Abstract
Experimental models have shown that lipoproteins from Mycobacterium tuberculosis induce apoptosis via Toll-like receptor 2 (TLR2) in the THP-1 cell line and in monocyte-derived macrophages from healthy volunteers. We found an increased percentage of circulating monocytes in patients with tuberculosis (TB) in comparison to healthy controls. Patients with TB showed a higher TLR2 and TLR4 expression density on monocytes, and a higher proportion of TLR2+ monocytes, as well as increased serum tumour necrosis factor-α level. In culture, monocytes from TB patients were more susceptible to death than monocytes from healthy controls. Moreover, death-susceptible monocytes were positive to both TLR2 and TLR4 at the start of culture. Freshly obtained monocytes from TB patients exhibited cleaved caspase 9 and denaturalized cytochrome c. For levels of caspase 8, apoptosis-regulating signal kinase 1, and phospho-p38 mitogen-activated protein kinase there was no difference between samples from TB patients and from healthy controls. The culture filtrate antigen extract from M. tuberculosis H37Rv strain induced the death of monocytes from patient with TB after a 4-hr incubation, which was abrogated by neutralizing antibodies for TLR2 but not TLR4. Similarly, Pam3CSK4, a synthetic agonist triacylated ligand to TLR2, also induced the death of monocytes, although it did not increase levels of cleaved caspase 9. Our findings suggest that monocytes from TB patients are more susceptible to death, probably through mitochondrial damage, and that cell death increases in the presence of mycobacterial antigen by a TLR2-dependent pathway.
Keywords: apoptosis, monocytes, Mycobacterium tuberculosis, Toll-like receptor 2
Introduction
Human tuberculosis (TB) is an infectious disease caused mainly by Mycobacterium tuberculosis, a slow-growing pathogenic intracellular bacillus. The World Health Organization estimates 9·4 million new cases of clinical disease and 1·3 million deaths each year; consequently, TB represents a serious public health problem.1 In the innate immune system response against M. tuberculosis infection, macrophages play an important role during the early interaction with mycobacteria.2 Human and murine macrophages recognize mycobacteria via toll-like receptor (TLR) proteins, which bind different mycobacterial ligands such as lipoproteins.3 The recognition of mycobacterial ligands by TLR2 or TLR4 causes activation of macrophages,3,4 which can control intracellular growth of the bacilli by producing pro-inflammatory cytokines and activating antimicrobial effector pathways.5,6 The interaction of TLR4 and TLR2 with diverse mycobacterial ligands triggers signalling that leads to activation of p38 mitogen-activated protein kinase (MAPK), apoptosis-regulating signal kinase 1 (ASK1) and p47phox pathways, as well as production of pro-inflammatory cytokines in human monocytes.7,8 There is evidence that the synthetic bacterial lipopeptide Pam3CSK4 induces TLR2-mediated apoptosis in the human pro-monocytic THP-1 cell line.9 Other studies have shown that a 19 000 molecular weight (MW) M. tuberculosis lipoprotein induces apoptosis in differentiated cells of the THP-1 cell line and monocyte-derived macrophages mediated by TLR2.10,11 The 19 000 MW lipoprotein-induced macrophage apoptosis is exerted through a pathway involving caspase 8, but not caspase 9.10 Similarly, a 38 000 MW M tuberculosis lipoprotein has been reported to be apoptogenic for human monocyte-derived macrophages through TLR2.12 In that study, apoptosis was dependent on both caspase 8 and caspase 9, implicating tumour necrosis factor-α (TNF-α) and its receptors TNFR1 and TNFR2, as well as Fas ligand and its receptor Fas.12 These data strongly suggest that mycobacterial antigens induce apoptosis via TLR2 in monocytes/macrophages from patients with TB. Our objective was to examine monocytes from patients with TB with respect to their percentage in peripheral blood, proportion of cell death in the absence of stimulus, percentage and density of TLR2 and TLR4 expression, evaluation of apoptosis-related molecules, and cell death through mycobacterial antigens via TLR2.
Materials and methods
Patients
The study population consisted of 54 patients with active TB, who were recruited from the Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas in Mexico City. Diagnosis of TB was based on clinical history, physical examination, chest X-rays and identification of acid-fast bacilli in sputum. In all the cases, the diagnosis was confirmed by M. tuberculosis growth in sputum culture. Patients were classified as having TB class 3 category I disease, according to the American Thoracic Society.13 The specific enrolment criteria were defined as adult individuals and absence of other diseases, such as multidrug-resistant TB, human immunodeficiency, pulmonary cancer or diabetes mellitus. The healthy control group consisted of 44 unrelated healthy volunteers, who had received the Mycobacterium bovis bacillus Calmette–Guérin vaccine during childhood. General data from TB patients and healthy controls are shown in Table 1. The institutional Medical Ethics Committee approved the study and all study participants provided written informed consent.
Table 1.
General data from healthy controls and patients with tuberculosis
| Healthy controls (n = 44) | Tuberculosis patients (n = 54) | |
|---|---|---|
| Age (years) | 31±7 | 41±17 |
| Gender | ||
| Male | 23 (52%) | 29 (54%) |
| Female | 21 (48%) | 25 (46%) |
| Tuberculosis | ||
| Pulmonary | − | 51 (94%) |
| Pleural | − | 3 (6%) |
Antibodies and reagents
Fluorescein isothiocyanate (FITC)-labelled mouse monoclonal antibodies (mAbs) to human CD14 (clone M5E2) and HLA-DR, -DP, -DQ (clone Tü39); phycoerythrin (PE)-labelled mAbs to CD86 (clone 2331/FUN-1) and CD14 (clone M5E2); PE-Cy5-labelled mAb to HLA-A, -B, -C (clone G46-2.6); AlexaFluor 488-labelled mAb to TLR2 (TLR2/CD282) (clone 11G7); allophycocyanin-labelled mAb to CD83 (clone HB15e); FITC-, AlexaFluor 488-, allophycocyanin-labelled mAbs (clone MOPC-21), and PE-labelled mAb (clone MOPC-31C) isotype controls; purified mAb (clone G155-178) as functional assay isotype control; and 7-aminoactinomycin-D (7-AAD) staining solution were purchased from BD Biosciences Pharmingen (San Diego, CA). The PE-labelled mAb to TLR4 (CD284) (clone HTA125) and functional blocking purified mAbs to TLR2 (clone TL2.1) and TLR4 (clone HTA125) were acquired from eBioscience Inc. (San Diego, CA). The PE-labelled mAb to CD80 (clone 2D10) was from BioLegend (San Diego, CA). Tripalmitoylated-CysSerLys4 (Pam3CSK4) lipopeptide, dipalmitoylated-CGDPKHPKSF fibroblast-stimulating lipopeptide-1 (FSL-1), and lipomannan of Mycobacterium smegmatis (LM-MS) were obtained from a human and mouse TLR2 agonist kit from InvivoGen (San Diego, CA). Mouse mAbs to human caspase 8 (clone 84131.11), caspase 9 (clone LAP6) and denaturalized cytochrome c (clone 7H8.2C12); sheep polyclonal antibodies to human ASK1; rabbit polyclonal antibody to human phospho-p38α MAP14-kinase; horseradish peroxidase-labelled goat anti-IgG rabbit and anti-IgG mouse polyclonal antibodies were acquired from R&D Systems Inc. (Minneapolis, MN). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was acquired from Invitrogen Co. (Camarillo, CA). A CD14+ cells positive isolation kit in a magnetic antibody cell sorting (MACS) system was acquired from Miltenyi Biotec (Bergisch Gladbach, Germany). Lymphoprep (Ficoll 1.077 density) was from Axis-Shield PoC As (Oslo, Norway). RPMI-1640 culture medium, trypan blue dye, p-formaldehyde, BSA fraction V, SDS, Tween 20, glycine, and salt reagents were all obtained from Sigma-Aldrich (St Louis, MO). Laemmli sample buffer for SDS–PAGE was acquired from Bio-Rad Laboratories (Hercules, CA). Sodium pyruvate, l-glutamine and 2-mercaptoethanol were from Gibco BRL (Rockville, MD) and penicillin/streptomycin solution was from In Vitro (Mexico City, Mexico). Fetal calf serum was from Hyclone Laboratories Inc. (Logan, UT). Culture filtrate antigen extract (CFAE) from M. tuberculosis H37Rv strain (ATCC 27294) was kindly provided by Dr Diana Aguilar, Instituto Nacional de Ciencias Médicas y de la Nutrición Salvador Zubiran in Mexico City.
Preparation of mycobacterial antigen
The CFAE from M. tuberculosis was obtained according to standard procedures. Briefly, bacteria were grown at 37° in Middlebrook 7H9 medium enriched with OADC (Difco Laboratories Inc., BD Diagnostic Systems, Detroit, MI) until the mid-logarithmic phase. Two rounds of filtration through 0·45-μm and 0·22-μm pore-size membranes (Millipore Corp., Billerica, MA) removed the bacillary material, the protein concentration was adjusted to 2 mg/ml and samples were stored at −20° until required.
Cells
Peripheral blood mononuclear cells (PBMC) were isolated from 30 ml heparinized whole blood by Ficoll density gradient centrifugation for 30 min at 500 g and 16°.14 After centrifugation, the interface cells were collected, washed three times in PBS (0·01 m sodium phosphate, 0·15 m sodium chloride, pH 7·2) and counted in a Neubauer chamber, determining cellular viability by trypan blue dye exclusion method.
Cell surface marker staining
The percentage of CD14 expression versus CD80, CD83, CD86, HLA-A, -B, -C, HLA-DR, -DP, -DQ, TLR2, and TLR4 expression in PBMC was analysed by double or triple immunofluorescence. Briefly, PBMC were suspended in PBS containing 0·2% BSA and 0·2% sodium azide (PBS-BSA buffer) and stained with fluorescent mAbs against the respective molecules for 15 min at 4°. After incubation, cells were washed twice in PBS-BSA buffer, fixed in 1%p-formaldehyde in PBS, and acquired with a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). In each case, 10 000 events were counted in linear mode for side scatter and log amplification for CD14 expression. Then, CD14+ cells were gated and analysed by cell surface molecule expression as described above. Fluorochrome-labelled isotype-matched control mAbs were used to evaluate background staining.
Purification of monocytes
The PBMC were suspended in PBS supplemented with 0·5% BSA and 2 mm EDTA at a concentration of 1 × 107 cells/0·1 ml and were incubated with microbeads coated with antibodies to CD14 in a MACS system. The purity percentage of CD14+ cells isolated from PBMC was always ≥ 90%, as measured by flow cytometry using FITC-labelled or PE-labelled anti-human CD14 mAbs.
Assessment of cell death
Purified CD14+ cells (1 × 105) were cultured without stimulus in 96-well flat-bottomed cell culture plates with ultra-low attachment surface (Costar Inc., Lowell, MA) in 100 μl RPMI-1640 culture medium supplemented with 1 mm sodium pyruvate, 2 mm l-glutamine, 50 μm 2-mercaptoethanol, 100 IU/ml penicillin, 100 μg/ml streptomycin and 10% heat-inactivated fetal calf serum for 24 and 48 hr at 37° in a humidified atmosphere containing 5% CO2. Ending the culture, cells were harvested, washed in PBS, and incubated with 3 μl 7-AAD solution for 20 min at 4° in the dark. The percentage of dead cells was measured through 7-AAD staining by flow cytometry. The percentage of cell death was calculated as follows: 15
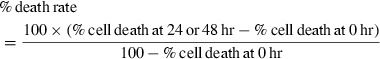 |
In another analysis, monocytes were stimulated with either 20 μg/ml CFAE from M. tuberculosis strain H37Rv or agonist ligands to TLR2, 0·5 μg/ml FSL-1 or Pam3CSK4 synthetic lipopeptide, or 5 ng/ml LM-MS during 4 hr under the same conditions. To establish the optimal dose of mycobacterial protein, antigen-induced cell death experiments were also carried out over a 4-hr period using from 10 to 50 μg/ml CFAE from M. tuberculosis. The percentage of antigen-induced specific death was calculated in similar way to that indicated above; where % specific death = 100 × (% cell death in the presence of CFAE − % cell death in the absence of CFAE)/(100 − % cell death in the absence of CFAE).
To inhibit antigen-induced monocyte death, cells were incubated with 3 μg TLR2-blocking mAb or 1 μg TLR4-blocking mAb for 20 min before mycobacterial antigen. The optimal dose of mAb to block TLRs was obtained by staining cells with fluorescent mAb to TLR2 or TLR4 after incubating with different protein concentrations of TLR-blocking mAbs. The absence of staining on monocytes pointed out the optimal dose of TLR2 or TLR4 blocking mAb. Isotype-matched mAbs to unrelated molecules were used as experimental controls.
Western blot
Purified CD14+ cells (1 × 105) were stimulated with mycobacterial antigen in the presence or absence of TLR2 blocking mAbs for 4 hr at 37° in a humidified atmosphere containing 5% CO2. In another analysis, cells were stimulated with agonist ligands to TLR2, Pam3CSK4, FSL-1 or LM-MS, under the same conditions. After stimulation, cells were washed once with ice-cold PBS and lysed in Laemmli sample buffer (Bio-Rad). All cellular protein extracts were separated by SDS–PAGE and transferred to 0·45-μm pore-size PVDF Immobilon-P membranes (Millipore Corp.) with 25 mm Tris-base (pH 8·0) containing 150 mm glycine and 20% (volume/volume) methanol.16 Membranes were blocked with 1% BSA in PBS and individually incubated with antibodies to caspase 8, caspase 9, denaturalized cytochrome c, ASK1 and phospho-p38α. Membranes were then washed twice with 0·5% Tween-20 in PBS. Protein bands were detected by incubating with horseradish peroxidase-labelled antibodies and visualized with enhanced chemiluminescence reagent (Thermo Scientific, Pierce Biotech., Rockford, IL) using a Kodak Biomax MR Film (Carrestream Health Inc. Rochester, NY). Band densities in the film were analysed by densitometry using the imagej 1.39c software (http://rsd.info.nih.gov/ij) as described by Miller (http://www.lukemiller.or/journal/2007/08/quantifying-westernblots-without.html).
Statistical analysis
Data were evaluated by graphpad prism 5 software (La Jolla, CA), using a Shapiro–Wilk test to discover population distributions. Student's t-test was performed for comparison of variables that were symmetrically distributed, and values are shown as mean ± standard deviation (SD). To compare groups, an analysis of variance test followed by Bonferroni's multiple comparison test was performed. Differences between groups were considered statistically significant at P < 0·05.
Results
Increased percentage of peripheral blood monocytes in TB patients
First, we assessed the proportion of CD14+ cells in PBMC from patients with TB and in healthy control individuals. The percentage of circulating monocytes was found to be increased, there was a twofold increase in the number of monocytes in TB patients (Fig. 1a), which showed a significant statistical difference compared with cells from healthy controls (18·9 ± 10·6% versus 9·3 ± 4·1%, respectively, P = 0·0005) (Fig. 1b). We also observed that monocytes from some TB patients exhibited a higher cell granularity than monocytes from healthy controls (Fig. 1a), which suggested a cell activation status. For this reason, we measured CD80, CD83, CD86, HLA-A, -B, -C, and HLA-DR, -DP, -DQ expression in freshly obtained monocytes, but no difference was found between study groups (Table 2). These data indicate that TB patients have an increased number of peripheral blood monocytes.
Figure 1.
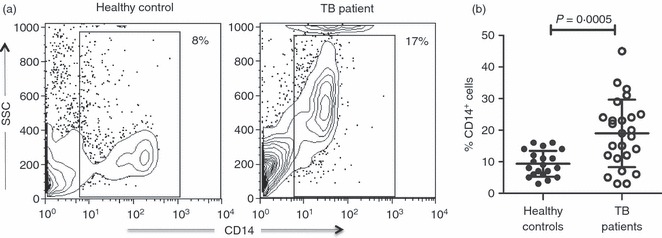
Cells from patients with tuberculosis (TB) show an increased percentage of monocytes. Freshly obtained peripheral blood mononuclear cells (PBMC) were incubated with FITC-labelled antibodies to human CD14 and analysed by flow cytometry. (a) Representative contour plot for side scatter and CD14 expression in PBMC from healthy controls and TB patients. Percentage is shown in gated CD14+ cells. (b) Proportion of CD14+ cells from 41 healthy controls and 51 TB patients. The bars denote mean ± standard deviation. The P-value of the difference between TB patients and healthy controls is shown.
Table 2.
Expression of activation molecules on monocytes
| Molecule | Healthy controls | Tuberculosis patients |
|---|---|---|
| CD80 | 39±23 | 27±10 |
| CD83 | 0.2±0.005 | 0.3±0.2 |
| CD86 | 69±16 | 71±3 |
| HLA-DR, DP, DQ | 33±11 | 46±8 |
| HLA-A, B, C | 90±12 | 91±18 |
The numbers denote mean percentage±SD.
Increased percentage and expression density of TLR2+ monocytes in TB patients
We next analysed the phenotype of monocytes from TB patients, evaluating TLR2 and TLR4 expression, which has been associated with the immune response to M. tuberculosis.7,17 An important dissimilarity was found in the TLR2 expression; the percentage of TLR2+ cells in gated CD14+ cells from TB patients was 1·4 times greater than in those cells from healthy controls (77·4 ± 22·7% versus 54·1 ± 28·5%, respectively, P =0·003) (Fig. 2a,c). In addition, mean fluorescence intensity (MFI) to TLR2 was also higher in monocytes from TB patients in comparison to cells from healthy controls (28·9 MFI ± 21·8 versus 16·8 MFI ± 12·5, respectively, P = 0·03) (Fig. 2b,d). However, there was no difference in the percentage of TLR4+ monocytes between both study groups. Whereas 41 ± 25·6% of monocytes were TLR4+ in patients, 36 ± 28% corresponded to those cells in healthy controls (Fig. 3a,c). On the contrary, as observed in Fig. 3(b,d), MFI to TLR4 exhibited an increase in monocytes from TB patients (19 ± 17) in comparison to cells from healthy controls (10 ± 6) that was statistically significant (P = 0·02). Interestingly, the percentage of TLR2+ monocytes was 1·9-fold higher than that of TLR4+ monocytes in TB patients (77·4 ± 22·7% versus 41 ± 25·6%, respectively), showing a significant difference (P = 0·0001) (Fig. 4a). Likewise, TLR2 expression was 1·5-fold higher than TLR4 expression in monocytes from TB patients (28·9 MFI ± 21·8 versus 19 ± 17, respectively; P = 0·01) (Fig. 4b). The data showed that patients with TB have a greater percentage of TLR2+ monocytes and a greater expression density of TLR2 and TLR4 than healthy controls. In addition, TLR2 expression was higher than TLR4 expression in the cells from TB patients.
Figure 2.
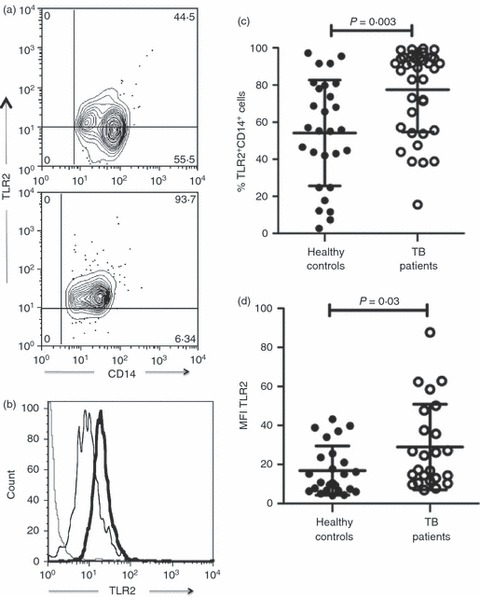
Monocytes from patients with tuberculosis (TB) display increased percentage and expression density to Toll-like receptor 2 (TLR2). Monocytes were isolated from peripheral blood mononuclear cells by magnetic bead-labelled antibodies, incubated with AlexaFluor 488-labelled antibodies and analysed by flow cytometry. (a) Representative contour plot for TLR2 versus CD14 expression in cells from healthy controls (upper panel) and TB patients (lower panel). (b) Representative histogram for TLR2 expression in cells from healthy controls (thin line) and TB patients (thick line). Dotted line indicates the background level of cells incubated with isotype-matched control antibody. (c) Proportion of TLR2+ CD14+ cells from 41 healthy controls and 51 TB patients. (d) Mean fluorescence intensity (MFI) to TLR2 expression in cells from healthy control and TB patients. The bars denote mean ± standard deviation. The P-value of the difference between TB patients and healthy controls is shown.
Figure 3.
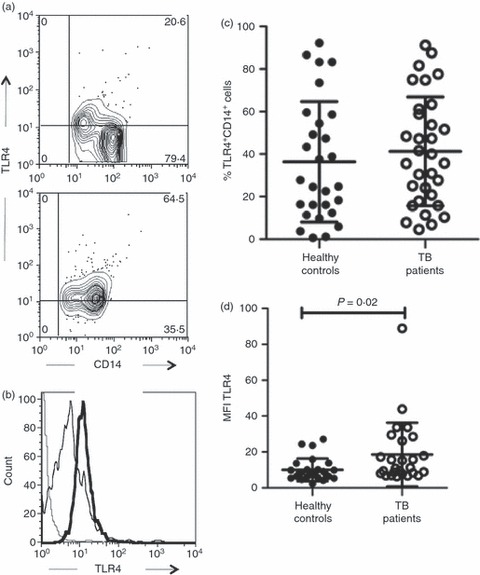
Monocytes from patients with tuberculosis (TB) show increased expression density to Toll-like receptor 4 (TLR4). Monocytes were isolated from peripheral blood mononuclear cells by magnetic bead-labelled antibodies, incubated with AlexaFluor 488-labelled antibodies and analysed by flow cytometry. (a) Representative contour plot for TLR4 versus CD14 expression in cells from healthy controls (upper panel) and TB patients (lower panel). (b) Representative histogram for TLR4 expression in cells from healthy controls (thin line) and TB patients (thick line). Dotted line indicates the background level of cells incubated with isotype-matched control antibody. (c) Proportion of TLR4+ CD14+ cells from 41 healthy controls and 51 TB patients. (d) Mean fluorescence intensity (MFI) to TLR4 expression in cells from healthy controls and TB patients. The bars denote mean ± standard deviation. The P-value of the difference between TB patients and healthy controls is shown.
Figure 4.
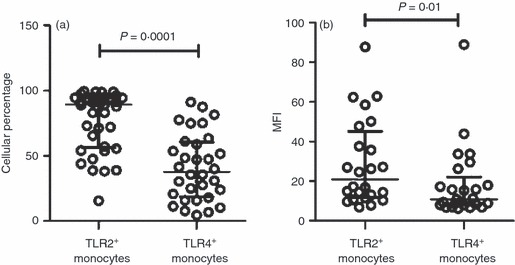
Percentage and expression density of Toll-like receptor 2 (TLR2) is higher than TLR4 in monocytes from patients with tuberculosis (TB). Monocytes were isolated from peripheral blood mononuclear cells by magnetic bead-labelled antibodies, incubated with AlexaFluor 488-labelled antibodies and analysed by flow cytometry. (a) Proportion of TLR2+ and TLR4+ monocytes. (b) Mean fluorescence intensity (MFI) to TLR2 and TLR4 in monocytes. Circles represent the measure of TLR2 or TLR4 in monocytes from each TB patient. The bars denote mean ± standard deviation. The P-value of the difference between groups is indicated.
Increased percentage of death in monocytes from TB patients
To obtain insights about the death of circulating monocytes in TB patients, we evaluated this in cell culture for 24 and 48 hr in the absence of stimulus. The percentage death of freshly purified monocytes at the beginning of culture was 3·4 ± 1·0% in healthy controls and 4·9 ± 1·9% in TB patients. Although, an increased death of monocytes from TB patients was observed at 24 hr, there was no significant difference in the comparison between TB patients and healthy controls (4·5 ± 1·9% versus 1·2 ± 1·2%, respectively) (Fig. 5). This observed increase in cell death was only statistically significant until 48 hr of culture (P = 0·01). The rate of monocyte death in patients with TB was 2·5-fold greater than that of cells from healthy controls (7·0 ± 2·9% versus 2·7 ± 0·5%, respectively). Under the same conditions, dying cells were positive to both TLR2 and TLR4 at the beginning of culture; but after 48 hr, the damaged cells corresponded to TLR2− TLR4− cells (Fig. 6). Although, it was less in healthy controls than in TB patients (Fig. 5), the type of cells undergoing death was similar in both study groups. Interestingly, TLR2 or TLR4 single-positive cells survived throughout the culture (Fig. 6). These results point out that TLR2+ TLR4+ monocytes from TB patients were more susceptible to death.
Figure 5.
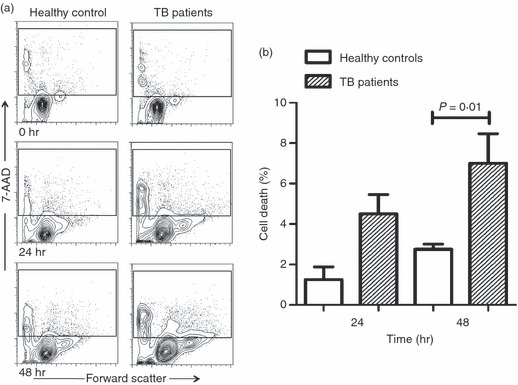
Monocytes from patients with tuberculosis (TB) show increased death rate in the absence of mycobacterial antigen. Monocytes were isolated from peripheral blood mononuclear cells by magnetic beads-labelled antibodies, and incubated with culture medium alone for 24 or 48 hr at 37°. After the culture, cells were stained by 7-aminoactinomycin-D (7-AAD) and analysed through flow cytometry. (a) Representative contour plots for forward scatter versus 7-AAD-stained cells from healthy controls (right panels) or TB patients (left panels). (b) The bars denote cell death rate in cells from healthy control patients (open bars) or TB patients (hatched bars). The P-value of the difference between bars is shown, n = 3.
Figure 6.
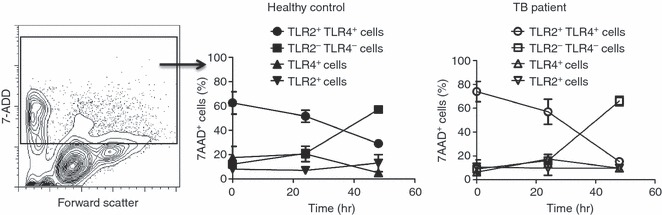
Monocytes positive to both Toll-like receptor 2 (TLR2) and TLR4 are cells in the death process. Monocytes were isolated from peripheral blood mononuclear cells by magnetic bead-labelled antibodies and stained by triple fluorescence before and after being cultured without stimulus for 24 or 48 hr at 37°. Cells were stained by AlexaFluor 488-labelled monoclonal antibody to TLR2, phycoerythrin (PE) -labelled monoclonal antibody to TLR4, and 7-aminoactinomycin-D (7-AAD) followed by flow cytometry analysis. Cells positive to 7-AAD were gated from contour plot for forward scatter versus 7-AAD staining (left panel), and analysed by TLR2 and/or TLR4 expression in healthy controls (medium panels) and patients with tuberculosis (TB) (right panel). Symbols represent the mean and standard deviation of cell subsets, n = 3.
Monocyte death in TB patients because of mycobacterial antigen via TLR2
We evaluated the phenomenon of cell death induced by TLR2, because our results indicate that there is an increase in TLR2 expression in monocytes from TB patients, and because other groups have demonstrated that TLR2 is linked to cell death induced by mycobacterial antigen in in vitro models.10–12,18 Purified monocytes were incubated with CFAE in the presence or absence of either anti-TLR2 or anti-TLR4 mAbs. No difference was observed between the percentage of death affecting cells from TB patients and those from healthy controls, when incubated with the medium alone (7·9 ± 3·5% versus 8·3 ± 5·3%, respectively). Hence, this cell death percentage was taken as the background rate when calculating the death rate caused specifically by stimulating CFAE. With this calculation, CFAE-incubated monocytes from TB patients showed a cell death rate 3·1-fold higher than from healthy controls (Fig. 7); the difference was statistically significant (7·2 ± 1·9% versus 2·3 ± 1·6%, respectively, P = 0·01). As expected, when cells from TB patients were incubated with CFAE in the presence of anti-TLR2 mAb, the cell death rate diminished significantly, 3·4-fold (P = 0·01), which was not observed in the presence of either anti-TLR4 mAb or isotype-matched control antibody (Fig. 7). Regarding healthy control cells no difference was observed under any of the conditions tested. These data indicate that mycobacterial antigens increase death of monocytes from patients with TB by a TLR2-dependent pathway, but not through TLR4.
Figure 7.
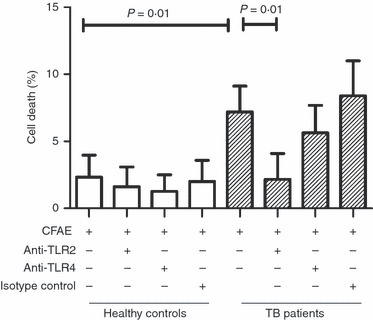
Monocytes from patients with tuberculosis (TB) undergo death induced through mycobacterial antigen via Toll-like receptor 2 (TLR2). Monocytes were isolated from peripheral blood mononuclear cells by magnetic bead-labelled antibodies, and incubated with culture filtrate antigen extract (CFAE) from Mycobacterium tuberculosis H37Rv strain in the presence or absence of anti-TLR2 or anti-TLR4 antibodies for 4 hr at 37°. The bars denote cell death rate in cells from healthy controls (open bars) or TB patients (hatched bars). The P-value of the difference between bars is shown, n = 5.
Monocytes from TB patients were positive to cleaved caspase 9 and denaturalized cytochrome c
To clarify whether monocyte death occurred through extrinsic or intrinsic pathways, we evaluated, by Western blot, the intracellular level of caspase 8 and caspase 9 in monocytes incubated for 4 hr in the presence or absence of CFAE and/or anti-TLR2 mAbs. The results showed unexpectedly a thick band corresponding to cleaved caspase 9 in TB patient cells incubated with the medium alone (Fig. 8a), which apparently diminished in the presence of CFAE or CFAE plus anti-TLR2 mAb, but the statistical analysis did not show any difference (Fig. 8c). In contrast, the sample from healthy controls exhibited a very thin band for cleaved caspase 9, which did not change in any of the tested conditions (Fig. 8a,c). To confirm mitochondrial injury by the intrinsic pathway, cytochrome c was evaluated by using an antibody that recognizes the denaturalized shape. We found high levels of denaturalized cytochrome c in samples from TB patients, and, contrary to caspase 9, the use of anti-TLR2 mAb had no effect, suggesting persistence of mitochondrial damage (Fig. 8a,b). Although in Fig. 8(a), cleaved caspase 8 is not observed, the density analysis showed background levels of this protein, with no difference between samples from TB patients and from healthy controls (Fig. 8d).
Figure 8.
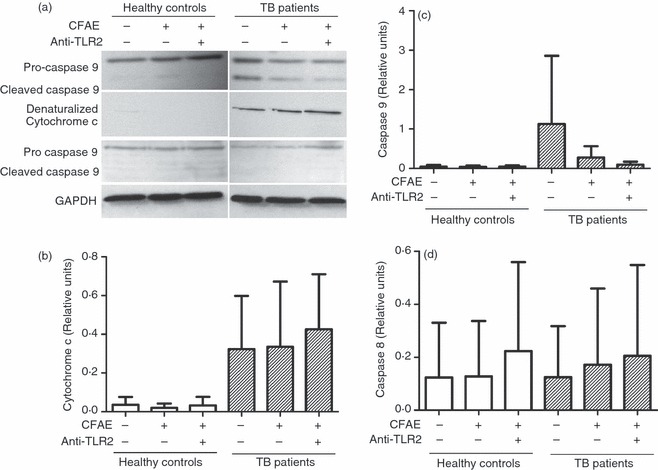
Monocytes from patients with tuberculosis (TB) exhibit cleaved caspase 9 and denaturalized cytochrome c. Monocytes from TB patients or healthy controls, were isolated from peripheral blood mononuclear cells by magnetic bead-labelled antibodies. (a) Western blot for pro-caspase 8, cleaved caspase 8, pro-caspase 9, cleaved caspase 9, and denaturalized cytochrome c of the supernatants of monocyte lysates after stimulation with medium alone or culture filtrate antigen extract (CFAE) from Mycobacterium tuberculosis H37Rv strain in the presence or absence of anti-Toll-like receptor 2 (TLR2) antibodies for 4 hr at 37°. Total protein was determined and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. (b–d) Band densities of denatured cytochrome c, cleaved caspase 9, and cleaved caspase 8 in the Western blots were normalized against GAPDH by densitometry analysis. Results are shown in relative units of concentration using the imagej 1.39c software in samples from healthy controls (open bars) and TB patients (hatched bars). Each bar represents the mean ± SD of three independent experiments.
Monocytes from patients with TB are exposed to high levels of serum TNF-α but show levels of ASK1 and phospho-p38 MAPK similar to healthy control monocytes
Yuk et al. demonstrated that M. bovis BCG stimulation in macrophages results in secretion of TNF-α in a TLR2-dependent and TLR4-dependent manner, which leads to activation of ASK1 upstream of the p38 MAPK.19 Hence, we decided to assess whether this pathway is triggered in monocytes from TB patients after incubation for 4 hr in the presence or absence of CFAE and/or anti-TLR2 mAb. Although we found increased TNF-α levels in serum from TB patients (1·4 ± 1·4 pg/ml versus 0·2 ± 0·08 pg/ml, P = 0·0001) (Fig. 9d) no difference was observed in either ASK1 or phospho-p38α levels (Fig. 9a–c). These observations suggest that even though monocytes from TB patients are exposed to high concentrations of serum TNF-α, this does not affect the levels of ASK1 and phospho-p38 MAPK in the cytoplasm.
Figure 9.
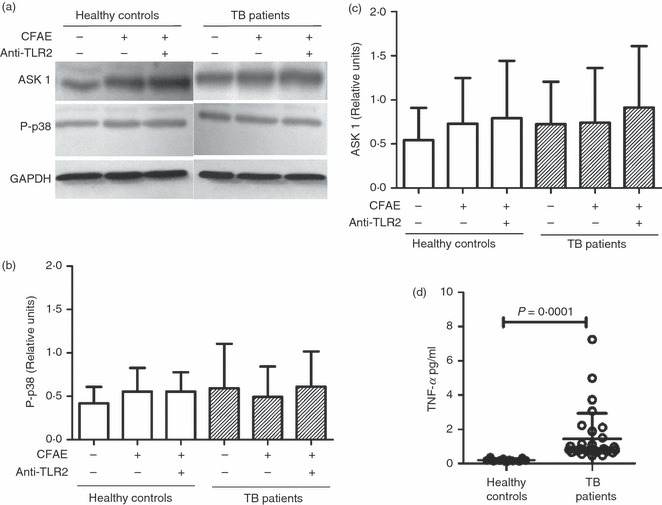
Monocytes from patients with tuberculosis (TB) patients and healthy controls exhibit similar apoptosis-regulating signal kinase 1 (ASK1) and phospho-p38 mitogen-activated protein kinase (MAPK) levels in spite of exposure to high levels of serum tumour necrosis factor-α (TNF-α). Monocytes from TB patients or healthy controls were isolated from peripheral blood mononuclear cells by magnetic beads-labelled antibodies. (a) Western blot for ASK-1 and phospho-p38 MAPK of the supernatants of monocyte lysates after stimulation with medium alone or culture filtrate antigen extract (CFAE) from Mycobacterium tuberculosis H37Rv strain in the presence or absence of anti-Toll-like receptor 2 (TLR2) antibodies for 4 hr at 37°. Total protein was determined and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. (b, c) Band densities of ASK-1 and phospho-p38 MAPK in the Western blots were normalized against GAPDH by densitometry analysis. Results are shown in relative units of concentration using the imagej 1.39c software in samples from healthy controls (open bars) and TB patients (hatched bars). Each bar represents the mean ± SD of three independent experiments. (d) TNF-α concentration in sera from 41 healthy controls and 51 TB patients. The P-value of the difference between TB patients and healthy controls is shown.
Pam3CSK4 synthetic lipopeptide induces monocyte death but does not increase caspase 9 level
To examine whether agonist ligands to TLR2 increase the cleaved caspase 9 level, monocytes were stimulated with either Pam3CSK4 or FSL-1 or LM-MS for 4 hr. As shown in Fig. 10a, the Pam3CSK4 synthetic lipopeptide induced a cell death comparable to that caused by CFAE on TB patient-monocytes. In contrast, the FSL-1 diacylated ligand induced a fivefold lower rate of cell death than the Pam3CSK4 triacylated ligand (1·0 ± 1·0% versus 5·0 ± 1·0%, respectively), which was statistically significant (P = 0·04). The LM-MS had no effect on TB patient monocytes, but, in monocytes from healthy controls the cell death rate was similar to that for FSL-1-induced death (Fig. 10a). On the other hand, the stimulation of monocytes by Pam3CSK4, FSL-1, or LM-MS showed pro-caspase 9 levels only in the group of healthy controls (Fig. 10b). Conversely, in unstimulated monocytes from healthy controls, the cleaved caspase 9 level was undetectable, whereas a thick band was observed in TB patients (Fig. 10b). Although to a minor extent for samples from healthy controls, the protein density analysis in Western blot revealed that cleaved caspase 9 levels were similar in any of the conditions tested in both study groups (Fig. 10c,d). Neither denaturalized cytochrome c levels nor cleaved caspase 8 levels showed differences after monocyte stimulation with any of the aforementioned ligands to TLR2 (data not shown). These findings indicate that monocyte death is augmented by addition of Pam3CSK4 triacylated lipopeptide, which did not cause modification in cleaved caspase 9 levels.
Figure 10.
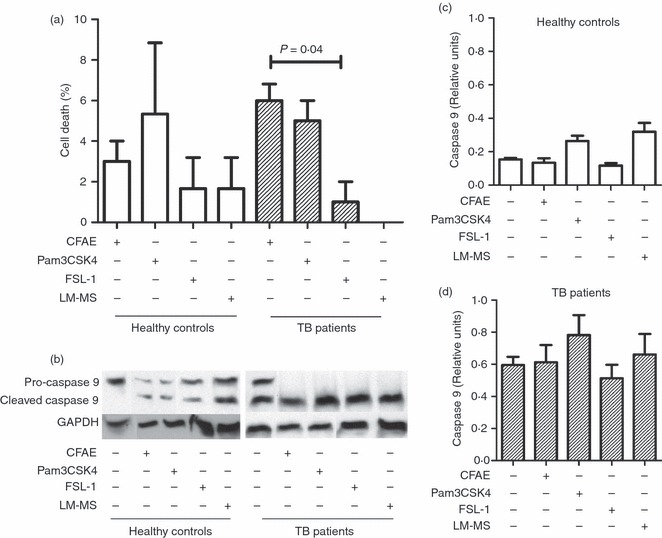
The synthetic lipopeptide Pam3CSK4 induces monocyte death but does not increase caspase 9 levels. Monocytes were isolated from peripheral blood mononuclear cells by magnetic bead-labelled antibodies, and cultured in the presence or absence of mycobacterial antigen (using culture filtrate antigen extract; CFAE) from Mycobacterium tuberculosis H37Rv strain or agonist ligand to Toll-like receptor 2 (TLR2) [Pam3CSK4, fibroblast-stimulating lipopeptide-1 (FSL-1), and lipomannan of Mycobacterium smegmatis (LM-MS)] for 4 hr at 37°. (a) Graphic showing death rate percentage in cells from healthy control (open bars) or patients with tuberculosis (TB) (hatched bars). The P-value of the difference between bars is shown, n = 3. (b) Western blot for pro-caspase 9, and cleaved caspase 9 of the supernatants of monocyte lysates after stimulation with medium alone, or CFAE, or ligands to TLR2. Total protein was determined and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. (c, d) Graphics representing band densities of cleaved caspase 9 in the Western blots normalized against GAPDH by densitometry analysis. Results are shown in relative units of concentration using the imagej 1.39c software in samples from healthy controls (open bars) and TB patients (hatched bars). Each bar represents the mean ± SD of three independent experiments.
Discussion
Innate immune defence mechanisms cooperate to eliminate infected cells but M. tuberculosis uses strategies to prevent macrophage apoptosis and the balance between survival and death in macrophages can determine the outcome of infection. However, these pathways still remain poorly understood in cells from TB patients. In this study we found a significant increase of peripheral blood monocytes in TB patients. This result is consistent with observations from Sánchez et al. and Janols et al.20,21, who reported an increase of circulating monocytes in TB patients in both percentage and absolute numbers, respectively. However, Sánchez et al.20 showed also a decreased expression of HLA-DR using an anti-HLA-DR mAb in monocytes from TB patients, which we did not find; perhaps, this difference is because we used an antibody recognizing the three major isoforms of HLA class II molecules (HLA-DR, -DP, -DQ).
Concerning TLR2 in monocytes from TB patients, we found an increased proportion of TLR2+ monocytes (77·4%) in patients with pulmonary tuberculosis. In contrast, another group reported approximately 50% of monocytes expressing TLR2 in patients with tuberculous pleuritis.22 The percentage difference might be the result of factors such as tuberculosis type or M. tuberculosis virulence. In this regard, Rocha-Ramirez et al.23 showed that lipid fractions can regulate TLR2 and TLR4 expression depending on the virulence grade of bacteria.
When we compared density expression, both TLR2 and TLR4 were significantly higher in monocytes from TB patients than in those cells from healthy controls. Moreover, the proportion of TLR2+ monocytes was higher than that of TLR4+ monocytes in TB patients. These data suggest a strong association between TRL2 expression and tuberculosis disease. It has been shown that TLR2-deficient mice succumb to M. tuberculosis infection,24 and that, human TLR2 polymorphism is associated with susceptibility to tuberculosis.25,26 However, the role of TLR2 in the protective immune response to tuberculosis remains controversial, because other authors have shown a TLR2-independent immune response to M. tuberculosis infection models.27,28 It has been demonstrated that TLR2 is required for the production of diverse cytokines, for example interleukin-12, TNF-α and interleukin-6, in response to diverse mycobacterial antigens in human monocytes or murine macrophages.3,5,7,29 However, different mycobacterial antigens can also induce apoptosis by TLR2 in either a human THP-1 monocytic line or monocyte-derived macrophages.10–12,30 We found that the mycobacterial antigens from M. tuberculosis H37Rv strain caused a 7·2% death rate in circulating monocytes from TB patients, which was abrogated by neutralizing antibodies for TLR2 but not TLR4. Our results suggest that mycobacterial antigens to TLR4 are not involved in inducing cell death in human tuberculosis.
An interesting finding from our study is that freshly obtained monocytes from TB patients showed denaturalized cytochrome c and cleaved caspase 9 levels in the absence of mycobacterial antigens. In contrast, we did not observe this difference when cleaved caspase 8 levels were analysed in the presence or absence of mycobacterial antigen and anti-TLR2 mAb, probably because the level of capase 8 comes from background cell death. Caspases are cytoplasmic cysteine proteases synthesized as zymogens (pro-caspases), which turn into active enzymes (cleaved caspases) after a limited proteolysis.31 Activated caspase 8 or caspase 9, in turn, cleaves effector pro-caspases propagating the death signalling cascade. The presence of cleaved caspase 8 corresponds to intracellular signalling from the apoptosis extrinsic pathway, whereas cleaved caspase 9 and cytochrome c levels are associated with mitochondrial injury (intrinsic pathway).31 Hence, levels of activated caspase 9 and cytochrome c in monocytes from patients with TB suggest a pre-apoptotic primed status, which is also suggested by the percentage of cell death that occurs during the 24–48 hr of culture in the absence of antigen. Perhaps this pre-apoptotic status induces monocytes from TB patients to respond rapidly to TLR2 ligation with CFAE, leading to cell death that is avoided by neutralizing antibodies for TLR2. Moreover, the cleaved caspase 9 level in unstimulated monocytes from TB patients was similar to that obtained for cells stimulated with CFAE or distinct agonist ligands to TLR2 (Figs 8 and 10). Similar results were observed when we analysed the denaturalized cytochrome c levels, which persisted under the same conditions. In contrast, the denaturalized cytochrome c and cleaved caspase 9 values in samples from healthy controls indicated a minimal level, which supports our previous result of low percentage of cell death at 24 or 48 hr of culture. Taken together, our results suggest the persistence of mitochondrial damage and activated caspase 9 interactions with other apoptotic process molecules.32
An important point that should be considered in our results is the standard deviation, which, although minimal in healthy controls, reached high values in samples from TB patients and showed great dispersion, perhaps because of intrinsic factors in the immune response.
In addition, the majority of unstimulated cells that were damaged at the beginning of culture corresponded to TLR2+ TLR4+ monocytes in both patients and controls. Our results suggest that interaction between TLR2-mediated signalling pathways and TLR4-mediated signalling pathways are involved in monocyte death, perhaps in the same way as reported in an M. tuberculosis infection model of murine macrophage lines.33
Based on reports indicating that 19 000 MW and 38 000 MW M. tuberculosis lipoproteins cause activation of caspase 8 and caspase 9 in both THP-1 cells and monocyte-derived macrophages through TLR2,10,12 we decided to evaluate whether a TLR2-dependent cell death pathway occurs in TB patient monocytes stimulated with mycobacterial antigens. Our results regarding activated caspase 8 levels were equivalent in samples from both TB patients and healthy controls; hence, caspase 8 does not seem to be required initially for death by TLR2 in monocytes from TB patients. On the other hand, cell death is prevented by the anti-TLR2 mAb decreasing cleaved caspase 9 levels, which suggests that caspase 9 is associated with TLR2-dependent cell death induced by antigens from M. tuberculosis.
Several authors have reported activation of ASK1 and p38 MAPK through TLR2 signalling in human primary monocytes stimulated with purified protein derivative tuberculin or murine macrophages stimulated with M. bovis BCG.8,19 Likewise, a previous report suggests that TNF-α plays an important role in the induction of macrophage apoptosis.34 For this reason, we examined concentrations of phospho-p38 MAPK and ASK1 in monocytes from patients with TB. However, no difference was observed in any conditions tested in spite of high serum TNF-α levels in patients with TB (Fig. 9).
Finally, another finding was that both CFAE and Pam3CSK4 induced the death of monocytes from TB patients in a similar way, whereas FSL-1 showed only a slight effect. This outcome could be the result of the ability of the cytoplasmic TLR2 domain to form functional heterodimeric complexes with TLR6 or TLR1.35 Previous reports have shown that Pam3CSK4 is a synthetic triacylated lipopeptide recognized by the TLR2/TLR1 heterodimer,36 whereas FSL-1 (Pam2CGDPKHPKSF) is a synthetic diacylated lipopeptide recognized by the TLR2/TLR6 heterodimer.37 These data suggest that triacylated lipopeptides from M. tuberculosis increase the death process in monocytes from TB patients.
Our report offers new insight into the cell death of monocytes from TB patients; probably, these cells have undergone mitochondrial injury, leading to a constant release of cytochrome c and production of cleaved caspase 9, thereby becoming more susceptible to death.
Acknowledgments
We are grateful to Dr Iris Estrada, Dr Gloria Soldevila and Dr Raul Mancilla for useful comments. This article is part of the requirements for the degree of PhD by Leslie Chávez-Galán in the program: Doctorado en Ciencias Biomédicas, Facultad de Medicina, Universidad Nacional Autónoma de México (UNAM), Mexico. This work was supported by grant 82434 and student scholarship 203164 from the Consejo Nacional de Ciencia y Tecnología, Mexico.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.World Health Organization. Global tuberculosis control – surveillance, planning, financing. WHO report 2008, WHO/HTM/TB/2008.393.
- 2.Lee J, Hartman M, Kornfeld H. Macrophage apoptosis in tuberculosis. Yonsei Med J. 2009;50:1–11. doi: 10.3349/ymj.2009.50.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–6. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 4.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–7. [PubMed] [Google Scholar]
- 5.Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci USA. 1999;96:14459–63. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thoma-Uszynski S, Stenger S, Takeuchi O, et al. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291:1544–7. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- 7.Jung SB, Yang CS, Lee JS, et al. The mycobacterial 38-kilodalton glycolipoprotein antigen activates the mitogen-activated protein kinase pathway and release of proinflammatory cytokines through Toll-like receptors 2 and 4 in human monocytes. Infect Immun. 2006;74:2686–96. doi: 10.1128/IAI.74.5.2686-2696.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang CS, Shin DM, Lee HM, et al. ASK1-p38 MAPK-p47phox activation is essential for inflammatory responses during tuberculosis via TLR2-ROS signaling. Cell Microbiol. 2008;10:741–54. doi: 10.1111/j.1462-5822.2007.01081.x. [DOI] [PubMed] [Google Scholar]
- 9.Aliprantis AO, Yang RB, Mark MR, et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–9. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 10.López M, Sly LM, Luu Y, Young D, Cooper H, Reiner NE. The 19-kDa Mycobacterium tuberculosis protein induces macrophage apoptosis through Toll-like receptor-2. J Immunol. 2003;170:2409–16. doi: 10.4049/jimmunol.170.5.2409. [DOI] [PubMed] [Google Scholar]
- 11.Ciaramella A, Cavone A, Santucci MB, et al. Induction of apoptosis and release of interleukin-1 beta by cell wall-associated 19-kDa lipoprotein during the course of mycobacterial infection. J Infect Dis. 2004;190:1167–76. doi: 10.1086/423850. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez A, Espinosa P, Esparza MA, Colon M, Bernal G, Mancilla R. Mycobacterium tuberculosis 38-kDa lipoprotein is apoptogenic for human monocyte-derived macrophages. Scand J Immunol. 2009;69:20–8. doi: 10.1111/j.1365-3083.2008.02193.x. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society and the Centers for Disease Control and Prevention, Diagnosis Standards and Classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–95. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 14.Boyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976;5:9–15. [PubMed] [Google Scholar]
- 15.Lecoeur H, Février M, Garcia S, Rivière Y, Gougeon ML. A novel flow cytometric assay for quantitation and multiparametric characterization of cell-mediated cytotoxicity. J Immunol Methods. 2001;253:177–87. doi: 10.1016/s0022-1759(01)00359-3. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher S, Winston SE, Fuller SA, Hurrell JG. Chapter 8 Unit 8.10. Immunoblotting and immunodetection. In: Coligan JE, Bierer B, Margulies DH, Shevach EM, Strober W, Coico R, editors. Current Protocols in Immunology. Somerset, NJ: Wiley Online Library; 2001. pp. 8.10.1–10.21. [Google Scholar]
- 17.Means TK, Jones BW, Schromm AB, et al. Differential effects of a Toll-like receptor antagonist on Mycobacterium tuberculosis-induced macrophage responses. J Immunol. 2001;166:4074–82. doi: 10.4049/jimmunol.166.6.4074. [DOI] [PubMed] [Google Scholar]
- 18.Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A. The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J. 2000;19:3325–36. doi: 10.1093/emboj/19.13.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuk JM, Shin DM, Yang CS, Kim KH, Rho J, Park JK, JO EK. Role of apoptosis-regulating signal kinase 1 in innate immune responses by Mycobacterium bovis bacillus Calmette-Guérin. Immunol Cell Biol. 2009;87:100–7. doi: 10.1038/icb.2008.74. [DOI] [PubMed] [Google Scholar]
- 20.Sánchez MD, García Y, Montes C, París SC, Rojas M, Barrera LF, Arias MA, García LF. Functional and phenotypic changes in monocytes from patients with tuberculosis are reversed with treatment. Microbes Infect. 2006;8:2492–500. doi: 10.1016/j.micinf.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Janols H, Bredberg A, Thuvesson I, Janciauskiene S, Grip O, Wullt M. Lymphocyte and monocyte flow cytometry immunophenotyping as a diagnostic tool in uncharacteristic inflammatory disorders. BMC Infect Dis. 2010;10:205. doi: 10.1186/1471-2334-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prabha C, Rajashree P, Sulochana DD. TLR2 and TLR4 expression on the immune cells of tuberculous pleural fluid. Immunol Lett. 2008;117:26–34. doi: 10.1016/j.imlet.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Rocha-Ramírez LM, Estrada-García I, López-Marín LM, et al. Mycobacterium tuberculosis lipids regulate cytokines, TLR-2/4 and MHC class II expression in human macrophages. Tuberculosis (Edinb) 2008;88:212–20. doi: 10.1016/j.tube.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Drennan MB, Nicolle D, Quesniaux VJ, et al. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am J Pathol. 2004;164:49–57. doi: 10.1016/S0002-9440(10)63095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben-Ali M, Barbouche MR, Bousnina S, Chabbou A, Dellagi K. Toll-like receptor 2 Arg677Trp polymorphism is associated with susceptibility to tuberculosis in Tunisian patients. Clin Diagn Lab Immunol. 2004;11:625–6. doi: 10.1128/CDLI.11.3.625-626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogus AC, Yoldas B, Ozdemir T, et al. The Arg753GLn polymorphism of the human toll-like receptor 2 gene in tuberculosis disease. Eur Respir J. 2004;23:219–23. doi: 10.1183/09031936.03.00061703. [DOI] [PubMed] [Google Scholar]
- 27.Hölscher C, Reiling N, Schaible UE, et al. Containment of aerogenic Mycobacterium tuberculosis infection in mice does not require MyD88 adaptor function for TLR2, -4 and -9. Eur J Immunol. 2008;38:680–94. doi: 10.1002/eji.200736458. [DOI] [PubMed] [Google Scholar]
- 28.McBride A, Bhatt K, Salgame P. Development of a secondary immune response to Mycobacterium tuberculosis is independent of Toll-like receptor 2. Infect Immun. 2011;79:1118–23. doi: 10.1128/IAI.01076-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pecora ND, Gehring AJ, Canaday DH, Boom WH, Harding CV. Mycobacterium tuberculosis LprA is a lipoprotein agonist of TLR2 that regulates innate immunity and APC function. J Immunol. 2006;177:422–9. doi: 10.4049/jimmunol.177.1.422. [DOI] [PubMed] [Google Scholar]
- 30.Dao DN, Kremer L, Guérardel Y, Molano A, Jacobs WR, Jr, Porcelli SA, Briken V. Mycobacterium tuberculosis lipomannan induces apoptosis and interleukin-12 production in macrophages. Infect Immun. 2004;72:2067–74. doi: 10.1128/IAI.72.4.2067-2074.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbas AK, Lichtman AH, Pillai S. Cellular and Molecular Immunology. 6th edn. Philadelphia, PA: Saunders Elsevier; 2007. [Google Scholar]
- 32.Slee EA, Harte MT, Kluck RM, et al. Ordering the cytochrome C-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–92. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sánchez D, Rojas M, Hernández I, Radzioch D, García LF, Barrera LF. Role of TLR2- and TLR4-mediated signaling in Mycobacterium tuberculosis-induced macrophage death. Cell Immunol. 2010;260:128–36. doi: 10.1016/j.cellimm.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Rojas M, Olivier M, Gros P, Barrera LF, García LF. TNF-α and IL-10 modulate the induction of apoptosis by virulent Mycobacterium tuberculosis in murine macrophages. J Immunol. 1999;162:6122–31. [PubMed] [Google Scholar]
- 35.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–71. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO. Crystal structure of the TLR1–TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–82. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Kang JK, Nan X, Jin MS, et al. Recognition of lipopeptide patterns by Toll-like receptor 2–Toll-like receptor 6 heterodimer. Immunity. 2009;31:873–84. doi: 10.1016/j.immuni.2009.09.018. [DOI] [PubMed] [Google Scholar]


