Abstract
BACKGROUND AND PURPOSE
Depression is the most common psychiatric disorder in Huntington's disease (HD) patients. Women are more prone to develop depression and such susceptibility might be related to 5-hydroxytryptaminergic (serotonergic) dysregulation.
EXPERIMENTAL APPROACH
We performed tests of depression-related behaviours on female R6/1 HD mice that had been chronically treated with sertraline or provided with running-wheels. Functional assessments of 5-HT1A and 5-HT2A receptors were performed by measuring behavioural and physiological responses following administration of specific agonists, in combination with analysis of hippocampal gene expression. Finally we assessed the effect of exercise on hippocampal cell proliferation.
KEY RESULTS
Female HD mice recorded increased immobility time in the forced-swimming test, reduced saccharin preference and a hyperthermic response to stress compared with wild-type animals. These alterations were improved by chronic sertraline treatment. Wheel-running also resulted in similar improvements with the exception of saccharin preference but failed to correct the hippocampal cell proliferation deficits displayed by HD mice. The benefits of sertraline treatment and exercise involved altered 5-HT1A autoreceptor function, as demonstrated by modulation of the exaggerated 8-OH-DPAT-induced hypothermia exhibited by female HD mice. On the other hand, sertraline treatment was unable to restore the reduced 5-HT1A and 5-HT2 heteroceptor function observed in HD animals.
CONCLUSIONS AND IMPLICATIONS
We report for the first time a crucial role for 5-HT1A autoreceptor function in mediating the sex-specific depressive-like phenotype of female R6/1 HD mice. Our data further support a differential effect of chronic sertraline treatment and exercise on hippocampal cell proliferation despite common behavioural benefits.
Keywords: depression, Huntington's disease, exercise, 5-HT, serotonin, behaviour, neurogenesis, sertraline
Introduction
Huntington's disease (HD) is an autosomal dominant neurodegenerative disorder caused by an abnormal expansion of CAG repeats in exon 1 of the huntingtin gene (The Huntington's Disease Collaborative Research Group, 1993). Clinical diagnosis of HD is determined on the basis of motor symptoms; however, the pre-motor stages of the disease are commonly associated with psychiatric manifestations including depression (Paulsen et al., 2005; Duff et al., 2007; Julien et al., 2007; Marshall et al., 2007).
Depression is one of the most prevalent causes of disability worldwide and is diagnosed in women more frequently than in men (Fava and Kendler, 2000; Kornstein et al., 2000; Marcus et al., 2008). Clinical studies indicate that mood disorders occur in close association with a dysregulation of 5-HT (serotonergic) neurotransmission involving the 5-HT transporter (5-HTT) (Grabe et al., 2005; Mizuno et al., 2006; Sjoberg et al., 2006; Brummett et al., 2008) and the 5-HT1A receptor (5-HT1AR) (Lemonde et al., 2003; Kishi et al., 2009). The 5-HT1AR is an inhibitory GPCR that is present in the dorsal raphe nucleus where it functions as an autoreceptor to control 5-hydroxytryptaminergic tone through negative feedback inhibition, and in broader brain regions that receive 5-hydroxytryptaminergic innervation including the cortex and the hippocampus where it functions as a heteroreceptor. Both 5-HT1A autoreceptors and heteroreceptors have been found to be differentially altered in depressed patients (Stockmeier et al., 1998; Sargent et al., 2000; Bhagwagar et al., 2004; Drevets et al., 2007; Miller et al., 2009; Sullivan et al., 2009) and rodent models of depression (van Gaalen et al., 2002; Overstreet, 2002; El Yacoubi et al., 2003; Renoir et al., 2008; Richardson-Jones et al., 2010).
The benefits of chronic treatment with selective 5-HT re-uptake inhibitors (SSRIs) are, in part, due to enhancement of 5-HT neurotransmission through the desensitization of the 5-HT1A autoreceptors (Dawson et al., 2002; Rossi et al., 2008), and are not observed in the absence of the 5-HT1AR (Santarelli et al., 2003). Chronic antidepressant treatment is also associated with an up-regulation of hippocampal neurogenesis, a process with a debatable role in the pathogenesis of depression (Petersen et al., 2008; Lucassen et al., 2010). Recent evidence suggests that the behavioural effects of SSRIs are mediated by neurogenesis-dependent and -independent mechanisms and this may vary depending on mouse strain and the precise behavioural paradigms employed (Santarelli et al., 2003; Holick et al., 2008; David et al., 2009).
Clinical evidence suggests that physical exercise has a positive relationship with the outcome of different mental diseases, such as depression, Alzheimer's disease and Parkinson's disease (Deslandes et al., 2009). In mice, running has antidepressant-like behavioural effects (Duman et al., 2008) with a recent study even suggesting that the neurogenic response to exercise might be greater than to antidepressant drugs (Marlatt et al., 2010). Further research is needed to evaluate the value of exercise in reducing depression in neurodegenerative disorders (Goodwin et al., 2008). There has yet to be a study of voluntary physical exercise as a means of alleviating depression in the context of HD, despite established benefits in slowing the progression of motor and cognitive deficits in mouse models of HD (Pang et al., 2006; van Dellen et al., 2008; but also see Potter et al., 2010).
The R6/1 and R6/2 transgenic mice were the first mouse models developed to study HD and expressed exon 1 of the human HD gene with around 115 and 150 CAG repeats, respectively (Mangiarini et al., 1996). The expression of various 5-HT receptors mRNA and protein levels are reduced in the R6 mouse models of HD (Yohrling et al., 2002; Pang et al., 2009). Using the forced-swimming test (FST) in which animals are individually placed into a beaker filled with water, we recently reported a female-specific depression-like behaviour in R6/1 HD mice before the onset of motor symptoms (Pang et al., 2009). Immobility in the FST is a commonly used measure of depression in rodents (Porsolt et al., 1977; Cryan et al., 2002).
HD animals have deficits in hippocampal neurogenesis, which are ameliorated by chronic treatment with SSRIs such as fluoxetine (Grote et al., 2005) and sertraline (Duan et al., 2008; Peng et al., 2008). However, chronic fluoxetine was ineffective in correcting the depression-like behaviour of HD animals (Pouladi et al., 2009). No study has assessed the effects of chronic sertraline in the context of depression in pre-symptomatic HD mice, despite it slowing down disease progression (Duan et al., 2008; Peng et al., 2008; Cheng et al., 2011) and having advantages over fluoxetine in terms of pharmacological selectivity (Bymaster et al., 2002), clinical efficacy and tolerance (Cipriani et al., 2009; 2010).
We assessed the depressive-like behaviours of female R6/1 HD following chronic sertraline treatment and voluntary wheel-running. Immobility in the FST (Porsolt et al., 1977; Cryan et al., 2002) and saccharin preference (Harkin et al., 2002) was used to assess despair- and anhedonic-like behaviours, respectively. As an index of stress reactivity, we measured the change of temperature induced by the tail-suspension test (TST) (Liu et al., 2003). We performed functional assessments of 5-HT2/5-HT1A auto- and/or heteroreceptor using selective 5-HT receptor agonists to uncover the physiological consequences of altered 5-HT receptor expression in the HD brain. Finally, we examined other physiological processes associated with depression pathology such as alterations of stress regulation and hippocampal cell proliferation.
Methods
Mice
R6/1 transgenic hemizygote males (Mangiarini et al., 1996) were originally obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and bred with CBB6 (CBA × N/C57/B6) F1 females to establish the R6/1 (HD) colony. Two distinct cohorts of mice were generated to complete the project. Cohort#1 was used to study the effect of chronic treatment with sertraline. Mice were chronically treated with either vehicle (1% Tween-20, 1 mL per 100 g body weight) or sertraline (10–20 mg·kg−1·day−1, i.p.) from 8 to 12 weeks of age. The last sertraline injection was given 24 h before the actual test. A second cohort (cohort#2) was used to study the effects of voluntary exercise. Animals housed in standard cages (15 × 30 × 12 cm) were compared with mice housed in large cages (25 × 37 × 16 cm) with elevated lids and provided with two running-wheels (RWs, 12 cm diameter) from 8 to 12 weeks of age. All animals used in this study were group-housed (two mice from each genotype per cage) and maintained on a 12 h light/dark cycle with access to food and water ad libitum. Therefore, we were not able to individually measure the total distance run during the 4 weeks with free access to RWs. For experimental purposes each cohort described above was subdivided into several groups designated as follows: group 1 (FST and then following a 2 day recovery period DPAT-induced hypothermia), group 2 (TST and then following a 2 day recovery period DPAT-induced hypothermia), group 3 [saccharin preference and then used for DOI-induced head-twitches or culled for 5-bromo-2′-deoxyuridine (BrdU)/GTP-γ-S labelling] and group 4 (corticosterone assay and gene expression).
All experiments were performed on female 12 week-old mice (unless specified) in accordance with the guidelines of the HFI Animal Ethics Committee and the National Health and Medical Research Council (NHMRC).
Drugs
[35S]-GTP-γ-S was purchased from GE Healthcare Europe (Orsay, France). (±)-8-OH-DPAT and (±)DOI were supplied by Sigma (Aldrich, NSW, Australia). Other compounds were sertraline (Pfizer Inc, CT, USA), WAY 100635 (Wyeth-Ayerst, Princeton, NJ, USA) and 5-carboxamidotryptamine (5-CT, Res Biochem Int, Natick, MA, USA).
Body temperature
Core temperature was measured at ambient temperature of 23 ± 1°C in gently restrained mice using a thermocouple probe (ID-Tech-Bioseb, France; 0.71 mm diameter).
Forced-swimming test
As previously described (Pang et al., 2009), mice were individually placed into a beaker (13 cm diameter) filled with 12 cm deep water (25–26°C) and video recorded for 300 s. Total immobility time of each mouse after the first 60 s was manually scored by an experienced experimenter blind to treatment and mouse genotype. A mouse was considered immobile when it stopped struggling and moved only to remain afloat or maintain balance. A total of 97 animals (43 from cohort#1 and 54 from cohort#2) were assessed on FST (n = 7–14 mice per group).
TST-induced hyperthermia
Mice were suspended by the tail for a 6 min session. TST-induced hyperthermia has been shown to be sensitive to antidepressants and may be an index of stress reactivity (Liu et al., 2003). The change of temperature induced by TST was expressed as the change between pre-TST (baseline, t = 0 min) and after-TST (t = 6 min) temperatures. A total of 105 animals (41 from cohort#1 and 64 from cohort#2) were assessed on TST (n = 10–17 mice per group).
Saccharin-preference test
The saccharin-preference test was performed according to a validated protocol (Harkin et al., 2002). On the 26th day of sertraline or exercise, animals were individually-housed (and the RWs removed) and allowed a 2 day habituation period before the actual test in which mice were exposed to both 0.1% saccharin and tap water solutions across 15 h overnight periods (18h00min–9h00min). Saccharin-preference scores were calculated as a percentage of total fluid intake. A total of 85 animals (40 controls, 26 sertraline and 19 RWs) were assessed on the saccharin-preference test.
8-OH-DPAT-induced hypothermia
As a pilot study, an additional group of mice (cohort#3: included 61 male and 63 female animals, n = 8–13 mice per group) were initially used to study the DPAT-induced hypothermia paradigm on naïve mice. Baseline temperatures were determined just before s.c. injection of the 5-HT1A receptor agonist 8-OH-DPAT (0.1 and 0.3 mg·kg−1) or vehicle (0.9% NaCl, 1 mL 100 g-1 body weight), and recorded every 10 min thereafter. The response to 8-OH-DPAT, which specifically involves 5-HT1A autoreceptors (Bill et al., 1991), was calculated as the decrease (from baseline) in body temperature over the 60 min period post injection. A total of 140 animals (83 from cohort#1 and 57 from cohort#2) were used to assess the effect of sertraline treatment and exercise on 8-OH-DPAT-induced hypothermia (n = 10–15 mice per group).
8-OH-DPAT and DOI-induced change of serum corticosterone levels
Mice naïve to behavioural testing were killed 30 min after acute administration of the 5-HT1A receptor agonist 8-OH-DPAT (0.3 mg·kg−1, s.c.), the 5-HT2 receptor agonist (±)DOI (1 mg·kg−1, i.p.) or vehicle (0.9% NaCl, 1 mL 100 g−1 body weight) between 8 h30min and 10h00 min. Blood was collected via cardiac puncture and serum corticosterone levels were determined by immunoassay according to manufacturer's instructions (Cayman Chemical Company, Ann Arbor, MI, USA). A total of 75 animals (33 from cohort#1 and 42 from cohort#2) were used for analysis of corticosterone levels.
DOI-induced head-twitches
Mice were administered vehicle (0.9% NaCl, 1 mL 100 g−1 body weight) or the 5-HT2A/C receptor agonist (±)DOI (1 mg·kg−1, i.p.). Fifteen minutes after drug administration, mice were observed over a 15 min period for the number of head-twitches. A total of 52 animals (12–15 mice per group) were used to assess behavioural effect of DOI.
Quantitative autoradiography of 5-HT1A-mediated [35S]-GTP-γ-S binding
Following the saccharin-preference test, mice were decapitated and their brains were frozen by immersion in isopentane chilled at −30°C with dry ice, then stored at −80°C. Coronal sections (20 µm thick) were cut at −20°C, and thaw-mounted onto gelatin-coated slides. Autoradiographic measurement of 5-HT1A receptor-stimulated [35S]-GTP-γ-S binding within the CA1 area of the hippocampus was performed according to Froger et al. (2004). This experimental approach has been previously used to measure the ability of the 5-HT1A receptor to activate G proteins (Froger et al., 2004; Hensler et al., 2010). Briefly, brain sections were pre-incubated then incubated with 0.05 nM [35S]-GTP-γ-S (1000 Ci·mmol−1) either in the absence (basal conditions) or the presence (stimulated conditions) of 5-CT, a non-selective 5-HT1 receptor agonist with nanomolar affinity for 5-HT1A receptors at 1 µM. Non-specific binding was determined in the presence of 10 µM WAY 100635 to block 5-HT1A receptors. OD was measured on Biomax MR autoradiographic films (Kodak, Rochester, NY, USA) using computerized image software (Samba). 5-CT-stimulated [35S]-GTP-γ-S binding is expressed as OD over the baseline (ODstimulated-ODbasal). A total of 24 animals (six mice per group) were used for [35S]-GTP-γ-S binding.
Real-time PCR for quantification of mRNA expression
As previously described (Pang et al., 2009), mice were killed for dissection of hippocampus. Total RNA was isolated using Qiagen RNeasy extraction kits (Qiagen, NSW, Australia) and stored at −80°C. RNA concentration and quality were determined using a Nanodrop spectrophotometer. cDNA was reverse transcribed from 1 mg of total RNA per sample using Applied Biosciences Reverse Transcription kits (PE Applied Biosystems, Foster City, CA, USA) with random hexamers. The reverse transcription PCR conditions were 25°C – 10 min, 48°C – 30 min and 95°C – 5 min. cDNA products were stored at −20°C. Quantitative real-time PCR was performed using SYBR Green PCR master mix (Sigma-Aldrich) on a PE ABI Prism 7500 Light Cycler system (PE Applied Biosystems). All primer pairs were optimized for working concentrations before use and primer sequences are as follows: 5-HT1AR F: CCC CAA CGA GTG CAC CAT, R: GCG CCG AAA GTG GAG TAG AT; 5-HTT F: CTT CAG CCC CGG ATG GTT, R: GTG GAC TCA TCA AAA AAC TGC AAA; 5-HT2AR F: CAC TGT GAA GCG AGG CAT AA, R: AAG CCG GAA GTT GTA GCA GA; glucocorticoid receptor (GR) F: AGG CCG CTC AGT GTT TTC TA, R: TAC AGC TTC CAC ACG TCA GC; cyclophilin F: CCC ACC GTG TTC TTC GAC A, R: CCA GTG CTC AGA GCT CGA AA. Cyclophilin was used as the endogenous housekeeping gene as it is not altered in the R6/1 mouse line at this age (M.S. Zajac, unpubl. data). Real-time PCR conditions were 50°C – 2 min, 95°C – 10 min, followed by 40 cycles of 95°C – 15 s and 60°C – 1 min. All reactions were performed on five to eight individual subjects per group, each in triplicate. Melt curve analyses were performed to ensure that only one reaction product was obtained. A total of 44 animals (15 from cohort#1 and 29 from cohort#2) were used for gene expression analysis.
Hippocampal cell proliferation
Identification and quantification of proliferating cells in the dentate gyrus was performed according to Grote et al. (2005) on 13 animals [five standard-housed (SH) and eight RWs]. BrdU (Sigma-Aldrich, NSW, Australia) was dissolved in 0.9% saline administered (50 mg·kg−1, i.p.) over 12 consecutive days starting 4 weeks before the brains were examined. Mice were deeply anaesthetized for transcardial perfusion with 0.1 M PBS followed by 4% paraformaldehyde. Brains were sectioned in the coronal plane on a cryostat at 20 µm intervals, thaw-mounted onto slides and stored at −80°C until further use. Sections were quenched in 1 M PBS for 10 min, treated with 2 M HCl at 37°C for 30 min, rinsed in PBS for 5 min, blocked with Cas-block (Invitrogen, USA) for 2 h at room temperature then incubated with sheep anti-BrdU antibody (1:500, Exalpha Biologicals Inc, MA, USA) in 50% Cas-block overnight at room temperature. Slides were rinsed in PBS, and then incubated with a biotinylated rabbit anti-sheep antibody (1:500, Vector Labs, CA, USA) in 50% Cas-block for 2–3 h at room temperature. Sections were rinsed in PBS and visualization of BrdU-positive cells was by reaction with Vectorstain Elite ABC Reagent (Vector Labs, CA, USA) followed by DAB peroxidase solution (DakoCytomation, Code K3466, CA, USA). Finally, sections were counterstained with cresyl violet and mounted in DPX. For each brain, six sections, each at 200 µm intervals (1: 10 series), commencing at the most rostral section, were selected. The dentate gyrus was imaged and manually highlighted according to a mouse brain atlas (Paxinos & Franklin, 2nd edition, 2001) at 10× magnification and the area was automatically calculated by the Zeiss Axiovision 4.5 software (Carl Zeiss Microimaging Inc, USA). Volumetric estimations were determined by summing the areas of the dentate gyrus and adjusting for sampling frequency and section thickness. Counts of BrdU-positive cells were performed at 40× magnification from the same sections used for volume estimation. The absolute number of cells immunoreactive for BrdU were counted on each section and totalled for all sections. All volumetric and cell count analyses were performed blind to genotype and experimental group.
Statistical analysis
Statistical analyses were performed using spss statistics 17.0 and GraphPad Prism 5.0. Two-way anovas were used to examine possible effect of genotype and/or treatments. To determine specific group differences, in the case of significant main effects (or interaction), the two-way anovas were followed by Fisher's least significant difference or Bonferroni post hoc tests. In all cases, the significance level was set at P < 0.05.
Results
Differential effects of chronic antidepressant treatments on depressive-related behaviours in female HD mice
Forced-swimming test. There was an overall genotype effect [F(1,37)= 27.7, P < 0.001] on FST performances of cohort#1 (Figure 1A). We also found an effect of sertraline treatment [F(1,37)= 3.7, P < 0.05] but no interaction [F(1,37)= 2.7, P = 0.08]. Post hoc tests revealed that HD mice (in both vehicle and sertraline10 groups) had greater immobility times compared with WT animals (P < 0.01). Immobility time of HD mice was lowered by chronic treatment with sertraline at the high dose of 20 mg·kg−1 (P < 0.05) but not following treatment with 10 mg·kg−1. In the wheel-running study (cohort#2), the two-way anova revealed a significant interaction between genotype and housing condition [F(1,50)= 18.2, P < 0.001] (Figure 1B). Post hoc tests revealed significant increase in immobility time of SH HD mice compared with WT animals (P < 0.001). However, this behavioural difference was no longer observed when the RW groups were compared. Compared with SH conditions, RW decreased immobility time in HD mice (P < 0.05) but increased this measure in WT animals (P < 0.01). We did not find any effect of genotype on spontaneous locomotor activity (data not shown).
Figure 1.
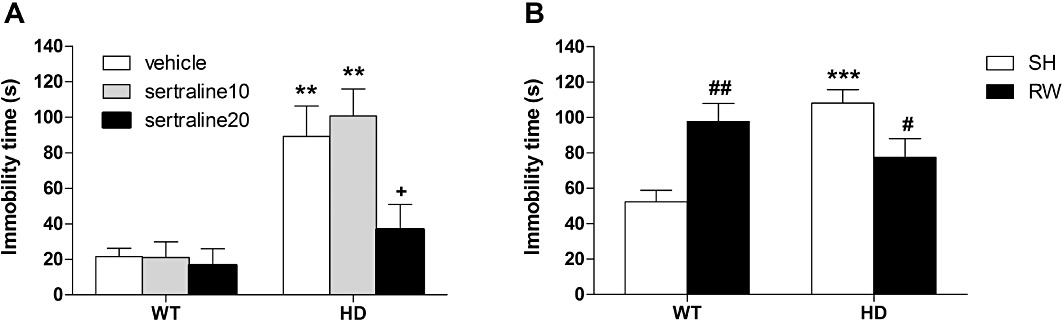
Effect of chronic treatment with sertraline or exercise on the FST. Using the FST we found that control female HD mice exhibited augmented immobility time when compared with WT animals. This despair-like behaviour displayed by HD mice was prevented by (A) chronic treatment with sertraline at the high dose of 20 mg·kg−1 and (B) RW. Values represent means (±SEM) of n = 7–14 mice per group. WT versus HD: **P < 0.01, ***P < 0.001; vehicle versus sertraline 20: +P < 0.05; SH versus RW: #P < 0.05, ##P < 0.01.
TST-induced hyperthermia. In both cohort#1 (Figure 2A) and cohort#2 (Figure 2B), we found that HD mice were significantly more sensitive to TST-induced hyperthermia [F(1,37)= 32.1, P < 0.001; F(1,60)= 12.6, P < 0.001 respectively]. Post hoc analysis revealed a significant increase in stress response of control HD mice compared with WT animals for both studies (P < 0.001). There was a significant effect of sertraline [F(1,37)= 4.6, P < 0.05] in cohort#1 and a significant interaction between genotype and housing condition [F(1,60)= 5.7, P < 0.05] in cohort#2. Indeed, the impaired stress response exhibited by HD animal was no longer observed in the mice exposed to RWs (Figure 2B).
Figure 2.
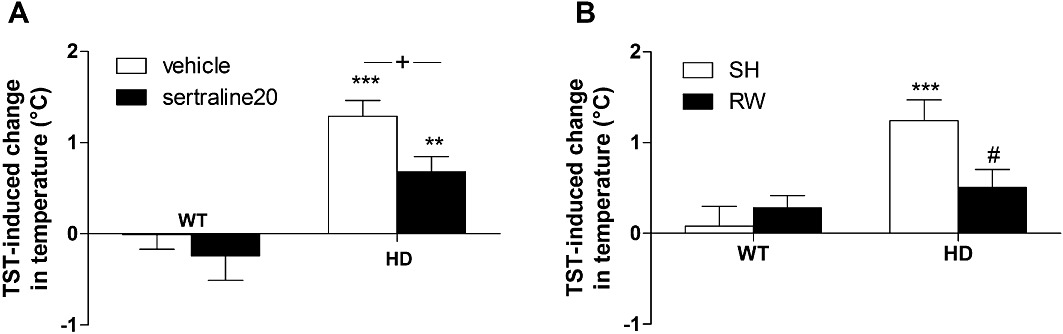
Effect of chronic treatment with sertraline or exercise on the TST-induced hypothermia. Measuring the body temperature before and after a 6 min TST, we found an increased TST-induced hyperthermia in control HD mice compared with WT animals. This impaired ‘stress response’ displayed by HD mice was attenuated by (A) chronic treatment with sertraline and (B) RW. Values represent means (±SEM) of n = 10–17 mice per group. WT versus HD: **P < 0.01, ***P < 0.001; vehicle versus sertraline 20: +P < 0.05; SH versus RW: #P < 0.05.
Saccharin-preference test. As animals chronically injected with vehicle (controls for cohort#1) showed similar saccharin preference and total fluid intake when compared with naïve SH mice (controls for cohort#2) we pooled the data into a unique ‘controls’ group (Figure 3). The examination of saccharin preference (Figure 3A) revealed a significant effect of treatments [F(2,79)= 9.4, P < 0.001] and an interaction with the genotype [F(2,79)= 4.4, P < 0.05]. Control HD mice showed decreased preference for saccharin solution compared with WT control animals (P < 0.05). Chronic sertraline treatment (but not RWs) increased saccharin preference of HD mice (P < 0.05). There was an overall genotype difference for total fluid intake [F(1,79)= 21.2, P < 0.001] but no treatment effect [F(1,79)= 0.03, P = 0.82] (Figure 3B).
Figure 3.
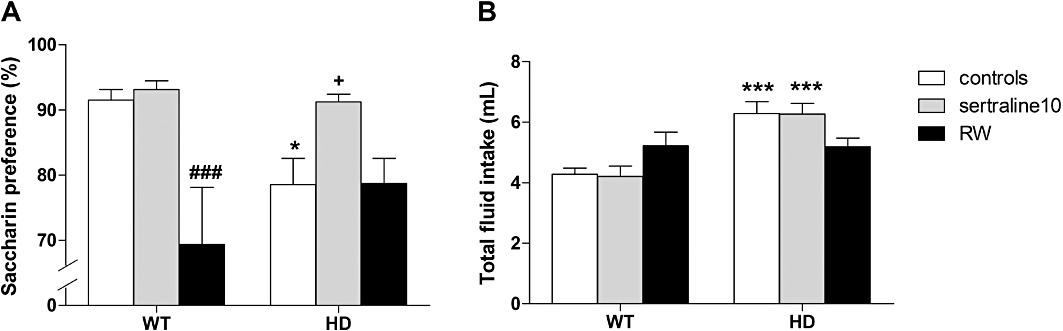
Effect of chronic treatment with sertraline or exercise on the saccharin-preference test. (A) We found that control HD mice showed decreased preference for saccharin solution when compared with WT animals. This anhedonia-like behaviour displayed by HD mice was prevented by chronic treatment with sertraline but not RW. Regarding the total fluid intake (B), we also found an overall genotype difference but no effect of treatments. Values represent means (±SEM) of n = 19–21 controls and n = 9–14 mice for each treatment. WT versus HD: *P < 0.05, ***P < 0.001; vehicle versus sertraline 20: +P < 0.05; SH versus RW: ###P < 0.001.
Chronic antidepressant treatments prevented the exaggerated 5-HT1A autoreceptor function exhibited by female HD mice
Sex-specific effect of HD mutation on hypothermia induced by 8-OH-DPAT. In vivo evaluation of 5-HT1A autoreceptor function was determined measuring the effect of the 5-HT1A receptor agonist 8-OH-DPAT on body temperature (cohort#3). There were no differences in baseline temperatures recorded between the sexes or genotypes before the administration of 8-OH-DPAT (data not shown), which significantly decreased rectal temperature in a dose-dependent manner in male (Figure S1A) and female (Figure 4A) mice compared with saline-injected animals. Area under curve (AUC) analysis for temperature changes 1 h post injection (Figures S1B and 4B) revealed an overall effect of treatment in male [F(2,55)= 47.4, P < 0.001] and female [F(2,57)= 71.1, P < 0.001] groups. However, there was a further genotype effect [F(1,57)= 20.9, P < 0.001] for female mice only. Post hoc analysis showed that female HD mice exhibited an augmented hypothermic response to both doses of 8-OH-DPAT (P < 0.01).
Figure 4.
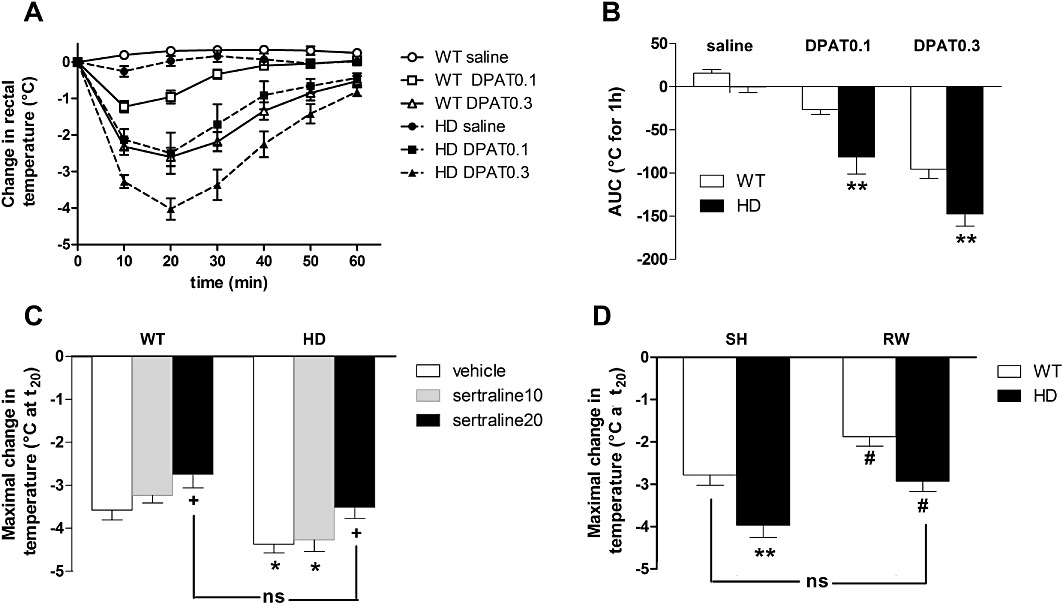
Effect of chronic treatment with sertraline or exercise on the 8-OH-DPAT-induced hypothermia. Using the 8-OH-DPAT-induced hypothermia we found that administration of the 5-HT1A agonist decreased rectal temperature [compared with baseline temperature (t0) and expressed in °C] in a dose- and time-dependent manner (A). The maximal hypothermic response was observed 20 min after 8-OH-DPAT administration. Considering the AUC for 1 h post injection, we found a significant overall effect of treatment and genotype (B). Indeed, compared with WT animals, HD female mice exhibited augmented hypothermia for both doses of 8-OH-DPAT (0.1 and 0.3 mg·kg−1). On analysing the maximal change in temperature 20 min (t20) after 8-OH-DPAT (0.3 mg·kg−1) injection, we found that (C) chronic treatment with sertraline and (D) RW prevented the exaggerated hypothermic response exhibited by control HD mice. Values represent means (±SEM) of n = 8–15 mice per group. WT versus HD: *P < 0.05, **P < 0.01, ***P < 0.001; vehicle versus sertraline 20: +P < 0.05; SH versus RW: #P < 0.05. ns, not significant.
Effects of chronic treatment with sertraline or exercise on the exaggerated 5-HT1A autoreceptor function exhibited by female HD mice. As shown in Figure 4C (looking at cohort#1), the maximum hypothermic response observed 20 min post 8-OH-DPAT (0.3 mg·kg−1, s.c.) was analysed in WT/HD vehicle- (respectively −3.57 ± 0.23 and −4.37 ± 0.20°C, P < 0.05), WT/HD sertraline10 (respectively −3.23 ± 0.18 and −4.27 ± 0.27°C, P < 0.01) and WT/HD sertraline20 (respectively −2.74 ± 0.32 and −3.51 ± 0.26°C, ns) treated mice. A two-way anova revealed an overall effect of both genotype [F(1,77)= 17.9, P < 0.001] and sertraline treatment [F(2,77)= 5.7, P < 0.01] but no significant interaction [F(2,77)= 0.17, P = 0.85]. Chronic treatment with 20 mg·kg−1 sertraline reduced the 8-OH-DPAT response in both WT and HD animals (P < 0.05). We conducted a similar experimental approach to study the effect of RW (cohort#2) on 5-HT1A autoreceptor function (Figure 4D). We found a significant effect of both genotype [F(1,53)= 20, P < 0.001] and exercise [F(1,53)= 15.1, P < 0.001] and no interaction [F(1,53)= 0.06, P = 0.80]. Exercise reduced the 8-OH-DPAT response in both WT and HD animals (P < 0.05). Interestingly the exaggerated hypothermic response exhibited by SH HD mice (P < 0.01) was no longer observed in RW animals.
Female HD mice displayed reduced 5-HT1A and 5-HT2A heteroreceptor function
Corticosterone levels. On measuring baseline corticosterone levels in serum of mice chronically injected (cohort#1), we found a significant effect of both genotype [F(1,29)= 7.5, P < 0.05] and sertraline [F(2,29)= 4.5, P < 0.01] but no interaction [F(1,29)= 0.02, ns]. Chronic vehicle-injected female HD mice had higher corticosterone levels at baseline than WT animals (P < 0.05; Figure 5A). Chronic treatment with sertraline increased corticosterone levels of WT mice (P < 0.05). Interestingly, the genotype-dependent effect of chronic vehicle injection was not observed in male animals (data not shown). We investigated the effect of 5-HT1A or 5-HT2 receptor activation on corticosterone levels of SH animals as well as the effect of wheel-running. We found a significant treatment effect [F(3,34)= 8.2, P < 0.001] and an interaction with the genotype [F(3,34)= 4.1, P < 0.05]. Post hoc tests showed significant increases in corticosterone levels of WT mice following acute administration of 8-OH-DPAT (0.3 mg·kg−1) and DOI (1 mg·kg−1) (Figure 5B). Both pharmacological responses were blunted in HD animals. In contrast to our findings in chronically injected mice (Figure 5A), WT and HD animals had similar levels of corticosterone following acute saline injections. Finally, we found that exercise on RWs increased corticosterone levels in both WT (P < 0.001) and HD mice (P < 0.05).
Figure 5.
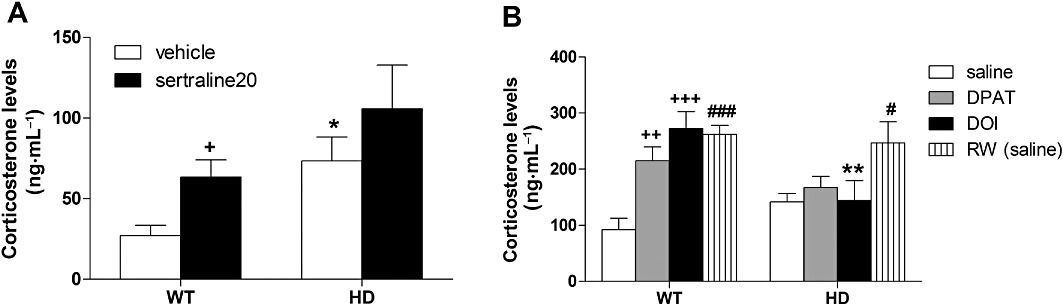
Effect of chronic treatment with sertraline or exercise on baseline or pharmacologically-induced corticosteone release. (A) On measuring baseline corticosterone levels in serum of mice chronically injected with vehicle (n = 12), we found that female HD mice exhibited higher baseline levels of corticosterone when compared with WT animals. We also find an effect of chronic sertraline regardless of the genotype (n = 4–5 per group). (B) Analysing the effect of 5-HT1A or 5-HT2 receptor activation on corticosterone levels of SH animals as well as the effect of RW, we found that 8-OH-DPAT and DOI injection increased corticosterone in WT but not in HD animals. Exercise induced corticosterone release regardless of the genotype. Values represent means (±SEM) of n = 4–7 mice for each treatment conditions. WT versus HD: *P < 0.05, **P < 0.01; effect of pharmacological treatments (sertraline/DOI/DPAT): +P < 0.05, ++P < 0.01, +++P < 0.001; SH versus RW: #P < 0.05, ###P < 0.001.
DOI-induced head-twitches. Compared with saline-injected mice (not shown), the 5-HT2A/2C receptor agonist DOI induced head-twitches in all animals (Figure 6A). A two-way anova revealed an overall effect of both genotype [F(1,48)= 68.5, P < 0.001] and sertraline treatment [F(1,48)= 9.6, P < 0.01] with a significant interaction between both factors [F(1,48)= 4.4, P < 0.05]. Post hoc tests showed that HD mice exhibited fewer head-twitches when compared with WT (P < 0.001) and that chronic treatment with sertraline reduced the DOI response only in WT mice (P < 0.01).
Figure 6.
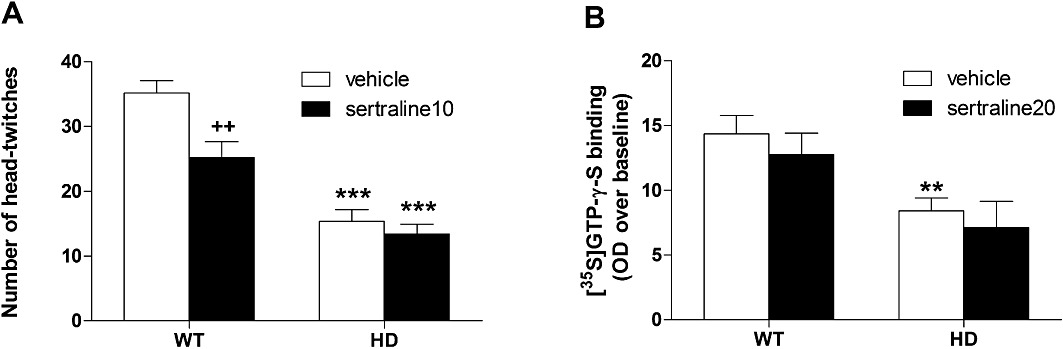
Effect of chronic treatment with sertraline on the 5-HT2/5-HT1A heteroreceptor function. (A) On measuring the number of head-twitches induced by DOI injection (n = 12–15 mice per group), we found that HD mice exhibited fewer head-twitches when compared with WT and that chronic treatment with sertraline reduced DOI response only in WT mice. (B) The 5-HT1AR-mediated [35S]-GTP-γ-S binding (n = 6 mice per group) was increased by the non-selective 5-HT receptor agonist 5-CT, and expressed as OD over basal condition. This binding was reduced in HD animals but not affected by sertraline treatment. WT versus HD: **P < 0.01, ***P < 0.001; vehicle versus sertraline10 (chronic treatment): ++P < 0.01.
5-HT1A receptor-G protein coupling. We used the 5HT1AR agonist 5-CT to stimulate [35S]-GTP-γ-S binding and so assess the function of 5-HT1A heteroreceptor function within the hippocampus of HD animals. In all groups of mice, the potent non-selective 5-HT receptor agonist 5-CT induced an increase (compared with basal level) in [35S]-GTP-γ-S labelling in the hippocampus. This stimulation could be prevented by the selective 5-HT1A receptor antagonist WAY 100635 (10 µM) (‘non-specific’ condition, data not shown). Analysis of OD measurements found an overall effect of genotype [F(1,20)= 13.6, P < 0.01] but no effect of sertraline [F(1,20)= 0.82, P = 0.38] or interaction [F(1,20)= 0.01, P = 0.92]. Within the group of vehicle-treated animals, [35S]-GTP-γ-S labelling in female HD mice was reduced by ∼40% (P < 0.001) compared with WT animals (Figure 6B).
Sertraline and exercise effects on hippocampal gene expression
5-HT1AR. There was a consistent significant genotype effect in both studies of sertraline treatment [F(1,11)= 247, P < 0.001] and wheel-running [F(1,25)= 43, P < 0.001]. Post hoc tests revealed significant reduction of 5-HT1AR mRNA levels in HD mice (P < 0.001) (Figure 7A and B). Interestingly, we found a significant effect of exercise [F(1,25)= 6.9, P < 0.05] but no effect of chronic sertraline [F(1,11)= 2.45, P = 0.15]. Post hoc tests analysis showed that 5-HT1AR mRNA levels of wheel-running HD mice were lower compared with SH HD animals (Figure 7B, P < 0.05).
Figure 7.
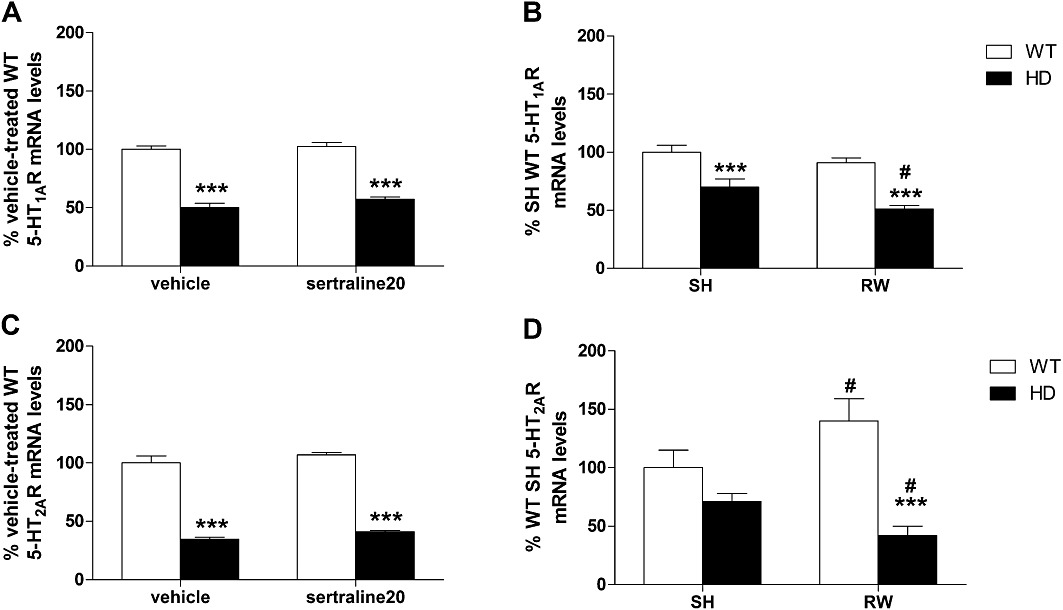
Effect of chronic treatment with sertraline or exercise on hippocampal gene expression. Hippocampal mRNA levels of 5-HT1A receptor (5-HT1AR) and 5-HT2A receptor (5-HT2AR) were measured following chronic treatment with (A/C) sertraline (20 mg·kg−1) or (B/D) voluntary physical exercise. Both 5-HT1AR and 5-HT2AR mRNA levels in control HD mice were decreased when compared with paired WT animals. In the RW groups, 5-HT2AR were increased in WT but decreased in HD mice when compared with SH animals. Regardless of the genotype, we found no effect of chronic treatment with sertraline for all the genes studied. Values represent means (±SEM) of n = 3–4 mice per group. WT versus HD: ***P < 0.001; SH versus RW: #P < 0.05.
5-HT2AR. There was an overall significant effect of the HD mutation on 5-HT2AR mRNA levels in both cohort#1 [F(1,11)= 335, P < 0.001] and cohort#2 [F(1,22)= 24, P < 0.001]. Post hoc tests revealed a reduction in 5-HT2AR mRNA levels in HD mice (P < 0.001) (Figure 7C). There was no effect of chronic sertraline treatment [F(1,11)= 3.38, P = 0.09] but a significant interaction between genotype and exercise [F(1,22)= 7.0, P < 0.05]. Post hoc analysis showed that wheel-running increased 5-HT2AR mRNA levels in WT mice (P < 0.05) but decreased 5-HT2AR mRNA in HD animals (P < 0.05).
We did not find any effects of genotype or treatments (sertraline and exercise) on either 5-HTT or GR gene expression (Figure S2)
Effects of genotype and exercise on hippocampal cell proliferation
On assessing the effects of exercise on hippocampal cell proliferation (Figure 8A), we found significant effects of genotype [F(1,9)= 183, P < 0.001] and exercise [F(1,9)= 90, P < 0.001], as well as a significant interaction [F(1,9)= 72, P < 0.001]. Post hoc tests showed that the total number of BrdU-positive cells in SH animals was decreased by 80% in female HD mice compared with WT animals (P < 0.05). Interestingly, exercise had no effect on HD mice but increased cell proliferation in WT animals (+300%, P < 0.001). Similarly, there were significant effects of genotype [F(1,9)= 7.4, P < 0.05] and exercise [F(1,9)= 11.1, P < 0.05] on dentate gyrus volume (Figure 8B), as well as a significant genotype–exercise interaction [F(1,9)= 6.3, P < 0.05]. Wheel-running significantly increased dentate gyrus volume in WT animals (P < 0.001) but had no effect on HD mice. However, there was no apparent volume difference between HD and WT SH animals.
Figure 8.
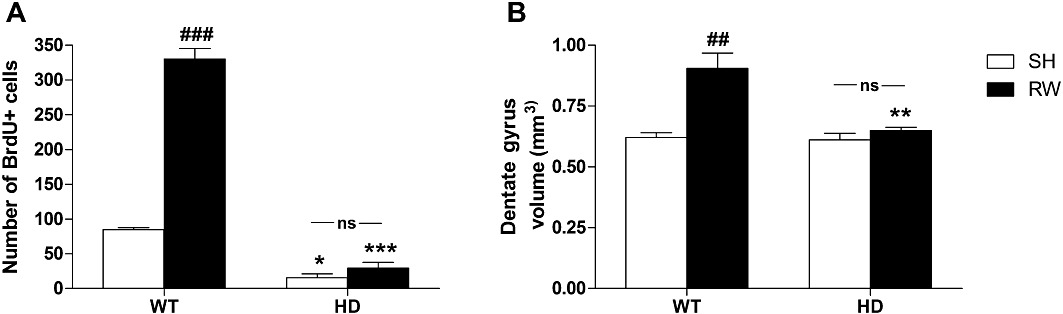
Effect of exercise on the hippocampal cell proliferation process. On comparing (A) the number of cells labelled for BrdU in the dentate gyrus of mice SH or exposed to RWs, we found a significant interaction between genotype and exercise. The total number of BrdU-positive cells was decreased in female HD mice when compared with WT animals. Exercise had no effect on HD mice but increased cell proliferation in WT animals. (B) Exercise had no effect on HD mice but significantly increased dentate gyrus volume in WT animals. Values represent means (±SEM) of n = 3–5 mice per group. WT versus HD: *P < 0.05, **P < 0.01, ***P < 0.001; SH versus RW: ##P < 0.01, ###P < 0.001. ns, not significant.
Discussion and conclusions
Depression is a common psychiatric symptom during the pre-motor stage of HD (Paulsen et al., 2005; Duff et al., 2007; Julien et al., 2007; Marshall et al., 2007). Gender is a significant risk factor for developing depression (Fava and Kendler, 2000; Kornstein et al., 2000; Marcus et al., 2008). While HD is an autosomal dominant condition that affects both males and females equally, there is yet to be a study of sexual dimorphism in the development and presentation of depression in HD patients. Using the R6/1 transgenic mouse model of HD, we had previously described female-specific depression-like behaviours in pre-motor symptomatic HD mice (Pang et al., 2009). However, the sex-dependent mechanism(s) mediating this phenotype remained unclear and had not been functionally assessed. Here we have investigated the behavioural and molecular effects of a pharmacological (chronic sertraline) and a non-pharmacological (voluntary physical exercise) antidepressant approach, by assessing 5-hydroxytryptaminergic neurotransmission, stress response and hippocampal plasticity.
Of all animal models, the FST remains one of the most used tools for screening antidepressant activity (Petit-Demouliere et al., 2005). Consistent with previous findings (Pang et al., 2009), female HD mice exhibited increased immobility time in the FST. We have now shown that female HD animals display anhedonic behaviour (a key feature of depression) given their reduced preference for saccharin solution and also have an abnormal physiological response to acute stress as demonstrated by the exaggerated TST-induced hyperthermia. Interestingly, these depression-related responses were corrected following chronic sertraline treatment and are unlikely to be due to any confounding effects on spontaneous locomotor activity (Reith and Fischette, 1991; T. Renoir, unpubl. data).
This study is the first to show that chronic SSRI treatment can correct the depressive-like phenotype of pre-motor symptomatic HD mice. In a previous study on the R6/1 line, Grote et al. (2005) had found improvements following chronic fluoxetine; however, the interpretation of the behavioural tests in that study was confounded by the use of late-stage HD animals that were highly motor symptomatic by 20 weeks of age. Pouladi et al. (2009) studied the effect of antidepressant treatment in the YAC transgenic model of HD and found no beneficial effects but that study was limited due to the use of only a few HD animals and the lack of appropriate WT controls.
Voluntary physical exercise delays the onset and progression of motor and cognitive deficits in HD mice (Pang et al., 2006; van Dellen et al., 2008). We now provide evidence of an antidepressive effect of exercise, which reduced FST immobility time and the TST-induced hyperthermic response of HD mice. The beneficial effects of physical activity in alleviating depressive mood are well-known and exercise is often suggested as a form of therapy for depressed patients (Babyak et al., 2000; Strawbridge et al., 2002; Dunn et al., 2005). It has also been studied in several rodent models of depression (Solberg et al., 1999; Greenwood et al., 2003; Adlard and Cotman, 2004; Bjornebekk et al., 2008; 2010). Interestingly, wheel-running did not prevent the reduced saccharin preference of HD mice, suggesting that exercise may exert differential effects on ‘despair’ versus ‘anhedonia’-like behaviours. However, it is worth noting that for experimental reasons, mice were individually-housed without RWs during the saccharin-preference test, introducing a potential confounder. Indeed, wheel-running may have similarities with addictive behaviours and a sudden stop in exercise could induce a withdrawal-like status in rodents (Hoffmann et al., 1987). As suggested by a study carried out in female rats, the loss of sensitivity to opioids under exercise conditions may actually be fully reversed when the animals do not have access to RWs anymore (Smith and Lyle, 2006). This potential anhedonic-like behaviour induced by a brief period of exercise deprivation (observed in our WT animals) has also been reported by healthy women (who exercise at least four times per week) during a 72 h exercise abstinence (Niven et al., 2008).
Wheel-running was originally reported as having antidepressive effects in WT rodents (Duman et al., 2008; Trejo et al., 2008); however, recent evidence suggests otherwise (Arunrut et al., 2009; Fuss et al., 2010). Our findings of increased FST immobility times and reduced saccharin preference by wheel-running WT support the latter reports. As previously suggested, it is likely that specific experimental conditions such as animal strain as well as mode or intensity of the exercise may account for the mixed effect of exercise on FST (Clark et al., 2011; Dubreucq et al., 2010). Interestingly, mouse lines specifically bred for their high levels of wheel-running (HR lines) have been shown to be more immobile in the FST (Malisch et al., 2009). As social isolation would have confounded the assessments of neurogenesis and affective-behaviour (Ago et al., 2008; Ibi et al., 2008; Lukkes et al., 2009), our study design involved two RWs shared by four animals. Therefore, we were unable to quantify the distance run by individual mice and have no evidence that our WT mice overwork when RWs are presented in this context; however, genotype effect on the running pattern is still a possibility that needs to be addressed in further studies. Consistent with previous studies (Droste et al., 2003; Fediuc et al., 2006), we found that exercise increased baseline corticosterone levels without changing hippocampal GR gene expression. These effects were observed in both genotype and therefore are unlikely to explain per se the opposite behavioural outcomes.
Consistent with other mouse models of HD (Kohl et al., 2007; Potter et al., 2010; Simpson et al., 2011), we observed an altered neurogenesis in HD animals, which was not restored by wheel-running. In contrast, chronic administration of SSRIs including sertraline has been shown to promote cell proliferation in HD animals (Grote et al., 2005; Duan et al., 2008; Peng et al., 2008), possibly through brain-derived neurotrophic factor (BDNF) and GR mechanisms (Anacker et al., 2011). Therefore, despite a common antidepressant-like behavioural effect, chronic SSRI treatment and wheel-running have different effects on hippocampal cell turnover. This finding supports the hypothesis that the behavioural effects of some antidepressant treatments may involve neurogenesis-independent mechanisms (Holick et al., 2008; Trejo et al., 2008; David et al., 2009). Our findings also indicate that hippocampal neurogenesis is not solely regulated by BDNF levels, as running prevented the decrease in hippocampal BDNF mRNA in female HD animals (Zajac et al., 2010).
The 5-HT1A receptor has been implicated in the increased susceptibility of females to developing depression (Szewczyk et al., 2009). Here, we report for the first time that female, but not male, HD mice have exaggerated hypothermic responses to the 5-HT1A receptor agonist 8-OH-DPAT, thereby identifying 5-HT1A autoreceptor hypersensitization as the female-specific molecular change associated with the depressive phenotype. Indeed, the specific involvement of 5-HT1A autoreceptors in 8-OH-DPAT-induced hypothermia is well-established in the mouse (Bill et al., 1991). Similar animal studies have also found a causal relationship between 5-HT1A autoreceptor function and depressive-like behaviours (Renoir et al., 2008; Richardson-Jones et al., 2010). However, a sensitization of 5-HT7 receptors in HD mice cannot be excluded, as this receptor has also been reported to be involved in 8-OH-DPAT-induced hypothermia (Hedlund et al., 2004) and this could be a focus of future studies. Selective 5-HT7 receptor antagonists have recently been proposed as a new class of antidepressants (Mnie-Filali et al., 2007). Desensitization of the 5-HT1A autoreceptor is essential for enhancement of 5-HT transmission following chronic SSRI treatment (Maj and Moryl, 1992; Dawson et al., 2002; Rossi et al., 2008). Consistent with the antidepressive effects of chronic sertraline treatment and wheel-running, both treatment approaches corrected the 5-HT1A receptor hypersensitivity in the female HD mice. Our results provide a mechanistic rationale for future attempts to target the dysfunction of 5-HT1A autoreceptor as a means of treating HD-associated depression.
Consistent with observations in depressed patients (Meltzer and Maes, 1995; Stockmeier et al., 1998; Shapira et al., 2000; Riedel et al., 2002; Miller et al., 2009; Sullivan et al., 2009) and several mouse models of depression (McKittrick et al., 1995; Overstreet, 2002; El Yacoubi et al., 2003; Mato et al., 2007; Richardson-Jones et al., 2010), we also found that 5-HT1A heteroreceptor function was reduced in female HD animals. Indeed, [35S]-GTP-γ-S binding stimulated by the 5-HT1AR agonist 5-CT was reduced within the hippocampus of HD animals. Using a similar experimental approach in a mouse model of depression, Hensler et al. (2010) suggested that hippocampal 5-HT1ARs were regulated by corticosterone. Supporting the evidence of a possible desensitization of hippocampal 5-HT1AR in patients with major depression (Shapira et al., 2000; Riedel et al., 2002; but also see Navines et al., 2007; Miller et al., 2009), we found the corticosterone response to the 5-HT1AR agonist 8-OH-DPAT was attenuated in female HD mice.
We also observed attenuated behavioural and hormonal responses to the 5-HT2A receptor agonist DOI in our HD animals. Similar observations have been reported in other mouse models relevant to depression (Mato et al., 2007), including mice lacking the 5-HT transporter (Rioux et al., 1999) with functional interactions between 5-HT2A and presynaptic 5-HT1A receptors (Fox et al., 2010). 5-HT2A function was also reduced in WT animals after chronic sertraline. Whether the decrease in 5-HT2A receptor function in depressed patients represents a pathological trait or alternatively a consequence of antidepressant medication remains unclear (Messa et al., 2003; Mintun et al., 2004).
Finally, we report a novel genotype-dependent response to both acute (TST-induced hyperthermia) and chronic stress (chronically saline-injected female HD mice exhibited higher baseline levels of corticosterone compared with WT animals). A sex-specific increased hypothalamic-pituitary-adrenal (HPA) axis reactivity has recently been reported in women with chronic major depressive disorder (Chopra et al., 2009). Also, augmented levels of cortisol and corticosterone have been found in HD patients (Aziz et al., 2009) and in R6/2 HD mice (Bjorkqvist et al., 2006), respectively. These could be due to dysregulation of 5-hydroxytryptaminergic neurotransmission (Hery et al., 2000; Froger et al., 2004). It is worth noting that corticosterone levels of chronically injected mice were still in the low range (<100 ng·mL−1), especially when compared with the values reported by Bjorkqvist et al. (∼400 ng·mL−1) or naive animals either challenged with 5-HT1A/5-HT2 agonists or exposed to RWs (corticosterone levels ∼250 ng·mL−1).
Collectively, our findings indicate that the manifestation of the depressive-like behaviours exhibited by pre-motor symptomatic female HD mice is attributable to a dysfunction of the 5-HT1A autoreceptor. Chronic sertraline treatment and physical activity corrected that dysfunction and prevented the depressive-like behaviours. Our data also suggest that the antidepressant effect of running on HD mice is independent of hippocampal cell proliferation.
Acknowledgments
This work was funded by NHMRC Project Grants (AJH), philanthropic support from a Pfizer Australia Research Fellowship (AJH), NHMRC-INSERM Exchange Fellowship (TR), ARC Future Fellowship (AJH) and University of Melbourne Research Scholarships (MSZ and XD). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Glossary
- 5-CT
5-carboxamidotryptamine
- 5-HTT
5-HT transporter
- AUC
area under curve
- BDNF
brain-derived neurotrophic factor
- BrdU
5-bromo-2′-deoxyuridine
- FST
forced-swimming test
- GR
glucocorticoid receptor
- HD
Huntington's disease
- HPA
hypothalamic-pituitary-adrenal
- RW
running-wheel
- SH
standard-housed
- SSRIs
selective 5-HT re-uptake inhibitors
- TST
tail-suspension test
- WT
wild-type
Conflicts of interest
The authors do not have any conflicts of interests to disclose.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Effect of HD mutation on the8-OH-DPAT-induced hypothermia in male animals. (A) Using the8-OH-DPAT-induced hypothermia we found that administration of the5-HT1A agonist decreased rectal temperature [comparedwith baseline temperature (t0) and expressed in°C] in a dose- and time-dependent manner in all animals. (B)Considering the AUC for 1 h post injection, we also found asignificant overall effect of DPAT treatment but no effect ofgenotype. Values represent means (±SEM) of n =8–11 mice per group.
Figure S2 Effect of chronic treatment withsertraline or exercise on hippocampal gene expression. HippocampalmRNA levels of 5-HT transporter (5-HTT) and GR were measuredfollowing chronic treatment with (A/C) sertraline (20mg·kg−1) or (B/D) voluntary physicalexercise (RW). None gene expression was affected by either thegenotype or the treatment conditions. Values represent means(±SEM) of n = 3–4 mice per group.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 2004;124:985–992. doi: 10.1016/j.neuroscience.2003.12.039. [DOI] [PubMed] [Google Scholar]
- Ago Y, Arikawa S, Yata M, Yano K, Abe M, Takuma K, et al. Antidepressant-like effects of the glucocorticoid receptor antagonist RU-43044 are associated with changes in prefrontal dopamine in mouse models of depression. Neuropharmacology. 2008;55:1355–1363. doi: 10.1016/j.neuropharm.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Anacker C, Zunszain PA, Cattaneo A, Carvalho LA, Garabedian MJ, Thuret S, et al. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol Psychiatry. 2011;16:738–750. doi: 10.1038/mp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunrut T, Alejandre H, Chen M, Cha J, Russo-Neustadt A. Differential behavioral and neurochemical effects of exercise, reboxetine and citalopram with the forced swim test. Life Sci. 2009;84:584–589. doi: 10.1016/j.lfs.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz NA, Pijl H, Frolich M, van der Graaf AW, Roelfsema F, Roos RA. Increased hypothalamic-pituitary-adrenal axis activity in Huntington's disease. J Clin Endocrinol Metab. 2009;94:1223–1228. doi: 10.1210/jc.2008-2543. [DOI] [PubMed] [Google Scholar]
- Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62:633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, Cowen PJ. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Mol Psychiatry. 2004;9:386–392. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- Bill DJ, Knight M, Forster EA, Fletcher A. Direct evidence for an important species difference in the mechanism of 8-OH-DPAT-induced hypothermia. Br J Pharmacol. 1991;103:1857–1864. doi: 10.1111/j.1476-5381.1991.tb12342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkqvist M, Petersen A, Bacos K, Isaacs J, Norlen P, Gil J, et al. Progressive alterations in the hypothalamic-pituitary-adrenal axis in the R6/2 transgenic mouse model of Huntington's disease. Hum Mol Genet. 2006;15:1713–1721. doi: 10.1093/hmg/ddl094. [DOI] [PubMed] [Google Scholar]
- Bjornebekk A, Mathe AA, Gruber SH, Brene S. Housing conditions modulate escitalopram effects on antidepressive-like behaviour and brain neurochemistry. Int J Neuropsychopharmacol. 2008;11:1135–1147. doi: 10.1017/S1461145708008912. [DOI] [PubMed] [Google Scholar]
- Bjornebekk A, Mathe AA, Brene S. The antidepressant effects of running and escitalopram are associated with levels of hippocampal NPY and Y1 receptor but not cell proliferation in a rat model of depression. Hippocampus. 2010;20:820–828. doi: 10.1002/hipo.20683. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Boyle SH, Siegler IC, Kuhn CM, Ashley-Koch A, Jonassaint CR, et al. Effects of environmental stress and gender on associations among symptoms of depression and the serotonin transporter gene linked polymorphic region (5-HTTLPR) Behav Genet. 2008;38:34–43. doi: 10.1007/s10519-007-9172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Zhang W, Carter PA, Shaw J, Chernet E, Phebus L, et al. Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology (Berl) 2002;160:353–361. doi: 10.1007/s00213-001-0986-x. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Peng Q, Hou Z, Aggarwal M, Zhang J, Mori S, et al. Structural MRI detects progressive regional brain atrophy and neuroprotective effects in N171-82Q Huntington's disease mouse model. Neuroimage. 2011;56:1027–1034. doi: 10.1016/j.neuroimage.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra KK, Ravindran A, Kennedy SH, Mackenzie B, Matthews S, Anisman H, et al. Sex differences in hormonal responses to a social stressor in chronic major depression. Psychoneuroendocrinology. 2009;34:1235–1241. doi: 10.1016/j.psyneuen.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- Cipriani A, La Ferla T, Furukawa TA, Signoretti A, Nakagawa A, Churchill R, et al. Sertraline versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2010;(4):CD006117. doi: 10.1002/14651858.CD006117.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Brzezinska WJ, Rhodes JS. Genetic influences on exercise-induced adult hippocampal neurogenesis across 12 divergent mouse strains. Genes Brain Behav. 2011;10:345–353. doi: 10.1111/j.1601-183X.2010.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson LA, Nguyen HQ, Smith DL, Schechter LE. Effect of chronic fluoxetine and WAY-100635 treatment on serotonergic neurotransmission in the frontal cortex. J Psychopharmacol. 2002;16:145–152. doi: 10.1177/026988110201600205. [DOI] [PubMed] [Google Scholar]
- van Dellen A, Cordery PM, Spires TL, Blakemore C, Hannan AJ. Wheel running from a juvenile age delays onset of specific motor deficits but does not alter protein aggregate density in a mouse model of Huntington's disease. BMC Neurosci. 2008;9:34. doi: 10.1186/1471-2202-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslandes A, Moraes H, Ferreira C, Veiga H, Silveira H, Mouta R, et al. Exercise and mental health: many reasons to move. Neuropsychobiology. 2009;59:191–198. doi: 10.1159/000223730. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, et al. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droste SK, Gesing A, Ulbricht S, Muller MB, Linthorst AC, Reul JM. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology. 2003;144:3012–3023. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- Duan W, Peng Q, Masuda N, Ford E, Tryggestad E, Ladenheim B, et al. Sertraline slows disease progression and increases neurogenesis in N171-82Q mouse model of Huntington's disease. Neurobiol Dis. 2008;30:312–322. doi: 10.1016/j.nbd.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreucq S, Marsicano G, Chaouloff F. Emotional consequences of wheel running in mice: which is the appropriate control? Hippocampus. 2010 doi: 10.1002/hipo.20778. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Stout JC. Psychiatric symptoms in Huntington's disease before diagnosis: the predict-HD study. Biol Psychiatry. 2007;62:1341–1346. doi: 10.1016/j.biopsych.2006.11.034. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Bouali S, Popa D, Naudon L, Leroux-Nicollet I, Hamon M, et al. Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. Proc Natl Acad Sci USA. 2003;100:6227–6232. doi: 10.1073/pnas.1034823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Kendler KS. Major depressive disorder. Neuron. 2000;28:335–341. doi: 10.1016/s0896-6273(00)00112-4. [DOI] [PubMed] [Google Scholar]
- Fediuc S, Campbell JE, Riddell MC. Effect of voluntary wheel running on circadian corticosterone release and on HPA axis responsiveness to restraint stress in Sprague-Dawley rats. J Appl Physiol. 2006;100:1867–1875. doi: 10.1152/japplphysiol.01416.2005. [DOI] [PubMed] [Google Scholar]
- Fox MA, Stein AR, French HT, Murphy DL. Functional interactions between 5-HT2A and presynaptic 5-HT1A receptor-based responses in mice genetically deficient in the serotonin 5-HT transporter (SERT) Br J Pharmacol. 2010;159:879–887. doi: 10.1111/j.1476-5381.2009.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froger N, Palazzo E, Boni C, Hanoun N, Saurini F, Joubert C, et al. Neurochemical and behavioral alterations in glucocorticoid receptor-impaired transgenic mice after chronic mild stress. J Neurosci. 2004;24:2787–2796. doi: 10.1523/JNEUROSCI.4132-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss J, Ben Abdallah NM, Vogt MA, Touma C, Pacifici PG, Palme R, et al. Voluntary exercise induces anxiety-like behavior in adult C57BL/6J mice correlating with hippocampal neurogenesis. Hippocampus. 2010;20:364–376. doi: 10.1002/hipo.20634. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Reul JH, Gesing A, Stenzel-Poore MP, Holsboer F, Steckler T. Mice overexpressing CRH show reduced responsiveness in plasma corticosterone after a5-HT1A receptor challenge. Genes Brain Behav. 2002;1:174–177. doi: 10.1034/j.1601-183x.2002.10305.x. [DOI] [PubMed] [Google Scholar]
- Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2008;23:631–640. doi: 10.1002/mds.21922. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Lange M, Wolff B, Volzke H, Lucht M, Freyberger HJ, et al. Mental and physical distress is modulated by a polymorphism in the 5-HT transporter gene interacting with social stressors and chronic disease burden. Mol Psychiatry. 2005;10:220–224. doi: 10.1038/sj.mp.4001555. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, et al. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote HE, Bull ND, Howard ML, van Dellen A, Blakemore C, Barlett PF, et al. Cognitive disorders and neurogenesis deficits in Huntington's disease mice are rescued by fluoxetine. Eur J Neurosci. 2005;22:2081–2088. doi: 10.1111/j.1460-9568.2005.04365.x. [DOI] [PubMed] [Google Scholar]
- Harkin A, Houlihan DD, Kelly JP. Reduction in preference for saccharin by repeated unpredictable stress in mice and its prevention by imipramine. J Psychopharmacol. 2002;16:115–123. doi: 10.1177/026988110201600201. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur J Pharmacol. 2004;487:125–132. doi: 10.1016/j.ejphar.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Hensler JG, Vogt MA, Gass P. Regulation of cortical and hippocampal 5-HT(1A) receptor function by corticosterone in GR+/− mice. Psychoneuroendocrinology. 2010;35:469–474. doi: 10.1016/j.psyneuen.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hery M, Semont A, Fache MP, Faudon M, Hery F. The effects of serotonin on glucocorticoid receptor binding in rat raphe nuclei and hippocampal cells in culture. J Neurochem. 2000;74:406–413. doi: 10.1046/j.1471-4159.2000.0740406.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Thoren P, Ely D. Effect of voluntary exercise on open-field behavior and on aggression in the spontaneously hypertensive rat (SHR) Behav Neural Biol. 1987;47:346–355. doi: 10.1016/s0163-1047(87)90461-4. [DOI] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Ibi D, Takuma K, Koike H, Mizoguchi H, Tsuritani K, Kuwahara Y, et al. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J Neurochem. 2008;105:921–932. doi: 10.1111/j.1471-4159.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- Julien CL, Thompson JC, Wild S, Yardumian P, Snowden JS, Turner G, et al. Psychiatric disorders in preclinical Huntington's disease. J Neurol Neurosurg Psychiatry. 2007;78:939–943. doi: 10.1136/jnnp.2006.103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Tsunoka T, Ikeda M, Kawashima K, Okochi T, Kitajima T, et al. Serotonin 1A receptor gene and major depressive disorder: an association study and meta-analysis. J Hum Genet. 2009;54:629–633. doi: 10.1038/jhg.2009.84. [DOI] [PubMed] [Google Scholar]
- Kohl Z, Kandasamy M, Winner B, Aigner R, Gross C, Couillard-Despres S, et al. Physical activity fails to rescue hippocampal neurogenesis deficits in the R6/2 mouse model of Huntington's disease. Brain Res. 2007;1155:24–33. doi: 10.1016/j.brainres.2007.04.039. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, et al. Gender differences in chronic major and double depression. J Affect Disord. 2000;60:1–11. doi: 10.1016/s0165-0327(99)00158-5. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Peprah D, Gershenfeld HK. Tail-suspension induced hyperthermia: a new measure of stress reactivity. J Psychiatr Res. 2003;37:249–259. doi: 10.1016/s0022-3956(03)00004-9. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, et al. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: implications for depression and antidepressant action. Eur Neuropsychopharmacol. 2010;20:1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Watt MJ, Lowry CA, Forster GL. Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front Behav Neurosci. 2009;3:18. doi: 10.3389/neuro.08.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj J, Moryl E. Effects of sertraline and citalopram given repeatedly on the responsiveness of 5-HT receptor subpopulations. J Neural Transm Gen Sect. 1992;88:143–156. doi: 10.1007/BF01244819. [DOI] [PubMed] [Google Scholar]
- Malisch JL, Breuner CW, Kolb EM, Wada H, Hannon RM, Chappell MA, et al. Behavioral despair and home-cage activity in mice with chronically elevated baseline corticosterone concentrations. Behav Genet. 2009;39:192–201. doi: 10.1007/s10519-008-9246-8. [DOI] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- Marcus SM, Kerber KB, Rush AJ, Wisniewski SR, Nierenberg A, Balasubramani GK, et al. Sex differences in depression symptoms in treatment-seeking adults: confirmatory analyses from the Sequenced Treatment Alternatives to Relieve Depression Study. Compr Psychiatry. 2008;49:238–246. doi: 10.1016/j.comppsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt MW, Lucassen PJ, van Praag H. Comparison of neurogenic effects of fluoxetine, duloxetine and running in mice. Brain Res. 2010;1341:93–99. doi: 10.1016/j.brainres.2010.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J, White K, Weaver M, Flury Wetherill L, Hui S, Stout JC, et al. Specific psychiatric manifestations among preclinical Huntington disease mutation carriers. Arch Neurol. 2007;64:116–121. doi: 10.1001/archneur.64.1.116. [DOI] [PubMed] [Google Scholar]
- Mato S, Aso E, Castro E, Martin M, Valverde O, Maldonado R, et al. CB1 knockout mice display impaired functionality of 5-HT1A and 5-HT2A/C receptors. J Neurochem. 2007;103:2111–2120. doi: 10.1111/j.1471-4159.2007.04961.x. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Serotonin receptor binding in a colony model of chronic social stress. Biol Psychiatry. 1995;37:383–393. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Maes M. Effects of ipsapirone on plasma cortisol and body temperature in major depression. Biol Psychiatry. 1995;38:450–457. doi: 10.1016/0006-3223(94)00370-i. [DOI] [PubMed] [Google Scholar]
- Messa C, Colombo C, Moresco RM, Gobbo C, Galli L, Lucignani G, et al. 5-HT(2A) receptor binding is reduced in drug-naive and unchanged in SSRI-responder depressed patients compared to healthy controls: a PET study. Psychopharmacology (Berl) 2003;167:72–78. doi: 10.1007/s00213-002-1379-5. [DOI] [PubMed] [Google Scholar]
- Miller JM, Brennan KG, Ogden TR, Oquendo MA, Sullivan GM, Mann JJ, et al. Elevated serotonin 1A binding in remitted major depressive disorder: evidence for a trait biological abnormality. Neuropsychopharmacology. 2009;34:2275–2284. doi: 10.1038/npp.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, Sheline YI, Moerlein SM, Vlassenko AG, Huang Y, Snyder AZ. Decreased hippocampal 5-HT2A receptor binding in major depressive disorder: in vivo measurement with [18F]altanserin positron emission tomography. Biol Psychiatry. 2004;55:217–224. doi: 10.1016/j.biopsych.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Aoki M, Shimada Y, Inoue M, Nakaya K, Takahashi T, et al. Gender difference in association between polymorphism of serotonin transporter gene regulatory region and anxiety. J Psychosom Res. 2006;60:91–97. doi: 10.1016/j.jpsychores.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Mnie-Filali O, Lambas-Senas L, Zimmer L, Haddjeri N. 5-HT7 receptor antagonists as a new class of antidepressants. Drug News Perspect. 2007;20:613–618. doi: 10.1358/dnp.2007.20.10.1181354. [DOI] [PubMed] [Google Scholar]
- Navines R, Gomez-Gil E, Martin-Santos R, de Osaba MJ, Escolar G, Gasto C. Hormonal response to buspirone is not impaired in major depression. Hum Psychopharmacol. 2007;22:389–395. doi: 10.1002/hup.862. [DOI] [PubMed] [Google Scholar]
- Niven A, Rendell E, Chisholm L. Effects of 72-h of exercise abstinence on affect and body dissatisfaction in healthy female regular exercisers. J Sports Sci. 2008;26:1235–1242. doi: 10.1080/02640410802027378. [DOI] [PubMed] [Google Scholar]
- Overstreet DH. Behavioral characteristics of rat lines selected for differential hypothermic responses to cholinergic or serotonergic agonists. Behav Genet. 2002;32:335–348. doi: 10.1023/a:1020262205227. [DOI] [PubMed] [Google Scholar]
- Pang TY, Stam NC, Nithianantharajah J, Howard ML, Hannan AJ. Differential effects of voluntary physical exercise on behavioral and brain-derived neurotrophic factor expression deficits in Huntington's disease transgenic mice. Neuroscience. 2006;141:569–584. doi: 10.1016/j.neuroscience.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Pang TY, Du X, Zajac MS, Howard ML, Hannan AJ. Altered serotonin receptor expression is associated with depression-related behavior in the R6/1 transgenic mouse model of Huntington's disease. Hum Mol Genet. 2009;18:753–766. doi: 10.1093/hmg/ddn385. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Nehl C, Hoth KF, Kanz JE, Benjamin M, Conybeare R, et al. Depression and stages of Huntington's disease. J Neuropsychiatry Clin Neurosci. 2005;17:496–502. doi: 10.1176/jnp.17.4.496. [DOI] [PubMed] [Google Scholar]
- Peng Q, Masuda N, Jiang M, Li Q, Zhao M, Ross CA, et al. The antidepressant sertraline improves the phenotype, promotes neurogenesis and increases BDNF levels in the R6/2 Huntington's disease mouse model. Exp Neurol. 2008;210:154–163. doi: 10.1016/j.expneurol.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A, Wortwein G, Gruber SH, Mathe AA. Escitalopram reduces increased hippocampal cytogenesis in a genetic rat depression model. Neurosci Lett. 2008;436:305–308. doi: 10.1016/j.neulet.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl) 2005;177:245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Potter M, Yuan C, Ottenritter C, Mughal M, van Praag H. Exercise is not beneficial and may accelerate symptom onset in a mouse model of Huntington's disease. PLoS Curr. 2010;2:RRN1201. doi: 10.1371/currents.RRN1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouladi MA, Graham RK, Karasinska JM, Xie Y, Santos RD, Petersen A, et al. Prevention of depressive behaviour in the YAC128 mouse model of Huntington disease by mutation at residue 586 of huntingtin. Brain. 2009;132:919–932. doi: 10.1093/brain/awp006. [DOI] [PubMed] [Google Scholar]
- Reith ME, Fischette CT. Sertraline and cocaine-induced locomotion in mice. II. Chronic studies. Psychopharmacology (Berl) 1991;103:306–313. doi: 10.1007/BF02244283. [DOI] [PubMed] [Google Scholar]
- Renoir T, Paizanis E, Yacoubi ME, Saurini F, Hanoun N, Melfort M, et al. Differential long-term effects of MDMA on the serotoninergic system and hippocampal cell proliferation in 5-HTT knock-out vs. wild-type mice. Int J Neuropsychopharmacol. 2008;11:1149–1162. doi: 10.1017/S1461145708009048. [DOI] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel WJ, Klaassen T, Griez E, Honig A, Menheere PP, van Praag HM. Dissociable hormonal, cognitive and mood responses to neuroendocrine challenge: evidence for receptor-specific serotonergic dysregulation in depressed mood. Neuropsychopharmacology. 2002;26:358–367. doi: 10.1016/S0893-133X(01)00361-X. [DOI] [PubMed] [Google Scholar]
- Rioux A, Fabre V, Lesch KP, Moessner R, Murphy DL, Lanfumey L, et al. Adaptive changes of serotonin 5-HT2A receptors in mice lacking the serotonin transporter. Neurosci Lett. 1999;262:113–116. doi: 10.1016/s0304-3940(99)00049-x. [DOI] [PubMed] [Google Scholar]
- Rossi DV, Burke TF, McCasland M, Hensler JG. Serotonin-1A receptor function in the dorsal raphe nucleus following chronic administration of the selective serotonin reuptake inhibitor sertraline. J Neurochem. 2008;105:1091–1099. doi: 10.1111/j.1471-4159.2007.05201.x. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, et al. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Shapira B, Newman ME, Gelfin Y, Lerer B. Blunted temperature and cortisol responses to ipsapirone in major depression: lack of enhancement by electroconvulsive therapy. Psychoneuroendocrinology. 2000;25:421–438. doi: 10.1016/s0306-4530(99)00067-0. [DOI] [PubMed] [Google Scholar]
- Simpson JM, Gil-Mohapel J, Pouladi MA, Ghilan M, Xie Y, Hayden MR, et al. Altered adult hippocampal neurogenesis in the YAC128 transgenic mouse model of Huntington disease. Neurobiol Dis. 2011;41:249–260. doi: 10.1016/j.nbd.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Sjoberg RL, Nilsson KW, Nordquist N, Ohrvik J, Leppert J, Lindstrom L, et al. Development of depression: sex and the interaction between environment and a promoter polymorphism of the serotonin transporter gene. Int J Neuropsychopharmacol. 2006;9:443–449. doi: 10.1017/S1461145705005936. [DOI] [PubMed] [Google Scholar]
- Smith MA, Lyle MA. Chronic exercise decreases sensitivity to mu opioids in female rats: correlation with exercise output. Pharmacol Biochem Behav. 2006;85:12–22. doi: 10.1016/j.pbb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Solberg LC, Horton TH, Turek FW. Circadian rhythms and depression: effects of exercise in an animal model. Am J Physiol. 1999;276(1 Pt 2):R152–R161. doi: 10.1152/ajpregu.1999.276.1.R152. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge WJ, Deleger S, Roberts RE, Kaplan GA. Physical activity reduces the risk of subsequent depression for older adults. Am J Epidemiol. 2002;156:328–334. doi: 10.1093/aje/kwf047. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Ogden RT, Oquendo MA, Kumar JS, Simpson N, Huang YY, et al. Positron emission tomography quantification of serotonin-1A receptor binding in medication-free bipolar depression. Biol Psychiatry. 2009;66:223–230. doi: 10.1016/j.biopsych.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk B, Albert PR, Burns AM, Czesak M, Overholser JC, Jurjus GJ, et al. Gender-specific decrease in NUDR and 5-HT1A receptor proteins in the prefrontal cortex of subjects with major depressive disorder. Int J Neuropsychopharmacol. 2009;12:155–168. doi: 10.1017/S1461145708009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Llorens-Martin MV, Torres-Aleman I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci. 2008;37:402–411. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Yohrling IG, Jiang GC, DeJohn MM, Robertson DJ, Vrana KE, Cha JH. Inhibition of tryptophan hydroxylase activity and decreased 5-HT1A receptor binding in a mouse model of Huntington's disease. J Neurochem. 2002;82:1416–1423. doi: 10.1046/j.1471-4159.2002.01084.x. [DOI] [PubMed] [Google Scholar]
- Zajac MS, Pang TY, Wong N, Weinrich B, Leang LS, Craig JM, et al. Wheel running and environmental enrichment differentially modify exon-specific BDNF expression in the hippocampus of wild-type and pre-motor symptomatic male and female Huntington's disease mice. Hippocampus. 2010;20:621–636. doi: 10.1002/hipo.20658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


