Abstract
It is well established that T regulatory (Treg) cells counteract tumour immunity. However, conflicting results describing the role of Treg cells in haematological tumours warrant further investigations to clarify the interactions between Treg cells and the tumour. B-cell malignancy derives from different stages of B-cell development and differentiation in which T cells play a profound role. The transformed B cell may still be in need of T-cell help to thrive but simultaneously they may be recognized and destroyed by cytotoxic lymphocytes. Recent reports demonstrate that Treg cells can suppress and even kill B cells as part of their normal function to rescue the body from autoimmunity. An emerging body of evidence points out that Treg cells not only inhibit tumour-specific T cells but may also have a role in suppressing the progression of the B-cell tumour. In this review, we discuss the origin and function of Treg cells and their role in patients with B-cell tumours.
Keywords: B-cell leukaemia, B-cell lymphoma, cytotoxic regulatory cells, immune regulation, T regulatory cells
Introduction
The immune system plays an important role in tumour transformation and progression as chronic inflammation generates reactive oxygen species (ROS), which increase DNA damage and thereby cause genomic instability and accumulation of mutations.1 However, the adaptive responses may instead detect and destroy tumour cells because of their aberrant expression of various tumour-associated, or tumour-specific, proteins. Indeed, there is a positive correlation between the number of infiltrating T cells and patient survival in many solid cancers.2 Nevertheless, immune cells in the tumour area are often anergized by the presence of immunosuppressive cells and cytokines. In haematological cancers, the tumour cell itself is an immune cell that complicates the cell interactions in the tumour microenvironment. The T regulatory (Treg) cells, for example, restrain unwanted immune responses (autoimmunity, post-infection inflammation). These cells increase during cancer progression and have been correlated to a worse prognosis in many malignancies.3–5 However, the role of Treg cells in haematological tumours is debated. Contradicting results in B-cell malignancies demonstrate that Treg cells can be associated with both poor prognosis and increased survival.6–13 As immunosuppressors, Treg cells may act against the attacking effector lymphocytes as well as against the B-cell-derived tumour. In this review, we will discuss Treg cells and their complex role in haematological tumours.
The birth of T regulatory cells
The suppressive capacity of lymphocytes has been known to man for decades. As early as the 1970s, Gershon and Kondo14,15 discovered that T cells pre-treated with high doses of antigen became tolerant and that tolerance could be passed on to surrounding T and B cells. Subsequent studies aimed at characterizing these cells were undertaken but no specific markers were available and the existence of the cells was hard to prove.16 It was not until 1995, when Sakaguchi et al.17 suggested that murine suppressive T cells could be identified by their high expression of CD25 [interleukin-2 receptor α (IL-2Rα)], that the interest in this subset resurfaced. They demonstrated that mice developed autoimmune diseases upon depletion of CD25+ cells. Further, these mice reacted strongly to skin transplants and this reaction could be hampered by the reinfusion of CD4+ CD25+ cells.17 During the following years suppressive T cells were rebranded as regulatory T cells.16
Treg cell-associated markers
Discrepancies in the results from published studies evaluating Treg cells in B-cell malignancies as well as in other studies may be the result of the difficulty of finding one good marker for identification of the cells. CD4+ CD25+ cells were identified as Treg cells in humans a couple of years after Sakaguchi's milestone paper.17–21 However, as CD25 is also up-regulated on activated T cells, it is not a specific marker.22 Even so, because of the lack of a more unique cell surface marker, CD25 has been used extensively to sort Treg cells for experimental evaluation. Most Treg cells express CD25 (70–80%23) but the existence of CD25-negative Treg cells has been reported by us and others.24–26 Other markers are needed to encompass all or certain populations of Treg cells. Additional markers expressed by Treg cells are cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4),27,28 glucocorticoid-induced tumour necrosis factor receptor29 and lymphocyte-activation gene 3.30 These markers are constitutively or highly expressed on Treg cells but suffer the same fate as CD25 by being up-regulated on conventional T cells upon activation.26,27
The transcription factor Forkhead box P3 (FoxP3) is considered the most specific marker for Treg cells.31–33 FoxP3 acts both as a repressor and an activator of gene transcription and binds over 700 genes.34 It is known to repress the gene expression of IL-2, CD127 (IL7R), tumour necrosis factor-α and interferon-γ, and to enhance the expression of CD25 and CTLA-4.31–33,35–38 Given the fact that CD127 is a cell surface marker it can be used to distinguish the CD25high CD127low Treg cells from the CD25high CD127high activated T cells.39 This may be crucial because some studies have shown that FoxP3 can be transiently up-regulated in human effector T cells that do not exhibit regulatory functions.40–43 However, as several of these studies were performed with an antibody of questioned specificity (PCH101, eBiosciences, San Diego, CA) it is hard to interpret these results.40,42–44 Whether the transient FoxP3 expression confers transient regulatory functions on T cells is still a matter of debate and may be difficult to investigate because of the short timespan of FoxP3 expression.
Treg cell development and subtypes
Natural Treg cells originate in the thymus but Treg cells can be induced from naive T cells in the periphery as well. These two cell populations have been hard to distinguish from one another because they are very similar, both phenotypically and functionally. However, recent studies identified Helios, a zinc finger transcription factor, as being highly expressed in the natural Treg cells but not in peripherally induced Treg cells.45,46 Natural Treg cells are selected in the thymus in the same way as conventional T cells. They can be either CD4+ or CD8+ but the CD4+ subclass dominates. Their T-cell receptors have high affinity for self antigens, meaning that these are in the border of thymic elimination.47 Because they are reactive with auto-peptides they are likely to have a role in preventing autoimmunity. Nevertheless, considering the thymic involution and the fact that Treg cells do not decrease with age, inducible Treg cells must also be an important source of Treg cells in adults. Although natural Treg cells are important in protection against autoimmunity, inducible Treg cells are thought to protect the surrounding tissues in an ongoing immune response. If the B-cell tumour is producing antibodies that react to autoantigens, natural Treg cells may play an important role in hampering tumour cell progression whereas both natural and inducible Treg cells may restrain tumour-reactive T cells. Considering that the malignant B cells have an aberrant behaviour compared with normal B cells (regarding proliferation and apoptosis resistance), it is possible that the malignant B cells are also targets for the inducible Treg cells.
Inducible Treg cells, also known as adaptive Treg cells, are induced from naive T cells by antigenic stimulation in combination with factors that are not optimal for effector T-cell generation, such as high levels of IL-10, IL-2 and transforming growth factor-β (TGF-β).48 Retinoic acid has been shown to induce Treg cells even in the presence of inflammatory cytokines.49 Several subsets of inducible Treg cells have been described, including CD4+ Tr1 and Th3 Treg cells, as well as CD8+ Treg cells. Recently, T follicular regulatory cells were described in mice, and these Treg cells were shown to regulate germinal centre reactions.50–52 This CXCR5+ subset is, however, most likely induced from natural Treg cells.50,51 The environment defines the Treg cell phenotype because it has been demonstrated that transcriptional regulators associated with different types of T helper cell responses (Th1, Th2, Th17) shape the Treg cell response.53 Further, Treg cell status is not at a differentiation endpoint because studies have shown that these cells can transform into effector cells, lose their regulatory function and produce IL-17 or interferon-γ.54,55 Hence, the plasticity of these cells allows for the multitask functions needed to balance between immune regulation and activation. However, these characteristics makes it difficult to pinpoint certain Treg cell subtypes and their intermediate phases, and it complicates the role of these cells in haematological malignancies because their status may either regulate or stimulate the tumour cell. The environment in B-cell tumours will, hence, determine the subtypes of Treg cells present in the tumour.
Treg cell effector functions
Treg cells are able to suppress a wide range of immune cells. The suppression can either be direct or mediated through secondary immune cells. To exert their function Treg cells need to be activated in an antigen-dependent manner but are then able to suppress nearby immune cells by antigen-dependent mechanisms or, more commonly, independently of specificity. Hence, an immune effector cell does not need to share the same specificity as the Treg cell in order to be suppressed. Treg cells are able to suppress target cells by the release of inhibitory cytokines such as IL-10, TGF-β and IL-35.56 Interleukin-10 induces a long-lasting anergy in both CD4+ and CD8+ T cells and down-regulates the expression of co-stimulatory molecules, adhesion molecules and MHC class II on antigen-presenting cells.57–60 The TGF-β is able to inhibit T-cell proliferation by disturbing IL-2 production and can block differentiation of naive T cells.61 Interleukin-35 suppresses T-cell proliferation62 and can stimulate Treg cells to proliferate and produce high levels of IL-10.63
Treg cells are also able to inhibit T cells by interfering with their metabolism. By expressing the ectozymes CD39 and CD73, Treg cells generate adenosine, which suppresses effector T cells by binding to the adenosine receptor 2A.64,65 Further, cAMP can be transported from Treg cells to T cells through gap junctions where they inhibit proliferation and IL-2 production.66,67 Prostaglandin E2, which is generated by cyclooxygenase-2, can be secreted by Treg cells68 and mediates its suppressive function by increasing the level of cAMP, which further suppresses the T cells.69 Recently, we suggested that Treg cells are able to release CD25 to further deprive its microenvironment of IL-2 and by this means inhibit the proliferation of conventional T cells.70 Treg cells can also deprive T cells of thiols, such as cysteine, that are provided to T cells in the dendritic cell vicinity.71 The Treg cells are able to induce indoleamine 2,3-dioxygenase production by dendritic cells in a CTLA-4-dependent manner.72 Indoleamine 2,3-dioxygenase is an enzyme that catabolizes tryptophan into the toxic kynurenine metabolites 3-HAA (3-Hydroxyanthranilic acid) and QUIN (Quinolinic acid), which induce apoptosis in Th173 and Th2 cells.74
Treg cells are able to suppress a wide range of immune cells by induction of apoptosis. This killing is generally mediated by Fas–Fas ligand interaction75,76 or through the release of perforins/granzymes.77–82 Grossman et al.78 demonstrated that natural Treg cells preferentially express granzyme A upon activation, in contrast to inducible Treg cells, which express granzyme B.78 Treg cells can also kill T cells by using the TRAIL–DR5 pathway.83 Also, galectin-1, which can induce apoptosis in target cells, is up-regulated on Treg cells.84 By killing their target, Treg cells can control CD4+78 and CD8+75,78,80 T cells, monocytes,78 dendritic cells,78 B cells76,79,81 and natural killer cells.80
Treg cells in B-cell-derived tumours
The role of Treg cells is more complex in haematological cancers compared with non-haematological cancers because the tumour is derived from the immune system. The normal behaviour of antigen-presenting cells, such as the B cell, in lymph nodes or at sites of inflammation is to interact with T cells including Treg cells. It is possible that Treg cells have a dual role in patients with leukaemia or lymphoma. On one hand, they may suppress anti-tumour immune responses mediated by T cells but on the other hand they may regulate the malignant immune cell, either directly or by interfering with T-cell help (Fig. 1). Some studies have investigated the relationship between the number of Treg cells and patient outcome in B-cell lymphoma. Several of these showed that patients with a high number of tumour infiltrating FoxP3+ cells (Treg cells) have a better survival than patients with few Foxp3+ cells.7–13
Figure 1.
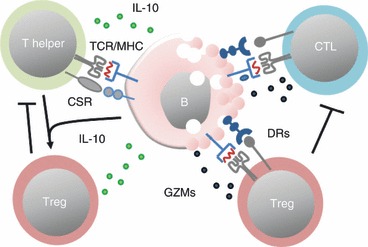
Interactions between T cells and malignant B cells. The malignant B cells may require T helper cells for sustained growth but the helper cells may also be part of the control of malignant cells by direct cytotoxicity or by stimulating anti-tumour immunity. Helper cells can interact via T-cell receptor (TCR)/MHC-II and via co-stimulatory receptors (CSR). The malignant B cells can release substances [such as interleukin-10 (IL-10)] that promote the conversion of T helper cells into regulatory T (Treg) cells that suppress both T helper cells and cytotoxic T cells (CTL). The Treg cells can also recognize MHC-II on the B cells. If cytotoxic, they may interact via death receptors (DRs) to induce B-cell apoptosis. The CTLs recognize MHC-I on malignant B cells and induce apoptosis via DRs. GZMs, granzymes.
The tumour microenvironment is beneficial for the maintenance and expansion of Treg cells because of the presence of IL-10, TGF-β and immature dendritic cells,85,86 and these factors are present in both non-haematological and haematological cancers. Although, IL-10 is an immunosuppressive molecule for effector T cells, it is stimulatory for B cells. Hence, IL-10 may drive both Treg cells and the B-cell tumour. In B-cell lymphoma it was shown that patients with elevated levels of both IL-10 and tumour necrosis factor-α were less likely to respond well to treatment.87 In follicular lymphoma, the tumour B cells were shown to convert nearby CD4+ T cells into FoxP3+ Treg cells in an antigen-specific manner.88
During different steps of B-cell development, T cells control the fate of the B cell by either killing it89 or promoting its survival by up-regulating anti-apoptotic molecules as well as inducing its proliferation. B-cell malignancies arise during different stages of B-cell maturation90 and several of them originate from germinal centre-derived B cells. The B-cell tumour in patients with chronic lymphocytic leukaemia (CLL) dies rapidly in vitro, which highlights the importance of the tumour micro-milieu in tumour survival. It has been shown that CLL cell survival in vitro can be supported by stromal accessory cells.91 As a result of the close contact of B cells and T helper cells it is likely that T-cell help is an important feature of tumour progression. Hence, by suppressing T helper cells in the tumour vicinity through the effector mechanisms discussed above, Treg cells may block tumour cell progression. Correspondingly, studies have shown that Treg cells are able to regulate B cells by interfering with their need for T-cell help in germinal centres.92,93 However, a study on Hodgkin's lymphoma demonstrated that many Treg cells in combination with few Th2 cells correlated with increased risk of relapse.6
Treg cells are also able to regulate B cells directly by induction of apoptosis.76,79,81 In a study recently published by our group, we demonstrated that FoxP3+ Treg cells in patients with B-cell leukaemia or lymphoma, expressed cytolytic markers and were able to kill malignant B cells in vitro.94 The same phenomenon has been noted in patients with systemic lupus erythematosus. In that study, Treg cells were able to regulate malignant autoantibody producing B cells.95 In CLL, at least half of the patients have tumour cells with somatically mutated immunoglobulin heavy chain variable genes and more than 20% express homologous stereotyped B-cell receptors. These findings indicate that a certain antigen may have caused the disease onset.96 It is not clear if this agent (or agents) still drives the disease. Some antigens suggested are present on apoptotic cells, or bacteria.97,98 It has been proposed that CLL is driven by autoantigens and CLL cells were shown to produce autoantibodies.99,100 Since then, CLL has been connected to several different autoimmune conditions.101 By controlling CLL cells, Treg cells may exert their natural function as suppressors of autoimmunity. As an interesting parallel; studies have shown that several autoimmune diseases associated with autoantibody production have Treg cells at a decreased level of function.102–106 Treg cells controlling B cells may suppress the B cells in an antigen-specific manner (T-cell receptor–MHC-II-restricted) because malignant B cells express MHC-II and killing via death receptor ligands or granzyme release is commonly regulated via T-cell antigen recognition. However, other mechanisms exerted by the Treg cells may be used.
Even if several studies show a positive correlation between FoxP3 and survival in B-cell malignancy, there are also studies demonstrating that Treg cells are associated with a worse outcome in these patients.6 The discrepancy may lay in methods of Treg cell detection. For example, the PCH101 antibody can mistakenly also stain activated T cells.41 Hence, some of the detected FoxP3+ cells may have been activated T cells which at least in other cancers have been consistently shown to be beneficial. As a result of the promiscuous phenotype of Treg cells, these cells may also represent an intermediate phenotype on their way to transform into effector T cells. Indeed, FoxP3– T cells in patients with leukaemia or lymphoma also displayed markers of cytolysis94 demonstrating the active participation of the immune system to combat the malignant B cell. Clearly, further investigations are needed to elucidate the role of Treg cells, and T cells in general, in patients with haematological tumours such as B-cell malignancy.
Conclusion
Treg cells exist as many subtypes changing their wardrobe depending on the ongoing immunological scenario. The role of Treg cells in solid non-haematopoietic cancers is to suppress tumour immunity probably through their importance in inhibiting immune activity to self cells. In haematological tumours the role of Treg cells may be more complex because the Treg cells on the one hand create a tumour-supporting environment by blocking ongoing immune attacks in the tumour milieu, and on the other hand may kill the tumour by recognizing tumour antigens on MHC-II on the tumour cell leading to the traditional ‘kiss of death’. Understanding the basic interactions between T cells, Treg cells and normal B cells will give new insights into the various immune responses occurring in patients with B-cell-derived tumours.
Acknowledgments
The Loskog research group is supported by grants from AFA Insurance, the Swedish Childhood Cancer Society, The Swedish Society of Cancer, The Swedish Research Council, Åke Wiberg Fund, Ellen Bachrach Fund, and LeukemiaNet. C Lindqvist is supported by grants from the foundations of G. G. Claéson, A. Karlsson and L. Eriksson at the Medical Faculty at Uppsala University.
Disclosures
The authors declare no conflict of interest.
References
- 1.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oble DA, Loewe R, Yu P, Mihm MC., Jr Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human melanoma. Cancer Immunol. 2009;9:3. [PMC free article] [PubMed] [Google Scholar]
- 3.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi N, Hiroaka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–11. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 5.Mougiakakos D, Johansson CC, Trocme E, All-Ericsson C, Economou MA, Larsson O, Seregard S, Kiessling E. Intratumoral forkhead box P3-positive regulatory T cells predict poor survival in cylooxygenase-2-positive uveal melanoma. Cancer. 2010;116:2224–33. doi: 10.1002/cncr.24999. [DOI] [PubMed] [Google Scholar]
- 6.Schreck S, Friebel D, Buettner M, Distel L, Grabenbauer G, Young LS, Niedobitek G. Prognostic impact of tumour-infiltrating Th2 and regulatory T cells in classical Hodgkins lymphoma. Hematol Oncol. 2009;27:31–9. doi: 10.1002/hon.878. [DOI] [PubMed] [Google Scholar]
- 7.Alvaro T, Lejeune M, Salvado MT, et al. Outcome in Hodgkin's lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11:1467–73. doi: 10.1158/1078-0432.CCR-04-1869. [DOI] [PubMed] [Google Scholar]
- 8.Carreras J, Lopez-Guillermo A, Fox BC, et al. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108:2957–64. doi: 10.1182/blood-2006-04-018218. [DOI] [PubMed] [Google Scholar]
- 9.Lee NR, Song EK, Jang KY, et al. Prognostic impact of tumor infiltrating FOXP3 positive regulatory T cells in diffuse large B-cell lymphoma at diagnosis. Leuk Lymphoma. 2008;49:247–56. doi: 10.1080/10428190701824536. [DOI] [PubMed] [Google Scholar]
- 10.Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin's lymphoma. Haematologica. 2008;93:193–200. doi: 10.3324/haematol.11702. [DOI] [PubMed] [Google Scholar]
- 11.Wahlin BE, Aggarwal M, Montes-Moreno S, et al. A unifying microenvironment model in follicular lymphoma: outcome is predicted by programmed death-1-positive, regulatory, cytotoxic, and helper T cells and macrophages. Clin Cancer Res. 2010;16:637–50. doi: 10.1158/1078-0432.CCR-09-2487. [DOI] [PubMed] [Google Scholar]
- 12.Koreishi AF, Saenz AJ, Persky DO, Cui H, Moskowitz A, Moskowitz CH, Teruya-Feldstein J. The role of cytotoxic and regulatory T cells in relapsed/refractory Hodgkin lymphoma. Appl Immunohistochem Mol Morphol. 2010;18:206–11. doi: 10.1097/PAI.0b013e3181c7138b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelley TW, Pohlman B, Elson P, Hsi ED. The ratio of FOXP3+ regulatory T cells to granzyme B+ cytotoxic T/NK cells predicts prognosis in classical Hodgkin lymphoma and is independent of bcl-2 and MAL expression. Am J Clin Pathol. 2007;128:958–65. doi: 10.1309/NB3947K383DJ0LQ2. [DOI] [PubMed] [Google Scholar]
- 14.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18:723–37. [PMC free article] [PubMed] [Google Scholar]
- 15.Gershon RK, Kondo K. Infectious immunological tolerance. Immunology. 1971;21:903–14. [PMC free article] [PubMed] [Google Scholar]
- 16.Basten A, Fazekas de St Groth B. Special regulatory T-cell review: T-cell dependent suppression revisited. Immunology. 2008;123:33–9. doi: 10.1111/j.1365-2567.2007.02772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 18.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+ CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 19.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4+ CD25+ T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–10. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4+ CD25+ T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taams LS, Vukmanovic-Stejic M, Smith J, et al. Antigen-specific T cell suppression by human CD4+ CD25+ regulatory T cells. Eur J Immunol. 2002;32:1621–30. doi: 10.1002/1521-4141(200206)32:6<1621::AID-IMMU1621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 22.Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+ CD25+ regulatory T cells. Semin Immunol. 2004;16:89–98. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Mays LE, Chen YH. Maintaining immunological tolerance with Foxp3. Cell Res. 2007;17:904–18. doi: 10.1038/cr.2007.84. [DOI] [PubMed] [Google Scholar]
- 24.Fransson M, Burman J, Lindqvist C, Atterby C, Fagius J, Loskog A. T regulatory cells lacking CD25 are increased in MS during relapse. Autoimmunity. 2010;43:590–7. doi: 10.3109/08916930903541190. [DOI] [PubMed] [Google Scholar]
- 25.Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25− subpopulations. J Immunol. 2000;165:3105–10. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- 26.Bonelli M, Savitskaya A, Steiner CW, Rath E, Smolen JS, Scheinecker C. Phenotypic and functional analysis of CD4+ CD25− Foxp3+ T cells in patients with systemic lupus erythematosus. J Immunol. 2009;182:1689–95. doi: 10.4049/jimmunol.182.3.1689. [DOI] [PubMed] [Google Scholar]
- 27.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+ CD4+ regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25+ CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4+ CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 30.Camisaschi C, Casati C, Rini F, et al. LAG-3 expression defines a subset of CD4+ CD25high Foxp3+ regulatory T cells that are expanded at tumor sites. J Immunol. 2010;184:6545–51. doi: 10.4049/jimmunol.0903879. [DOI] [PubMed] [Google Scholar]
- 31.Yagi H, Nomura T, Nakamura K, et al. Crucial role of FOXP3 in the development and function of human CD25+ CD4+ regulatory T cells. Int Immunol. 2004;16:1643–56. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 32.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 33.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 34.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–40. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 35.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bettelli E, Dastrange M, Oukka M. Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci. 2005;102:5138–43. doi: 10.1073/pnas.0501675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Z, Song X, Berezov A, Li B, Greene MI. Structural aspects of the FOXP3 regulatory complex as an immunopharmacological target. Int Immunopharmacol. 2009;9:518–20. doi: 10.1016/j.intimp.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Z, Song X, Li B, Greene MI. FOXP3 and its partners: structural and biochemical insights into the regulation of FOXP3 activity. Immunol Res. 2008;42:19–28. doi: 10.1007/s12026-008-8029-x. [DOI] [PubMed] [Google Scholar]
- 39.Banham AH. Cell-surface IL-7 receptor expression facilitates the purification of FOXP3+ regulatory T cells. Trends Immunol. 2006;27:541–4. doi: 10.1016/j.it.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–38. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 41.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+ FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–90. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Passerini L, Allan SE, Battaglia M, Di Nunzio S, Alstad AN, Levings MK, Roncarolo MG, Bacchetta R. STAT5-signaling cytokines regulate the expression of FOXP3 in CD4+ CD25+ regulatory T cells and CD4+ CD25− effector T cells. Int Immunol. 2008;20:421–31. doi: 10.1093/intimm/dxn002. [DOI] [PubMed] [Google Scholar]
- 43.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–54. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 44.Pillai V, Karandikar NJ. Attack on the clones? Human FOXP3 detection by PCH101, 236A/E7, 206D, and 259D reveals 259D as the outlier with lower sensitivity. Blood. 2008;111:463–4. doi: 10.1182/blood-2007-09-111823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Getnet D, Grosso JF, Goldberg MV, et al. A role for the transcription factor Helios in human CD4+ CD25+ regulatory T cells. Mol Immunol. 2010;47:1595–600. doi: 10.1016/j.molimm.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–41. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bettini ML, Vignali DA. Development of thymically derived natural regulatory T cells. Ann N Y Acad Sci. 2010;1183:1–12. doi: 10.1111/j.1749-6632.2009.05129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+ CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–74. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Linterman MA, Pierson W, Lee SK, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–82. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung Y, Tanaka S, Chu F, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–8. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alexander CM, Tygrett LT, Boyden AW, Wolniak KL, Legge KL, Waldschmidt TJ. T regulatory cells participate in the control of germinal centre reactions. Immunology. 2011;133:452–68. doi: 10.1111/j.1365-2567.2011.03456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campbell DJ, Cock MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–29. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+ CD25− Foxp3− T cells of are self-induced to become Th17 cells in the absence of exogenous TGF-β. J Immunol. 2007;178:6725–9. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 55.Zhou X, Bailey-Bucktrout SL, Jeker LT, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–7. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shalev I, Schmelzle M, Robson SC, Levy G. Making sense of regulatory T cell suppressive function. Semin Immunol. 2011;23:282–92. doi: 10.1016/j.smim.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinbrink K, Graulich E, Kubsch S, Knop J, Enk AH. CD4+ and CD8+ anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood. 2002;99:2468–76. doi: 10.1182/blood.v99.7.2468. [DOI] [PubMed] [Google Scholar]
- 58.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 59.Willems F, Marchant A, Delville JP, Gerard C, Delvaux A, Velu T, de Boer M, Goldman M. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur J Immunol. 1994;24:1007–9. doi: 10.1002/eji.1830240435. [DOI] [PubMed] [Google Scholar]
- 60.de Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 62.Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–9. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 63.Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–9. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 64.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 66.Bopp T, Becker C, Klein M, et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med. 2007;204:1303–10. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Novak TJ, Rothenberg EV. cAMP inhibits induction of interleukin 2 but not of interleukin 4 in T cells. Proc Natl Acad Sci USA. 1990;87:9353–7. doi: 10.1073/pnas.87.23.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mahic M, Yaqub S, Johansson CC, Tasken K, Aandahl EM. FOXP3+ CD4+ CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J Immunol. 2006;177:246–54. doi: 10.4049/jimmunol.177.1.246. [DOI] [PubMed] [Google Scholar]
- 69.Goto T, Herberman RB, Maluish A, Strong DM. Cyclic AMP as a mediator of prostaglandin E-induced suppression of human natural killer cell activity. J Immunol. 1983;130:1350–5. [PubMed] [Google Scholar]
- 70.Lindqvist CA, Christiansson LH, Simonsson B, Enblad G, Olsson-Stromberg U, Loskog AS. T regulatory cells control T-cell proliferation partly by the release of soluble CD25 in patients with B-cell malignancies. Immunology. 2010;131:371–6. doi: 10.1111/j.1365-2567.2010.03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Angelini G, Gardella S, Ardy M, et al. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci. 2002;99:1491–6. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fallarino F, Grohmann U, Hwang KW, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–12. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 73.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–77. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 74.Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-kappaB activation. Nat Rev. 2007;7:817–23. doi: 10.1038/nri2163. [DOI] [PubMed] [Google Scholar]
- 75.Strauss L, Bergmann C, Whiteside TL. Human circulating CD4+ CD25high Foxp3+ regulatory T cells kill autologous CD8+ but not CD4+ responder cells by Fas-mediated apoptosis. J Immunol. 2009;182:1469–80. doi: 10.4049/jimmunol.182.3.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Janssens W, Carlier V, Wu B, VanderElst L, Jacquemin MG, Saint-Remy JM. CD4+ CD25+ T cells lyse antigen-presenting B cells by Fas–Fas ligand interaction in an epitope-specific manner. J Immunol. 2003;171:4604–12. doi: 10.4049/jimmunol.171.9.4604. [DOI] [PubMed] [Google Scholar]
- 77.Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104:2840–8. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- 78.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 79.Mahnke K, Bedke T, Enk AH. Regulatory conversation between antigen presenting cells and regulatory T cells enhance immune suppression. Cell Immunol. 2007;250:1–13. doi: 10.1016/j.cellimm.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 80.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–46. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 81.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+ CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925–32. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boissonnas A, Scholer-Dahirel A, Simon-Blancal V, et al. Foxp3+ T cells induce perforin-dependent dendritic cell death in tumor-draining lymph nodes. Immunity. 2010;32:266–78. doi: 10.1016/j.immuni.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 83.Ren X, Ye F, Jiang Z, Chu Y, Xiong S, Wang Y. Involvement of cellular death in TRAIL/DR5-dependent suppression induced by CD4+ CD25+ regulatory T cells. Cell Death Differ. 2007;14:2076–84. doi: 10.1038/sj.cdd.4402220. [DOI] [PubMed] [Google Scholar]
- 84.Garin MI, Chu CC, Golshayan D, Cernuda-Morollon E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4+ CD25+ T cells. Blood. 2007;109:2058–65. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- 85.Croci DO, Zacarias Fluck MF, Rico MJ, Matar P, Rabinovich GA, Scharovsky OG. Dynamic cross-talk between tumor and immune cells in orchestrating the immunosuppressive network at the tumor microenvironment. Cancer Immunol Immunother. 2007;56:1687–700. doi: 10.1007/s00262-007-0343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Poggi A, Zocchi MR. Mechanisms of tumor escape: role of tumor microenvironment in inducing apoptosis of cytolytic effector cells. Arch Immunol Ther Exp (Warsz) 2006;54:323–33. doi: 10.1007/s00005-006-0038-7. [DOI] [PubMed] [Google Scholar]
- 87.Lech-Maranda E, Bienvenu J, Broussais-Guillaumot F, Warzocha K, Michallet AS, Robak T, Coiffier B, Salles G. Plasma TNF-alpha and IL-10 level-based prognostic model predicts outcome of patients with diffuse large B-cell lymphoma in different risk groups defined by the international prognostic index. Arch Immunol Ther Exp (Warsz) 2010;58:131–41. doi: 10.1007/s00005-010-0066-1. [DOI] [PubMed] [Google Scholar]
- 88.Ai WZ, Hou JZ, Zeiser R, et al. Follicular lymphoma B cells induce the conversion of conventional CD4+ T cells to T-regulatory cells. Int J Cancer. 2009;124:239–44. doi: 10.1002/ijc.23881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choe J, Kim HS, Zhang X, Armitage RJ, Choi YS. Cellular and molecular factors that regulate the differentiation and apoptosis of germinal center B cells. Anti-Ig down-regulates Fas expression of CD40 ligand-stimulated germinal center B cells and inhibits Fas-mediated apoptosis. J Immunol. 1996;157:1006–16. [PubMed] [Google Scholar]
- 90.Kuppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat Rev Cancer. 2005;5:251–62. doi: 10.1038/nrc1589. [DOI] [PubMed] [Google Scholar]
- 91.Schulz A, Toedt G, Zenz T, Stilgenbauer S, Lichter P, Seiffert M. Inflammatory cytokines and signaling pathways are associated with survival of primary chronic lymphocytic leukemia cells in vitro: a dominant role of CCL2. Haematologica. 2011;96:408–16. doi: 10.3324/haematol.2010.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lim HW, Hillsamer P, Kim CH. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J Clin Invest. 2004;114:1640–9. doi: 10.1172/JCI22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu HY, Quintana FJ, Weiner HL. Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4+ CD25− LAP+ regulatory T cell and is associated with down-regulation of IL-17+ CD4+ ICOS+ CXCR5+ follicular helper T cells. J Immunol. 2008;181:6038–50. doi: 10.4049/jimmunol.181.9.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lindqvist CA, Christiansson LH, Thörn I, et al. Both CD4+ FoxP3+ and CD4+ FoxP3– T cells from patients with B-cell malignancy express cytolytic markers and kill autologous leukaemic B cells in vitro. Immunology. 2011;133:296–306. doi: 10.1111/j.1365-2567.2011.03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iikuni N, Lourenco EV, Hahn BH, La Cava A. Cutting edge: regulatory T cells directly suppress B cells in systemic lupus erythematosus. J Immunol. 2009;183:1518–22. doi: 10.4049/jimmunol.0901163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Caligaris-Cappio F. Inflammation, the microenvironment and chronic lymphocytic leukemia. Haematologica. 2011;96:353–5. doi: 10.3324/haematol.2010.039446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Catera R, Silverman GJ, Hatzi K, et al. Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation. Mol Med. 2008;14:665–74. doi: 10.2119/2008-00102.Catera. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lanemo Myhrinder A, Hellqvist E, Sidorova E, et al. A new perspective: molecular motifs on oxidized LDL, apoptotic cells, and bacteria are targets for chronic lymphocytic leukemia antibodies. Blood. 2008;111:3838–48. doi: 10.1182/blood-2007-11-125450. [DOI] [PubMed] [Google Scholar]
- 99.Borche I, Liam A, Binet JL, Dighiero G. Evidence that chronic lymphocytic leukemia B lymphocytes are frequently committed to production of natural autoantibodies. Blood. 1990;76:562. [PubMed] [Google Scholar]
- 100.Sthoeger ZM, Wakai M, Tse DB. Production of autoantibodies by CD5-expressing B lymphocytes in patients with chronic lymphocytic leukemia. J Exp Med. 1989;169:255–68. doi: 10.1084/jem.169.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ansell P, Simpson J, Lightfoot T, et al. Non-Hodgkin lymphoma and autoimmunity: does gender matter? Int J Cancer. 2011;129:460–6. doi: 10.1002/ijc.25680. [DOI] [PubMed] [Google Scholar]
- 102.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–85. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boyer O, Saadoun D, Abriol J, Dodille M, Piette JC, Cacoub P, Klatzmann D. CD4+ CD25+ regulatory T cell deficiency in patients with hepatitis C-mixed cryoglobulinemia vasculitis. Blood. 2004;103:3428–30. doi: 10.1182/blood-2003-07-2598. [DOI] [PubMed] [Google Scholar]
- 104.Balandina A, Lecart S, Dartevelle P, Saoudi A, Berrih-Aknin S. Functional defect of regulatory CD4+ CD25+ T cells in the thymus of patients with myasthenia gravis. Blood. 2005;105:735–41. doi: 10.1182/blood-2003-11-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+ CD25hi T-regulatory cells. Blood. 2006;108:253–61. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+ CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–88. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]


