Abstract
The pattern-recognition receptor (PRR) family includes Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD) -like receptors (NLRs), RIG-I-like receptors (RLRs), C-type lectin receptors (CLRs) and the receptor for advanced glycation end products (RAGE). They recognize various microbial signatures or host-derived danger signals and trigger an immune response. Eosinophils are multifunctional leucocytes involved in the pathogenesis of several inflammatory processes, including parasitic helminth infection, allergic diseases, tissue injury and tumour immunity. Human eosinophils express several PRRs, including TLR1–5, TLR7, TLR9, NOD1, NOD2, Dectin-1 and RAGE. Receptor stimulation induces survival, oxidative burst, activation of the adhesion system and release of cytokines (interleukin-1β, interleukin-6, tumour necrosis factor-α and granulocyte–macrophage colony-stimulating factor), chemokines (interleukin-8 and growth-related oncogene-α) and cytotoxic granule proteins (eosinophil cationic protein, eosinophil-derived neurotoxin, eosinophil peroxidase and major basic protein). It is also evident that eosinophils play an immunomodulatory role by interacting with surrounding cells. The presence of a broad range of PRRs in eosinophils indicates that they are not only involved in defence against parasitic helminths, but also against bacteria, viruses and fungi. From a clinical perspective, eosinophilic PRRs seem to be involved in both allergic and malignant diseases by causing exacerbations and affecting tumour growth, respectively.
Keywords: C-type lectin receptor, nucleotide-binding oligomerization domain-like receptor, pattern-recognition receptor, receptor for advanced glycation end products, RIG-I-like receptor, Toll-like receptor
Introduction
Eosinophils are multifunctional leucocytes involved in the pathogenesis of several inflammatory processes, like allergic diseases, tissue injury and tumour immunity.1 Traditionally, their primary function has been to provide protection against parasitic helminths. However, there are few data to support this in vivo, and it is now known that their role in parasitic infections can be both protective and pathogenic.2,3 Eosinophils mature in the bone marrow, are released into the circulation where they constitute 1–3% of the circulating leucocytes, and are eventually recruited to various tissues.4 Their development is governed by interleukin-3 (IL-3), IL-5 and granulocyte–macrophage colony-stimulating factor (GM-CSF). Under homeostatic conditions, eosinophils predominantly reside in the gastrointestinal tract and traffic into sites of inflammation by the action of T helper type 2 cytokines (e.g. IL-4, IL-5 and IL-13), adhesion molecules and chemokines (e.g. eotaxin and RANTES).1,5–8 Eosinophils possess secondary granules containing four toxic basic granule proteins; eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN), eosinophil peroxidase (EPO) and major basic protein (MBP). In addition, they release reactive oxygen species, leukotrienes, prostaglandins and a range of cytokines and chemokines.7 In turn, these molecules cause epithelial damage, smooth muscle constriction, increased vascular permeability and recruitment of inflammatory cells.1,7,9
Emerging evidence shows that eosinophils are not only involved in defence against parasites, but also in the recognition and killing of bacteria, viruses and fungi. Linch et al.10 have recently reported that murine eosinophils have a role in the clearance of Pseudomonas aeruginosa. It has also been shown in vitro that human eosinophils are activated by Haemophilus influenzae,11 and are able to kill Escherichia coli and Staphylococcus aureus.12,13 Respiratory syncytial virus and pneumonia virus of mice have been demonstrated to infect human and murine eosinophils and elicit pro-inflammatory responses.14 Moreover, extracts from Alternaria alternata, an environmental airborne fungus, have been found to induce activation and degranulation of human eosinophils.15 Recently, several reports have documented the presence of Toll-like receptors (TLRs) in eosinophils,16,17 suggesting that these receptors are responsible for their antimicrobial properties. The TLRs belong to a large family of receptors, called pattern-recognition receptors (PRRs). Except for the TLRs, the PRR family also includes the nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), retinoic acid-inducible gene I (RIG-I) -like receptors (RLRs), C-type lectin receptors (CLRs) and the receptor for advanced glycation end products (RAGE).18 Common for all PRRs is that they recognize and respond to specific microbial components called pathogen-associated molecular patterns (PAMPs) or to endogenous molecules produced by injured or dying cells called danger-associated molecular patterns (DAMPs).18,19 Upon recognition of PAMPs or DAMPs, an immune reaction is elicited to protect the host from infection.
In this paper, we will describe the different PRR families and summarize what is known about human eosinophil responses to PAMPs and DAMPs. We will also discuss the role of eosinophilic PRRs in allergic airway disease and tumour growth.
Pattern-recognition receptors
In 1997, a human homologue to the Toll protein in Drosophila was identified by Medzhitov et al.,20 later designated TLR4. Since then, the TLR family has grown rapidly and led to the discovery of several novel pathogen sensors, including the NLRs, RLRs and CLRs. Briefly, by recognizing PAMPs and DAMPs, the PRRs instruct the immune system to distinguish between harmful and harmless microorganisms and subsequently signal the invasion by pathogens that can cause tissue damage.21 It should also be mentioned that probiotic bacteria, such as bifidobacteria and lactobacilli, mediate beneficial health effects partly through their ability to modulate the immune response by activating PRRs.22,23 To ensure effective detection and clearance of an infection, the PRRs are located in different cellular compartments. The TLRs are positioned on the cell surface (TLR1, -2, -4, -5, -6 and -10) where they primarily detect bacterial proteins, lipoproteins and polysaccharides, as well as in endosomes (TLR3, -7, -8 and -9) where they detect viral nucleic acids.24–26 In contrast, the NLRs and RLRs are found in the cytosol where they sense mainly bacterial peptidoglycan and viral dsRNA, respectively.27–29 The CLRs can be membrane-bound or secreted and respond mainly to fungal components.21 RAGE is another receptor included in the PRR family that deserves attention in the context of eosinophil responses to DAMPs. It is a transmembrane receptor responsible for the recognition of endogenous ligands generated upon cell death and tissue injury.30 A schematic outline of the PRR members and their ligands is presented in Fig. 1.
Figure 1.

Schematic outline of the pattern-recognition receptors (PRRs) and their ligands. The Toll-like receptors (TLRs) are located at the cell surface or in endosomes where they sense bacterial proteins and viral nucleic acids, respectively. The nucleotide-binding oligomerization domain (NOD) -like receptors (NLRs) and retinoic acid-inducible gene I (RIG-I) -like receptors (RLRs) are positioned in the cytoplasm where they detect bacterial structures, danger signals and viral RNA. The C-type lectin receptor (CLR) member Dectin-1 is a transmembrane receptor that binds yeast cell wall components, whereas receptor for advanced glycation end products (RAGE) is a transmembrane receptor responsible for the recognition of high-motility group box 1 (HMGB1). All PRRs activate downstream signalling cascades, involving nuclear factor-κB (NF-κB) and interferon (IFN) regulatory factors (IRFs) with the resultant release of pro-inflammatory cytokines and type I IFNs.
The TLR family
The human TLR family has 10 members (TLR1 to TLR10), whereas in mice there are 12 receptors.18 The TLRs are type I integral membrane glycoproteins, characterized by leucine-rich repeat motifs in the recognition domain and a Toll IL-1 receptor domain that constitutes the cytoplasmic signalling domain.26,31 TLR2 acts as a heterodimer in concert with TLR1 or TLR6, and mediates responses to lipoproteins, lipoteichoic acids, peptidoglycan and zymosan. Depending on the heterodimer, TLR2 can discriminate between diacyl (TLR1/2) and triacyl (TLR2/6) lipopeptides. There are indications that TLR10 also has the ability to form heterodimers with TLR1 and TLR2, but the specific ligands have not yet been identified.24 TLR3 is involved in the recognition of dsRNA from viruses and the synthetic dsRNA analogue poly(I:C).32 TLR4 is the main lipopolysaccharide (LPS) receptor. However, it is dependent on cooperation with LPS-binding protein, CD14 and MD-2.33 TLR5 recognizes flagellin, an essential component of the bacterial flagella.34 TLR7 and TLR8 mediate responses to viral ssRNA, and also to immunomodulatory drugs such as the imidazoquinolines imiquimod (R-837) and resiquimod (R-848).35 TLR9 responds to bacterial and viral DNA containing unmethylated CpG motifs.36 All TLRs except TLR3 signal through the myeloid differentiation factor 88 (MyD88) -dependent pathway to activate nuclear factor-κB (NF-κB), which ultimately regulates the transcription of pro-inflammatory genes. To some extent, TLR7, -8 and -9 also trigger the production of type I interferons (IFNs) through MyD88-dependent activation of IFN regulatory factors (IRFs). In contrast, the MyD88-independent pathway, selectively used by TLR3 (and to some extent TLR4), uses Toll-receptor-associated activator of interferon as an adaptor to activate IRF-3 leading to the transcription of type I IFNs.19,24,37,38
The NLR family
The NLR family consists of more than 20 cytoplasmic pathogen and danger sensors.39 Four subfamilies have been approved based on the N-terminal domain: NLR family, acidic domain containing (NLRA); NLR family, BIR domain containing (NLRB); NLR family, CARD domain containing (NLRC) and NLR family, pyrin domain containing (NLRP).40–42 The ligands for most NLRs remain unknown and their physiological functions are poorly understood. The best characterized proteins are so far the NLRC members NOD1 and NOD2, and NLRP3 (also known as NALP3). NOD1 detects bacterial cell wall peptidoglycan containing γ-d-glutamyl-meso diaminopimelic acid (iE-DAP) found primarily in Gram-negative bacteria, whereas NOD2 recognizes the muramyldipeptide (MDP) MurNAc-L-Ala-D-isoGln that is conserved in peptidoglycans of Gram-positive and Gram-negative bacteria.43–45 Both NOD1 and NOD2 activate NF-κB-dependent transcription of pro-inflammatory cytokines through the recruitment of receptor-interacting protein 2.29 In contrast, NLRP3 responds to a range of DAMPs, such as necrotic cells, uric acid, ATP, biglycan and hyaluronan and activates the caspase-1-dependent inflammasome through apoptosis-associated speck-like protein containing a CARD.46–50 The NLRP3 inflammasome mediates cell death and production of IL-1β and IL-18 through the cleavage of pro-IL-1β and pro-IL-18 into their active secreted forms.51 Additionally, recent studies demonstrate a role for the NLRP3 inflammasome in the recognition of aluminium salts (alum), a mechanism that can be of critical importance for the adjuvant effect of alum in vaccination.52,53
The RLR family
The RLR family comprises three RNA helicases; RIG-I, melanoma differentiation associated gene-5 (MDA-5) and laboratory of genetics and physiology-2 (LGP-2). The RLRs are composed of two CARDs, a central DEAD box helicase/ATPase domain and a C-terminal regulatory domain.18 Unlike TLRs that recognize viruses in the endosomal compartment (i.e. ‘outside’ the cell), the RLRs are located in the cytoplasm and mediate responses to viruses that replicate inside the cell.28,29,54 RIG-I recognizes ssRNA that has a triphosphate moiety in its 5′-terminus,55 along with short blunt dsRNA, whereas long dsRNA and long poly(I:C) are detected preferentially by MDA-5.56 No ligand is yet defined for LGP-2, but it has been described as a regulator of the RIG-I-mediated and MDA-5-mediated immune responses.19,28 All RLRs transmit their signal through a common adaptor protein, IFN promoter stimulator-1, to activate NF-κB, mitogen-activated protein kinases (MAPKs) and IRFs for induction of type I IFNs and inflammatory cytokines.18,28
The CLR family
The CLRs are transmembrane receptors characterized by the presence of a carbohydrate-binding domain. The best member is Dectin-1, but other receptors that will not be further mentioned here, such as DC-SIGN, Mincle, langerin, siglecs and the mannose receptor, are also included.19,21,57–59 Dectin-1 recognizes β-glucans, the major fungal cell wall component, and zymosan.60 In contrast to the murine Dectin-1, the human homologue termed β-glucan receptor (βGR) can be divided into two major (and several minor) isoforms –βGR-A and βGR-B.61 The CLR signalling is largely mediated by a spleen tyrosine kinase (Syk) -dependent activation of MAPKs and NF-κB with the resultant generation of pro-inflammatory cytokines.18
Receptor for advanced glycation end products
RAGE is a transmembrane receptor responsible for the recognition of advanced glycation end products, non-enzymatically glycated or oxidated proteins, lipids and nucleic acids, that are formed in the environment of oxidant stress and hyperglycaemia. One prototypic DAMP recognized by RAGE is the high mobility group box 1 (HMGB1). Upon binding, RAGE initiates cellular signals that activate NF-κB with the subsequent induction of pro-inflammatory cytokines. However, the adaptor protein transmitting the signals is unknown.30
Expression and function of PRRs in human eosinophils
The first studies on TLRs in eosinophils were published in the early 2000s, but since then, surprisingly few studies have been carried out. Overall, the expression of PRRs in eosinophils is relatively low compared with neutrophils that express high levels of most PRRs.16,17,62–65 A presentation of the PRR expression in human eosinophils is provided in Table 1.
Table 1.
Expression of pattern recognition receptors (PRRs) in human eosinophils and their activators
| PRR | Activator | Expression | References |
|---|---|---|---|
| Toll-like receptors (TLRs) | |||
| TLR1 | Triacylated lipopeptides | ++ | 16, 17 |
| TLR2 | Lipoprotein, peptidoglycan | −/+ | 16, 17, 68, 75 |
| TLR3 | Poly(I:C) | −/+ | 16, 17, 71 |
| TLR4 | Lipopolysaccharide | −/+ | 16, 17, 68, 72, 75, 78, 79 |
| TLR5 | Flagellin | ++ | 16, 17 |
| TLR6 | Diacylated lipopeptides | −/+ | 16, 17 |
| TLR7 | ssRNA | +++ | 16, 17, 70, 81 |
| TLR8 | ssRNA | − | 16, 17, 81 |
| TLR9 | CpG-DNA | ++ | 16, 17, 70, 73, 85, 88 |
| TLR10 | Unknown | + | 16 |
| Nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) | |||
| NOD1 | iE-DAP | + | 64 |
| NOD2 | MDP | ++ | 64 |
| NLRP3 | Uric acid, alum, ATP | − | 64 |
| Retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) | |||
| RIG-I | Short dsRNA, 5′-triphosphate dsRNA | ++ | 64 |
| MDA-5 | Long dsRNA | − | 64 |
| LGP-2 | Unknown | ND | |
| C-type lectin receptors (CLRs) | |||
| Dectin-1 | β-glucan, zymosan | ++ | 11, 65, 90 |
| Others | |||
| RAGE | HMGB1 | ++ | 93 |
+, ++, +++ and −indicate the intensity of the expression of each PRR; ND, not determined.
TLR responses
In an early study by Muzio et al.,66 mRNA expression of TLR1, TLR2, TLR4 and TLR5 was demonstrated in polymorphonuclear leucocytes, and this has since been confirmed by other groups. Today, mRNA or proteins for all TLRs except TLR8 have been found in eosinophils, although at varying intensities.16,17,67–75 In particular, there has been some controversy regarding the presence or absence of TLR3, TLR4 and TLR6. TLR7 is the only receptor that has been found to be highly active in all studies.
TLR2 forms heterodimers with either TLR1 or TLR6 and discriminates between different PAMPs depending on which protein it associates with. Three groups have shown that expression of TLR2 in human eosinophils is absent, and that Pam3CSK4 (ligand for TLR1/TLR2) and peptidoglycan (TLR2) are unable to induce activation.16,68,75 In contrast, Wong et al.17 have detected mRNA and proteins for TLR1, TLR2 and TLR6, and have shown that peptidoglycan can activate eosinophils by up-regulating cell surface expression of intracellular adhesion molecule 1 (ICAM-1) and CD18, and induce the release of IL-1β, IL-6, IL-8, growth-related oncogene α (GRO-α), ECP and superoxides. These effects seem to be mediated by a combined action of extracellular signal-regulated kinase (ERK), phosphoinositide 3-kinase (PI3K) and NF-κB pathways. In another comprehensive study by the same group, peptidoglycan is found to enhance eosinophil survival, induce the expression of ICAM-1 and CD18, correspondingly suppress the expression of ICAM-3 and l-selectin, and enhance the chemokine ligand 5 (CCL5)-induced migration. It is also demonstrated that the signals transmitted through ERK are mediated by the focal adhesion kinase.76 Moreover, Driss et al.77 have reported that Mycobacterium bovis bacillus Calmette–Guérin (BCG) activates human eosinophils and that TLR2 plays a major role in the activation process. They show that both live BCG and lipomannan purified from M. bovis BCG attract eosinophils and promote synthesis of reactive oxygen species, EPO, ECP, tumour necrosis factor-α (TNF-α) and α-defensin by activating MyD88-, p38 MAPK- and NF-κB-dependent pathways. In addition, they have observed a heterogeneous donor-dependent and inducible expression of TLR2 and TLR4.
In contrast to the majority of studies showing no or low levels of TLR3,16,17,76 our group has demonstrated the presence of TLR3 mRNA and protein in eosinophils from bone marrow and peripheral blood. In this study, the TLR3 expression is found to be higher in bone marrow-derived cells than in circulating cells and down-regulated in both compartments during symptomatic allergic rhinitis and in the presence of IL-4 and IL-5. It also shows that poly(I:C) has the ability to induce an increase in the expression of CD11b and the release of IL-8, effects found to be mediated via the p38 MAPK and NF-κB signalling pathways. Further, IL-5 is proven to augment the poly(I:C)-induced response.71 In support of this finding, TLR3 mRNA has been detected in murine splenic eosinophils.69 However, it should be noted that the responses to poly(I:C) are relatively weak compared with the effects seen by other TLRs that are functionally active in eosinophils.
The immunostimulatory properties of LPS were known long before the discovery of TLR4. In an early study, an activation of human eosinophils in response to LPS was demonstrated, manifested by an induction in survival and secretion of GM-CSF, TNF-α and IL-8.78 A more recent study reveals expression of TLR4 mRNA and that the LPS-induced eosinophil activation in terms of TNF-α and ECP release is dependent on CD14.72 Presence of TLR4 mRNA has also been detected in eosinophils isolated from the spleens of naive hypereosinophilic mice.69 In contrast, another group has demonstrated that eosinophils are unresponsive to LPS and do not express TLR4 or CD14 proteins.68 Meerschaert et al.79 have shown that the LPS-induced activation is monocyte-dependent by comparing CD14-depleted and non-CD14-depleted eosinophil preparations, suggesting an indirect effect of LPS on eosinophil functions. In line with this, most recent data on the topic show no eosinophil-activating capability of LPS.16,17,76 Moreover, recent observations from our laboratory show that TLR4 in eosinophils is up-regulated in patients with allergic rhinitis during the pollen season.80
While one study found no evidence for TLR5 mRNA in human eosinophils,16 another has detected the TLR5 protein using flow cytometry and Western blot.17 The latter also demonstrates that the TLR5 ligand flagellin is capable of inducing eosinophil activation in terms of chemokine and cytokine secretion (IL-1β, IL-6, IL-8, GRO-α), superoxide generation, prolongation of eosinophil survival and activation of the adhesion system (ICAM-1, CD18, ICAM-3, l-selectin) with a subsequent increase in CCL5-induced migration. These effects are found to be mediated by NF-κB and a focal adhesion kinase-dependent activation of ERK signalling pathways.17,76
TLR7 is the most prominent TLR in eosinophils, highly expressed at both mRNA and protein levels, whereas expression of TLR8 is lacking.16,17,69,70 This is in contrast to neutrophils, which selectively express TLR8 but not TLR7.16,81 The first study to investigate TLRs in eosinophils demonstrated that R-848 is the main PAMP to activate human eosinophils via the TLR system. This report is based on the ability of R-848 to regulate the expression of CD11b and l-selectin, enhance the viability, increase the generation of superoxide and activate the p38 MAPK pathway. They further show that IFN-γ up-regulates the mRNA levels of several TLRs and synergistically affects the CD11b and l-selectin expression.16 Following this study, several investigators have confirmed and complemented these data. One group has shown that R-837 regulates the expression of ICAM-1, CD18, ICAM-3 and l-selectin, and subsequently enhances the CCL5-induced migration, induces the release of IL-1β, IL-6, IL-8, GRO-α and superoxides and prolongs survival by activating the NF-κB, PI3K, p38 MAPK and ERK signalling pathways.17,76 In support of this, our group has verified the R-837-induced IL-8 secretion, prolongation in survival and activation of the adhesion system and subsequent increase in chemotactic migration. We also showed that priming with IL-5, and to a lesser extent IL-4, sensitizes eosinophils for TLR7 activation and that the responses of eosinophils to R-837 are higher in atopic patients with seasonal allergic rhinitis than in healthy non-allergic controls.70 Moreover, Hiraguchi et al.82 have investigated the ability of Procaterol (a short-acting β2-agonist) and Budesonide (a corticosteroid), which are commonly used for virus-induced asthma, on the TLR7-induced eosinophil activation. They report that a combination of the two drugs inhibits eosinophil effector functions by decreasing CD11b expression, superoxide generation and IL-8 release. It should also be mentioned that even though R-837 affects several eosinophil functions, two independent studies have failed to detect release of the granule proteins ECP and EDN in response to stimulation.17,70 Moreover, Phipps et al.69 demonstrate that murine eosinophils express TLR7 that can be activated by ssRNA stimulation. By infecting the airways of hypereosinophilic (IL-5 transgenic) mice with respiratory syncytial virus or by the adoptive transfer of MyD88-sufficient, but not MyD88-deficient, eosinophils to wild-type mice, they show that eosinophils mediate accelerated respiratory syncytial virus clearance via MyD88-dependent pathways.
TLR9 recognizes bacterial and viral, but not vertebrate, DNA that is characterized by unmethylated CpG sequences.83,84 Even though eosinophil expression of TLR9 has been demonstrated by several investigators,16,17,70,73,85 there has been some controversy as to the ability of CpG to activate human eosinophils. This might be explained by the great variation in the structure of CpG, with three different classes (types A, B and C, based on the backbone composition), each characterized by numerous sequence variations.86,87 Although three studies are unable to see any effect of CpG,16,17,76 our group has demonstrated that CpG prolongs survival, regulates the expression of CD11b, l-selectin and CD69 leading to facilitated IL-8-induced migration, and enhances the release of IL-8 and EDN. Also, conditioned media from CpG-treated eosinophils is shown to promote airway epithelial damage by inducing cell death and cytokine release. It is also demonstrated that priming with IL-5 and histamine increases the responsiveness to CpG stimulation and that the TLR9-induced responses are higher in patients with allergic rhinitis compared with healthy non-allergic controls in terms of IL-8 and EDN release. However, the heightened responsiveness in allergic patients was not a result of a higher TLR9 expression.70 In another study, we found a higher expression of TLR9 in eosinophils derived from bone marrow than from peripheral blood.85 In support of this, Ilmarinen et al.73 have found that bacterial DNA delays eosinophil apoptosis in a TLR9-dependent fashion by activating PI3K and NF-κB signalling cascades. Moreover, Lotfi et al.88 show that CpG promotes eosinophil-induced dendritic cell maturation (up-regulation of CD80, CD83, CD86 and HLA-DR) by releasing MBP that is taken up and internalized by the dendritic cells. Direct cell–cell interaction appears to enhance this maturation process, but is not obligatory.
Responses induced by cytosolic PRRs: NLRs and RLRs
In contrast to the TLR system, limited information is available regarding eosinophil expression of NLRs and RLRs, except that murine eosinophils lack NLRP3.89 We have recently demonstrated that human eosinophils express mRNA and protein for NOD1, NOD2 and RIG-I, but not NLRP3 or MDA-5. Stimulation with iE-DAP and MDP, ligands for NOD1 and NOD2, respectively, induces secretion of IL-8, regulates the expression of CD11b, l-selectin and CD69 and facilitates IL-8-induced and eotaxin-induced migration. MDP also promotes the release of EDN. No effects are seen upon stimulation with alum or poly(I:C)/LyoVec, agonists for NLRP3 and RIG-I/MDA-5, respectively, and no differences in the expression or activation profile upon NOD1 or NOD2 engagement have been observed in patients with allergic rhinitis and non-allergic controls. We also show that the NOD1-induced and NOD2-induced activation is dependent on the NF-κB pathway. Notably, the responses to iE-DAP and MDP are modest compared with the TLR-induced activation, although they can be enhanced in the presence of IL-5 and GM-CSF, but not IFN-γ.64
CLR responses
While Yoon et al.90 have found no signs of Dectin-1 in human eosinophils, either on the cell surface or in the intracellular compartment, two groups have detected several Dectin-1 isoforms, including βGR-A and βGR-B on human eosinophils.11,65 The former group also demonstrates that non-typeable H. influenzae activates eosinophils to produce IL-8, promote oxidative burst and induce up-regulation of several genes regulating, for example, signal transduction, cell division and cytokine/chemokine release. Blocking experiments with the soluble glucan derivates laminarin and scleroglucan show this activation to be dependent on β-glucan receptors.11 Early studies conducted before the identification of Dectin-1 show that zymosan induces superoxide anion generation,91 and that unopsonized zymosan and glucan particles activate eosinophils to produce leukotriene C4 through a glucan recognition mechanism.92 Moreover, Inoue et al.15 have cultured eosinophils with intact fungi. They demonstrate that cells obtained from patients with clinical allergy or asthma release ∼ 70% more EDN compared with cells from normal individuals upon stimulation with culture extracts from the environmental fungus A. alternata.
RAGE mediates responses to DAMPs
RAGE is one of the most prominent DAMP sensors by responding to a range of signals derived from dying or damaged cells. Lotfi et al.93 have demonstrated that human eosinophils express RAGE and that necrotic material from tumour cell lysates induces oxidative burst and release of MBP and EPO. In addition, they show that HMGB1 induces degranulation and oxidative burst and serves as a chemoattractant and an eosinophil survival factor. Blocking experiments reveal that the HMGB1-induced activation is RAGE-dependent.
Concluding remarks
Eosinophils express a range of PRRs that upon recognition of pathogens or host-derived danger signals trigger an immune reaction by inducing release of pro-inflammatory cytokines, chemokines, cytotoxic granule proteins, leukotrienes and reactive oxygen species, by delaying apoptosis and by activating the adhesion system and cellular trafficking. In turn, these mediators contribute to airway inflammation and hyper-reactivity. Also, eosinophils have an immunomodulatory role by interacting with surrounding cells. An overview of the eosinophil responses to various PRR activators is given in Fig. 2.
Figure 2.
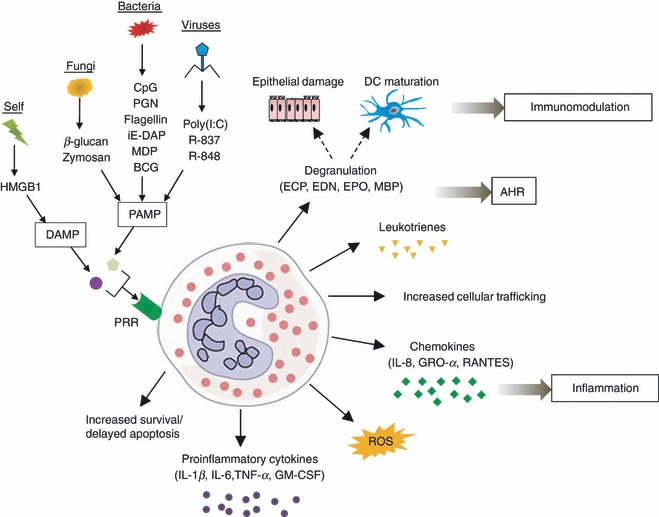
Eosinophil responses to pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs). Eosinophils are activated by various stimuli, leading to increased survival/delayed apoptosis, increased cellular trafficking and production of reactive oxygen species (ROS), pro-inflammatory cytokines, chemokines, granule proteins and leukotrienes, which in turn induce inflammation, airway hyper-reactivity (AHR) and immunomodulation. IL-1β, interleukin-1β; TNF-α, tumour necrosis factor-α; GM-CSF, granulocyte–macrophage colony-stimulating factor; GRO-α, growth-related oncogene-α; ECP, eosinophil cationic protein; EDN, eosinophil-derived neurotoxin; EPO, eosinophil peroxidise; and MBP, major basic protein.
The function of each PRR in eosinophils has been studied mainly in isolated single cell systems that do not mirror the natural biological environment in which different cell types, receptors and mediators interact. The PRR expression can be regulated at sites of infections either directly by microbial components or indirectly by cytokines. IFN-γ, IL-4, IL-5 and GM-CSF have, for example, been shown to alter the PRR expression in eosinophils and the responsiveness to ligand stimulation.16,64,70,71 Moreover, it is being increasingly acknowledged that efficient detection and clearance of microbes involve a cross-talk between multiple pathogen sensors. This is enabled by the overlapping detection capacities of TLRs, NLRs, RLRs and CLRs combined with their diverse compartmental distribution.
Traditionally, eosinophils are regarded as a key player in the immune response against parasitic helminths by the release of cytotoxic granule proteins, cytokines and lipid mediators. However, the recent discovery of a broad set of functional PRRs in eosinophils suggests that the innate immunity exerted by eosinophils reaches well beyond parasites to include bacteria, viruses and filamentous fungi. It is also clear that eosinophils not only have a direct effector function in our microbial defence, but also an instrumental role in modulating immune responses by interacting with other cells, such as inducing epithelial damage and dendritic cell maturation.
Strong evidence points to a role of the eosinophilic PRR system in allergic airway disease. A differential expression of TLR3 and TLR4 has been found in various allergic settings. Also, the responses induced by TLR7 and TLR9 are higher in eosinophils from patients with allergic rhinitis compared with healthy controls, and the activation via TLR3, TLR7, TLR9, NOD1 and NOD2 is augmented in the presence of a T helper type 2 cytokine milieu. Elevated levels of serum IL-4 and IL-5 have been reported in patients with allergic rhinitis, bronchial asthma and atopic dermatitis,70,94,95 suggesting that eosinophils from such patients may be more responsive to PAMPs or may have a lower threshold for activation. Likewise, from a clinical perspective it is well-known that the increased responsiveness of the airways seen during periods of symptomatic rhinitis or asthma is further enhanced if the patient simultaneously suffers from a respiratory tract infection. It might be that eosinophils, accumulated in the airways during such periods, are activated by bacterial, viral or fungal components to release mediators that in turn aggravate the allergic inflammation and cause tissue damage. However, the picture is not clear as several TLR7 and TLR9 agonists have shown promising results in animal models of allergic asthma in terms of preventing airway eosinophilia, release of T helper type 2 cytokines and airway reactivity.96–102
Peripheral blood eosinophilia and tumour-associated tissue eosinophilia are linked to a good prognosis for some types of cancer (e.g. gastrointestinal and head and neck cancer), whereas the opposite is true for other tumour types (e.g. oral squamous cell carcinoma).103,104 Within the tumour microenvironment, the ongoing tissue damage and cell death generate DAMPs, such as HMGB1, S100, hyaluronan, heat-shock proteins, heparan and syndecan, many of which bind to PRRs. These can both serve as eosinophil chemoattractants and survival factors and elicit eosinophil effector functions.93,104 Although it is clear that eosinophils are a common tumour infiltrate, it is still not known whether they limit tumour growth through their destructive effector functions or whether they promote tumour growth and metastasis through their immunoregulatory and remodelling activities.
Taken together, the effector functions exerted by eosinophils upon recognition of PAMPs and DAMPs affect the clinical presentation of both allergic airway disease and cancer. Careful manipulation of the eosinophilic PRR system might therefore be of therapeutic use in future management of both atopy-related and cancer-related diseases.
References
- 1.Hogan SP, Rosenberg HF, Moqbel R, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–50. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 2.Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. 2004;113:30–7. doi: 10.1016/j.jaci.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 3.Fabre V, Beiting DP, Bliss SK, et al. Eosinophil deficiency compromises parasite survival in chronic nematode infection. J Immunol. 2009;182:1577–83. doi: 10.4049/jimmunol.182.3.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broide D, Sriramarao P. Eosinophil trafficking to sites of allergic inflammation. Immunol Rev. 2001;179:163–72. doi: 10.1034/j.1600-065x.2001.790116.x. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard C, Rothenberg ME. Biology of the eosinophil. Adv Immunol. 2009;101:81–121. doi: 10.1016/S0065-2776(08)01003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303–10. doi: 10.1016/j.jaci.2007.03.048. quiz 11-2. [DOI] [PubMed] [Google Scholar]
- 7.Trivedi SG, Lloyd CM. Eosinophils in the pathogenesis of allergic airways disease. Cell Mol Life Sci. 2007;64:1269–89. doi: 10.1007/s00018-007-6527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wardlaw AJ, Brightling C, Green R, Woltmann G, Pavord I. Eosinophils in asthma and other allergic diseases. Br Med Bull. 2000;56:985–1003. doi: 10.1258/0007142001903490. [DOI] [PubMed] [Google Scholar]
- 9.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 10.Linch SN, Kelly AM, Danielson ET, Pero R, Lee JJ, Gold JA. Mouse eosinophils possess potent antibacterial properties in vivo. Infect Immun. 2009;77:4976–82. doi: 10.1128/IAI.00306-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahren IL, Eriksson E, Egesten A, Riesbeck K. Nontypeable Haemophilus influenzae activates human eosinophils through β-glucan receptors. Am J Respir Cell Mol Biol. 2003;29:598–605. doi: 10.1165/rcmb.2002-0138OC. [DOI] [PubMed] [Google Scholar]
- 12.Persson T, Andersson P, Bodelsson M, Laurell M, Malm J, Egesten A. Bactericidal activity of human eosinophilic granulocytes against Escherichia coli. Infect Immun. 2001;69:3591–6. doi: 10.1128/IAI.69.6.3591-3596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svensson L, Wenneras C. Human eosinophils selectively recognize and become activated by bacteria belonging to different taxonomic groups. Microbes Infect. 2005;7:720–8. doi: 10.1016/j.micinf.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Dyer KD, Percopo CM, Fischer ER, Gabryszewski SJ, Rosenberg HF. Pneumoviruses infect eosinophils and elicit MyD88-dependent release of chemoattractant cytokines and interleukin-6. Blood. 2009;114:2649–56. doi: 10.1182/blood-2009-01-199497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue Y, Matsuwaki Y, Shin SH, Ponikau JU, Kita H. Nonpathogenic, environmental fungi induce activation and degranulation of human eosinophils. J Immunol. 2005;175:5439–47. doi: 10.4049/jimmunol.175.8.5439. [DOI] [PubMed] [Google Scholar]
- 16.Nagase H, Okugawa S, Ota Y, et al. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J Immunol. 2003;171:3977–82. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- 17.Wong CK, Cheung PF, Ip WK, Lam CW. Intracellular signaling mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am J Respir Cell Mol Biol. 2007;37:85–96. doi: 10.1165/rcmb.2006-0457OC. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Kumagai Y, Akira S. Identification and functions of pattern-recognition receptors. J Allergy Clin Immunol. 2010;125:985–92. doi: 10.1016/j.jaci.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 20.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 21.Sato S, St-Pierre C, Bhaumik P, Nieminen J. Galectins in innate immunity: dual functions of host soluble β-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs) Immunol Rev. 2009;230:172–87. doi: 10.1111/j.1600-065X.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- 22.Plantinga TS, van Maren WW, van Bergenhenegouwen J, et al. Differential Toll-like receptor recognition and induction of cytokine profile by Bifidobacterium breve and Lactobacillus strains of probiotics. Clin Vaccine Immunol. 2011;18:621–8. doi: 10.1128/CVI.00498-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss G, Rasmussen S, Zeuthen LH, Nielsen BN, Jarmer H, Jespersen L, Frokiaer H. Lactobacillus acidophilus induces virus immune defence genes in murine dendritic cells by a Toll-like receptor-2-dependent mechanism. Immunology. 2010;131:268–81. doi: 10.1111/j.1365-2567.2010.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker LC, Prince LR, Sabroe I. Translational mini-review series on Toll-like receptors: networks regulated by Toll-like receptors mediate innate and adaptive immunity. Clin Exp Immunol. 2007;147:199–207. doi: 10.1111/j.1365-2249.2006.03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 26.Pandey S, Agrawal DK. Immunobiology of Toll-like receptors: emerging trends. Immunol Cell Biol. 2006;84:333–41. doi: 10.1111/j.1440-1711.2006.01444.x. [DOI] [PubMed] [Google Scholar]
- 27.Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–59. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–37. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Creagh EM, O'Neill LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006;27:352–7. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Lin L. RAGE on the Toll Road? Cell Mol Immunol. 2006;3:351–8. [PubMed] [Google Scholar]
- 31.Takeda K, Akira S. Toll receptors and pathogen resistance. Cell Microbiol. 2003;5:143–53. doi: 10.1046/j.1462-5822.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 32.Bowie AG. Translational mini-review series on Toll-like receptors: recent advances in understanding the role of Toll-like receptors in anti-viral immunity. Clin Exp Immunol. 2007;147:217–26. doi: 10.1111/j.1365-2249.2006.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoshino K, Takeuchi O, Kawai T, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 34.Hayashi F, Smith KD, Ozinsky A, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 35.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 36.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 37.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 38.Muzio M, Polentarutti N, Bosisio D, Prahladan MK, Mantovani A. Toll-like receptors: a growing family of immune receptors that are differentially expressed and regulated by different leukocytes. J Leukoc Biol. 2000;67:450–6. doi: 10.1002/jlb.67.4.450. [DOI] [PubMed] [Google Scholar]
- 39.Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. NOD-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250–7. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 40.Ting JP, Lovering RC, Alnemri ES, et al. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–7. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carneiro LA, Magalhaes JG, Tattoli I, Philpott DJ, Travassos LH. NOD-like proteins in inflammation and disease. J Pathol. 2008;214:136–48. doi: 10.1002/path.2271. [DOI] [PubMed] [Google Scholar]
- 42.Kaparakis M, Philpott DJ, Ferrero RL. Mammalian NLR proteins; discriminating foe from friend. Immunol Cell Biol. 2007;85:495–502. doi: 10.1038/sj.icb.7100105. [DOI] [PubMed] [Google Scholar]
- 43.Chamaillard M, Hashimoto M, Horie Y, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–7. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 44.Girardin SE, Boneca IG, Carneiro LA, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300:1584–7. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 45.Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–72. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 46.Mariathasan S, Weiss DS, Newton K, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–32. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 47.Iyer SS, Pulskens WP, Sadler JJ, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:20388–93. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 49.Yamasaki K, Muto J, Taylor KR, et al. NLRP3/cryopyrin is necessary for interleukin-1β (IL-1β) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J Biol Chem. 2009;284:12762–71. doi: 10.1074/jbc.M806084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Babelova A, Moreth K, Tsalastra-Greul W, et al. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem. 2009;284:24035–48. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muruve DA, Petrilli V, Zaiss AK, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–7. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 52.Kool M, Petrilli V, De Smedt T, et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–9. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 53.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–6. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 55.Hornung V, Ellegast J, Kim S, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–7. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 56.Kato H, Takeuchi O, Mikamo-Satoh E, et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–10. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeuchi O, Akira S. Toll-like receptors; their physiological role and signal transduction system. Int Immunopharmacol. 2001;1:625–35. doi: 10.1016/s1567-5769(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 58.Willment JA, Brown GD. C-type lectin receptors in antifungal immunity. Trends Microbiol. 2008;16:27–32. doi: 10.1016/j.tim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Lee MS, Kim YJ. Pattern-recognition receptor signaling initiated from extracellular, membrane, and cytoplasmic space. Mol Cells. 2007;23:1–10. [PubMed] [Google Scholar]
- 60.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 61.Willment JA, Gordon S, Brown GD. Characterization of the human β-glucan receptor and its alternatively spliced isoforms. J Biol Chem. 2001;276:43818–23. doi: 10.1074/jbc.M107715200. [DOI] [PubMed] [Google Scholar]
- 62.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–9. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 63.Ekman AK, Cardell LO. The expression and function of Nod-like receptors in neutrophils. Immunology. 2009;130:55–63. doi: 10.1111/j.1365-2567.2009.03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mansson Kvarnhammar A, Petterson T, Cardell L. NOD-like receptors and RIG-I-like receptors in human eosinophils: activation by NOD1 and NOD2 agonists. Immunology. 2011;134:314–5. doi: 10.1111/j.1365-2567.2011.03492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willment JA, Marshall AS, Reid DM, Williams DL, Wong SY, Gordon S, Brown GD. The human β-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur J Immunol. 2005;35:1539–47. doi: 10.1002/eji.200425725. [DOI] [PubMed] [Google Scholar]
- 66.Muzio M, Bosisio D, Polentarutti N, et al. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 67.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–61. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 68.Sabroe I, Jones EC, Usher LR, Whyte MK, Dower SK. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol. 2002;168:4701–10. doi: 10.4049/jimmunol.168.9.4701. [DOI] [PubMed] [Google Scholar]
- 69.Phipps S, Lam CE, Mahalingam S, et al. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110:1578–86. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 70.Mansson A, Cardell LO. Role of atopic status in Toll-like receptor (TLR)7- and TLR9-mediated activation of human eosinophils. J Leukoc Biol. 2009;85:719–27. doi: 10.1189/jlb.0808494. [DOI] [PubMed] [Google Scholar]
- 71.Mansson A, Fransson M, Adner M, Benson M, Uddman R, Bjornsson S, Cardell LO. TLR3 in human eosinophils: functional effects and decreased expression during allergic rhinitis. Int Arch Allergy Immunol. 2010;151:118–28. doi: 10.1159/000236001. [DOI] [PubMed] [Google Scholar]
- 72.Plotz SG, Lentschat A, Behrendt H, et al. The interaction of human peripheral blood eosinophils with bacterial lipopolysaccharide is CD14 dependent. Blood. 2001;97:235–41. doi: 10.1182/blood.v97.1.235. [DOI] [PubMed] [Google Scholar]
- 73.Ilmarinen P, Hasala H, Sareila O, Moilanen E, Kankaanranta H. Bacterial DNA delays human eosinophil apoptosis. Pulm Pharmacol Ther. 2009;22:167–76. doi: 10.1016/j.pupt.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 74.Komiya A, Nagase H, Okugawa S, et al. Expression and function of toll-like receptors in human basophils. Int Arch Allergy Immunol. 2006;140(Suppl 1):23–7. doi: 10.1159/000092707. [DOI] [PubMed] [Google Scholar]
- 75.Mattsson E, Persson T, Andersson P, Rollof J, Egesten A. Peptidoglycan induces mobilization of the surface marker for activation marker CD66b in human neutrophils but not in eosinophils. Clin Diagn Lab Immunol. 2003;10:485–8. doi: 10.1128/CDLI.10.3.485-488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheung PF, Wong CK, Ip WK, Lam CW. FAK-mediated activation of ERK for eosinophil migration: a novel mechanism for infection-induced allergic inflammation. Int Immunol. 2008;20:353–63. doi: 10.1093/intimm/dxm146. [DOI] [PubMed] [Google Scholar]
- 77.Driss V, Legrand F, Hermann E, et al. TLR2-dependent eosinophil interactions with mycobacteria: role of α-defensins. Blood. 2009;113:3235–44. doi: 10.1182/blood-2008-07-166595. [DOI] [PubMed] [Google Scholar]
- 78.Takanaski S, Nonaka R, Xing Z, O'Byrne P, Dolovich J, Jordana M. Interleukin 10 inhibits lipopolysaccharide-induced survival and cytokine production by human peripheral blood eosinophils. J Exp Med. 1994;180:711–5. doi: 10.1084/jem.180.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meerschaert J, Busse WW, Bertics PJ, Mosher DF. CD14+ cells are necessary for increased survival of eosinophils in response to lipopolysaccharide. Am J Respir Cell Mol Biol. 2000;23:780–7. doi: 10.1165/ajrcmb.23.6.4171. [DOI] [PubMed] [Google Scholar]
- 80.Ekman AK, Virtala R, Fransson M, Adner M, Benson M, Jansson L, Cardell L. A systemic up-regulation of TLR4 causes LPS augmentation of nasal cytokine release in allergic rhinitis. Int Arch Allergy Immunol. 2012 doi: 10.1159/000335196. (In press) [DOI] [PubMed] [Google Scholar]
- 81.Janke M, Poth J, Wimmenauer V, et al. Selective and direct activation of human neutrophils but not eosinophils by Toll-like receptor 8. J Allergy Clin Immunol. 2009;123:1026–33. doi: 10.1016/j.jaci.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 82.Hiraguchi Y, Tanida H, Hosoki K, Nagao M, Tokuda R, Fujisawa T. Inhibition of eosinophil activation mediated by a Toll-like receptor 7 ligand with a combination of procaterol and budesonide. Int Arch Allergy Immunol. 2011;155(Suppl 1):85–9. doi: 10.1159/000327438. [DOI] [PubMed] [Google Scholar]
- 83.Wagner H. Interactions between bacterial CpG-DNA and TLR9 bridge innate and adaptive immunity. Curr Opin Microbiol. 2002;5:62–9. doi: 10.1016/s1369-5274(02)00287-4. [DOI] [PubMed] [Google Scholar]
- 84.Krug A, Rothenfusser S, Hornung V, et al. Identification of CpG oligonucleotide sequences with high induction of IFN-α/β in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–63. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 85.Fransson M, Benson M, Erjefalt JS, et al. Expression of Toll-like receptor 9 in nose, peripheral blood and bone marrow during symptomatic allergic rhinitis. Respir Res. 2007;8:17. doi: 10.1186/1465-9921-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hartmann G, Battiany J, Poeck H, et al. Rational design of new CpG oligonucleotides that combine B cell activation with high IFN-α induction in plasmacytoid dendritic cells. Eur J Immunol. 2003;33:1633–41. doi: 10.1002/eji.200323813. [DOI] [PubMed] [Google Scholar]
- 87.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–84. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 88.Lotfi R, Lotze MT. Eosinophils induce DC maturation, regulating immunity. J Leukoc Biol. 2008;83:456–60. doi: 10.1189/jlb.0607366. [DOI] [PubMed] [Google Scholar]
- 89.Guarda G, Zenger M, Yazdi AS, et al. Differential expression of NLRP3 among hematopoietic cells. J Immunol. 2011;186:2529–34. doi: 10.4049/jimmunol.1002720. [DOI] [PubMed] [Google Scholar]
- 90.Yoon J, Ponikau JU, Lawrence CB, Kita H. Innate antifungal immunity of human eosinophils mediated by a β2 integrin, CD11b. J Immunol. 2008;181:2907–15. doi: 10.4049/jimmunol.181.4.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sedgwick JB, Vrtis RF, Gourley MF, Busse WW. Stimulus-dependent differences in superoxide anion generation by normal human eosinophils and neutrophils. J Allergy Clin Immunol. 1988;81:876–83. doi: 10.1016/0091-6749(88)90945-1. [DOI] [PubMed] [Google Scholar]
- 92.Mahauthaman R, Howell CJ, Spur BW, Youlten LJ, Clark TJ, Lessof MH, Lee TH. The generation and cellular distribution of leukotriene C4 in human eosinophils stimulated by unopsonized zymosan and glucan particles. J Allergy Clin Immunol. 1988;81:696–705. doi: 10.1016/0091-6749(88)91041-x. [DOI] [PubMed] [Google Scholar]
- 93.Lotfi R, Herzog GI, DeMarco RA, et al. Eosinophils oxidize damage-associated molecular pattern molecules derived from stressed cells. J Immunol. 2009;183:5023–31. doi: 10.4049/jimmunol.0900504. [DOI] [PubMed] [Google Scholar]
- 94.Matsumoto T, Miike T, Yamaguchi K, Murakami M, Kawabe T, Yodoi J. Serum levels of soluble IL-2 receptor, IL-4 and IgE-binding factors in childhood allergic diseases. Clin Exp Immunol. 1991;85:288–92. doi: 10.1111/j.1365-2249.1991.tb05720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ohshima Y, Katamura K, Miura M, Mikawa H, Mayumi M. Serum levels of interleukin 4 and soluble CD23 in children with allergic disorders. Eur J Pediatr. 1995;154:723–8. doi: 10.1007/BF02276715. [DOI] [PubMed] [Google Scholar]
- 96.Grela F, Aumeunier A, Bardel E, et al. The TLR7 agonist R848 alleviates allergic inflammation by targeting invariant NKT cells to produce IFN-γ. J Immunol. 2011;186:284–90. doi: 10.4049/jimmunol.1001348. [DOI] [PubMed] [Google Scholar]
- 97.Quarcoo D, Weixler S, Joachim RA, Stock P, Kallinich T, Ahrens B, Hamelmann E. Resiquimod, a new immune response modifier from the family of imidazoquinolinamines, inhibits allergen-induced Th2 responses, airway inflammation and airway hyper-reactivity in mice. Clin Exp Allergy. 2004;34:1314–20. doi: 10.1111/j.1365-2222.2004.02023.x. [DOI] [PubMed] [Google Scholar]
- 98.Duechs MJ, Hahn C, Benediktus E, et al. TLR agonist mediated suppression of allergic responses is associated with increased innate inflammation in the airways. Pulm Pharmacol Ther. 2011;24:203–14. doi: 10.1016/j.pupt.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 99.Xirakia C, Koltsida O, Stavropoulos A, Thanassopoulou A, Aidinis V, Sideras P, Andreakos E. Toll-like receptor 7-triggered immune response in the lung mediates acute and long-lasting suppression of experimental asthma. Am J Respir Crit Care Med. 2010;181:1207–16. doi: 10.1164/rccm.200908-1255OC. [DOI] [PubMed] [Google Scholar]
- 100.Biffen M, Matsui H, Edwards S, et al. Biological characterisation of a novel class of toll-like receptor 7 (TLR7) agonists designed to have reduced systemic activity. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01790.x. Nov 29. doi: 10.1111/j.1476-5381.2011.01790.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fanucchi MV, Schelegle ES, Baker GL, et al. Immunostimulatory oligonucleotides attenuate airways remodeling in allergic monkeys. Am J Respir Crit Care Med. 2004;170:1153–7. doi: 10.1164/rccm.200404-533OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Broide D, Schwarze J, Tighe H, et al. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J Immunol. 1998;161:7054–62. [PubMed] [Google Scholar]
- 103.Samoszuk M. Eosinophils and human cancer. Histol Histopathol. 1997;12:807–12. [PubMed] [Google Scholar]
- 104.Lotfi R, Lee JJ, Lotze MT. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): role in the inflammatory response within tumors. J Immunother. 2007;30:16–28. doi: 10.1097/01.cji.0000211324.53396.f6. [DOI] [PubMed] [Google Scholar]


