Abstract
Highly differentiated CD8+ CD28− CD27− T cells have short telomeres, defective telomerase activity and reduced capacity for proliferation. In addition, these cells express increased levels of inhibitory receptors and display defective Akt(ser473) phosphorylation following activation. It is not known whether signalling via programmed death 1 (PD-1) contributes to any of the attenuated differentiation-related functional changes in CD8+ T cells. To address this we blocked PD-1 signalling during T-cell receptor (TCR) activation using antibodies against PD-1 ligand 1 (PDL1) and PDL2. This resulted in a significant enhancement of Akt(ser473) phosphorylation and TCR-induced proliferative activity of highly differentiated CD8+ CD28− CD27− T cells. In contrast, the reduced telomerase activity in these cells was not altered by blockade of PDL1/2. We also demonstrate that PD-1 signalling can inhibit the proliferative response in primary human CD8+ T cells from both young and older humans. These data collectively highlight that some, but not all, functional changes that arise during progressive T-cell differentiation and during ageing are maintained actively by inhibitory receptor signalling.
Keywords: age, differentiation, human, PD-1, T cell
Introduction
The immune system undergoes dramatic restructuring with age, with an increase in the proportion of dysfunctional T cells and a decline in immune responses.1,2 These changes are associated with an increase in frequency and severity of infections and autoimmune diseases, and a higher occurrence of malignancies in older adults.3 This immune decline is manifested at the cellular level by a marked decline in the number of naive T cells as a result of thymic atrophy.4,5 This reduced thymic output leads to the ongoing requirement for peripheral expansion of naive and memory T cells to maintain the T-cell pool and this leads to the accumulation of oligoclonal expansions of functionally impaired T cells in older subjects.6,7 These defects include decreased interleukin-2 synthesis,8,9 reduced proliferation, in part because of defects in cell signalling10 and in particular loss of the ability to phosphorylate Akt at the ser473 site9 and excessive telomere erosion.11
One of the key phenotypic changes that occurs in T cells during ageing and differentiation is the loss of the cell surface co-stimulatory molecules CD27 and CD28, with the phenotypic shift being more pronounced on the CD8+ than the CD4+ T-cell subset.12–14 Initially, it was thought that the loss of CD28 was a major factor in the reduced activation and function of these cells;15 however, there is considerable redundancy in co-stimulatory receptor usage in highly differentiated T cells and alternative receptors may be engaged instead to promote T-cell activation in CD28− CD8+ populations.9,16,17 The increase in highly differentiated CD8+ CD28− CD27− T cells during ageing1,5,14 with their functional defects, may explain the decreased efficiency of the immune system in older individuals.18 However, it is not clear how the decreased functionality is controlled. We hypothesize here that the decreased functionality of highly differentiated primary human T cells may be directly regulated by inhibitory signals, as the inhibitory receptors killer cell lectin-like receptor G1 (KLRG1),17,19 programmed death 1 (PD-1),20–22 cytotoxic T lymphocyte antigen 4 (CTLA-4),23,24 T cell immunoglobulin mucin 3 (TIM-3)25–27 and lymphocyte activation gene 3 (LAG-3)23,28 have all been shown to induce T-cell unresponsiveness. Although these inhibitory receptors have been strongly associated with T-cell exhaustion, the expression of these receptors per se does not indicate that a T cell is exhausted. For example, human T cells that express PD-1, CTLA-4 and other inhibitory receptors can exhibit functional activity after activation.29 However, late stage, differentiated T-cell populations can express relatively high levels of these inhibitory receptors compared with undifferentiated cells, suggesting their potential to affect T-cell function in older humans in whom these cells accumulate.
PD-1 signalling during T-cell stimulation has been shown to inhibit phosphoinositide 3-kinase (PI3K) activation through the binding of either SH2 domain-containing protein tyrosine phosphatase 1 (SHP-1) or SHP-2 to the immunoreceptor tyrosine switch motif.30 A key downstream effector of PI3K is the serine-threonine kinase Akt which, in response to PI3K activation, phosphorylates and regulates the activity of a number of targets including kinases, transcription factors and other regulatory molecules.31 The activation of Akt requires the binding of its pleckstrin homology domain to the phosphoinositide products of PI3K resulting in its recruitment to the plasma membrane. Once there, Akt activation is controlled by phosphorylation at two different sites, Thr308 and Ser473. We have previously shown that highly differentiated CD8+ CD28− CD27− T cells are unable to phosphorylate Akt(ser473), with the Thr308 phosphorylation site being unaffected.9 We demonstrate here that the blockade of PD-1 signalling restored the defective Akt(ser473) phosphorylation in highly differentiated CD8+ CD28− CD27− T cells. This indicates that the defective Akt phosphorylation is not a passive consequence of antigen-driven differentiation of CD8+ T cells but is instead actively maintained by inhibitory receptor signalling.
We demonstrate here that the defective Akt(ser473) phosphorylation and proliferation of highly differentiated CD8+ CD28− CD27− T cells are actively regulated by PD-1 signalling and that these defects can be reversed by blocking the interaction of this molecule with its ligand. Furthermore, the combined use of PD-1, CTLA-4 and KLRG1 blockade did not enhance proliferation, indicating that these molecules operate via a similar signalling pathway. However, PD-1 blockade did not reverse the telomerase activity defect in these cells after activation, indicating that other Akt-independent mechanisms are involved in telomerase down-regulation in these cells. The manipulation of inhibitory signals mediated by PD-1 and other inhibitory receptors on T cells may be potentially useful for increasing selective T-cell functions during immunotherapeutic regimens such as vaccination in older subjects.
Methods
Blood sample collection and isolation
Heparinized peripheral blood samples were taken from healthy volunteers. Where the data are stratified by age, young is defined as individuals between 20 and 35 years (median age 30) and old as people over 65 years (median age 78). All samples were obtained in accordance with the ethical committee of Royal Free and University College Medical School. Old donors did not have any co-morbidity and were not on any immunosuppressive drugs and retained mobility and independence. Peripheral blood mononuclear cells were isolated using Ficoll–Hypaque (Amersham Biosciences, Amersham, UK) and either analysed immediately or cryopreserved as described previously.17
Flow cytometric analysis and cell sorting
Five-colour flow cytometric analysis was performed using the following antibodies: phycoerythrin (PE) -conjugated anti-PD-1 (kind gift from G. Freeman, Dept Medical Oncology, Dana-Faber Cancer Institute, USA), anti-CTLA-4 PE (clone BN13), peridinin chlorophyll protein-conjugated anti-CD8 (clone SK1), FITC-conjugated anti-CD27 (clone M-T271), allophycocyanin-H7-conjugated anti-CD27 (clone M-T271) allophycocyanin-conjugated anti-CD28 (clone CD28.2), PE-Cy7-conjugated anti-CD45RA (clone L48), PE-Cy7-conjugated anti-CCR7 (clone 3D12), all from BD Biosciences (Oxford, UK). All samples were run using LSRII and analysed using FlowJo software (Treestar, Ashland, OR).
CD8+ T cells were purified by negative selection using the VARIOMACS system (Miltenyi Biotec, Bisley, UK) according to the manufacturer's instructions. Negatively selected CD8+ T cells were stained with anti-CD28 biotin (clone CD28.2; BD Biosciences), washed, and then incubated with anti-biotin microbeads according to the manufacturer's instructions. Positively selected cells were CD8+ CD28+ CD27+. The CD8+ CD28− fraction was further separated into CD8+ CD28− CD27+ and CD8+ CD28− CD27− using CD27 microbeads (Miltenyi Biotec).
Inhibitory receptor blockade
Inhibitory receptors on purified CD8+ T cells and CD28/27-defined CD8+ subsets were blocked using either 10 μg/ml anti-CTLA-4 (clone BN13; BD Biosciences), anti-PDL1, anti-PDL2 (kind gift from G. Freeman), anti-E-cadherin (clone 67A4; Chemicon, Watford, UK) or isotype controls, anti-IgG2a (clone MG2a-53), anti-IgG2b (clone MPC-11) (both from Abcam, Cambridge, UK), anti-IgG1 (clone MOPC31c; Sigma, Gillingham, UK) at the start of culture during the 3-day stimulation period with anti-CD3 (purified OKT3, 0·5 μg/ml) and irradiated antigen-presenting cells (APCs), in a 1 : 1 ratio. Irradiated APCs are used as a source of multiple co-stimulatory signals to activated T-cell subsets sorted on the presence or absence of CD28.9
Proliferation assays
CD8+ T cells were stimulated with anti-CD3 (purified OKT3, 0·5 μg/ml) and irradiated APCs, in a 1 : 1 ratio, for 3 days and proliferation was assessed by [3H]thymidine incorporation. Proliferation was expressed either as the mean [3H]thymidine incorporation (counts/min; c.p.m.) of triplicate wells ± SD or a proliferation index was calculated. The proliferation index was determined by calculating the ratio of c.p.m. in cells proliferating in response to anti-CD3 stimulation versus the c.p.m. in cells proliferating in response to antibody blockade.
Western blot analysis
Purified CD8+ CD28/27 populations were activated in the presence of anti-CD3 (0·5 μg/ml) and irradiated APCs for 3 days. After which time cell lysates were obtained by sonicating cells in 50 mm Tris–HCl (pH 7·5), 2 mm EGTA, 0·1% Triton X-100 buffer. Lysates from 2 × 106 cells were fractionated on SDS–polyacrylamide electrophoresis gels and analysed by immunoblotting with anti-pAkt1/2/3 (Ser473), anti-pAkt (Thr308), anti-Akt, anti-Cyclin D1/2/3 or anti-β-actin, all from Santa Cruz Biotechnology (Wembley, UK) using the ECL Advanced Western Blotting Detection kit (Amersham Biosciences), according to the protocol provided by the manufacturer.
Measurement of telomerase activity
Purified CD8+ T-cell populations (2 × 105 cells) were snap-frozen after stimulation for 3 days with anti-CD3 (purified OKT3, 0·5 μg/ml), and irradiated APCs. Telomerase activity was determined using the TeloTAGGG telomerase ELISA kit from Roche (Burgess Hill, UK) according to the protocol provided by the manufacturer. The absolute numbers of CD8+ T cells were enumerated using Trypan blue (Sigma) and Ki67 analysis. ELISAs was performed with samples adjusted to 500 Ki67+ T cells per reaction as described previously.9
Statistical analysis
Statistical significance was evaluated using a paired one-way Student's t-test. Differences were considered significant when P was < 0·05.
Results
The expression of PD-1 on CD8+ T cells from young and old donors
Three main subsets of CD8+ T cells can be identified on the basis of CD28 and CD27 expression (Fig. 1a): CD28+ CD27+ (relatively undifferentiated), CD28− CD27+ (intermediate differentiation) and CD28− CD27− (highly differentiated), as described previously.9,32–35 Although human CD8+ T cells can be identified and isolated using combinations of different sets of surface markers such as CCR7 and CD45RA,35 the use of CD28/CD27 enabled the isolation of sufficient CD8+ cells from three discrete stages of differentiation for functional analysis. The percentage of highly differentiated CD28− CD27− T cells within the CD8+ T-cell pool increases significantly during ageing (Fig. 1b; P < 0·0001). These highly differentiated CD28− CD27− T cells show reduced proliferative responses to stimulation and have the shortest telomeres, resulting from a reduced ability to up-regulate telomerase following activation. However, it is not known how the functional defects in these highly differentiated cells are regulated.9
Figure 1.
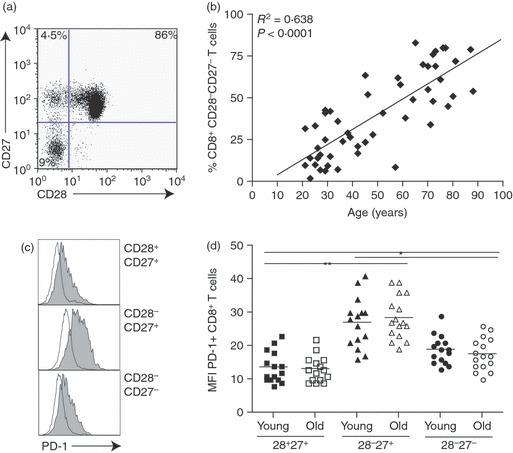
Changes occurring to CD8+ T cells with age and differentiation. (a) CD28/CD27 staining using purified peripheral blood mononuclear cells isolated from a young donor (< 35 years) gated on CD8+ T cells. (b) Graph showing the correlation between highly differentiated CD28− CD27− T cells, expressed as a percentage of CD8+ T cells, and age. (c) Programmed death 1 (PD-1) staining on CD28/CD27-defined CD8+ T-cell subsets from a young individual. (d) Graph showing the mean fluorescence intensity (MFI) of CD8+ T cells expressing PD-1 in young and old donors on CD28/CD27-defined subsets. Horizontal lines depict mean values and P-values were calculated using one-way paired Student's t-test.
PD-1 is the most investigated inhibitory receptor but little is known about how the expression of this molecule changes during human T-cell differentiation. When we examined the expression of PD-1 on CD28/CD27-defined CD8+ T-cell subsets, we found the highest levels on the intermediate (CD28− CD27+) subsets (Fig. 1c). The highly differentiated population expressed significantly higher levels of this molecule than undifferentiated CD28/CD27 CD8+ T cells (Fig. 1c). Although we found significant differences in the level of PD-1 expression between the three CD28/CD27 subsets (Fig. 1d), we found there to be no difference in the expression of PD-1 with age (data not shown), supporting previous observations.17
PD-1 blockade reverses defective Akt(ser473) phosphorylation in highly differentiated CD8+ T cells
Activation of the kinase Akt is essential for many cellular functions and is controlled by phosphorylation of this molecule at two different sites, Thr308 and Ser473.36,37 We have reported that highly differentiated CD8+ CD28− CD27− T cells are unable to phosphorylate Akt at the Ser473 phosphorylation site.9 We showed, by Western blot analysis, that in the presence of PDL1/2 blockade, there is an enhancement of Akt(ser473) phosphorylation in CD8+ CD28− CD27− T cells following a 3-day stimulation with anti-CD3 and autologous irradiated APCs (Fig. 2a,b). This increase reached the level of undifferentiated CD8+ CD28+ CD27+ T cells. We found there to be no effect on Akt at the Thr308 site following anti-PDL1/2 block (Fig. 2c).
Figure 2.
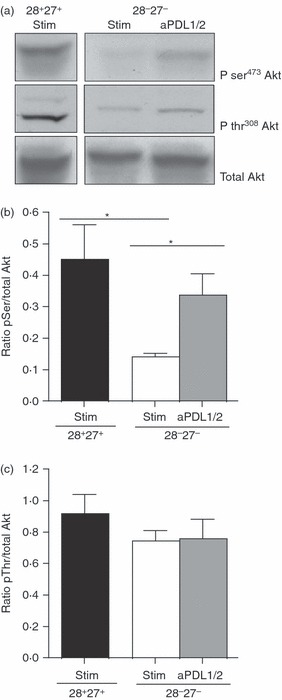
Programmed death 1 (PD-1) blockade increases Ser473 Akt phosphorylation. (a) Immunoblots of pSer473, pThr308 and total Akt for CD8+ CD28/CD27 subsets following a 3-day incubation with 10 μg/ml anti-PD-1 ligand 1/2 (PDL1/2) antibody block. The CD28/CD27 T cells were isolated from a young donor (< 35 years) (b) Densitometric analysis showing the ratio between pSer Akt/total Akt in CD8+ CD28+ CD27+ T cells and CD8+ CD28− CD27− T cells following anti-PDL1/2 blockade. (c) Densitometric analysis showing the ratio between pThr Akt/total Akt following anti-PDL1/2 blockade in CD28/CD27-defined subsets. Graphs show the mean ± SE for three donors and P-values were calculated using one-way paired Student's t-test.
PD-1 blockade enhances CD8+ T-cell proliferation
The PI3K/Akt signalling pathway has been shown to play a key role in regulating cellular proliferation38. Therefore, we investigated whether the proliferative defect in highly differentiated cells could be reversed through blockade of PD-1 signalling, We found that the addition of anti-PDL1/2 significantly enhanced the proliferative capacity of unfractionated CD8+ T cells measured by [3H]thymidine incorporation (representative example Fig. 3a) in both young (Fig. 3b, P < 0·05) and old (Fig. 3c, P < 0·01) subjects. We next investigated the effect of anti-PDL1/2 block on CD28/CD27-defined CD8+ T-cell subpopulations. We found that the addition of anti-PDL1/2 blocking antibodies significantly increased proliferative activity in all three populations isolated from young individuals (Fig. 3d). The greatest enhancement observed was among the intermediate CD28− CD27+ CD8+ T-cell subset (P < 0·01). CD28/CD27-defined CD8+ T-cell populations from old individuals also responded to the anti-PDL1/2 blocking antibodies, with significantly enhanced proliferation being observed in the intermediate CD28− CD27+ and highly differentiated CD28− CD27− populations (P < 0·01 and P < 0·05, respectively; Fig. 3e).
Figure 3.
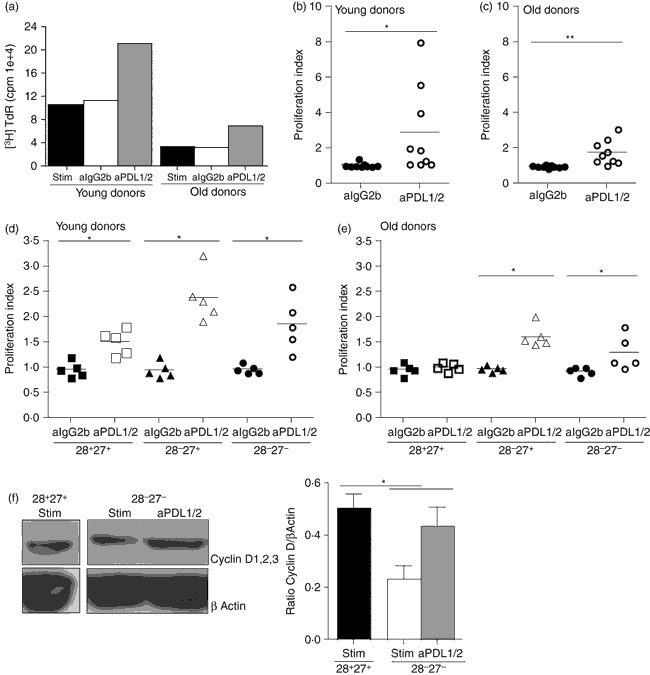
Blockade of programmed death 1 (PD-1) causes increased proliferation in CD8+ T cells. (a) Representative example of anti-PD-1 ligand 1/2 (PDL1/2) blockade in CD8+ T cells isolated from young (< 35 years) and old (> 65 years) donors measured after 3 days by [3H]thymidine incorporation. Graphs showing proliferation of CD8+ T cells following a 3-day incubation with 10 μg/ml anti-PDL1/2 antibody block measured by [3H]thymidine incorporation, in young (b) and old (c) donors. Data showing the effect of 10 μg/ml anti-PDL1/2 antibody block on proliferative capacity in CD8+ CD28/CD27-defined T-cell subsets in young (d) and old (e) donors. Proliferation index was calculated by determining the ratio between cells proliferating in the presence of 0·5 μg/ml anti-CD3 stimulation versus 10 μg/ml anti-IgG2b isotype control or anti-PDL1/2 block. Horizontal lines depict mean values. (f) Immunoblots of cyclin D1,2,3 and β actin for CD8+ CD28/CD27 subsets, from young individuals, following a 3-day incubation with 10 μg/ml anti-PDL1/2 antibody block. Together with densitometric analysis showing the ratio between cyclin D1,2,3/β actin in CD8+ CD28+ CD27+ T cells and CD8+ CD28− CD27− T cells following anti-PDL1/2 blockade. Graphs show the mean ± SE for three donors and P-values were calculated using the Student's t-test.
We then investigated the change in expression of cyclin D1,2,3 by Western blot analysis after activation of the CD8+ T cells in the presence of anti-PDL1/2 blockade (Fig. 3f). After activation, cyclin D1,2,3 was significantly increased in the highly differentiated CD28+ CD27+ subset (Fig. 3f, P < 0·05), confirming the change in proliferative responses as determined by [3H]thymidine incorporation after blocking PD-1 signalling (Fig. 2).
It is evident from both the unfractionated CD8+ T cells and the CD28/CD27 subsets isolated from old individuals that the T cells are hypoproliferative compared with cells taken from younger donors (Fig. 3a and data not shown). Furthermore, the enhanced proliferation upon anti-PDL1/2 blockade was of a much less than that observed in the young (Fig. 3b,c). This suggests that other age-related defects contribute towards the characteristic dysfunctional proliferative responses of highly differentiated CD8+ T cells in old individuals.
It has been demonstrated that the combined blockade of PDL1 with other co-inhibitory receptors such as TIM-3,26,27 CTLA-439 or LAG-323,40 was more effective than targeting either pathway alone in restoring T-cell responses. We therefore investigated whether we could enhance the proliferative response of human CD8+ T cells further by blocking PD-1 together with other inhibitory receptors. Expression of both CTLA-4 (Fig. 4a) and KLRG117 was increased on CD8+ T cells during ageing. We showed previously that blocking KLRG1 could enhance proliferative activity of CD8+ T cells and also induce Akt(ser473) phosphorylation.17 However, when we blocked PD-1, KLRG1 and CTLA-4 simultaneously we observed no additive enhancement in proliferative ability of the CD8+ T cells (Fig. 4b). We also found that, like the blockade of PD-1 and KLRG1 signalling, blocking CTLA-4 signalling also enhanced Akt(ser473) phosphorylation in CD8+ CD28− CD27− T cells following activation (Fig. 4c,d) but again, no additive effect was found when all three receptors were blocked simultaneously. This suggests that PD-1, KLRG1 and CTLA-4 may act on the same pathway to inhibit the proliferative response of human CD8+ T cells.
Figure 4.
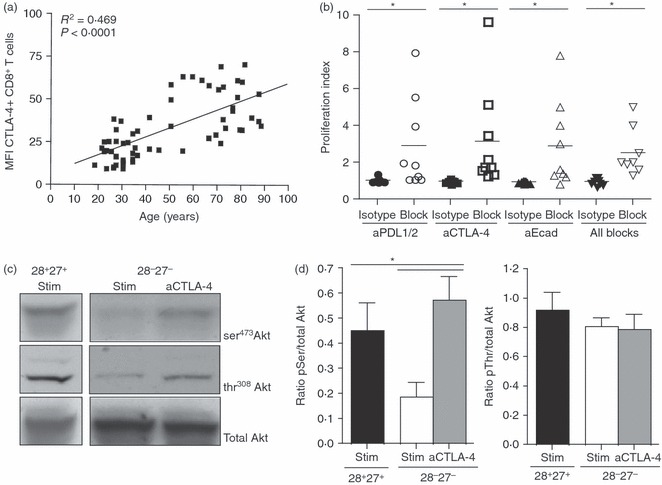
Combined use of inhibitory receptor antibody block causes no additive enhancement in proliferative ability on CD8+ T cells. (a) Graph showing the correlation between cytotoxic T-lymphocyte antigen 4 (CTLA-4) expression on CD8+ T cells and age. (b) Data showing the effect of 10 μg/ml anti-programmed death 1 ligand 1/2 (PDL1/2), anti-CTLA-4, anti-E-cadherin or all three antibody blocks on proliferative capacity in CD8+ T-cell subsets in young donors. Proliferation index was calculated by determining the ratio between cells proliferating in the presence of 0·5 μg/ml anti-CD3 stimulation versus 10 μg/ml anti-IgG2b isotype control or anti-inhibitory receptor block. Horizontal lines depict mean values. (c) Immunoblots of pSer473, pThr308 and total Akt for CD8+ CD28/CD27 subsets, from young donors, following a 3-day incubation with 10 μg/ml anti-CTLA-4 antibody block. (d). Densitometric analysis showing the ratio between pSer Akt/total Akt and pThr Akt/total Akt in CD8+ CD28+ CD27+ T cells and CD8+ CD28− CD27− T cells following anti-CTLA-4 blockade. Graphs show the mean ± SE for three donors and all P-values were calculated using one-way paired Student's t-test.
PD-1 blockade does not enhance telomerase activity
We previously showed telomerase activity to be defective in CD8+ CD28− CD27− T cells9 compared with their early (P < 0·005) and intermediate (P < 0·05) differentiated counterparts (Fig. 5). However, contrary to a previous report41, we find no enhancement of telomerase activity following anti-PDL1/2 blockade.
Figure 5.
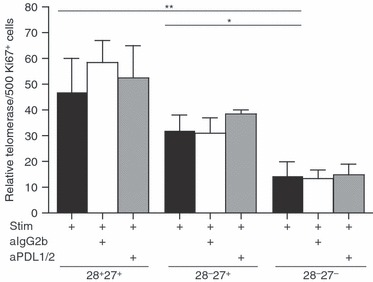
No increase in telomerase expression following programmed death 1 (PD-1) blockade. Graph showing telomerase activity after 3 days following 10 μg/ml anti-PD-1 ligand 1/2 (PDL1/2) antibody blockade in CD8+ CD28/27-defined T-cell subsets from young donors. Telomerase activity was determined using the TeloTAGGG telomerase ELISA kit from Roche. Graph shows the mean ± SE for three donors and P-values were calculated using the Student's t-test.
Discussion
We show here that the proliferative defect in highly differentiated human CD8+ CD28− CD27− T cells can be restored through the interruption of the PD-1 signalling pathway in both young and old individuals. Furthermore, the combined blockade of PD-1, CTLA-4 and KLRG1 did not provide additional enhancement of T-cell responses. This finding differs from reports where the combined targeting of PDL1/CTLA-4,39 PDL1/interleukin-1042 and PDL1/LAG-323 during chronic viral infections, and of PDL1/TIM-326 for the treatment of advanced melanoma have all been shown to be more effective than PDL1 blockade alone. This suggests that in healthy individuals with no ongoing illness the combined use of inhibitory receptor blockade will be unlikely to boost T-cell responses. However, it is possible that manipulation of other signalling pathways such as p38 mitogen-activated protein kinase (MAPK), which engages distinct signalling components to PD-1,43,44 may provide an additional enhancement to certain functional responses of CD8+ T cells.
We observed that the increased phosphorylation of Akt by PDL1/2 blockade caused an increase in CD8+ T-cell proliferative responses, in agreement with other findings.45,46 Moreover, we found that by combining blockade of PD-1 with CTLA-4 and KLRG1 we were unable to see any additional enhancement to the T-cell proliferative response in CD8+ T cells from either young or old donors. Both CTLA-430,47 and KLRG117,48 also inhibit the PI3K pathway but via different phosphatases to PD-1. CTLA-4 acts by recruiting SHP-2 and PP2A30 and KLRG1 mediates its effects through the recruitment of SHIP-1 and SHP-2.48 As all three inhibitory molecules mediate their inhibition via the PI3K pathway, one might not expect to see an enhancement in T-cell responses in healthy individuals, other reports showing that the combined blockade of several inhibitory receptors further boost T-cell responses may be the result of the ongoing pressure of antigenic load or tumour burden driving CD8+ T cells to exhaustion.
When we examined which of the CD28/CD27-defined CD8+ T-cell subsets responded to the anti-PDL1/2 blockade, we found that all three subsets responded in CD8+ T cells from young donors and only the intermediate and late differentiated CD8+ T cells responded from old individuals, with no significant difference observed between the proliferative responses of any of the subsets. This observation may seem at odds with our expression data, where we identified the intermediate CD8+ CD28− CD27+ T cells as expressing the highest levels of PD-1. However, differing PD-1 expression levels may engage distinct intracellular targets.49 For example, PD-1 binds the SH2 domain-containing protein tyrosine phosphatases SHP-1 and SHP-2 in naive T cells, but in exhausted cells the high levels of PD-1 expression can recruit additional signalling molecules.49 Furthermore the cytokine microenvironment can also control expression of PD-1, with the common γ-chain cytokines and interferon-α inducing PD-1, the differing response to PDL1/2 blockade in the CD28/CD27-defined subsets may reflect the different levels of cytokine receptor expression in the subsets.35 Alternatively, PDL1 may be interacting with CD80 (B7-1), as both molecules are induced on activated T cells and the interaction between PDL1/CD80 has been shown to inhibit T-cell activation.50 However, the extent to which these highly differentiated CD8+ CD28− CD27− T cells express CD80 remains to be determined.
We then investigated whether PDL1/2 blockade merely affected G0/G1 cell-cycle checkpoints and the frequency of cells entering mitosis, or whether it actively enhanced telomerase activity protecting the CD8+ T cells from senescence. We found no enhancement in telomerase activity following blockade of the PD-1 pathway, again this is contrary to published data demonstrating an increase in telomerase activity following blockade of the PDL1/2 pathway in HIV-specific CD8+ T cells following specific peptide stimulation.41 However, telomerase activity increases in proliferating cells and without standardizing the telomerase assay to 500 Ki67+ cells per sample, as we have done, there remains the possibility that their observation merely reflects the increased proliferation of these cells. Apart from Akt, other key signalling molecules such as extracellular signal-regulated kinase (ERK) and nuclear factor-κB are activated following PI3K activation.38 In one study, a small molecule inhibitor that activates the ERK/MAPK pathway enhanced proliferation, telomerase activity and replicative lifespan of T cells from HIV-infected patients.51 Furthermore, we have recently shown that the small molecule p38 MAPK inhibitor, BIRB796 increased telomerase activity in highly differentiated CD4+ CD45RA+ CD27− T cells.44 Ongoing studies in our laboratory are directed at understanding the role of p38 MAPK signalling in CD8+ T cells.
It is well recognized that older humans have decreased T-cell responses3,52,53 and it is possible that modulating the signalling through certain inhibitory receptors like PD-1 may boost these responses. We observed a significant increase in the proliferative response in CD8+ T cells isolated from old individuals after PDL1/2 blockade but this enhancement was less than in young individuals (1·5-fold to threefold increase compared with 1·5-fold to eightfold increase, respectively). This suggests that other age-related defects are contributing to the poor proliferative responses of CD8+ T cells from old donors and these remain to be clarified. The current challenge is to identify the extent to which other signalling pathways such as the MAPKs are involved in the reduction of certain functions in highly differentiated T cells and whether they can be manipulated either alone or in combination with other targets to improve immune responsiveness during ageing.
Acknowledgments
The authors would like to acknowledge the supply of PD-1 reagents from Prof. Gordon Freeman. This work was supported by the Biotechnology and Biological Sciences Research Council (A.N.A.).
Disclosures
The authors have no conflicting financial interests.
References
- 1.Akbar AN, Beverley PC, Salmon M. Will telomere erosion lead to a loss of T-cell memory? Nat Rev Immunol. 2004;4:737–43. doi: 10.1038/nri1440. [DOI] [PubMed] [Google Scholar]
- 2.Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495–9. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wick G, Jansen-Durr P, Berger P, Blasko I, Grubeck-Loebenstein B. Diseases of aging. Vaccine. 2000;18:1567–83. doi: 10.1016/s0264-410x(99)00489-2. [DOI] [PubMed] [Google Scholar]
- 4.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 5.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–9. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 6.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200:1347–58. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akbar AN, Fletcher JM. Memory T cell homeostasis and senescence during aging. Curr Opin Immunol. 2005;17:480–5. doi: 10.1016/j.coi.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Pawelec G. Immunity and ageing in man. Exp Gerontol. 2006;41:1239–42. doi: 10.1016/j.exger.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Plunkett FJ, Franzese O, Finney HM, et al. The loss of telomerase activity in highly differentiated CD8+CD28–CD27– T cells is associated with decreased Akt (Ser473) phosphorylation. J Immunol. 2007;178:7710–9. doi: 10.4049/jimmunol.178.12.7710. [DOI] [PubMed] [Google Scholar]
- 10.Larbi A, Dupuis G, Khalil A, Douziech N, Fortin C, Fulop T., Jr Differential role of lipid rafts in the functions of CD4+ and CD8+ human T lymphocytes with aging. Cell Signal. 2006;18:1017–30. doi: 10.1016/j.cellsig.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Iancu EM, Speiser DE, Rufer N. Assessing ageing of individual T lymphocytes: mission impossible? Mech Ageing Dev. 2008;129:67–78. doi: 10.1016/j.mad.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Effros RB, Boucher N, Porter V, et al. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp Gerontol. 1994;29:601–9. doi: 10.1016/0531-5565(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 13.Goronzy JJ, Lee WW, Weyand CM. Aging and T-cell diversity. Exp Gerontol. 2007;42:400–6. doi: 10.1016/j.exger.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czesnikiewicz-Guzik M, Lee WW, Cui D, et al. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–18. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005;205:147–57. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 16.Waller EC, McKinney N, Hicks R, Carmichael AJ, Sissons JG, Wills MR. Differential costimulation through CD137 (4-1BB) restores proliferation of human virus-specific “effector memory” (CD28– CD45RAHI) CD8+ T cells. Blood. 2007;110:4360–6. doi: 10.1182/blood-2007-07-104604. [DOI] [PubMed] [Google Scholar]
- 17.Henson SM, Franzese O, Macaulay R, et al. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood. 2009;113:6619–28. doi: 10.1182/blood-2009-01-199588. [DOI] [PubMed] [Google Scholar]
- 18.Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, et al. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol. 2005;175:8218–25. doi: 10.4049/jimmunol.175.12.8218. [DOI] [PubMed] [Google Scholar]
- 19.Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1) Blood. 2002;100:3698–702. doi: 10.1182/blood-2002-02-0657. [DOI] [PubMed] [Google Scholar]
- 20.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 21.Petrovas C, Casazza JP, Brenchley JM, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–92. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 23.Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufmann DE, Kavanagh DG, Pereyra F, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–54. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 25.Jones RB, Ndhlovu LC, Barbour JD, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–79. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fourcade J, Sun Z, Benallaoua M, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–86. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–94. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wherry EJ, Ha SJ, Kaech SM, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–84. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 30.Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donahue AC, Fruman DA. PI3K signaling controls cell fate at many points in B lymphocyte development and activation. Semin Cell Dev Biol. 2004;15:183–97. doi: 10.1016/j.semcdb.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 32.Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, Nayak L, Moss PA. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169:1984–92. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 33.van Baarle D, Tsegaye A, Miedema F, Akbar A. Significance of senescence for virus-specific memory T cell responses: rapid ageing during chronic stimulation of the immune system. Immunol Lett. 2005;97:19–29. doi: 10.1016/j.imlet.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 34.van Lier RA, ten Berge IJ, Gamadia LE. Human CD8+ T-cell differentiation in response to viruses. Nat Rev Immunol. 2003;3:931–9. doi: 10.1038/nri1254. [DOI] [PubMed] [Google Scholar]
- 35.Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–83. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 36.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–51. [PMC free article] [PubMed] [Google Scholar]
- 37.Jacinto E, Facchinetti V, Liu D, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–37. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 38.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamoto N, Cho H, Shaked A, et al. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–80. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lichterfeld M, Mou D, Cung TD, et al. Telomerase activity of HIV-1-specific CD8+ T cells: constitutive up-regulation in controllers and selective increase by blockade of PD ligand 1 in progressors. Blood. 2008;112:3679–87. doi: 10.1182/blood-2008-01-135442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brooks DG, Ha SJ, Elsaesser H, Sharpe AH, Freeman GJ, Oldstone MB. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc Natl Acad Sci U S A. 2008;105:20428–33. doi: 10.1073/pnas.0811139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol. 2011;11:289–95. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 44.Di Mitri D, Azevedo RI, Henson SM, et al. Reversible senescence in human CD4+ CD45RA+ CD27– memory T cells. J Immunol. 2011;187:2093–100. doi: 10.4049/jimmunol.1100978. [DOI] [PubMed] [Google Scholar]
- 45.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 46.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–45. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 47.Riley JL, June CH. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- 48.Tessmer MS, Fugere C, Stevenaert F, Naidenko OV, Chong HJ, Leclercq G, Brossay L. KLRG1 binds cadherins and preferentially associates with SHIP-1. Int Immunol. 2007;19:391–400. doi: 10.1093/intimm/dxm004. [DOI] [PubMed] [Google Scholar]
- 49.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–25. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–22. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parish ST, Wu JE, Effros RB. Modulation of T lymphocyte replicative senescence via TNF-α inhibition: role of caspase-3. J Immunol. 2009;182:4237–43. doi: 10.4049/jimmunol.0803449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayward AR, Buda K, Levin MJ. Immune response to secondary immunization with live or inactivated VZV vaccine in elderly adults. Viral Immunol. 1994;7:31–6. doi: 10.1089/vim.1994.7.31. [DOI] [PubMed] [Google Scholar]
- 53.Stepanova L, Naykhin A, Kolmskog C, Jonson G, Barantceva I, Bichurina M, Kubar O, Linde A. The humoral response to live and inactivated influenza vaccines administered alone and in combination to young adults and elderly. J Clin Virol. 2002;24:193–201. doi: 10.1016/s1386-6532(01)00246-3. [DOI] [PubMed] [Google Scholar]


