Abstract
Several studies implicate Toll-like receptors (TLRs) in alcohol-induced neuroinflammatory processes. The work reported by Wu et al., in this issue of the British Journal of Pharmacology, indicates that TLR4 along with its intracellular adaptor protein, MyD88, may play crucial roles in the acute actions of alcohol. The deletions of TLR4 or MyD88 gene or pharmacological inhibition of TLR4 by (+)-naloxone were able to attenuate alcohol-induced sedation, motor impairment and acute alcohol-induced increases in IkBα protein levels in the hippocampus of mice. These results clearly suggest that TLR4-MyD88 signalling may play a causal role in the mediation of the behavioural effects of acute alcohol.
LINKED ARTICLE
This article is a commentary on Wu et al., pp. 1319–1329 of this issue. To view this paper visit http://dx.doi.org/10.1111/j.1476-5381.2011.01572.x
Keywords: alcohol, TLR4, MyD88, NF-κB, sedation, motor impairment, hippocampus, neuroinflammation
Understanding the mechanisms underlying the acute actions of alcohol is crucial in order to identify, in more detail, the behavioural processes of alcohol tolerance and dependence and thus to advance the treatment of alcohol abuse disorders (Koob, 2003; Pandey et al., 2008; Crews et al., 2011). Alcohol addictive processes develop from a series of neuroadaptive changes leading to tolerance, withdrawal and relapse (Koob, 2003). Studies indicate that GABA, NMDA and other neurotransmitter systems may be involved in the acute and chronic effects of ethanol (Koob, 2003). Recently, a role for the innate immune system has been implicated in ethanol-induced neuroinflammatory processes leading to brain damage (Crews et al., 2011). Toll-like receptors (TLRs) have been shown to be involved in these neuroinflammatory responses to chronic and acute ethanol exposure (Alfonso-Loeches et al., 2010; Crews et al., 2011; Pascual et al., 2011). One of the TLRs, TLR4, has been shown to initiate the innate immune response by activating MyD88-dependent and -independent signalling pathways. MyD88 is an intracellular adaptor protein that interacts with TLR4 in response to LPS exposure and activates protein kinases and gene transcription factors, such as NF-κB, that can lead to increased expression of genes related to inflammation (Laird et al., 2009; Alfonso-Loeches et al., 2010; Crews et al., 2011).
Acute ethanol exposure produces a variety of behavioural modifications, such as anxiolytic effects, sedation and motor impairment (Pandey et al., 2008; Wu et al., 2011; 2012). The studies published in this issue of the British Journal of Pharmacology by Wu et al. (2012) on TLR4-MyD88 signalling in relation to the behavioural responses of ethanol, clearly establish causal roles for TLR4 and MyD88 genes in alcohol-induced sedation and motor impairment in mice. They found that acute ethanol produced a sedative response in a dose-dependent manner and motor impairment, as measured by the rotor rod procedure in wild-type (+/+) mice, which was attenuated in TLR4 and MyD88 deficient (−/−) mice. Interestingly, these behavioural responses in wild-type mice were significantly attenuated by treatment with a TLR4 antagonist (+)-naloxone (Figure 1). They did not observe any effects of acute ethanol on p38, JNK or Erk 1/2 phosphorylation. NF-κB is considered an important pro-inflammatory transcription factor and one of the genes that is activated by NF-κB codes for IκBα (Ferreiro and Komives, 2010). Wu et al. (2012) found that IκBα protein levels were increased due to acute ethanol exposure in the hippocampus of wild-type (+/+) mice but not in TLR4 and MyD88 deficient (−/−) mice. These results suggest that TLR4-MyD88 signalling may be important in the molecular mechanisms underlying the actions of alcohol.
Figure 1.
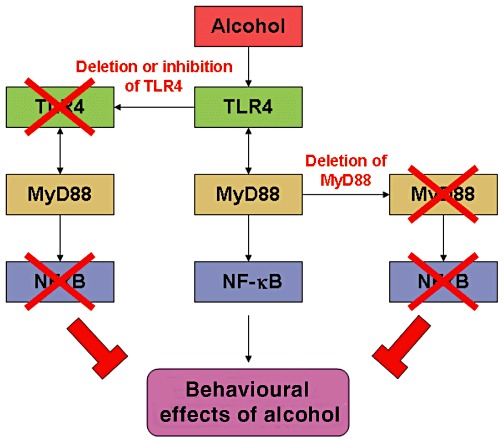
Molecular model depicting the actions of acute ethanol on TLR4-MyD88 signalling. As shown by Wu et al. (2012), activation of TLR4-MyD88 signalling by alcohol may mediate sedative and motor impairment effects, as they were attenuated by the deletion of TLR4 and MyD88 genes, or by the pharmacological blocking of TLR4 with (+)-naloxone.
Several studies have supported this suggestion. A deficiency of TLR4 has been shown to protect against alcohol-induced glial activation and prevent induction of inflammatory processes including activation of NF-κB pathways and apoptosis in mice (Alfonso-Loeches et al., 2010). Interestingly, the motor ataxic effects of ethanol were enhanced in rats after 24 h, but not after 2 h of LPS exposure (Drugan et al., 2007). Also, a single LPS injection increased innate immune gene expression in the brain (Crews et al., 2011) and alcohol consumption in mice (Blednov et al., 2011). Another interesting study pointed out the interactions between GABAA-α2 and TLR4 in relation to binge drinking in alcohol-preferring (P) rats. Knocking down the expression of GABAA-α2 by small interfering RNA (siRNA) in the central nucleus of amygdala (CeA) also decreased TLR4 expression and decreased binge drinking in P rats. Furthermore, siRNA knock down of TLR4 expression directly decreased binge drinking in P rats (Liu et al., 2011). Deletion of certain genes related to chemokine networks resulted in altered alcohol-related behaviours in mice (Blednov et al., 2005) and treatment with minocycline (blocker of pro-inflammatory microglial activation) and IL-1 receptor antagonists reduced the sedative effects of acute ethanol in mice (Wu et al., 2011). Studies conducted in mice and human post-mortem brains suggest that levels of a key innate immune chemokine, CCL2 (MCP-1), were increased in mice chronically treated with ethanol and also in several brain regions of human alcoholics (Crews et al., 2011). A dysregulation in the NF-κB system has also been observed in the prefrontal cortex of human alcoholics (Okvist et al., 2007). Taken together, these studies suggest that altered innate immune gene expression along with its interaction with TLR4-MyD88 signalling and NF-κB may play a neuroadaptive role in the development of alcoholism.
Anxiety that occurs during withdrawal from chronic ethanol exposure plays a fundamental role in relapse and may therefore maintain the continued abuse of alcohol (Koob, 2003). One recent study (Pascual et al., 2011) demonstrated a role for TLR4 in anxiety-like behaviours developed in wild-type mice during abstinence after chronic ethanol exposure, as they were abolished in TLR4 deficient (-/-) mice. Alcohol also activated TLR4 receptors in glial cells and caused the induction of pro-inflammatory cytokines in the brain (Alfonso-Loeches et al., 2010). In the absence of TLR4, these responses may not be initiated by alcohol; therefore, one can assume that anxiety-like behaviours were not seen in TLR4 deficient (-/-) mice, during withdrawal after chronic exposure.
Another interesting observation reported was that epigenetic modifications, such as changes in histone acetylation, were not observed in brain regions of TLR4 deficient (-/-) mice. Activity of histone acetyltransferases (HAT) and acetylation of histones H4 and H3 were decreased in several brain regions of wild-type mice during abstinence (15 days after a 5 month period of ethanol drinking). Reduced HAT activity and histone acetylation were not observed in the brains of TLR4 deficient (-/-) mice during abstinence after chronic ethanol exposure in mice (Pascual et al., 2011). We reported that decreased histone (H3 and H4) acetylation in the CeA and medial nucleus of amygdala (MeA) may be involved in the development of anxiety-like behaviours during withdrawal after chronic ethanol exposure in rats. Acute ethanol exposure increased histone acetylation in the CeA and MeA and produced anxiolytic effects in rats (Pandey et al., 2008). As mentioned previously, a deficiency in TLR4 in mice prevented changes in histone acetylation in the brain during the abstinence phase after chronic ethanol exposure (Pascual et al., 2011), suggesting the possibility that TLR4 may interact with the chromatin architecture and may also be involved in the regulation of innate immune gene expression and the neurobiology of alcohol addiction.
In summary, the work conducted by Wu et al. (2012) along with other studies (Crews et al., 2011; Pascual et al., 2011) of the interactions between TLR4 and ethanol, suggests that TLR4 is an important molecular target in the brain that may mediate the behavioural responses related to acute and chronic ethanol exposure. Alcohol-induced activation of TLR4-MyD88 signalling may lead to activation of the NF-κB gene transcription factor and, along with chromatin remodelling, may modify the expression of chemokines and cytokines, which in turn can regulate neuroinflammatory processes. Further work is needed to explore the links between TLR4 signalling and chromatin remodelling and interactions with the NF-κB gene transcription factor and innate immune gene expression in brain regions implicated in cognition, reward and anxiety during alcoholism.
Acknowledgments
The research work in the laboratory of Dr Pandey was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (AA-010005; AA-013341; AA-016690; AA-019971) and the Department of Veterans Affairs (Merit Review Grant; Research Career Scientist Award).
Glossary
- CeA
central nucleus of amygdale
- Erk1/2
extracellular signal regulated kinase 1/2
- IkBa
inhibitor of kB
- JNK
c-Jun N-terminal kinase
- LPS
Lipopolysaccharide
- MeA
medial nucleus of amygdale
- MyD88
myeloid differentiation primary response gene 88
- TLR4
Toll-like receptor 4
Conflict of interest
None.
References
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Bergeson SE, Walker D, Ferreira VM, Kuziel WA, Harris RA. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav Brain Res. 2005;165:110–125. doi: 10.1016/j.bbr.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun. 2011;25:S92–S105. doi: 10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L. Inductions of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun. 2011;25:S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugan RC, Wiedholz LM, Holt A, Kent S, Christianson JP. Environmental and immune stressors enhance alcohol-induced motor ataxia in rat. Pharmacol Biochem Behav. 2007;86:125–131. doi: 10.1016/j.pbb.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Ferreiro DU, Komives EA. Molecular mechanisms of system control of NF-κB signaling by IkBα. Biochemistry. 2010;49:1560–1567. doi: 10.1021/bi901948j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Laird MH, Rhee SH, Perkins DJ, Medvedev AE, Piao W, Fenton MJ, et al. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J Leukoc Biol. 2009;85:966–977. doi: 10.1189/jlb.1208763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL, Jr, et al. Binge alcohol drinking is associated with GABAAα2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proc Natl Acad Sci USA. 2011;108:4465–4470. doi: 10.1073/pnas.1019020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okvist A, Johansson S, Kuzmin A, Bazov I, Merino-Martinez R, Ponomarev I, et al. Neuroadaptations in human chronic alcoholics : dysregulation of the NF-κB system. PLoS One. 2007;2:e930. doi: 10.1371/journal.pone.0000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Baliño P, Alfonso-Loeches S, Aragón CM, Guerri C. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav Immun. 2011;25:S80–S91. doi: 10.1016/j.bbi.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Wu Y, Lousberg EL, Moldenhauer LM, Hayball JD, Robertson SA, Coller JK, et al. Attenuation of microglial and IL-1 signaling protects mice from acute alcohol-induced sedation and/or motor impairment. Brain Behav Immun. 2011;25:S155–S164. doi: 10.1016/j.bbi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Wu Y, Lousberg EL, Moldenhauer LM, Hayball JD, Coller JK, Rice KC, et al. Inhibiting the TLR4-MyD88 signaling cascade by genetic or pharmacologic strategies reduces acute alcohol dose-induced sedation and motor impairment in mice. Br J Pharmacol. 2012;165:1319–1329. doi: 10.1111/j.1476-5381.2011.01572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


