Abstract
BACKGROUND AND PURPOSE
Deficient transmission at the glutamate NMDA receptor is considered a key component of the pathophysiology of schizophrenia. However, the effects of antipsychotic drugs on the release of the endogenous NMDA receptor partial agonist, d-serine, remain to be clarified.
EXPERIMENTAL APPROACH
We determined the interaction between antipsychotic drugs (clozapine and haloperidol) and transmission-modulating toxins (tetanus toxin, fluorocitrate, tetrodotoxin) on the release of L-glutamate and d-serine in the medial prefrontal cortex (mPFC) of freely moving rats, using microdialysis, and primary cultures of astrocytes using extreme high-pressure liquid chromatography.
KEY RESULTS
Release of L-glutamate and d-serine in the mPFC and in cultured astrocytes was inhibited by tetanus toxin (a synaptobrevin inhibitor) and fluorocitrate (a glial toxin), whereas tetrodotoxin (a voltage-sensitive Na+ blocker) inhibited depolarization-induced L-glutamate release in the mPFC without affecting that of d-serine. Clozapine (1 and 5 mg·kg−1), but not haloperidol (0.5 and 1 mg·kg−1), dose-dependently increased L-glutamate and d-serine release from both astrocytes and mPFC. Clozapine-induced release of L-glutamate and d-serine was also reduced by tetanus toxin and fluorocitrate. Tetrodotoxin reduced clozapine-induced mPFC L-glutamate release but not that of d-serine. Clozapine-induced L-glutamate release preceded clozapine-induced d-serine release. MK-801 (a NMDA receptor antagonist) inhibited the delayed clozapine-induced L-glutamate release without affecting that of d-serine.
CONCLUSIONS AND IMPLICATIONS
Clozapine predominantly activated glial exocytosis of d-serine, and this clozapine-induced d-serine release subsequently enhances neuronal L-glutamate release via NMDA receptor activation. The enhanced d-serine associated glial transmission seems a novel mechanism of action of clozapine but not haloperidol.
Keywords: antipsychotics, clozapine, haloperidol, microdialysis, L-glutamate, d-serine
Introduction
There are two major hypotheses on the pathophysiology of schizophrenia, dopaminergic dysfunction and glutamatergic hypofunction associated with the glutamate NMDA receptor (Javitt, 2007; Lieberman et al., 2008; Labrie and Roder, 2010; receptor nomenclature follows Alexander et al., 2011). The most compelling link between NMDA receptor function and schizophrenia is the ability of NMDA receptor antagonists, for example, phencyclidine and ketamine, to induce schizophrenia-like positive and negative symptoms in healthy volunteers (Krystal et al., 1994; Malhotra et al., 1996; Lieberman et al., 2008) and to exacerbate psychosis in schizophrenia patients (Malhotra et al., 1997). Moreover, NMDA antagonist-induced psychosis models exhibit certain features of schizophrenia, such as negative symptoms and cognitive deficits, more closely than the amphetamine/dopamine psychosis model (Krystal et al., 2003).
Electrophysiological studies have demonstrated that several atypical antipsychotics (clozapine, olanzapine, risperidone and quetiapine), but not haloperidol, preferentially enhance NMDA receptor-mediated transmission (Arvanov et al., 1997; Ninan et al., 2003; Kargieman et al., 2007). In contrast to these electrophysiological studies, in vivo microdialysis studies have demonstrated that zotepine and quetiapine increase extracellular glutamate levels in the medial prefrontal cortex (mPFC), whereas neither haloperidol, risperidone nor blonanserin have such an effect (Abekawa et al., 2007; Yamamura et al., 2009a,b; Ohoyama et al., 2011). Indeed, several clinical studies demonstrated that NMDA receptor partial agonists, d-cycloserine and d-serine, significantly improve primary negative symptoms of schizophrenia when used as adjuvants to conventional typical and atypical antipsychotics (Evins et al., 2002; Heresco-Levy et al., 2005; Shim et al., 2008; Tsai and Lin, 2010). In contrast, such effects are not observed when d-cycloserine or d-serine are used as adjuvants to clozapine (Goff et al., 1999; Tsai and Lin, 2010). These results suggest that clozapine, in comparison with other antipsychotics, could have different effects on glutamate-mediated transmission in vivo. Based on these preclinical and clinical studies, we hypothesized that the mechanism of action of clozapine included enhancement of transmission mediated through d-serine.
L-glutamate and d-serine are chemical transmitters that mediate astrocyte-neurone signalling (Parpura and Zorec, 2009). d-serine has been identified as a major glial transmitter in the CNS and serves as an endogenous ligand for the glycine site of NMDA receptors (Parpura and Zorec, 2009). Indeed, in vitro experiments using cultured cortical astrocytes showed Ca2+-dependent and soluble N-ethylmaleimide sensitive factor attachment protein receptors (SNARE)-associated release of d-serine (Mothet et al., 2005). However, recent studies demonstrated that d-serine and serine racemases were present in neurones as well as in astrocytes (Kartvelishvily et al., 2006), though the serine racemase, which synthesizes d-serine, is mostly neuronal (Kartvelishvily et al., 2006; Miya et al., 2008). Furthermore, an early study using microdialysis failed to demonstrate any depolarization-induced d-serine release in the mPFC (Hashimoto et al., 1995). Therefore, the mechanisms underlying release of d-serine remain to be clarified.
In the present study, we studied the release mechanisms of L-glutamate and d-serine in the mPFC using in vivo microdialysis and from astrocytes, using primary cultures of these cells. Furthermore, to test our hypothesis, whether clozapine does enhance D-serine release or not, the effects of clozapine and haloperidol on L-glutamate and d-serine release in primary cultured astrocytes and in the mPFC of freely moving rats were assessed using microdialysis with extreme high-pressure liquid-chromatography (xLC).
Methods
Experimental animals
All animal care and experimental procedures were in accordance with the Ethical Guidelines established by Institutional Animal Care and Use Committee at Mie University. Male Sprague-Dawley rats (SLC, Shizuoka, Japan) were housed under conditions of constant temperature at 22 ± 2°C with 12–12 h light-dark cycle.
Primary astrocyte culture
Cortical astrocyte cultures were prepared from neonatal Sprague-Dawley rats (n = 8) killed by decapitation at 0–24 h of age and the cerebral hemispheres were removed under the dissecting microscope. Cortices were placed into a dish containing 10 mL Dulbecco's modified Eagle's medium (DMEM, Sigma) at 4°C. Tissue was chopped until very fine using scissors and then triturated briefly with a 1 mL micropipette. The suspension was filtered using 70 µm nylon mesh (BD, Franklin Lakes, NJ, USA) and centrifuged at 100×g for 3 min at 4°C. The pellet was resuspended in 10 mL DMEM supplemented with 10% fetal calf serum (Invitrogen, Carlsbad, CA, USA). The suspension was centrifuged at 100×g for 3 min at 4°C and the pellet was resuspended in 12 mL DMEM with 10% fetal calf serum again. The resuspension step was repeated three times. The cortical suspensions were plated into a number of 75 cm3 tissue culture flasks equivalent to the number of animals used. Flasks were kept at 37°C in a CO2 incubator (95% air and 5% CO2). The medium was replaced with fresh culture medium (DMEM containing 10% fetal calf serum) after 48 h and subsequently changed every 6 days. After 14 days in vitro (DIV14), contaminating cells were removed by shaking in a standard incubator for 16 h at 200 r.p.m. The medium was removed and discarded, and the flask contents were harvested and reseeded to yield double the original number of flasks. To study the effects of synaptobrevin on glial release of L-glutamate and d-serine, on DIV20, astrocytes were incubated in fresh culture medium containing tetanus toxin (3 µg·mL−1) for 24 h (pre-incubation with tetanus toxin). This pre-incubation step was omitted in other experiments (Murakami et al., 2001; Okada et al., 2001; Mothet et al., 2005). Astrocytes were removed from flasks by trypsinization and seeded onto translucent PET membrane (8 µm) 24-well plates (BD) at a density of 105 cells·cm−2 for experimentation, on DIV21.
The astrocytes were washed three times in artificial cerebrospinal fluid (ACSF) containing, in mM: NaCl 130, KCl 5.4, CaCl2 1.8, MgCl2 1, and glucose 5.5, and buffered with 20 mM HEPES buffer to pH 7.3, and were then incubated in ACSF buffered with 100% O2 for 30 min recovery time at 35°C (washing with ACSF). To determine the glial transmitter release, astrocytes were incubated in 0.1 mL ASCF containing with or without the following agents: 1 µM tetrodotoxin (TTX), 1 mM fluorocitrate, 2–200 nM clozapine or 1–100 nM haloperidol for 60 min (pre-incubation). After the pre-incubation, the transmitter release studies were carried out in quadruplicate at 35°C using the discontinuous method (Zhu et al., 2006). To determine the depolarization-induced glial transmitter release, astrocytes were incubated in 0.1 mL ASCF containing 100 mM K+ (HKACSF). The released transmitter level in each sample (10 min·fraction−1) was measured using xLC techniques (see below). The detailed study designs are described in the Results section.
Preparation of microdialysis system
Male Sprague-Dawley rats, weighing 250–300 g (n = 90), were placed in a stereotaxic frame and kept under 1.8% isoflurane anaesthesia. Before inserting the microdialysis probe, all rats used in this study were pretreated with a microinjection of 0.3 µL modified Ringer's solution (MRS; composition see below) with or without 1 ng tetanus toxin (Okada et al., 2001; 2005). The concentric I-shaped direct insertion type dialysis probes (D-I-7-03: 0.2 mm diameter, 2 mm exposed membrane; Eicom, Kyoto, Japan) were implanted in the mPFC (A =+3.2 mm, L =−0.8 mm, V =−6.0 mm relative to bregma) (Yamamura et al., 2009b; 2011; Ohoyama et al., 2011).
The perfusion experiments commenced 24 h after recovery from isoflurane anaesthesia (Okada et al., 2001). The perfusion rate was set at 1 µL·min−1, using MRS (composition (in mM): 145 Na+, 2.7 K+, 1.2 Ca2+, 1.0 Mg2+ and 154.4 Cl-, and buffered with 2 mM phosphate buffer and 1.1 mM Tris buffer to pH 7.4) (Okada et al., 2001; Yamamura et al., 2009b).
Initially, the perfusate was MRS with or without 1 mM fluorocitrate, and the extracellular neurotransmitter levels were measured at least 8 h after starting perfusion (the microdialysis samples were collected in 20 min intervals). The coefficients of variation for extracellular levels of each neurotransmitter became less than 5% over a period of 60 min (stabilization) and control data were then obtained over another 60 min. To study the sensitivity of L-glutamate and d-serine to TTX, the perfusate was switched to MRS containing 1 µM TTX (Okada et al., 2001; 2004). After stabilization, the perfusate was switched to MRS containing 100 mM K+ (HKMRS) with the same agents to study sensitivity to K+. The ionic compositions of these perfusates were modified and isotonicity was maintained by an equimolar change of Na+ (Okada et al., 2001; 2004). To study the effects of antipsychotic drugs, the rats were given clozapine (1 or 5 mg·kg−1, i.p.) (Kapur et al., 2003) or haloperidol (0.5 or 1 mg·kg−1, i.p.) (Yamamura et al., 2009b). According to Kapur and colleagues, the dose of clozapine that approximates the clinically comparable dopamine D2 receptor occupancy is 5–15 mg·kg−1 in a single-dose model (Kapur et al., 2003). Each dialysate was injected into the xLC apparatus. The detailed study designs are described in the Results section.
Extreme high–pressure liquid chromatography (xLC)
The dialysis sample and incubated MRS were filtered with 0.2 µm syringe filters (Millex, Millipore, Billerica, MA, USA) before analysis. The levels of L-glutamate and d-serine in dialyzed and incubated MRS were determined by xLC (dual xLC 3185PU, Jasco, Tokyo, Japan) with fluorescence detection (xLC3120FP, Jasco) after derivatization with isobutyryl-L-cysteine and o-phthalaldehyde. Derivatizing reagent solutions were prepared by dissolving isobutyryl-L-cysteine (2 mg) and o-phthalaldehyde (1 mg) in 0.1 mL ethanol followed by the addition of 0.9 mL 0.2 M sodium borate buffer (pH 9.0). The reagent solutions were filtered with 0.2 µm syringe filters and prepared freshly every second day and stored at 4°C when not in use. Automated pre-column derivatization was carried out by drawing up a 5 µL aliquot of sample, standard or blank solution and 5 µL of derivatizing reagent solution, and holding in the reaction vials for 5 min before injection (xLC3059AS, Jasco). Derivatized samples (5µL) were injected by auto sampler (xLC3059AS, Jasco).
The analytical column (Inertsil ODS-4, particle 2 µm, 50 × 2.1 mm, GL Science, Tokyo) was maintained at 45°C and the flow rate of the mobile phase was set at 500 µL·min−1. A linear gradient elution program was performed over 10 min with mobile phase A (0.05 M citrate buffer, pH 5.0) and B (0.05 M citrate buffer containing 30% acetonitrile and 30% methanol, pH 3.5). Representative chromatograms of amino acid analysis using xLC with a fluorescence detector (excitation and emission wavelengths were 345 and 455 nm respectively) are presented in Figure 1.
Figure 1.
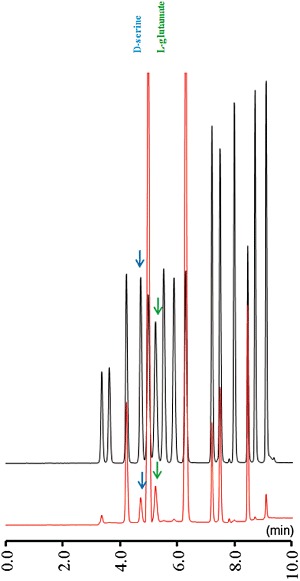
Typical chromatograms of amino acid analysis using extreme high-pressure liquid chromatography equipped with a fluorescence detector. The chromatograms were obtained from 5 µL of a standard solution containing 50 pmol·5 µL−1 of L-glutamate and d-serine (black line) and prefrontal perfusate (red line). The quantification limits for L-glutamate and d-serine were 10 and 25 fmol·5 µL−1 respectively.
Statistical analysis
Values were expressed as mean ± SEM. The effects of tetanus toxin, TTX and fluorocitrate on the release of L-glutamate and d-serine in the mPFC were compared using repeated measurements anova with Tukey's multiple comparison. The effects of tetanus toxin, TTX and fluorocitrate on area under curve values of basal and K+-evoked release of L-glutamate and d-serine in the mPFC were compared using the Student's t-test. The effects of tetanus toxin, TTX and fluorocitrate on K+-evoked release of L-glutamate and d-serine in the mPFC were compared using multivariate anova (manova) with Tukey's multiple comparisons. The effects of tetanus toxin, TTX and fluorocitrate on the release of L-glutamate and d-serine from primary cultured astrocytes were also compared using the Student's t-test. The concentration-dependent effects of AMPA and NMDA on release of L-glutamate and d-serine from primary cultured astrocytes were compared by one-way anova with Tukey's multiple comparisons.
The dose-dependent effects of systemic administration of haloperidol and clozapine on the extracellular levels of L-glutamate and d-serine were analysed by manova with Tukey's multiple comparisons. The effects of tetanus toxin, TTX and fluorocitrate on antipsychotic-induced release of L-glutamate and d-serine in the mPFC were compared using two-way anova or manova with Tukey's multiple comparisons. The effects of perfusion with MK-801 on clozapine-induced release of L-glutamate and d-serine in the mPFC were compared using manova with Tukey's multiple comparisons. A P value less than 0.05 was considered statistically significant.
Materials
The atypical antipsychotic drugs, clozapine and haloperidol; the synaptobrevin inhibitor tetanus toxin; the glial toxin fluorocitrate; NMDA and the NMDA receptor inhibitor dizocilpine (MK-801) were all purchased from Sigma (St. Louis, MO, USA). The voltage-sensitive Na+ channel inhibitor tetrodotoxin (TTX) and [2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid] (AMPA) were obtained from Wako Chemicals (Osaka, Japan). Clozapine, haloperidol and MK-801 were dissolved in MRS or ACSF containing less than 0.1% vol·vol−1 acetic acid. The pH of the final concentrations was adjusted to 7.0 with phosphate buffer. Tetanus toxin, fluorocitrate, TTX and AMPA were diluted directly in MRS or ACSF.
Results
Cultured astrocytes
Effects of TTX, fluorocitrate and tetanus toxin on release of L-glutamate and d-serine from primary cultures of astrocytes. To study the synaptobrevin-associated glial release of L-glutamate and d-serine, on DIV20, astrocytes were incubated in fresh culture medium containing tetanus toxin (3 µg·mL−1) for 24 h (pre-incubation with tetanus toxin). On DIV21, after washing with ACSF, the L-glutamate and d-serine released from astrocytes was determined. During sample collection, astrocytes were incubated in ACSF. To study the effects of fluorocitrate and TTX on glial transmitter release, on DIV21, after washing with ACSF, astrocytes were incubated in ACSF containing 1 mM fluorocitrate (pre-incubation with fluorocitrate) or 1 µM TTX (pre-incubation with TTX) for 60 min before sample collection (Ninan et al., 2003; Martin et al., 2007). During sample collection, astrocytes were incubated in ACSF containing 1 mM fluorocitrate or 1 µM TTX.
Tetanus toxin (3 µg·mL−1) and fluorocitrate (1 mM), but not TTX (1 µM), significantly reduced L-glutamate release from primary cultured astrocytes (P < 0.01, Figure 2A). Treatment with tetanus toxin (P < 0.05) and fluorocitrate (P < 0.01), but not with TTX, also reduced d-serine release from primary cultured astrocytes (Figure 2B).
Figure 2.
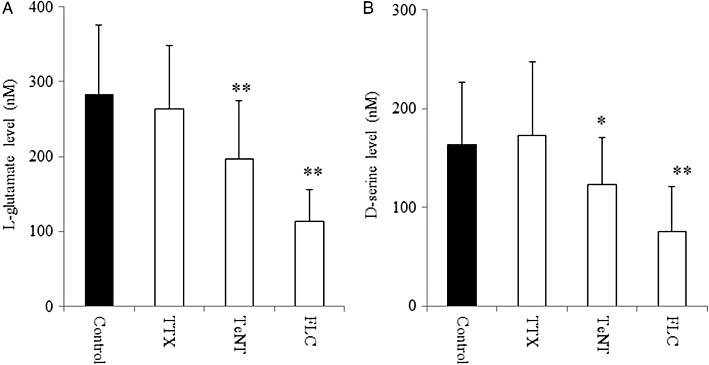
Effects of TTX, tetanus toxin and fluorocitrate on basal release of L-glutamate and d-serine of primary cultured astrocytes. Effects of tetanus toxin (TeNT; 3 µg·mL−1), TTX (1 µM) and fluorocitrate (FLC; 1 mM) on basal release of (A) L-glutamate and (B) d-serine from primary cultured astrocytes. Data are mean ± SEM (n = 6). *P < 0.05, **P < 0.01 significantly different from control, by Student's t-test.
Effects of TTX, fluorocitrate and tetanus toxin on stimulation-induced release of L-glutamate and d-serine from primary cultured astrocytes. To study the K+-evoked release of L-glutamate and d-serine from glia, after the pre-incubation with ACSF, astrocytes were incubated in 100 mM K+ containing ACSF (HKACSF). The K+-evoked stimulation did not affect the release of L-glutamate and d-serine from primary cultured astrocytes (data not shown).
To study the effects of AMPA and NMDA on glial transmitter release, astrocytes were incubated in ACSF containing AMPA (10–300 µM) or NMDA (10–300 µM), after washing with ACSF. AMPA increased the release of L-glutamate [one-way anova: FDose(4,25) = 2.9 (P < 0.05)] and d-serine [anova: FDose(4,25) = 3.9 (P < 0.05)] from primary cultures of astrocytes (Figure 3A,B). Neither release of L-glutamate nor that of D-serine from primary cultured astrocytes was affected by NMDA (10-300 µM) (Figure 3A and B).
Figure 3.
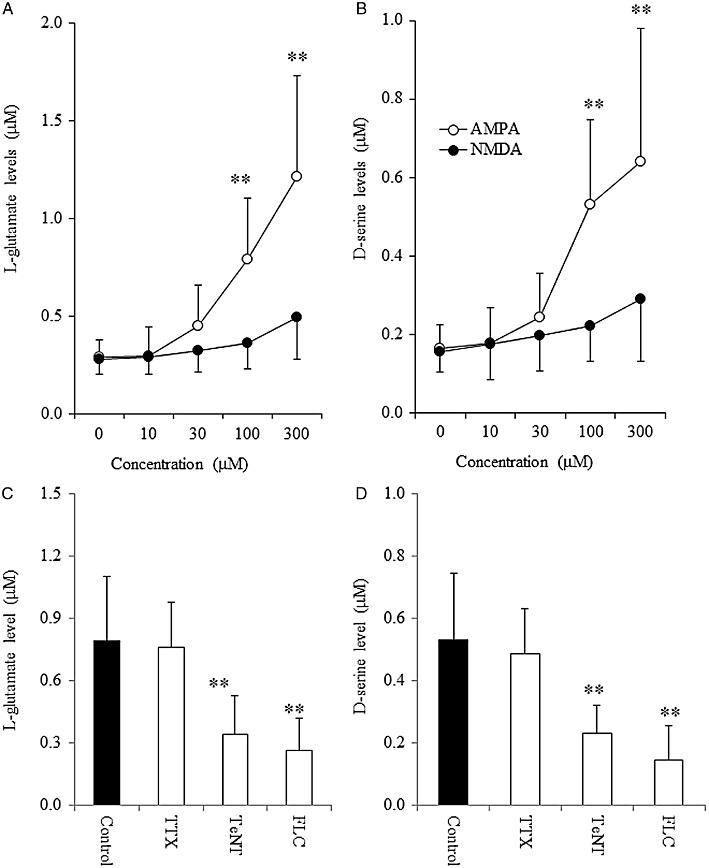
Effects of AMPA, NMDA, TTX, tetanus toxin and fluorocitrate on L-glutamate and d-serine release from primary cultured astrocytes. Concentration-dependent effects of AMPA (10–300 µM) and NMDA (10–300 µM) on release of (A) L-glutamate and (B) d-serine from primary cultured astrocytes. Data are mean ± SEM (n = 6). **P < 0.01 versus control, by one-way anova with Tukey's multiple comparisons. Effects of tetanus toxin (TeNT; 3 µg·mL−1), TTX (1 µM) and fluorocitrate (FLC; 1 mM) on 100 µM AMPA-evoked release of (C) L-glutamate and (D) d-serine from primary cultured astrocytes. Data are mean ± SEM (n = 6). **P < 0.01 significantly different from control, by Student's t-test.
To study the effects of tetanus toxin on AMPA-induced glial release of L-glutamate and d-serine, after the pre-incubation with tetanus toxin, washout with ACSF and pre-incubation with ACSF, astrocytes were incubated in ASCF containing 100 µM AMPA. To study the effects of fluorocitrate and TTX on AMPA-induced glial release of L-glutamate and d-serine, after the pre-incubation with fluorocitrate or TTX, astrocytes were incubated in ASCF containing 1 mM fluorocitrate or 1 µM TTX with 100 µM AMPA. AMPA-induced release of L-glutamate and d-serine was reduced by tetanus toxin (3 µg·mL−1) and fluorocitrate (1 mM) (P < 0.01), but not affected by TTX (1 µM) (Figure 3C and D).
Effects of tetanus toxin and fluorocitrate on clozapine-induced release of L-glutamate and d-serine from primary cultured astrocytes. To study the effects of clozapine and haloperidol on glial transmitter release, after the pre-incubation with ACSF, astrocytes were incubated in ACSF containing clozapine (2–200 nM) or haloperidol (1–100 nM) (Ninan et al., 2003). Clozapine increased the release of L-glutamate [repeated anova: F(3,15) = 88.6, P < 0.01] and d-serine [repeated anova: F(3,15) = 56.8, P < 0.01] in a concentration-dependent manner, whereas haloperidol did not affect release of L-glutamate or d-serine (Figure 4). At low concentration (2 nM), clozapine had no effect on the release of L-glutamate or d-serine, whereas the release of both was increased at higher concentrations (20 nM, P < 0.05 and 200 nM, P < 0.01) (Figure 4).
Figure 4.
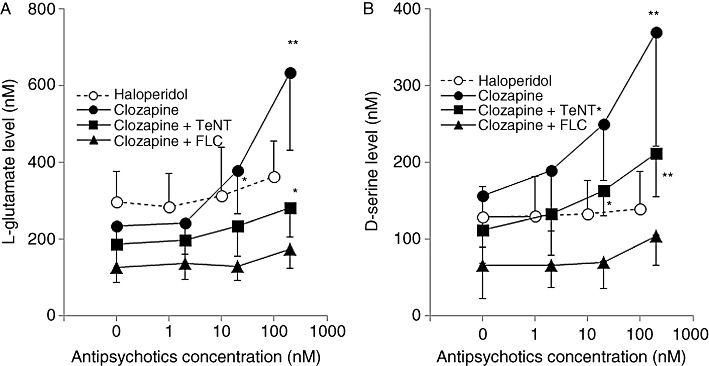
Interaction between antipsychotic drugs and transmission-modulating agents on releases of L-glutamate and d-serine of primary cultured astrocytes. (A) Concentration-dependent effects of clozapine (0, 2, 20 and 200 nM) and haloperidol (0, 1, 10 and 100 nM) on L-glutamate release, and the effects of tetanus toxin (TeNT; 3 µg·mL−1) and fluorocitrate (FLC; 1 mM) on dose-dependent clozapine-induced L-glutamate release from primary cultured astrocytes. (B) Concentration-dependent effects of clozapine and haloperidol on D-serine release and effects of tetanus toxin and fluorocitrate on dose-dependent clozapine-induced d-serine release from primary cultured astrocytes. Data are mean ± SEM (n = 6). P < 0.05, **P < 0.01 significantly different from control: 0 nM, by repeated measurement anova with Tukey's multiple comparisons.
To study the effects of tetanus toxin on clozapine-induced glial release of L-glutamate and d-serine, after the pre-incubation with tetanus toxin, astrocytes were incubated in ASCF containing 2–200 nM clozapine. After the cleavage of synaptobrevin by tetanus toxin (3 µg·mL−1), clozapine, at the highest concentration (200nM), increased the release of L-glutamate [repeated anova: F(3,15) = 36.2, P < 0.01] and d-serine [repeated anova: F(3,15) = 78.5, P < 0.01] but neither 2 nM nor 20 nM clozapine was effective (Figure 4).
To study the effects of fluorocitrate and TTX on clozapine-induced glial release of L-glutamate and d-serine, after the pre-incubation with fluorocitrate or TTX, astrocytes were incubated in ASCF containing 1 mM fluorocitrate or 1 µM TTX with 2–200 nM clozapine. Incubation with 1 mM fluorocitrate abolished the clozapine-induced release of L-glutamate and d-serine (Figure 4). TTX (1 µM) did not affect the stimulatory effects of clozapine on L-glutamate or d-serine release (data not shown).
In vivo microdialysis
Effects of TTX, fluorocitrate and tetanus toxin on basal and K+-evoked release of L-glutamate and d-serine in the mPFC. The basal extracellular levels of L-glutamate and d-serine in the mPFC were 2.15 ± 0.34 and 1.28 ± 0.23 µM, respectively (not corrected for in vitro dialysis probe recovery).
To study the synaptobrevin-associated release of L-glutamate and d-serine in the mPFC, 24 h after microinjection of tetanus toxin (1 ng·0.3 µL−1), the perfusion experiment was started. The extracellular neurotransmitter levels were measured at least 8 h after starting perfusion. Microinjection of tetanus toxin (1 ng·0.3 µL−1) did not affect the basal extracellular levels of L-glutamate or d-serine in the mPFC (Figure 5A,B).
Figure 5.
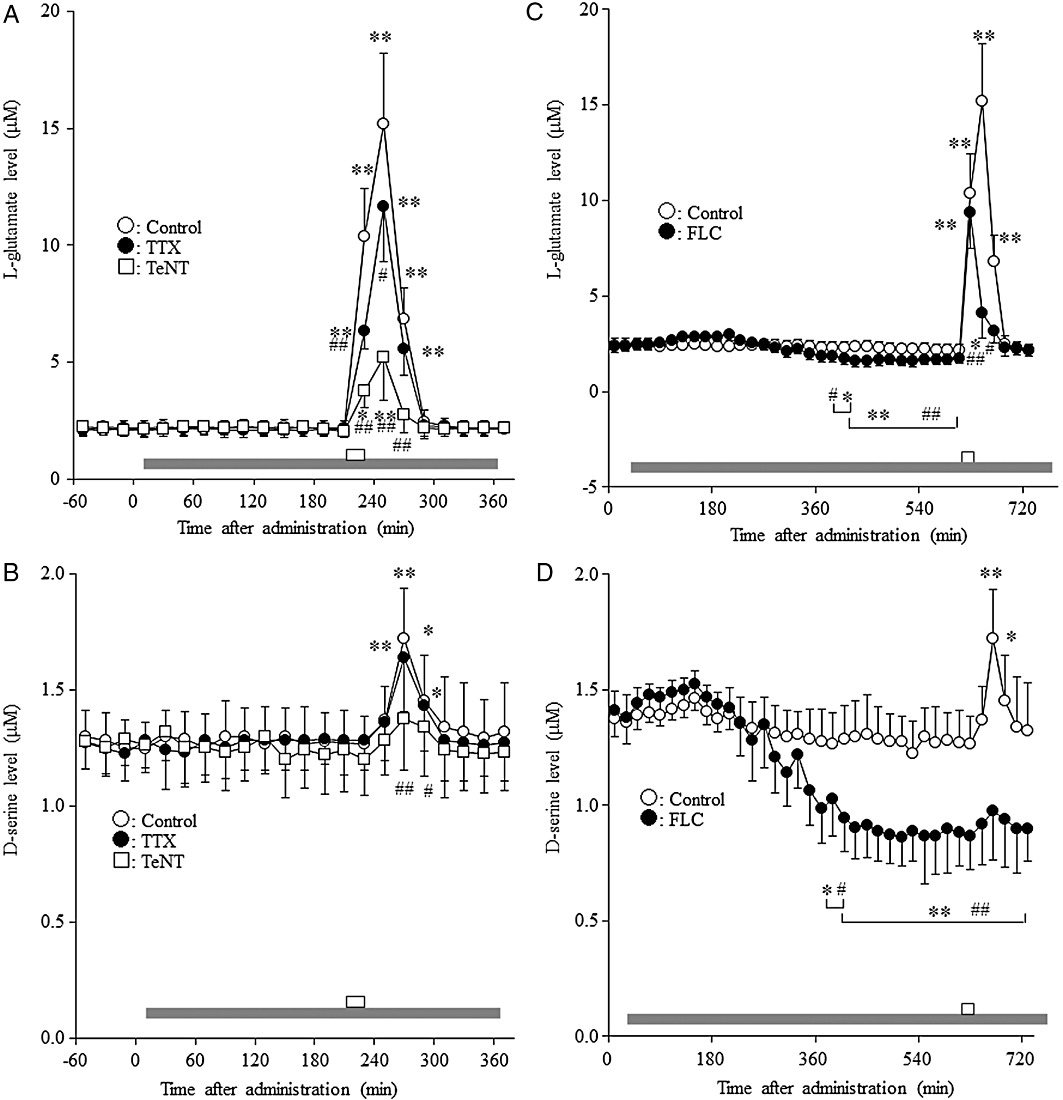
Effects of TTX, fluorocitrate and tetanus toxin on basal and K+-evoked release of L-glutamate and d-serine in the mPFC. Effects of perfusion with 1 µM TTX and microinjection of 1 ng·0.3 µL−1 tetanus toxin (TeNT) on the basal and K+-evoked release of (A) L-glutamate and (B) d-serine in the mPFC. Effects of perfusion with 1 mM fluorocitrate (FLC) on the basal and K+-evoked release of (C) L-glutamate and (D) d-serine in the mPFC. Grey horizontal bars: perfusion with MRS containing 1 µM TTX or 1 mM fluorocitrate; open horizontal bar: perfusion with K+, 100 mM (HKMRS) for 20 min. Data are means ± SEM (n = 6) of extracellular levels. The effects of microinjection of tetanus toxin and perfusion with TTX and fluorocitrate were compared using manova with Tukey's multiple comparisons (*P < 0.05; **P < 0.01 significantly different from pre-perfusion period and #P < 0.05, ##P < 0.01 significantly different from control).
To study the effects of TTX and fluorocitrate on basal release of L-glutamate and d-serine, after the confirming of stabilization, the perfusion medium was switched from MRS to MRS containing 1 mM fluorocitrate or 1 µM TTX. Perfusion with 1 µM TTX did not affect the basal extracellular levels of L-glutamate or d-serine in the mPFC (Figure 5A,B). Perfusion with 1 mM fluorocitrate reduced the frontal extracellular levels of L-glutamate [repeated anova: F(26,130) = 311.9, P < 0.01] and d-serine [repeated anova: F(26,130) = 88.4, P < 0.01] (Figure 5C,D). However, the inhibitory effects of fluorocitrate on the release of L-glutamate and d-serine reached at steady state only after 6 h of fluorocitrate perfusion (Figure 5C,D).
After these measurements, the effects of TTX and fluorocitrate on depolarization-induced release of L-glutamate and d-serine were studied. The perfusion medium was switched from MRS containing 1 mM fluorocitrate or 1 µM TTX to HKMRS containing the same agent. The 100 mM K+-evoked stimulation (perfusion with HKMRS for 20 min) increased the extracellular level of L-glutamate in the mPFC [repeated anova: F(6,30) = 39.5, P < 0.01] (Figure 5A,C). The magnitude of K+-evoked L-glutamate reached a peak level at 40 min after the start of K+-evoked stimulation (Figure 5A). Perfusion with 1 µM TTX [manova: FTTX(1,10) = 5.5 (P < 0.05), FTime(6,60) = 270.0 (P < 0.01), FDose × Time(6,60) = 11.0 (P < 0.01)] or 1 mM fluorocitrate [manova: Ffluorocitrate(1,10) = 18.6 (P < 0.01), FTime(6,60) = 201.7 (P < 0.01), FDose × Time(6,60) = 64.5 (P < 0.01)] reduced K+-evoked L-glutamate release (Figure 5A,C). Perfusion with fluorocitrate did not affect the K+-evoked L-glutamate release at 20 min after the start of K+-evoked stimulation, but reduced it in the following periods (at 40 and 60 min) (Figure 5C). The extracellular levels of d-serine [repeated anova: F(6,30) = 18.6, P < 0.01] were also increased by HKMRS perfusion (Figure 5B,D). The magnitude of K+-evoked d-serine reached a peak level at 60 min after the start of K+-evoked stimulation (Figure 5B,D). Perfusion with fluorocitrate inhibited the K+-evoked d-serine release [manova: Ffluorocitrate (1,10) = 23.0 (P < 0.01), FTime(6,60) = 83.7 (P < 0.01), FDose × Time(6,60) = 33.7 (P < 0.01)], whereas perfusion with TTX did not affect this release (Figure 5B,D).
To study the effects of tetanus toxin on depolarization-induced release of L-glutamate and d-serine, the perfusion medium was switched from MRS to HKMRS. Microinjection of tetanus toxin (1 ng·0.3 µL−1) reduced K+-evoked release of L-glutamate [manova: Ftetanus toxin(1,10) = 32.5 (P < 0.01), FTime(6,60) = 166.3 (P < 0.01), FDose × Time(6,60) = 65.6 (P < 0.01)] and d-serine [manova: Ftetanus toxin (1,10) = 1.3 (P < 0.01), FTime(6,60) = 110.1 (P < 0.01), FDose × Time(6, 60) = 24.2 (P < 0.01)] (Figure 5A,B).
Effects of systemic administration of haloperidol and clozapine on extracellular levels of L-glutamate and d-serine in the mPFC. To study the effects of systemic administration of haloperidol and clozapine on release of L-glutamate and d-serine, after stabilization, the rats were given clozapine (1 or 5 mg·kg−1, i.p.) (Kapur et al., 2003) or haloperidol (0.5 or1 mg·kg−1, i.p.) (Yamamura et al., 2009b). Systemic administration of clozapine dose-dependently increased the extracellular levels of L-glutamate [manova: FDose(2,15) = 7.9 (P < 0.01), FTime(9,135) = 259.8 (P < 0.01), FDose × Time(18,135) = 152.9 (P < 0.01)] and d-serine [manova: FDose(2,15) = 7.7 (P < 0.01), FTime(9,135) = 81.2 (P < 0.01), FDose × Time(18,135) = 60.1 (P < 0.01)]; however, neither systemic administration of 0.5 mg·kg−1 (data not shown) nor 1 mg·kg−1 of haloperidol affected these levels (Figure 6A,B). Clozapine (5 mg·kg−1 i.p.) induced a rise in extracellular d-serine levels earlier (60 min after clozapine injection) than that of L-glutamate (140 min after clozapine).
Figure 6.
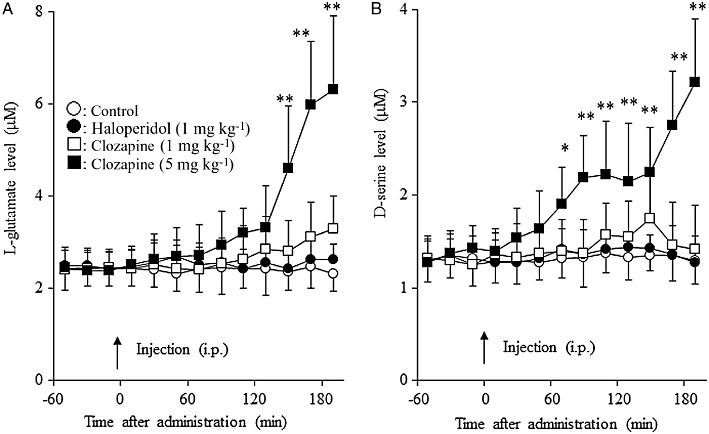
Effects of clozapine and haloperidol on extracellular levels of L-glutamate and d-serine in the mPFC. Effects of systemic administration of clozapine (1 or 5 mg·kg−1, i.p.) and haloperidol (1 mg·kg−1, i.p.) on the extracellular levels of (A) L-glutamate and (B) d-serine in the mPFC. After stabilization of extracellular levels of L-glutamate and d-serine (pretreatment), clozapine (1 mg·kg−1, 5 mg·kg−1) or haloperidol (1 mg·kg−1) was injected i.p. *P < 0.05, **P < 0.01 significantly different from pretreatment, by manova with Tukey's multiple comparison.
Effects of TTX, fluorocitrate and tetanus toxin on clozapine-induced release of L-glutamate and d-serine in the mPFC. To study the effects of TTX and fluorocitrate on release of L-glutamate and d-serine induced by antipsychotic drugs, perfusion was commenced with MRS containing 1 µM TTX or 1 mM fluorocitrate. After stabilization, the rats were given clozapine (5 mg·kg−1, i.p.) or haloperidol (1 mg·kg−1, i.p.). To study the effects of tetanus toxin on antipsychotics-induced release of L-glutamate and d-serine, 24 h after the microinjection of tetanus toxin (1 ng·0.3 µL−1), the rats were given clozapine (5 mg·kg−1, i.p.) or haloperidol (1 mg·kg−1, i.p.).
Haloperidol or clozapine interacted with transmission-modulating toxins (TTX, tetanus toxin or fluorocitrate) on L-glutamate release [two-way anova: FAntipsychotic(2,75) = 74.8 (P < 0.01), FToxin(4,75) = 25.7 (P < 0.01), FAntipsychotic × Toxin(8,75) = 10.6 (P < 0.01)]. Perfusion with 1 µM TTX (P < 0.05), microinjection of tetanus toxin (1 ng 0.3·µL−1) (P < 0.01) and perfusion with 1 mM fluorocitrate (P < 0.01) reduced the stimulatory effect of clozapine on extracellular L-glutamate levels (Figure 7A,C). Furthermore, haloperidol or clozapine also interacted with transmission-modulating toxins on d-serine release [two-way anova: FAntipsychotic(2,75) = 79.35 (P < 0.01), FToxin(4,75) = 22.3 (P < 0.01), FAntipsychotic × Toxin(8,75) = 7.9 (P < 0.01)]. Tetanus toxin (P < 0.01) and fluorocitrate (P < 0.01), but not TTX, reduced the stimulatory effects of clozapine on extracellular d-serine level (Figure 7B,D).
Figure 7.
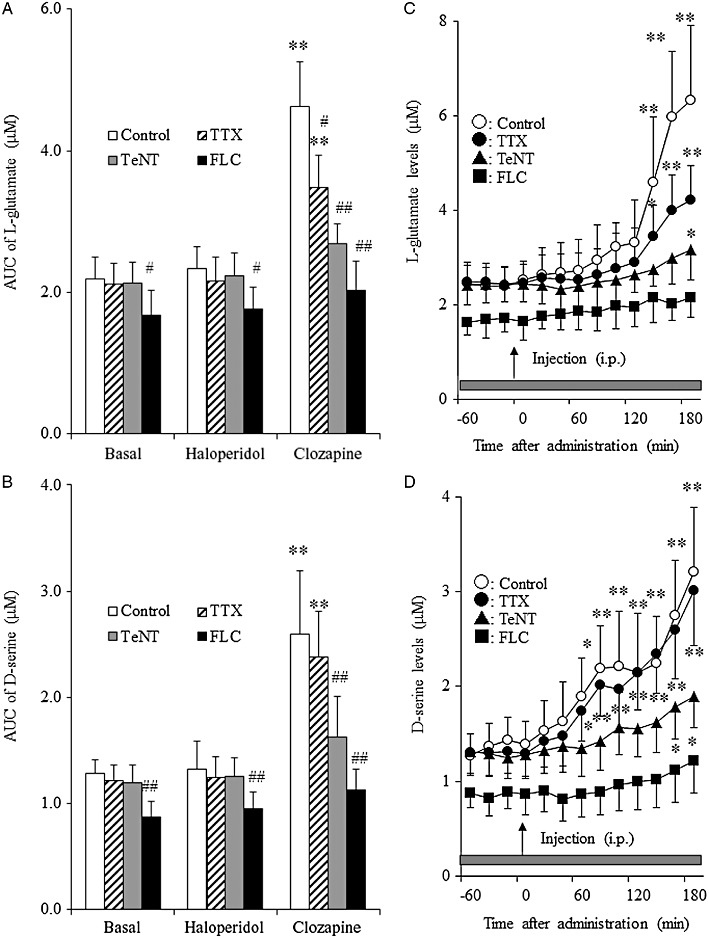
Interaction between antipsychotic drugs and transmission-modulating agents on extracellular levels of L-glutamate and d-serine in the mPFC. Effects of microinjection of 1 ng·0.3 µL−1 tetanus toxin (TeNT), perfusion with 1 µM TTX or 1 mM fluorocitrate (FLC) on antipsychotic (5 mg·kg−1 clozapine and 1 mg·kg−1 haloperidol)-induced release of (A) L-glutamate and (B) d-serine in the mPFC. Data are mean ± SEM (n = 6). *P < 0.05, **P < 0.01 versus control, by two-way anova with Tukey's multiple comparisons. Effects of microinjection of 1 ng·0.3 µL−1 tetanus toxin, perfusion with 1 µM TTX or 1 mM fluorocitrate on 5 mg·kg−1 clozapine-induced release of (C) L-glutamate and (D) d-serine in the mPFC. Data are mean ± SEM (n = 6). *P < 0.05, **P < 0.01 significantly different from pretreatment period, by manova with Tukey's multiple comparison. AUC, area under curve.
Effects of MK-801 on clozapine-induced release of L-glutamate and d-serine in the mPFC. In these experiments, the perfusion medium was commenced with MRS containing 50 µM MK-801. After stabilization, the rats were given clozapine (5 mg·kg−1, i.p.). The interaction between systemic administration of clozapine and perfusion with 50 µM MK-801 on the extracellular L-glutamate levels in the mPFC [manova; FMK-801(1,10) = 2.7, P > 0.1; FTime(9,90) = 113.1, P < 0.01; FMK-801 × Time(9,90) = 22.7, P < 0.01] is shown in Figure 8A. Antagonism of the NMDA receptor reduced clozapine-induced L-glutamate release (P < 0.01). In contrast, the NMDA antagonist did not affect clozapine-induced increases of the extracellular d-serine levels in the mPFC [manova; FMK-801(1, 10) = 0.3, P > 0.1; FTime(9, 90) = 174.1, P < 0.01; FMK-801 × Time(9, 90) = 1.6, P > 0.1] (Figure 8B).
Figure 8.
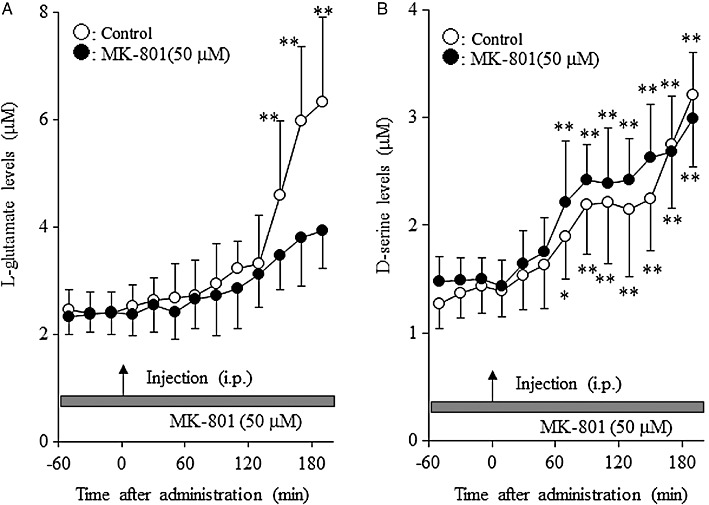
Effects of MK-801 on clozapine-induced release of L-glutamate and d-serine in the mPFC. Effects of perfusion of MK-801 (50 µM) on 5 mg·kg−1 clozapine-induced release of (A) L-glutamate and (B) d-serine in the mPFC. Data are mean ± SEM (n = 6). *P < 0.05, **P < 0.01 significantly different from pretreatment period, by manova with Tukey's multiple comparisons.
Discussion and conclusions
The present study demonstrated that the atypical antipsychotic drug, clozapine, enhanced transmission associated with L-glutamate and d-serine; but that the classical antipsychotic drug, haloperidol, had no such effects. Systemic administration of clozapine dose-dependently increased the release of L-glutamate and d-serine in the mPFC both in vivo and in primary cultured astrocytes. A large body of evidence supports a central role for dysfunction of the NMDA receptor in the pathophysiology of schizophrenia (Lieberman et al., 2008; Labrie and Roder, 2010). Both L-glutamate and d-serine are well established as major endogenous NMDA receptor agonists (Parpura and Zorec, 2009), and are two of the most recognized amino acid transmitters that mediate astrocyte-neurone signalling (Parpura and Zorec, 2009; Labrie and Roder, 2010). In spite of this literature, the exact mechanisms of neuronal and glial exocytosis of d-serine in vivo remain to be clarified; d-serine has been detected in both astrocytes and neurones in the brain (Kartvelishvily et al., 2006; Williams et al., 2006; Miya et al., 2008), with distribution patterns similar to those of the NMDA receptor (Schell et al., 1997).
Origin of release of L-glutamate and d -serine in the mPFC
In the present study, microdialysis experiments demonstrated that the basal extracellular levels of L-glutamate and d-serine in the mPFC were not sensitive to TTX and tetanus toxin, but increased by K+-evoked stimulation (Figure 5). The glial toxin, fluorocitrate, reduced the basal extracellular levels of these transmitters (Figure 5). Furthermore, the basal release of L-glutamate and d-serine from primary cultures of astrocytes was also decreased by tetanus toxin and fluorocitrate, but not by TTX (Figure 2). These results suggest that the extracellular levels of L-glutamate and d-serine detected by microdialysis during the resting stage, are predominantly of glial, rather than neuronal, origin.
The K+-evoked release of L-glutamate and d-serine in the mPFC was decreased by tetanus toxin and fluorocitrate; however, TTX inhibited the K+-evoked release of L-glutamate without affecting that of d-serine (Figure 5). The magnitudes of K+-evoked release of L-glutamate and d-serine reached peak levels at 40 and 60 min respectively (Figure 5). The early phase of K+-evoked L-glutamate release (20 min after the start of K+-evoked stimulation) was inhibited by TTX but not by fluorocitrate; however, the late phase (40–60 min after the start of K+-evoked stimulation) was inhibited by fluorocitrate (Figure 5). Furthermore, tetanus toxin inhibited both early and late phases of K+-evoked L-glutamate release (Figure 5). Thus, these pharmacological demonstrations suggest that the depolarization-induced release of L-glutamate probably represents both early neuronal and delayed glial release, whereas that of d-serine predominantly represents delayed glial release.
To clarify our hypothesis, the effects of TTX, tetanus toxin and fluorocitrate on release of L-glutamate and d-serine from primary cultured astrocytes was examined. In the present study, AMPA, but not NMDA or K+-evoked stimulation, increased the release of L-glutamate and d-serine from primary cultured astrocytes (Figure 3), as reported previously (Schell et al., 1995). Fluorocitrate, but not TTX, inhibited both basal and AMPA-induced release of L-glutamate and d-serine from cultured astrocytes (Figures 2 and 3). Voltage-activated Na+ currents have been observed in only 5% of glial fibrillary acidic protein positive astrocytes using the slice patch-clamp technique (Sontheimer and Waxman, 1993). Taken together with the results of the electrophysiological study, the present results indicate that TTX-sensitive Na+ channels do not play important roles in transmitter release from glia.
The expression of SNARE, including synaptobrevin, in cultured astrocytes has been demonstrated by immunohistochemistry, and glial d-serine release was regulated by a Ca2+-dependent SNARE complex (Schell et al., 1995; Mothet et al., 2005). Similar to these results, the present study showed that tetanus toxin inhibited both basal and AMPA-induced release of L-glutamate and d-serine in cultured astrocytes (Figures 2 and 3). Therefore, the release of L-glutamate and d-serine was, at least in part, regulated by a glial exocytosis mechanism. The present results indicate several mechanisms of release of L-glutamate and d-serine. The release of L-glutamate monitored by microdialysis comprised both neuronal and glial sources, whereas that of d-serine was probably glial release. The glial release of L-glutamate and d-serine is regulated by the SNARE complex, as observed in neurones (Okada et al., 2001).
Mechanisms of clozapine-induced release of L-glutamate and d -serine
In the present study, systemic administration of a therapeutically relevant dose of clozapine (5 mg·kg−1, i.p.) (Kapur et al., 2003) increased the extracellular d-serine level in the mPFC, but a lower dose of clozapine (1 mg·kg−1, i.p.) (Kapur et al., 2003) had no such effect (Figure 6). Furthermore, haloperidol did not alter extracellular d-serine levels (Figure 6). Clozapine-induced d-serine release in the mPFC was reduced by tetanus toxin and fluorocitrate but was not affected by TTX (Figure 7). As in the microdialysis experiments, clozapine-induced d-serine release from cultured astrocytes was also inhibited by tetanus toxin and fluorocitrate but was not affected by TTX (Figure 4). Fluorocitrate abolished the clozapine-induced d-serine release from cultured astrocytes (Figure 4). Therefore, clozapine-induced d-serine release is probably mainly of glial origin. Similarly, in cultured astrocytes, clozapine-induced L-glutamate release was also inhibited by tetanus toxin and fluorocitrate (fluorocitrate abolished clozapine-induced release), but not by TTX (Figure 7). In contrast to the results of cultured astrocytes, clozapine-induced L-glutamate release in the mPFC was reduced by TTX, tetanus toxin and fluorocitrate (Figure 7). These findings suggest that clozapine probably activates both neuronal and glial release of L-glutamate. However, the onset of clozapine-induced L-glutamate release (140 min after clozapine administration) was preceded by clozapine-induced d-serine release (60 min after clozapine administration) (Figures 6–8). The delayed clozapine-induced L-glutamate release was reduced by MK-801, whereas the latter had no effect on clozapine-induced d-serine release (Figure 8). Therefore, the clozapine-induced d-serine release leads to a subsequent increase in L-glutamate release via activation of NMDA receptors.
Several randomized controlled clinical studies have indicated that the NMDA receptor partial agonists, d-cycloserine and d-serine, significantly improve the negative symptoms of schizophrenia when used as adjuvants to conventional antipsychotics (Evins et al., 2002; Heresco-Levy et al., 2005; Tsai and Lin, 2010), whereas their clinical advantages were trivial in patients with schizophrenia treated with clozapine (Goff et al., 1999; Tsai and Lin, 2010). In contrast, a recent clinical study could not demonstrate the effectiveness of d-cycloserine (Buchanan et al., 2007). Several meta-analyses showed the effectiveness of d-serine against negative symptoms, but the magnitude of the effect was moderate (Tuominen et al., 2006; Buchanan et al., 2007; Tsai and Lin, 2010). Based on the clinical evidence, d-serine can improve the negative symptoms of schizophrenia though its effectiveness is limited (Shim et al., 2008; Tsai and Lin, 2010). Furthermore, the effectiveness of d-serine against positive symptoms and cognitive deficits remains to be clarified (Tuominen et al., 2006; Buchanan et al., 2007; Tsai and Lin, 2010). The present study demonstrated the stimulatory effects of clozapine on glial d-serine release; however, alternatively, the present results could suggest that activation of glial transmission is a novel target for atypical antipsychotics (Lieberman et al., 2008). To clarify this possibility, we plan to determine the effects of other atypical antipsychotics on glial transmission.
In conclusion, the present study demonstrated that the extracellular d-serine levels in the mPFC were regulated by a glial exocytosis mechanism rather than neuronal exocytosis. Clozapine at a therapeutically relevant dose increased the extracellular d-serine levels in the mPFC, whereas neither haloperidol nor a lower than therapeutically relevant dose of clozapine affected extracellular d-serine levels. The clozapine-induced d-serine release was also regulated by a glial exocytosis mechanism. Furthermore, the clozapine-induced d-serine release led to a secondary increase in neuronal L-glutamate release in the mPFC. The stimulatory effects of clozapine on glial transmission are, at least partially, involved in the antipsychotic action of clozapine.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Science and Culture (18390316 and 18659330), a Grant from the Mitsubishi Pharma Research Foundation, and a Grant from the Japan Epilepsy Research Foundation. We thank Prof F.G. Issa (http://www.word-medex.com.au) for the careful reading and editing of the manuscript.
Glossary
- ACSF
artificial cerebrospinal fluid
- AMPA
2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid
- DMEM
Dulbecco's modified Eagle's medium
- HKACSF
100 mM K+ containing ACSF artificial cerebrospinal fluid
- HKMRS
100 mM K+ containing modified Ringer's solution
- MANOVA
multivariate analysis of variance
- mPFC
medial prefrontal cortex
- MRS
modified Ringer's solution
- SNARE
soluble N-ethylmaleimide sensitive factor attachment protein receptors
- TTX
tetrodotoxin
- xLC
extreme high-pressure liquid chromatography
Conflict of interest
The authors state no conflict of interest.
References
- Abekawa T, Ito K, Koyama T. Different effects of a single and repeated administration of clozapine on phencyclidine-induced hyperlocomotion and glutamate releases in the rat medial prefrontal cortex at short- and long-term withdrawal from this antipsychotic. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:261–271. doi: 10.1007/s00210-007-0154-x. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edn. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanov VL, Liang X, Schwartz J, Grossman S, Wang RY. Clozapine and haloperidol modulate N-methyl-D-aspartate- and non-N-methyl-D-aspartate receptor-mediated neurotransmission in rat prefrontal cortical neurons in vitro. J Pharmacol Exp Ther. 1997;283:226–234. [PubMed] [Google Scholar]
- Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, et al. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164:1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- Evins AE, Amico E, Posever TA, Toker R, Goff DC. D-Cycloserine added to risperidone in patients with primary negative symptoms of schizophrenia. Schizophr Res. 2002;56:19–23. doi: 10.1016/s0920-9964(01)00220-1. [DOI] [PubMed] [Google Scholar]
- Goff DC, Henderson DC, Evins AE, Amico E. A placebo-controlled crossover trial of d-cycloserine added to clozapine in patients with schizophrenia. Biol Psychiatry. 1999;45:512–514. doi: 10.1016/s0006-3223(98)00367-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Oka T, Nishikawa T. Extracellular concentration of endogenous free d-serine in the rat brain as revealed by in vivo microdialysis. Neuroscience. 1995;66:635–643. doi: 10.1016/0306-4522(94)00597-x. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Ebstein R, Vass A, Lichtenberg P, Bar G, et al. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol Psychiatry. 2005;57:577–585. doi: 10.1016/j.biopsych.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther. 2003;305:625–631. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- Kargieman L, Santana N, Mengod G, Celada P, Artigas F. Antipsychotic drugs reverse the disruption in prefrontal cortex function produced by NMDA receptor blockade with phencyclidine. Proc Natl Acad Sci USA. 2007;104:14843–14848. doi: 10.1073/pnas.0704848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartvelishvily E, Shleper M, Balan L, Dumin E, Wolosker H. Neuron-derived d-serine release provides a novel means to activate N-methyl-D-aspartate receptors. J Biol Chem. 2006;281:14151–14162. doi: 10.1074/jbc.M512927200. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- Labrie V, Roder JC. The involvement of the NMDA receptor d-serine/glycine site in the pathophysiology and treatment of schizophrenia. Neurosci Biobehav Rev. 2010;34:351–372. doi: 10.1016/j.neubiorev.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Bymaster FP, Meltzer HY, Deutch AY, Duncan GE, Marx CE, et al. Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev. 2008;60:358–403. doi: 10.1124/pr.107.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, et al. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996;14:301–307. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, et al. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- Martin ED, Fernandez M, Perea G, Pascual O, Haydon PG, Araque A, et al. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia. 2007;55:36–45. doi: 10.1002/glia.20431. [DOI] [PubMed] [Google Scholar]
- Miya K, Inoue R, Takata Y, Abe M, Natsume R, Sakimura K, et al. Serine racemase is predominantly localized in neurons in mouse brain. J Comp Neurol. 2008;510:641–654. doi: 10.1002/cne.21822. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter d-serine. Proc Natl Acad Sci USA. 2005;102:5606–5611. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Okada M, Kawata Y, Zhu G, Kamata A, Kaneko S. Determination of effects of antiepileptic drugs on SNAREs-mediated hippocampal monoamine release using in vivo microdialysis. Br J Pharmacol. 2001;134:507–520. doi: 10.1038/sj.bjp.0704285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninan I, Jardemark KE, Wang RY. Differential effects of atypical and typical antipsychotic drugs on N-methyl-D-aspartate- and electrically evoked responses in the pyramidal cells of the rat medial prefrontal cortex. Synapse. 2003;48:66–79. doi: 10.1002/syn.10189. [DOI] [PubMed] [Google Scholar]
- Ohoyama K, Yamamura S, Hamaguchi T, Nakagawa M, Motomura E, Shiroyama T, et al. Effect of novel atypical antipsychotic, blonanserin, on extracellular neurotransmitter level in rat prefrontal cortex. Eur J Pharmacol. 2011;653:47–57. doi: 10.1016/j.ejphar.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Okada M, Nutt DJ, Murakami T, Zhu G, Kamata A, Kawata Y, et al. Adenosine receptor subtypes modulate two major functional pathways for hippocampal serotonin release. J Neurosci. 2001;21:628–640. doi: 10.1523/JNEUROSCI.21-02-00628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Zhu G, Yoshida S, Hirose S, Kaneko S. Protein kinase associated with gating and closing transmission mechanisms in temporoammonic pathway. Neuropharmacology. 2004;47:485–504. doi: 10.1016/j.neuropharm.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Okada M, Yoshida S, Zhu G, Hirose S, Kaneko S. Biphasic actions of topiramate on monoamine exocytosis associated with both soluble N-ethylmaleimide-sensitive factor attachment protein receptors and Ca(2+)-induced Ca(2+)-releasing systems. Neuroscience. 2005;134:233–246. doi: 10.1016/j.neuroscience.2005.03.045. [DOI] [PubMed] [Google Scholar]
- Parpura V, Zorec R. Gliotransmission: exocytotic release from astrocytes. Brain Res Rev. 2009;63:83–92. doi: 10.1016/j.brainresrev.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH. d-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci USA. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MJ, Brady RO, Jr, Molliver ME, Snyder SH. d-serine as a neuromodulator: regional and developmental localizations in rat brain glia resemble NMDA receptors. J Neurosci. 1997;17:1604–1615. doi: 10.1523/JNEUROSCI.17-05-01604.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim SS, Hammonds MD, Kee BS. Potentiation of the NMDA receptor in the treatment of schizophrenia: focused on the glycine site. Eur Arch Psychiatry Clin Neurosci. 2008;258:16–27. doi: 10.1007/s00406-007-0757-8. [DOI] [PubMed] [Google Scholar]
- Sontheimer H, Waxman SG. Expression of voltage-activated ion channels by astrocytes and oligodendrocytes in the hippocampal slice. J Neurophysiol. 1993;70:1863–1873. doi: 10.1152/jn.1993.70.5.1863. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 2010;16:522–537. doi: 10.2174/138161210790361452. [DOI] [PubMed] [Google Scholar]
- Tuominen HJ, Tiihonen J, Wahlbeck K. Glutamatergic drugs for schizophrenia. Cochrane Database Syst Rev. 2006;(2):CD003730. doi: 10.1002/14651858.CD003730.pub2. [DOI] [PubMed] [Google Scholar]
- Williams SM, Diaz CM, Macnab LT, Sullivan RK, Pow DV. Immunocytochemical analysis of d-serine distribution in the mammalian brain reveals novel anatomical compartmentalizations in glia and neurons. Glia. 2006;53:401–411. doi: 10.1002/glia.20300. [DOI] [PubMed] [Google Scholar]
- Yamamura S, Ohoyama K, Hamaguchi T, Kashimoto K, Nakagawa M, Kanehara S, et al. Effects of quetiapine on monoamine, GABA, and glutamate release in rat prefrontal cortex. Psychopharmacology (Berl) 2009a;206:243–258. doi: 10.1007/s00213-009-1601-9. [DOI] [PubMed] [Google Scholar]
- Yamamura S, Ohoyama K, Hamaguchi T, Nakagawa M, Suzuki D, Matsumoto T, et al. Effects of zotepine on extracellular levels of monoamine, GABA and glutamate in rat prefrontal cortex. Br J Pharmacol. 2009b;157:656–665. doi: 10.1111/j.1476-5381.2009.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura S, Abe M, Nakagawa M, Ochi S, Ueno S, Okada M. Different actions for acute and chronic administration of mirtazapine on serotonergic transmission associated with raphe nuclei and their innervation cortical regions. Neuropharmacology. 2011;60:550–560. doi: 10.1016/j.neuropharm.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Zhu G, Okada M, Yoshida S, Mori F, Hirose S, Wakabayashi K, et al. Involvement of Ca(2+)-induced Ca2+ releasing system in interleukin-1beta-associated adenosine release. Eur J Pharmacol. 2006;532:246–252. doi: 10.1016/j.ejphar.2005.12.085. [DOI] [PubMed] [Google Scholar]


