Abstract
The observation that human monocytes cultured in the presence of the chemokine CCL18 showed increased survival, led us to profile cytokine expression in CCL18-stimulated versus control cultures. CCL18 caused significantly increased expression of chemokines (CXCL8, CCL2, CCL3 and CCL22), interleukin-10 (IL-10) and platelet-derived growth factor, but no up-regulation of M1 cytokines IL-1β or IL-12. CCL18-stimulated monocytes matured into cells with morphological resemblance to IL-4-stimulated macrophages, and expressed the monocyte marker CD14 as well the M2 macrophage markers CD206 and 15-lipoxygenase, but no mature dendritic cell markers (CD80, CD83 or CD86). Functionally, CCL18-stimulated macrophages showed a high capacity for unspecific phagocytosis and for pinocytosis, which was not associated with an oxidative burst. These findings suggest that CCL18-activated macrophages stand at the cross-roads between inflammation and its resolution. The chemokines that are produced in response to CCL18 are angiogenic and attract various leucocyte populations, which sustain inflammation. However, the capacity of these cells to remove cellular debris without causing oxidative damage and the production of the anti-inflammatory IL-10 will initiate termination of the inflammatory response. In summary, CCL18 induces an M2 spectrum macrophage phenotype in the absence of IL-4.
Keywords: chemokines/monokines, macrophages/monocytes, phagocytosis
Introduction
Monocytes and macrophages (MΦ) play an important role in innate and adaptive immunity. Following release from the bone marrow, monocytes circulate in the bloodstream for 24–48 hr before they are recruited to tissues, a migration that is mediated by the co-operation between integrins1,2 and chemokines, primarily CCL2 and CX3CL1.3–5 Once in the tissue, monocytes show amazing plasticity. Depending on the stimuli they receive from the surrounding environment, they can develop into myeloid dendritic cells or various forms of MΦ, which can acquire distinctly different functions depending on the specific signals provided by the milieu.6 For example ‘classically activated MΦ’ or M1 MΦ stimulated by interferon-γ followed by lipopolysaccharide (LPS) exposure, phagocytose pathogens and pathogen components; suitable peptide antigens are processed and loaded onto MHC class II molecules for T-cell presentation. This is associated with the production of reactive oxidant species both through NADPH oxidase activation7 and up-regulation of inducible nitric oxide synthase (iNOS),8 the production of cytotoxic cytokines including interleukin-1β (IL-1β), IL-6, tumour necrosis factor-α (TNF-α) and IL-129 and release of various matrix metalloproteinases which degrade extracellular matrix. The combination of these responses renders them potent microbe killers, but may also cause extensive tissue damage.
In contrast, IL-4-stimulated, so-called M2, MΦ10 are able to phagocytose without oxidant production11 and fail to produce IL-1β and IL-12.9,12 They do, however, release various chemokines11 including CCL1813 and CCL22,14 the anti-inflammatory IL-10, several fibrotic factors including platelet-derived growth factor (PDGF) and transforming growth factor -β,15 and extracellular matrix proteins including fibronectin.16 CCL18 production by MΦ is highly up-regulated in the presence of fibroblasts, partially because of the collagen produced by the fibroblasts.17
Chemokines are best known for their role in leucocyte trafficking. Although they have many additional physiological and pathophysiological functions ranging from angiogenesis to metastasis, most of these functions involve cell migration and are short-term, as would be anticipated from activation of mostly Gi-protein-coupled 7-transmembrane spanning receptors. However, there are an increasing number of examples in which chemokines showed long-ranging effects on haematopoietic cells including haematopoietic stem cells,18,19 monocytes20 and dendritic cells.21
CCL18 is also referred to as pulmonary and activation-regulated chemokine (PARC),22 DC-CK-1,23 alternative macrophage activation associated CC-chemokine (AMAC-1) and macrophage inflammatory protein 4 (MIP-4), and is a member of the CC-chemokine family. It is most closely related to CCL3 with which it shares 64% sequence identity. However, it does not activate the same receptors as CCL3, which is a ligand for both CCR1 and CCR5, and so far the identity of the CCL18 receptor(s) on leucocytes has not been elucidated, although a recent report describes a CCL18 receptor on breast cancer cells, which is not a G-protein-coupled receptor, but instead a membrane-associated phosphatidylinositol transfer protein called PITPNM3.24
CCL18 is produced primarily by cells of myeloid origin. It has been detected at high levels in alveolar MΦ22 and IL-4-activated or IL-13-activated M2 MΦ,13,25 and in lower amounts in various lymphatic tissues,22 in dendritic cells,23,26 bone-marrow22 and adherent monocytes and eosinophils.27
In spite of various reports which suggest a role for CCL18 in a variety of chronic, often T helper type 2 (Th2) -mediated diseases17,28–33 it has been difficult to assess its function in vivo, because it appears to be present only in primates rendering studies in rodents problematic, although a recent report indicates that adeno-virally transferred CCL18 induced transient lung fibrosis in mice.34
In vitro, it has been shown that CCL18 is chemotactic for lymphocytes,22 in particular for naive T cells,23 Th2 cells28 and B cells35 as well as for immature dendritic cells.26 While freshly isolated monocytes do not respond to CCL18,22,27 maturing monocytes/MΦ cultured for 3–4 days acquire transient responsiveness to CCL1827 including increased survival.19 Here we show a novel function for CCL18 as a maturation factor for monocytes, which leads to the development of MΦ in the M2 spectrum, which produce high concentrations of chemokines and IL-10, insignificant levels of cytotoxic cytokines and reactive oxidant species, and which are potent phagocytic cells.
Materials and methods
Reagents
Recombinant CCL18 was purified as described with some modifications.19 Our original construct contained two additional amino acids at the N-terminus, which might influence ligand affinity, so the construct was redesigned to obtain a recombinant protein that is identical to natural CCL18 consisting of 69 amino acids.36 Endotoxin was determined with the Limulus amoebocyte assay (Cambrex, East Rutherford, NJ) and was < 0·1 ng/μg protein in all CCL18 preparations used. Vitronectin and fibronectin were purified from human serum as previously described,37 rat tail type 1 collagen was from Millipore (Jaffrey, NH), and human umbilical cord hyaluronic acid (HA) was from Sigma-Aldrich (St Louis, MO).
Mouse FITC-anti-CD14, FITC-anti-CD80, FITC-anti-CD11b, FITC-anti-CD11c, allophycocyanin (APC)-anti-CD83, FITC-anti-CD86, CyChrome-anti-HLA-DR, anti-iNOS and isotype controls were purchased from Becton Dickinson Biosciences (San Diego, CA), FITC-anti-CD163 and APC-anti-CD206 were from BioLegend (San Diego, CA). Goat anti-PITPNM3/NIR1 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Guinea pig anti-15-lipoxygenase was a gift from Dr Y. Miller (UCSD). Secondary FITC and phycoerythrin (PE) antibodies were from Santa Cruz Biotechnology, and rhodamine Red-X-conjugated donkey anti-guinea pig IgG was supplied by Jackson ImmunoResearch (West Grove, PA).
Cell isolation and culture
Monocytes were purified from fresh human blood (San Diego Blood Bank) by Ficoll and Percoll gradient centrifugation followed by adhesion to tissue culture plastic as previously described27 and were cultured in RPMI-1640 containing 10% AB serum (complete media). Following 72 hr in culture, adherent cells were dissociated on ice with cell dissociation buffer (Invitrogen, Carlsbad, CA), centrifuged and resuspended in complete media or serum-free RPMI-1640 at a concentration of 106 cells/ml. For cytokine/chemokine detection, supernatants were harvested after 20 hr in serum-free media. For MΦ maturation experiments, cells (cultured for 48 hr) were stimulated in full media with 40 nm CCL18, 20 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF), 20 ng/ml IL-4, a combination of the same concentrations of GM-CSF and IL-4 to produce immature dendritic cells, 50 ng/ml macrophage colony-stimulating factor, 20 ng/ml interferon-γ (IFN-γ) (both R&D Systems, Minneapolis, MN), 100 ng/ml LPS (Escherichia coli; Sigma) or no addition for an additional 4 days.
Immuno-detection of phospho-ERK1/2, phospho-Akt, and iNOS
Monocytes (day 3) which had been serum-starved overnight, were stimulated with 100 nm CCL18 for the indicated times, cell lysates were prepared in modified radio-immune precipitation buffer (50 mm Tris–HCl, pH 7·4, 10% glycerol, 1% nonidet P-40, 150 mm NaCl, 5 mm MgCl2, 2 mm EDTA, 2 mm PMSF, 2 μg/ml leupeptin, 2 μg/ml aprotinin, 2 mm sodium pyrophosphate, 2 mm sodium vanadate and 10 mm NaF) and clarified by centrifugation. Nuclear and cytoplasmic fractions were prepared as previously described.38 These samples were resolved by SDS–PAGE, transferred to nitrocellulose membranes, blocked with 4% dry milk in Tris-buffered saline-Tween, and exposed to anti-phospho-extracellular signal-regulated kinase (ERK) 1/2 or anti-phospho-Akt antibody (Cell Signaling, Danvers, MA). Antibody binding was detected using horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (ECL Plus; GE Healthcare, Piscataway, NJ). Phospho-blots were re-probed with total ERK1/2 or Akt antibody (Cell Signaling) to assure equal loading. iNOS blots were reprobed with anti-β-actin as a loading control.
For the detection of C7, MΦ supernatants were concentrated fivefold, underwent electrophoresis and were transferred to nitrocellulose as described and developed using rabbit anti-human anti-C7 polyclonal antibody.
FACS analysis
FACS detection of surface molecules followed a general FACS protocol.19 The cells were harvested using cell dissociation buffer at 4° with gentle scraping. For the cell maturation studies various MΦ stimuli were added on day 2, and MΦ/dendritic cell marker antibodies (see Materials and methods) were used to characterize cells on day 6. FACS analysis was performed on a FACSCAN or LSRII instrument (Becton-Dickinson) with Cellquest Pro or Diva software. For the integrin expression studies, MΦ were stimulated for 1 hr with 40 nm CCL18 or control buffer on day 3, harvested as described above and stained with anti-CD11b and anti-CD11c antibody.
Immunofluorescence staining
For confocal microscopy, MΦ (day 2) were seeded on collagen-coated cover slips, and cultured as described above for 4 days in the presence or absence of CCL18 or IL-4. Following fixation with paraformaldehyde, cells were permeabilized with 0·2% Triton-X 100 in PBS and Fc receptors were blocked with Fc Blocking Reagent (Miltenyi Biotec, Auburn, CA). Following incubation with anti-15-lipoxygenase 1 (LOX-1) antibody overnight, the cells were stained with rhodamine Red-X conjugated donkey anti-guinea pig IgG. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI), and the slides were mounted with Slow Fade reagent (Invitrogen) and inspected on an Olympus FV1000 confocal microscope.
Cytokine/chemokine protein detection
Monocytes (day 3) were stimulated with 40 nm CCL18 for 20 hr in serum-free conditions. ELISA kits from BioLegend (IL-6, CXCL8, TNF-α) or R&D Systems (CCL3 and CCL5 and CCL22) were used for cytokine/chemokine quantification in the culture supernatants. Additional cytokines (CCL2, IL-1β, IL-10, IL-12, IL-13 and IFN-γ) were quantified by Q-Plex arrays (BioLegend).
For the CCL18 detection assays 35-mm Falcon tissue culture plates were coated with vitronectin, fibronectin, collagen type 1 or hyaluronic acid (all at 100 μg/ml) in PBS at 37° for 2 hr, followed by two washes with PBS. Monocytes (day 3, 106 cells/ml) were plated on these plates in macrophage serum-free (MSF) media (Invitrogen) in the presence or absence of 20 ng/ml IL-4. Supernatants were collected 48 hr later and CCL18 was detected by ELISA using mouse monoclonal CCL18 antibody (R&D Systems) as coating antibody and biotinylated rabbit-anti-CCL18 as detection antibody.27
Unspecific phagocytosis and pinocytosis assay
Unspecific phagocytosis was determined as described.20 On day 6 of culture, MΦ were removed with cell dissociation buffer at 4°, resuspended at 5 × 105 cells/ml in phenol-red-free RPMI-1640 containing 25 mm HEPES (RPMI-B). Aliquots (400 μl) were plated on glass cover slips in a 24-well tissue culture plate, centrifuged for 1 min at 300 g, and pre-incubated for 1 hr at 37°. Fluoresbrite YG carboxylate microspheres (1·75 μm) (Polysciences, Warrington, PA) were added at a ratio of 10 beads/cell. Following 30 min incubation at 37°, the samples were thoroughly washed, fixed with paraformaldehyde, stained with DAPI and examined by confocal microscopy. Internalized beads were counted in at least 400 cells for each condition.
For pinocytosis assays adherent MΦ (day 6) were similarly incubated in RPMI-B for 1 hr, FITC-dextran (10 μg/ml; 40 000 MW, Invitrogen) was added, the cells were incubated for 1hr at 37°, thoroughly washed at 4°, brought into suspension with cell dissociation buffer, fixed with paraformaldehyde, and fluorescence intensity was determined on a FACSCAN. To account for non-specific binding, background controls, which were incubated at 4° instead of at 37°, were subtracted.
Oxidative burst
Macrophages cultured for 6 days were brought into suspension as described above, centrifuged, and resuspended at 5 × 105 cells/ml in phenol-red-free RPMI-B containing 90 μg/ml lucigenin (9,9′-bis-N-methylacridiniumnitrate; Roche Diagnostics, Indianapolis, IN).20 Aliquots of cell suspension (200 μl) were pipetted into a white 96-well plate (Nunc) and warmed to 37°. Following the addition of 100 ng/ml PMA, luminescence was recorded on a FLUOstar Optima plate reader. Data are expressed as luminescence units.
Results
Responsiveness of cultured monocytes to CCL18
To ascertain that the cultured monocytes responded to CCL18, phosphorylation of ERK1/2 – a pathway that was previously shown to be activated by CCL18 in fibroblasts38 – was determined in monocytes cultured for 3 days and stimulated with CCL18. As shown in Fig. 1(a), CCL18 induced a robust phospho-ERK1/2 response. As we had previously observed that CCL18 had long-ranging effects on cultured monocytes, including increased survival,19 we also asked whether ERK1/2 phosphorylation was associated with nuclear translocation of ERK1/2, which will result in transcriptional activation. Phosphorylated ERK1/2 could be detected in both the cytoplasmic and nuclear fractions (Fig. 1b) suggestive of transcriptional activation. A weaker, but reproducible response could also be shown for Akt phosphorylation (Fig. 1c). In contrast, freshly isolated monocytes did not show ERK1/2 or Akt phosphorylation, when stimulated with CCL18 (results not shown).
Figure 1.
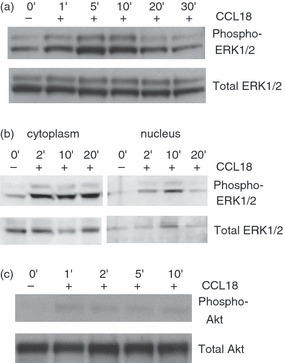
Effect of CCL18 on phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 and Akt. Monocytes cultured for 3 days were stimulated with 100 nm CCL18 for the indicated times. Cell lysates were prepared and immunoblotted. The blots were first probed with anti-phospho-ERK1/2 (a, b) or phospho-Akt (c), then re-probed with anti-total ERK1/2 or anti-Akt respectively. One experiment representative of four. In (a) and (c) whole cell lysates were used, whereas (b) shows cytoplasmic and nuclear fractions.
Chemokine/cytokine production by MΦ stimulated with CCL18
Cytokine production is an important MΦ function, which is up-regulated following mitogen-activated protein kinase activation. Therefore, we previously used antibody arrays to determine cytokine and chemokine accumulation over a period of 20 hr following CCL18 stimulation.19 Over this period CCL18 induced a reproducible increase in the growth factor PDGF-BB19 and in the chemokines CCL2, CCL3, CXCL1 and CXCL8 and a moderate increase of CCL5.19 Here production of selected chemokines was quantified by ELISA and CCL18-mediated up-regulation of these chemokines was slightly higher than for IL-4 with a statistically significant up-regulation of CCL2, CCL3, CCL22 and CXCL8 (Fig. 2a). Chemokine production was considerably lower however than in cells stimulated with GM-CSF or LPS (Fig. 2a). The TNF-α was significantly up-regulated in the presence of CCL18, from 6 ± 5 pg/ml in controls to 70 ± 6 pg/ml in CCL18 treated culture supernatants, but the extent of the up-regulation was an order of magnitude lower than seen in the presence of M1 stimuli such as LPS, which augmented TNF-α to 770 pg/ml (Fig. 2b). Several inflammatory cytokines – IL-1β and IL-6 (both shown in Fig. 2b), IL-12 and IL-13 (results not shown) – responded only minimally to CCL18 stimulation (< 1·5-fold increase). However, the anti-inflammatory IL-10 was significantly increased following CCL18 treatment (Fig. 2b). This chemokine/cytokine pattern resembled that described for IL-4-stimulated M2-spectrum MΦ.11,39
Figure 2.
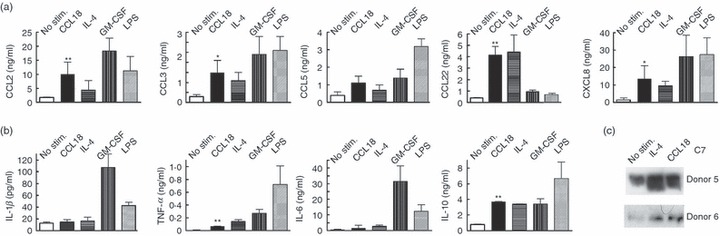
Effect of CCL18 on mediator production by cultured monocytes. Effect of CCL18 (40 nm) and known macrophage (MΦ) activators [interleukin-4 (IL-4), granulocyte–macrophage colony-stimulating factor (GM-CSF) and lipopolysaccharide (LPS)] on chemokine production by cultured MΦ. Monocytes were cultured for 3 days and stimulated with the respective activating agent in serum-free RPMI-1640 for 20 hr. Concentrations of chemokines (a) or cytokines (b) were detected in MΦ supernatants by commercial ELISA or Q-Plex array (n = 3 to n = 9). (a) Paired Student's t-test indicated that there was a statistically significant difference (*P < 0·05 or **P < 0·01) between unstimulated and CCL18-stimulated supernatants for CCL2, CCL3, CCL22 and CXCL8, but not for CCL5. All other stimuli used also showed a statistically significant increase compared with controls. (b) No difference between unstimulated and CCL18-stimulated supernatants was seen for interleukin-1β (IL-1β) and IL-6. Tumour necrosis factor-α (TNF-α) and IL-10 were significantly up-regulated in the presence of CCL18 (P < 0·01). The concentration of TNF-α detected in the presence of CCL18 was however only a fraction of the response seen with cells stimulated with GM-CSF or LPS. IL-4 induced a statistically significant increase of TNF-α, IL-6 and IL-10, but not of IL-1β. GM-CSF and LPS induced a statistically significant increase of all the shown cytokines. (c) Effect of CCL18 on C7 production by MΦ: MΦ were treated as described in (a) and C7 in the supernatants was detected by Western blot. Two donors representative of six are shown.
Previous gene arrays had indicated up-regulation of complement component C7 in CCL18-stimulated monocytes;19 this result was verified on the protein level by Western blotting of C7 in the supernatants of CCL18-stimulated monocytes. Again, both CCL18 and IL-4 responded in a similar fashion, causing up-regulation of C7 (Fig. 2c), which was not seen with LPS or GM-CSF.
Expression of M2 markers by CCL18-stimulated MΦ
Because of the similar effects of CCL18 and IL-4, it was asked whether CCL18-stimulated MΦ express surface markers of M2 MΦ. CCL18-stimulated MΦ expressed the monocyte-specific CD14 (Fig. 3a), which is absent in myeloid dendritic cells, the haemoglobin/haptoglobin complex receptor CD163, which is expressed by macrophage colony-stimulating factor-stimulated MΦ and to a lesser degree by M2 MΦ (Fig. 3b), high concentrations of the MHC class II antigen, HLA-DR (Fig. 3c), which is expressed at high levels in both M1 and M2 MΦ,39 and the mannose receptor CD206 (Fig. 3d), which is a specific marker for M2 polarization.12,40 In contrast, there was no evidence for expression of the myeloid dendritic cell markers CD80, CD83 or CD86 in MΦ cultured with CCL18 (results not shown) Taken together, these results indicate that CCL18-activated MΦ share many features with IL-4-stimulated MΦ, although with some quantitative differences.
Figure 3.
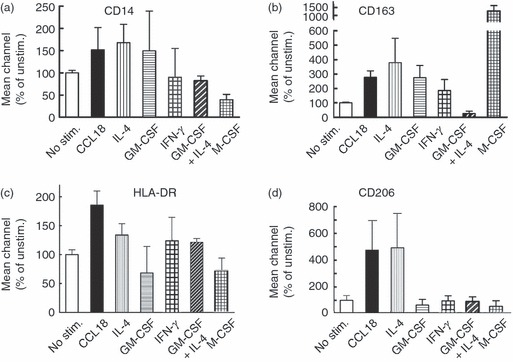
Effect of CCL18 on macrophage (MΦ) cell surface markers. Cultured monocytes (Day 2) were stimulated with 40 nm CCL18, 20 ng/ml interleukin-4 (IL-4), 20 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF), alone or in combination, 20 ng/ml interferon-γ (IFN-γ), or 50 ng/ml macrophage colony-stimulating factor. On day 6 cells were harvested, stained with FITC-anti-CD14 and Cy5-anti-HLA-DR or FITC-anti-CD163 and allophycocyanin-anti-CD206 antibody and analysed by FACS. (a–d) Bars represent average values (– isotype control) ± SD of mean fluorescence intensities of three consecutive donors for CD14, CD163, HLA-DR and CD206 as indicated. Only CCL18-stimulated and IL-4-stimulated cells showed high expression of the mannose receptor CD206. Representative FACS histograms of these results are shown in the Supplementary material, Fig. S1.
Another protein that is up-regulated in M2 MΦ and that plays an important role in eicosanoid formation is 15-LOX.12,41 As 15-LOX is expressed largely in the cytosol, it was detected by confocal microscopy in permeabilized cells. Both CCL18 and IL-4-stimulated MΦ showed increased 15-LOX expression (Fig. 4) compared with unstimulated cells. Again this was not seen in cells stimulated with LPS or GM-CSF (results not shown).
Figure 4.
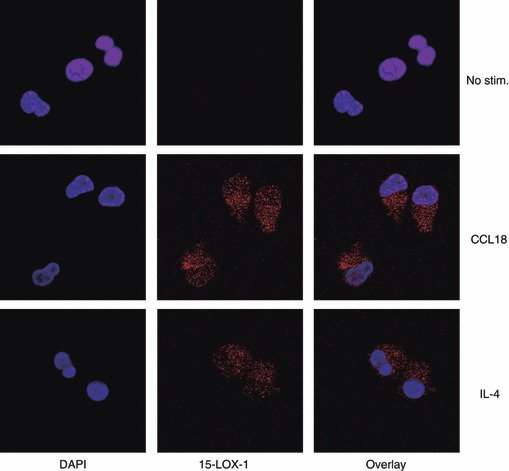
Effect of CCL18 on expression of 15-lipoxygenase (15-LOX). Cultured monocytes (day 2) were stimulated with 40 nm CCL18, 20 ng/ml interleukin-4 (IL-4), or buffer only. On day 6 adherent cells were fixed with paraformaldehyde, permeabilized with 0·2% Triton-X 100, and stained with anti-15-LOX. DAPI was used to counterstain nuclei. Images were taken on an Olympus FV1000 confocal microscope with a × 60 objective and the same exposure conditions for all images.
Functional characterization of CCL18-stimulated MΦ
Expression of high levels of CD163 and CD206 suggested that CCL18-activated cells may show potent non-specific phagocytosis. Therefore phagocytosis of latex particles was determined in MΦ, which had been stimulated with CCL18, IL-4, GM-CSF or IFN-γ. CCL18 was a potent stimulus of non-specific phagocytosis as shown in Fig. 5(a). Similar results were seen with IL-4, but with none of the other stimuli. CCL18 also supported pinocytosis of FITC-dextran to a similar level as IL-4 (Fig. 5b), which again was not seen with GM-CSF or IFN-γ. These results suggest that CCL18-activated MΦ may play a role in the removal of cellular debris, which is an important facet of MΦ function during the resolution of an inflammatory response.
Figure 5.
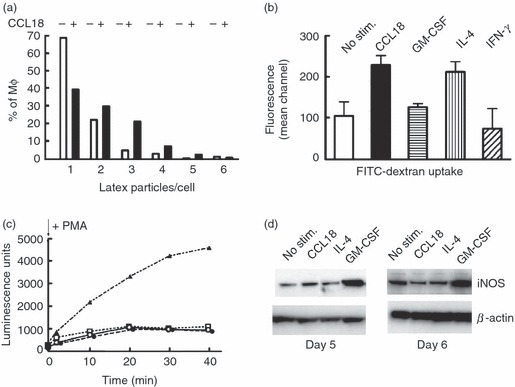
Cellular functions of macrophages (MΦ) stimulated with different mediators including CCL18. (a) Unspecific phagocytosis assay. The samples were prepared as described in the Materials and methods. Latex particles per cell were counted in 400 cells per condition by confocal microscopy and are plotted as number of particles per cell. The number of phagocytosed particles was greatly increased in the presence of CCL18. One experiment representative of three is shown. (b) Pinocytosis assay: cells were prepared as described in (a), but instead of latex beads, FITC dextran was added. Following 1 hr of incubation at 37°, cells were dissociated on ice, washed and analysed on a FACSCAN. Values for cells incubated at 4° were subtracted, and the background-subtracted value for unstimulated cells was defined as 100%. Mean ± SD of three experiments in duplicate. There was a statistically significant increase in fluorescence intensity in CCL18-stimulated and in interleukin-4 (IL-4) -stimulated MΦ. (c) Oxidative burst: samples were prepared as described in the Materials and methods section. Following the addition of 100 ng/ml PMA, luminescence was recorded for 40 min. No addition: ○; cells cultured in the presence of: 40 nm CCL18: •; 20 ng/ml IL-4: □; 20 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF): ▴. One experiment representative of three is shown. (d) inducible nitric oxide synthase (iNOS) expression: MΦ cell lysates were prepared on day 5 or day 6 and Western blots were probed with anti-iNOS antibody and reprobed with anti-β-actin to assure equal loading. One experiment representative of three is shown.
A further characteristic of IL-4-activated MΦ is the low level of reactive oxidant species formed by these cells. Classically primed MΦ undergo an oxidative burst when stimulated with TNF-α, PMA or LPS, and they up-regulate iNOS,38 leading to NO formation. Using MΦ cultured as described for Fig. 3, reactive oxidant production was determined following stimulation with PMA.20 Oxidant production was minimal in CCL18-cultured or IL-4-cultured cells, and two orders of magnitude higher in cells matured in the presence of GM-CSF (Fig. 5c).
As NO production is difficult to detect in human MΦ, iNOS protein expression was used to determine the potential for NO production by these cells. Whereas GM-CSF (Fig. 5d) and IFN-γ (results not shown) induced increased iNOS expression, no such effect was seen with CCL18 or IL-4 (Fig. 5d). These results indicate that MΦ matured in the presence of CCL18 will not produce injurious quantities of reactive oxidants.
Effect of extracellular matrix components on CCL18 production
Macrophages (days 2–4) not only mature to M2 spectrum MΦ in response to CCL18, but M2 MΦ are also known to produce CCL18,13 and the effect of IL-4 on CCL18 production was greatly enhanced, when the MΦ were cultured on collagen.17 We therefore asked whether adhesion molecules in general had an effect on CCL18 production by these cells. As expected there was an increase of CCL18 production, when IL-4-stimulated MΦ were cultured on collagen (Fig. 6a). More surprisingly, all additional adhesion molecules tested (fibronectin, vitronectin and hyaluronic acid) caused a significant increase of CCL18 production in the presence of IL-4 (Fig. 6a). Interestingly, two of the adhesion molecules (vitronectin and hyaluronic acid) brought about a significant increase in CCL18 production even in the absence of IL-4 (Fig. 6b), suggesting the possibility that increased concentrations of extracellular matrix molecules present in fibrotic conditions may be sufficient to up-regulate MΦ CCL18 expression and tilt them towards an M2 phenotype.
Figure 6.
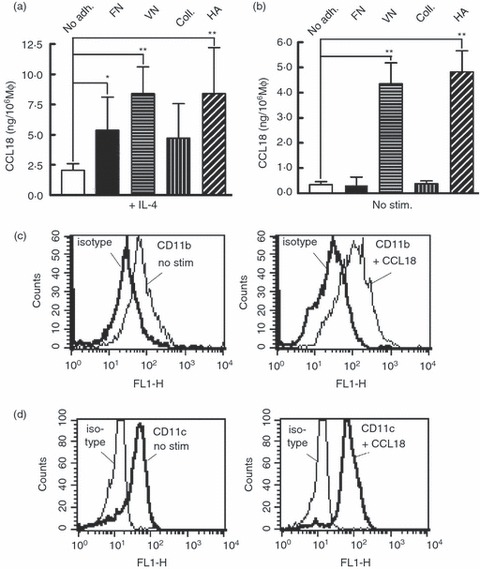
Effect of adhesion molecules on CCL18 production by macrophages (MΦ): MΦ were plated on the indicated adhesion molecules on day 3 and CCL18 was detected in the supernatants by ELISA 48 hr later. In (a), 20 ng/ml interleukin-4 (IL-4) was added at the time of plating, no addition was made in (b). Statistically significant differences (Student's t test, n = 4) are indicated: *P < 0·05 or **P < 0·01. (c, d) Effect of CCL18 on integrin cell surface expression by MΦ: monocytes (day 3) were stimulated with 40 nm CCL18 for 1 hr and expression of CD11b (c) and CD11c (d) was detected by FACS (one experiment representative of two is shown).
As we had described earlier that CCL18 stimulation of MΦ on day 3 increased adhesion of these cells to fibronectin and vitronectin,19 we also determined whether CCL18 would cause up-regulation of integrin expression on the cell surface, and found by FACS analysis that CD11b and CD11c were both up-regulated about twofold following stimulation with CCL18 (196 ± 14% for CD11b and 210 ± 19% for CD11c, mean and SD of two experiments in duplicate, Fig. 6c,d), a behaviour shared with many other chemokines.42
However, it was also recently reported that there is a CCL18 receptor expressed by breast cancer cells, which is not a G-protein-coupled receptor, but instead a phosphatidylinositol transfer protein called PITPNM3,24 which caused integrin clustering in these cells.24 We therefore asked whether PITPNM3 was responsible for CCL18 activation of MΦ, but could not find any evidence that this gene/protein was expressed by monocyte/macrophages either on the mRNA level (results not shown) or on the protein expression level as shown by FACS in the Supplementary material, Fig. S2. In contrast, MDA-MB-231 breast cancer cells clearly expressed this protein (Fig. S2). Therefore PITPNM3 does not appear to be the receptor that is responsible for CCL18-dependent monocyte activation.
Discussion
The results reported here indicate that CCL18 has a profound influence on MΦ maturation, driving these cells to an M2-spectrum phenotype. Although it is unusual that a chemokine has such effects, there is precedence for similar, but distinct, effects by other chemokines. CCL3 and CCL5 have been shown to increase antigen-presenting function of MΦ,43 and CCR7 ligands inhibit apoptotic signals in mature dendritic cells.44 CXCL4 added to freshly isolated monocytes protected from apoptosis and promoted MΦ maturation20,21 similar to our observation with cultured monocytes and CCL18.19 However, the specific type of MΦ maturation differed for the two chemokines. While CCL18-activated cells appeared spheroid19 and expressed high levels of HLA-DR as well as CD206 (Fig. 3), CXCL4-stimulated cells acquired a dendritic cell morphology and lost HLA-DR expression.20
Although CCL18 had these long-ranging effects on MΦ maturation, it is also clear that CCL18 does not activate circulating monocytes as we and others previously reported.22,27 Monocytes undergo a strict time-regulated schedule in which they respond to different chemokines: circulating monocytes are CCR2 negative at the time of their release from the bone marrow, then become CCR2 positive between approximately 24 and 48 hr, which is concomitant with their extravasation.45 Thereafter, the CCR2 is rapidly down-regulated. The period of time during which monocytes/MΦ respond to CCL18 (days 3–427) appears to coincide with this time following CCR2 down-regulation, and it appears that monocytes, which have just transmigrated to tissues, respond to CCL18. It is possible that the high concentrations of CCL18 in plasma (at least 20 ng/ml) desensitize its receptor, but it is equally likely that the expression of the CCL18 receptor is timed to follow the expression of CCR2, and meant to exert its effect on monocyte/MΦ, which have reached their tissue destination.
Although a CCL18 receptor has recently been described on breast cancer cells,24 which is not a G-protein-coupled receptor, but the phosphatidylinositol transfer protein PITPNM3, PITPNM3 does not appear to be the receptor mediating the effects of CCL18 on monocytes/MΦ, as there was no evidence for the expression of this gene/protein at any time between days 0 and 5 of culture (Fig. S2). For that matter we could not detect this receptor on lymphocytes either, indicating that the search for the elusive CCL18 receptor on leucocytes continues. Furthermore, all of the short-term effects of CCL18 on monocytes/MΦ, which include calcium mobilization,27 actin polymerization,27 ERK1/2 phosphorylation (Fig. 1) and chemokine up-regulation (Fig. 2), are characteristic for classical G-protein-coupled chemokine receptors.
The presence of high concentrations of CCL18 in the bronchoalveolar lavage fluid of patients with fibrotic lung disease17,29,46 and chronic asthma28 suggests that excessive concentrations of CCL18 may play a role in pulmonary tissue remodelling. This role has been mostly interpreted as a cross-talk between fibroblasts and lymphocytes, and the results presented here indicate that the MΦ may play an essential role in a triangular scenario between all three of these cell types.
The chemokine pattern produced in the presence of CCL18 (Fig. 2) resembles that described for monocytes stimulated with the pro-fibrotic Th2 cytokines IL-4 or IL-13.39,47–49 Both IL-4 and IL-13 induce CCL18 production in MΦ,13,28 and this effect is synergistically increased in the presence of IL-10, which shows little effect on its own.17 The MΦ-derived CCL18 production increases significantly, when alveolar MΦ are co-cultured with fibroblasts,17 and various extracellular matrix components, which are deposited in increased amounts in fibrotic disease states,50,51 similarly augmented CCL18 production by MΦ (Fig. 6a). This is a generalization of the previous observation that collagen type 1, which is produced by CCL18-activated fibroblasts,38 is a potent inducer of CCL18 production by alveolar MΦ – again in synergy with IL-4 and IL-10,17 thus creating an amplification cycle: MΦ exposed to a Th2 milieu (IL-4 or IL-13) will produce CCL18, which will cause increased fibroblast proliferation and collagen synthesis,38 which in turn increases the CCL18 production by the MΦ. At the same time CCL18 activates the MΦ to produce PDGF,19 which is a fibroblast growth factor known to play a role in fibrotic lung disease.52 It also induces IL-10 production by the MΦ (Fig. 2b), which can be fibrotic by itself53 and further augments CCL18 production by MΦ25. Interestingly, IL-10 was recently shown to induce an M2 type of MΦ,54 and could be responsible for at least a portion of the observed response to CCL18. This intricate CCL18-dependent interplay between Th2 lymphocytes, MΦ and fibroblasts is summarized in Fig. 7. In addition, CCL18 itself may contribute to the M2 MΦ cell-type in an autocrine fashion.
Figure 7.
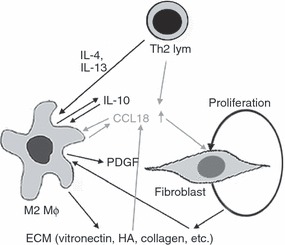
Schematic presentation of the CCL18-mediated cross-talk between macrophages (MΦ), fibroblasts and lymphocytes. Interleukin-4 (IL-4), IL-13, adhesion molecules and co-cultured fibroblasts increase CCL18 production by MΦ. CCL18 in turn activates maturing MΦ to acquire M2 spectrum traits. During this maturation various chemokines, IL-10 and platelet-derived growth factor (PDGF) are produced. IL-10 in combination with IL-4 or IL-13 enhances CCL18 production by MΦ and has been shown to cause the acquisition of M2 traits. Both PDGF and CCL18 cause increased fibroblast proliferation and collagen synthesis and CCL18 and CCL22 chemoattract T helper type 2 lymphocytes. See Discussion for further explanation.
Monocytes stimulated with CCL18 showed increased production of the chemokines CXCL1, CXCL5, CXCL8, CCL2, CCL3 and CCL22 (Fig. 2a and ref. 19). In a recent report, it was pointed out that MΦ produce either CCL18 or CCL3 in an exclusive manner,55 which seems to contradict our results. It should be pointed out, however that our chemokine detection was on day 4 of culture, whereas the cited report detected chemokines on day 12 of culture. At least some of the chemokines induced by CCL18, primarily CCL2,56 CXCL5 and CXCL857 have been shown to contribute to fibrotic lung disease and to the tissue remodelling seen in chronic asthma. CXCL1, CXCL5, CXCL8, CCL2, CCL3 and CCL22 are, however best known as chemotactic factors for neutrophils, monocytes, and activated lymphocytes, and one would therefore postulate that CCL18-activated MΦ cause an inflammatory response in vivo. CXCL1, CXCL5 and CXCL8 are also angiogenic through activation of endothelial CXCR2,58,59 and there is an angiogenic component in fibrotic diseases.57
In spite of these apparent fibrotic/pro-inflammatory effects of CCL18, CCL18 appears to stand at the cross-roads between inflammation and its resolution as supported by the following observations: (i) IL-10 was up-regulated in the presence of CCL18 (Fig. 2b), and although IL-10 has some fibrotic effects, it is best known for its capacity to inhibit production of Th1 cytokines.60 (ii) CCL18-stimulated MΦ produced low or no cytotoxic cytokines including IL-1β, IL-12 and TNF-α, which again would prevent a Th1 response. (iii) CD163, which is up-regulated in CCL18-activated MΦ (Fig. 3b) plays a role in haemoglobin removal and has been shown to have an anti-inflammatory function.61 (iv) The high capacity for non-specific phagocytosis seen in CCL18 activated MΦ (Fig. 5a) would suggest that these cells can remove cellular debris and apoptotic cells in the absence of a respiratory burst (Fig. 5c). (v) Complement component C7 was up-regulated by CCL18, and taken in light of novel findings that endothelial cell-bound C7 may serve an anti-inflammatory role by scavenging the terminal complement complexes,62 the observed induction of C7 supports the notion that one biological role of CCL18 is to inhibit potentially injurious molecules associated with inflammation. CCL18 apparently has pleiotrophic effects. This Janus-faced behaviour of CCL18 was also observed in vivo, where CCL18 caused mild lung fibrosis on its own,34 but attenuated bleomycin-induced fibrosis.34
Specific markers for human M2 MΦ are scarce, but CD206 has long been recognized as a marker for IL-4-activated MΦ,10 and is known to play a negative regulatory role in cytokine release from MΦ.10 The presence of CD206 on CCL18-stimulated MΦ (Fig. 3a,e) therefore emphasizes the resemblance between IL-4 and CCL18 activated MΦ. It remains to be determined whether CCL18 can serve as an autocrine factor in the maturation of MΦ to an M2 cell type and if and how much of the IL-4-induced cell type is the result of an indirect, autocrine effect of CCL18.
Finally, 15-LOX is another M2 marker12 induced by CCL18 (Fig. 4) with paradoxical pro- and anti-inflammatory effects.7 The pro-inflammatory effects have been seen mostly in the context of atherosclerosis, as 15-LOX oxidizes low-density lipoprotein. It is interesting in this respect that it was recently suggested that CCL18 may be a potential diagnostic parameter in patients with coronary artery disease.33 But 15-LOX also leads to increased production of the anti-inflammatory lipoxin A4 and decreased leukotriene B4 production with consequent diminished neutrophil activation.7 Finally, 15-LOX may contribute directly to the M2 maturation of CCL18-stimulated MΦ as it produces endogenous ligands for the peroxisome proliferator-activated receptor-γ (PPAR-γ),63 and activation of PPAR-γ in turn is required for maturation of M2 MΦ.64
CCL18 induced robust and relatively long-lasting ERK1/2 phosphorylation (Fig. 1). Such prolonged activation is generally needed for transcriptional activation, and transcriptional activation must occur to initiate the complex series of events, which allows MΦ maturation to a cell type in the M2 MΦ spectrum. It is not clear, how CCL18 can induce an MΦ maturation process that closely resembles signal transducer and activator of transcription 6 (STAT6) -mediated effects, which are the main signalling pathways of IL-4 and IL-13. STAT6 activation depends on binding of IL-4 or IL-13 to the α-chain of the IL-4 receptor, but STAT6 is not downstream of activation of G-protein-coupled receptors. There are, however, certain downstream signalling mechanisms including JAK2 and Egr-1 pathways, which are shared between STAT6 and G-protein-coupled receptor signalling, and which could account for the effect of CCL18.
In summary, CCL18 is an MΦ differentiation factor, which shares many similarities with the response evoked by IL-4. The responsiveness to CCL18 seems to appear only after monocytes have emigrated to tissues, so the effects of CCL18 in vivo may not result in increased recruitment of circulating monocytes, but rather in profound effects on the composition of the surrounding milieu and in MΦ that show a limited inflammatory response. Overall these findings suggest a role for CCL18 at the resolution stage of the inflammatory process.
Acknowledgments
This work was supported by Tobacco-Related Disease Research Program (TRDRP) grants 13RT-0083 to IUS, and 16RT-0134 to S.K.K., National Institutes of Health grants R21094878 to I.U.S., R21DK067084 to SKK and MSNRI 4061 to R.G.D. We thank Dr Y. Miller for the anti-15-LOX antibody.
Glossary
- 15-LOX
15-lipoxygenase
- APC
allophycocyanin
- DAPI
4,6-diamidino-2-phenylindole
- HRP
horseradish peroxidase
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- MΦ
macrophage
- PE
phycoerythrin
- PMA
12-phorbol 13-myristate acetate
- PMSF
phenylmethylsulphonyl fluoride
- RIPA buffer
radio-immune precipitation buffer
- TBS
TRIS-buffered saline
- TIMP
tissue inhibitor of metalloproteases
- VEGF
vascular endothelial growth factor
Disclosure
None.
Supporting Information
Figure S1: Representative FACS histograms of CD163 (top panel), HLA-DR (middle panel) and CD206 (bottom panel) stained cells cultured as indicated in Fig. 3. The grey-shaded histograms show the isotype control for each stimulus.
Figure S2: Lack of PITPNM3 expression by monocytes/Mϕs: Mϕs were harvested daily from day 1 to day 5, and PITPNM3cell surface expression was detected by FACS (1 experiment representative of 2). As a positive control MDA-MB231 breast cancer cells were similarly stained with PITPNM3 antibody (last panel).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than about missing material) should be directed to the corresponding author for the article.
References
- 1.Keizer GD, Te Velde AA, Schwarting R, Figdor CG, De Vries JE. Role of p150,95 in adhesion, migration, chemotaxis and phagocytosis of human monocytes. Eur J Immunol. 1987;17:1317–22. doi: 10.1002/eji.1830170915. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez-Madrid F, Simon P, Thompson S, Springer TA. Mapping of antigenic and functional epitopes on the α- and β-subunits of two related mouse glycoproteins involved in cell interactions, LFA-1 and Mac-1. J Exp Med. 1983;158:586–602. doi: 10.1084/jem.158.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–61. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med. 1997;186:1757–62. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, Gabuzda D. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. 2003;197:1701–7. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon S. The macrophage. Bioessays. 1995;17:977–86. doi: 10.1002/bies.950171111. [DOI] [PubMed] [Google Scholar]
- 7.Cox D, Greenberg S. Phagocytic signaling strategies: Fc gamma receptor-mediated phagocytosis as a model system. Semin Immunol. 2001;13:339–45. doi: 10.1006/smim.2001.0330. [DOI] [PubMed] [Google Scholar]
- 8.Duffield JS, Erwig LP, Wei X, Liew FY, Rees AJ, Savill JS. Activated macrophages direct apoptosis and suppress mitosis of mesangial cells. J Immunol. 2000;164:2110–9. doi: 10.4049/jimmunol.164.4.2110. [DOI] [PubMed] [Google Scholar]
- 9.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–12. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 10.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–92. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon S. Alternative activation of macrophages. Nature Rev Immunol. 2003;3:23–6. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 12.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–11. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 13.Kodelja V, Muller C, Politz O, Hakij N, Orfanos CE, Goerdt S. Alternative macrophage activation associated CC-chemokine-1, a novel structural homologue of macrophage inflammatory protein-1α with a Th2-associated expression pattern. J Immunol. 1998;160:1411–8. [PubMed] [Google Scholar]
- 14.Andrew DP, Chang M, McNinch J, Wathen ST, Rihanek M, Tseng J, Spellberg JP, Elias CG. STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. J Immunol. 1998;161:5027–38. [PubMed] [Google Scholar]
- 15.Song E, Ouyang N, Horbelt M, Antus B, Wang M, Exton MS. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell Immunol. 2000;204:19–28. doi: 10.1006/cimm.2000.1687. [DOI] [PubMed] [Google Scholar]
- 16.Gratchev A, Guillot P, Hakiy N, Politz O, Orfanos CE, Schledzewski K, Goerdt S. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein β IG-H3. Scand J Immunol. 2001;53:386–92. doi: 10.1046/j.1365-3083.2001.00885.x. [DOI] [PubMed] [Google Scholar]
- 17.Prasse A, Pechkovsky DV, Toews GB, et al. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med. 2006;173:781–92. doi: 10.1164/rccm.200509-1518OC. [DOI] [PubMed] [Google Scholar]
- 18.Broxmeyer HE, Cooper S, Kohli L, Hangoc G, Lee Y, Mantel C, Clapp DW, Kim CH. Transgenic expression of stromal cell-derived factor-1/CXC chemokine ligand 12 enhances myeloid progenitor cell survival/antiapoptosis in vitro in response to growth factor withdrawal and enhances myelopoiesis in vivo. J Immunol. 2003;170:421–9. doi: 10.4049/jimmunol.170.1.421. [DOI] [PubMed] [Google Scholar]
- 19.Wimmer A, Khaldoyanidi S, Judex M, Serobyan N, DiScipio RG, Schraufstatter IU. CCL18/PARC stimulates hematopoiesis in long-term bone marrow cultures indirectly through its effect on monocytes. Blood. 2006;108:3722–9. doi: 10.1182/blood-2006-04-014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheuerer B, Ernst M, Durrbaum-Landmann I, Fleischer J, Grage-Griebenow E, Brandt E, Flad HD, Petersen F. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood. 2000;95:1158–66. [PubMed] [Google Scholar]
- 21.Fricke I, Mitchell D, Petersen F, Bohle A, Bulfone-Paus S, Brandau S. Platelet factor 4 in conjunction with IL-4 directs differentiation of human monocytes into specialized antigen-presenting cells. FASEB J. 2004;18:1588–90. doi: 10.1096/fj.03-1435fje. [DOI] [PubMed] [Google Scholar]
- 22.Hieshima K, Imai T, Baba M, et al. A novel human CC chemokine PARC that is most homologous to macrophage-inflammatory protein-1 α/LD78 α and chemotactic for T lymphocytes, but not for monocytes. J Immunol. 1997;159:1140–9. [PubMed] [Google Scholar]
- 23.Adema GJ, Hartgers F, Verstraten R, et al. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature. 1997;387:713–7. doi: 10.1038/42716. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Yao Y, Gong C, et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 2011;19:541–55. doi: 10.1016/j.ccr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Lieshout AW, van der Voort R, le Blanc LM, Roelofs MF, Schreurs BW, van Riel PL, Adema GJ, Radstake TR. Novel insights in the regulation of CCL18 secretion by monocytes and dendritic cells via cytokines, toll-like receptors and rheumatoid synovial fluid. BMC Immunol. 2006;7:23. doi: 10.1186/1471-2172-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vulcano M, Struyf S, Scapini P, et al. Unique regulation of CCL18 production by maturing dendritic cells. J Immunol. 2003;170:3843–9. doi: 10.4049/jimmunol.170.7.3843. [DOI] [PubMed] [Google Scholar]
- 27.Schraufstatter IU, Takamori H, Sikora L, Sriramarao P, DiScipio RG. Eosinophils and monocytes produce pulmonary and activation-regulated chemokine (PARC/CCL18), which activates cultured monocytes/macrophages. Am J Physiol Lung Cell Mol Physiol. 2004;286:L494–501. doi: 10.1152/ajplung.00323.2002. [DOI] [PubMed] [Google Scholar]
- 28.de Nadai P, Charbonnier AS, Chenivesse C, et al. Involvement of CCL18 in allergic asthma. J Immunol. 2006;176:6286–93. doi: 10.4049/jimmunol.176.10.6286. [DOI] [PubMed] [Google Scholar]
- 29.Pardo A, Smith KM, Abrams J, et al. CCL18/DC-CK-PARC upregulation in hypersensitivity pneumonitis. J Leukoc Biol. 2001;70:610–6. [PubMed] [Google Scholar]
- 30.Pivarcsi A, Gombert M, Dieu-Nosjean MC, et al. CC chemokine ligand 18, an atopic dermatitis-associated and dendritic cell-derived chemokine, is regulated by staphylococcal products and allergen exposure. J Immunol. 2004;173:5810–7. doi: 10.4049/jimmunol.173.9.5810. [DOI] [PubMed] [Google Scholar]
- 31.Prasse A, Probst C, Bargali E, et al. Serum CC-chemokine ligand 18 concentration predicts outcome in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:717–23. doi: 10.1164/rccm.200808-1201OC. [DOI] [PubMed] [Google Scholar]
- 32.Radstake TRDJ, van der Voort R, ten Brummelhuis M, et al. Increased expression of CCL18, CCL19, and CCL17 by dendritic cells from patients with rheumatoid arthritis and regulation by Fc gamma receptors. Ann Rheum Dis. 2004;64:359–67. doi: 10.1136/ard.2003.017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Sutter J, Struyf S, Van de Veire NR, Philippé J, De Buyzere M, Van Damme J. Cardiovascular determinants and prognostic significance of CC chemokine ligand-18 (CCL18/PARC) in patients with stable coronary artery disease. J Mol Cell Cardiol. 2010;49:894–6. doi: 10.1016/j.yjmcc.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Luzina IG, Papadimitriou JC, Anderson R, Pochetuhen K, Atamas SP. Induction of prolonged infiltration of T lymphocytes and transient T lymphocyte-dependent collagen deposition in mouse lungs following adenoviral gene transfer of CCL18. Arthritis Rheum. 2006;54:2643–55. doi: 10.1002/art.21950. [DOI] [PubMed] [Google Scholar]
- 35.Lindhout E, Vissers JLM, Hartgers FC, Huijbens RJF, Scharenberg NM, Figdor CG, Adema GJ. The dendritic cell-specific CC-chemokine DC-CK1 is expressed by germinal center dendritic cells and attracts CD38-negative mantle zone B lymphocytes. J Immunol. 2001;166:3284–9. doi: 10.4049/jimmunol.166.5.3284. [DOI] [PubMed] [Google Scholar]
- 36.DiScipio RG, Khaldoyanidi SK, Schraufstatter IU. Expression of soluble proteins in Escherichia coli by linkage with the acidic propiece of eosinophil major basic protein. Protein Expr Purif. 2011;79:72–80. doi: 10.1016/j.pep.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 37.Deryugina EI, Ratnikov B, Monosov E, Postnova TI, DiScipio R, Smith JW, Strongin AY. MT1-MMP initiates activation of pro-MMP-2 and integrin αv, β3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res. 2001;263:209–23. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- 38.Atamas SP, Luzina IG, Choi J, et al. Pulmonary and activation-regulated chemokine stimulates collagen production in lung fibroblasts. Am J Respir Cell Mol Biol. 2003;29:743–9. doi: 10.1165/rcmb.2003-0078OC. [DOI] [PubMed] [Google Scholar]
- 39.Littman BH, Dastvan FF, Carlson PL, Sanders KM. Regulation of monocyte/macrophage C2 production and HLA-DR expression by IL-4 (BSF-1) and IFN-γ. J Immunol. 1989;142:520–55. [PubMed] [Google Scholar]
- 40.Anthony RM, Urban JF, Alem F, Hamed HA, Rozo CT, Boucher JL, Van Rooijen N, Gause WC. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–60. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy BD, Romano M, Chapman HA, Reilly JJ, Drazen J, Serhan CN. Human alveolar macrophages have 15-lipoxygenase and generate 15(S)-hydroxy-5,8,11-cis-13-trans-eicosatetraenoic acid and lipoxins. J Clin Invest. 1993;92:1572–9. doi: 10.1172/JCI116738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang Y, Beller DI, Frendl G, Graves DT. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J Immunol. 1992;148:2423–8. [PubMed] [Google Scholar]
- 43.Lee JK, Kim JK, Lee YR, Kim HS, Im SA, Kim K, Lee CK. Exposure to chemokines during maturation modulates antigen presenting cell function of mature macrophages. Cell Immunol. 2005;234:1–8. doi: 10.1016/j.cellimm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez-Sanchez N, Riol-Blanco L, de la Rosa G, et al. Chemokine receptor CCR7 induces intracellular signaling that inhibits apoptosis of mature dendritic cells. Blood. 2004;104:619–25. doi: 10.1182/blood-2003-11-3943. [DOI] [PubMed] [Google Scholar]
- 45.Xu H, Manivannan A, Dawson R, Crane IJ, Mack M, Sharp P, Liversidge J. Differentiation to the CCR2+ inflammatory phenotype in vivo is a constitutive, time-limited property of blood monocytes and is independent of local inflammatory mediators. J Immunol. 2005;175:6915–23. doi: 10.4049/jimmunol.175.10.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luzina IG, Atamas SP, Wise R, Wigley FM, Xiao HQ, White B. Gene expression in bronchoalveolar lavage cells from scleroderma patients. Am J Respir Cel Mol Biol. 2002;26:549–57. doi: 10.1165/ajrcmb.26.5.4683. [DOI] [PubMed] [Google Scholar]
- 47.Zhu Z, Ma B, Zheng T, Homer RJ, Lee CG, Charo IF, Noble P, Elias JA. IL-13-induced chemokine responses in the lung: role of CCR2 in the pathogenesis of IL-13-induced inflammation and remodeling. J Immunol. 2002;168:2953–62. doi: 10.4049/jimmunol.168.6.2953. [DOI] [PubMed] [Google Scholar]
- 48.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13 α2 receptor is involved in induction of TGF-β1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 49.Jakubzick C, Choi ES, Joshi BH, Keane MP, Kunkel SL, Puri RK, Hogaboam CM. Therapeutic attenuation of pulmonary fibrosis via targeting of IL-4- and IL-13-responsive cells. J Immunol. 2003;171:2684–93. doi: 10.4049/jimmunol.171.5.2684. [DOI] [PubMed] [Google Scholar]
- 50.Pohl WR, Conlan MG, Thompson AB, Ertl RF, Romberger DJ, Mosher DF, Rennard SI. Vitronectin in bronchoalveolar lavage fluid is increased in patients with interstitial lung disease. Am Rev Respir Dis. 1991;143:1369–75. doi: 10.1164/ajrccm/143.6.1369. [DOI] [PubMed] [Google Scholar]
- 51.Teder P, Nettelbladt O, Heldin P. Characterization of the mechanism involved in bleomycin-induced increased hyaluronan production in rat lung. Am J Respir Cell Mol Biol. 1995;12:181–9. doi: 10.1165/ajrcmb.12.2.7532420. [DOI] [PubMed] [Google Scholar]
- 52.Nagaoka I, Trapnell BC, Crystal RG. Up-regulation of platelet-derived growth factor-A and -B gene expression in alveolar macrophages of individuals with idiopathic pulmonary fibrosis. J Clin Invest. 1990;85:2023–7. doi: 10.1172/JCI114669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barbarin V, Xing Z, Delos M, Lison D, Huaux F. Pulmonary overexpression of IL-10 augments lung fibrosis and Th2 responses induced by silica particles. Am J Physiol Lung Cell Mol Physiol. 2005;288:L841–8. doi: 10.1152/ajplung.00329.2004. [DOI] [PubMed] [Google Scholar]
- 54.Prasse A, Germann M, Pechkovsky DV, et al. IL-10-producing monocytes differentiate to alternatively activated macrophages and are increased in atopic patients. J Allergy Clin Immunol. 2007;119:464–71. doi: 10.1016/j.jaci.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 55.Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481–9. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baran CP, Opalek JM, McMaken S, et al. Important roles for M-CSF, CCL2 and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:78–89. doi: 10.1164/rccm.200609-1279OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strieter RM, Belperio JA, Keane MP. CXC chemokines in angiogenesis related to pulmonary fibrosis. Chest. 2002;122:298S–301S. doi: 10.1378/chest.122.6_suppl.298s. [DOI] [PubMed] [Google Scholar]
- 58.Addison CL, Daniel TO, Burdick MD, et al. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR(+) CXC chemokine-induced angiogenic activity. J Immunol. 2000;165:5269–77. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- 59.Schraufstatter IU, Trieu K, Zhao M, Rose DM, Terkeltaub RA, Burger M. IL-8 mediated cell migration in endothelial cells depends on cathepsin B activity and transactivation of the EGF-receptor. J Immunol. 2003;171:6714–22. doi: 10.4049/jimmunol.171.12.6714. [DOI] [PubMed] [Google Scholar]
- 60.Moore KW, Vieira P, Fiorentino DF, Trounstine ML, Khan TA, Mosmann TR. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein–Barr virus gene BCRFI. Science. 1990;248:1230–4. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- 61.Abraham NG, Drummond G. CD163-mediated hemoglobin-heme uptake activates macrophage HO-1, providing an antiinflammatory function. Circ Res. 2006;99:911–4. doi: 10.1161/01.RES.0000249616.10603.d6. [DOI] [PubMed] [Google Scholar]
- 62.Bossi F, Rizzi L, Bulla R, et al. C7 is expressed on endothelial cells as a trap for the assembling terminal complement complex and may exert anti-inflammatory function. Blood. 2009;113:3640–8. doi: 10.1182/blood-2008-03-146472. [DOI] [PubMed] [Google Scholar]
- 63.Huang JT, Welch JS, Ricote M, et al. Interleukin-4-dependent production of PPAR-γ ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–82. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 64.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPAR-γ controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–20. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


