Abstract
Class switching and plasma cell differentiation occur at a high level within all mucosa-associated lymphoid tissues. The different classes of membrane immunoglobulin heavy chains are associated with the Igα/Igβ heterodimer within the B-cell receptor (BCR). Whether BCR isotypes convey specific signals adapted to the corresponding differentiation stages remains debated but IgG and IgA membranes have been suggested to promote plasma cell differentiation. We investigated the impact of blocking expression of the IgA-class BCR through a ‘αΔtail’ targeted mutation, deleting the Cα immunoglobulin gene membrane exon. This allowed us to evaluate to what extent class switching and plasma cell differentiation can be concurrent processes, allowing some αΔtail+/+ B cells with an IgM BCR to directly differentiate into IgA plasma cells and yield serum secreted IgA in spite of the absence of membrane IgA+ B lymphocytes. By contrast, in secretions the secretory IgA was very low, indicating that J-chain-positive plasma cells producing secretory IgA overwhelmingly differentiate from previously class-switched membrane IgA+ memory B cells. In addition, although mucosa-associated lymphoid tissues are a major site for plasma cell accumulation, αΔtail+/+ mice showed that the gut B-cell lineage homeostasis is not polarized toward plasma cell differentiation through a specific influence of the membrane IgA BCR.
Keywords: B cell receptor, lamina propria, membrane IgA
Introduction
Immunoglobulin A is considered a major actor in specific mucosal immunity. It is abundantly secreted by plasma cells that accumulate within mucosa-associated lymphoid tissues (MALT), at sites where both class switch recombination (CSR) to the IgA class and plasma cell differentiation are strongly stimulated by the cytokine microenvironment as well as by interaction with T cells.1–6 Consequently, IgA is the most abundantly synthesized immunoglobulin in mammals.7 IgA plasma cells probably differentiate from lymphocytes expressing a B-cell receptor (BCR) that includes membrane IgA (mIgA). This membrane-anchored form of the molecule features the highly conserved membrane anchoring domain of the α heavy chain and an intracellular tail of unknown function.8–11
Similarly to all other mIg, the mIgA associates with a transducing module made up of the disulphide-linked Igα/Igβ (CD79a/CD79b) heterodimer to compose the IgA class-BCR.12 BCR signalling has been studied in detail for the μ heavy chain and its dual role in pre-B-cell or B-cell survival (tonic signal in the absence of any antigen) along with B-cell activation upon antigen-mediated BCR cross-linking (triggering plasma cell differentiation and antibody secretion).13,14 Requirement of a B lymphocyte stage expressing a BCR of a given class before secretion of antibodies of the same class has been studied for IgE and IgG1. In the case of IgE, deletion of the membrane anchoring domain prevented the expression of IgE as a membrane-anchored molecule resulting in a 95–98% reduction of IgE production in vivo, but barely affected IgE secretion during the short lipopolysaccharide/interleukin-4 (LPS/IL-4) stimulations carried out in vitro.15 In fact, this knock-out affected both the primary and secondary responses that required the presence of mIgE-expressing memory cells, indicating that the production of specific antibodies of the IgE class requires an IgE class-specific BCR to be first expressed. Similar results were obtained regarding the stage of B cells that carry membrane-type γ1 heavy chain: although this stage appeared to be dispensable in vitro for LPS/IL-4 induction of IgG1 antibodies, it was shown to be crucial in vivo for optimal differentiation of antigen-specific IgG1-secreting plasma cells, in both primary and secondary specific responses.16 As the γ membrane anchoring region has been shown to play a role in optimizing antigen internalization as well as in processing and presentation to T cells, the phenotype observed in mice carrying a mutation of the γ1 heavy chain tail region could be a result of both a disturbed interaction with T cells in the course of antigen presentation and a putative defective stimulation towards plasma cell differentiation.16 Deletion of the membrane anchoring region has also been studied in the case of IgM. Absence of the μ chain membrane anchoring region in μMT (membrane tail deficient) mice was initially reported to result in a severe B-cell defect in the C57BL/6 background.17,18 However, this defect appeared to be incomplete in the BALB/c background where low expression of class-switched BCR and class-switched antibodies was demonstrated, suggesting that CSR can occur in the absence of expression of the IgM BCR.17,19 In the C57BL/6 background, it was even shown that aged μMT animals finally accumulate plasma cells in the MALT despite the apparent absence of lymphocytes carrying a BCR, suggesting that B-cell progenitors can undergo CSR to IgA and differentiate into IgA-secreting B cells (ASCs) in the absence of mIgM/mIgD.17,18
To date, little is known regarding the potentially specialized function of mIgA that could eventually confer specific properties on mucosal or memory mIgA+ cells in comparison with naive mIgM+ cells. It is often assumed that about half of the IgA-producing B cells are involved in T-cell-independent B1 responses, so that alongside the BCR, their development would rely in a large part on signals given by Toll-like receptors and other cytokine receptors in the MALT microenvironment. Cross-linking of mIgA raises the intracellular calcium concentration and supports B-cell activation so that mIgA+ B cells residing in the MALT can mediate IgA responses to local immunization.20,21 In addition, we have recently shown that replacing IgM expression with IgA expression in naive B cells results in the IgA BCR actively promoting plasma cell differentiation.22
We intended to check whether, as in ε and γ1 chains, expression of the membrane form of the α immunoglobulin heavy chain was required for generating IgA-ASC. This experiment also allowed us to check whether expression of the α class BCR was responsible for the plasma cell accumulation that normally characterizes MALT tissue and if so whether this knock-out would eventually result in the attrition of the gut plasma cell compartment. Consequently, we generated mutant mice in which the membrane exon downstream of the constant α region (Cα) was replaced by a floxed neomycin gene (αΔtail mice).
Methods
Mice
Animal experimentation was in accordance with international guidelines. EIIa-cre transgenic mice were a kind gift from Dr Heiner Westphal, used under a non-commercial research license agreement from Dupont Pharma (Wilmington, DE).
Gene targeting
The αΔtail construct included an 8-kb α mouse genomic fragment as a 5′ arm (from a SalI site 3 kb upstream of the Sα region to a HindIII downstream of CH3 secreted-form transcript polyadenylation signal) and a 3 kb long 3′ arm (a genomic fragment originating from downstream of the Cα gene membrane exon). A 1·5-kb NotI–NotI fragment encompassing a neomycin resistance gene flanked by loxP sites was fixed between both arms. E14 ES cells were transfected with linearized vector and selected using G418 (200 μg/ml). Recombinant clones were identified by Southern blot with an external 5′ probe (570 bp, a BamHI/EcoRI fragment located upstream of Sα). After the injection of recombinant ES clones in C57BL/6 blastocysts, the male chimeras were mated with C57BL/6 females and germline transmission of the mutation was checked by Southern blot with an internal probe (500 bp, CH3 fragment, Fig. 1, middle). Homozygous mutant αΔtailneo/αΔtailneo mice were mated with EIIa-cre transgenic mice. The progeny was checked by Southern blot for the occurrence of Cre-mediated deletion, yielding the αΔtail mutant allele.
Figure 1.
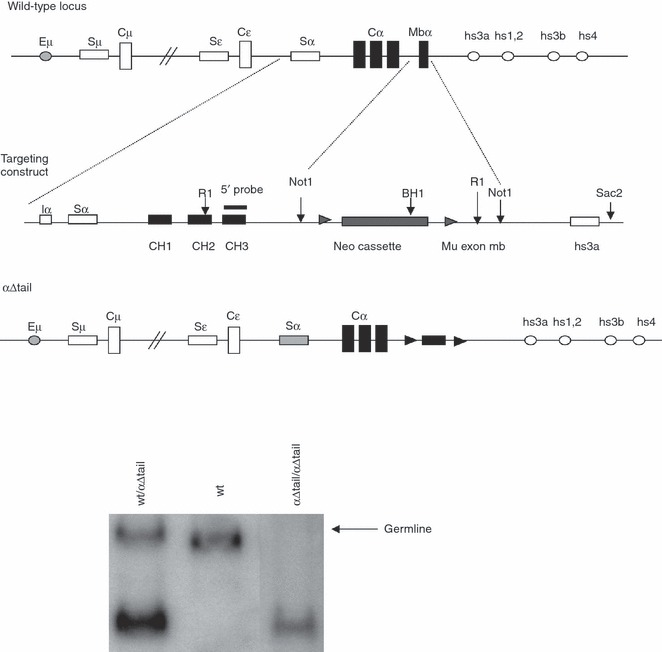
Replacement of the Cα gene membrane exon with a deletable neo cassette. (a) Top: structure of the targeted locus (not to scale), showing an unrearranged IgH locus and the extent of the deletion within the Cα gene. Middle: structure of the targeting vector in which Cα only includes the CH1, CH2, CH3 whereas the membrane α exon is replaced by a neor cassette flanked by two loxP sites. Bottom: the resulting locus is able to support the production of transcripts for the secreted form of the immunoglobulin α heavy chain but not for the membrane-anchored form, whereas the neo gene can be deleted by cre-mediated recombination. (b) Southern blot analysis of tail DNA from representative wild-type (wt), heterozygous, or homozygous mutant mice.
Cell flow cytometry
Cells from 6- to 8-week-old mice were stained with antibodies conjugated to FITC, phycoerythrin or allophycocyanin: anti-IgM (eB121-15F9), anti-IgD (11-26), anti-B220 (RA3-6B2), anti-mouse κ chains (187.1), anti-IgA (all from BD Biosciences Pharmingen, Le Pont-de-Claix, France, Southern Biotechnologies, Birmingham, AL or e-bioscience, San Diego, CA). Cells were analysed on a Beckman Coulter FC500 apparatus (Beckman Coulter, Fullerton, CA).
Antibody analysis in biologic samples
Mouse immunoglobulin classes and subclasses were measured using ELISA on plates coated and revealed with 1 μg/ml isotype-specific goat antibodies (Southern Biotechnologies). Mouse sera were assayed at 1 : 6, 1 : 36, 1 : 216 and 1 : 1296 dilutions.
In vitro stimulation assays
For these experiments, cells from αΔtail+/+ and control mice were stimulated for 2–4 days with 20 μg/ml LPS from Salmonella typhimurium (Sigma, St Louis, MO) with or without the addition of 5 ng/ml transforming growth factor-β (TGF-β; R&D Systems, Minneapolis, MN) in RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum. Cells were collected for RNA and supernatants were analysed for IgA secretion by ELISA.
Western blot and immunoprecipitation
Serum proteins were separated by non-reducing SDS–PAGE (10%) and transferred onto polyvinylidene difluoride membranes (Millipore, Molsheim, France). Membranes were blocked in 5% milk Tris-buffered saline-Tween, incubated with goat anti-mouse IgA (Southern Biotechnologies), and revealed with horseradish peroxidase-labelled anti-goat immunoglobulin (Dako, Glostrup, Denmark) by chemiluminescence (ECL, Pierce, Rockford, IL). Serum proteins were immunoprecipitated with goat anti-mouse J-chain (Santa-cruz Biotech, Santa-Cruz, CA), analysed by Western blots with anti-mouse IgA and revealed with horseradish peroxidase-labelled anti-goat immunoglobulin TrueBlot (eBioscience) by chemiluminescence (ECL, Pierce).
Germline transcripts
Total RNA was prepared with TRI Reagent (Ambion, Austin, TX), according to the manufacturer's protocol from wild-type (wt) or αΔtail spleen cells cultured for 3 days. Reverse transcription was carried out for 2 hr with a high-capacity cDNA RT kit (Applied Biosystems, Foster City, CA) with 2 μg RNA. Serial dilution of cDNA was carried out 1 : 1, 1 : 5, 1 : 25, and 1 : 125 for all transcripts. Transcripts from the mouse β-actin gene were used as internal loading control. Amplifications were performed with 2 μl cDNA template with hybridization at 58° over 25 cycles for β-actin; at 59° over 35 cycles for α; and at 55° over 35 cycles for μ.
Immunofluorescence and immunohistochemistry
For immunofluorescence, organs were frozen in liquid nitrogen. Cryosections of 8 μm were fixed with cold methanol for 10 min and permeabilized in PBS 0·15% Triton X-100 for 20 min at room temperature.
Blocking of unspecific and of Fc receptor labelling was carried out by incubation with PBS/3% BSA (Sigma Aldrich) for 45 min at 37°, followed by rat anti-mouse CD16/CD32 (Mouse BD Fc block; BD Biosciences, dilution 1/100, 5 min, 4°) incubation. Immunoglobulin staining was carried out using Alexa 488-goat anti-mouse κ light chain, FITC or rat anti-mouse IgA (BD-Pharmingen) for 45 min at 37°, then slides were washed in PBS and stained with Dapi for 1 min, Slides or cells were washed in PBS, mounted in Moviol (Merck, Nottingham, UK) and observed on an LSM 510 confocal microscope (Carl Zeiss, Jena, Germany).
Immunohistochemistry was performed on 4-μm paraffin-embedded tissue sections. Samples were pre-treated by microwave incubation in citrate buffer pH6·0 with 0·05% Tween 20. Sections were then incubated for 2 hr at room temperature with the following antibodies: anti-mouse B220 (clone RA3 6B2; BD Biosciences) or anti-CD138 (clone 281-2; BD Biosciences), 1 : 50 in Tris-buffered saline/0·05% Tween. A secondary horseradish peroxidase-conjugated rabbit anti-rat IgG (Dako) was used to reveal primary antibodies for 45 min at room temperature.
Acquisitions were carried out on a Zeiss LSM 510 microscope and then analysed with the Image J software (National Institutes of Health, Bethesda, MD) as follows: the complete tissue section surface was measured using the threshold tool; in the same way, but using a higher threshold, positive staining (B220+ or CD138+ total surface) was evaluated on each section. Finally ratios of B220+ : CD138+ stained areas were calculated.
Statistical analysis
Results are expressed as mean ± SEM (standard error of the mean), and overall differences between variables were evaluated by a two-tailed unpaired Student's t-test using Prism GraphPad software (Graphpad, San Diego, CA).
Results
Early B-cell differentiation is largely normal in mutant animals
To block expression of mIgA in B cells, the gene portion encoding the Cα membrane anchoring domain was deleted within the IgH locus (Fig. 1). A neor cassette flanked with loxP sites was inserted as a replacement of the Cα gene membrane exon and was then removed by mating mutants with the Cre transgenic mice (Fig. 1, middle and bottom).
Early B-cell compartments in mutant mice were analysed by flow cytometry. In comparison with wt mice, early B-cell maturation appeared normal in αΔtail+/+ mice. The total number of bone marrow lineage B220+ cells was similar to that in wt controls (26·67 ± 5·085, n = 3 for wt, 21·13 ± 3·839, n = 3 for αΔtail+/+), IgM/IgD expressing cells in αΔtail+/+ bone marrow was similar to that in wt (Fig. 2a) and showed normal absolute values for the CD117+/B220+ pro-B compartment, the CD43+/B220+ pro-B/early pre-B compartment and the B220+/CD25+ pre-B compartment (data not shown). In the periphery, the B220+ cells were similar to wt controls (62·90 ± 0·8591, n = 6 for wt, 67·46 ± 2·152, n = 5 for αΔtail+/+) and the homozygous mutation did not affect the number of surface IgM/IgD expressing cells in the spleen (Fig. 2b).
Figure 2.
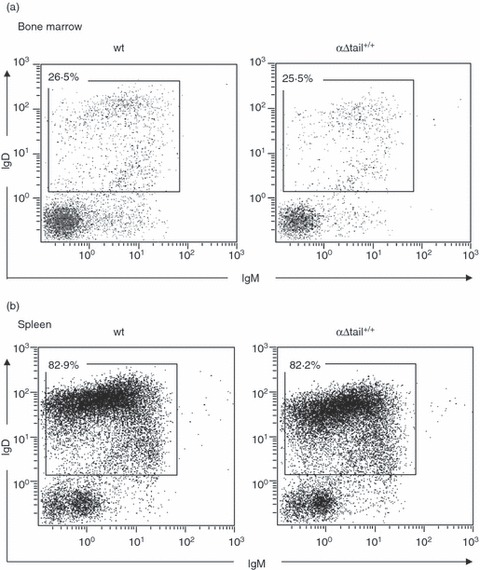
Normal development of B cells in mutant mice. Bone marrow (a) and (b) spleen from 6- to 8-week-old wild-type (wt; left) or αΔtail+/+ mice (right) were removed and cell suspensions were stained for the indicated markers and analysed by flow cytometry. The plots were gated on B220-positive cells and showed the normal IgM/IgD population cells in wt (left) or αΔtail+/+ mice (right). All numbers represent the percentage of cells within the designated gates. Cells were labelled in the presence of rat anti-mouse CD16/CD32 (Mouse Fc Block; BD Biosciences) to block unspecific Fc receptor staining and were first gated on lymphocytes according to forward scatter/side scatter criteria. The plots are representative of several similar experiments.
In vivo IgA switching and IgA secretion in homozygous αΔtail+/+ mice
To assess the effects of targeted deletion at the IgA locus on the expression of IgA in vivo, IgA levels of the αΔtail+/+ mice were measured in the serum and gastrointestinal secretions by ELISA. A low level of serum IgA was detectable in these mice (228·0 ± 33·89, n = 5 for wt, 9·220 ± 4·548, n = 5 for αΔtail+/+) (Fig. 3a, right). In addition, the production of secretory IgA transported into digestive secretions was very low and was maintained at around 1·7 μg/ml in the jejunum fluid (1·7 ± 0·6 μg/ml, n = 5) instead of 1058 ± 163·1 μg/ml in wt mice (n = 5) (Fig. 3b, right). By contrast IgM levels in digestive secretions were significantly higher in homozygous mutant animals than in the wt controls (2·380 ± 0·7415 μg/ml, for αΔtail+/+ mice and 0·6800 ± 0·2024 μg/ml for wt) (Fig. 3b, left). Serum IgG levels were normal in homozygous mutant animals (Fig. 3a).
Figure 3.
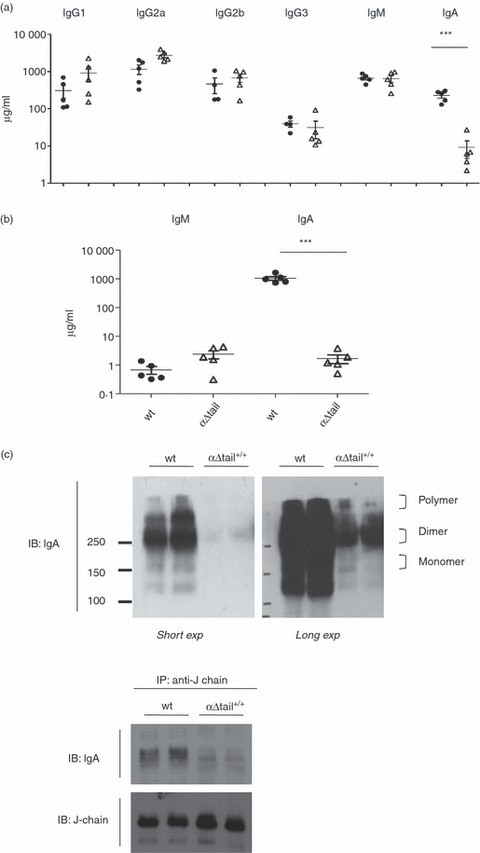
Analysis of mouse immunoglobulin production in sera and secretions. (a) Total endogenous immunoglobulin production was estimated by ELISA in sera from αΔtail+/+ (Δ) compared with wild-type (wt) mice (•). (b) Amount of total IgA and IgM antibodies in digestive secretion of wt (•) and α-Δtail+/+ mice (Δ), as determined by ELISA; n = 5 per group. For all figure, asterisks mark statistically significant differences with controls (Student's t-test, *** for P < 0·001). The vertical axis is logarithmic and values are indicated as μg/ml. (c) (Top) Protein expression level of polymeric, dimeric and monomeric IgA forms in sera was examined by non-reducing SDS–PAGE (two individual sera per group). The protein expressions shown are both a short exposure (short exp) and a long exposure (long exp) of the same blot. The positions of different forms were indicated. (Bottom) Sera from wt and αΔtail+/+ mice were subjected to immunoprecipitation (IP) using anti-mouse J-chain and the precipitates were subjected to immunoblotting (IB) with indicated antibodies (two individual sera per group).
To determine the dimeric and monomeric forms of IgA, immunoglobulins circulating in serum were separated by non-reducing SDS–PAGE. Monomeric IgA demonstrated single bands at a molecular weight of 150 000 whereas dimeric forms in samples showed bands at 360 000 (Fig. 3c, up). To test whether the dimeric IgA assembled correctly with endogenous mouse J-chain, we performed immunoprecipitation of J-chain from serum, followed by immunodetection using an anti-mouse IgA. In mutant mice, IgA was immunoprecipitated with anti J-chain (Fig. 3c, bottom), and indicated that few circulating IgA can dimerize and bind the J-chain.
LPS/TGF-β stimulation and in vitro generation of IgA antibody-secreting cells
We evaluated the amount of IgA-producing cells generated in vitro during a short-term culture independent of both antigen stimulation and BCR signalling. Splenocytes were stimulated with LPS and TGF-β for 4 days. Supernatants were then harvested and analysed for IgA content by isotype-specific ELISA. As we expected, IgA secretion was altered in LPS/TGF-β (33·2 ± 3·9 μg/ml, n = 5, instead of 260·9 ± 83·68 μg/ml, n = 5 for wt) (Fig. 4a). Secretion of IgG2b, IgG3 and IgM was normal, as expected (data not shown). To test class switching in vitro, we used molecular markers for CSR from the μ-chain to the α-chain: α-germline transcripts (Iα-Cα), production of which is a prerequisite for CSR, and Iμ-Cα transcripts that are expressed from the IgH locus after μ-chain to α-chain switching; we quantified those transcripts after 3 days of in vitro stimulation. The results showed that IgA CSR occurred in such conditions (Fig. 4b).
Figure 4.
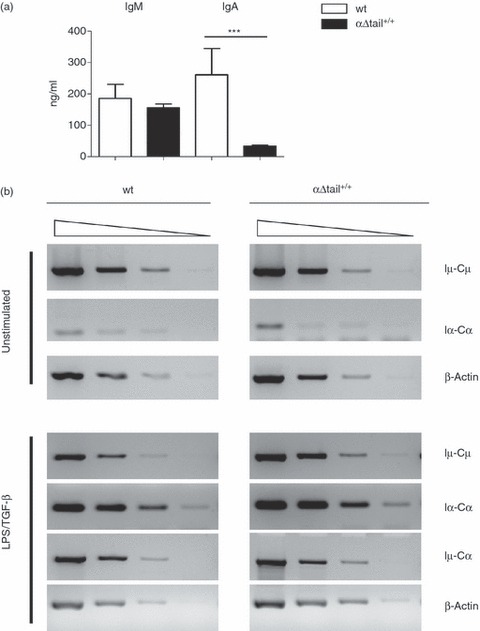
In vitro activation of mutant B cells. (a) Evaluation by ELISA of in vitro IgA secretion after lipopolysaccharide (LPS) plus transforming growth factor- β (TGF-β) stimulation during 4 days of splenocytes from αΔtail+/+ mutant mice compared with wild-type (wt). (b) Semi-quantitative RT-PCR experiments were performed on RNA harvested at day 3 from unstimulated and in vitro-stimulated B cells with LPS plus TGF-β. Serial dilutions of template cDNA are performed at 1 : 1, 1 : 5, 1 : 25, and 1 : 125 for all transcripts. Germline (Iμ-Cμ, Iα-Cα) and class-switched (Iμ-Cα) transcripts were analysed on such serial dilutions. Transcription of the β-actin gene was used as a control.
IgA-secreting cells and global plasma cell amount in mutant animals MALT
Cell cytometry revealed fewer B cells expressing mIgA in Peyer's patches (Fig. 5a,b). We also evaluated IgA plasma cells in lymphoid tissues. Hence, tissues were analysed by immunofluorescence for the presence of intracellular immunoglobulin, showing that fewer IgA-positive plasma cells were present in the lamina propria of mutant animals than in wt mice (Fig. 6). By contrast, the global amount of plasma cells infiltrating the lamina propria along the intestinal crypts did not appear to be affected in mutant mice when MALT tissues were examined by immunofluorescence with anti-κ-chain antibodies (Fig. 6b). No global difference was observed either when tissues were analysed by immunohistochemistry with anti-CD138 and anti-B220 antibodies (Fig. 7), to stain plasma cells and B cells independently of the heavy chain class. In the intestinal mucosae, the ratio of CD138+ cells/total area (7·4 ± 5·3% in wt versus 7·4 ± 5·9% in mutant animals) and the ratio of B220+ cells/total area (3·0 ± 2·3% in wt versus 4·0 ± 1·4% in mutant animals) did not significantly differ between wt and mutant mice, suggesting that plasma cell differentiation might proceed at a similar efficiency in both mutant and wt mice (Fig. 5c).
Figure 5.
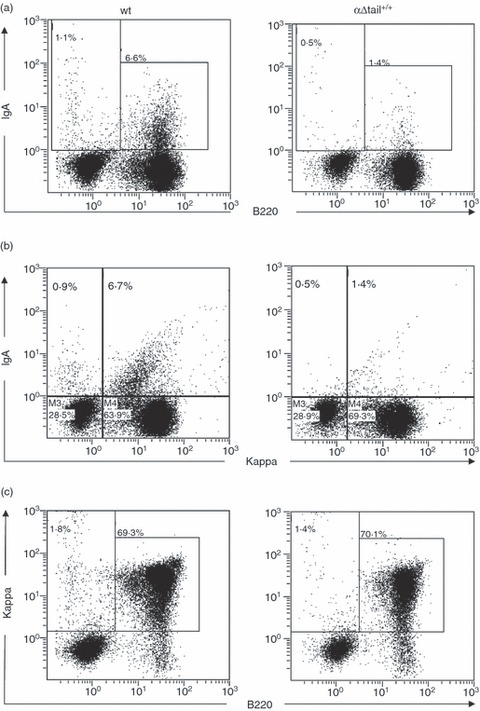
Expression of IgA in primary cells from Peyer's patches. Peyer's patches from αΔtail+/+ and wild-type (wt) mice were collected and cell suspensions were treated in saponin for intracellular staining with indicated surfaces markers and analysed by flow cytometry. Dot plot showed expression of mIgA+ B220+ B cells (a), κ light chain+ mIgA+ cells (b) and κ+ B220+ B cells (c) in Peyer's patches from αΔtail+/+ mice by comparison with wt.
Figure 6.
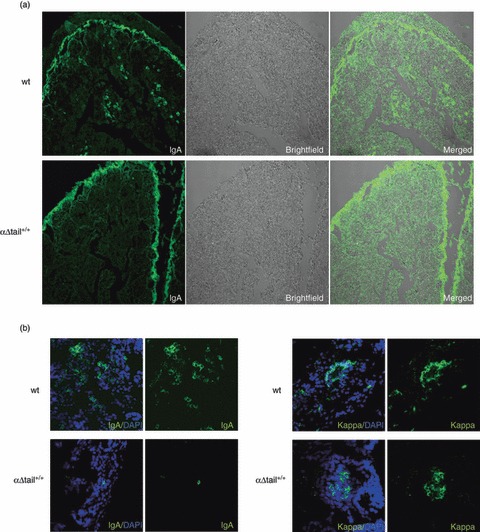
Evaluation of the amount of IgA-secreting B cells (ASC) versus Igκ-secreting cells in mice small intestine. (a) Sections of small intestine villosities stained for IgA (left) or observed by light microscopy (middle) from wild-type (wt) or αΔtail+/+mice show IgA plasma cells only in wt tissues (as usual, the brush border is artefactually labelled with any fluorescent antibody) (original magnification × 10). (b) IgA versus Igκ labelling (Dapi counterstaining in blue) and representative profiles by confocal microscopy in wt and homozygous αΔtail+/+ tissues (original magnification × 63).
Figure 7.
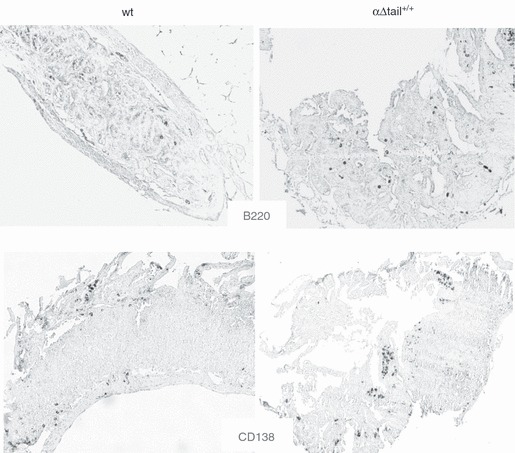
B220 and CD138 expression in α-Δtail+/+ versus wild-type (wt) mice in intestinal mucosae. The anti-mouse B220 (up) and anti-CD138 (down) stained cells in formalin-fixed, paraffin-embedded tissue sections of small intestine and representatives profiles were observed by light microscopy (original magnification × 10).
Discussion
We wished to block the expression of mIgA during B-cell differentiation by deleting the exon that encodes the membrane-anchoring domain of IgA within the Cα immunoglobulin gene. As expected, early B-cell maturation was normal in homozygous mutant animals, with absolute numbers of B cells accumulating in all of the peripheral lymphoid organs of the homozygous mutant mice, including spleen follicles, marginal zone, lymph nodes, Peyer's patches and in the peritoneum B1 compartment. Lack of mIgA expression in peripheral B cells strongly altered but did not abrogate the in vivo production of IgA antibodies, whereas the IgA serum level was cut by about 20-fold. Part of normal serum IgA might therefore come from recently switched and stimulated IgM+ naïve B cells simultaneously undergoing CSR to IgA and plasma cell differentiation, and hence bypassing the need for an IgA class BCR.18,23
Strikingly, the defect appeared much more severe when the IgA level was evaluated in digestive secretions, falling by about 500-fold. This more profound alteration of digestive rather than serum IgA levels indicates that in physiology, IgA production in the gut overwhelmingly relies on mIgA+ memory cells.23,24
Another likely feature of mIgA-driven B-cell differentiation in wt animals is to promote plasma cell differentiation in peripheral organs where mIgA+ cells are abundant, i.e. in the MALT. The propensity of mIgA+ B cells to undergo plasma cell differentiation was recently shown in a model where B cells were forced to prematurely express mIgA instead of mIgM and IgD.22 By contrast, in the mutant homozygous mice described herein, the total amount of plasma cells in the MALT was grossly normal in the small intestine lamina propria, as estimated by tissue sections. Although IgA plasma cells were almost absent, they were replaced by plasma cells producing other immunoglobulin classes. Patients with IgA deficiency often show increased levels of IgM in mucosal secretions, compensating the lack of IgA, and a similar mechanism probably occurs in the IgA-deficient mice. This may lead to forced differentiation of B cells into IgM plasma cells under conditions that would normally favour the generation of IgA plasma cells. Hence, it appears likely that the abundance of plasma cells within the gut-associated lymphoid tissues rather reflects the local concentration of mediators stimulating plasma cell differentiation, instead of being specifically boosted by signalling peculiarities from the IgA-class BCR.
Finally, we show that IgA ASC differentiation in vitro largely, but still partially, relies on the preceding expression of the IgA class BCR on B lymphocytes, because short-term stimulation of αΔtail+/+ mIgM cells by mitogens induced in vitro IgA secretion, albeit with a 10-fold reduced level. This reduction was significant and indicates that contrary to IgG1 and IgE, in vitro Toll-like receptor stimulation of B cells poorly allows a direct differentiation of mIgM+ B lymphocytes into class-switched plasma cells producing IgA.15,16
Therefore, the sum of these observations supports the concept that IgA secretion mostly relies on the differentiation of previously class-switched mIgA+ lymphocytes, especially with regard to the gut production of secretory IgA. Even if such cells are intrinsically prone to plasma cell differentiation, this feature does not account for the strong plasma cell infiltration of MALT, because similar plasma cell amounts are found in the absence of mIgA expression.
Acknowledgments
We thank Shyann Teli for critical reading of the manuscript, Nadine Cogné for blastocyst injection and Angélique Guillaudeau for help with immunofluorescent staining. This work was supported by grants from Ligue Nationale contre le Cancer and Conseil Régional du Limousin. R.A. was supported by a fellowship from Fondation pour la Recherche Médicale.
Disclosure
The authors have no conflict of interest and no disclosures.
References
- 1.Lebman DA, Lee FD, Coffman RL. Mechanism for transforming growth factor β and IL-2 enhancement of IgA expression in lipopolysaccharide-stimulated B cell cultures. J Immunol. 1990;144:952–9. [PubMed] [Google Scholar]
- 2.McIntyre TM, Kehry MR, Snapper CM. Novel in vitro model for high-rate IgA class switching. J Immunol. 1995;154:3156–61. [PubMed] [Google Scholar]
- 3.Harriman GR, Kunimoto DY, Elliott JF, Paetkau V, Strober W. The role of IL-5 in IgA B cell differentiation. J Immunol. 1988;140:3033–9. [PubMed] [Google Scholar]
- 4.Ishioka C, Yoshida A, Kimata H, Mikawa H. Vasoactive intestinal peptide stimulates immunoglobulin production and growth of human B cells. Clin Exp Immunol. 1992;87:504–8. doi: 10.1111/j.1365-2249.1992.tb03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Defrance T, Vanbervliet B, Briere F, Durand I, Rousset F, Banchereau J. Interleukin 10 and transforming growth factor β cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med. 1992;175:671–82. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunimoto DY, Harriman GR, Strober W. Regulation of IgA differentiation in CH12LX B cells by lymphokines. IL-4 induces membrane IgM-positive CH12LX cells to express membrane IgA and IL-5 induces membrane IgA-positive CH12LX cells to secrete IgA. J Immunol. 1988;141:713–20. [PubMed] [Google Scholar]
- 7.Brandtzaeg P. Do salivary antibodies reliably reflect both mucosal and systemic immunity? Ann N Y Acad Sci. 2007;1098:288–311. doi: 10.1196/annals.1384.012. [DOI] [PubMed] [Google Scholar]
- 8.Kerr MA. The structure and function of human IgA. Biochem J. 1990;271:285–96. doi: 10.1042/bj2710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flanagan JG, Lefranc MP, Rabbitts TH. Mechanisms of divergence and convergence of the human immunoglobulin α1 and α2 constant region gene sequences. Cell. 1984;36:681–8. doi: 10.1016/0092-8674(84)90348-9. [DOI] [PubMed] [Google Scholar]
- 10.Word CJ, Mushinski JF, Tucker PW. The murine immunoglobulin α gene expresses multiple transcripts from a unique membrane exon. EMBO J. 1983;2:887–98. doi: 10.1002/j.1460-2075.1983.tb01518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cogne M, Preud'homme JL. Gene deletions force nonsecretory α-chain disease plasma cells to produce membrane-form α-chain only. J Immunol. 1990;145:2455–8. [PubMed] [Google Scholar]
- 12.Neuberger MS, Patel KJ, Dariavach P, Nelms K, Peaker CJ, Williams GT. The mouse B-cell antigen receptor: definition and assembly of the core receptor of the five immunoglobulin isotypes. Immunol Rev. 1993;132:147–61. doi: 10.1111/j.1600-065x.1993.tb00841.x. [DOI] [PubMed] [Google Scholar]
- 13.Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu Rev Immunol. 1997;15:453–79. doi: 10.1146/annurev.immunol.15.1.453. [DOI] [PubMed] [Google Scholar]
- 14.Venkitaraman AR, Williams GT, Dariavach P, Neuberger MS. The B-cell antigen receptor of the five immunoglobulin classes. Nature. 1991;352:777–81. doi: 10.1038/352777a0. [DOI] [PubMed] [Google Scholar]
- 15.Achatz G, Nitschke L, Lamers MC. Effect of transmembrane and cytoplasmic domains of IgE on the IgE response. Science. 1997;276:409–11. doi: 10.1126/science.276.5311.409. [DOI] [PubMed] [Google Scholar]
- 16.Kaisho T, Schwenk F, Rajewsky K. The roles of gamma 1 heavy chain membrane expression and cytoplasmic tail in IgG1 responses. Science. 1997;276:412–5. doi: 10.1126/science.276.5311.412. [DOI] [PubMed] [Google Scholar]
- 17.Hasan M, Polic B, Bralic M, Jonjic S, Rajewsky K. Incomplete block of B cell development and immunoglobulin production in mice carrying the muMT mutation on the BALB/c background. Eur J Immunol. 2002;32:3463–71. doi: 10.1002/1521-4141(200212)32:12<3463::AID-IMMU3463>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 18.Macpherson AJ, Lamarre A, McCoy K, Harriman GR, Odermatt B, Dougan G, Hengartner H, Zinkernagel RM. IgA production without mu or delta chain expression in developing B cells. Nat Immunol. 2001;2:625–31. doi: 10.1038/89775. [DOI] [PubMed] [Google Scholar]
- 19.Orinska Z, Osiak A, Lohler J, Bulanova E, Budagian V, Horak I, Bulfone-Paus S. Novel B cell population producing functional IgG in the absence of membrane IgM expression. Eur J Immunol. 2002;32:3472–80. doi: 10.1002/1521-4141(200212)32:12<3472::AID-IMMU3472>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 20.Leduc I, Drouet M, Bodinier MC, Helal A, Cogne M. Membrane isoforms of human immunoglobulins of the A1 and A2 isotypes: structural and functional study. Immunology. 1997;90:330–6. doi: 10.1111/j.1365-2567.1997.00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandtzaeg P, Farstad IN, Johansen FE, Morton HC, Norderhaug IN, Yamanaka T. The B-cell system of human mucosae and exocrine glands. Immunol Rev. 1999;171:45–87. doi: 10.1111/j.1600-065X.1999.tb01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duchez S, Amin R, Cogne N, Delpy L, Sirac C, Pascal V, Corthesy B, Cogne M. Premature replacement of mu with α immunoglobulin chains impairs lymphopoiesis and mucosal homing but promotes plasma cell maturation. Proc Natl Acad Sci USA. 2010;107:3064–9. doi: 10.1073/pnas.0912393107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki K, Meek B, Doi Y, Honjo T, Fagarasan S. Two distinctive pathways for recruitment of naive and primed IgM+ B cells to the gut lamina propria. Proc Natl Acad Sci USA. 2005;102:2482–6. doi: 10.1073/pnas.0409539102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413:639–43. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]


