OATPs in humans, Oatps in rodents) are multispecific transporters located in numerous epithelia throughout the body. They mediate the cellular uptake of a broad range of substrates, including bile acids, steroid conjugates and numerous xenobiotics.
Tissue distribution
Protein expression for OATPs is summarized in Figure 1. OATP1A2 is widely distributed throughout the body, with the highest mRNA expression in the brain, liver, lung, kidney and testes (Kullak-Ublick et al., 1995; Steckelbroeck et al., 2004). With this distribution, it is thought that OATP1A2 could play a critical role in the absorption, distribution and excretion of xenobiotics. OATP1A2 protein has been localized to the brush border membrane of enterocytes in the duodenum (Glaeser et al., 2007), where it may mediate the absorption of xenobiotics. Within the liver, OATP1A2 is exclusively expressed in cholangiocytes (Lee et al., 2005) and may be involved in the reabsorption of xenobiotics excreted into the bile. In the kidney, OATP1A2 is expressed at the apical membrane of the distal nephron (Lee et al., 2005), where it could be responsible for either the reabsorption from or the secretion of xenobiotics into urine. OATP1A2 is also expressed at the luminal membrane of the endothelial cells of brain capillaries (Bronger et al., 2005) and is thought to be part of the blood–brain barrier. OATP1B1 and OATP1B3 are both selectively expressed in the liver (Abe et al., 1999; 2001; Hsiang et al., 1999; Konig et al., 2000a,b), where they are localized to the basolateral membrane of hepatocytes (Konig et al., 2000b; Abe et al., 2001; Kullak-Ublick et al., 2001; Cui et al., 2003). OATP1B1 is expressed in hepatocytes throughout the lobule, while OATP1B3 is primarily expressed around the central vein (Konig et al., 2000a); consistent with this pattern, expression levels of OATP1B1 mRNA in liver homogenate are higher overall than are levels of OATP1B3 (Michalski et al., 2002; Briz et al., 2006). OATP1C1 mRNA expression was originally localized to the brain and testes (Pizzagalli et al., 2002), and OATP1C1 protein has been detected at the basolateral membrane of choroid plexus epithelial cells (Roberts et al., 2008) and to the Leydig cells of the testes (Pizzagalli et al., 2002).
Figure 1.
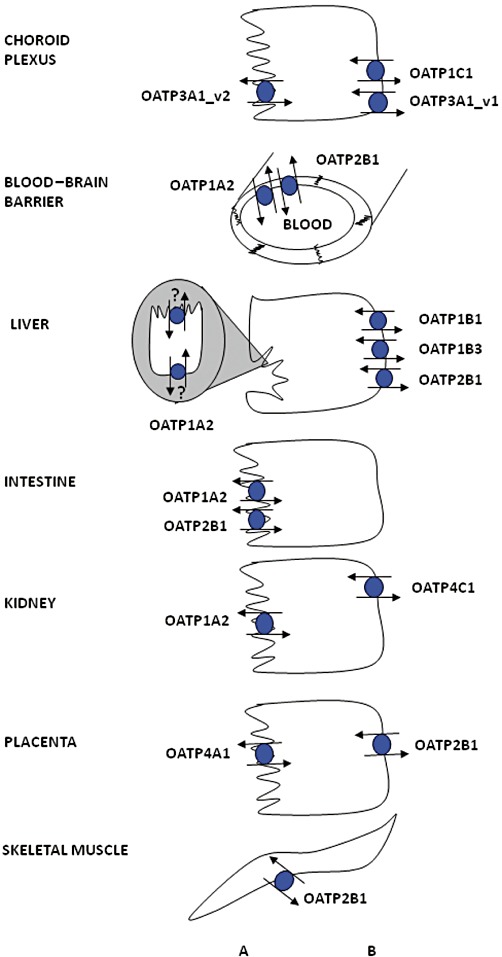
Expression of OATPs in selected human epithelial cells. For more details, see the text. OATP1A2 expression in cholangiocytes has been demonstrated, but it has not yet been localized to a distinct cell membrane. (A) apical; (B) basolateral.
OATP2A1, also known as the prostaglandin transporter (PGT), is ubiquitously expressed throughout the body (Nomura et al., 2004; 2005). As shown by Northern blot analysis, mRNA of OATP2A1 was found in several tissues including brain, colon, heart, kidney, liver, lung, ovary, pancreas, placenta, prostate, skeletal muscle, spleen and small intestine (Schuster, 2002). Recently, OATP2A1 protein expression was shown in the upper gastrointestinal tract, localized in the pyloric glands of the antrum and parietal cells of the gastric corpus (Mandery et al., 2010). OATP2A1 is thought to be involved in terminating prostaglandin signalling by transporting prostaglandins into cells (Nomura et al., 2004; 2005). OATP2B1 is also widely expressed throughout the body (Tamai et al., 2000; Kullak-Ublick et al., 2001). The highest levels of mRNA are found in the liver, where the protein is located at the basolateral membrane of hepatocytes (Kullak-Ublick et al., 2001). Protein expression has also been reported at the apical membrane of intestinal epithelial cells (Kobayashi et al., 2003), at the basolateral membrane of syncytiotrophoblasts in the placenta (St-Pierre et al., 2002), in epidermal keratinocytes (Schiffer et al., 2003), in the myoepithelium surrounding ductal epithelial cells in human mammary gland (Pizzagalli et al., 2003), in vascular endothelial cells in the heart (Grube et al., 2006b), in skeletal muscle (Knauer et al., 2010) and at the luminal membrane of the endothelial cells of the blood–brain barrier (Bronger et al., 2005).
OATP3A1 mRNA levels are highest in testes, brain and heart followed by lung, spleen, peripheral blood leukocytes and thyroid gland (Adachi et al., 2003; Huber et al., 2007). OATP3A1 mRNA expression has also been shown in human epidermal keratinocytes (Schiffer et al., 2003). OATP3A1 has two splice variants, which have cell type-specific expression. OATP3A1_v1 was localized to the germ cells of testes, at the basolateral membrane of the choroid plexus and in neuroglial cells of the grey matter in the frontal cortex, while OATP3A1_v2 was localized in the Sertoli cells of the testes, at the apical and sub-apical membrane in choroid plexus and in cell bodies and axons of the neurons in the frontal cortex (Huber et al., 2007).
OATP4A1 has been detected in several tissues with the highest levels of mRNA found in the heart and placenta, followed by lung, liver, skeletal muscle, kidney and pancreas (Tamai et al., 2000; Fujiwara et al., 2001). OATP4A1 protein was localized to the apical membrane of syncytiotrophoblasts in the placenta (Sato et al., 2003). OATP4C1 was initially thought to be a kidney-specific OATP, based on Northern blot analysis (Mikkaichi et al., 2004). Based on the localization of rat Oatp4c1, it is assumed that human OATP4C1 is also localized at the basolateral membrane of proximal tubule cells. A recent microarray suggests that OATP4C1 may also be expressed in the liver, although this has not yet been verified by RT-PCR or protein analysis (Bleasby et al., 2006). This microarray also contains the only determination of OATP5A1 expression to date, showing possible expression in fetal brain, prostate, skeletal muscle and thymus. OATP6A1 mRNA has been shown mainly in the testes, with low expression in spleen, brain, fetal brain and placenta (Suzuki et al., 2003; Lee et al., 2004).
Substrate specificity
The mechanism of OATP-mediated transport remains controversial. It is well established that transport is ATP- and sodium-independent, but the driving force for transport is still under investigation. OATPs are capable of bidirectional transport, and several studies have suggested that they work as electroneutral exchangers. Evidence suggests that individual OATPs/Oatps may exchange their substrates for intracellular bicarbonate (Satlin et al., 1997; Leuthold et al., 2009), glutathione (Li et al., 1998; Franco and Cidlowski, 2006) or glutathione conjugates (Li et al., 2000). However, it appears that there could be differences among the different OATPs/Oatps with respect to the exact transport mechanism: for example, transport mediated by OATP1B1 and OATP1B3 is not affected by glutathione (Mahagita et al., 2007).
OATP-mediated transport can also be affected by pH. Several studies have shown that OATP2B1 transport activity is increased at acidic pH (Kobayashi et al., 2003; Nozawa et al., 2004a; Sai et al., 2006; Varma et al., 2011). As OATP2B1 is expressed in the small intestine, this phenomenon could result in both increased transport of substrates and a broader substrate range and thus improve OATP2B1-mediated drug absorption. However, this effect seems to be substrate dependent and can be caused by both increased affinity (decreased Km) and increased turnover rate (Vmax) (Nozawa et al., 2004a; Leuthold et al., 2009). It has been proposed that the mechanism of increased substrate affinity is caused by the protonation of a conserved histidine residue at the extracellular end of transmembrane domain 3 (Leuthold et al., 2009). Transport of estrone-3-sulphate by OATP1B1 and OATP1B3 has previously been shown to be independent of the extracellular pH and of the membrane potential (Mahagita et al., 2007). However, a recent report demonstrates that these two transporters are influenced in different ways by both pH and the membrane potential (Martinez-Becerra et al., 2011).
To determine the driving force of OATP-mediated transport, additional studies are clearly needed. By using membrane vesicles isolated from cells that overexpress individual OATPs, the exact composition of the buffers on both sides of the plasma membrane can be controlled. Based on such experiments, an exact delineation of the involved driving forces and exchange mechanisms should be possible.
Most OATPs transport a broad range of compounds. The transported substrates are summarized for family OATP1 in Table 2, for family OATP2 in Table 3 and for all the remaining OATPs in Table 4 (no substrates have yet been identified for OATP5A1 or OATP6A1). Although the majority of substrates are anions, some OATPs can also transport neutral and cationic compounds (Bossuyt et al., 1996). In general, substrates are amphipathic molecules with molecular weights greater than 350 Daltons and include bile acids, conjugated steroids, thyroid hormones, linear and cyclic peptides and mushroom toxins as well as numerous drugs, including statins, sartans, antibiotics and anticancer drugs. Many of these compounds (e.g. estrone-3-sulphate, estradiol-17β-glucuronide or bromosulphophthalein) are substrates of multiple OATPs and are therefore commonly used as model substrates. However, some substrates appear to be more specific; for example, cholecystokinin-octapeptide (CCK-8) is selectively transported by OATP1B3 (Ismair et al., 2001), while digoxin seems to be mainly transported by OATP4C1 (Mikkaichi et al., 2004).
Table 2.
Substrates of human OATP1 family
If available, apparent affinity (Km) values are listed. This table is an updated and extended version of a similar table published in Hagenbuch and Gui (2008).
ACU154: metabolite of PKI166, an epidermal growth factor receptor kinase inhibitor; Bamet-R2: cis-diammine-chloro-cholylglycinate-platinum(II); Bamet-UD2: cis-diammine-bisursodeoxycholate-platinum(II); BDE47: 2,2′,4,4′-Tetrabromodiphenyl ether; BDE99: 2,2′,4,4′,5-pentabromodiphenyl ether; BDE153: 2,2′,4,4′,5,5′-hexabromodiphenyl ether; BQ-123: cyclic pentapeptide endothelin receptor antagonist; CDCA-NBD: chenodeoxycholyl-(Nε-NBD)-lysine; CRC220: peptidomimetic thrombin inhibitor; Flutax-2: paclitaxel, Oregon Green® 488 conjugate; Gd-B20790: gadolonium-18-((3-(2-carboxylbutyl)-2,4,6-triiodophenyl)amino)-3,6,9-tris(carboxymethyl)-11,18-dioxo-3,6,9,12-tetrazaoctadecanoic acid; Ro 48–5033: Bosentan metabolite; SN-38: 7-ethyl-10-hydroxycamptothecin (active metabolite of irinotecan); S-8921G: methyl 1-(3,4-dimethoxyphenyl)-(3-ethylvaleryl)-4-hydroxy-6,7,8-trimethoxy-2-naphthoate glucuronide (inhibitor of the ilial apical sodium-dependent bile acid transporter); TR-14035: α4β1/α4β7 integrin dual antagonist.
Table 3.
Substrates of human OATP2 family
If available, apparent affinity (Km) values are listed. This table is an updated and extended version of a similar table published in Hagenbuch and Gui (2008).
BDE47: 2,2′,4,4′-Tetrabromodiphenyl ether; BDE99: 2,2′,4,4′,5-pentabromodiphenyl ether; BDE153: 2,2′,4,4′,5,5′-hexabromodiphenyl ether; CP-671,305: (+)-2-[4-((2-(benzol[1,3]dioxol-5-yloxy)-pyridine-3-carbonyl]-amino)-methyl)-3-fluoro-phenoxy]-propionic acid; M17055: 7-chloro-2,3-dihydro-1-(2-methylbenzoyl)-4(1H)-quinolinone 4-oxime-O-sulphonic acid.
Table 4.
Substrates of human OATP families 3–6
If available, apparent affinity (Km) values are listed.
BQ-123: cyclic pentapeptide endothelin receptor antagonist.
It has been suggested that substrates are transported through a central positively charged pore in OATPs via a rocker-switch mechanism (Meier-Abt et al., 2005). A pharmacophore model developed for OATP1B1 based on published apparent Km values of OATP substrates suggests that substrates contain two hydrogen bond acceptors, one hydrogen bond donor and two hydrophobic regions (Chang et al., 2005). A CoMFA (comparative molecular field analysis) model calculated based on 25 competitive inhibitors suggested that the substrate binding site for estradiol-17β-glucuronide on OATP1B1 consists of a large hydrophobic region with basic residues at both ends (Gui et al., 2009). However, such analyses are complicated by the indication that OATPs have multiple substrate binding sites or translocation pathways. OATP1B1 has biphasic saturation kinetics for estrone-3-sulphate, suggesting the presence of both a high-affinity, low-capacity binding site and a low-affinity, high-capacity binding site (Tamai et al., 2001; Noe et al., 2007; Gui and Hagenbuch, 2009). Similarly, OATP4C1 was recently shown to have distinct binding sites for estrone-3-sulphate and digoxin (Yamaguchi et al., 2010). In addition, inhibition studies have shown that compounds can have stimulatory, inhibitory or no effect on OATP-mediated transport, depending on the model substrate used (Gui et al., 2008; Roth et al., 2011a,b).
Regulation of expression
Expression of OATPs is largely controlled by transcriptional regulation. Constitutive OATP1B1 expression in hepatocytes appears to be dependent on HNF1α (Jung and Kullak-Ublick, 2003; Furihata et al., 2007), while OATP1B3 is likely regulated by HNF3β (Vavricka et al., 2004). There is also evidence that additional signals may be involved in OATP1B expression, including Stat5 (Wood et al., 2005) and transcription factors activated by hepatocyte growth factor (Le Vee et al., 2009), IFN-γ (Le Vee et al., 2011) and IL-1β (Le Vee et al., 2008). The mechanisms for regulating OATP expression are likely to vary by tissue type. For example, OATP1A2 expression is up-regulated in response to increased bile acid levels (Kullak-Ublick et al., 1997a), which would affect expression levels in the small intestine and liver. In breast carcinoma tissues and cell lines, however, OATP1A2 expression is significantly associated with the steroid and xenobiotic receptor (SXR) expression (Miki et al., 2006).
Regulation of OATPs can also occur at the protein level. As most OATPs contain a PDZ consensus sequence (Wang et al., 2005a), and the carboxy-terminus of OATP1A2 has been shown to interact with PDZ proteins (Kato et al., 2004), membrane localization of OATPs may be due to interactions with PDZ proteins. A recent study with rat Oatp1a1 demonstrated that in addition to the interaction with PDZ proteins, phosphorylation affected membrane expression (Choi et al., 2011). It has also been shown that activation of PKC leads to the phosphorylation of OATP2B1 and a reduced Vmax for substrates, suggesting that the protein is internalized upon phosphorylation (Kock et al., 2010).
Transporter structure
Human OATPs range in size from 643 to 724 amino acids, with the exception of the as yet uncharacterized OATP5A1, which contains 848 amino acids. OATPs are predicted to contain 12 transmembrane domains, with both termini located intracellularly. Although hydropathy models predict either a 10- or 12-domain topology, the 12-transmembrane domain model was confirmed for rat Oatp1a1 (Wang et al., 2008). The second and fifth extracellular loops contain multiple predicted and/or confirmed N-glycosylation sites, although the exact location varies by protein. The large fifth extracellular loop contains many conserved cysteines, which have been shown to be involved in disulphide bonds and are important for the surface expression of OATP2B1 (Hanggi et al., 2006). Similar to most other mammalian transport proteins, there is no crystal structure available for any of the OATPs so far. Therefore, homology modelling has been used to construct putative three-dimensional models of OATPs; this aids in the generation of theoretical predictions that can then be tested experimentally (Meier-Abt et al., 2005; Gui and Hagenbuch, 2008; Glaeser et al., 2010). Based on such models, several conserved positively charged amino acids that line the putative substrate pore were studied, and amino acids R57, K361 and R580 in OATP1B1 (Weaver and Hagenbuch, 2010) and K41, R580 and K361 in OATP1B3 (Glaeser et al., 2010; Mandery et al., 2011) were shown to be important for substrate transport. These amino acids are highlighted in the predicted three-dimensional structure of OATP1B1 shown in Figure 2A. Additional experiments using chimeras between OATP1B1 and OATP1B3 identified transmembrane domains 8 and 9 for OATP1B1 (Miyagawa et al., 2009) and transmembrane domain 10 for both OATP1B1 (Gui and Hagenbuch, 2009) and OATP1B3 (Gui and Hagenbuch, 2008) to be important for substrate transport and expression. However, to identify the individual amino acids that are involved in substrate translocation, additional experiments such as cysteine scanning mutagenesis and eventually crystallography or NMR studies are needed. Because there is evidence that different substrates are handled slightly differently by at least OATP1B1 and OATP1B3 (Gui et al., 2008; Roth et al., 2011a,b), such experiments will have to be performed for multiple model substrates.
Figure 2.
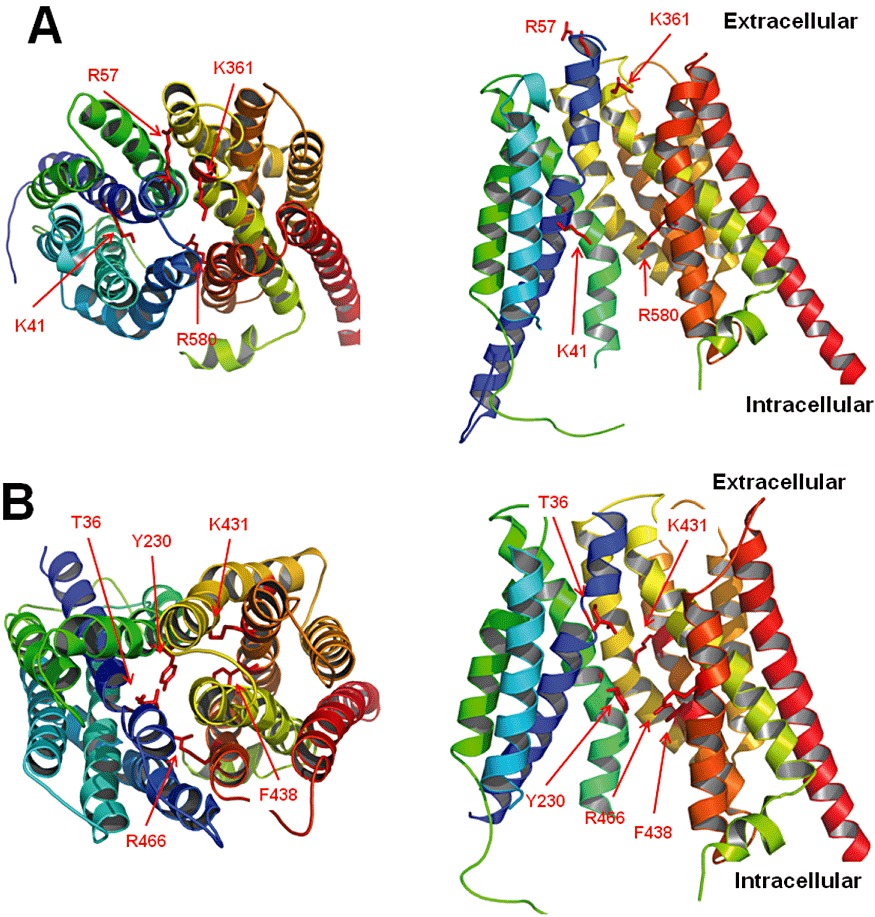
Homology models of members of the SLCO (OATP1B1) and the SCL22A (OAT1) families. The models were generated using Phyre2 (Kelley and Sternberg, 2009) and are based on the E. coli glycerol-3-phosphate transporter. (A) OATP1B1 is shown viewed from the extracellular side (left) and from within the lipid bilayer (right). For clarity, transmembrane domains 2 and 4 are omitted in the right panel. Amino acids mentioned in the text are indicated. (B) OAT1 is shown viewed from the extracellular side (left) and from within the lipid bilayer (right). For clarity, transmembrane domains 2 and 4 are omitted in the right panel. The indicated amino acids were identified as important for the function of OAT1 and face the putative aqueous pore (Hong et al., 2004; Perry et al., 2006; Rizwan et al., 2007).
Pathology and clinical significance
There are only a few links between disease states and altered function of OATPs; however, there have been many studies showing associations between altered OATP expression levels and disease states, and documenting effects of different alleles and single-nucleotide polymorphisms (SNPs) in OATPs on drug disposition.
Neonates with the OATP1B1 polymorphism N130D (found in OATP1B1*1b and *15) are at a higher risk for developing severe hyperbilirubinaemia (Huang et al., 2004; Buyukkale et al., 2011). Adults with the OATP1b1*15 haplotypes also have higher serum bilirubin levels (Ieiri et al., 2004), though there is no associated pathology. However, OATP expression is often altered in disease states. Cholestasis results in decreased mRNA levels of OATP1A2, OATP1B1 and OATP1B3 in whole livers (Keitel et al., 2005; Chen et al., 2008; Congiu et al., 2009). Placental expression of OATP1A2 mRNA is increased in patients with intrahepatic cholestatis of pregnancy (Cui et al., 2009). OATP1B1 is also reduced in patients with severe versus mild viral hepatitis (Oswald et al., 2001). Inflammatory bowel disease is associated with higher OATP2B1 and OATP4A1 levels in ileum and colon (Wojtal et al., 2009), and OATP4A1 is also up-regulated in polycystic ovarian syndrome (Plaza et al., 2010).
Of particular interest are several SNPs in OATP1B1, which have demonstrated the importance of this transporter in the disposition of certain drugs. The N130D allele, found in both OATP1B1*1b and *15, is associated with altered pharmacokinetics of pravastatin and pitavastatin (Nishizato et al., 2003; Mwinyi et al., 2004; Niemi et al., 2004; Chung et al., 2005; Wen and Xiong, 2010). The V174A allele, which is found in both OATP1B1*5 and OATP1B1*15, is associated with an attenuated cholesterol lowering effect of multiple statins (Tachibana-Iimori et al., 2004) as well as with an increased systemic exposure of the anti-diabetic nateglinide (Zhang et al., 2006) and the HIV protease inhibitor lopinavir (Hartkoorn et al., 2010). However, the V174 allele is unrelated to the pharmacokinetics of rosiglitazone and pioglitazone (Kalliokoski et al., 2008), torasemide (Werner et al., 2008), mycophenolic acid (Miura et al., 2007) or telmisartan (Miura et al., 2009). Polymorphisms in other OATPs, while less studied, can also affect drug pharmacokinetics. It has recently been shown that OATP1A2 polymorphisms are associated with imatinib clearance (Yamakawa et al., 2011), and polymorphisms in OATP2B1 are associated with the pharmacokinetics of fexofenadine (Akamine et al., 2010). Functional OATP polymorphisms are reviewed in detail by Kalliokoski and Niemi (2009) and Konig (2011).
Many cancer tissues and cell lines have altered expression of OATPs. For example, the normally liver-exclusive OATP1B3 is also expressed in gastric, colon and pancreatic cancers (Abe et al., 2001; Lee et al., 2008), as well as cancers of the lung (Monks et al., 2007), breast (Muto et al., 2007) and prostate (Hamada et al., 2008), whereas it has a reduced expression in hepatocellular carcinomas (Kinoshita and Miyata, 2002; Cui et al., 2003; Vavricka et al., 2004). Similarly, most of the other OATPs have been shown to have altered expression in different types of cancers. Because OATPs are known to transport hormones and their conjugates, which are thought to play a role in the enhanced proliferation or chemo-resistance of some cancers, the overexpression of OATPs may provide a survival benefit to these cells. The role of OATPs in cancer is discussed further in the reviews by Obaidat et al. (2012) and Wlcek et al. (2011).
One of the primary pathologies caused by OATPs is likely to be adverse drug–drug or drug–food interactions. Treatment with cyclosporine, an inhibitor of OATP-mediated transport, is associated with increased plasma concentrations of statins (Neuvonen et al., 2006). Cyclosporine also increases the plasma concentration of bosentan, as does rifampicin, both of which inhibit OATP-mediated bosentan uptake at clinically relevant concentrations (Treiber et al., 2007; van Giersbergen et al., 2007). Both rifamycin SV and rifampicin reduce bromosulphophthalein (BSP) elimination in humans and inhibit in vitro uptake of BSP by OATP1A2, OATP1B1, OATP1B3 and OATP2B1 (Vavricka et al., 2002). Macrolide antibiotics also inhibit uptake of BSP and pravastatin by OATP1B1 and OATP1B3 in vitro (Seithel et al., 2007). In addition, cyclosporine, saquinavir, indinavir and rifamycin SV inhibit uptake of estradiol-17β-glucuronide by OATP1B1 with potencies that correlate with the incidence of hyperbilirubinaemia associated with those four drugs (Campbell et al., 2004).
There are also reports on potential drug–food interactions occurring at OATPs, particularly for OATP1A2 and OATP2B1, which are expressed at the luminal membrane of enterocytes. Fruit juices decrease the oral bioavailability of fexofenadine in humans (Dresser et al., 2002; 2005; Glaeser et al., 2007). It has been shown that fexofenadine is a substrate of OATP1A2, and that uptake of fexofenadine by OATP1A2 is inhibited by naringin, a component of grapefruit (Bailey et al., 2007). In addition, many flavonoids affect OATP-mediated uptake of the model substrates estrone-3-sulphate, estradiol-17β-glucuronide and dehydroepiandrosterone-3-sulphate (DHEAS), suggesting that possible drug–food interactions could occur especially in patients taking ‘healthy’ dietary supplements in addition to their prescribed medications (Wang et al., 2005b; Roth et al., 2011b).
OATs
Organic anion transporters (OATs in humans, Oats in rodents) are another family of multispecific transporters and are encoded by the SLC22/Slc22 gene superfamily. They mediate the transport of a diverse range of low molecular weight substrates including steroid hormone conjugates, biogenic amines, various drugs and toxins.
Tissue distribution
Documented protein expression for OATs is summarized in Figure 3. Organic anion transporters are expressed in membranes of different tissues throughout the body. OAT1 was the first identified human OAT (Reid et al., 1998), with mRNA expression at highest levels in the kidney, followed by skeletal muscle, brain and placenta (Hosoyamada et al., 1999). At the protein level, OAT1 is expressed at the basolateral membrane of proximal tubules (Hosoyamada et al., 1999; Motohashi et al., 2002; Ljubojevic et al., 2007) and in the plasma membrane of skeletal muscle cells (Takeda et al., 2004). Membrane localization of human OAT1 in the choroid plexus has not yet been investigated, but Oat1 expression has been localized to the apical membrane of mouse and rat choroid plexus (Pritchard et al., 1999; Alebouyeh et al., 2003; Sykes et al., 2004). OAT2 mRNA has the highest expression levels in the liver with lower levels also seen in kidney (Sekine et al., 1998; Sun et al., 2001; Hilgendorf et al., 2007). Protein expression of OAT2 has been identified at the basolateral membrane of proximal tubules (Enomoto et al., 2002b), and it is assumed to be expressed at the basolateral membrane of human hepatocytes based on findings in rodents. OAT3 mRNA has highest expression levels in the kidney with lower levels in brain (Cha et al., 2001; Hilgendorf et al., 2007). OAT3 mRNA expression has also been shown in adrenal tissue and the human adrenal cell line NCI-H295R, and functional studies suggest the protein is expressed (Asif et al., 2005). OAT3 protein has been localized to the basolateral membrane of proximal tubules in the kidney (Cha et al., 2001). OAT4 mRNA is expressed in kidney and placenta (Cha et al., 2000; Bleasby et al., 2006), with protein identified at the apical membrane of renal proximal tubules (Babu et al., 2002; Ekaratanawong et al., 2004) and at the basolateral membrane of syncytiotrophoblasts in the placenta (Ugele et al., 2003). Similarly to OAT3, OAT4 mRNA expression and function has also been shown in adrenal tissue and the human adrenal cell line NCI-H295R (Asif et al., 2005). Little is known about human OAT5, although Northern blot analysis demonstrates mRNA expression in the liver (Sun et al., 2001). The recently characterized OAT7 has been shown to be exclusively expressed in adult and fetal liver, where its expression has been localized to the basolateral membrane of hepatocytes (Shin et al., 2007). OAT10 mRNA has been shown to have highest expression levels in the kidney followed by brain, heart, small intestine and colon (Nishiwaki et al., 1998; Bahn et al., 2008). URAT1, which was previously named the renal-specific transporter ‘RST’, has mRNA expression in both adult and fetal kidney (Enomoto et al., 2002a); more recently, mRNA was also identified in vascular smooth muscle cells (Price et al., 2006). Using immunohistochemistry, URAT1 protein has been localized to the apical membrane of renal proximal tubules (Enomoto et al., 2002a).
Figure 3.
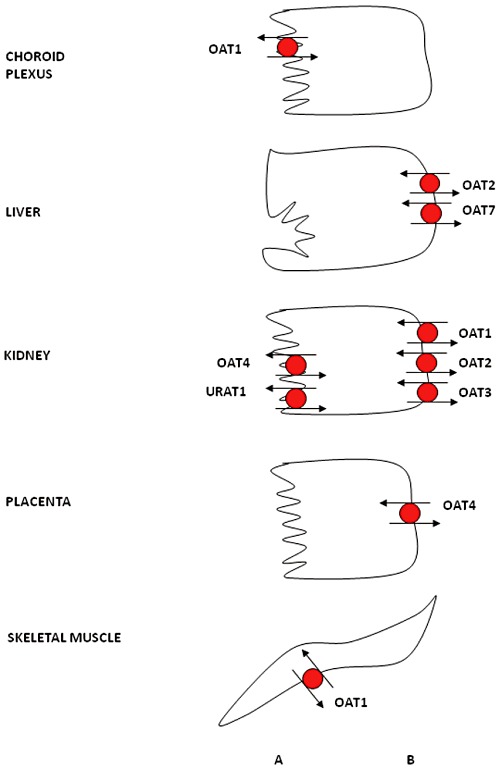
Expression of OATs in different human epithelia. For more details, see the text. OAT1 localization in the choroid plexus and OAT2 localization in the liver is inferred from rodent data. (A) apical; (B) basolateral.
Substrate specificity
The first cloning of human OAT1 was reported in 1998 (Reid et al., 1998). Additional reports in 1999 described the initial functional characterization of human OAT1 as a multispecific organic anion-dicarboxylate exchanger (Cihlar et al., 1999; Hosoyamada et al., 1999; Lu et al., 1999; Race et al., 1999). The best characterized OATs, OAT1 and OAT3, have been shown to transport organic anions against a negative membrane potential in exchange for the counter ion α-ketoglutarate. The α-ketoglutarate gradient is maintained by the secondary active sodium-dicarboxylate co-transporter, which utilizes the sodium gradient maintained by the primary active Na+/K+ ATPase (see Figure 4). Therefore, transport of these OATs has been termed ‘tertiary active.’ Unlike OAT1 and OAT3, human OAT7 exhibits a unique exchange mechanism using short chain fatty acids such as butyrate as counter ions for the transport of sulphate conjugates (Shin et al., 2007). OAT1 is primarily known for its high affinity transport of p-aminohippurate (PAH) from renal tubule cells with apparent affinity (Km) values reported in the low micro-molar range (Hosoyamada et al., 1999). OAT3 can also transport PAH but with slightly lower affinity than OAT1 (Cha et al., 2001). Aside from PAH, OAT1 has been shown to transport prostaglandins, α-ketoglutarate, NSAIDs, antivirals and anticancer drugs. The uricosuric drug, probenecid, is a potent inhibitor of OAT1 transport (Sweet et al., 1997; Cihlar et al., 1999; Hosoyamada et al., 1999; Lu et al., 1999; Race et al., 1999). A more comprehensive review of all OAT substrates and inhibitors can be found in tables of recently published reviews by VanWert et al. (2010) and Burckhardt and Burckhardt (2011).
Figure 4.
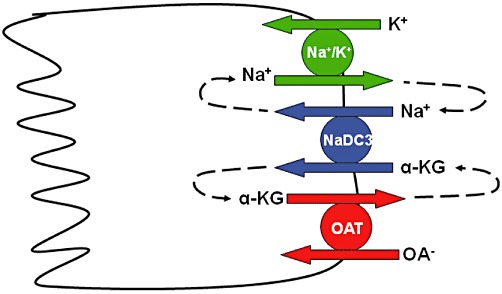
Cartoon of tertiary active transport mechanism for OAT-mediated uptake of organic anions. The primary active Na+/K+-ATPase generates the sodium gradient that is used by the secondary active Na+-dicarboxylate cotransporter (NaDC3) to maintain a high intracellular concentration of α-ketoglutarate, which is used to drive uptake of other organic anions by OAT1 and OAT3.
Regulation of expression
Transcriptional regulation of OATs has been studied by several groups and multiple transcription factors have been implicated. HNF-1α and/or HNF-1β have been shown to affect expression of human OAT1 (Saji et al., 2008), OAT3 (Kikuchi et al., 2006) and URAT1 (Kikuchi et al., 2007), while HNF-4α seems to be involved in human OAT2 expression (Popowski et al., 2005). In addition, for both OAT3 (Kikuchi et al., 2006) and URAT1 (Kikuchi et al., 2007), epigenetic mechanisms of regulation have been identified. At the protein level, PKC activation results in internalization and thus functional inhibition of human OAT1 in frog oocytes, HEK293 cells and Cos-7 cells (Wolff et al., 2003; Zhang et al., 2008). Activation of PKA resulted in stimulation of PAH uptake into opossum kidney cells, indicating that OAT1 could be stimulated by agents that activate PKA (Sauvant et al., 2001; 2002); however, these effects seem to depend on the agents used to stimulate the kinase (Sauvant et al., 2006). Additional studies are needed to investigate what consequences and effects drugs that inhibit or stimulate activation of protein kinases have on human OATs (VanWert et al., 2010).
Transporter structure
The size of OATs ranges from 542 amino acids for human OAT3 to 563 amino acids for OAT1. Like OATPs, OATs are predicted to have 12 transmembrane domains with intracellular amino and carboxy-termini. There is a large extracellular loop between transmembrane domains 1 and 2, as well as a large intracellular loop between transmembrane domains 6 and 7. The large extracellular loop contains potential N-glycosylation sites, while the large intracellular loop and the carboxy-terminus contain putative phosphorylation sites. The extra- and intracellular locations of the different loops were experimentally supported for human OAT1 (Hong et al., 2007).
As is the case for the OATPs, there is so far no crystal structure available for any of the OATs. Therefore, homology modelling has been used to predict the putative three-dimensional structure of human OAT1 on the basis of the bacterial glycerol-3-phosphate transporter and the lactose permease (Perry et al., 2006). Several groups have used site-directed mutagenesis coupled with functional experiments to investigate the role of individual amino acids identified, for example, from polymorphism studies (Bleasby et al., 2005; Fujita et al., 2005; Erdman et al., 2006; Zhou et al., 2010). Some of these amino acids are highlighted in the predicted three-dimensional structure of OAT1 shown in Figure 2B. For more details, please see Burckhardt and Burckhardt (2011).
More recently, a molecular dynamics simulation was performed for OAT1 based on the homology model developed. The data indicate that during the 100 ns simulation one pair of transmembrane domains in each half of the transporter tilt, suggesting a possible involvement in the opening and closing of the transporter (Tsigelny et al., 2011). However, such molecular simulation based on a homology model will most likely improve once a crystal structure is available.
Pathology and clinical significance
Knockout mice for Oat1 (Eraly et al., 2006) and Oat3 (Sweet et al., 2002) have been generated and are both viable and fertile. Characterization of Oat1 null mice showed decreased in vivo transport of Oat1 substrates such as PAH and furosemide, but not of estrone-3-sulphate, a substrate of Oat3 (Eraly et al., 2006). Renal slices from Oat3 null mice demonstrated decreased transport of estrone-3-sulphate, taurocholate and PAH (Sweet et al., 2002). These animal models are important tools to investigate whether Oat1 or Oat3 is primarily responsible for the transport of common drug substrates, but potential species differences must be considered when extrapolating to the human situation (Nigam et al., 2007).
For both OAT1 and OAT3, a lower than average mutation rate has been described (Urban et al., 2006). Although several non-synonymous polymorphisms exist for both transporters, only a few have been shown to affect the transport function (Srimaroeng et al., 2008). The only member of the OAT family for which mutations have been linked to a disease is URAT1. The first characterized mutation that was shown to result in familial idiopathic hypouricaemia is a missense mutation leading to a premature stop codon (W258X). It was first reported by Enomoto et al. (2002a), and later additional mutations have been described in patients with hypouricaemia (Anzai et al., 2007).
Because of the broad substrate specificity of OATs, drug–drug interactions are possible especially with drugs that are eliminated by OAT1 or OAT3 in the kidneys. The interaction between probenecid and methotrexate that was first described in 1978 (Aherne et al., 1978a,b) can today be explained by probenecid's inhibition of OAT3- and OAT1-mediated methotrexate transport (Nozaki et al., 2007). Similarly, co-administration of probenecid with furosemide and other loop diuretics can decrease the potency of these diuretics by reducing their OAT-mediated secretion in the proximal tubule (Burckhardt and Burckhardt, 2011). However, such drug–drug interactions are not always detrimental: for instance, co-administration with probenecid is used to decrease the OAT1- and OAT3-mediated renal elimination of penicillin and other β-lactam antibiotics (Burckhardt and Burckhardt, 2011).
OCTs
In addition to the OATs described above, the SLC22A family also contains the organic cation transporters (OCT1, OCT2 and OCT3) and the organic cation and carnitine transporters (OCT6, OCTN1 and OCTN2). Like the OATPs and OATs, OCTs are multispecific uptake transporters expressed in numerous epithelia throughout the body.
Tissue distribution
Protein expression of OCTs is summarized in Figure 5. OCT1 is usually considered to be a liver-specific transporter, along with OATP1B1 and OATP1B3. However, weak expression of OCT1 mRNA has been seen in other tissues, such as heart, skeletal muscle, kidney, brain and placenta (Gorboulev et al., 1997; Zhang et al., 1997). In the liver, OCT1 protein is localized to the basolateral membrane of hepatocytes (Nies et al., 2008). Furthermore, OCT1 protein was localized to the luminal membrane of lung epithelial cells (Lips et al., 2005). Although rodent Oct1 protein has been identified at the basolateral membrane of enterocytes and proximal tubule epithelial cells (Karbach et al., 2000), in situ hybridization did not detect OCT1 expression in human kidney (Gorboulev et al., 1997). OCT2 is generally considered to be a kidney transporter, though mRNA is expressed at low levels in other tissues such as spleen, placenta, small intestine and brain (Gorboulev et al., 1997). OCT2 protein is mainly localized to the luminal membrane of the distal convoluted tubules (Gorboulev et al., 1997). OCT2 has also been identified in the pyramidal cells of the cerebral cortex and hippocampus (Busch et al., 1998) as well as the luminal membrane of lung epithelia (Lips et al., 2005). OCT3, also known as the extraneuronal monoamine transporter (EMT), has the widest tissue distribution of the OCTs, with strong mRNA expression in liver, placenta, kidney and skeletal muscle, and weaker signals in lung, heart and brain (Wu et al., 2000). OCT3 protein expression has been confirmed on the basolateral membrane of hepatocytes (Nies et al., 2009), the basal membranes of trophoblasts (Sata et al., 2005), the apical membrane of enterocytes (Muller et al., 2005) and the luminal membrane of lung epithelial cells (Lips et al., 2005).
Figure 5.
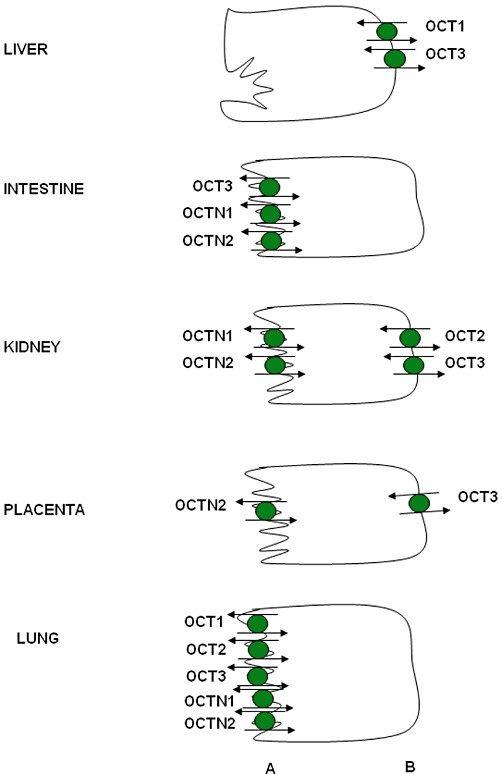
Expression of OCTs in human epithelial cells. For more details, see the text. Localization of OCTN1 in the kidney is concluded based on rodent data. (A) apical; (B) basolateral.
OCTN1, which was first cloned from a human fetal liver cDNA library, is expressed at the mRNA level in fetal liver, kidney and lung (Tamai et al., 1997). In adults, mRNA is strongly expressed in kidney, trachea and bone marrow, and is weakly expressed in skeletal muscle, prostate, lung, pancreas, placenta, heart, uterus, spleen and spinal cord, as well as several cancer cell lines (Tamai et al., 1997). OCTN2 mRNA expression is highest in heart, placenta, skeletal muscle, kidney and pancreas, though it is also expressed in brain, lung and liver (Wu et al., 1998). Within the kidney, two different transcript sizes (3.5 and 4.0 kb) were detected for OCTN2 (Wu et al., 1998). OCTN2 protein expression has been identified at the apical membrane of proximal tubules in kidney (Masuda et al., 2006) and the apical membrane of syncytiotrophoblasts in placenta (Grube et al., 2005). Both OCTN1 and OCTN2 are also expressed in bronchial epithelial cells, with the protein mainly localized to the apical membrane (Horvath et al., 2007). OCT6 (CT2) was originally cloned from a human testis cDNA library and has been localized to Sertoli cells and epithelial cells of the epididymis (Enomoto et al., 2002c). Expression of OCT6 mRNA is also seen in liver, hematopoietic cells and some cancer cell lines (Gong et al., 2002).
Substrate specificity
OCT1, OCT2 and OCT3 mediate the passive facilitated diffusion of a broad range of organic cations down their electrochemical gradients. As such, transport may occur in either direction, is independent of either sodium or pH, and transport of charged substrates is always electrogenic. Although OCT transporter action is independent of pH, affinity for certain substrates does depend on their degree of ionization, leading to increased transport of those substrates at reduced pH (Barendt et al., 2002). Substrates include a wide variety of structurally unrelated small organic cations, both endogenous and exogenous, including many drugs. An extensive list of OCT1-3 substrates and inhibitors is included in a recent review on the importance of organic cation transporters in drug therapy (Nies et al., 2011). Among these substrates are catecholamines, monoamine neurotransmitters and several antiviral drugs.
MPP (1-methyl-4-phenylpyridinium) is a commonly used model substrate for all three transporters; TEA (tetraethylammonium) is also commonly used for OCT1 and OCT2, although it is not a good substrate for OCT3 (Grundemann et al., 1998). A pharmacophore model developed for OCT1 suggests that substrates contain a positive ionizable site, a hydrophobic site and two hydrogen bond acceptor sites (Moaddel et al., 2007). A splice variant of OCT1 that lacks the carboxy-terminus of the protein was found to be non-functional for MPP transport (Hayer et al., 1999). Alternatively, a somewhat longer splice variant of OCT2 that also contains a premature stop codon is found in human kidney, producing a protein which can still transport TEA, although it is less functional for MPP or cimetidine and cannot transport guanidine (Urakami et al., 2002).
OCTN1, OCTN2 and OCT6 are all cation and carnitine transporters. OCTN1 transport activity can be affected by both sodium and proton gradients, depending on the substrate transported. OCTN2 also mediates both sodium-dependent and sodium-independent uptake, depending on the substrate (Koepsell et al., 2007). In addition to carnitine, TEA is also a substrate of both transporters and is frequently used as a model substrate. OCTN2 appears to have different binding sites for TEA and L-carnitine, as several mutations have been found to inhibit carnitine but not TEA transport (Seth et al., 1999). OCT6 has a much more limited substrate specificity than other organic cation transporters. Carnitine transport was found to be bidirectional and not fully dependent on extracellular sodium, although transport was altered by both sodium and pH (Enomoto et al., 2002c).
Regulation of expression
OCT regulation appears to vary depending on transporter, species and tissue localization; therefore, it remains an area of active research. Regulation of OCTs can occur at the transcriptional or protein level. OCT1 has two response elements for HNF-4α, which interacts with them and activates transcription; this activation can be inhibited through SHP (Saborowski et al., 2006). The OCT2 promoter region contains putative androgen receptor elements and steroid hormones increased both mRNA levels and activity of OCT2 in MDCK cells (Shu et al., 2001). OCTN1 transcription was altered by both the RUNX1 transcription factor and TNF-αin vitro (Tokuhiro et al., 2003).
OCT proteins contain phosphorylation sites for PKA, PKC, PKG and tyrosine kinase, and activation of these kinases can alter the activity of OCT1 and OCT2. OCT3 doesn't seem to be affected by PKA, PKC or PKG, despite several conserved target sequences; however, its activity is altered by both the MAP kinase pathway and the calcium–calmodulin pathway. PDZ family members interact with OCTN1 and OCTN2, and the interaction between PDZK1 and OCTN2 has been shown to stimulate transport (Kato et al., 2005). The targeting of OCTN1 and OCTN2 to brush border membrane of enterocytes has also been shown to be regulated by PDZ domain proteins. Detailed summaries of the current knowledge of OCT regulation can be found in recent reviews by Ciarimboli and Schlatter (2005), Koepsell et al. (2007) and Ciarimboli (2008).
Transporter structure
The organic cation transporting proteins contain between 543 and 557 amino acids. All are predicted to contain 12 transmembrane domains with intracellular amino and carboxy-termini. A large extracellular loop between the first and second transmembrane domains contains potential N-glycosylation sites, and a large intracellular loop between transmembrane domains 6 and 7 contains multiple putative phosphorylation sites. OCT1 and OCT2 have 70% amino acid identity to each other, and approximately 50% identity with OCT3 (Gorboulev et al., 1997; Zhang et al., 1997; Grundemann et al., 1998). OCTN1 and OCTN2, which share 77% identity with each other, and 31–37% identity with OCT1-3, also contain an ATP/GTP binding motif in the second intracellular loop (Tamai et al., 1997; Wu et al., 1998). OCT6, also called CT2, has 36%, 38% and 37% identity to OAT1, OCT1 and OCTN2 respectively (Enomoto et al., 2002c).
As has been done for the OATPs and OATs, homology modelling has been used to predict the putative three-dimensional structure of OCT1 (Popp et al., 2005). According to this model, substrates seem to interact with OCT1 within a region rather than at a single binding site (Koepsell, 2011). Additional experiments demonstrated that five amino acids in the substrate binding region can interact with both extracellular and intracellular substrates and are thus likely part of the translocation pathway (Volk et al., 2009; Koepsell, 2011). Furthermore, rat Oct1 has been expressed in a cell-free system, purified and reconstituted into proteoliposomes for functional characterization (Keller et al., 2008). Production of OCT1 in such a cell-free system could be the first step towards the crystallization of this important drug transporter.
Pathology and clinical significance
OCT1, OCT2 and OCT3 knockout mice have been generated and have no obvious phenotype (Jonker et al., 2001; 2003; Zwart et al., 2001). Similarly, no known polymorphisms in OCTs are associated with human pathologies. OCT1 has 18 SNPs that alter amino acids – six have reduced transport activity and one has increased activity (Kerb et al., 2002). OCT2 has ten variants: with the exception of a premature stop codon, all are functionally active, though substrate selectivity and the ability to transport may be slightly altered (Koepsell et al., 2007). Five non-synonymous polymorphisms have been identified in OCT3, three of which show reduced transport activity (Sakata et al., 2010). As with the OATPs, it seems that the greatest risk of pathology associated with the organic cation transporters is that of adverse drug–drug interactions. OCT1 polymorphisms have been associated with altered pharmacokinetics of the anti-diabetic metformin and the tyrosine kinase inhibitor imatinib, while a wide range of drugs have been implicated in potential drug–drug interactions as reviewed by Fahrmayr et al. (2010).
OCTN proteins, however, have been directly indicated in pathologies. Mutations in the gene cluster that contains the OCTN1 and OCTN2 genes have been associated with autoimmune diseases. OCTN1 variant L503F is associated with familial and sporadic inflammatory bowel disease (Lin et al., 2010). Functionally, this variant has altered substrate specificity with a significantly increased affinity for the common model substrate TEA (Urban et al., 2007). Systemic carnitine deficiency, which is caused by a lack of active reabsorption of carnitine in the kidney, has been associated with multiple mutations that cause low or impaired function of OCTN2 (Lahjouji et al., 2001).
Conclusion
Proteins encoded by the SLCO and SLC22A superfamilies are expressed in nearly every epithelium of the body, where they play a significant role in the absorption, distribution and elimination of drugs and other xenobiotics. Many members of these superfamilies transport a broad range of structurally diverse compounds, and several examples have been documented where transport proteins of the SLCO or SLC22A gene families were involved in adverse or intended drug–drug as well as drug–food interactions. Future studies should focus on the elucidation of the three-dimensional structure of these important drug uptake transporters because this will allow to predict and prevent such drug-related pathologies as well as to rationally design drugs targeted to individual tranpsorters. Overall, such studies will lead to a better and safer drug therapy.
Acknowledgments
BH is supported by grants from the National Institutes of Health (RR021940 and GM077336).
Glossary
- ABC
ATP-binding cassette
- BSP
bromosulphophthalein
- CCK-8
cholecystokinin-octapeptide
- CoMFA
comparative molecular field analysis
- HNF
hepatocyte nuclear factor
- MPP
1-methyl-4-phenylpyridinium
- NSAID
non-steroidal anti-inflammatory drug
- OAT
organic anion transporter
- OATP
organic anion transporting polypeptide
- OCT
organic cation transporter
- OCTN
organic cation and carnitine transporter
- PAH
p-aminohippurate
- SHP
small heterodimer partner
- SLC
solute carrier
- SNP
single nucleotide polymorphism
- SXR
steroid and xenobiotic receptor
- TEA
tetraethylammonium
- URAT
urate transporter
Disclosure statement
The authors have nothing to disclose.
References
- Abe T, Kakyo M, Tokui T, Nakagomi R, Nishio T, Nakai D, et al. Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1. J Biol Chem. 1999;274:17159–17163. doi: 10.1074/jbc.274.24.17159. [DOI] [PubMed] [Google Scholar]
- Abe T, Unno M, Onogawa T, Tokui T, Kondo TN, Nakagomi R, et al. LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology. 2001;120:1689–1699. doi: 10.1053/gast.2001.24804. [DOI] [PubMed] [Google Scholar]
- Adachi H, Suzuki T, Abe M, Asano N, Mizutamari H, Tanemoto M, et al. Molecular characterization of human and rat organic anion transporter OATP-D. Am J Physiol Renal Physiol. 2003;285:F1188–F1197. doi: 10.1152/ajprenal.00402.2002. [DOI] [PubMed] [Google Scholar]
- Aherne GW, Piall E, Marks V, Mould G, White WF. Prolongation and enhancement of serum methotrexate concentrations by probenecid. Br Med J. 1978a;1:1097–1099. doi: 10.1136/bmj.1.6120.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aherne W, Piall E, Marks V. Radioimmunoassay of methotrexate: use of 75Se-labelled methotrexate. Ann Clin Biochem. 1978b;15:331–334. doi: 10.1177/000456327801500179. [DOI] [PubMed] [Google Scholar]
- Akamine Y, Miura M, Sunagawa S, Kagaya H, Yasui-Furukori N, Uno T. Influence of drug-transporter polymorphisms on the pharmacokinetics of fexofenadine enantiomers. Xenobiotica. 2010;40:782–789. doi: 10.3109/00498254.2010.515318. [DOI] [PubMed] [Google Scholar]
- Alebouyeh M, Takeda M, Onozato ML, Tojo A, Noshiro R, Hasannejad H, et al. Expression of human organic anion transporters in the choroid plexus and their interactions with neurotransmitter metabolites. J Pharmacol Sci. 2003;93:430–436. doi: 10.1254/jphs.93.430. [DOI] [PubMed] [Google Scholar]
- Annaert P, Ye ZW, Stieger B, Augustijns P. Interaction of HIV protease inhibitors with OATP1B1, 1B3, and 2B1. Xenobiotica. 2010;40:163–176. doi: 10.3109/00498250903509375. [DOI] [PubMed] [Google Scholar]
- Anzai N, Kanai Y, Endou H. New insights into renal transport of urate. Curr Opin Rheumatol. 2007;19:151–157. doi: 10.1097/BOR.0b013e328032781a. [DOI] [PubMed] [Google Scholar]
- Asif AR, Steffgen J, Metten M, Grunewald RW, Muller GA, Bahn A, et al. Presence of organic anion transporters 3 (OAT3) and 4 (OAT4) in human adrenocortical cells. Pflugers Arch. 2005;450:88–95. doi: 10.1007/s00424-004-1373-3. [DOI] [PubMed] [Google Scholar]
- Babu E, Takeda M, Narikawa S, Kobayashi Y, Enomoto A, Tojo A, et al. Role of human organic anion transporter 4 in the transport of ochratoxin A. Biochim Biophys Acta. 2002;1590:64–75. doi: 10.1016/s0167-4889(02)00187-8. [DOI] [PubMed] [Google Scholar]
- Badagnani I, Castro RA, Taylor TR, Brett CM, Huang CC, Stryke D, et al. Interaction of methotrexate with organic-anion transporting polypeptide 1A2 and its genetic variants. J Pharmacol Exp Ther. 2006;318:521–529. doi: 10.1124/jpet.106.104364. [DOI] [PubMed] [Google Scholar]
- Bahn A, Hagos Y, Reuter S, Balen D, Brzica H, Krick W, et al. Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13) J Biol Chem. 2008;283:16332–16341. doi: 10.1074/jbc.M800737200. [DOI] [PubMed] [Google Scholar]
- Bailey DG, Dresser GK, Leake BF, Kim RB. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther. 2007;81:495–502. doi: 10.1038/sj.clpt.6100104. [DOI] [PubMed] [Google Scholar]
- Baldes C, Koenig P, Neumann D, Lenhof HP, Kohlbacher O, Lehr CM. Development of a fluorescence-based assay for screening of modulators of human organic anion transporter 1B3 (OATP1B3) Eur J Pharm Biopharm. 2006;62:39–43. doi: 10.1016/j.ejpb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Barendt WM, Wright SH. The human organic cation transporter (hOCT2) recognizes the degree of substrate ionization. J Biol Chem. 2002;277:22491–22496. doi: 10.1074/jbc.M203114200. [DOI] [PubMed] [Google Scholar]
- Bleasby K, Hall LA, Perry JL, Mohrenweiser HW, Pritchard JB. Functional consequences of single nucleotide polymorphisms in the human organic anion transporter hOAT1 (SLC22A6) J Pharmacol Exp Ther. 2005;314:923–931. doi: 10.1124/jpet.105.084301. [DOI] [PubMed] [Google Scholar]
- Bleasby K, Castle JC, Roberts CJ, Cheng C, Bailey WJ, Sina JF, et al. Expression profiles of 50 xenobiotic transporter genes in humans and pre-clinical species: a resource for investigations into drug disposition. Xenobiotica. 2006;36:963–988. doi: 10.1080/00498250600861751. [DOI] [PubMed] [Google Scholar]
- Bossuyt X, Muller M, Meier PJ. Multispecific amphipathic substrate transport by an organic anion transporter of human liver. J Hepatol. 1996;25:733–738. doi: 10.1016/s0168-8278(96)80246-7. [DOI] [PubMed] [Google Scholar]
- Briz O, Serrano MA, Rebollo N, Hagenbuch B, Meier PJ, Koepsell H, et al. Carriers involved in targeting the cytostatic bile acid-cisplatin derivatives cis-diammine-chloro-cholylglycinate-platinum(II) and cis-diammine-bisursodeoxycholate-platinum(II) toward liver cells. Mol Pharmacol. 2002;61:853–860. doi: 10.1124/mol.61.4.853. [DOI] [PubMed] [Google Scholar]
- Briz O, Serrano MA, MacIas RI, Gonzalez-Gallego J, Marin JJ. Role of organic anion-transporting polypeptides, OATP-A, OATP-C and OATP-8, in the human placenta-maternal liver tandem excretory pathway for foetal bilirubin. Biochem J. 2003;371:897–905. doi: 10.1042/BJ20030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briz O, Romero MR, Martinez-Becerra P, Macias RI, Perez MJ, Jimenez F, et al. OATP8/1B3-mediated cotransport of bile acids and glutathione: an export pathway for organic anions from hepatocytes? J Biol Chem. 2006;281:30326–30335. doi: 10.1074/jbc.M602048200. [DOI] [PubMed] [Google Scholar]
- Bronger H, Konig J, Kopplow K, Steiner HH, Ahmadi R, Herold-Mende C, et al. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Res. 2005;65:11419–11428. doi: 10.1158/0008-5472.CAN-05-1271. [DOI] [PubMed] [Google Scholar]
- Burckhardt G, Burckhardt BC. In vitro and in vivo evidence of the importance of organic anion transporters (OATs) in drug therapy. Handb Exp Pharmacol. 2011;201:29–104. doi: 10.1007/978-3-642-14541-4_2. [DOI] [PubMed] [Google Scholar]
- Busch AE, Karbach U, Miska D, Gorboulev V, Akhoundova A, Volk C, et al. Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol Pharmacol. 1998;54:342–352. doi: 10.1124/mol.54.2.342. [DOI] [PubMed] [Google Scholar]
- Buyukkale G, Turker G, Kasap M, Akpinar G, Arisoy E, Gunlemez A, et al. Neonatal Hyperbilirubinemia and Organic Anion Transporting Polypeptide-2 Gene Mutations. Am J Perinatol. 2011;28:619–626. doi: 10.1055/s-0031-1276736. [DOI] [PubMed] [Google Scholar]
- Campbell SD, de Morais SM, Xu JJ. Inhibition of human organic anion transporting polypeptide OATP 1B1 as a mechanism of drug-induced hyperbilirubinemia. Chem Biol Interact. 2004;150:179–187. doi: 10.1016/j.cbi.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, et al. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem. 2000;275:4507–4512. doi: 10.1074/jbc.275.6.4507. [DOI] [PubMed] [Google Scholar]
- Cha SH, Sekine T, Fukushima JI, Kanai Y, Kobayashi Y, Goya T, et al. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol Pharmacol. 2001;59:1277–1286. doi: 10.1124/mol.59.5.1277. [DOI] [PubMed] [Google Scholar]
- Chang C, Pang KS, Swaan PW, Ekins S. Comparative pharmacophore modeling of organic anion transporting polypeptides: a meta-analysis of rat Oatp1a1 and human OATP1B1. J Pharmacol Exp Ther. 2005;314:533–541. doi: 10.1124/jpet.104.082370. [DOI] [PubMed] [Google Scholar]
- Chen HL, Liu YJ, Wu SH, Ni YH, Ho MC, Lai HS, et al. Expression of hepatocyte transporters and nuclear receptors in children with early and late-stage biliary atresia. Pediatr Res. 2008;63:667–673. doi: 10.1203/PDR.0b013e318170a6b5. [DOI] [PubMed] [Google Scholar]
- Chi Y, Schuster VL. The prostaglandin transporter PGT transports PGH(2) Biochem Biophys Res Commun. 2010;395:168–172. doi: 10.1016/j.bbrc.2010.03.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Murray JW, Wolkoff AW. PDZK1 binding and serine phosphorylation regulate subcellular trafficking of organic anion transport protein 1a1. Am J Physiol Gastrointest Liver Physiol. 2011;300:G384–G393. doi: 10.1152/ajpgi.00500.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu XY, Bleasby K, Yabut J, Cai X, Chan GH, Hafey MJ, et al. Transport of the dipeptidyl peptidase-4 inhibitor sitagliptin by human organic anion transporter 3, organic anion transporting polypeptide 4C1, and multidrug resistance P-glycoprotein. J Pharmacol Exp Ther. 2007;321:673–683. doi: 10.1124/jpet.106.116517. [DOI] [PubMed] [Google Scholar]
- Chung JY, Cho JY, Yu KS, Kim JR, Oh DS, Jung HR, et al. Effect of OATP1B1 (SLCO1B1) variant alleles on the pharmacokinetics of pitavastatin in healthy volunteers. Clin Pharmacol Ther. 2005;78:342–350. doi: 10.1016/j.clpt.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Ciarimboli G. Organic cation transporters. Xenobiotica. 2008;38:936–971. doi: 10.1080/00498250701882482. [DOI] [PubMed] [Google Scholar]
- Ciarimboli G, Schlatter E. Regulation of organic cation transport. Pflugers Arch. 2005;449:423–441. doi: 10.1007/s00424-004-1355-5. [DOI] [PubMed] [Google Scholar]
- Cihlar T, Lin DC, Pritchard JB, Fuller MD, Mendel DB, Sweet DH. The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol Pharmacol. 1999;56:570–580. doi: 10.1124/mol.56.3.570. [DOI] [PubMed] [Google Scholar]
- Congiu M, Mashford ML, Slavin JL, Desmond PV. Coordinate regulation of metabolic enzymes and transporters by nuclear transcription factors in human liver disease. J Gastroenterol Hepatol. 2009;24:1038–1044. doi: 10.1111/j.1440-1746.2009.05800.x. [DOI] [PubMed] [Google Scholar]
- Cui T, Liu Y, Men X, Xu Z, Wu L, Liu S, et al. Bile acid transport correlative protein mRNA expression profile in human placenta with intrahepatic cholestasis of pregnancy. Saudi Med J. 2009;30:1406–1410. [PubMed] [Google Scholar]
- Cui Y, Konig J, Leier I, Buchholz U, Keppler D. Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6. J Biol Chem. 2001;276:9626–9630. doi: 10.1074/jbc.M004968200. [DOI] [PubMed] [Google Scholar]
- Cui Y, Konig J, Nies AT, Pfannschmidt M, Hergt M, Franke WW, et al. Detection of the human organic anion transporters SLC21A6 (OATP2) and SLC21A8 (OATP8) in liver and hepatocellular carcinoma. Lab Invest. 2003;83:527–538. doi: 10.1097/01.lab.0000065015.02412.48. [DOI] [PubMed] [Google Scholar]
- Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27:866–871. [PubMed] [Google Scholar]
- van der Deure WM, Hansen PS, Peeters RP, Kyvik KO, Friesema EC, Hegedus L, et al. Thyroid hormone transport and metabolism by organic anion transporter 1C1 and consequences of genetic variation. Endocrinology. 2008;149:5307–5314. doi: 10.1210/en.2008-0430. [DOI] [PubMed] [Google Scholar]
- Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, et al. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11–20. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]
- Dresser GK, Kim RB, Bailey DG. Effect of grapefruit juice volume on the reduction of fexofenadine bioavailability: possible role of organic anion transporting polypeptides. Clin Pharmacol Ther. 2005;77:170–177. doi: 10.1016/j.clpt.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Ekaratanawong S, Anzai N, Jutabha P, Miyazaki H, Noshiro R, Takeda M, et al. Human organic anion transporter 4 is a renal apical organic anion/dicarboxylate exchanger in the proximal tubules. J Pharmacol Sci. 2004;94:297–304. doi: 10.1254/jphs.94.297. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002a;417:447–452. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Takeda M, Shimoda M, Narikawa S, Kobayashi Y, Yamamoto T, et al. Interaction of human organic anion transporters 2 and 4 with organic anion transport inhibitors. J Pharmacol Exp Ther. 2002b;301:797–802. doi: 10.1124/jpet.301.3.797. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Wempe MF, Tsuchida H, Shin HJ, Cha SH, Anzai N, et al. Molecular identification of a novel carnitine transporter specific to human testis. Insights into the mechanism of carnitine recognition. J Biol Chem. 2002c;277:36262–36271. doi: 10.1074/jbc.M203883200. [DOI] [PubMed] [Google Scholar]
- Eraly SA, Vallon V, Vaughn DA, Gangoiti JA, Richter K, Nagle M, et al. Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. J Biol Chem. 2006;281:5072–5083. doi: 10.1074/jbc.M508050200. [DOI] [PubMed] [Google Scholar]
- Erdman AR, Mangravite LM, Urban TJ, Lagpacan LL, Castro RA, de la Cruz M, et al. The human organic anion transporter 3 (OAT3; SLC22A8): genetic variation and functional genomics. Am J Physiol Renal Physiol. 2006;290:F905–F912. doi: 10.1152/ajprenal.00272.2005. [DOI] [PubMed] [Google Scholar]
- Fahrmayr C, Fromm MF, Konig J. Hepatic OATP and OCT uptake transporters: their role for drug-drug interactions and pharmacogenetic aspects. Drug Metab Rev. 2010;42:380–401. doi: 10.3109/03602530903491683. [DOI] [PubMed] [Google Scholar]
- Fehrenbach T, Cui Y, Faulstich H, Keppler D. Characterization of the transport of the bicyclic peptide phalloidin by human hepatic transport proteins. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:415–420. doi: 10.1007/s00210-003-0814-4. [DOI] [PubMed] [Google Scholar]
- Fischer WJ, Altheimer S, Cattori V, Meier PJ, Dietrich DR, Hagenbuch B. Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicol Appl Pharmacol. 2005;203:257–263. doi: 10.1016/j.taap.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Franco R, Cidlowski JA. SLCO/OATP-like transport of glutathione in FasL-induced apoptosis: glutathione efflux is coupled to an organic anion exchange and is necessary for the progression of the execution phase of apoptosis. J Biol Chem. 2006;281:29542–29557. doi: 10.1074/jbc.M602500200. [DOI] [PubMed] [Google Scholar]
- Franke RM, Baker SD, Mathijssen RH, Schuetz EG, Sparreboom A. Influence of solute carriers on the pharmacokinetics of CYP3A4 probes. Clin Pharmacol Ther. 2008;84:704–709. doi: 10.1038/clpt.2008.94. [DOI] [PubMed] [Google Scholar]
- Fujino H, Saito T, Ogawa S, Kojima J. Transporter-mediated influx and efflux mechanisms of pitavastatin, a new inhibitor of HMG-CoA reductase. J Pharm Pharmacol. 2005;57:1305–1311. doi: 10.1211/jpp.57.10.0009. [DOI] [PubMed] [Google Scholar]
- Fujita T, Brown C, Carlson EJ, Taylor T, de la Cruz M, Johns SJ, et al. Functional analysis of polymorphisms in the organic anion transporter, SLC22A6 (OAT1) Pharmacogenet Genomics. 2005;15:201–209. doi: 10.1097/01213011-200504000-00003. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Adachi H, Nishio T, Unno M, Tokui T, Okabe M, et al. Identification of thyroid hormone transporters in humans: different molecules are involved in a tissue-specific manner. Endocrinology. 2001;142:2005–2012. doi: 10.1210/endo.142.5.8115. [DOI] [PubMed] [Google Scholar]
- Furihata T, Satoh T, Yamamoto N, Kobayashi K, Chiba K. Hepatocyte nuclear factor 1 alpha is a factor responsible for the interindividual variation of OATP1B1 mRNA levels in adult Japanese livers. Pharm Res. 2007;24:2327–2332. doi: 10.1007/s11095-007-9458-2. [DOI] [PubMed] [Google Scholar]
- Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J Pharmacol Exp Ther. 2000;294:73–79. [PubMed] [Google Scholar]
- Gao B, Huber RD, Wenzel A, Vavricka SR, Ismair MG, Reme C, et al. Localization of organic anion transporting polypeptides in the rat and human ciliary body epithelium. Exp Eye Res. 2005;80:61–72. doi: 10.1016/j.exer.2004.08.013. [DOI] [PubMed] [Google Scholar]
- van Giersbergen PL, Treiber A, Schneiter R, Dietrich H, Dingemanse J. Inhibitory and inductive effects of rifampin on the pharmacokinetics of bosentan in healthy subjects. Clin Pharmacol Ther. 2007;81:414–419. doi: 10.1038/sj.clpt.6100075. [DOI] [PubMed] [Google Scholar]
- Glaeser H, Bailey DG, Dresser GK, Gregor JC, Schwarz UI, McGrath JS, et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81:362–370. doi: 10.1038/sj.clpt.6100056. [DOI] [PubMed] [Google Scholar]
- Glaeser H, Mandery K, Sticht H, Fromm MF, Konig J. Relevance of conserved lysine and arginine residues in transmembrane helices for the transport activity of organic anion transporting polypeptide 1B3. Br J Pharmacol. 2010;159:698–708. doi: 10.1111/j.1476-5381.2009.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Lu X, Xu Y, Swiderski CF, Jordan CT, Moscow JA. Identification of OCT6 as a novel organic cation transporter preferentially expressed in hematopoietic cells and leukemias. Exp Hematol. 2002;30:1162–1169. doi: 10.1016/s0301-472x(02)00901-3. [DOI] [PubMed] [Google Scholar]
- Gorboulev V, Ulzheimer JC, Akhoundova A, Ulzheimer-Teuber I, Karbach U, Quester S, et al. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 1997;16:871–881. doi: 10.1089/dna.1997.16.871. [DOI] [PubMed] [Google Scholar]
- Grube M, Meyer Zu Schwabedissen H, Draber K, Prager D, Moritz KU, Linnemann K, et al. Expression, localization, and function of the carnitine transporter octn2 (slc22a5) in human placenta. Drug Metab Dispos. 2005;33:31–37. doi: 10.1124/dmd.104.001560. [DOI] [PubMed] [Google Scholar]
- Grube M, Kock K, Karner S, Reuther S, Ritter CA, Jedlitschky G, et al. Modification of OATP2B1-mediated transport by steroid hormones. Mol Pharmacol. 2006a;70:1735–1741. doi: 10.1124/mol.106.026450. [DOI] [PubMed] [Google Scholar]
- Grube M, Kock K, Oswald S, Draber K, Meissner K, Eckel L, et al. Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin Pharmacol Ther. 2006b;80:607–620. doi: 10.1016/j.clpt.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Grundemann D, Schechinger B, Rappold GA, Schomig E. Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nat Neurosci. 1998;1:349–351. doi: 10.1038/1557. [DOI] [PubMed] [Google Scholar]
- Gui C, Hagenbuch B. Amino acid residues in transmembrane domain 10 of organic anion transporting polypeptide 1B3 are critical for cholecystokinin octapeptide transport. Biochemistry. 2008;47:9090–9097. doi: 10.1021/bi8008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui C, Hagenbuch B. Role of transmembrane domain 10 for the function of organic anion transporting polypeptide 1B1. Protein Sci. 2009;18:2298–2306. doi: 10.1002/pro.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui C, Miao Y, Thompson L, Wahlgren B, Mock M, Stieger B, et al. Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3. Eur J Pharmacol. 2008;584:57–65. doi: 10.1016/j.ejphar.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui C, Wahlgren B, Lushington GH, Hagenbuch B. Identification, Ki determination and CoMFA analysis of nuclear receptor ligands as competitive inhibitors of OATP1B1-mediated estradiol-17beta-glucuronide transport. Pharmacol Res. 2009;60:50–56. doi: 10.1016/j.phrs.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui C, Obaidat A, Chaguturu R, Hagenbuch B. Development of a cell-based high-throughput assay to screen for inhibitors of organic anion transporting polypeptides 1B1 and 1B3. Curr Chem Genomics. 2010;4:1–8. doi: 10.2174/1875397301004010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Gui C. Xenobiotic transporters of the human organic anion transporting polypeptide (OATP) family. Xenobiotica. 2008;7–8:778–801. doi: 10.1080/00498250801986951. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–665. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- Hamada A, Sissung T, Price DK, Danesi R, Chau CH, Sharifi N, et al. Effect of SLCO1B3 haplotype on testosterone transport and clinical outcome in caucasian patients with androgen-independent prostatic cancer. Clin Cancer Res. 2008;14:3312–3318. doi: 10.1158/1078-0432.CCR-07-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanggi E, Grundschober AF, Leuthold S, Meier PJ, St-Pierre MV. Functional analysis of the extracellular cysteine residues in the human organic anion transporting polypeptide, OATP2B1. Mol Pharmacol. 2006;70:806–817. doi: 10.1124/mol.105.019547. [DOI] [PubMed] [Google Scholar]
- Hartkoorn RC, Kwan WS, Shallcross V, Chaikan A, Liptrott N, Egan D, et al. HIV protease inhibitors are substrates for OATP1A2, OATP1B1 and OATP1B3 and lopinavir plasma concentrations are influenced by SLCO1B1 polymorphisms. Pharmacogenet Genomics. 2010;20:112–120. doi: 10.1097/FPC.0b013e328335b02d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayer M, Bonisch H, Bruss M. Molecular cloning, functional characterization and genomic organization of four alternatively spliced isoforms of the human organic cation transporter 1 (hOCT1/SLC22A1) Ann Hum Genet. 1999;63:473–482. doi: 10.1017/S0003480099007770. [DOI] [PubMed] [Google Scholar]
- Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell AL, Karlsson J. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos. 2007;35:1333–1340. doi: 10.1124/dmd.107.014902. [DOI] [PubMed] [Google Scholar]
- Hirano M, Maeda K, Shitara Y, Sugiyama Y. Contribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humans. J Pharmacol Exp Ther. 2004;311:139–146. doi: 10.1124/jpet.104.068056. [DOI] [PubMed] [Google Scholar]
- Hirano M, Maeda K, Shitara Y, Sugiyama Y. Drug-drug interaction between pitavastatin and various drugs via OATP1B1. Drug Metab Dispos. 2006;34:1229–1236. doi: 10.1124/dmd.106.009290. [DOI] [PubMed] [Google Scholar]
- Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, et al. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006;130:1793–1806. doi: 10.1053/j.gastro.2006.02.034. [DOI] [PubMed] [Google Scholar]
- Ho RH, Choi L, Lee W, Mayo G, Schwarz UI, Tirona RG, et al. Effect of drug transporter genotypes on pravastatin disposition in European- and African-American participants. Pharmacogenet Genomics. 2007;17:647–656. doi: 10.1097/FPC.0b013e3280ef698f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Zhou F, You G. Critical amino acid residues in transmembrane domain 1 of the human organic anion transporter hOAT1. J Biol Chem. 2004;279:31478–31482. doi: 10.1074/jbc.M404686200. [DOI] [PubMed] [Google Scholar]
- Hong M, Tanaka K, Pan Z, Ma J, You G. Determination of the external loops and the cellular orientation of the N- and the C-termini of the human organic anion transporter hOAT1. Biochem J. 2007;401:515–520. doi: 10.1042/BJ20061171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath G, Schmid N, Fragoso MA, Schmid A, Conner GE, Salathe M, et al. Epithelial organic cation transporters ensure pH-dependent drug absorption in the airway. Am J Respir Cell Mol Biol. 2007;36:53–60. doi: 10.1165/rcmb.2006-0230OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoyamada M, Sekine T, Kanai Y, Endou H. Molecular cloning and functional expression of a multispecific organic anion transporter from human kidney. Am J Physiol. 1999;276:F122–F128. doi: 10.1152/ajprenal.1999.276.1.F122. [DOI] [PubMed] [Google Scholar]
- Hsiang B, Zhu Y, Wang Z, Wu Y, Sasseville V, Yang WP, et al. A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J Biol Chem. 1999;274:37161–37168. doi: 10.1074/jbc.274.52.37161. [DOI] [PubMed] [Google Scholar]
- Hu S, Franke RM, Filipski KK, Hu C, Orwick SJ, de Bruijn EA, et al. Interaction of imatinib with human organic ion carriers. Clin Cancer Res. 2008;14:3141–3148. doi: 10.1158/1078-0432.CCR-07-4913. [DOI] [PubMed] [Google Scholar]
- Huang MJ, Kua KE, Teng HC, Tang KS, Weng HW, Huang CS. Risk factors for severe hyperbilirubinemia in neonates. Pediatr Res. 2004;56:682–689. doi: 10.1203/01.PDR.0000141846.37253.AF. [DOI] [PubMed] [Google Scholar]
- Huber RD, Gao B, Sidler Pfandler MA, Zhang-Fu W, Leuthold S, Hagenbuch B, et al. Characterization of two splice variants of human organic anion transporting polypeptide 3A1 isolated from human brain. Am J Physiol Cell Physiol. 2007;292:C795–C806. doi: 10.1152/ajpcell.00597.2005. [DOI] [PubMed] [Google Scholar]
- Ieiri I, Suzuki H, Kimura M, Takane H, Nishizato Y, Irie S, et al. Influence of common variants in the pharmacokinetic genes (OATP-C, UGT1A1, and MRP2) on serum bilirubin levels in healthy subjects. Hepatol Res. 2004;30:91–95. doi: 10.1016/j.hepres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Ishiguro N, Maeda K, Kishimoto W, Saito A, Harada A, Ebner T, et al. Predominant contribution of OATP1B3 to the hepatic uptake of telmisartan, an angiotensin II receptor antagonist, in humans. Drug Metab Dispos. 2006;34:1109–1115. doi: 10.1124/dmd.105.009175. [DOI] [PubMed] [Google Scholar]
- Ismair MG, Stieger B, Cattori V, Hagenbuch B, Fried M, Meier PJ, et al. Hepatic uptake of cholecystokinin octapeptide by organic anion-transporting polypeptides OATP4 and OATP8 of rat and human liver. Gastroenterology. 2001;121:1185–1190. doi: 10.1053/gast.2001.28704. [DOI] [PubMed] [Google Scholar]
- Jonker JW, Wagenaar E, Mol CA, Buitelaar M, Koepsell H, Smit JW, et al. Reduced hepatic uptake and intestinal excretion of organic cations in mice with a targeted disruption of the organic cation transporter 1 (Oct1 [Slc22a1]) gene. Mol Cell Biol. 2001;21:5471–5477. doi: 10.1128/MCB.21.16.5471-5477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker JW, Wagenaar E, Van Eijl S, Schinkel AH. Deficiency in the organic cation transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of organic cations. Mol Cell Biol. 2003;23:7902–7908. doi: 10.1128/MCB.23.21.7902-7908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D, Kullak-Ublick GA. Hepatocyte nuclear factor 1 alpha: a key mediator of the effect of bile acids on gene expression. Hepatology. 2003;37:622–631. doi: 10.1053/jhep.2003.50100. [DOI] [PubMed] [Google Scholar]
- Kalgutkar AS, Feng B, Nguyen HT, Frederick KS, Campbell SD, Hatch HL, et al. Role of transporters in the disposition of the selective phosphodiesterase-4 inhibitor (+)-2-[4-({[2-(benzo[1,3]dioxol-5-yloxy)-pyridine-3-carbonyl]-amino}-methy l)-3-fluoro-phenoxy]-propionic acid in rat and human. Drug Metab Dispos. 2007;35:2111–2118. doi: 10.1124/dmd.107.016162. [DOI] [PubMed] [Google Scholar]
- Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009;158:693–705. doi: 10.1111/j.1476-5381.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliokoski A, Neuvonen M, Neuvonen PJ, Niemi M. No significant effect of SLCO1B1 polymorphism on the pharmacokinetics of rosiglitazone and pioglitazone. Br J Clin Pharmacol. 2008;65:78–86. doi: 10.1111/j.1365-2125.2007.02986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai N, Lu R, Satriano JA, Bao Y, Wolkoff AW, Schuster VL. Identification and characterization of a prostaglandin transporter. Science. 1995;268:866–869. doi: 10.1126/science.7754369. [DOI] [PubMed] [Google Scholar]
- Karbach U, Kricke J, Meyer-Wentrup F, Gorboulev V, Volk C, Loffing-Cueni D, et al. Localization of organic cation transporters OCT1 and OCT2 in rat kidney. Am J Physiol Renal Physiol. 2000;279:F679–F687. doi: 10.1152/ajprenal.2000.279.4.F679. [DOI] [PubMed] [Google Scholar]
- Kato K, Shirasaka Y, Kuraoka E, Kikuchi A, Iguchi M, Suzuki H, et al. Intestinal absorption mechanism of tebipenem pivoxil, a novel oral carbapenem: involvement of human OATP family in apical membrane transport. Mol Pharmacol. 2010;7:1747–1756. doi: 10.1021/mp100130b. [DOI] [PubMed] [Google Scholar]
- Kato Y, Yoshida K, Watanabe C, Sai Y, Tsuji A. Screening of the interaction between xenobiotic transporters and PDZ proteins. Pharm Res. 2004;21:1886–1894. doi: 10.1023/b:pham.0000045244.83999.43. [DOI] [PubMed] [Google Scholar]
- Kato Y, Sai Y, Yoshida K, Watanabe C, Hirata T, Tsuji A. PDZK1 directly regulates the function of organic cation/carnitine transporter OCTN2. Mol Pharmacol. 2005;67:734–743. doi: 10.1124/mol.104.002212. [DOI] [PubMed] [Google Scholar]
- Kato Y, Miyazaki T, Kano T, Sugiura T, Kubo Y, Tsuji A. Involvement of influx and efflux transport systems in gastrointestinal absorption of celiprolol. J Pharm Sci. 2009;98:2529–2539. doi: 10.1002/jps.21618. [DOI] [PubMed] [Google Scholar]
- Katz DA, Carr R, Grimm DR, Xiong H, Holley-Shanks R, Mueller T, et al. Organic anion transporting polypeptide 1B1 activity classified by SLCO1B1 genotype influences atrasentan pharmacokinetics. Clin Pharmacol Ther. 2006;79:186–196. doi: 10.1016/j.clpt.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Keitel V, Burdelski M, Warskulat U, Kuhlkamp T, Keppler D, Haussinger D, et al. Expression and localization of hepatobiliary transport proteins in progressive familial intrahepatic cholestasis. Hepatology. 2005;41:1160–1172. doi: 10.1002/hep.20682. [DOI] [PubMed] [Google Scholar]
- Keller T, Schwarz D, Bernhard F, Dotsch V, Hunte C, Gorboulev V, et al. Cell free expression and functional reconstitution of eukaryotic drug transporters. Biochemistry. 2008;47:4552–4564. doi: 10.1021/bi800060w. [DOI] [PubMed] [Google Scholar]
- Kelley L, Sternberg M. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- Kerb R, Brinkmann U, Chatskaia N, Gorbunov D, Gorboulev V, Mornhinweg E, et al. Identification of genetic variations of the human organic cation transporter hOCT1 and their functional consequences. Pharmacogenetics. 2002;12:591–595. doi: 10.1097/00008571-200211000-00002. [DOI] [PubMed] [Google Scholar]
- Kikuchi R, Kusuhara H, Hattori N, Shiota K, Kim I, Gonzalez FJ, et al. Regulation of the expression of human organic anion transporter 3 by hepatocyte nuclear factor 1alpha/beta and DNA methylation. Mol Pharmacol. 2006;70:887–896. doi: 10.1124/mol.106.025494. [DOI] [PubMed] [Google Scholar]
- Kikuchi R, Kusuhara H, Hattori N, Kim I, Shiota K, Gonzalez FJ, et al. Regulation of tissue-specific expression of the human and mouse urate transporter 1 gene by hepatocyte nuclear factor 1 alpha/beta and DNA methylation. Mol Pharmacol. 2007;72:1619–1625. doi: 10.1124/mol.107.039701. [DOI] [PubMed] [Google Scholar]
- Kindla J, Muller F, Mieth M, Fromm MF, Konig J. Influence of non-steroidal anti-inflammatory drugs on Organic Anion Transporting Polypeptide (OATP) 1B1- and OATP1B3-mediated drug transport. Drug Metab Dispos. 2011;39:1047–1053. doi: 10.1124/dmd.110.037622. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Miyata M. Underexpression of mRNA in human hepatocellular carcinoma focusing on eight loci. Hepatology. 2002;36:433–438. doi: 10.1053/jhep.2002.34851. [DOI] [PubMed] [Google Scholar]
- Knauer MJ, Urquhart BL, Meyer zu Schwabedissen HE, Schwarz UI, Lemke CJ, Leake BF, et al. Human skeletal muscle drug transporters determine local exposure and toxicity of statins. Circ Res. 2010;106:297–306. doi: 10.1161/CIRCRESAHA.109.203596. [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther. 2003;306:703–708. doi: 10.1124/jpet.103.051300. [DOI] [PubMed] [Google Scholar]
- Kock K, Koenen A, Giese B, Fraunholz M, May K, Siegmund W, et al. Rapid modulation of the organic anion transporting polypeptide 2B1 (OATP2B1, SLCO2B1) function by protein kinase C-mediated internalization. J Biol Chem. 2010;285:11336–11347. doi: 10.1074/jbc.M109.056457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsell H. Substrate recognition and translocation by polyspecific organic cation transporters. Biol Chem. 2011;392:95–101. doi: 10.1515/BC.2011.009. [DOI] [PubMed] [Google Scholar]
- Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Furukawa T, Ikeda R, Takumi S, Nong Q, Aoyama K, et al. Involvement of mitogen-activated protein kinase signaling pathways in microcystin-LR-induced apoptosis after its selective uptake mediated by OATP1B1 and OATP1B3. Toxicol Sci. 2007;97:407–416. doi: 10.1093/toxsci/kfm054. [DOI] [PubMed] [Google Scholar]
- Konig J. Uptake transporters of the human OATP family: molecular characteristics, substrates, their role in drug-drug interactions, and functional consequences of polymorphisms. Handb Exp Pharmacol. 2011;201:1–28. doi: 10.1007/978-3-642-14541-4_1. [DOI] [PubMed] [Google Scholar]
- Konig J, Cui Y, Nies AT, Keppler D. Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem. 2000a;275:23161–23168. doi: 10.1074/jbc.M001448200. [DOI] [PubMed] [Google Scholar]
- Konig J, Cui Y, Nies AT, Keppler D. A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest Liver Physiol. 2000b;278:G156–G164. doi: 10.1152/ajpgi.2000.278.1.G156. [DOI] [PubMed] [Google Scholar]
- Kopplow K, Letschert K, Konig J, Walter B, Keppler D. Human hepatobiliary transport of organic anions analyzed by quadruple-transfected cells. Mol Pharmacol. 2005;68:1031–1038. doi: 10.1124/mol.105.014605. [DOI] [PubMed] [Google Scholar]
- Kraft ME, Glaeser H, Mandery K, Konig J, Auge D, Fromm MF, et al. The prostaglandin transporter OATP2A1 is expressed in human ocular tissues and transports the antiglaucoma prostanoid latanoprost. Invest Ophthalmol Vis Sci. 2010;51:2504–2511. doi: 10.1167/iovs.09-4290. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Hagenbuch B, Stieger B, Schteingart CD, Hofmann AF, Wolkoff AW, et al. Molecular and functional characterization of an organic anion transporting polypeptide cloned from human liver. Gastroenterology. 1995;109:1274–1282. doi: 10.1016/0016-5085(95)90588-x. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Beuers U, Fahney C, Hagenbuch B, Meier PJ, Paumgartner G. Identification and functional characterization of the promoter region of the human organic anion transporting polypeptide gene. Hepatology. 1997a;26:991–997. doi: 10.1002/hep.510260429. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Glasa J, Boker C, Oswald M, Grutzner U, Hagenbuch B, et al. Chlorambucil-taurocholate is transported by bile acid carriers expressed in human hepatocellular carcinomas. Gastroenterology. 1997b;113:1295–1305. doi: 10.1053/gast.1997.v113.pm9322525. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Fisch T, Oswald M, Hagenbuch B, Meier PJ, Beuers U, et al. Dehydroepiandrosterone sulfate (DHEAS): identification of a carrier protein in human liver and brain. FEBS Lett. 1998;424:173–176. doi: 10.1016/s0014-5793(98)00168-9. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, et al. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology. 2001;120:525–533. doi: 10.1053/gast.2001.21176. [DOI] [PubMed] [Google Scholar]
- Lahjouji K, Mitchell GA, Qureshi IA. Carnitine transport by organic cation transporters and systemic carnitine deficiency. Mol Genet Metab. 2001;73:287–297. doi: 10.1006/mgme.2001.3207. [DOI] [PubMed] [Google Scholar]
- Lamhonwah AM, Ackerley CA, Tilups A, Edwards VD, Wanders RJ, Tein I. OCTN3 is a mammalian peroxisomal membrane carnitine transporter. Biochem Biophys Res Commun. 2005;338:1966–1972. doi: 10.1016/j.bbrc.2005.10.170. [DOI] [PubMed] [Google Scholar]
- Lau YY, Huang Y, Frassetto L, Benet LZ. effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther. 2007;81:194–204. doi: 10.1038/sj.clpt.6100038. [DOI] [PubMed] [Google Scholar]
- Le Vee M, Gripon P, Stieger B, Fardel O. Down-regulation of organic anion transporter expression in human hepatocytes exposed to the proinflammatory cytokine interleukin 1beta. Drug Metab Dispos. 2008;36:217–222. doi: 10.1124/dmd.107.016907. [DOI] [PubMed] [Google Scholar]
- Le Vee M, Lecureur V, Moreau A, Stieger B, Fardel O. Differential regulation of drug transporter expression by hepatocyte growth factor in primary human hepatocytes. Drug Metab Dispos. 2009;37:2228–2235. doi: 10.1124/dmd.109.028035. [DOI] [PubMed] [Google Scholar]
- Le Vee M, Jouan E, Moreau A, Fardel O. Regulation of drug transporter mRNA expression by interferon-gamma in primary human hepatocytes. Fundam Clin Pharmacol. 2011;25:99–103. doi: 10.1111/j.1472-8206.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- Lee SY, Williamson B, Caballero OL, Chen YT, Scanlan MJ, Ritter G, et al. Identification of the gonad-specific anion transporter SLCO6A1 as a cancer/testis (CT) antigen expressed in human lung cancer. Cancer Immun. 2004;4:13–21. [PubMed] [Google Scholar]
- Lee W, Glaeser H, Smith LH, Roberts RL, Moeckel GW, Gervasini G, et al. Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): implications for altered drug disposition and central nervous system drug entry. J Biol Chem. 2005;280:9610–9617. doi: 10.1074/jbc.M411092200. [DOI] [PubMed] [Google Scholar]
- Lee W, Belkhiri A, Lockhart AC, Merchant N, Glaeser H, Harris EI, et al. Overexpression of OATP1B3 confers apoptotic resistance in colon cancer. Cancer Res. 2008;68:10315–10323. doi: 10.1158/0008-5472.CAN-08-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letschert K, Keppler D, Konig J. Mutations in the SLCO1B3 gene affecting the substrate specificity of the hepatocellular uptake transporter OATP1B3 (OATP8) Pharmacogenetics. 2004;14:441–452. doi: 10.1097/01.fpc.0000114744.08559.92. [DOI] [PubMed] [Google Scholar]
- Letschert K, Faulstich H, Keller D, Keppler D. Molecular characterization and inhibition of amanitin uptake into human hepatocytes. Toxicol Sci. 2006;91:140–149. doi: 10.1093/toxsci/kfj141. [DOI] [PubMed] [Google Scholar]
- Leuthold S, Hagenbuch B, Mohebbi N, Wagner CA, Meier PJ, Stieger B. Mechanisms of pH-gradient driven transport mediated by organic anion polypeptide transporters. Am J Physiol Cell Physiol. 2009;296:C570–C582. doi: 10.1152/ajpcell.00436.2008. [DOI] [PubMed] [Google Scholar]
- Li L, Meier PJ, Ballatori N. Oatp2 mediates bidirectional organic solute transport: a role for intracellular glutathione. Mol Pharmacol. 2000;58:335–340. doi: 10.1124/mol.58.2.335. [DOI] [PubMed] [Google Scholar]
- Li LQ, Lee TK, Meier PJ, Ballatori N. Identification of glutathione as a driving force and leukotriene C-4 as a substrate for oatp1, the hepatic sinusoidal organic solute transporter. J Biol Chem. 1998;273:16184–16191. doi: 10.1074/jbc.273.26.16184. [DOI] [PubMed] [Google Scholar]
- Lin Z, Nelson L, Franke A, Poritz L, Li TY, Wu R, et al. OCTN1 variant L503F is associated with familial and sporadic inflammatory bowel disease. J Crohns Colitis. 2010;4:132–138. doi: 10.1016/j.crohns.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Lips KS, Volk C, Schmitt BM, Pfeil U, Arndt P, Miska D, et al. Polyspecific cation transporters mediate luminal release of acetylcholine from bronchial epithelium. Am J Respir Cell Mol Biol. 2005;33:79–88. doi: 10.1165/rcmb.2004-0363OC. [DOI] [PubMed] [Google Scholar]
- Liu L, Cui Y, Chung AY, Shitara Y, Sugiyama Y, Keppler D, et al. Vectorial transport of enalapril by Oatp1a1/Mrp2 and OATP1B1 and OATP1B3/MRP2 in rat and human livers. J Pharmacol Exp Ther. 2006;318:395–402. doi: 10.1124/jpet.106.103390. [DOI] [PubMed] [Google Scholar]
- Ljubojevic M, Balen D, Breljak D, Kusan M, Anzai N, Bahn A, et al. Renal expression of organic anion transporter OAT2 in rats and mice is regulated by sex hormones. Am J Physiol Renal Physiol. 2007;292:F361–F372. doi: 10.1152/ajprenal.00207.2006. [DOI] [PubMed] [Google Scholar]
- Lu R, Chan BS, Schuster VL. Cloning of the human kidney PAH transporter: narrow substrate specificity and regulation by protein kinase C. Am J Physiol. 1999;276:F295–F303. doi: 10.1152/ajprenal.1999.276.2.F295. [DOI] [PubMed] [Google Scholar]
- Lu WJ, Tamai I, Nezu J, Lai ML, Huang JD. Organic anion transporting polypeptide-C mediates arsenic uptake in HEK-293 cells. J Biomed Sci. 2006;13:525–533. doi: 10.1007/s11373-006-9071-0. [DOI] [PubMed] [Google Scholar]
- Maeda K, Ieiri I, Yasuda K, Fujino A, Fujiwara H, Otsubo K, et al. Effects of organic anion transporting polypeptide 1B1 haplotype on pharmacokinetics of pravastatin, valsartan, and temocapril. Clin Pharmacol Ther. 2006a;79:427–439. doi: 10.1016/j.clpt.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Maeda K, Kambara M, Tian Y, Hofmann AF, Sugiyama Y. Uptake of ursodeoxycholate and its conjugates by human hepatocytes: role of Na(+)-taurocholate cotransporting polypeptide (NTCP), organic anion transporting polypeptide (OATP) 1B1 (OATP-C), and oatp1B3 (OATP8) Mol Pharm. 2006b;3:70–77. doi: 10.1021/mp050063u. [DOI] [PubMed] [Google Scholar]
- Maeda T, Takahashi K, Ohtsu N, Oguma T, Ohnishi T, Atsumi R, et al. Identification of influx transporter for the quinolone antibacterial agent levofloxacin. Mol Pharm. 2007;4:85–94. doi: 10.1021/mp060082j. [DOI] [PubMed] [Google Scholar]
- Mahagita C, Grassl SM, Piyachaturawat P, Ballatori N. Human organic anion transporter 1B1 and 1B3 function as bidirectional carriers and do not mediate GSH-bile acid cotransport. Am J Physiol Gastrointest Liver Physiol. 2007;293:G271–G278. doi: 10.1152/ajpgi.00075.2007. [DOI] [PubMed] [Google Scholar]
- Mandery K, Bujok K, Schmidt I, Wex T, Treiber G, Malfertheiner P, et al. Influence of cyclooxygenase inhibitors on the function of the prostaglandin transporter organic anion-transporting polypeptide 2A1 expressed in human gastroduodenal mucosa. J Pharmacol Exp Ther. 2010;332:345–351. doi: 10.1124/jpet.109.154518. [DOI] [PubMed] [Google Scholar]
- Mandery K, Sticht H, Bujok K, Schmidt I, Fahrmayr C, Balk B, et al. Functional and structural relevance of conserved positively charged lysine residues in organic anion transporting polypeptide 1B3. Mol Pharmacol. 2011;80:400–406. doi: 10.1124/mol.111.071282. [DOI] [PubMed] [Google Scholar]
- Martinez-Becerra P, Briz O, Romero MR, Macias RI, Perez MJ, Sancho-Mateo C, et al. Further characterization of the electrogenicity and pH sensitivity of the human organic anion-transporting polypeptides OATP1B1 and OATP1B3. Mol Pharmacol. 2011;79:596–607. doi: 10.1124/mol.110.069971. [DOI] [PubMed] [Google Scholar]
- Masuda S, Terada T, Yonezawa A, Tanihara Y, Kishimoto K, Katsura T, et al. Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J Am Soc Nephrol. 2006;17:2127–2135. doi: 10.1681/ASN.2006030205. [DOI] [PubMed] [Google Scholar]
- Meier PJ, Eckhardt U, Schroeder A, Hagenbuch B, Stieger B. Substrate specificity of sinusoidal bile acid and organic anion uptake systems in rat and human liver. Hepatology. 1997;26:1667–1677. doi: 10.1002/hep.510260641. [DOI] [PubMed] [Google Scholar]
- Meier-Abt F, Faulstich H, Hagenbuch B. Identification of phalloidin uptake systems of rat and human liver. Biochim Biophys Acta. 2004;1664:64–69. doi: 10.1016/j.bbamem.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Meier-Abt F, Mokrab Y, Mizuguchi K. Organic anion transporting polypeptides of the OATP/SLCO superfamily: identification of new members in nonmammalian species, comparative modeling and a potential transport mode. J Membr Biol. 2005;208:213–227. doi: 10.1007/s00232-005-7004-x. [DOI] [PubMed] [Google Scholar]
- Michalski C, Cui Y, Nies AT, Nuessler AK, Neuhaus P, Zanger UM, et al. A naturally occurring mutation in the SLC21A6 gene causing impaired membrane localization of the hepatocyte uptake transporter. J Biol Chem. 2002;277:43058–43063. doi: 10.1074/jbc.M207735200. [DOI] [PubMed] [Google Scholar]
- Miki Y, Suzuki T, Kitada K, Yabuki N, Shibuya R, Moriya T, et al. Expression of the steroid and xenobiotic receptor and its possible target gene, organic anion transporting polypeptide-A, in human breast carcinoma. Cancer Res. 2006;66:535–542. doi: 10.1158/0008-5472.CAN-05-1070. [DOI] [PubMed] [Google Scholar]
- Mikkaichi T, Suzuki T, Onogawa T, Tanemoto M, Mizutamari H, Okada M, et al. Isolation and characterization of a digoxin transporter and its rat homologue expressed in the kidney. Proc Natl Acad Sci U S A. 2004;101:3569–3574. doi: 10.1073/pnas.0304987101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Satoh S, Inoue K, Kagaya H, Saito M, Inoue T, et al. Influence of SLCO1B1, 1B3, 2B1 and ABCC2 genetic polymorphisms on mycophenolic acid pharmacokinetics in Japanese renal transplant recipients. Eur J Clin Pharmacol. 2007;63:1161–1169. doi: 10.1007/s00228-007-0380-7. [DOI] [PubMed] [Google Scholar]
- Miura M, Satoh S, Inoue K, Saito M, Habuchi T, Suzuki T. Telmisartan pharmacokinetics in Japanese renal transplant recipients. Clin Chim Acta. 2009;399:83–87. doi: 10.1016/j.cca.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Miyagawa M, Maeda K, Aoyama A, Sugiyama Y. The eighth and ninth transmembrane domains in organic anion transporting polypeptide 1B1 affect the transport kinetics of estrone-3-sulfate and estradiol-17beta-D-glucuronide. J Pharmacol Exp Ther. 2009;329:551–557. doi: 10.1124/jpet.108.148411. [DOI] [PubMed] [Google Scholar]
- Moaddel R, Ravichandran S, Bighi F, Yamaguchi R, Wainer IW. Pharmacophore modelling of stereoselective binding to the human organic cation transporter (hOCT1) Br J Pharmacol. 2007;151:1305–1314. doi: 10.1038/sj.bjp.0707341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks NR, Liu S, Xu Y, Yu H, Bendelow AS, Moscow JA. Potent cytotoxicity of the phosphatase inhibitor microcystin LR and microcystin analogues in OATP1B1- and OATP1B3-expressing HeLa cells. Mol Cancer Ther. 2007;6:587–598. doi: 10.1158/1535-7163.MCT-06-0500. [DOI] [PubMed] [Google Scholar]
- van Montfoort JE, Hagenbuch B, Fattinger KE, Muller M, Groothuis GM, Meijer DK, et al. Polyspecific organic anion transporting polypeptides mediate hepatic uptake of amphipathic type II organic cations. J Pharmacol Exp Ther. 1999;291:147–152. [PubMed] [Google Scholar]
- Motohashi H, Sakurai Y, Saito H, Masuda S, Urakami Y, Goto M, et al. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am Soc Nephrol. 2002;13:866–874. doi: 10.1681/ASN.V134866. [DOI] [PubMed] [Google Scholar]
- Mougey EB, Feng H, Castro M, Irvin CG, Lima JJ. Absorption of montelukast is transporter mediated: a common variant of OATP2B1 is associated with reduced plasma concentrations and poor response. Pharmacogenet Genomics. 2009;19:129–138. doi: 10.1097/FPC.0b013e32831bd98c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Lips KS, Metzner L, Neubert RH, Koepsell H, Brandsch M. Drug specificity and intestinal membrane localization of human organic cation transporters (OCT) Biochem Pharmacol. 2005;70:1851–1860. doi: 10.1016/j.bcp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Muto M, Onogawa T, Suzuki T, Ishida T, Rikiyama T, Katayose Y, et al. Human liver-specific organic anion transporter-2 is a potent prognostic factor for human breast carcinoma. Cancer Sci. 2007;98:1570–1576. doi: 10.1111/j.1349-7006.2007.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwinyi J, Johne A, Bauer S, Roots I, Gerloff T. Evidence for inverse effects of OATP-C (SLC21A6) 5 and 1b haplotypes on pravastatin kinetics. Clin Pharmacol Ther. 2004;75:415–421. doi: 10.1016/j.clpt.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Nakagomi-Hagihara R, Nakai D, Kawai K, Yoshigae Y, Tokui T, Abe T, et al. OATP1B1, OATP1B3, and mrp2 are involved in hepatobiliary transport of olmesartan, a novel angiotensin II blocker. Drug Metab Dispos. 2006;34:862–869. doi: 10.1124/dmd.105.008888. [DOI] [PubMed] [Google Scholar]
- Nakai D, Nakagomi R, Furuta Y, Tokui T, Abe T, Ikeda T, et al. Human liver-specific organic anion transporter, LST-1, mediates uptake of pravastatin by human hepatocytes. J Pharmacol Exp Ther. 2001;297:861–867. [PubMed] [Google Scholar]
- Nakakariya M, Shimada T, Irokawa M, Maeda T, Tamai I. Identification and species similarity of OATP transporters responsible for hepatic uptake of beta-lactam antibiotics. Drug Metab Pharmacokinet. 2008;23:347–355. doi: 10.2133/dmpk.23.347. [DOI] [PubMed] [Google Scholar]
- Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80:565–581. doi: 10.1016/j.clpt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Niemi M, Schaeffeler E, Lang T, Fromm MF, Neuvonen M, Kyrklund C, et al. High plasma pravastatin concentrations are associated with single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide-C (OATP-C, SLCO1B1) Pharmacogenetics. 2004;14:429–440. doi: 10.1097/01.fpc.0000114750.08559.32. [DOI] [PubMed] [Google Scholar]
- Nies AT, Herrmann E, Brom M, Keppler D. Vectorial transport of the plant alkaloid berberine by double-transfected cells expressing the human organic cation transporter 1 (OCT1, SLC22A1) and the efflux pump MDR1 P-glycoprotein (ABCB1) Naunyn Schmiedebergs Arch Pharmacol. 2008;376:449–461. doi: 10.1007/s00210-007-0219-x. [DOI] [PubMed] [Google Scholar]
- Nies AT, Koepsell H, Winter S, Burk O, Klein K, Kerb R, et al. Expression of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology. 2009;50:1227–1240. doi: 10.1002/hep.23103. [DOI] [PubMed] [Google Scholar]
- Nies AT, Koepsell H, Damme K, Schwab M. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb Exp Pharmacol. 2011;201:105–167. doi: 10.1007/978-3-642-14541-4_3. [DOI] [PubMed] [Google Scholar]
- Nigam SK, Bush KT, Bhatnagar V. Drug and toxicant handling by the OAT organic anion transporters in the kidney and other tissues. Nat Clin Pract Nephrol. 2007;3:443–448. doi: 10.1038/ncpneph0558. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Kubo Y, Kato Y, Sai Y, Ogihara T, Tsuji A. Characterization of the uptake mechanism for a novel loop diuretic, M17055, in Caco-2 cells: involvement of organic anion transporting polypeptide (OATP)-B. Pharm Res. 2007;24:90–98. doi: 10.1007/s11095-006-9127-x. [DOI] [PubMed] [Google Scholar]
- Nishiwaki T, Daigo Y, Tamari M, Fujii Y, Nakamura Y. Molecular cloning, mapping, and characterization of two novel human genes, ORCTL3 and ORCTL4, bearing homology to organic-cation transporters. Cytogenet Cell Genet. 1998;83:251–255. doi: 10.1159/000015197. [DOI] [PubMed] [Google Scholar]
- Nishizato Y, Ieiri I, Suzuki H, Kimura M, Kawabata K, Hirota T, et al. Polymorphisms of OATP-C (SLC21A6) and OAT3 (SLC22A8) genes: consequences for pravastatin pharmacokinetics. Clin Pharmacol Ther. 2003;73:554–565. doi: 10.1016/S0009-9236(03)00060-2. [DOI] [PubMed] [Google Scholar]
- Noe J, Portmann R, Brun ME, Funk C. Substrate-dependent drug-drug interactions between gemfibrozil, fluvastatin and other organic anion-transporting peptide (OATP) substrates on OATP1B1, OATP2B1, and OATP1B3. Drug Metab Dispos. 2007;35:1308–1314. doi: 10.1124/dmd.106.012930. [DOI] [PubMed] [Google Scholar]
- Nomura T, Lu R, Pucci ML, Schuster VL. The two-step model of prostaglandin signal termination: in vitro reconstitution with the prostaglandin transporter and prostaglandin 15 dehydrogenase. Mol Pharmacol. 2004;65:973–978. doi: 10.1124/mol.65.4.973. [DOI] [PubMed] [Google Scholar]
- Nomura T, Chang HY, Lu R, Hankin J, Murphy RC, Schuster VL. Prostaglandin signaling in the renal collecting duct: release, reuptake, and oxidation in the same cell. J Biol Chem. 2005;280:28424–28429. doi: 10.1074/jbc.M408286200. [DOI] [PubMed] [Google Scholar]
- Nozaki Y, Kusuhara H, Kondo T, Iwaki M, Shiroyanagi Y, Nakayama H, et al. Species difference in the inhibitory effect of nonsteroidal anti-inflammatory drugs on the uptake of methotrexate by human kidney slices. J Pharmacol Exp Ther. 2007;322:1162–1170. doi: 10.1124/jpet.107.121491. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Tamai I, Sai Y, Nezu J, Tsuji A. Contribution of organic anion transporting polypeptide OATP-C to hepatic elimination of the opioid pentapeptide analogue [D-Ala2, D-Leu5]-enkephalin. J Pharm Pharmacol. 2003;55:1013–1020. doi: 10.1211/0022357021440. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. J Pharmacol Exp Ther. 2004a;308:438–445. doi: 10.1124/jpet.103.060194. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Sugiura S, Nakajima M, Goto A, Yokoi T, Nezu J, et al. Involvement of organic anion transporting polypeptides in the transport of troglitazone sulfate: implications for understanding troglitazone hepatotoxicity. Drug Metab Dispos. 2004b;32:291–294. doi: 10.1124/dmd.32.3.291. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Minami H, Sugiura S, Tsuji A, Tamai I. Role of organic anion transporter OATP1B1 (OATP-C) in hepatic uptake of irinotecan and its active metabolite, 7-ethyl-10-hydroxycamptothecin: in vitro evidence and effect of single nucleotide polymorphisms. Drug Metab Dispos. 2005;33:434–439. doi: 10.1124/dmd.104.001909. [DOI] [PubMed] [Google Scholar]
- Obaidat A, Roth M, Hagenbuch B. The expression and function of organic anion transporting polypeptides in cancer. Annu Rev Pharmacol Toxicol. 2012;52:135–151. doi: 10.1146/annurev-pharmtox-010510-100556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostendorp RL, van de Steeg E, van der Kruijssen CM, Beijnen JH, Kenworthy KE, Schinkel AH, et al. Organic anion-transporting polypeptide 1B1 mediates transport of Gimatecan and BNP1350 and can be inhibited by several classic ATP-binding cassette (ABC) B1 and/or ABCG2 inhibitors. Drug Metab Dispos. 2009;37:917–923. doi: 10.1124/dmd.108.024901. [DOI] [PubMed] [Google Scholar]
- Oswald M, Kullak-Ublick GA, Paumgartner G, Beuers U. Expression of hepatic transporters OATP-C and MRP2 in primary sclerosing cholangitis. Liver. 2001;21:247–253. doi: 10.1034/j.1600-0676.2001.021004247.x. [DOI] [PubMed] [Google Scholar]
- Oswald S, Konig J, Lutjohann D, Giessmann T, Kroemer HK, Rimmbach C, et al. Disposition of ezetimibe is influenced by polymorphisms of the hepatic uptake carrier OATP1B1. Pharmacogenet Genomics. 2008;18:559–568. doi: 10.1097/FPC.0b013e3282fe9a2c. [DOI] [PubMed] [Google Scholar]
- Pacyniak E, Roth M, Hagenbuch B, Guo GL. Mechanism of polybrominated diphenyl ether uptake into the liver: PBDE congeners are substrates of human hepatic OATP transporters. Toxicol Sci. 2010;115:344–353. doi: 10.1093/toxsci/kfq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasanen MK, Neuvonen M, Neuvonen PJ, Niemi M. SLCO1B1 polymorphism markedly affects the pharmacokinetics of simvastatin acid. Pharmacogenet Genomics. 2006;16:873–879. doi: 10.1097/01.fpc.0000230416.82349.90. [DOI] [PubMed] [Google Scholar]
- Pascolo L, Cupelli F, Anelli PL, Lorusso V, Visigalli M, Uggeri F, et al. Molecular mechanisms for the hepatic uptake of magnetic resonance imaging contrast agents. Biochem Biophys Res Commun. 1999;257:746–752. doi: 10.1006/bbrc.1999.0454. [DOI] [PubMed] [Google Scholar]
- Perry JL, Dembla-Rajpal N, Hall LA, Pritchard JB. A three-dimensional model of human organic anion transporter 1: aromatic amino acids required for substrate transport. J Biol Chem. 2006;281:38071–38079. doi: 10.1074/jbc.M608834200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Yee SW, Woillard JB, Lebranchu Y, Le Meur Y, Giacomini KM, et al. The role of organic anion-transporting polypeptides and their common genetic variants in mycophenolic acid pharmacokinetics. Clin Pharmacol Ther. 2010;87:100–108. doi: 10.1038/clpt.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli F, Hagenbuch B, Stieger B, Klenk U, Folkers G, Meier PJ. Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter. Mol Endocrinol. 2002;16:2283–2296. doi: 10.1210/me.2001-0309. [DOI] [PubMed] [Google Scholar]
- Pizzagalli F, Varga Z, Huber RD, Folkers G, Meier PJ, St-Pierre MV. Identification of steroid sulfate transport processes in the human mammary gland. J Clin Endocrinol Metab. 2003;88:3902–3912. doi: 10.1210/jc.2003-030174. [DOI] [PubMed] [Google Scholar]
- Plaza F, Gabler F, Romero C, Vantman D, Valladares L, Vega M. The conversion of dehydroepiandrosterone into androst-5-ene-3beta,17beta-diol (androstenediol) is increased in endometria from untreated women with polycystic ovarian syndrome. Steroids. 2010;75:810–817. doi: 10.1016/j.steroids.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Popowski K, Eloranta JJ, Saborowski M, Fried M, Meier PJ, Kullak-Ublick GA. The human organic anion transporter 2 gene is transactivated by hepatocyte nuclear factor-4 alpha and suppressed by bile acids. Mol Pharmacol. 2005;67:1629–1638. doi: 10.1124/mol.104.010223. [DOI] [PubMed] [Google Scholar]
- Popp C, Gorboulev V, Muller TD, Gorbunov D, Shatskaya N, Koepsell H. Amino acids critical for substrate affinity of rat organic cation transporter 1 line the substrate binding region in a model derived from the tertiary structure of lactose permease. Mol Pharmacol. 2005;67:1600–1611. doi: 10.1124/mol.104.008839. [DOI] [PubMed] [Google Scholar]
- Price KL, Sautin YY, Long DA, Zhang L, Miyazaki H, Mu W, et al. Human vascular smooth muscle cells express a urate transporter. J Am Soc Nephrol. 2006;17:1791–1795. doi: 10.1681/ASN.2006030264. [DOI] [PubMed] [Google Scholar]
- Pritchard JB, Sweet DH, Miller DS, Walden R. Mechanism of organic anion transport across the apical membrane of choroid plexus. J Biol Chem. 1999;274:33382–33387. doi: 10.1074/jbc.274.47.33382. [DOI] [PubMed] [Google Scholar]
- Race JE, Grassl SM, Williams WJ, Holtzman EJ. Molecular cloning and characterization of two novel human renal organic anion transporters (hOAT1 and hOAT3) Biochem Biophys Res Commun. 1999;255:508–514. doi: 10.1006/bbrc.1998.9978. [DOI] [PubMed] [Google Scholar]
- Reid G, Wolff NA, Dautzenberg FM, Burckhardt G. Cloning of a human renal p-aminohippurate transporter, hROAT1. Kidney Blood Press Res. 1998;21:233–237. doi: 10.1159/000025863. [DOI] [PubMed] [Google Scholar]
- Rizwan AN, Krick W, Burckhardt G. The chloride dependence of the human organic anion transporter 1 (hOAT1) is blunted by mutation of a single amino acid. J Biol Chem. 2007;282:13402–13409. doi: 10.1074/jbc.M609849200. [DOI] [PubMed] [Google Scholar]
- Roberts LM, Woodford K, Zhou M, Black DS, Haggerty JE, Tate EH, et al. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology. 2008;149:6251–6261. doi: 10.1210/en.2008-0378. [DOI] [PubMed] [Google Scholar]
- Roth M, Araya JJ, Timmermann BN, Hagenbuch B. Isolation of modulators of the liver specific Organic Anion Transporting Polypeptides (OATPs) 1B1 and 1B3 from Rollinia emarginata Schlecht (Annonaceae) J Pharmacol Exp Ther. 2011a;339:624–632. doi: 10.1124/jpet.111.184564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M, Timmermann BN, Hagenbuch B. Interactions of green tea catechins with organic anion-transporting polypeptides. Drug Metab Dispos. 2011b;39:920–926. doi: 10.1124/dmd.110.036640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saborowski M, Kullak-Ublick GA, Eloranta JJ. The human organic cation transporter-1 gene is transactivated by hepatocyte nuclear factor-4alpha. J Pharmacol Exp Ther. 2006;317:778–785. doi: 10.1124/jpet.105.099929. [DOI] [PubMed] [Google Scholar]
- Sai Y, Kaneko Y, Ito S, Mitsuoka K, Kato Y, Tamai I, et al. Predominant contribution of organic anion transporting polypeptide OATP-B (OATP2B1) to apical uptake of estrone-3-sulfate by human intestinal Caco-2 cells. Drug Metab Dispos. 2006;34:1423–1431. doi: 10.1124/dmd.106.009530. [DOI] [PubMed] [Google Scholar]
- Saji T, Kikuchi R, Kusuhara H, Kim I, Gonzalez FJ, Sugiyama Y. Transcriptional regulation of human and mouse organic anion transporter 1 by hepatocyte nuclear factor 1 alpha/beta. J Pharmacol Exp Ther. 2008;324:784–790. doi: 10.1124/jpet.107.128249. [DOI] [PubMed] [Google Scholar]
- Sakamoto S, Kusuhara H, Horie K, Takahashi K, Baba T, Ishizaki J, et al. Identification of the transporters involved in the hepatobiliary transport and intestinal efflux of methyl 1-(3,4-dimethoxyphenyl)-3-(3-ethylvaleryl)-4-hydroxy-6,7,8-trimethoxy-2-na phthoate (S-8921) glucuronide, a pharmacologically active metabolite of S-8921. Drug Metab Dispos. 2008;36:1553–1561. doi: 10.1124/dmd.108.020511. [DOI] [PubMed] [Google Scholar]
- Sakata T, Anzai N, Kimura T, Miura D, Fukutomi T, Takeda M, et al. Functional analysis of human organic cation transporter OCT3 (SLC22A3) polymorphisms. J Pharmacol Sci. 2010;113:263–266. doi: 10.1254/jphs.09331sc. [DOI] [PubMed] [Google Scholar]
- Sandhu P, Lee W, Xu X, Leake BF, Yamazaki M, Stone JA, et al. Hepatic uptake of the novel antifungal agent caspofungin. Drug Metab Dispos. 2005;33:676–682. doi: 10.1124/dmd.104.003244. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Suzuki H, Ito K, Abe T, Sugiyama Y. Transcellular transport of organic anions across a double-transfected Madin-Darby canine kidney II cell monolayer expressing both human organic anion-transporting polypeptide (OATP2/SLC21A6) and Multidrug resistance-associated protein 2 (MRP2/ABCC2) J Biol Chem. 2002;277:6497–6503. doi: 10.1074/jbc.M109081200. [DOI] [PubMed] [Google Scholar]
- Sata R, Ohtani H, Tsujimoto M, Murakami H, Koyabu N, Nakamura T, et al. Functional analysis of organic cation transporter 3 expressed in human placenta. J Pharmacol Exp Ther. 2005;315:888–895. doi: 10.1124/jpet.105.086827. [DOI] [PubMed] [Google Scholar]
- Satlin LM, Amin V, Wolkoff AW. Organic anion transporting polypeptide mediates organic anion/HCO3- exchange. J Biol Chem. 1997;272:26340–26345. doi: 10.1074/jbc.272.42.26340. [DOI] [PubMed] [Google Scholar]
- Sato K, Sugawara J, Sato T, Mizutamari H, Suzuki T, Ito A, et al. Expression of Organic Anion Transporting Polypeptide E (OATP-E) in human placenta. Placenta. 2003;24:144–148. doi: 10.1053/plac.2002.0907. [DOI] [PubMed] [Google Scholar]
- Satoh H, Yamashita F, Tsujimoto M, Murakami H, Koyabu N, Ohtani H, et al. Citrus juices inhibit the function of human organic anion-transporting polypeptide OATP-B. Drug Metab Dispos. 2005;33:518–523. doi: 10.1124/dmd.104.002337. [DOI] [PubMed] [Google Scholar]
- Sauvant C, Holzinger H, Gekle M. Modulation of the basolateral and apical step of transepithelial organic anion secretion in proximal tubular opossum kidney cells. Acute effects of epidermal growth factor and mitogen-activated protein kinase. J Biol Chem. 2001;276:14695–14703. doi: 10.1074/jbc.M007046200. [DOI] [PubMed] [Google Scholar]
- Sauvant C, Holzinger H, Gekle M. Short-term regulation of basolateral organic anion uptake in proximal tubular OK cells: EGF acts via MAPK, PLA(2), and COX1. J Am Soc Nephrol. 2002;13:1981–1991. doi: 10.1097/01.asn.0000024437.62046.af. [DOI] [PubMed] [Google Scholar]
- Sauvant C, Holzinger H, Gekle M. Prostaglandin E2 inhibits its own renal transport by downregulation of organic anion transporters rOAT1 and rOAT3. J Am Soc Nephrol. 2006;17:46–53. doi: 10.1681/ASN.2005070727. [DOI] [PubMed] [Google Scholar]
- Schiffer R, Neis M, Holler D, Rodriguez F, Geier A, Gartung C, et al. Active influx transport is mediated by members of the organic anion transporting polypeptide family in human epidermal keratinocytes. J Invest Dermatol. 2003;120:285–291. doi: 10.1046/j.1523-1747.2003.12031.x. [DOI] [PubMed] [Google Scholar]
- Schuster VL. Prostaglandin transport. Prostaglandins Other Lipid Mediat. 2002;68–69:633–647. doi: 10.1016/s0090-6980(02)00061-8. [DOI] [PubMed] [Google Scholar]
- Seithel A, Eberl S, Singer K, Auge D, Heinkele G, Wolf NB, et al. The influence of macrolide antibiotics on the uptake of organic anions and drugs mediated by OATP1B1 and OATP1B3. Drug Metab Dispos. 2007;35:779–786. doi: 10.1124/dmd.106.014407. [DOI] [PubMed] [Google Scholar]
- Sekine T, Cha SH, Tsuda M, Apiwattanakul N, Nakajima N, Kanai Y, et al. Identification of multispecific organic anion transporter 2 expressed predominantly in the liver. FEBS Lett. 1998;429:179–182. doi: 10.1016/s0014-5793(98)00585-7. [DOI] [PubMed] [Google Scholar]
- Seth P, Wu X, Huang W, Leibach FH, Ganapathy V. Mutations in novel organic cation transporter (OCTN2), an organic cation/carnitine transporter, with differential effects on the organic cation transport function and the carnitine transport function. J Biol Chem. 1999;274:33388–33392. doi: 10.1074/jbc.274.47.33388. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Fuse K, Okudaira K, Nishigaki R, Maeda K, Kusuhara H, et al. Contribution of OATP (organic anion-transporting polypeptide) family transporters to the hepatic uptake of fexofenadine in humans. Drug Metab Dispos. 2005;33:1477–1481. doi: 10.1124/dmd.105.004622. [DOI] [PubMed] [Google Scholar]
- Shin HJ, Anzai N, Enomoto A, He X, Kim K, Endou H, et al. Novel liver-specific organic anion transporter OAT7 that operates the exchange of sulfate conjugates for short chain fatty acid butyrate. Hepatology. 2007;45:1046–1055. doi: 10.1002/hep.21596. [DOI] [PubMed] [Google Scholar]
- Shirasaka Y, Kuraoka E, Spahn-Langguth H, Nakanishi T, Langguth P, Tamai I. Species difference in the effect of grapefruit juice on intestinal absorption of talinolol between human and rat. J Pharmacol Exp Ther. 2010;332:181–189. doi: 10.1124/jpet.109.159756. [DOI] [PubMed] [Google Scholar]
- Shitara Y, Itoh T, Sato H, Li AP, Sugiyama Y. Inhibition of transporter-mediated hepatic uptake as a mechanism for drug-drug interaction between cerivastatin and cyclosporin A. J Pharmacol Exp Ther. 2003;304:610–616. doi: 10.1124/jpet.102.041921. [DOI] [PubMed] [Google Scholar]
- Shu Y, Bello CL, Mangravite LM, Feng B, Giacomini KM. Functional characteristics and steroid hormone-mediated regulation of an organic cation transporter in Madin-Darby canine kidney cells. J Pharmacol Exp Ther. 2001;299:392–398. [PubMed] [Google Scholar]
- Smith NF, Acharya MR, Desai N, Figg WD, Sparreboom A. Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol Ther. 2005;4:815–818. doi: 10.4161/cbt.4.8.1867. [DOI] [PubMed] [Google Scholar]
- Srimaroeng C, Perry JL, Pritchard JB. Physiology, structure, and regulation of the cloned organic anion transporters. Xenobiotica. 2008;38:889–935. doi: 10.1080/00498250801927435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckelbroeck S, Nassen A, Ugele B, Ludwig M, Watzka M, Reissinger A, et al. Steroid sulfatase (STS) expression in the human temporal lobe: enzyme activity, mRNA expression and immunohistochemistry study. J Neurochem. 2004;89:403–417. doi: 10.1046/j.1471-4159.2004.02336.x. [DOI] [PubMed] [Google Scholar]
- St-Pierre MV, Hagenbuch B, Ugele B, Meier PJ, Stallmach T. Characterization of an organic anion-transporting polypeptide (OATP-B) in human placenta. J Clin Endocrinol Metab. 2002;87:1856–1863. doi: 10.1210/jcem.87.4.8431. [DOI] [PubMed] [Google Scholar]
- Su Y, Zhang X, Sinko PJ. Human organic anion-transporting polypeptide OATP-A (SLC21A3) acts in concert with P-glycoprotein and multidrug resistance protein 2 in the vectorial transport of Saquinavir in Hep G2 cells. Mol Pharm. 2004;1:49–56. doi: 10.1021/mp0340136. [DOI] [PubMed] [Google Scholar]
- Sun W, Wu RR, van Poelje PD, Erion MD. Isolation of a family of organic anion transporters from human liver and kidney. Biochem Biophys Res Commun. 2001;283:417–422. doi: 10.1006/bbrc.2001.4774. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Onogawa T, Asano N, Mizutamari H, Mikkaichi T, Tanemoto M, et al. Identification and characterization of novel rat and human gonad specific organic anion transporters. Mol Endocrinol. 2003;17:1203–1215. doi: 10.1210/me.2002-0304. [DOI] [PubMed] [Google Scholar]
- Sweet DH, Wolff NA, Pritchard JB. Expression cloning and characterization of ROAT1. The basolateral organic anion transporter in rat kidney. J Biol Chem. 1997;272:30088–30095. doi: 10.1074/jbc.272.48.30088. [DOI] [PubMed] [Google Scholar]
- Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J Biol Chem. 2002;277:26934–26943. doi: 10.1074/jbc.M203803200. [DOI] [PubMed] [Google Scholar]
- Sykes D, Sweet DH, Lowes S, Nigam SK, Pritchard JB, Miller DS. Organic anion transport in choroid plexus from wild-type and organic anion transporter 3 (Slc22a8)-null mice. Am J Physiol Renal Physiol. 2004;286:F972–F978. doi: 10.1152/ajprenal.00356.2003. [DOI] [PubMed] [Google Scholar]
- Tachibana-Iimori R, Tabara Y, Kusuhara H, Kohara K, Kawamoto R, Nakura J, et al. Effect of genetic polymorphism of OATP-C (SLCO1B1) on lipid-lowering response to HMG-CoA reductase inhibitors. Drug Metab Pharmacokinet. 2004;19:375–380. doi: 10.2133/dmpk.19.375. [DOI] [PubMed] [Google Scholar]
- Takada T, Weiss HM, Kretz O, Gross G, Sugiyama Y. Hepatic transport of PKI166, an epidermal growth factor receptor kinase inhibitor of the pyrrolo-pyrimidine class, and its main metabolite, ACU154. Drug Metab Dispos. 2004;32:1272–1278. doi: 10.1124/dmd.104.000497. [DOI] [PubMed] [Google Scholar]
- Takeda M, Noshiro R, Onozato ML, Tojo A, Hasannejad H, Huang XL, et al. Evidence for a role of human organic anion transporters in the muscular side effects of HMG-CoA reductase inhibitors. Eur J Pharmacol. 2004;483:133–138. doi: 10.1016/j.ejphar.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Sugiura T, Umeda S, Matsubara K, Horikawa M, Nakamichi N, et al. Pharmacokinetics and hepatic uptake of eltrombopag, a novel platelet-increasing agent. Drug Metab Dispos. 2011;39:1088–1096. doi: 10.1124/dmd.110.037960. [DOI] [PubMed] [Google Scholar]
- Tamai I, Yabuuchi H, Nezu J, Sai Y, Oku A, Shimane M, et al. Cloning and characterization of a novel human pH-dependent organic cation transporter, OCTN1. FEBS Lett. 1997;419:107–111. doi: 10.1016/s0014-5793(97)01441-5. [DOI] [PubMed] [Google Scholar]
- Tamai I, Nezu J, Uchino H, Sai Y, Oku A, Shimane M, et al. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun. 2000;273:251–260. doi: 10.1006/bbrc.2000.2922. [DOI] [PubMed] [Google Scholar]
- Tamai I, Nozawa T, Koshida M, Nezu J, Sai Y, Tsuji A. Functional characterization of human organic anion transporting polypeptide B (OATP-B) in comparison with liver-specific OATP-C. Pharm Res. 2001;18:1262–1269. doi: 10.1023/a:1013077609227. [DOI] [PubMed] [Google Scholar]
- Tirona RG, Leake BF, Wolkoff AW, Kim RB. Human organic anion transporting polypeptide-C (SLC21A6) is a major determinant of rifampin-mediated pregnane X receptor activation. J Pharmacol Exp Ther. 2003;304:223–228. doi: 10.1124/jpet.102.043026. [DOI] [PubMed] [Google Scholar]
- Tokuhiro S, Yamada R, Chang X, Suzuki A, Kochi Y, Sawada T, et al. An intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid arthritis. Nat Genet. 2003;35:341–348. doi: 10.1038/ng1267. [DOI] [PubMed] [Google Scholar]
- Treiber A, Schneiter R, Hausler S, Stieger B. Bosentan is a substrate of human OATP1B1 and OATP1B3: inhibition of hepatic uptake as the common mechanism of its interactions with cyclosporin A, rifampicin, and sildenafil. Drug Metab Dispos. 2007;35:1400–1407. doi: 10.1124/dmd.106.013615. [DOI] [PubMed] [Google Scholar]
- Tsigelny IF, Kovalskyy D, Kouznetsova VL, Balinskyi O, Sharikov Y, Bhatnagar V, et al. Conformational changes of the multispecific transporter Organic Anion Transporter 1 (OAT1/SLC22A6) suggests a molecular mechanism for initial stages of drug and metabolite transport. Cell Biochem Biophys. 2011;61:251–259. doi: 10.1007/s12013-011-9191-7. [DOI] [PubMed] [Google Scholar]
- Tsuda-Tsukimoto M, Maeda T, Iwanaga T, Kume T, Tamai I. Characterization of hepatobiliary transport systems of a novel alpha4beta1/alpha4beta7 dual antagonist, TR-14035. Pharm Res. 2006;23:2646–2656. doi: 10.1007/s11095-006-9102-6. [DOI] [PubMed] [Google Scholar]
- Ugele B, St-Pierre MV, Pihusch M, Bahn A, Hantschmann P. Characterization and identification of steroid sulfate transporters of human placenta. Am J Physiol Endocrinol Metab. 2003;284:E390–E398. doi: 10.1152/ajpendo.00257.2002. [DOI] [PubMed] [Google Scholar]
- Urakami Y, Akazawa M, Saito H, Okuda M, Inui K. cDNA cloning, functional characterization, and tissue distribution of an alternatively spliced variant of organic cation transporter hOCT2 predominantly expressed in the human kidney. J Am Soc Nephrol. 2002;13:1703–1710. doi: 10.1097/01.asn.0000019413.78751.46. [DOI] [PubMed] [Google Scholar]
- Urban TJ, Sebro R, Hurowitz EH, Leabman MK, Badagnani I, Lagpacan LL, et al. Functional genomics of membrane transporters in human populations. Genome Res. 2006;16:223–230. doi: 10.1101/gr.4356206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban TJ, Yang C, Lagpacan LL, Brown C, Castro RA, Taylor TR, et al. Functional effects of protein sequence polymorphisms in the organic cation/ergothioneine transporter OCTN1 (SLC22A4) Pharmacogenet Genomics. 2007;17:773–782. doi: 10.1097/FPC.0b013e3281c6d08e.. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan S, Camenisch G, Schuetz H, Reynolds C, Yeh CM, Bizot MN, et al. Pharmacokinetics of the oral direct renin inhibitor aliskiren in combination with digoxin, atorvastatin, and ketoconazole in healthy subjects: the role of P-glycoprotein in the disposition of aliskiren. J Clin Pharmacol. 2008;48:1323–1338. doi: 10.1177/0091270008323258. [DOI] [PubMed] [Google Scholar]
- VanWert AL, Gionfriddo MR, Sweet DH. Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm Drug Dispos. 2010;31:1–71. doi: 10.1002/bdd.693. [DOI] [PubMed] [Google Scholar]
- Varma MV, Rotter CJ, Chupka J, Whalen KM, Duignan DB, Feng B, et al. pH-sensitive interaction of HMG-CoA reductase inhibitors (statins) with organic anion transporting polypeptide 2B1. Mol Pharm. 2011;8:1303–1313. doi: 10.1021/mp200103h. [DOI] [PubMed] [Google Scholar]
- Vavricka SR, Van Montfoort J, Ha HR, Meier PJ, Fattinger K. Interactions of rifamycin SV and rifampicin with organic anion uptake systems of human liver. Hepatology. 2002;36:164–172. doi: 10.1053/jhep.2002.34133. [DOI] [PubMed] [Google Scholar]
- Vavricka SR, Jung D, Fried M, Grutzner U, Meier PJ, Kullak-Ublick GA. The human organic anion transporting polypeptide 8 (SLCO1B3) gene is transcriptionally repressed by hepatocyte nuclear factor 3beta in hepatocellular carcinoma. J Hepatol. 2004;40:212–218. doi: 10.1016/j.jhep.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Volk C, Gorboulev V, Kotzsch A, Muller TD, Koepsell H. Five amino acids in the innermost cavity of the substrate binding cleft of organic cation transporter 1 interact with extracellular and intracellular corticosterone. Mol Pharmacol. 2009;76:275–289. doi: 10.1124/mol.109.054783. [DOI] [PubMed] [Google Scholar]
- Vormfelde SV, Toliat MR, Schirmer M, Meineke I, Nurnberg P, Brockmoller J. The polymorphisms Asn130Asp and Val174Ala in OATP1B1 and the CYP2C9 allele *3 independently affect torsemide pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;83:815–817. doi: 10.1038/sj.clpt.6100404. [DOI] [PubMed] [Google Scholar]
- Walker AL, Franke RM, Sparreboom A, Ware RE. Transcellular movement of hydroxyurea is mediated by specific solute carrier transporters. Exp Hematol. 2011;39:446–456. doi: 10.1016/j.exphem.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Wang JJ, Xiao Y, Murray JW, Novikoff PM, Angeletti RH, et al. Interaction with PDZK1 is required for expression of organic anion transporting protein 1A1 on the hepatocyte surface. J Biol Chem. 2005a;280:30143–30149. doi: 10.1074/jbc.M503969200. [DOI] [PubMed] [Google Scholar]
- Wang P, Hata S, Xiao Y, Murray JW, Wolkoff AW. Topological assessment of oatp1a1: a 12-transmembrane domain integral membrane protein with three N-linked carbohydrate chains. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1052–G1059. doi: 10.1152/ajpgi.00584.2007. [DOI] [PubMed] [Google Scholar]
- Wang X, Wolkoff AW, Morris ME. Flavonoids as a novel class of human organic anion-transporting polypeptide OATP1B1 (OATP-C) modulators. Drug Metab Dispos. 2005b;33:1666–1672. doi: 10.1124/dmd.105.005926. [DOI] [PubMed] [Google Scholar]
- Weaver YM, Hagenbuch B. Several conserved positively charged amino acids in OATP1B1 are involved in binding or translocation of different substrates. J Membr Biol. 2010;236:279–290. doi: 10.1007/s00232-010-9300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Xiong Y. OATP1B1 388A>G polymorphism and pharmacokinetics of pitavastatin in Chinese healthy volunteers. J Clin Pharm Ther. 2010;35:99–104. doi: 10.1111/j.1365-2710.2009.01071.x. [DOI] [PubMed] [Google Scholar]
- Werner D, Werner U, Meybaum A, Schmidt B, Umbreen S, Grosch A, et al. Determinants of steady-state torasemide pharmacokinetics: impact of pharmacogenetic factors, gender and angiotensin II receptor blockers. Clin Pharmacokinet. 2008;47:323–332. doi: 10.2165/00003088-200847050-00003. [DOI] [PubMed] [Google Scholar]
- Wlcek K, Svoboda M, Riha J, Zakaria S, Olszewski U, Dvorak Z, et al. The analysis of organic anion transporting polypeptide (OATP) mRNA and protein patterns in primary and metastatic liver cancer. Cancer Biol Ther. 2011;11:801–811. doi: 10.4161/cbt.11.9.15176. [DOI] [PubMed] [Google Scholar]
- Wojtal KA, Eloranta JJ, Hruz P, Gutmann H, Drewe J, Staumann A, et al. Changes in mRNA expression levels of solute carrier transporters in inflammatory bowel disease patients. Drug Metab Dispos. 2009;37:1871–1877. doi: 10.1124/dmd.109.027367. [DOI] [PubMed] [Google Scholar]
- Wolff NA, Thies K, Kuhnke N, Reid G, Friedrich B, Lang F, et al. Protein kinase C activation downregulates human organic anion transporter 1-mediated transport through carrier internalization. J Am Soc Nephrol. 2003;14:1959–1968. doi: 10.1097/01.asn.0000079040.55124.25. [DOI] [PubMed] [Google Scholar]
- Wood M, Ananthanarayanan M, Jones B, Wooton-Kee R, Hoffman T, Suchy FJ, et al. Hormonal regulation of hepatic organic anion transporting polypeptides. Mol Pharmacol. 2005;68:218–225. doi: 10.1124/mol.104.010371. [DOI] [PubMed] [Google Scholar]
- Wu X, Prasad PD, Leibach FH, Ganapathy V. cDNA sequence, transport function, and genomic organization of human OCTN2, a new member of the organic cation transporter family. Biochem Biophys Res Commun. 1998;246:589–595. doi: 10.1006/bbrc.1998.8669. [DOI] [PubMed] [Google Scholar]
- Wu X, Huang W, Ganapathy ME, Wang H, Kekuda R, Conway SJ, et al. Structure, function, and regional distribution of the organic cation transporter OCT3 in the kidney. Am J Physiol Renal Physiol. 2000;279:F449–F458. doi: 10.1152/ajprenal.2000.279.3.F449. [DOI] [PubMed] [Google Scholar]
- Yamada A, Maeda K, Kamiyama E, Sugiyama D, Kondo T, Shiroyanagi Y, et al. Multiple human isoforms of drug transporters contribute to the hepatic and renal transport of olmesartan, a selective antagonist of the angiotensin II AT1-receptor. Drug Metab Dispos. 2007;35:2166–2176. doi: 10.1124/dmd.107.017459. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Okada M, Akitaya S, Ohara H, Mikkaichi T, Ishikawa H, et al. Transport of fluorescent chenodeoxycholic acid via the human organic anion transporters OATP1B1 and OATP1B3. J Lipid Res. 2006;47:1196–1202. doi: 10.1194/jlr.M500532-JLR200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Sugie M, Okada M, Mikkaichi T, Toyohara T, Abe T, et al. Transport of estrone 3-sulfate mediated by organic anion transporter OATP4C1: estrone 3-sulfate binds to the different recognition site for digoxin in OATP4C1. Drug Metab Pharmacokinet. 2010;25:314–317. doi: 10.2133/dmpk.25.314. [DOI] [PubMed] [Google Scholar]
- Yamakawa Y, Hamada A, Shuto T, Yuki M, Uchida T, Kai H, et al. Pharmacokinetic impact of SLCO1A2 polymorphisms on imatinib disposition in patients with chronic myeloid leukemia. Clin Pharmacol Ther. 2011;90:157–163. doi: 10.1038/clpt.2011.102. [DOI] [PubMed] [Google Scholar]
- Yamashiro W, Maeda K, Hirouchi M, Adachi Y, Hu Z, Sugiyama Y. Involvement of transporters in the hepatic uptake and biliary excretion of valsartan, a selective antagonist of the angiotensin II AT1-receptor, in humans. Drug Metab Dispos. 2006;34:1247–1254. doi: 10.1124/dmd.105.008938. [DOI] [PubMed] [Google Scholar]
- Youngblood GL, Sweet DH. Identification and functional assessment of the novel murine organic anion transporter Oat5 (Slc22a19) expressed in kidney. Am J Physiol Renal Physiol. 2004;287:F236–F244. doi: 10.1152/ajprenal.00012.2004. [DOI] [PubMed] [Google Scholar]
- Zhang L, Dresser MJ, Gray AT, Yost SC, Terashita S, Giacomini KM. Cloning and functional expression of a human liver organic cation transporter. Mol Pharmacol. 1997;51:913–921. doi: 10.1124/mol.51.6.913. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Hong M, Duan P, Pan Z, Ma J, You G. Organic anion transporter OAT1 undergoes constitutive and protein kinase C-regulated trafficking through a dynamin- and clathrin-dependent pathway. J Biol Chem. 2008;283:32570–32579. doi: 10.1074/jbc.M800298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, He YJ, Han CT, Liu ZQ, Li Q, Fan L, et al. Effect of SLCO1B1 genetic polymorphism on the pharmacokinetics of nateglinide. Br J Clin Pharmacol. 2006;62:567–572. doi: 10.1111/j.1365-2125.2006.02686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Zhu L, Cui PH, Church WB, Murray M. Functional characterization of nonsynonymous single nucleotide polymorphisms in the human organic anion transporter 4 (hOAT4) Br J Pharmacol. 2010;159:419–427. doi: 10.1111/j.1476-5381.2009.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Verhaagh S, Buitelaar M, Popp-Snijders C, Barlow DP. Impaired activity of the extraneuronal monoamine transporter system known as uptake-2 in Orct3/Slc22a3-deficient mice. Mol Cell Biol. 2001;21:4188–4196. doi: 10.1128/MCB.21.13.4188-4196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


