Abstract
Understanding the thymic processes that support the generation of functionally competent and self-tolerant lymphocytes requires dissection of the T-cell receptor (TCR) response to ligands of different affinities. In spatially segregated regions of the thymus, with unique expression of proteases and cytokines, TCR affinity guides a number of cell fate decisions. Yet affinity alone does not explain the selection paradox. Increasing evidence suggests that the ‘altered peptide’ model of the 1980s together with the affinity model might best explain how the thymus supports conventional and regulatory T-cell development. Development of new tools to study the strength of TCR signals perceived by T cells, novel regulatory T-cell transgenic mice, and tetramer enrichment strategies have provided an insight into the nature of TCR signals perceived during thymocyte development. These topics are discussed and support for the prevailing hypotheses is presented.
Keywords: affinity, natural killer T cell, regulatory T cell, T-cell receptor, thymus
Introduction
The ability of antigen receptors to engage self-ligands with varying affinity is crucial for lymphocyte maturation at multiple stages of development. In the absence of sensitivity to strong or weak antigen receptor signals, the homeostasis of the immune system is compromised and the risk of autoimmunity and/or infection ensues. T-cell receptor (TCR) recognition of ‘self’ ligands is required for T-cell development and survival (a process known as positive selection) but also poses the possibility of inducing cell death (negative selection) or regulatory T-cell development (agonist selection). Therefore, the affinity of the receptor–ligand interaction is crucial for determining lymphocyte fate.
Our understanding of the role of TCR affinity has relied largely on antigen receptor transgenic models that assume that monoclonal populations of cells behave in analogous ways to polyclonal populations. However, there is mounting evidence to suggest that this is not the case. Moreover, over the past 20 years we have come to appreciate that T-lymphocyte development generates a numerically small but functionally important population of cells with regulatory or suppressive functions via a process known as agonist selection. The strength of signal perceived by this population of cells during development is proposed by transgenic models but remains a controversial topic within the field. Although the role of TCR signal strength in γδ T-cell development is of great interest,1–3 the focus of this review will be αβ T cells. This review summarizes recent work supporting the role of TCR signal strength in positive, negative and agonist selection.
Thymic anatomy and lymphocyte development
Establishment of a functionally competent immune system that can respond to pathogens yet tolerate self-antigens happens during lymphocyte development. In the case of T cells, the thymus serves this specialized function. In the thymus, progenitor survival and lineage commitment require the TCR to interact with self-peptide MHC ligands on epithelial cells in the thymic cortex. These receptor–ligand interactions occur over a great range of affinities both because of the diversity of the TCR combining site amino acids, and because of the diversity of self-peptides displayed by each MHC allele. Quantifiable differences in TCR affinity for peptide–MHC (pMHC) complexes result in diametrically different selection outcomes, establishing the basis for positive and negative selection.4 Weak TCR signals support positive selection whereas strong, agonist, signals support the removal of a potentially self-reactive TCR through negative selection.
The thymus can be divided into two main compartments, the cortex, where immature thymocytes reside until they undergo positive selection, and the medulla, where negative selection occurs and where thymocytes undergo functional maturation before they exit from the thymus. Each region is composed of specialized epithelial cells named for the region in which they reside; cortical thymic epithelial cells (cTECs) or medullary thymic epithelial cells (mTECs). Each subset of epithelial cells is tasked with specific roles in establishing a competent T-cell repertoire. This is most evident by the differences in antigen-processing and presentation machinery in cTECs compared with mTECs or other antigen-presenting cells.5 For example, CD8 T-cell-positive selection requires MHC Class I and peptide processing by proteasomes. Interestingly, cTECs express a unique catalytic subunit of the proteosome, β5t.6 In mice deficient for this protein, CD8 T cells fail to develop.6 The cTECs also express unique proteases required for Class II antigen presentation, cathepsin L7 and thymus-specific serine protease.8 Analogous to β5t, the absence of cathepsin L or thymus-specific serine protease from the cortex results in a loss of CD4 T cells. Together, these data illustrate the distinctive function of cTECs in presenting pMHC complexes that support the positive selection of lymphocytes.
Interestingly, evolution also generated a system to allow thymocytes to ‘see’ a large array of self-peptides during development. This snapshot of ‘self’ happens in the medulla of the thymus via a specialized population of epithelial cells, mTECs. These cells express a gene know as autoimmune regulator (AIRE). AIRE is a transcriptional regulator that permits expression of a diverse array of strictly tissue-restricted peripheral antigens within the thymus to eliminate T cells with too strong an affinity for any of these antigens.9 Hence, thymocytes must express TCRs with incredible sensitivity to pMHC signals received during maturation to protect the host from allowing a self-reactive TCR to survive.10 Moreover, it is also true that thymocytes must express TCRs with the ability to transduce signals for very weak, low-affinity pMHC molecules to support positive selection. If they fail to recognize self pMHC and provide a TCR-specific survival signal, they undergo apoptosis via a process known as death by neglect.4 Therefore, a thymocyte's fate is ultimately determined by its specificity and affinity for self pMHC.
However, this process of segregating weak and strong signals into life or death fates is not simple. In fact, there is mounting evidence that strong TCR signals in developing thymocytes also support positive selection of some unique T cells – a process termed ‘agonist selection’.
TCR affinity and altered peptide models: they're not mutually exclusive
The basis of positive and negative selection relies on the premise that weak to moderate TCR signals support T-cell development whereas strong TCR signals support the culling of potentially self-reactive lymphocytes. Although this idea appears straightforward, the field has been challenged to explain how diametrically different cell fates could be supported by similar processes and generate a competent immune repertoire capable of responding to peripheral pMHC molecules. Nearly 25 years ago, in 1987, the hypothesis was put forward that the thymus supported positive selection by presenting unique pMHC molecules that would not be encountered anywhere else in the body.11 This idea was termed the ‘altered peptide’ model. Although an intriguing idea, when splenic antigen-presenting cells were shown to present many of the same MHC II peptides as those found on cTECs, the idea was dismissed.12 Moreover, peripheral ligands were subsequently identified that supported positive selection of thymocytes and homeostasis of peripheral T cells.13–15 Over time, the ‘altered peptide’ model fell out of favour.
An alternative to the altered peptide model is that positive and negative selection could be explained by differences in the affinity or avidity of a given pMHC for a developing thymocyte. Although early evidence showed that low concentrations of a high-affinity ligand could support positive selection in thymic organ culture,16,17 further analysis suggested that such cells are not functionally normal.18–20 Hence the prevailing model explaining the selection paradox is that variations in affinity influence the fate of the developing thymocyte.4,21,22 It was recently illustrated that this affinity window for positive versus negative selection is narrow and that remarkably small changes in the affinity of the TCR signal re-direct the fate of immature thymocytes.23 Interestingly, thymocytes are far more sensitive to various TCR affinities than mature T cells,10,24 so even minor changes in affinity can have a significant influence on the fate of the immature thymocyte. Analogous work in Class II restricted model systems has also generally confirmed the affinity model.14,15,25 Naturally occurring selecting peptides were ultimately identified for various Class I restricted13,26–28 and Class II restricted TCRs.15 Moreover, the affinity hypothesis has been validated in the polyclonal endogenous environment using a novel TCR signalling reporter mouse.29
Data supporting an affinity model of selection were accumulating in compelling fashion but interesting findings were also emerging that have called for a reconsideration of the prematurely dismissed ‘altered peptide’ model. Indeed, this model together with the affinity model may ultimately best explain the paradox of how the thymus supports the selection of a functional T-cell repertoire. As previously discussed, the epithelial cells of the cortex express unique proteases that are not present in other antigen-presenting cells in the body. For example, epithelial cells of the thymic cortex uniquely express the proteasomal subunit β5t whereas other thymic antigen-presenting cells express β5 or β5i.6 Because the proteasome is responsible for the generation of peptides for loading into MHC I molecules, it was hypothesized that inclusion of a different catalytic subunit would alter the nature of the peptides produced. Indeed, evidence to date supports this idea.30
What is the role of these unique peptides? Typically, proteasomes containing either β5 or β5i generate peptides with hydrophobic C-termini. Proteasomes containing β5t, on the other hand, did not efficiently cleave substrates after hydrophobic residues, at least in vitro. This may potentially be important because C-terminal hydrophobicity allows for strong binding between the peptide and MHC grooves, thereby stabilizing the pMHC complex. It is possible that stable pMHC complexes allow for longer and possibly stronger interactions with T cells than less stable ones, and that the specialized role of the β5t-containing proteasome in cortical epithelial cells is to generate less stable pMHC complexes.31 Alternatively, all of the unique proteolysis genes expressed in cortical epithelial cells may simply serve to create a non-redundant pool of peptides, to maximize the number of clones that are positively selected, but not subsequently negatively selected.32 Further work will be required to distinguish these possibilities.
Differential TCR signals drive positive and negative selection
Negative selection requires up-regulation of the pro-apoptotic Bcl-2 family member, Bim. Two recent reports illustrate the fascinating role of antigen affinity and location within the thymus. In the HY TCR model of ubiquitous male antigen presentation, Bim deficiency failed to rescue cells from negative selection.33,34 However, in a different TCR model (OT-I/Rip-mOVA) where antigen presentation was limited to the medulla, T cells deficient in Bim were rescued from negative selection.29 The relevant difference in outcome is the result of the anatomic location of negative selection, because even in the same model system self-reactive T cells could not be rescued if deletion occurred early (in the cortex), but it was rescued if deletion occurred later (in the medulla).35 At first glance, these data appear contradictory. In a simple threshold model of selection (Fig. 1, left), low-affinity interactions trigger sufficient intracellular signals to induce survival and maturation, whereas higher affinity interactions are required to exceed the threshold for induction of apoptosis. Bim deficiency should therefore rescue T cells from deletion, regardless of the anatomic context. However, multiple studies have suggested that low-affinity TCR signals are biochemically unique,23,36,37 particularly regarding sustained extracellular signal-regulated kinase activation. High-affinity TCR signals do not generate a similar signal, possibly because of the induction of mitogen-activated protein kinase phosphatases, which shorten the duration of extracellular signal-regulated kinase signals. This suggests a sustained signalling model of selection (Fig. 1, right) where signal duration is a crucial factor. Hence, in situations of exposure to high-affinity ligands in the thymic cortex, even when acute apoptosis is prevented by Bim deficiency, the cell will fail positive selection, and ultimately will not mature to the single-positive (SP) stage, or survive beyond the ordinary lifespan of a double-positive (DP) thymocyte (which is not regulated by Bim). In other words, Bim deficiency at this stage fails to rescue this population of T cells because they have failed to be positively selected. However, in models where agonist ligand expression is restricted to the medulla, thymocytes are first positively selected on low-affinity ligands in the cortex. After maturing and migrating to the medulla they encounter high-affinity ligands and subsequently undergo negative selection in a Bim-dependent fashion. In this case Bim deficiency can rescue a mature, self-reactive T-cell population that subsequently populates the peripheral tissues (A. Suen, T.A. Baldwin, manuscript submitted).29
Figure 1.
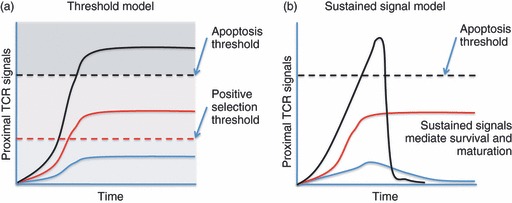
Threshold versus sustained signalling models of thymic selection. (a) A simplistic model to explain how T-cell receptor (TCR) signal strength can influence positive/negative selection invokes a higher threshold for negative selection, and a lower threshold for positive selection. Clones with TCRs that recognize and transduce a signal sufficient to promote survival and differentiation but not strong enough to trigger apoptosis are positively selected by the process. Importantly, in this model if apoptosis were prevented, high-affinity interactions would lead to positive selection. (b) Evidence suggests that stronger proximal TCR signals induce more negative feedback. A sustained signalling model proposes that low-affinity TCR interactions trigger positive selection because they are sustained over time. High-affinity signals can trigger apoptosis. However, in this model if apoptosis were prevented, high-affinity interactions would still not lead to positive selection because of the lack of a sustained TCR signal.
The affinity of a developing thymocyte's TCR not only drives positive or negative selection, but may also influence the helper/killer lineage choice. There has been much debate as to how DP thymocytes become either an MHC class II restricted CD4 single-positive (CD4SP) T cell or an MHC class I restricted CD8 single-positive (CD8SP) T cell. Evidence to date suggests that it is determined by the strength and duration of signals perceived by the TCR, as reviewed in ref. 38, with CD4 T cells generally experiencing stronger self signals than CD8 T cells.29
Conventional and non-conventional T-cell selection
It is well established that there are three distinct lineage ‘choices’ for a developing thymocyte: death by neglect when the progenitor fails to generate a TCR that recognizes self pMHC, positive selection when the TCR has a low affinity for self pMHC, and negative selection when the TCR has a high affinity for self pMHC. Each of these fates reinforces the concept that the TCR is highly sensitive to affinity and that it is in the best interest of the organism to eliminate any potentially self-reactive and high-affinity T cells. However, negative selection is not perfect and some high affinity TCRs may replace their TCR α chains to generate a new lower affinity TCR (a process known as receptor editing).39 Alternatively, thymocytes expressing high-affinity TCRs can be rendered functionally inactive, a process known as anergy.40 Either of these outcomes results in a cell with a TCR that is no longer self-reactive and theoretically has undergone an alternative form of negative selection.4
Nonetheless, there has been a long-standing debate about the role of TCR signal strength in γδ,41 and some αβ T cell subsets: these include CD4+ CD25+ Foxp3+ regulatory T cells (Treg cells),42 natural killer T cells (NKT cells),43 CD8αα intestinal intraepithelial lymphocytes,44 and RORγt+ natural T helper type 17 cells.45 For αβ T cells, this lineage choice, known as ‘agonist’ selection, diverts thymocytes with high-affinity TCRs to one of a number of distinct regulatory-like T-cell subsets. Agonist selection is based on the idea that the selecting self-peptide is an activating (agonist) ligand found in the thymus.46 In addition, these populations express elevated phenotypic markers like CD69 and CD44, which supports this notion.
The idea that Treg-cell differentiation would be ‘instructed’ by self-reactive TCR specificities seems intuitive. Treg cells have an ‘antigen-experienced’ phenotype in the absence of any intentional exposure to foreign antigens. Furthermore, recombination-activating gene (RAG) -deficient mice that express transgenes encoding foreign antigen-specific TCRs generally do not have Foxp3+ Treg cells. Yet when these mice are crossed with mice expressing the cognate foreign antigen, Treg cells do appear, highlighting a possible role of high-affinity TCR signals in Treg-cell development.47,48
The important question of the affinity of TCRs in polyclonal Treg-cell differentiation has been more controversial. Conceptually, it would seem that the repertoires of the conventional CD4 population would be distinctly different from that of the regulatory T-cell population if they were selected on distinct high-affinity self-ligands. And although there are robust data to suggest that this is the case,49 it has also been argued that the repertoires are mostly overlapping50 and that Treg cells may develop independently of TCR specificity. Recently, however, two groups addressed this question by creating TCR transgenic mice using TCR genes cloned from natural Treg cells.51,52
The Treg TCR transgenic mice were generated by cloning the naturally occurring TCR from Treg cells.51,52 The initial hypothesis suggested that a Treg TCR transgenic mouse would have dramatically increased numbers of Foxp3+ Treg cells. Unexpectedly, both groups reported that very few cells expressed Foxp3 in RAG-deficient mice expressing a Treg-cell-derived TCR transgene. Yet when these progenitors were mixed with a polyclonal population of T cells, they found a striking inverse correlation between the precursor frequency of TCR transgenic cells and the propensity of those cells to give rise to Foxp3+ Treg cells. This result suggests that there is a ‘niche’ that limits Treg-cell development. Interestingly, in the same type of mixed bone marrow chimeras created with TCRs cloned from the non-Treg-cell repertoire, Foxp3+ cells were not found, even at low frequencies of TCR transgenic precursor cells. Overall, these results suggest that TCR specificity does influence Treg-cell potential but that Treg cells compete for a limiting niche during development, be it ligand or growth factors, or something else. Moreover, these Treg TCR transgenic T cells were shown to be more self-reactive than those clones derived from non-Treg TCRs. These data are consistent with the idea that Treg cells express higher affinity TCRs for self pMHC49 than conventional CD4+ T cells. Using a TCR signalling reporter mouse, it was shown that strong TCR signalling was only perceived at low precursor frequency, suggesting that competition occurs at the level of recognition of high-affinity ligands, which are presumably not abundant.29 Furthermore, polyclonal Treg cells continued to perceive higher-affinity TCR signals in the periphery when compared with CD4+ CD25− conventional T cells.29
In light of these data, we propose a model for Treg-cell development from thymocyte progenitors with Class II-restricted TCRs (Fig. 2). In this model, a certain low threshold of TCR affinity for self pMHC in the cortex is required for positive selection. Selected cells move to the medulla, and encounter distinct antigen-presenting cells including mTEC and dendritic cells. Depending on the affinity of those interactions, and the availability of cytokines such as interleukin-253 or transforming growth factor-β,54 the progenitor may differentiate into a mature naive CD4 T cell or a Foxp3+ regulatory T cell. Stronger TCR interactions increase the propensity to give rise to Treg cells, although this probably never reaches 100%, as the strongest interactions will also cull progenitors from the repertoire via clonal deletion (Fig. 2).
Figure 2.
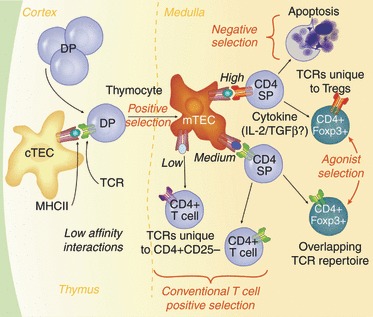
T regulatory (Treg) cells integrate cytokine and antigen receptor signal strength cues during thymic development. CD4+ CD8+ double-positive (DP) thymocytes undergo positive selection on cortical thymic epithelial cells (cTECs) presenting low-affinity peptide–MHC complex (pMHC) ligands in the thymic cortex, then migrate to the medulla where they encounter novel pMHC ligands on medullary thymic epithelial cells (mTECs) and dendritic cells. Those clones that encounter high-affinity pMHC ligands will be triggered to undergo apoptosis. However, clones that coincidently encounter survival cytokines such as interleukin-2 (IL-2) or transforming growth factor β (TGFβ), could be rescued and directed to become a Treg cell. Such clones would express TCRs unique to the Treg-cell population. Clones with a slightly lower relative affinity (medium) could either develop into a conventional CD4+ T cell in the absence of a cytokine encounter, or into a regulatory T cell with exposure to IL-2. Hence, some TCRs in the Treg-cell repertoire would overlap with those of conventional CD4+ T cells.
As with Treg-cell development, the role of TCR signal strength in NKT-cell lineage commitment is still under investigation. NKT-cell development is unique in that rare progenitors with a canonical Va14+ TCR require encounter with self-lipid–CD1d complexes presented by other thymocytes.55 Their selection strictly requires co-stimulation via SLAM (signalling lymphocyte-activation molecule) family members. Evidence for the hypothesis that NKT cells are selected on high-affinity self-ligands in the thymus began to accumulate after it was observed that this population of T cells had an activated phenotype in the thymus. However, the lack of dependence on CD1d for peripheral maintenance of NKT cells56 suggested that they might not be overtly self-reactive. Yet, NKT cells can rapidly produce the cytokines interleukin-4 and interferon-γ. The production of these cytokines within hours after TCR activation suggests previous ‘priming’ by an agonist ligand and it was proposed that this ligand was a positively selecting thymic self-lipid ligand. However, exposure to the very strong NKT cell agonist α-galactosylceramide led to negative selection,57,58 so any potential agonist ligand must not be as stimulatory as α-galactosylceramide. The lipid isoglobotrihexosylceramide (iGb3) was identified as a potential selecting ligand,59 and indeed this lipid can stimulate NKT cells, although it is currently a matter of debate whether iGb3 is a physiologically relevant or non-redundant selecting lipid. The earliest NKT cell progenitors, termed ‘stage 0’, expressed very high levels of green fluorescent protein in the recently developed TCR signalling reporter mouse, suggesting that these progenitors experienced high-affinity TCR signals at the time of selection.29 Interestingly, NKT cells did not retain high levels of green fluorescent protein in the periphery, suggesting that stimulatory lipids are not continuously encountered by NKT cells, as they are by Treg T cells. This may turn out to be fundamental for the biology of NKT cells, as current evidence suggests that infection induces the display of stimulatory self-lipids that triggers NKT-cell activation and cytokine production.60,61
Conclusion
Until recently, the prevailing hypothesis for thymocyte development relied exclusively on the role of TCR affinity in directing T-cell selection and dismissed the role of altered peptide repertoires. In light of recent data on the unique expression of proteasomal machinery restricted to cortical epithelial cells and subsequently unique pMHC complexes presented in the cortex, the ‘altered peptide’ model, together with the affinity model, may best describe the general selection paradox. Moreover, as we begin to appreciate other unique signals provided by distinct thymic microenviroments, such as co-stimulatory molecules, self-lipids and cytokines, we can begin to piece together how functionally diverse populations of naive and regulatory T cells are all produced by this complex organ. An important focus for the future will be to confirm and extend findings from TCR transgenic models in the polyclonal repertoire. New tools, such as tetramer enrichment62,63 and signalling reporters29 will certainly aid this effort.
Acknowledgments
This work was supported by the National Institutes of Health (grant RO1 AI39560 to K.A. Hogquist and grant T32 AI007313 to A.E. Moran).
Disclosures
The authors declare no conflicts of interest.
References
- 1.Haks MC, Lefebvre JM, Lauritsen JP, Carleton M, Rhodes M, Miyazaki T, Kappes DJ, Wiest DL. Attenuation of γδTCR signaling efficiently diverts thymocytes to the αβ lineage. Immunity. 2005;22:595–606. doi: 10.1016/j.immuni.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Kreslavsky T, Garbe AI, Krueger A, von Boehmer H. T cell receptor-instructed αβ versus γδ lineage commitment revealed by single-cell analysis. J Exp Med. 2008;205:1173–86. doi: 10.1084/jem.20072425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciofani M, Zuniga-Pflucker JC. Determining γδ versus αβ T cell development. Nat Rev Immunol. 2010;10:657–63. doi: 10.1038/nri2820. [DOI] [PubMed] [Google Scholar]
- 4.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–76. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 5.Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009;9:833–44. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- 6.Murata S, Sasaki K, Kishimoto T, Niwa S, Hayashi H, Takahama Y, Tanaka K. Regulation of CD8+ T cell development by thymus-specific proteasomes. Science. 2007;316:1349–53. doi: 10.1126/science.1141915. [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa T, Roth W, Wong P, et al. Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science. 1998;280:450–3. doi: 10.1126/science.280.5362.450. [DOI] [PubMed] [Google Scholar]
- 8.Gommeaux J, Gregoire C, Nguessan P, Richelme M, Malissen M, Guerder S, Malissen B, Carrier A. Thymus-specific serine protease regulates positive selection of a subset of CD4+ thymocytes. Eur J Immunol. 2009;39:956–64. doi: 10.1002/eji.200839175. [DOI] [PubMed] [Google Scholar]
- 9.Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self shadow within the thymus by the AIRE protein. Science. 2002;298:1395–401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 10.Davey GM, Schober SL, Endrizzi BT, Dutcher AK, Jameson SC, Hogquist KA. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med. 1998;188:1867–74. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marrack P, Kappler J. The T cell receptor. Science. 1987;238:1073–9. doi: 10.1126/science.3317824. [DOI] [PubMed] [Google Scholar]
- 12.Marrack P, Ignatowicz L, Kappler JW, Boymel J, Freed JH. Comparison of peptides bound to spleen and thymus class II. J Exp Med. 1993;178:2173–83. doi: 10.1084/jem.178.6.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogquist KA, Tomlinson AJ, Kieper WC, McGargill MA, Hart MC, Naylor S, Jameson SC. Identification of a naturally occurring ligand for thymic positive selection. Immunity. 1997;6:389–99. doi: 10.1016/s1074-7613(00)80282-4. [DOI] [PubMed] [Google Scholar]
- 14.Lo WL, Felix NJ, Walters JJ, Rohrs H, Gross ML, Allen PM. An endogenous peptide positively selects and augments the activation and survival of peripheral CD4+ T cells. Nat Immunol. 2009;10:1155–61. doi: 10.1038/ni.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebert PJ, Jiang S, Xie J, Li QJ, Davis MM. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nat Immunol. 2009;10:1162–9. doi: 10.1038/ni.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashton-Rickardt PG, Van Kaer L, Schumacher TN, Ploegh HL, Tonegawa S. Peptide contributes to the specificity of positive selection of CD8+ T cells in the thymus. Cell. 1993;73:1041–9. doi: 10.1016/0092-8674(93)90281-t. [DOI] [PubMed] [Google Scholar]
- 17.Sebzda E, Wallace VA, Mayer J, Yeung RS, Mak TW, Ohashi PS. Positive and negative thymocyte selection induced by different concentrations of a single peptide. Science. 1994;263:1615–8. doi: 10.1126/science.8128249. [DOI] [PubMed] [Google Scholar]
- 18.Hogquist KA, Jameson SC, Bevan MJ. Strong agonist ligands for the T cell receptor do not mediate positive selection of functional CD8+ T cells. Immunity. 1995;3:79–86. doi: 10.1016/1074-7613(95)90160-4. [DOI] [PubMed] [Google Scholar]
- 19.Hogquist KA, Bonnevier JL. Development of peptide-selected CD8 T cells in fetal thymic organ culture occurs via the conventional pathway. J Immunol. 1998;161:3896–901. [PubMed] [Google Scholar]
- 20.Yamagata T, Mathis D, Benoist C. Self-reactivity in thymic double-positive cells commits cells to a CD8 αα lineage with characteristics of innate immune cells. Nat Immunol. 2004;5:597–605. doi: 10.1038/ni1070. [DOI] [PubMed] [Google Scholar]
- 21.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 22.Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NR. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–20. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 23.Daniels MA, Teixeiro E, Gill J, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–9. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 24.Lucas B, Stefanová I, Yasutomo K, Dautigny N, Germain RN. Divergent changes in the sensitivity of maturing T cells to structurally related ligands underlies formation of a useful T cell repertoire. Immunity. 1999;10:367–76. doi: 10.1016/s1074-7613(00)80036-9. [DOI] [PubMed] [Google Scholar]
- 25.Williams CB, Engle DL, Kersh GJ, Michael White J, Allen PM. A kinetic threshold between negative and positive selection based on the longevity of the T cell receptor–ligand complex. J Exp Med. 1999;189:1531–44. doi: 10.1084/jem.189.10.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santori FR, Kieper WC, Brown SM, et al. Rare, structurally homologous self-peptides promote thymocyte positive selection. Immunity. 2002;17:131–42. doi: 10.1016/s1074-7613(02)00361-8. [DOI] [PubMed] [Google Scholar]
- 27.Sasada T, Ghendler Y, Neveu JM, Lane WS, Reinherz EL. A naturally processed mitochondrial self-peptide in complex with thymic MHC molecules functions as a selecting ligand for a viral-specific T cell receptor. J Exp Med. 2001;194:883–92. doi: 10.1084/jem.194.7.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Q, Bazemore Walker CR, Girao C, Opferman JT, Sun J, Shabanowitz J, Hunt DF, Ashton-Rickardt PG. Specific recognition of thymic self-peptides induces the positive selection of cytotoxic T lymphocytes. Immunity. 1997;7:221–31. doi: 10.1016/s1074-7613(00)80525-7. [DOI] [PubMed] [Google Scholar]
- 29.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–89. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nitta T, Murata S, Sasaki K, et al. Thymoproteasome shapes immunocompetent repertoire of CD8+ T cells. Immunity. 2010;32:29–40. doi: 10.1016/j.immuni.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Ziegler A, Muller CA, Bockmann RA, Uchanska-Ziegler B. Low-affinity peptides and T-cell selection. Trends Immunol. 2009;30:53–60. doi: 10.1016/j.it.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Hogquist KA, Xing Y. Why CD8+ T cells need diversity when growing up. Immunity. 2010;32:5–6. doi: 10.1016/j.immuni.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Hu Q, Sader A, Parkman JC, Baldwin TA. Bim-mediated apoptosis is not necessary for thymic negative selection to ubiquitous self-antigens. J Immunol. 2009;183:7761–7. doi: 10.4049/jimmunol.0902181. [DOI] [PubMed] [Google Scholar]
- 34.Kovalovsky D, Pezzano M, Ortiz BD, Sant'Angelo DB. A novel TCR transgenic model reveals that negative selection involves an immediate, Bim-dependent pathway and a delayed, Bim-independent pathway. PLoS One. 2010;5:e8675. doi: 10.1371/journal.pone.0008675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suen A, Baldwin TA. Differential requirement for Bim during thymic clonal deletion to ubiquitous versus tissue restricted antigens. Proc Natl Acad Sci USA. 2012 doi: 10.1073/pnas.1114834109. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNeil LK, Starr TK, Hogquist KA. A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc Natl Acad Sci U S A. 2005;102:13574–9. doi: 10.1073/pnas.0505110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das J, Ho M, Zikherman J, Govern C, Yang M, Weiss A, Chakraborty AK, Roose JP. Digital signaling and hysteresis characterize ras activation in lymphoid cells. Cell. 2009;136:337–51. doi: 10.1016/j.cell.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4– versus CD8– lineage choice. Nat Rev Immunol. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGargill MA, Derbinski JM, Hogquist KA. Receptor editing in developing T cells. Nat Immunol. 2000;1:336–41. doi: 10.1038/79790. [DOI] [PubMed] [Google Scholar]
- 40.Ramsdell F, Fowlkes BJ. Clonal deletion versus clonal anergy: the role of the thymus in inducing self tolerance. Science. 1990;248:1342–8. doi: 10.1126/science.1972593. [DOI] [PubMed] [Google Scholar]
- 41.Pennington DJ, Silva-Santos B, Hayday AC. γδ T cell development – having the strength to get there. Curr Opin Immunol. 2005;17:108–15. doi: 10.1016/j.coi.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Fontenot JD, Rudensky AY. Molecular aspects of regulatory T cell development. Semin Immunol. 2004;16:73–80. doi: 10.1016/j.smim.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Gapin L. The making of NKT cells. Nat Immunol. 2008;9:1009–11. doi: 10.1038/ni0908-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leishman AJ, Gapin L, Capone M, Palmer E, MacDonald HR, Kronenberg M, Cheroutre H. Precursors of functional MHC class I- or class II-restricted CD8 αα+ T cells are positively selected in the thymus by agonist self-peptides. Immunity. 2002;16:355–64. doi: 10.1016/s1074-7613(02)00284-4. [DOI] [PubMed] [Google Scholar]
- 45.Marks BR, Nowyhed HN, Choi JY, Poholek AC, Odegard JM, Flavell RA, Craft J. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat Immunol. 2009;10:1125–32. doi: 10.1038/ni.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baldwin TA, Hogquist KA, Jameson SC. The fourth way? Harnessing aggressive tendencies in the thymus. J Immunol. 2004;173:6515–20. doi: 10.4049/jimmunol.173.11.6515. [DOI] [PubMed] [Google Scholar]
- 47.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+ CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–6. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 48.Wirnsberger G, Hinterberger M, Klein L. Regulatory T-cell differentiation versus clonal deletion of autoreactive thymocytes. Immunol Cell Biol. 2011;89:45–53. doi: 10.1038/icb.2010.123. [DOI] [PubMed] [Google Scholar]
- 49.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–10. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 50.Pacholczyk R, Kern J, Singh N, Iwashima M, Kraj P, Ignatowicz L. Nonself-antigens are the cognate specificities of Foxp3+ regulatory T cells. Immunity. 2007;27:493–504. doi: 10.1016/j.immuni.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, Luo J, Rudensky AY, Hsieh CS. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol. 2009;10:610–7. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leung MW, Shen S, Lafaille JJ. TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. J Exp Med. 2009;206:2121–30. doi: 10.1084/jem.20091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burchill MA, Yang J, Vang KB, Farrar MA. Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol Lett. 2007;114:1–8. doi: 10.1016/j.imlet.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ouyang W, Beckett O, Ma Q, Li MO. Transforming growth factor-β signaling curbs thymic negative selection promoting regulatory T cell development. Immunity. 2010;32:642–53. doi: 10.1016/j.immuni.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 56.McNab FW, Berzins SP, Pellicci DG, Kyparissoudis K, Field K, Smyth MJ, Godfrey DI. The influence of CD1d in postselection NKT cell maturation and homeostasis. J Immunol. 2005;175:3762–8. doi: 10.4049/jimmunol.175.6.3762. [DOI] [PubMed] [Google Scholar]
- 57.Hayakawa Y, Berzins SP, Crowe NY, Godfrey DI, Smyth MJ. Antigen-induced tolerance by intrathymic modulation of self-recognizing inhibitory receptors. Nat Immunol. 2004;5:590–6. doi: 10.1038/ni1069. [DOI] [PubMed] [Google Scholar]
- 58.Chun T, Page MJ, Gapin L, et al. CD1d-expressing dendritic cells but not thymic epithelial cells can mediate negative selection of NKT cells. J Exp Med. 2003;197:907–18. doi: 10.1084/jem.20021366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou D, Mattner J, Cantu C, III, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–9. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 60.Mattner J, Debord KL, Ismail N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–9. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 61.Darmoise A, Teneberg S, Bouzonville L, Brady RO, Beck M, Kaufmann SH, Winau F. Lysosomal α-galactosidase controls the generation of self lipid antigens for natural killer T cells. Immunity. 2010;33:216–28. doi: 10.1016/j.immuni.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–5. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 63.Chu HH, Moon JJ, Takada K, et al. Positive selection optimizes the number and function of MHCII-restricted CD4+ T cell clones in the naive polyclonal repertoire. Proc Natl Acad Sci U S A. 2009;106:11241–5. doi: 10.1073/pnas.0902015106. [DOI] [PMC free article] [PubMed] [Google Scholar]


