Abstract
B-cell activation is triggered by the binding of antigen to the B-cell receptor (BCR). The early molecular events triggered by BCR binding of ligand have been well-characterized both biochemically and using optical microscopy techniques to visualize B-cell activation as it happens. However, we understand much less about the BCR before activation. For this reason, this review will address recent advances in our view of the structure, organization and dynamics of the resting, unstimulated BCR. These parameters have important implications for our understanding of the initiation of B-cell activation and will be discussed in the context of current models for BCR activation. These models include the conformation-induced oligomerization model, in which binding of antigen to monomeric BCR induces a pulling or twisting force causing conformational unmasking of a clustering interface in the Cμ4 domain. Conversely, the dissociation activation model proposes that BCRs exist in auto-inhibitory oligomers on the resting B-cell surface and binding of antigen promotes the dissociation of the BCR oligomer exposing phosphorylation residues within Igα/Igβ. Finally, the collision coupling model suggests that BCR are segregated from activating co-receptors or kinases and activation is associated with changes in BCR mobility on the cell surface, which allows for the functional interaction of these elements.
Keywords: B-cell activation, B-cell receptor, B lymphocytes, receptor dynamics, receptor organization
B-cell activation is triggered by the binding of ligand (referred to as antigen) to the B-cell receptor (BCR), which initiates a cascade of intracellular signalling leading to the internalization of antigen for processing and presentation to T cells.1 The intracellular signalling pathways initiated upon ligand binding have been well characterized biochemically,2–4 and in recent years have been investigated using optical microscopy techniques in live cells, which has provided important new insights. These studies have been well covered in an excellent review5 and will not be discussed here. Instead, this review will focus on recent work investigating the unstimulated or ‘resting’ BCR in terms of both organization and dynamics, and the implications of these in terms of current models of BCR activation.
Structure and organization of the BCR
Structure of the BCR
Mature B cells express two BCR isotypes, IgM and IgD. The BCR is composed of membrane immunoglobulin (mIg); a structure of four (in the case of IgD) or five (IgM) immunoglobulin domains in the heavy chain linked by a hinge, and a short intracellular domain consisting of just three amino acids: lysine, valine, lysine (KVK). The mIg itself does not contain any signalling motifs but instead is linked to the Igα/Igβ heterodimer, which contains immunoreceptor tyrosine-based activation motifs (ITAM); a conserved sequence of four amino acids in which a tyrosine is separated from a leucine or isoleucine by any two amino acids (YxxL/I) and generally repeated twice in the cytoplasmic domain of ITAM-containing proteins separated by between 7 and 12 amino acids, giving it the signature YxxL7–12YxxL.6 The BCR complex was originally believed to be composed of a ‘sheath’ of Igα/Igβ; that is, each mIg was non-covalently bound on each side to Igα/Igβ chains, thus ‘sheathing’ the mIg.7 However, this 1 : 2 stoichiometry of BCR : Igα/Igβ was never experimentally tested and subsequent biochemical studies demonstrated that the BCR was composed of a single mIg and one associated Igα/Igβ chain.8 This was subsequently confirmed using a quantitative microscopy approach, which compared the ratio of fluorescence intensity of labelled mIg to Igα/Igβ across a range of expression levels.9 So, although the term Igα/Igβ sheath occasionally still persists in the literature, and can be confusing for newcomers to B-cell biology, the structure of the BCR as being composed of a single mIg and associated Igα/Igβ chain in a 1 : 1 stoichiometry can be agreed upon. However, whether the ‘resting’ BCR on the B-cell surface consists of a single complex of mIg and Igα/Igβ (monomer) or a higher order structure consisting of several mIg and Igα/Igβ (oligomer) is still debated.
Monomeric BCR and the conformation-induced oligomerization model
The first evidence suggesting that the BCR is a monomer on the cell surface of live cells was provided by Förster (Fluorescence) Resonance Energy Transfer (FRET) studies conducted by Tolar et al.9 FRET is a technique in which the transfer of excited state energy from a donor fluorophore to an acceptor fluorophore only occurs if molecules are within close proximity, on the scale of < 10 nm, making it a powerful technique for examining inter- (and intra-) molecular interactions.10 To determine if the BCR existed as higher order structures, Tolar et al. measured FRET between Igα and Igα fused to donor and acceptor fluorophores – i.e. Igα-cyan fluorescent protein and Igα-yellow fluorescent protein (YFP). For both IgM-BCR and IgG-BCR, FRET efficiency between Igα and Igα was negligible in resting unstimulated B cells, suggesting that IgM and IgG BCR exist as monomers on the cell surface.9 However, it should be noted that the FRET efficiency even between Igα and Igβ was relatively low in these studies, despite being in a complex; if interaction within a defined heterodimer is difficult to measure, then perhaps using this technique to detect higher order structures is problematic. Indeed, although FRET is a highly sensitive molecular ruler, it is also highly sensitive to the ratio between donor and acceptor and the orientation of fluorophores and consequently, lack of FRET does not necessarily rule out a protein–protein interaction.
In contrast, a rapid increase in FRET between donor and acceptor labelled ectodomains of individual BCR was observed upon cross-linking with a polyvalent soluble antigen, suggesting rapid oligomerization (or clustering of the BCR), consistent with BCR microclusters formed in response to membrane-bound antigen.11 To probe the intracellular organization of the BCR, intracellularly labelled mIg and Igα were labelled with donor and acceptor fluorophores; a rapid increase in FRET was observed upon receptor cross-linking.9 This was followed by a subsequent decrease in FRET between the cytoplasmic domains, despite the fact that the ectodomains remain stably clustered. Based on these results, the authors suggested that the cytoplasmic domains of the BCR undergo a conformational change from a closed to an open form, which probably permits the association of kinases. This research group has also used a single molecule approach to examine the formation of ligand-induced BCR oligomers. Using mutated IgM BCR consisting of only the Cμ4 domain, they demonstrated spontaneous clustering of this construct.12 Moreover, when expressed with Igα/Igβ, clustering of the Cμ4 domain led to the spontaneous recruitment of the kinase Syk into these clusters. Consequently, it was proposed that the Cμ4 domain contains a homotypic clustering interface that is not accessible in mIg in the ‘resting’ state. The conformation-induced oligomerization model (Fig. 1) proposes that binding of antigen induces a conformational unmasking of the clustering interface in the Cμ4 domain; a pulling or twisting force induces either a conformational change within Cμ4 or a reorientation of the Cμ3 domain to allow access to the Cμ4 clustering interface.13 Unfortunately, structural studies of the Fc region of IgM are not available, and will be necessary to determine if the conformational changes suggested by this model are likely.
Figure 1.
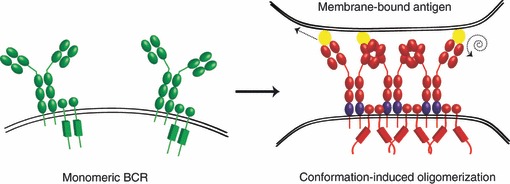
Schematic diagram of the monomeric B-cell receptor (BCR) and the conformation-induced oligomerization model. In resting B cells, the BCR is a monomer in a closed, inactive (green BCR) conformation at the B-cell surface (left panel). Upon binding of antigen (yellow), a pulling or twisting force (indicated by dashed arrows) induces a conformational change in the Cμ4 domain (indicated in purple) to expose a clustering interface and the opening of the cytoplasmic domains for the initiation of signalling (red BCR).
Oligomeric BCR and the dissociation activation model
Reth and colleagues8 first proposed the oligomeric BCR model based on studies using blue native–polyacrylamide gel electrophoresis, a technique that had previously been used to identify components of supramolecular protein complexes.14 These studies found that under low percentage detergent lysing conditions the BCR of both the IgM and the IgD class runs as a large molecular complex.8 Furthermore, BCR oligomers were found to be class-specific, that is, oligomers only contain molecules of the same class. Interestingly, IgM complexes were much more sensitive to the stringency of lysing conditions,8 indicating that they are probably less stable than IgD complexes. This is consistent with an earlier study in which IgD was shown to be more stably associated with Igα/Igβ; a feature attributed to the δm transmembrane (TM) region.15 This is an important observation because it suggests that the overall structure of these two isotypes may be different; an important consideration in our interpretation of class-specific experiments. Indeed, the TM region of mIg contains two sides; the sequence of one side is conserved in all immunoglobulin classes and is involved in the binding of Igα/Igβ, whereas the sequence on the other side is evolutionarily conserved but class specific. Schamel and Reth,8 through mutational analysis, showed that the size of IgD complexes was dependent on these class-specific amino acids in the TM region of mIg, suggesting that these amino acids are involved in oligomerization. This model, however, was not widely accepted within the field, perhaps because it emerged at a time when classical biochemical techniques were experiencing intense scrutiny because of the possibility of artefacts as a result of the specific composition of the lysis buffer.
This group has recently used a synthetic biology approach to examine the structure of the ‘resting’ BCR; essentially building a BCR in a cell that does not express either a BCR or any associated signalling molecules, such as Drosophila S2 cells, a common model for this type of reconstitution experiment. This approach was combined with a bifluorescence complementation assay (BiFC); an elegant method in which the assembly of a complete YFP, detected as a fluorescent signal, from the N-terminal and C-terminal half domains, which have been tagged to proteins of interest, is evidence for dimerization. The half domains of YFP and cyan fluorescent protein were fused to either Igα or Igβ, with formation of the Igα/Igβ heterodimer serving as a positive control; or to two different Igα, with formation of a complete YFP indicative of BCR oligomerization. Using this technique, combined with immunoprecipitation and flow cytometry to quantify the relation between total BCR complexes and YFP fluorescence, Yang and Reth16 detected a high BiFC efficiency for both IgM-BCR and IgD-BCR. The authors then focused on IgD, mutating the conserved but class-specific amino acids in the TM domain as in the previous study;8 however, there was no difference in the BiFC efficiency of IgD complexes,16 which was attributed to the fact that this mutant could still form dimers in earlier biochemical assays.8 Yang and Reth16 therefore searched for additional mutations that may affect IgD oligomers and found that a cysteine-to-serine mutation in the linker region of Igα, which prevents formation of a covalent disulphide bridge between Igα and Igβ, did not affect the BiFC efficiency of Igα : Igβ, but combined with the IgD TM mutations resulted in a 10-fold decrease in BiFC efficiency. Thus, IgD oligomerization is dependent on both IgD TM residues and the disulphide bridge link between Igα and Igβ. This monomeric mutant also presents an opportunity to examine the effect of BCR structure on BCR signalling. Indeed, Yang and Reth16 found that this monomeric mutant of IgD BCR is more active and less stably expressed on the cell surface. Conversely, a BiFC stabilized BCR-dimer is less active in signalling and is internalized more slowly.16 Taken together, these observations led to the proposal of the dissociation activation model (Fig. 2). According to the dissociation activation model, most BCR are in tight oligomers that are auto-inhibitory on the resting B-cell surface. The binding of a polyvalent antigen to the BCR will promote the dissociation of the BCR oligomer; consequently exposing the ITAMs within Igα/Igβ for phosphorylation and the conserved class-specific amino acids of the TM region to the lipid environment, which may help to target the BCR to specialized membrane compartments such as lipid rafts.17 This model is consistent with the observation that a polyvalent soluble antigen is necessary to activate the BCR, but does not account for the observation that a monomeric membrane-bound antigen is able to activate B cells.18
Figure 2.
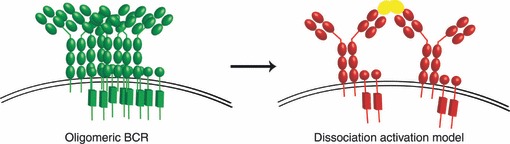
Schematic diagram of the oligomeric B-cell receptor (BCR) and the dissociation activation model. In resting B cells, the BCR is an auto-inhibited (green BCR) oligomer at the B-cell surface (left panel). Binding of polyvalent antigen (yellow) promotes the dissociation of BCR oligomers and the exposure of immunoreceptor tyrosine-based activation motif residues for phosphorylation and signalling (red BCR).
It is important to note that the model of oligomeric cell surface receptors is not without precedent. Indeed, there is evidence that many cell surface proteins are dimers or oligomers in the resting, unstimulated state, including MHC class I19 and class II,20 epidermal growth factor receptor,21 interleukin-2 and interleukin-15,22,23 and several G protein-coupled receptors.24–29 Many of these studies have used flow cytometry-based FRET, a powerful technique for examining protein oligomerization but providing no spatial information. To overcome this, researchers have combined this technique with transmission electron microscopy to visualize proteins at high resolution. Interestingly, these studies suggest that proteins are not only present as oligomers, but as higher order ‘protein islands’ on the cell surface,19,30 which in some cases may be co-clustered31 or segregated32 from selected functional elements; suggesting that higher order receptor organization may be an important mechanistic factor in receptor function.
Although the approach taken by Reth and colleagues is both elegant and technologically impressive, it is not without its limitations. Specifically, this technique is still ultimately a biochemical assay because while the complete YFP may form in vivo, cells are still lysed and complexes eluted from a population of cells to derive the BiFC efficiency. Hence, spatial information about the organization of BCR oligomers is lost. Moreover, BiFC efficiency, at least as determined here, cannot distinguish between a dimer and a higher order oligomer; nor does it tell us anything about the proportion of BCR as monomer, dimer or higher order oligomer or how big these structures may be. Finally, because the formation of complete YFP is irreversible, this assay cannot provide information on the stability or dynamics of these structures. For these reasons, these parameters remain open questions and additional methods will need to be developed and used to provide a complete picture of the overall structure or organization of the BCR in the ‘resting’ B cell. Some of these limitations could be overcome using new optical microscopy techniques that break the diffraction-limited resolution of confocal and epifluorescence microscopy, including stimulated emission depletion,33 photoactivated localization microscopy (PALM),34 stochastic optical reconstruction microscopy,35 or near-field scanning optical microscopy (NSOM).36 Indeed, PALM37,38 and NSOM with single-molecule sensitivity39,40 have recently been applied to examine the nanoscale distribution of membrane proteins and quantify various parameters of spatial organization including the fraction of monomer versus oligomer, and the size and packing density of higher order structures. These are important parameters to be unravelled for a better picture of the organization of the BCR in the resting state.
Dynamics of the BCR and the collision coupling model
In addition to the structure and organization of the ‘resting’ BCR, another important parameter to consider is how the BCR moves within the membrane and the potential mechanisms that regulate this movement. We have recently used single particle tracking techniques to examine the movement of IgM, IgD and IgG BCR isotypes in resting B cells.41 We found that BCR of all isotypes display a degree of restricted steady-state diffusion. This restriction in diffusion was partly the result of the actin cytoskeleton, which appeared to create boundaries for BCR diffusion. Interestingly, the diffusion of IgD was 10-fold slower than that of IgM; IgD being almost completely immobilized, indicating a fundamental difference in the steady-state of these two BCR isotypes. The diffusion of both IgM and IgD was increased by depolymerization of the actin cytoskeleton, although not to the same degree. Hence, it may be that there are additional mechanisms that regulate the diffusion of IgD, or perhaps this difference is the result of differences in the organization of IgD into higher order clusters that are more stable than IgM, as suggested by Schamel and Reth.8 Indeed, it should be noted that because of the low labelling density used to visualize single molecules, we cannot say if the molecules we track are present as a monomers or higher order structures on the cell surface.
Interestingly, we also found that BCR diffusion was correlated with the concentration of intercellular adhesion molecule 1 present in planar lipid bilayers (B. Treanor, D. Depoil, and F.D. Batista, unpublished results); an observation that may explain the difference in BCR diffusion in resting B cells observed by Tolar et al.12 This is probably the result of increased association of the actin cytoskeleton with the plasma membrane mediated by adhesion receptors or interactions with ezrin-radixin-moesin proteins. Importantly, this observation raises the question of what is a ‘resting’ B cell? In vivo, B cells will be circulating through the blood and migrating through lymph nodes, and as a result the actin cytoskeleton will be undergoing constant dynamic reorganization. How this dynamic reorganization of the actin cytoskeleton during cell migration will impact on BCR dynamics, and perhaps even the organization of the BCR, is an important question yet to be addressed. Moreover, in light of our observations (discussed below), this has important implications for B-cell activation.
The observation of restricted steady-state diffusion of the BCR raised the question of whether this has some functional significance for BCR activation. Surprisingly, we found that simply altering the actin cytoskeleton was sufficient to induce robust signalling, comparable to that induced by cross-linking the BCR; resulting not only in early signalling events such as calcium flux, but also in activation of downstream signalling cascades including Akt, and the mitogen-activated protein kinase pathway.41 Remarkably we found that even brief treatment of primary naive B cells with actin depolymerizing agents was sufficient to induce up-regulation of co-stimulatory molecules. Signalling was strongly correlated with changes in BCR diffusion, in particular, with a reduction in the proportion of very slowly diffusing (or immobile) BCR. Importantly, this robust intracellular signalling upon disruption of actin was observed in the absence of any BCR ligand. However, the signal induced by actin alteration was abrogated in B cells lacking key BCR signalling molecules such as phospholipase C-γ2 and Vav, strongly suggesting that this signal is initiated by the BCR. This ligand-independent BCR activation is not necessarily surprising given that the low-level constitutive (tonic) signal necessary for B-cell survival appears to be ligand independent;42 this observation does, however, have important implications for understanding both tonic43 and ligand-induced BCR activation.
So, how might signalling be initiated by disruption of the actin cytoskeleton and an increase in BCR diffusion? And can we reconcile these observations with the models of BCR activation discussed above? It may be that the BCR oligomers proposed by Reth and colleagues are dependent on the actin cytoskeleton and that disruption of actin is sufficient to dissociate these auto-inhibitory oligomers. Reth and colleagues did not examine if BCR oligomers are actin-dependent but it has been shown in T cells that ‘protein islands’ are connected to the actin cytoskeleton and at least partially depend on it for their formation and/or maintenance.30 It is more difficult to reconcile our observations with the conformation-induced oligomerization model because it is difficult to envisage how simply disrupting actin in the absence of any ligand could cause a conformational change in the Cμ4 domain. Alternatively, we proposed a model in which disruption of the diffusion barrier defined by the actin cytoskeleton increases the mobile fraction of the BCR and so increases the probability that the BCR will encounter an activated kinase or co-receptor41 (Fig. 3); an idea reminiscent of the collision coupling or mobile receptor hypothesis of receptor activation.44,45 In support of such a model, it has recently been shown that destabilization of the actin cytoskeleton causes an increase in serotonin receptor diffusion and this is strongly correlated with the efficiency of ligand-induced signalling.46 This model would imply that BCRs are segregated from activating co-receptors or kinases, and that disruption of the actin cytoskeleton allows for the functional interaction of these elements. Such segregation in the resting state and concatenation upon activation has recently been shown for pre-existing ‘protein islands’ of T-cell receptor and the key adaptor linker for activation of T cells.32 It remains to be determined if BCR are physically separated from functional co-elements on the resting B-cell surface. It could also be that the actin cytoskeleton effectively immobilizes BCR with negative regulators of BCR signalling (Fig. 3), such as the protein-arginine-methyl-transferase1, which has recently been shown to co-localize with the resting BCR,47 and disruption of actin releases this inhibitory interaction.
Figure 3.
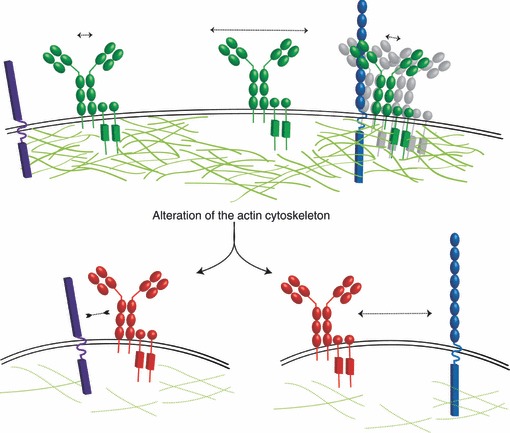
Schematic diagram of the collision coupling/dissociation model. In resting B cells (upper panel), the actin cytoskeleton defines the diffusion dynamics of the B-cell receptor (BCR); there are both mobile (indicated by long dashed arrow) and immobile (short dashed arrow) BCR. It is not known from single particle tracking studies if the BCR is a monomer or in a higher order structure (grey BCR). Upon alteration of the actin cytoskeleton (lower panels), in the absence of any ligand, BCR signalling can be initiated (red BCR). The collision coupling model proposes that the actin cytoskeleton may limit the interaction between the BCR and functional co-elements such as a key co-receptor (purple); hence, alteration of the diffusion barrier releases the BCR leading to an increase in BCR diffusion and so to an increase in the probability that the BCR will encounter a functional co-element. Alternatively, the actin cytoskeleton may immobilize BCR and inhibitory molecules (blue) together and alteration of actin releases this inhibitory interaction.
In addition, as briefly alluded to earlier, the context of a ‘resting’ B cell could have important implications for both the organization and dynamics of the BCR, and consequently for B-cell activation. The dynamic reorganization of the actin cytoskeleton during cell migration could have a significant impact on these parameters. Moreover, other external stimuli, such as cytokines, may impact on BCR diffusion. Indeed, tumour necrosis factor-α and interferon-γ have been shown to affect the lateral diffusion of MHC class I in human endothelial cells.48 It remains to be determined how migration, cell polarization, or various cytokines impact on BCR diffusion but it is tempting to speculate that in some cases the diffusion dynamics of the BCR may be altered to either enhance or dampen BCR triggering.
Conclusion
The structure, organization and dynamics of the BCR in ‘resting’ B cells are critical for understanding BCR activation. The disparity in the models presented here illustrates the technical challenges of deciphering these parameters. Improved genetic, biochemical and optical microscopy techniques will no doubt continue to inform our view of the ‘resting’ BCR. Of note, it is worth remembering that the term BCR does not refer to a single entity, but rather to two separate entities, IgM and IgD (and indeed, IgG and IgA, or IgE in memory B cells); the evidence of their distinction is highlighted by the difference in their dynamics41 and stability;15 considering this may not only help to reconcile some of the disparities in these models, but may also illuminate distinct regulatory and functional mechanisms of these two BCR isotypes. Clearly, many questions remain with respect to the organization and dynamics of the BCR and how these parameters impact on BCR activation. In particular, future research will need to address how these parameters are influenced by the in vivo context of B cells, where migration, polarization and non-BCR-specific stimuli are integrated, all of which may impact on the organization and dynamics of the ‘resting’ BCR.
Disclosures
The author has no conflict of interest.
References
- 1.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–9. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 2.DeFranco AL. The complexity of signaling pathways activated by the BCR. Curr Opin Immunol. 1997;9:296–308. doi: 10.1016/s0952-7915(97)80074-x. [DOI] [PubMed] [Google Scholar]
- 3.Kurosaki T. Regulation of BCR signaling. Mol Immunol. 2011;48:1287–91. doi: 10.1016/j.molimm.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu Rev Immunol. 1997;15:453–79. doi: 10.1146/annurev.immunol.15.1.453. [DOI] [PubMed] [Google Scholar]
- 5.Harwood NE, Batista FD. New insights into the early molecular events underlying B cell activation. Immunity. 2008;28:609–19. doi: 10.1016/j.immuni.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–4. [PubMed] [Google Scholar]
- 7.Hombach J, Tsubata T, Leclercq L, Stappert H, Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature. 1990;343:760–2. doi: 10.1038/343760a0. [DOI] [PubMed] [Google Scholar]
- 8.Schamel WW, Reth M. Monomeric and oligomeric complexes of the B cell antigen receptor. Immunity. 2000;13:5–14. doi: 10.1016/s1074-7613(00)00003-0. [DOI] [PubMed] [Google Scholar]
- 9.Tolar P, Sohn HW, Pierce SK. The initiation of antigen-induced B cell antigen receptor signaling viewed in living cells by fluorescence resonance energy transfer. Nat Immunol. 2005;6:1168–76. doi: 10.1038/ni1262. [DOI] [PubMed] [Google Scholar]
- 10.Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–46. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- 11.Depoil D, Fleire S, Treanor BL, Weber M, Harwood NE, Marchbank KL, Tybulewicz VL, Batista FD. CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nat Immunol. 2008;9:63–72. doi: 10.1038/ni1547. [DOI] [PubMed] [Google Scholar]
- 12.Tolar P, Hanna J, Krueger PD, Pierce SK. The constant region of the membrane immunoglobulin mediates B cell-receptor clustering and signaling in response to membrane antigens. Immunity. 2009;30:44–55. doi: 10.1016/j.immuni.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tolar P, Pierce SK. A conformation-induced oligomerization model for B cell receptor microclustering and signaling. Curr Top Microbiol Immunol. 2010;340:155–69. doi: 10.1007/978-3-642-03858-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schagger H, von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem. 1991;199:223–31. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- 15.Schamel WW, Reth M. Stability of the B cell antigen receptor complex. Mol Immunol. 2000;37:253–9. doi: 10.1016/s0161-5890(00)00025-0. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Reth M. Oligomeric organization of the B-cell antigen receptor on resting cells. Nature. 2010;467:465–9. doi: 10.1038/nature09357. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Reth M. The dissociation activation model of B cell antigen receptor triggering. FEBS Lett. 2010;584:4872–7. doi: 10.1016/j.febslet.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 18.Fleire SJ, Goldman JP, Carrasco YR, Weber M, Bray D, Batista FD. B cell ligand discrimination through a spreading and contraction response. Science. 2006;312:738–41. doi: 10.1126/science.1123940. [DOI] [PubMed] [Google Scholar]
- 19.Damjanovich S, Vereb G, Schaper A, et al. Structural hierarchy in the clustering of HLA class I molecules in the plasma membrane of human lymphoblastoid cells. Proc Natl Acad Sci USA. 1995;92:1122–6. doi: 10.1073/pnas.92.4.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szollosi J, Horejsi V, Bene L, Angelisova P, Damjanovich S. Supramolecular complexes of MHC class I, MHC class II, CD20, and tetraspan molecules (CD53, CD81, and CD82) at the surface of a B cell line JY. J Immunol. 1996;157:2939–46. [PubMed] [Google Scholar]
- 21.Gadella TW, Jr, Jovin TM. Oligomerization of epidermal growth factor receptors on A431 cells studied by time-resolved fluorescence imaging microscopy. A stereochemical model for tyrosine kinase receptor activation. J Cell Biol. 1995;129:1543–58. doi: 10.1083/jcb.129.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenei A, Kormos J, Szentesi G, Veres AJ, Varga S, Bodnar A, Damjanovich S, Matyus L. Non-random distribution of interleukin receptors on the cell surface. Chemphyschem: A Eur J Chem Phys Phys Chem. 2009;10:1577–85. doi: 10.1002/cphc.200900242. [DOI] [PubMed] [Google Scholar]
- 23.Vamosi G, Bodnar A, Vereb G, et al. IL-2 and IL-15 receptor α-subunits are coexpressed in a supramolecular receptor cluster in lipid rafts of T cells. Proc Natl Acad Sci USA. 2004;101:11082–7. doi: 10.1073/pnas.0403916101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Comps-Agrar L, Kniazeff J, Norskov-Lauritsen L, et al. The oligomeric state sets GABAB receptor signalling efficacy. EMBO J. 2011;30:2336–49. doi: 10.1038/emboj.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harikumar KG, Morfis MM, Lisenbee CS, Sexton PM, Miller LJ. Constitutive formation of oligomeric complexes between family B G protein-coupled vasoactive intestinal polypeptide and secretin receptors. Mol Pharmacol. 2006;69:363–73. doi: 10.1124/mol.105.015776. [DOI] [PubMed] [Google Scholar]
- 26.Kang DS, Gustafsson C, Morgelin M, Leeb-Lundberg LM. B1 bradykinin receptor homo-oligomers in receptor cell surface expression and signaling: effects of receptor fragments. Mol Pharmacol. 2005;67:309–18. doi: 10.1124/mol.104.002840. [DOI] [PubMed] [Google Scholar]
- 27.Kuhn C, Bufe B, Batram C, Meyerhof W. Oligomerization of TAS2R bitter taste receptors. Chem Senses. 2010;35:395–406. doi: 10.1093/chemse/bjq027. [DOI] [PubMed] [Google Scholar]
- 28.Maurel D, Comps-Agrar L, Brock C, et al. Cell-surface protein–protein interaction analysis with time-resolved FRET and snap-tag technologies: application to GPCR oligomerization. Nat Methods. 2008;5:561–7. doi: 10.1038/nmeth.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miura S, Karnik SS, Saku K. Constitutively active homo-oligomeric angiotensin II type 2 receptor induces cell signaling independent of receptor conformation and ligand stimulation. J Biol Chem. 2005;280:18237–44. doi: 10.1074/jbc.M500639200. [DOI] [PubMed] [Google Scholar]
- 30.Lillemeier BF, Pfeiffer JR, Surviladze Z, Wilson BS, Davis MM. Plasma membrane-associated proteins are clustered into islands attached to the cytoskeleton. Proc Natl Acad Sci USA. 2006;103:18992–7. doi: 10.1073/pnas.0609009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bene L, Balazs M, Matko J, Most J, Dierich MP, Szollosi J, Damjanovich S. Lateral organization of the ICAM-1 molecule at the surface of human lymphoblasts: a possible model for its co-distribution with the IL-2 receptor, class I and class II HLA molecules. Eur J Immunol. 1994;24:2115–23. doi: 10.1002/eji.1830240928. [DOI] [PubMed] [Google Scholar]
- 32.Lillemeier BF, Mortelmaier MA, Forstner MB, Huppa JB, Groves JT, Davis MM. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2010;11:90–6. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eggeling C, Ringemann C, Medda R, et al. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature. 2009;457:1159–62. doi: 10.1038/nature07596. [DOI] [PubMed] [Google Scholar]
- 34.Betzig E, Patterson GH, Sougrat R, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–5. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 35.Rust MJ, Bates M, Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nat Methods. 2006;3:793–5. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oshikane Y, Kataoka T, Okuda M, Hara S, Inoue H, Nakano M. Observation of nanostructure by scanning near-field optical microscope with small sphere probe. Sci Technol Adv Mater. 2007;8:181–5. [Google Scholar]
- 37.Sengupta P, Jovanovic-Talisman T, Skoko D, Renz M, Veatch SL, Lippincott-Schwartz J. Probing protein heterogeneity in the plasma membrane using PALM and pair correlation analysis. Nat Methods. 2011;8:969–75. doi: 10.1038/nmeth.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherman E, Barr V, Manley S, et al. Functional nanoscale organization of signaling molecules downstream of the T cell antigen receptor. Immunity. 2011;35:705–20. doi: 10.1016/j.immuni.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Bakker BI, Bodnar A, van Dijk EM, et al. Nanometer-scale organization of the α subunits of the receptors for IL2 and IL15 in human T lymphoma cells. J Cell Sci. 2008;5:627–33. doi: 10.1242/jcs.019513. [DOI] [PubMed] [Google Scholar]
- 40.de Bakker BI, de Lange F, Cambi A, Korterik JP, van Dijk EM, van Hulst NF, Figdor CG, Garcia-Parajo MF. Nanoscale organization of the pathogen receptor DC-SIGN mapped by single-molecule high-resolution fluorescence microscopy. Chemphyschem: A Eur J Chem Phys Phys Chem. 2007;8:1473–80. doi: 10.1002/cphc.200700169. [DOI] [PubMed] [Google Scholar]
- 41.Treanor B, Depoil D, Gonzalez-Granja A, Barral P, Weber M, Dushek O, Bruckbauer A, Batista FD. The membrane skeleton controls diffusion dynamics and signaling through the B cell receptor. Immunity. 2010;32:187–99. doi: 10.1016/j.immuni.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–83. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 43.Treanor B, Batista FD. Organisation and dynamics of antigen receptors: implications for lymphocyte signalling. Curr Opin Immunol. 2010;22:299–307. doi: 10.1016/j.coi.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Jans DA. The mobile receptor hypothesis revisited: a mechanistic role for hormone receptor lateral mobility in signal transduction. Biochim Biophys Acta. 1992;4:271–6. doi: 10.1016/0304-4157(92)90001-q. [DOI] [PubMed] [Google Scholar]
- 45.Jacobs S, Cuatrecasas P. The mobile receptor hypothesis for cell membrane receptor action. Trends Biochem Sci. 1977;2:280–1. [Google Scholar]
- 46.Ganguly S, Pucadyil TJ, Chattopadhyay A. Actin cytoskeleton-dependent dynamics of the human serotonin 1A receptor correlates with receptor signaling. Biophys J. 2008;95:451–63. doi: 10.1529/biophysj.107.125732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Infantino S, Benz B, Waldmann T, Jung M, Schneider R, Reth M. Arginine methylation of the B cell antigen receptor promotes differentiation. J Exp Med. 2010;207:711–19. doi: 10.1084/jem.20091303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stolpen AH, Golan DE, Pober JS. Tumor necrosis factor and immune interferon act in concert to slow the lateral diffusion of proteins and lipids in human endothelial cell membranes. J Cell Biol. 1988;107:781–9. doi: 10.1083/jcb.107.2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]


