Abstract
Blood doping practices in sports have been around for at least half a century and will likely remain for several years to come. The main reason for the various forms of blood doping to be common is that they are easy to perform, and the effects on exercise performance are gigantic. Yet another reason for blood doping to be a popular illicit practice is that detection is difficult. For autologous blood transfusions, for example, no direct test exists, and the direct testing of misuse with recombinant human erythropoietin (rhEpo) has proven very difficult despite a test exists. Future blood doping practice will likely include the stabilization of the transcription factor hypoxia-inducible factor which leads to an increased endogenous erythropoietin synthesis. It seems unrealistic to develop specific test against such drugs (and the copies hereof originating from illegal laboratories). In an attempt to detect and limit blood doping, the World Anti-Doping Agency (WADA) has launched the Athlete Biological Passport where indirect markers for all types of blood doping are evaluated on an individual level. The approach seemed promising, but a recent publication demonstrates the system to be incapable of detecting even a single subject as ‘suspicious’ while treated with rhEpo for 10–12 weeks. Sad to say, the hope that the 2012 London Olympics should be cleaner in regard to blood doping seems faint. We propose that WADA strengthens the quality and capacities of the National Anti-Doping Agencies and that they work more efficiently with the international sports federations in an attempt to limit blood doping.
Keywords: Epo, exercise, rhEpo, transfusion, red blood cell, haemoglobin mass, performance, Hb, Htc, haematocrit, blood passport
Introduction
Blood doping practices in sports have been around for several decades and will likely remain for several years to come also. After the Olympic Games in Mexico City in 1968, focus was on the role of the Hb concentration and the total amount of Hb (nHb) for maximal oxygen uptake (VO2max) and endurance performance. In two studies by Ekblom and colleagues, it was convincingly demonstrated that the elevation in Hb concentration led to a higher VO2max and an improved performance (Ekblom et al., 1972; 1976) (Figure 1). The effect was equally apparent in subjects with low and high aerobic fitness, as well as whether the basal [Hb] was low, normal or high. This latter finding was surprising since the individuals with a high starting [Hb] point would enter the steep part of the [Hb]–blood viscosity curve (Pirofsky, 1953), and when reaching a certain threshold, this may limit maximal cardiac output and hence exercise performance. However, the elevation in VO2max as well as in power output was a function of increase in [Hb] regardless of its level. A few years later came the first accounts of blood manipulations in sports. In January 1984, Francesco Moser cycled 51.151 km in 1 h and thereby shattered the previous record set by Eddy Merkcx in 1972 by more than 1.5 km. Later, it was revealed that Moser had been ‘prepared’ by Dr Francesco Conconi by means of blood transfusions for this event. The same year, blood transfusions were used with success by the American track cycling team during the Olympic Games held in Los Angeles to win several medals. Blood transfusions were also practiced by Finish cross-country skiers in the 1970s and 1980s. Following the ‘transfusion era’, erythropoietin (Epo) abuse took over in the next decennium, as highlighted by the countless doping scandals during the Tour de France bicycle race in those years. The practice with red cell infusion has however received a renaissance as a timed supplement to Epo misuse in the last decade (Waddington and Smith, 2008). The reason why blood doping is a ‘popular’ means of cheating is that it is one of few feasible procedures to increase exercise performance substantially. Physiological factors usually associated to exercise performance such as the maximum pumping capacity of the heart, O2 extraction and utilization of the exercising muscles, exercise economy and sub-maximal lactate levels (Jacobs et al., 2011) are much more difficult to manipulate with, as compared to the arterial blood O2 transport capacity which is increased easily by means of blood transfusions or injections with recombinant human Epo (rhEpo). This is also the reason why much focus is given to the haemoglobin concentration ([Hb]; g·L−1 or mM), the total amount of whole body haemoglobin (nHb; g) and other blood variables in ongoing anti-doping work. With this review, we hope to increase the general understanding of blood doping practice by athletes. Throughout the manuscript, data will be given as presented in the original work. To convert haematocrit (Htc) to [Hb; mmol·L−1], divide by 4.7 (Figure 2A). Two prior reviews on the topic published some years ago in the British Journal of Pharmacology are recommended (Elliott, 2008; Spedding and Spedding, 2008).
Figure 1.
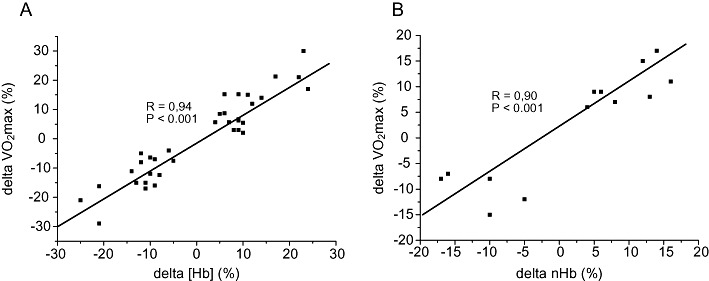
Influence of changes in (A) [Hb] and (B) nHb at the individual level on VO2max. All data have been collected by the authors during experimental work conducted at the Copenhagen Muscle Research Centre between 2002 and 2006.
Figure 2.
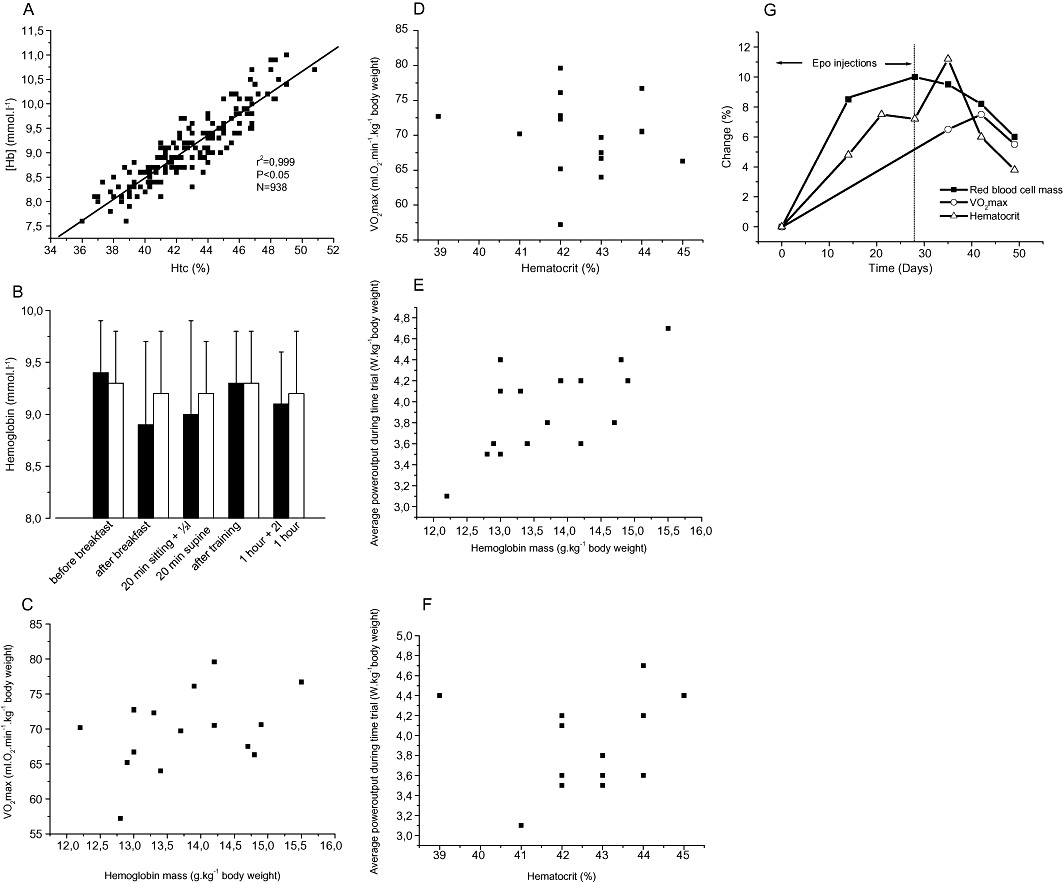
(A) Correlation between [Hb] (mmol·L−1) and haematocrit (%) values in world class cross-country skiers; (B) Effect of various daily activities in world class cross-country skiers (n = 12) on [Hb; mmol·L−1] on two separate days (black and white bars). ‘20 min sitting +½ L’ indicates that the athlete drank ½ L of water while sitting for 20 min; ‘after training’ indicates that the sample was obtained after normal endurance training of 2–3 h of duration without fluid intake; ‘1 h + 2 L’ indicates that the sample was taken 1 h after completion of the training bout and that 1 L of water was drunk during this period); (C–F) correlation between haemoglobin mass (g·kg−1 body weight) and haematocrit (%) with VO2max (mL·min−1·kg−1 body weight) and 26 km time trial performance (W−1·kg−1 body weight) in elite cyclists (adapted from (Jacobs et al., 2011); and (G) Time span for %-changes in haematocrit (Htc), haemoglobin mass (nHb) and maximal aerobic power (VO2max) (adapted from (Lundby et al., 2008a).
Performance gains following blood and Epo doping
In elite athletes (and also in normal humans), aerobic power and time trial performance correlate well with the individuals’ normal haemoglobin mass (Figure 2C and E), but not with Htc (Figure 2D and F), whereas increases in either value will increase performance (Ekblom et al., 1972; 1976) (Figure 1). This demonstrates that having a naturally high Htc does not guarantee a high performance level, but that manipulations with Htc will affect performance. On the contrary, a naturally high nHb is usually also associated with a high VO2max (Martino et al., 2002), and manipulations herewith will also alter performance. The performance gain associated to blood manipulations is far from negligible. The first observation in this regard was reported by Pace and colleagues (Pace et al., 1945) where Htc was increased from 47 to 59% by means of blood transfusions, and sub-maximal heart rate at a given workload decreased from 127 beats·min−1 to 113 beats·min−1 in hypoxia. A recent review on the topic revealed that VO2max is increased by 5–10% when [Hb] is raised 10% by blood transfusion, and that VO2max is diminished by the same magnitude if [Hb] is decreased by 10% with haemodilution (Calbet et al., 2006). As mentioned above, Ekblom and co-workers (Ekblom et al., 1972; 1976) concluded that VO2max would increase independent of base [Hb]; however, it must be assumed that, at some point, VO2max will not increase any further despite increases in [Hb]. Likely due to increased blood viscosity with increasing Htc (Pirofsky, 1953), VO2max and time trial performance do not increase linearly (or infinite) with increasing Htc. The increased VO2max following Epo injections are maintained for at least 3 weeks (Figure 2G) (Lundby et al., 2008a). This makes out-of-competition testing crucial (Figure 3) as the detection success in the weeks following termination of rhEpo treatment is virtually zero (Lundby et al., 2008a). Of note is also that sub-maximal exercise performance – such as time trial performance – is increased by much more following blood doping as compared to the increase in VO2max, and, in a recent study, time to exhaustion was increased by 50% in an approximately 30 min long bike time trial following 4 and 11 weeks of rhEpo treatment in normal healthy volunteers (Thomsen et al., 2007). Whether this is directly transferable to elite athletes remains unknown (within the scientific community), but seems likely. The increase in sub-maximal exercise performance following Epo injections is likely due to a reduced relative cardiovascular and metabolic stress, and at least part of the explanation seems to be related to a 2 mM reduction (from approximately 9 to 7 mM) in plasma lactate values during the time trial (Thomsen et al., 2007). Furthermore, rhEpo treatment also increases exercise performance at moderate altitudes (Robach et al., 2008), and, finally, it had for some time been speculated that rhEpo may increase performance by other means than by stimulating erythropoiesis, but this seems not to be the case (Lundby et al., 2008b; Rasmussen et al., 2010; Lundby and Olsen, 2011).
Figure 3.
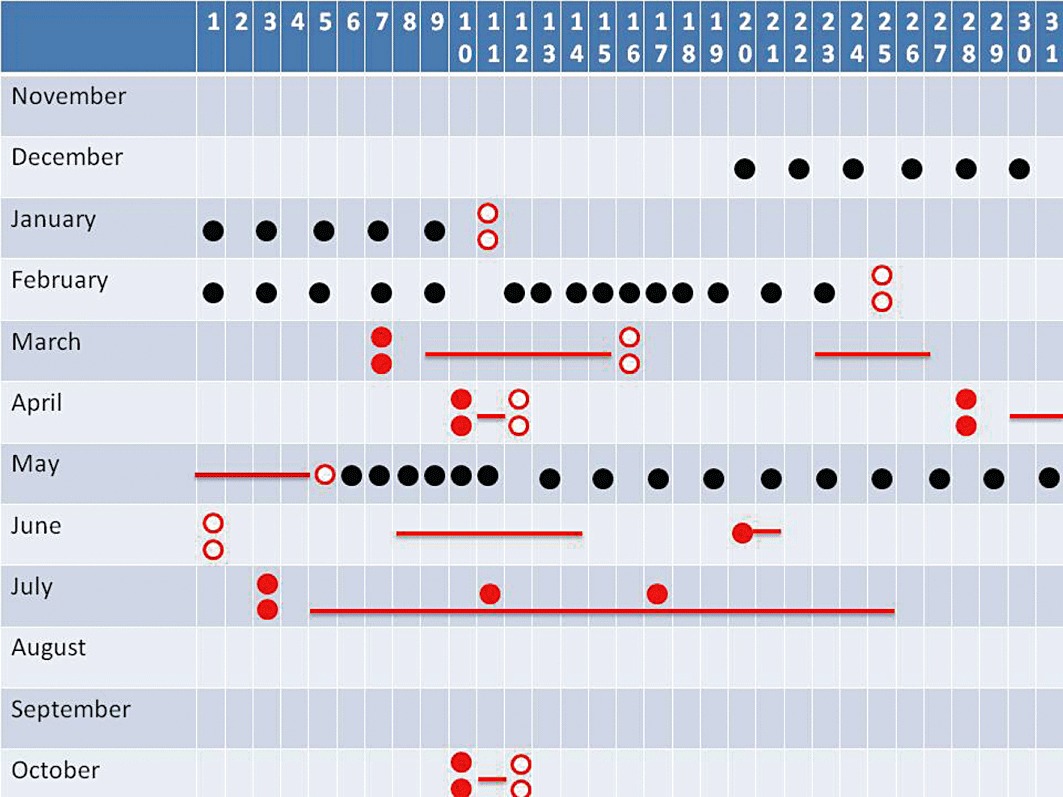
Based on testimonies from caught cyclists, confiscated diaries, etc., it is easy to unravel the blood doping strategy in present-day cycle sports. Here is the example of a cyclist aiming to perform well in Paris-Nice (seven stage race in March), a spring classic (one day race) in late April, and then in the Tour de France (three week stage race) in July, and also the world championships held in the autumn. Epo could then be abused in December, January and February in order to increase red cell mass. Once a sufficient red cell mass has been synthesized, one to three bags of blood are withdrawn and stored for later use. One to 2 days before Paris – Nice two blood bags will be infused and there will be likely no trace of Epo in blood or urine at this time point (Lundby et al., 2008a). Once the race is completed, the two blood bags will be withdrawn, stored and re-infused 1 to 2 days before the spring classic and again withdrawn and stored upon completion of the race (likely on the very same day). Epo injections are likely to occur in May where no major competitions are planned. Following 2–3 weeks of treatment, the gained blood is withdrawn and stored for later use. For the Tour de France in July, two blood bags are infused 1 to 2 days before the start, and then one blood bag is re-transfused on the two resting days. Since there is no direct doping test against autologous blood doping, this makes the doper very difficult to detect during competition, whereas a chance of getting caught exists during the Epo injection period by direct test, whereas the chance of getting caught by the blood passport seems minimal – again highlighting the importance of frequent out-of-competition testing. In this regard, it is surprising that a cyclist finishing within the very top in the 2010 world championships, and hence also a top contender for the 2011 championships, has not been tested since October 2010 (as of July 1st 2011, personal communication). Black circles indicate rhEPO injections, open red circles each indicate the withdrawal and storage of a one unit blood bag (i.e. two dots = two bags), and closed red circles each indicate the re-transfusion of one unit of blood. Red vertical lines indicate competitions. It should be kept in mind that this review only deals with blood doping and that substances such as growth hormone, IGF-1, anabolic steroids and doping ‘maskers’ are very likely also misused by this type of athlete.
In the absence of mountains and in an attempt to limit blood doping, some countries have built nitrogen houses where it is possible to expose athletes to hypoxia and hence stimulate altitude exposure. Such facilities are used with the intent to increase endogenous Epo production, and thereby stimulate the synthesis of red blood cell mass and thus exercise performance. The concept of Live High (i.e. living at altitude) – Train Low (LHTL) (i.e. training at sea level) was introduced by Levine and Stray-Gundersen in the early 1990s, and demonstrated feasible a few years later (Levine and Stray-Gundersen, 1997). It should be noted, however, that the performance gains following LHTL are limited to around 1% (Bonetti and Hopkins, 2009), which may of course be relevant for elite performance, but which is still miles away from the performance gains observed following, for example, rhEpo injections (Thomsen et al., 2007). Furthermore, the to date only placebo-controlled and double-blinded LHTL study using nitrogen houses demonstrated no performance gains following 4 weeks of LHTL (16 h per day at 3000 m) (Siebenmann et al., 2011). Thus, altitude training cannot be ‘offered’ as an alternative to blood doping to athletes. Of greater concern, however, is that altitude exposure seems misused by some athletes as a masking procedure since sports federations such as the International Cycling Union (ICU) exclude the analytical results of blood samples obtained for the Athlete Biological Passport (ABP) (see later) in conjugation to altitude exposure, and, hence, it cannot be ruled that some athletes may go to altitude with the specific aim to dope knowing that potential blood samples will not be used.
New substances mimicking the actions of Epo
A recent review (Jelkmann and Lundby, 2011) describes the potential for new substances developed to induce endogenous Epo synthesis and agents mimicking the actions of Epo. Very few of these, however, have been tested in humans, and, here, we have chosen only to include substances proven effective in humans.
The research group around Kai-Uwe Eckardt from Germany published a ground-breaking study. Using the prolyl hydroxylase inhibitor PHD-I FG-2216 provided by FibroGen in San Francisco, CA, USA, they proved that oral administration of this phase 1 drug increases endogenous Epo synthesis in healthy volunteers and haemodialysis (Bernhardt et al., 2010), and it is likely only a question of short time before other companies will launch similar products. Although presently unknown, it must be assumed that long-term administration of such drugs will also increase nHb and [Hb], and accordingly also exercise performance. At present, the physiological response to long-term suppression of hypoxia-inducible factor (HIF) breakdown in humans remains unknown, but it should be recognized that HIF controls the expression of a variety of prominent genes within angiogenesis and metabolism besides being the master switch for erythropoiesis. Provided that side effects are not occurring, the immense importance of this study for patients worldwide is obvious. From an anti-doping point of view, however, it is a setback. It will become virtually impossible to develop specific tests for every drug intended to block the breakdown of HIF or to stimulate the Epo receptor. Realizing that the ABP approach is unable to detect doping (Ashenden et al., 2011) with rhEpo injections (see later), it seems very unlikely that this would be the case for these new drugs.
Detection methods for blood transfusions
Although autologous blood transfusions (i.e. withdrawal and re-transfusions with one's own blood) are a prohibited method of the World Anti Doping Agency (WADA), there is no direct detection method for the procedure. Several non-direct methods have been proposed including the analysis of plastic residues (dioctyl phthalate) (Solymos et al., 2010) present in blood following storage in certain transfusion bags (but not all), gene expression changes (Pottgiesser et al., 2009), microRNAs and proteomics (ongoing projects) and the concept of the ABP first introduced some 10 years ago (Cazzola, 2000; Malcovati et al., 2003), perhaps with the addition of nHb to the ABP (Mørkeberg et al., 2011). Of these, only the ABP seems realistically applicable at the present time and will be discussed following the section on Detection of rhEpo injections since the ABP is also designed to detect rhEpo misuse.
Detection of rhEpo injections
The main difficulty in detecting doping with rhEpo is based on the fact that rhEpo is structurally very similar to endogenous Epo. However, endogenous Epo and rhEpo glycosylation can be distinguished by their charge (Wide and Bengtsson, 1990; Wide et al., 1995), isoelectric point, which is the method used in WADA-accredited laboratories (Lasne et al., 2002), molecular mass (Kohler et al., 2008; Reichel, 2011) or by interaction with specific lectins (Franco Fraguas et al., 2008; Lönnberg et al., 2012). The methods differ in how well they distinguish a certain type of glycosylation (the Epo isoform resolution), and how much Epo is required for analysis (the Epo detection sensitivity). For an in-depth description of the various test and procedures, the reader is encouraged to read a recent review (Reichel, 2011). Despite the efforts by WADA laboratories for a clean sport, rhEpo detection is very difficult to detect, especially when the injection frequency is kept low, albeit sufficient to increase exercise performance (Lundby et al., 2008a). This study also demonstrated inconsistencies in analytical results between two WADA-accredited laboratories. Hence, other methods are currently being investigated.
Ongoing work in regard to rhEpo detection
A proteomic approach seems feasible (Christensen et al., 2011; Fania et al., 2011) but still has a long way to go, as, for example, the influence of different training modalities, altitude exposure, etc. on the proteomic response needs to be clarified, as does the window of detection. Also, the analytical costs of this approach are not negligible. As for autologous blood doping, the CO re-breathing method was suggested to be included in anti-doping work to determine potential rhEpo-induced increases in haemoglobin mass, but was deemed non-suitable (Lundby and Robach, 2010) due to biological and analytical variations (Lundby and Robach, 2010; Eastwood et al., 2011) allowing room for changes in nHb which would still increase exercise performance tremendously, but also due to practical limitations of the method and concerns towards the investigated athletes.
One of the most promising approaches to detect rhEpo doping in current anti-doping work seems to be the ongoing work with membrane-assisted isoform immunoassays (MAIIA) developed by Maria Lonnberg and Jan Carlson from Uppsala in Sweden (Lönnberg et al., 2012). The method can be used to distinguish recombinant protein and peptide hormones from their endogenous counterparts by differences in protein carbohydrate structure while requiring only a small amount of each isoform. Besides possessing superior sensitivity, MAIIA is also faster and much cheaper to perform than the present isoelectric focusing (IEF) test used by WADA, and we expect this innovative technology to present a great alternative to the cumbersome IEF test.
Another potential addition to current anti-blood doping work could be hepcidin, a hormone produced in the liver which plays a key role in regulating iron balance. Recent data indicate urinary hepcidin suppression in healthy volunteers treated with low-dose rhEpo (Robach et al., 2009), and following a single rhEpo injection (5000 IU s.c.), it was observed that serum hepcidin levels were profoundly suppressed in six subjects starting 24 h after the injection, and remained suppressed for 2 weeks (Ashby et al., 2010). However, potential implementation of hepcidin assay for anti-doping purpose is limited by several problems: (i) This hormone is modulated by several other factors. On one hand, inflammation or iron loading increases hepcidin expression (Nicolas et al., 2002). These two conditions can occur in elite sports, which in turn may mask rhEpo-induced hepcidin suppression. On the other hand, hypoxia or iron deficiency is known to promote hepcidin suppression (Piperno et al., 2011), highlighting the risk of detecting false positive, particularly for iron-deficient athletes or athletes undergoing altitude training regimen. Evidence from animal studies suggest that massive erythropoietic stimulus would dominate over inflammation on hepcidin expression (Lasocki et al., 2008), but whether this holds true in humans treated with low dose rhEpo remains unknown; (ii) Hepcidin assay is currently performed through various techniques which often requires expensive equipment and dedicated skilled personnel (Kroot et al., 2009). Furthermore, a reference range for normal hepcidin values in the normal population is not available yet.
In summary, using hepcidin as a tool for anti-doping seems promising but still needs further work addressing: (i) the role of the main confounding factors on rhEpo-induced hepcidin suppression; and (ii) most importantly, the development of a rapid, low-cost and easy-to-use instrumental approach.
The ABP
The original idea for a biological passport for athletes was first published by Italian M. Cazzola (Cazzola, 2000), and much work in this regard was initially conducted especially by Australian researchers (Parisotto et al., 2000a,b; 2001). Although blood monitoring programs in single sports federations such as the ICU and the International Ski Federation are generally referred to as ‘Blood Passports’, these are not to be mistaken with WADA's more strictly regulated ‘Athlete Biological Passport’. Today's ABP statistical software system was largely developed by Pierre-Edouard Sottas (Sottas et al., 2011) and is based on personalized monitoring of biomarkers indicative of doping, and is hence not a direct test program for a given substance. The approach can be used for a variety of doping practices, but, here, we will focus on blood doping – the haematological module of the program. The overall idea is that if an athlete is doping by means of blood transfusions, rhEpo injections or any other method with the purpose to increase red blood cell mass, this will lead to changes in a variety of haematological parameters such as reticulocyte count and [Hb]. Besides, the analysed haematological values, also heterogeneous factors, such as age, sex and genotype, are included in the ABP as are information on altitude exposure and sample handling. The ABP is a multistep system where the first part is performed using a software system. The next step is completed by experts who evaluate the blood profiles flagged as abnormal by the ABP software, and only if these experts come to the conclusion that it is very likely that blood manipulations have occurred while excluding all other potential causes a disciplinary procedure is opened. In an anti-doping setting and using the ABP approach, Morkeberg and co-workers (Mørkeberg et al., 2011) achieved rather disappointing sensitivity rates of less than 20% in a controlled autologous blood transfusion study, whereas Pottgiesser et al. (2011) reported abnormal samples in eight of 11 transfused subjects when the probability level was set to 99% and five abnormal samples if probability level was set to 99.9% using a blinded doping control investigator. For such a scenario to be effective in real life, the authors concluded that a true expert group is required with specific knowledge in regard to competitions, strategies, training settings, etc. within the specific discipline. As such, the results are promising, but one concern in regard to the later study is that one subject triggered a false-positive result based on high initial [Hb]. Since all values flagged by the ABP software as being suspicious are submitted to the panel of experts, this would likely not have resulted in an anti-doping rule violation procedure, but emphasizes the importance of the quality of these experts. It would be preferable for them to have hands-on experience with blood manipulations gained in conjugation to their scientific or clinical work, and we encourage invited experts only to join doping-related panels if strictly within their field of expertise. The prosecuted athlete may of course question a given conclusion by enrolling his/her own expert panel.
Although the ABP sounds promising, limits and pitfalls have been put forward. Most recently, one of the main critics of the system, Guiseppe Banfi from Milano (Banfi, 2011), raised serious concerns in regard to, among others, the data source used to design the ABP, and furthermore stated that the variance used for cyclists is not correct and that the quality control of the used instruments is not completely assured. Based on the earlier discussion, the ABP seems the best currently available tool for detecting autologous blood manipulations, but it also needs to be acknowledged that the system in its present form is far from bulletproof, and that it is only an indirect evidence for blood manipulations, and, furthermore, that it is impossible to be 100% certain that all prosecuted athletes have in fact practiced doping. Therefore, we propose that ABP abnormalities can elicit a lighter ‘no start’ sanction as compared to the up to 2 years ban issued today (which for some athletes corresponds to ‘death sentence’). It should also be acknowledged that in a scenario as exemplified in Figure 2, where the transfusion and subsequent withdrawal could both be performed on the very same day as the competition (Spring Classic or World Championships), this would make it virtually impossible for the ABP to detect manipulations.
In addition to the ABP, Mørkeberg and co-workers investigated the possibility to include nHb to the algorithm, and demonstrated this to increase the sensitivity (Mørkeberg et al., 2011). Since, however, it cannot be ruled out that the amount of carbon monoxide (CO) given to the athlete in order to determine nHb will negatively affect exercise performance, or even potentially be of harm to the athlete's health, it seems very unlikely that the CO re-breathing method will be introduced into anti-doping work, and we certainly do not support such an implementation.
The ABP was designed also to detect rhEpo misuse. Since the reticulocyte count is however decreased after an initial increment to base values with continuous rhEpo stimulation (Lundby et al., 2007), the sensitivity of the ABP with long-term rhEpo should be questioned. Bornøet al. (2010) were the first to test the feasibility of a passport approach in order to detect rhEpo doping and reported a 58% detection rate in subjects injected with moderate rhEpo concentrations. Subsequently, Ashenden and co-workers (Ashenden et al., 2011) from Science and Industry Against Blood doping performed a study where ‘microdoses’ of rhEpo were injected in healthy volunteers for up to 12 weeks. The micro-dosing was sufficient to increase nHb by 113 g (approximately equivalent to the haemoglobin contained in two 450 mL bags of liquid or frozen stored blood) which will increase exercise performance tremendously (Thomsen et al., 2007; Lundby et al., 2008b). Disappointingly, the ABP software did not flag a single subject as being suspicious of doping, and the authors concluded that it is possible for athletes to misuse rhEpo without eliciting abnormal changes in the blood variables currently monitored by the ABP. A further concern raised by the study was that one sample before any rhEpo injections had occurred was designated ‘abnormal’. As mentioned above, in real life, this value would be evaluated by an expert panel before any prosecutions would be initiated, and we can only hope that the panel would make the right decision.
Htc and [Hb] in healthy humans
In order to discuss blood manipulations, one has to appreciate the reference values. At sea level, healthy males in the United States (n = 7426) have an Htc between 40 and 49% (5th and 95th percentile, respectively), whereas the normal limits for females (n = 7704) are 35 and 44%, with a slight tendency to increase following menopause. The 50th percentile for males and females in the ‘exercise competitive age’ is 44.5 and 39%, respectively. For young children, the normal limits are 33 and 40% (Fulwood et al., 1982). Based on the Second National Health and Nutrition Examination Survey (n = 2515), major [Hb] differences exist between black and white males (144.8 vs. 153.2 g·L−1), females (128.4 vs. 133.9 g·L−1) and children (120.3 vs. 126.8 g·L−1) (Perry et al., 1992). Smoking more than 10 cigarettes per day significantly increases [Hb], and when comparing never-smokers to smokers, an increase in [Hb] from 133 to 137 and from 152 to 156 g·L−1 is seen in females (n = 2454) and males (n = 2250), respectively (Nordenberg et al., 1990). Based on this and similar data, and in an attempt to limit doping practice, the upper Htc limit (cut-off value) allowed for males and females by most sports federations was set to 50 and 47%, respectively. The reference values used for these limits as noted earlier, however, are based on normal healthy individuals. With exercise training, haematological adaptations occur, leading to an initial expansion of plasma volume. However, after approximately 30 days of endurance training, the increase in red cell and plasma volume are approximately equal, and hence also [Hb] normalizes (Sawka et al., 2000). The haematological profile of an elite endurance athlete is less well described. To compare the distribution of blood haemoglobin levels in healthy blood donors and elite athletes specifically for anti-doping purposes, a retrospective (2001–2005) cohort study was performed in 85 846 Danish (sea level) blood donors (males = 36 962; females = 48 884, age 18–65 years) and compared to 1406 national team rowers (males = 1116; females = 290, age 16–32 years). In this data set, 3.9% of the male blood donors had a blood [Hb] of above 10.5 mM (corresponding to a Htc of 51%, i.e., above the cut-off value), and 1.6% of the females had a [Hb] above 9.7 mM (equivalent to a Htc of 47%, the female cut-off value). Surprisingly, this data set also demonstrated that the % distribution for the cut-off values are higher (P < 0.0001) in elite athletes, i.e., 10.4 and 8.3% for the male and females, respectively, and thereby demonstrating that high [Hb] levels in blood are seen regularly in normal people and especially in competitive athletes (Johansson et al., 2009). It needs to be stated, however, that it not can be ruled out that at least some of the athletes included in this study may have made use of doping practice, and that this could have affected the values. In another study, moderately trained subjects (n = 44) were compared to trained runners (n = 19), highly trained cyclists (n = 17) and world class cross-country skiers (n = 21), and in that study, only two of the moderately trained subjects surpassed the upper limit for [Hb], whereas none of the better trained subjects surpassed the upper accepted level. Data from this study also suggest that Htc in Olympic calibre athletes and non-athletes are rather similar, but that the nHb and plasma volumes are increased in highly trained athletes (Jelkmann and Lundby, 2011). Nonetheless, it needs to be acknowledged that some athletes may have blood values above the cut-off values, and this gives national sports federations the very important task to pinpoint such athletes from an early time point in their careers, and then to ensure the needed documentation in order to avoid a ‘no start’ penalty or potential mis-accreditaion of the athlete by sports colleagues or the press.
It is also important to realize that blood values undergo biological variation. Based on results from 12 studies of 638 normal healthy adults, the coefficient of within-subject biological variation of Htc is 3%. The normal within-subject biological variation (3%) and analytical variation (3%) may explain a relative change of approximately 12%, e.g., a change from 42 to 47% between two successive Htc values, measured with a time interval between 1 day and 1–2 months (Thirup, 2003). Partly due to haemodilution in warm weather, Htc often has a seasonal variation in normal healthy adults, and, based on results from 18 studies of 24 793 participants, the population mean is approximately 3% lower in summer than in winter. Population mean values that are 7% lower in summer than in winter have been found in some studies, although no seasonal effect may also be seen, especially in temperate climates (Thirup, 2003). Besides ambient temperature, also altitude exposure influences blood values. As compared to sea level, no changes in Htc or red cell mass (nHb) are usually observed with exposure to 1.600 m altitude (Weil et al., 1968), but starting from around 2350 m altitude (Schuler et al., 2007), an increase is noted which becomes gradually more increased with further altitude gain (Weil et al., 1968; Calbet et al., 2003; Lundby et al., 2006). Other external factors affecting plasma volume, and hence Htc and [Hb], include posture, hydration status and acute exercise (Harrison, 1985). In contrast to this, Schumacher and colleagues (Schumacher et al., 2010) concluded from a specifically anti-doping designed study performed on endurance-trained subjects (mostly cyclists) and appropriate controls that fluid intake and ambient temperature over the course of a ‘typical training day’ had no significant effect on [Hb]. The conducted training resulted in an average [Hb] increase of 0.46 g·dL−1 which however disappeared 2 h into recovery, and, thus, minor changes in [Hb] can be expected following training/racing, but unless a given athlete is extremely dehydrated (and hence not able to train/race in a meaningful manner) (Harrison, 1985), this likely resembles no limitation to anti-doping work. An example of a similar study approach in national team cross-country skiers is shown in Figure 2B where [Hb] is seen not to be significantly influenced by various interventions associated to normal life of such athletes. In more extreme exercise events (Pugh, 1969), however, a decrease in Htc may be observed secondary to an increase in plasma volume while red cell mass remains stable.
Is it worth it/future direction?
Citius, altius, fortius– the Olympic motto – is supposed to be reached solely by natural talent, proper training and diet. How true is that today? Probably not true at all. Today, it may be impossible to reach the top in a number of sports without using one or more suitable doping listed drug(s). In a recent Nature article, Don Catlin is cited to claim, ‘Everyone in cycling was doping’ (Callaway, 2011). Although Catlin may have gone too far, reasonable questions to be asked by the public are: ‘How can it be so bad with all the anti-doping activities and controls taking place?’, and if the situation is as bad as stated by Don Catlin, ‘is it really worth all the efforts and expenses?’
The answer to the first question has in part been given earlier. Valid and sensitive tests are lacking. The timing of when the tests are performed is not appropriate. In regard to blood manipulations, all of the above seem true. For the most commonly abused drug, anabolic steroids, the tests are there, but the timing is critical. The window for positive detection is quite long (weeks–months), but if anabolic steroids are used, they are taken in periods of hard muscle strength training, many months away from competition.
Indeed, many athletes in strength-demanding sports may only have misused these steroids when preparing for a career in the junior/young senior ranks, years ahead of a career with many successes as a senior. Just as Catlin states that all cyclists were doped, one could anticipate that all athletes in ‘strength’ events have at some point in their carrier been on a drug, which, linked with efficient training, enlarged their muscle mass and strength.
Thus, a quite dark account is painted of the doping situation in elite sports. Why do international sports federations, the International Olympic Committee (IOC) and governments continue to spend millions and millions of US dollars on anti-doping articles and controls? Do they have a choice? Probably not! Governments, regions and communities in most countries of the world support sports with tax money. There are many good reasons for this support. However, one may wonder whether, in a political perspective, the public accepts the support of leisure time activities and environments, which are not ‘drug free’, pretended or in reality.
In WADA's fight against doping, there are three criteria for a substance to be on the doping list:
Enhances performance.
Have harmful effects to the body.
Contradicts the spirit of sports.
The first and second reasons are quite logical, but the third is more difficult to define; however, it illustrates the wish to highlight sports as healthy, ‘clean’ and a good environment for youth development. International sports leaders are most likely well aware of the fact that another statement by Catlin also is correct: ‘No matter how sophisticated (test and controls) for every move to the right the other guys are moving to the left and it balances out again’. Nevertheless, they have to set money aside for anti-doping work. In other words, regardless of the effort and money spent, doping in elite sports (and among many leisure sports-active individuals) is still present and quite common.
It is understandable that IOC and WADA market another picture. Ahead of the Beijing Olympics, the message was that the competitions were the cleanest ever, and, indeed, the numbers of positive tests were few. However, that is, of course, no measure of the real misuse that has taken place for years and months during preparations for the games.
In the Nature article (Callaway, 2011), some WADA laboratory-related persons bring forward the hopes they and WADA have in regard to the ABP. The idea of accepting indirect evidence of doping has been discussed in various circles including WADA for more than a decade and was first formally discussed at a WADA meeting ahead of the Sydney Olympics.
Since then, WADA has promised to develop this approach, but it was not formally decided until late in 2009. The experience so far is primarily related to the control of blood manipulation. Still, the difficulties are more pronounced than the success of its implementation. In sports as cross-country skiing and speed skating, with only a limited number of top class athletes, the passport could in theory be quite effective, both in preventing doping procedures and also in order to detect misuse. The reason being that the athletes can be well characterized when entering the system and also followed not only during the competitive season but also in between. However, in sports like athletics or soccer, it is difficult to implement the ABP, whatever efforts are made. The critical issue is to establish the individual's ‘normal’ blood values as well as what is the normal or acceptable variation around the normal value. Another prerequisite for the blood pass to function is regular testing, all the year round. This cannot be handled by WADA and an international sports federation. Well-developed National Anti-Doping Agencies (NADAs) with extensive resources are needed, and WADA and not the least IOC should focus more on aiding the NADAs to become well-functioning. IOC also has the power to block for countries to be part of the international sporting community, if a NADA in a given country does not meet the standards. As long as this is not the case, our hopes are low for the ABP to help to limit doping in sports.
Glossary
- ABP
Athlete Biological Passport
- Epo
erythropoietin
- Htc
haematocrit
- LHTL
Live High – Train Low
- NADA
National Anti-Doping Agency
- nHb
total haemoglobin mass
- rhEpo
recombinant human erythropoietin
- VO2max
maximal oxygen uptake
- WADA
World Anti-Doping Agency
Conflicts of interest
None.
References
- Ashby DR, Gale DP, Busbridge M, Murphy KG, Duncan ND, Cairns TD, et al. Erythropoietin administration in humans causes a marked and prolonged reduction in circulating hepcidin. Haematologica. 2010;95:505–508. doi: 10.3324/haematol.2009.013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashenden M, Gough C, Garnham A, Gore C, Sharpe K. Current markers of the Athlete Blood Passport do not flag microdose EPO doping. Eur J Appl Physiol. 2011;111:2307–2314. doi: 10.1007/s00421-011-1867-6. [DOI] [PubMed] [Google Scholar]
- Banfi G. Limits and pitfalls of Athlete's Biological Passport. Clin Chem Lab Med. 2011;49:1417–1421. doi: 10.1515/CCLM.2011.633. [DOI] [PubMed] [Google Scholar]
- Bernhardt WM, Wiesener MS, Scigalla P, Chou J, Schmieder RE, Günzler V, et al. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J Am Soc Nephrol. 2010;21:2151–2156. doi: 10.1681/ASN.2010010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetti DL, Hopkins WG. Sea-level exercise performance following adaptation to hypoxia: a meta-analysis. Sports Med. 2009;39:107–127. doi: 10.2165/00007256-200939020-00002. [DOI] [PubMed] [Google Scholar]
- Bornø A, Aachmann-Andersen N, Munch-Andersen T, Hulston C, Lundby C. Screening for recombinant human erythropoietin using [Hb], reticulocytes, the OFF(hr score), OFF(z score) and Hb(z score): status of the Blood Passport. Eur J Appl Physiol. 2010;109:537–543. doi: 10.1007/s00421-010-1370-5. [DOI] [PubMed] [Google Scholar]
- Calbet JAL, Boushel R, Rådegran G, Søndergaard H, Wagner PD, Saltin B. Why is Vo2 max after altitude acclimatization still reduced despite normalization of arterial O2 content? Am J Physiol Regul Integr Comp Physiol. 2003;284:R304–R316. doi: 10.1152/ajpregu.00156.2002. [DOI] [PubMed] [Google Scholar]
- Calbet JAL, Lundby C, Koskolou M, Boushel R. Importance of hemoglobin concentration to exercise: acute manipulations. Respir Physiol Neurobiol. 2006;151:132–140. doi: 10.1016/j.resp.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Callaway E. Sports doping: racing just to keep up. Nature. 2011;475:283–285. doi: 10.1038/475283a. [DOI] [PubMed] [Google Scholar]
- Cazzola M. A global strategy for prevention and detection of blood doping with erythropoietin and related drugs. Haematologica. 2000;85:561–563. [PubMed] [Google Scholar]
- Christensen B, Sackmann-Sala L, Cruz-Topete D, Jørgensen JOL, Jessen N, Lundby C, et al. Novel serum biomarkers for erythropoietin use in humans: a proteomic approach. J Appl Physiol. 2011;110:149–156. doi: 10.1152/japplphysiol.00665.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood A, Sharpe K, Bourdon PC, Woolford SM, Saunders PU, Robertson EY, et al. Within subject variation in hemoglobin mass in elite athletes. Med Sci Sports Exerc. 2011 doi: 10.1249/MSS.0b013e318238ea7f. Sep 24. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Ekblom B, Goldbarg AN, Gullbring B. Response to exercise after blood loss and reinfusion. J Appl Physiol. 1972;33:175–180. doi: 10.1152/jappl.1972.33.2.175. [DOI] [PubMed] [Google Scholar]
- Ekblom B, Wilson G, Astrand PO. Central circulation during exercise after venesection and reinfusion of red blood cells. J Appl Physiol. 1976;40:379–383. doi: 10.1152/jappl.1976.40.3.379. [DOI] [PubMed] [Google Scholar]
- Elliott S. Erythropoiesis-stimulating agents and other methods to enhance oxygen transport. Br J Pharmacol. 2008;154:529–541. doi: 10.1038/bjp.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fania C, Vasso M, Torretta E, Robach P, Cairo G, Lundby C, et al. Setup for human sera MALDI profiling: the case of rhEPO treatment. Electrophoresis. 2011;32:1715–1727. doi: 10.1002/elps.201100134. [DOI] [PubMed] [Google Scholar]
- Franco Fraguas L, Carlsson J, Lönnberg M. Lectin affinity chromatography as a tool to differentiate endogenous and recombinant erythropoietins. J Chromatogr A. 2008;1212:82–88. doi: 10.1016/j.chroma.2008.10.036. [DOI] [PubMed] [Google Scholar]
- Fulwood R, Johnson CL, Bryner JD, Gunther EW, McGrath CR. Hematological and Nutritional Biochemistry References Data for Persons 6 Months-74 Years of Age: United States, 1976–1982. Bethesta: National Center for Health Statistics; 1982. [PubMed] [Google Scholar]
- Harrison MH. Effects on thermal stress and exercise on blood volume in humans. Physiol Rev. 1985;65:149–209. doi: 10.1152/physrev.1985.65.1.149. [DOI] [PubMed] [Google Scholar]
- Jacobs RA, Rasmussen P, Siebenmann C, Díaz V, Gassmann M, Pesta D, et al. Determinants of time trial performance and maximal incremental exercise in highly trained endurance athletes. J Appl Physiol. 2011;111:1422–1430. doi: 10.1152/japplphysiol.00625.2011. [DOI] [PubMed] [Google Scholar]
- Jelkmann W, Lundby C. Blood doping and its detection. Blood. 2011;118:2395–2404. doi: 10.1182/blood-2011-02-303271. [DOI] [PubMed] [Google Scholar]
- Johansson PI, Ullum H, Jensen K, Secher NH. A retrospective cohort study of blood hemoglobin levels in blood donors and competitive rowers. Scand J Med Sci Sports. 2009;19:92–95. doi: 10.1111/j.1600-0838.2008.00771.x. [DOI] [PubMed] [Google Scholar]
- Kohler M, Ayotte C, Desharnais P, Flenker U, Lüdke S, Thevis M, et al. Discrimination of recombinant and endogenous urinary erythropoietin by calculating relative mobility values from SDS gels. Int J Sports Med. 2008;29:1–6. doi: 10.1055/s-2007-989369. [DOI] [PubMed] [Google Scholar]
- Kroot JJ, Kemna EH, Bansal SS, Busbridge M, Campostrini N, Girelli D, et al. Results of the first international round robin for the quantification of urinary and plasma hepcidin assays: need for standardization. Haematologica. 2009;94:1748–1752. doi: 10.3324/haematol.2009.010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasne F, Martin L, Crepin N, de Ceaurriz J. Detection of isoelectric profiles of erythropoietin in urine: differentiation of natural and administered recombinant hormones. Anal Biochem. 2002;311:119–126. doi: 10.1016/s0003-2697(02)00407-4. [DOI] [PubMed] [Google Scholar]
- Lasocki S, Millot S, Andrieu V, Letteron P, Pilard N, Muzeau F, et al. Phlebotomies or erythropoietin injections allow mobilization of iron stores in a mouse model mimicking intensive care anemia. Crit Care Med. 2008;36:2388–2394. doi: 10.1097/CCM.0b013e31818103b9. [DOI] [PubMed] [Google Scholar]
- Levine BD, Stray-Gundersen J. ‘Living high-training low’: effect of moderate-altitude acclimatization with low-altitude training on performance. J Appl Physiol. 1997;83:102–112. doi: 10.1152/jappl.1997.83.1.102. [DOI] [PubMed] [Google Scholar]
- Lönnberg M, Andrén M, Birgegård G, Drevin M, Garle M, Carlsson J. Rapid detection of erythropoiesis-stimulating agents in urine and serum. Anal Biochem. 2012;420:101–114. doi: 10.1016/j.ab.2011.09.021. [DOI] [PubMed] [Google Scholar]
- Lundby C, Olsen NV. Effects of recombinant human erythropoietin in normal humans. J Physiol. 2011;589:1265–1271. doi: 10.1113/jphysiol.2010.195917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby C, Robach P. Assessment of total haemoglobin mass: can it detect erythropoietin-induced blood manipulations? Eur J Appl Physiol. 2010;108:197–200. doi: 10.1007/s00421-009-1259-3. [DOI] [PubMed] [Google Scholar]
- Lundby C, Sander M, van Hall G, Saltin B, Calbet JAL. Maximal exercise and muscle oxygen extraction in acclimatizing lowlanders and high altitude natives. J Physiol. 2006;573:535–547. doi: 10.1113/jphysiol.2006.106765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby C, Thomsen JJ, Boushel R, Koskolou M, Warberg J, Calbet JAL, et al. Erythropoietin treatment elevates haemoglobin concentration by increasing red cell volume and depressing plasma volume. J Physiol. 2007;578:309–314. doi: 10.1113/jphysiol.2006.122689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby C, Achman-Andersen NJ, Thomsen JJ, Norgaard AM, Robach P. Testing for recombinant human erythropoietin in urine: problems associated with current anti-doping testing. J Appl Physiol. 2008a;105:417–419. doi: 10.1152/japplphysiol.90529.2008. [DOI] [PubMed] [Google Scholar]
- Lundby C, Robach P, Boushel R, Thomsen JJ, Rasmussen P, Koskolou M, et al. Does recombinant human Epo increase exercise capacity by means other than augmenting oxygen transport? J Appl Physiol. 2008b;105:581–587. doi: 10.1152/japplphysiol.90484.2008. [DOI] [PubMed] [Google Scholar]
- Malcovati L, Pascutto C, Cazzola M. Hematologic passport for athletes competing in endurance sports: a feasibility study. Haematologica. 2003;88:570–581. [PubMed] [Google Scholar]
- Martino M, Gledhill N, Jamnik V. High VO2max with no history of training is primarily due to high blood volume. Med Sci Sports Exerc. 2002;34:966–971. doi: 10.1097/00005768-200206000-00010. [DOI] [PubMed] [Google Scholar]
- Mørkeberg J, Sharpe K, Belhage B, Damsgaard R, Schmidt W, Prommer N, et al. Detecting autologous blood transfusions: a comparison of three passport approaches and four blood markers. Scand J Med Sci Sports. 2011;21:235–243. doi: 10.1111/j.1600-0838.2009.01033.x. [DOI] [PubMed] [Google Scholar]
- Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordenberg D, Yip R, Binkin NJ. The effect of cigarette smoking on hemoglobin levels and anemia screening. JAMA. 1990;264:1556–1559. [PubMed] [Google Scholar]
- Pace N, Consolazio WV, Lozner FL. The effect of transfusions of red blood cells on the hypoxia tolerence of normal men. Science. 1945;102:589–591. doi: 10.1126/science.102.2658.589. [DOI] [PubMed] [Google Scholar]
- Parisotto R, Gore C, Emslie K, Ashenden M, Brugnara C, Howe C, et al. A novel method utilising markers of altered erythropoiesis for the detection of recombinant human erythropoietin abuse in athletes. Haematologica. 2000a;85:564–572. [PubMed] [Google Scholar]
- Parisotto R, Gore CJ, Hahn AG, Ashenden MJ, Olds TS, Martin DT, et al. Reticulocyte parameters as potential discriminators of recombinant human erythropoietin abuse in elite athletes. Int J Sports Med. 2000b;21:471–479. doi: 10.1055/s-2000-7421. [DOI] [PubMed] [Google Scholar]
- Parisotto R, Wu M, Ashenden MJ, Emslie KR, Gore CJ, Howe C, et al. Detection of recombinant human erythropoietin abuse in athletes utilizing markers of altered erythropoiesis. Haematologica. 2001;86:128–137. [PubMed] [Google Scholar]
- Perry GS, Byers T, Yip R, Margen S. Iron nutrition does not account for the hemoglobin differences between Blacks and Whites. J Nutr. 1992;122:1417–1424. doi: 10.1093/jn/122.7.1417. [DOI] [PubMed] [Google Scholar]
- Piperno A, Galimberti S, Mariani R, Pelucchi S, Ravasi G, Lombardi C, et al. Modulation of hepcidin production during hypoxia-induced erythropoiesis in humans in vivo: data from the HIGHCARE project. Blood. 2011;117:2953–2959. doi: 10.1182/blood-2010-08-299859. [DOI] [PubMed] [Google Scholar]
- Pirofsky B. The determination of blood viscosity in man by a method based on Poiseuille's law. J Clin Invest. 1953;32:292–298. doi: 10.1172/JCI102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottgiesser T, Schumacher YO, Funke H, Rennert K, Baumstark MW, Neunuebel K, et al. Gene expression in the detection of autologous blood transfusion in sports – a pilot study. Vox Sang. 2009;96:333–336. doi: 10.1111/j.1423-0410.2009.01169.x. [DOI] [PubMed] [Google Scholar]
- Pottgiesser T, Sottas P-E, Echteler T, Robinson N, Umhau M, Schumacher YO. Detection of autologous blood doping with adaptively evaluated biomarkers of doping: a longitudinal blinded study. Transfusion. 2011;51:1707–1715. doi: 10.1111/j.1537-2995.2011.03076.x. [DOI] [PubMed] [Google Scholar]
- Pugh LGCE. Blood volume changes in outdoor exercise of 8–10 hour duration. J Physiol. 1969;200:345–351. doi: 10.1113/jphysiol.1969.sp008697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen P, Foged EM, Krogh-Madsen R, Nielsen J, Nielsen TR, Olsen NV, et al. Effects of erythropoietin administration on cerebral metabolism and exercise capacity in men. J Appl Physiol. 2010;109:476–483. doi: 10.1152/japplphysiol.00234.2010. [DOI] [PubMed] [Google Scholar]
- Reichel C. Recent developments in doping testing for erythropoietin. Anal Bioanal Chem. 2011;401:463–481. doi: 10.1007/s00216-011-5116-y. [DOI] [PubMed] [Google Scholar]
- Robach P, Calbet JAL, Thomsen JJ, Boushel R, Mollard P, Rasmussen P, et al. The ergogenic effect of recombinant human erythropoietin on VO2max depends on the severity of arterial hypoxemia. PLoS ONE. 2008;3:e2996. doi: 10.1371/journal.pone.0002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robach P, Recalcati S, Girelli D, Gelfi C, Aachmann-Andersen NJ, Thomsen JJ, et al. Alterations of systemic and muscle iron metabolism in human subjects treated with low-dose recombinant erythropoietin. Blood. 2009;113:6707–6715. doi: 10.1182/blood-2008-09-178095. [DOI] [PubMed] [Google Scholar]
- Sawka MN, Convertino VA, Eichner EA, Schnieder SM, Young AJ. Blood volume: importance and adaptations to exercise training, environmental stresses, and trauma/sickness. Med Sci Sports Exerc. 2000;32:332–348. doi: 10.1097/00005768-200002000-00012. [DOI] [PubMed] [Google Scholar]
- Schuler B, Thomsen JJ, Gassmann M, Lundby C. Timing the arrival at 2340 m altitude for aerobic performance. Scand J Med Sci Sports. 2007;17:588–594. doi: 10.1111/j.1600-0838.2006.00611.x. [DOI] [PubMed] [Google Scholar]
- Schumacher YO, Wenning M, Robinson N, Sottas PE, Ruecker G, Pottgiesser T. Diurnal and exercise-related variability of haemoglobin and reticulocytes in athletes. Int J Sports Med. 2010;31:225–230. doi: 10.1055/s-0029-1243617. [DOI] [PubMed] [Google Scholar]
- Siebenmann C, Robach P, Jacobs RA, Rasmussen P, Nordsborg NB, Díaz V, et al. ‘Live high – train low’ using normobaric hypoxia: a double-blinded, placebo-controlled study. J Appl Physiol. 2011;112:106–117. doi: 10.1152/japplphysiol.00388.2011. [DOI] [PubMed] [Google Scholar]
- Solymos E, Guddat S, Geyer H, Flenker U, Thomas A, Segura J, et al. Rapid determination of urinary di(2-ethylhexyl) phthalate metabolites based on liquid chromatography/tandem mass spectrometry as a marker for blood transfusion in sports drug testing. Anal Bioanal Chem. 2010;401:517–528. doi: 10.1007/s00216-010-4589-4. [DOI] [PubMed] [Google Scholar]
- Sottas P-E, Robinson N, Rabin O, Saugy M. The Athlete Biological Passport. Clin Chem. 2011;57:969–976. doi: 10.1373/clinchem.2011.162271. [DOI] [PubMed] [Google Scholar]
- Spedding M, Spedding C. Drugs in sport: a scientist-athlete's perspective: from ambition to neurochemistry. Br J Pharmacol. 2008;154:496–501. doi: 10.1038/bjp.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirup P. Haematocrit: within-subject and seasonal variation. Sports Med. 2003;33:231–243. doi: 10.2165/00007256-200333030-00005. [DOI] [PubMed] [Google Scholar]
- Thomsen J, Rentsch R, Robach P, Calbet J, Boushel R, Rasmussen P, et al. Prolonged administration of recombinant human erythropoietin increases submaximal performance more than maximal aerobic capacity. Eur J Appl Physiol. 2007;101:481–486. doi: 10.1007/s00421-007-0522-8. [DOI] [PubMed] [Google Scholar]
- Waddington I, Smith A. An Introduction to Drugs in Sport: Addicted to Winning. Abingdon, Oxon: Routledge Chapman & Hall; 2008. [Google Scholar]
- Weil JV, Jamieson G, Brown DW, Grover RF. The red cell mass-arterial oxygen relationship in normal man: application to patients with chronic obstructive airway disease. J Clin Invest. 1968;47:1627–1639. doi: 10.1172/JCI105854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wide L, Bengtsson C. Molecular charge heterogeneity of human serum erythropoietin. Br J Haematol. 1990;76:121–127. doi: 10.1111/j.1365-2141.1990.tb07846.x. [DOI] [PubMed] [Google Scholar]
- Wide L, Bengtsson C, Berglund B, Ekblom B. Detection in blood and urine of recombinant erythropoietin administered to healthy men. Med Sci Sports Exerc. 1995;27:1569–1576. [PubMed] [Google Scholar]


