Abstract
Wild-type (WT) and CD1d−/− [without natural killer (NK) T cells] mice were treated with zymosan A to induce granuloma formation in the liver. Increased granuloma formation was seen in NKT-less mice on days 7 and 14 after administration. WT mice showed limited granuloma formation, and zymosan A eventually induced NKT cell accumulation as identified by their surface marker (e.g. CD1d-tetramer). Zymosan A augmented the expression of Toll-like receptor 2 on the cell surface of both macrophages and NKT cells. One possible reason for accelerated granuloma formation in NKT-less mice was increased production of interferon- γ (IFN-γ); a theory that was confirmed using IFN-γ−/− mice. Also, zymosan A increased interleukin-10 production in WT mice, which suppresses IFN-γ production. Taken together, these results suggest that NKT cells in the liver have the potential to suppress zymosan A-mediated granuloma formation.
Keywords: granuloma, interferon-γ, liver, natural killer T cells, zymosan A
Introduction
The liver is a unique immune organ, in terms of both the unconventional leucocyte populations that reside there and the immunosuppressive responses often induced. This is related to the fact that the liver has to process many foreign antigens transported via the portal vein. Another possible reason may be that the liver originates phylogenetically from the intestine, which is central to mucosal immunity. For example, the liver contains many unique leucocytes, including Kupffer cells, dendritic cells, natural killer T (NKT) cells,1–5 and intermediate T-cell receptor cells of extrathymic origin.6,7 These leucocyte populations seem to mediate liver-specific immune responses. Of these, hepatic dendritic cells are unique in terms of morphology and function because they are immunosuppressive (e.g. they are associated with immune tolerance in the liver).8–13
The NKT cell population is also unique, being most abundant in the liver, and recognizing some glycolipids in conjunction with the MHC class I-like molecule, CD1d.1–5 It is conceivable that NKT cells in the liver mediate some unknown function to maintain liver-specific immune responses. In recent studies, we noticed that CD1d−/− (NKT-less) mice are sensitive to zymosan A-induced granuloma formation in the liver. Therefore, in the present study, we investigated the possibility that NKT cells play a major role in suppressing granuloma formation in the liver.
Granulomatous diseases occur at various sites within the body; however, granuloma formation is very common in the liver. Because it is likely that many microbial antigens reach the liver via the portal vein,14–17 we speculated that NKT cells within the liver play a key role in regulating the immune responses in this organ.
Materials and methods
Mice
C57BL/6 [B6, wild-type (WT)] and CD1d−/−18 interferon-γ-deficient (IFN-γ−/−) mice19 were used at the age of 8–12 weeks. All these mice had a B6 background. The CD1d−/− mice lacked the generation of invariant NKT cells and almost all NK1.1+ CD3int cells (NKT cells including invariant NKT cells) are absent in these mice. We therefore refer to these CD1d−/− mice as NKT-less mice in the text. These mice were maintained at the animal facility of Niigata University (Niigata, Japan) under specific pathogen-free conditions.
Granuloma formation
Zymosan A (mainly β-1,3-glucan), which reacts with toll-like receptor 2 (TLR2)17 and dectin-1,20,21 was used to induce granulomas in the liver. Zymosan A (Sigma, St Louis, MO) was suspended in PBS, placed in a boiling water bath for 1 hr, then centrifuged for 30 min at 1300 g; the residue was resuspended in PBS. Zymosan A was injected intravenously at a dose of 1 mg/mouse in 200 μl PBS.
Histology of organs
Tissues for histology were fixed in 10% phosphate-buffered formalin and embedded in paraffin. Sections, 4 μm in thickness, were stained with haematoxylin & eosin.
Cell preparation
Hepatic mononuclear cells and splenic lymphocytes were isolated by a previously described method.6 Briefly, the liver (or the spleen) was removed, pressed through 200-gauge stainless steel mesh, and suspended in Eagle's minimal essential medium (Nissui Pharmaceutical, Tokyo, Japan) supplemented with 5 mm HEPES and 2% heat-inactivated newborn calf serum. After being washed once with medium, the cells were resuspended in 15 ml of 35% Percoll solution (Amersham Pharmacia Biotech, Piscataway, NJ) containing 100 U/ml heparin and centrifuged at 300 g for 15 min. The pellet was resuspended in erythrocyte lysing solution (155 mm NH4Cl, 10 mm KHCO3, 1 mm Na-EDTA, and 17 mm Tris–HCl; pH 7·3). Concanavalin A blasts and lipopolysaccharide blasts were prepared by using splenic lymphocytes as previously described.7
Immunofluorescence tests by a cell analyser
The surface phenotype was identified by using monoclonal antibodies (mAbs) in conjunction with two-colour or three-colour immunofluorescence tests.7 The mAbs used here included FITC-, phycoerythrin- (PE) or biotin-conjugated reagents of anti-CD3 (145-2C11), anti-CD4 (RM4-5), anti-CD8 (53-6.7), anti-Mac-1 (M1/70), anti-Gr-1 (RB6-8C5), anti-IFN-γ (XMG1.2, rat IgG1), isotype control (R3-34, rat IgG1) mAbs (BD Biosciences, San Diego, CA); anti-TLR2 (6C2) mAbs (eBioscience, San Diego, CA); and CD1d tetramer (ProImmune Ltd., Oxford, UK). Biotin-conjugated reagents were developed with Tri-Color-conjugated streptavidin (Caltag Laboratory, San Francisco, CA). To prevent non-specific binding of mAbs, CD32/16 (BD Biosciences) was added before staining with labelled mAbs. For the intracellular staining, Cytofix/CytoPerm Kit (BD Biosciences) was used. The fluorescence-positive cells were analysed by flow cytometry (FACScan; BD Biosciences). Dead cells were excluded by forward scatter, side scatter and propidium iodide gating.
Lymphocyte culture
Both WT and NKT-less mouse liver cells (1 × 106/ml) were cultured in complete RPMI-1640 medium containing 10% fetal calf serum in the presence of 100 μg/ml zymosan A in a 96-well microculture plate for 1, 3 and 5 days at 37°.
ELISA for the detention of IFN-γ, TNF-α and IL-10
Pooled sera and cultured supernatant fluid were used for measurement of the concentrations of IFN-γ, tumour necrosis factor-α (TNF-α) and interleukin-10 (IL-10) by ELISA using OptEIA mouse IFN-γ and IL-10 sets (BD Biosciences) and mouse TNF-α ELISA Ready-SET-Go! (eBioscience).
Reverse transcription-PCR and real-time PCR analysis
Total RNA was extracted from cells of WT and NKT-less mice. To detect mRNAs of cytokines, RNA was reverse transcribed using the primers of these genes and such cDNA was further amplified by PCR methods. Briefly, total RNA was prepared from cells with Isogen (Nippon Gene, Tokyo, Japan). The cDNA was synthesized using 1 μg RNA with a SuperScript β First-Stand Synthesis System for reverse transcription-PCR (RT-PCR) (Invitrogen, Carlsbad, CA) and oligo-dT 15 Primer (Promega, Madison, WI). The PCR amplification of synthesized cDNA was then conducted. Forward primers for IL-4, IL-10, IL-12p40, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were paired with a reverse primer for a constant region sequence that is shared by all T-cell receptor c clusters. The PCR was carried out with an initial denaturation for 10 min at 95°, followed by 40 cycles of 1 min at 95°, 1 min at 57°, and 1 min at 72°. Their sequences are as follows: IL-4 sense, 5′-CCAGCTAGTTGTCATCCTGC-3′; IL-4 antisense, 5′-GTGATGTGGACTTGGACTCA-3′; IL-10 sense, 5′-GGACAACATACTGCTAACCGG-3′; IL-10 antisense, 5′-ATATTTCGGAGAGAGGTACA-3′; IL-12p40 sense, 5′-CGTGCTCATGGCTGGTGCAAAG-3′; IL-12p40 antisense, 5′-CTTCATCTGCAAGTTCTTGGGC-3′; GAPDH sense, 5′-ACCACAGTCCATGAAATCAC-3′; GAPDH antisense, 5′-TCCACCACCCTGTTGCTGTA-3′. PCR products were visualized on 2% agarose gel stained with ethidium bromide under UV illumination.
To quantify the amount of IL-10 RNA, a real-time PCR, based on SYBR green fluorescence, was performed using SYBR Premix Ex Taq polymerase and Takara real-time Thermal Cycler Dice (Takara, Shiga, Japan). The following primers were used to specifically amplify respective genes: IL-10 sense, 5′-GCCAGAGCCACATGCTCCTA-3′; IL-10 antisense, 5′-GATAAGGCTTGGCAACCCAAGTAA-3′; GAPDH gene used as a control, GAPDH sense, 5′-TGTGTCCGTCGTGGAT CTGA-3′; GAPDH antisense, 5′-TTGCTGTTGAAGTCGCAGGAG-3′.
Statistical analysis
Significance of differences was determined by unpaired t-test or two-way analysis of variance. A value of P < 0·05 was considered to be significant.
Results
Predominant formation of granulomas in CD1d−/− mice
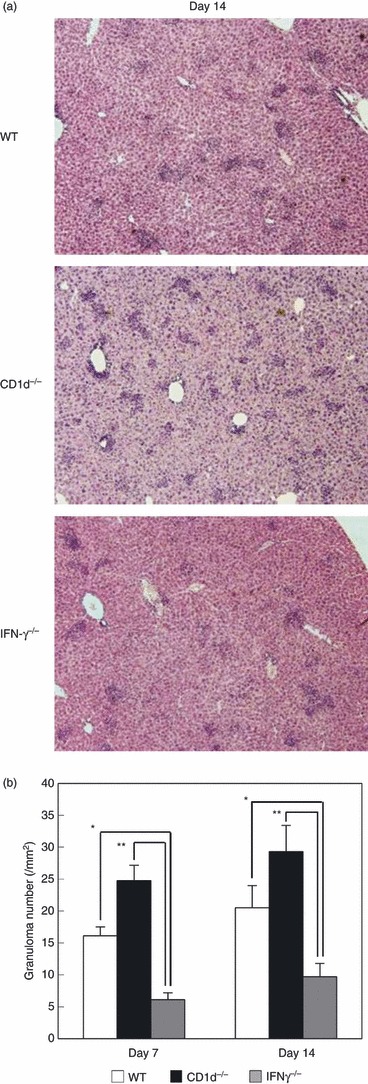
Mononuclear cells were isolated from the livers of WT and NKT-less mice and the number of mononuclear cells was counted (Fig. 1a). After the administration of zymosan A, the number of mononuclear cells increased up until day 7 before decreasing again. The number of cells was comparable in both mouse strains, but was slightly higher in NKT-less mice. During the same time period, granulomas formed in both mouse strains. The number of granulomas observed in liver sections was compared on days 7 and 14 (Fig. 1b) and the results showed a significantly higher number in NKT-less mice than in WT mice (P < 0·01). Representative images are shown in Fig. 1(c). On day 14 after zymosan A administration, many granulomas were seen in the livers of NKT-less mice along with some areas of necrosis. The granulomas disappeared spontaneously by day 28 in both strains of mice.
Figure 1.
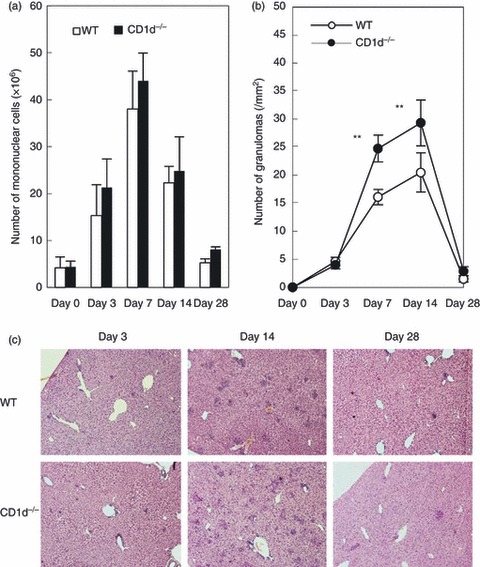
Granuloma formation in the livers of wild-type (WT) and CD1d−/− [natural killer T (NKT) cell-less] mice. (a) The number of mononuclear cells in the liver. (b) The number of granulomas in the liver. (c) Histology. WT and NKT-less mice were treated with zymosan A and various parameters were examined at the indicated time-points. Data represent the mean ± SD from four mice. **P < 0·01.
Temporal kinetics regarding the number of NKT cells and macrophages in the liver of mice treated with zymosan A
We next examined how NKT cells were regulated in the liver of WT mice after zymosan A administration (Fig. 2a). NKT cells were identified by their expression of CD1d-tetramer and CD3int. This cell population was completely absent from NKT-less mice (data not shown). On days 7 and 14, the proportion of NKT cells decreased markedly; however, the absolute number remained relatively constant (Fig. 2b). The reason for this was an increase in the total number of mononuclear cells within the liver.
Figure 2.
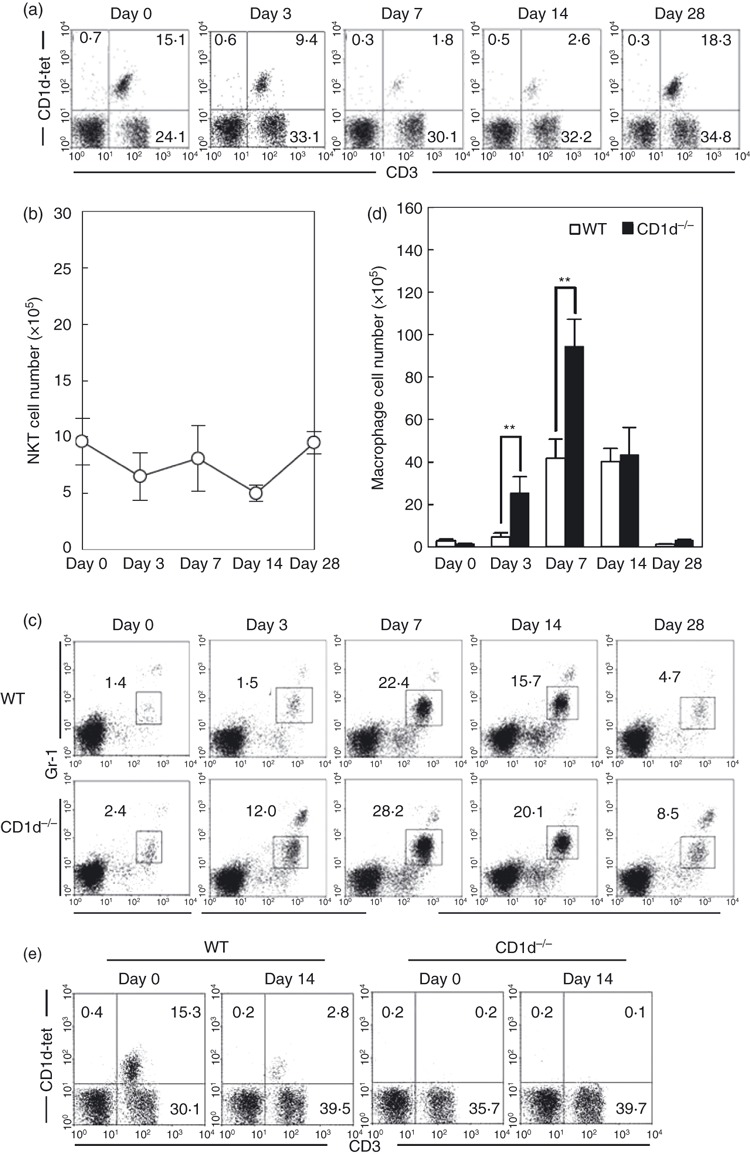
Identification of natural killer T (NKT) cells and macrophages. (a) Two-colour staining for CD3 and CD1d-tetramer. (b) Number of NKT cells. (c) Two-colour staining for Mac-1 and Gr-1. (d) Number of macrophages. (e) Identification of NKT cells in CD1d−/− mice. Mononuclear cells were isolated from the livers of wild-type (WT) and NKT-less mice. Immunofluorescence studies were done to identify NKT cells (CD3int CD1d-tetramer+) and macrophages (Gr-1int Mac-1+). The data represent the mean ± SD from four mice. **P < 0·01.
Next, we determined the proportion of macrophages (Gr-1int Mac-1+) in the livers of both strains of mice (Fig. 2c). Most of this population was F4/80 positive (data not shown). On days 7 and 14, the proportion of macrophages increased markedly in both strains; however, this increase was observed earlier (day 3), and to a greater extent, in NKT-less mice. The absolute number of macrophages was then calculated (Fig. 2d), and was found to be significantly higher in NKT-less mice than in WT mice, especially on days 3 and 7. In a final portion of this experiment (Fig. 2e), it was confirmed that NKT cells were rarely found in CD1d−/− mice irrespective of zymosan A treatment.
Augmented expression of TLR2 by NKT cells and macrophages
The expression of TLR2 (a receptor for β-1,3-glucan) by mononuclear cells in the livers of zymosan A-treated WT mice was examined using three-colour staining for NK1.1, CD3 and TLR2, or for Gr-1, Mac-1 and TLR2 (Fig. 3). The expression of TLR2 on NKT cells (NK1.1+ CD3int), macrophages (Gr-1int Mac-1+) and T cells (NK1.1− CD3high) was determined using gated analysis. On day 0, TLR2 was expressed at very low levels by NKT cells, but was positive in 59% of macrophages. After the administration of zymosan A TLR2 was expressed by 10% of NKT cells after 2 hr and on day 7. The majority of macrophages also showed increased levels of TLR2 expression after 2 hr and on day 7.
Figure 3.
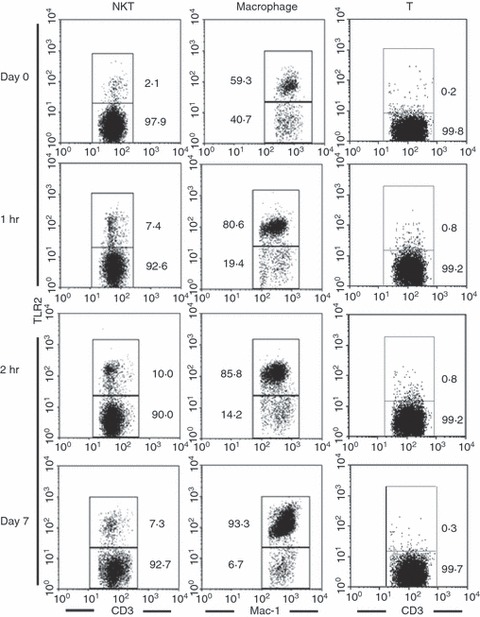
Expression of Toll-like receptor 2 (TLR2) on natural killer T (NKT) cells and macrophages. Wild-type (WT) mice were treated with zymosan A and mononuclear cells were isolated from the liver on the indicated days. Three-colour staining for TLR2, NK1.1 (or Gr-1) and CD3 (or Mac-1) was then performed. To show the expression of TLR2, the gated analysis was conducted for NKT cells (NK1.1+ CD3int), macrophages (Gr-1int Mac-1+), and T cells (NK1.1− CD3high). Representative results from three independent experiments are shown. The numbers in the figure represent the percentage of fluorescence-positive cells.
CD1d−/− mice treated with zymosan A show increased IFN-γ production
The results of our experiments revealed that NKT-less mice showed increased granuloma formation, and that these granulomas contained a higher number of macrophages after treatment with zymosan A. As macrophages are activated by cytokines such as IFN-γ and TNF-α, the levels of these cytokines were examined both in sera and in in vitro culture (Fig. 4). High levels of IFN-γ were detected in the sera of NKT-less mice treated with zymosan A (Fig. 4a), reaching a peak by day 14. In contrast, the level of TNF-α was similar between WT mice and NKT-less mice.
Figure 4.
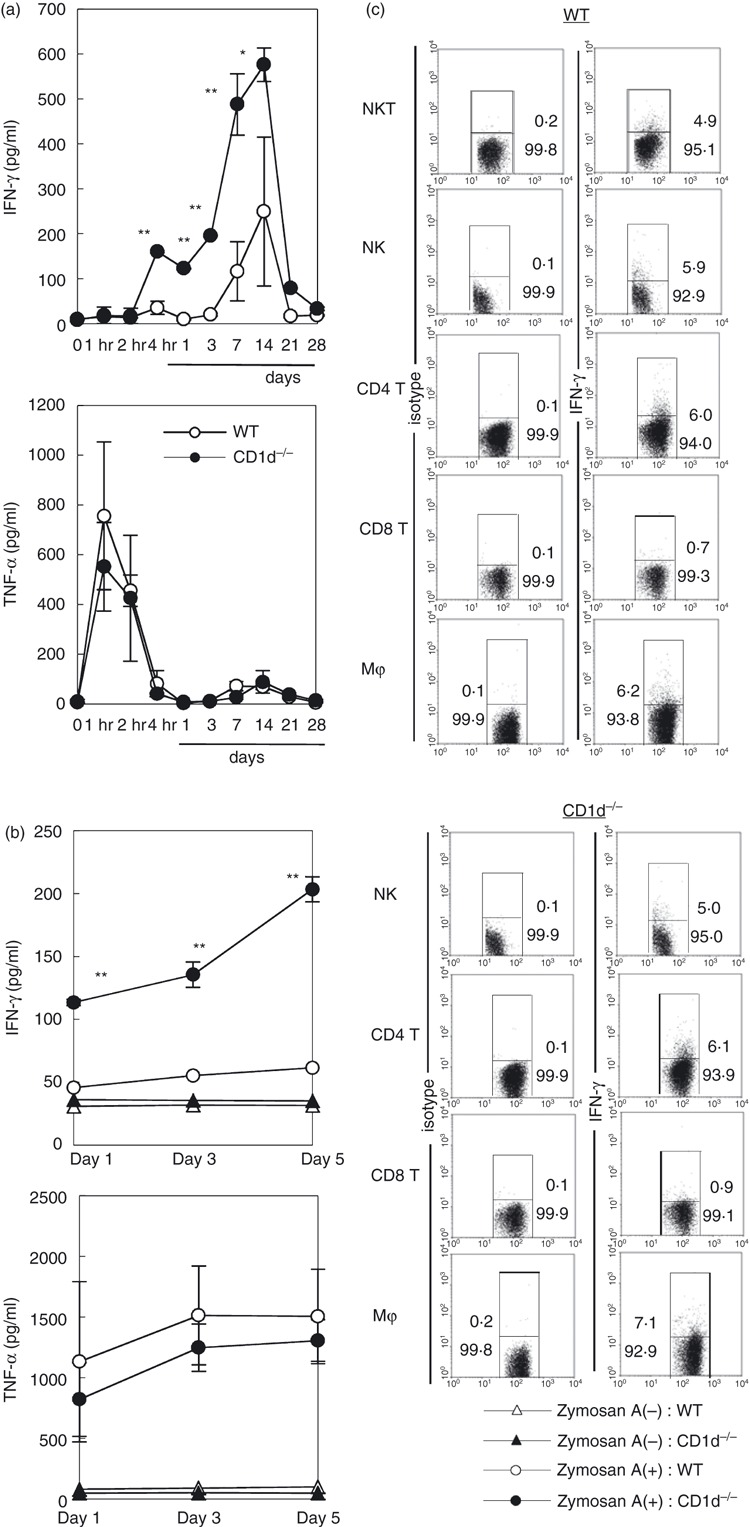
Interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) production in wild-type (WT) and CD1d−/− mice. (a) IFN-γ and TNF-α levels in the sera. (b) IFN-γ and TNF-α levels in the culture supernatants. (c) Cytoplasmic detection of IFN-γ and TNF-α in different leucocyte populations. Cytoplasmic cytokines were detected using immunofluorescence. Data represent the mean ± SD from four experiments.
Next, liver mononuclear cells were isolated from both WT mice and NKT-less mice and cultured with or without zymosan A (Fig. 4b). The levels of IFN-γ and TNF-α in the culture supernatants were then measured on the indicated days. Higher titres of IFN-γ were detected in NKT-less mice, whereas the levels of TNF-α were similar in both strains.
Next, the leucocyte subsets responsible for IFN-γ production in zymosan A-treated NKT-less mice were analysed (Fig. 4c). Liver mononuclear cells were isolated on day 14 and three-colour staining was performed (the third colour stained cytoplasmic IFN-γ). All the tested cell populations (except CD8+ T cells) contained IFN-γ-producing cells (between 4·9 and 7·1%). However, the difference between WT mice and NKT-less mice was insignificant.
IFN-γ−/− mice show decreased granuloma formation
It was hypothesized that IFN-γ was intimately involved in granuloma formation after zymosan A administration. To confirm this, tissue sections from IFN-γ−/− mice were prepared on day 14 and the numbers of granulomas in WT, NKT-less and IFN-γ−/− mice were compared (Fig. 5a). The results showed that granuloma formation was most pronounced in NKT-less mice, and least in IFN-γ−/− mice. The actual number of granulomas was compared on days 7 and 14 (Fig. 5b), and a large decrease in the number of granulomas was observed in IFN-γ−/− mice at both time points.
Figure 5.
Association of interferon-γ (IFN-γ) with granuloma formation. (a) Liver histology. (b) Number of granulomas. Wild-type (WT), CD1d−/− and IFN-γ−/− mice were treated with zymosan A. On the indicated days, hepatic tissue was isolated. The data represent the mean ± SD of the number of granulomas in four mice. *P < 0·05 and **P < 0·01.
WT mice show high levels of IL-10 after zymosan A treatment
Why did the deficiency of NKT cells result in increased production of IFN-γ and subsequent activation of macrophages? The answer to this question may involve IL-10, which is known to suppress IFN-γ production. Hence, IL-10 levels were measured in WT and NKT-less mice treated with zymosan A (Fig. 6a). High levels of IL-10 were detected in the sera of WT mice as early as 1 to 2 hr after zymosan A administration. However, only minimal levels were detected in NKT-less mice. Interleukin-10 was not detectable in the sera of either mouse strain between day 1 and day 28.
Figure 6.
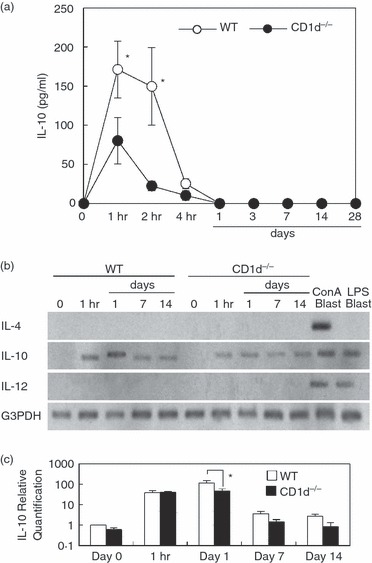
Increased interleukin-10 (IL-10) production in wild-type (WT) mice. (a) IL-10 levels in sera. (b) IL-10 mRNA expression. (c) Real-time PCR. RNA was isolated from concanavalin A (Con A) blasts and lipopolysaccharide (LPS) blasts were used as a positive control. Data represent the mean ± SD IL-10 concentration in the sera of four mice. For experiment (b), representative results are shown. *P < 0·05.
Increased IL-10 production was confirmed using RT-PCR (Fig. 6b). The signal for IL-10 mRNA increased between 1 hr and 1 day after zymosan A administration in WT mice, but not in NKT-less mice. Both IL-4 mRNA and IL-12 mRNA were examined as controls. The signal for IL-4 mRNA was only detected in concanavalin A blasts, whereas that for IL-12 mRNA was detected in both concanavalin A blasts and lipopolysaccharide blasts. Real-time PCR was also conducted (Fig. 6c). The data confirmed the result from Fig. 6(b).
Discussion
In the present study, we showed that CD1d−/− mice had an unusually high number of granulomas in the liver after zymosan A treatment. This animal model of zymosan A-induced granuloma formation is linked to activation of macrophages in WT mice.22–26 At this time, TLR217 and dectin-120, 21 might be associated with such activation. These are known to be binding sites for many bacterial products, including zymosan A. However, we did not analysed which molecules of TLR2 or dectin-1 played a major role for zymosan A-induced granuloma formation in this protocol. Compared with WT mice, NKT-less mice treated with zymosan A showed an increase in the macrophage population in the liver (both in terms of number and proportion). As NKT cells in WT mice treated with zymosan A eventually down-regulated their expression of T-cell receptors on the cell surface, it is conceivable that activated NKT cells modulated the magnitude of zymosan A-mediated granuloma formation in the liver.
We first assessed the production of IFN-γ by mononuclear cells in the liver. This is because IFN-γ is the most important cytokine involved in macrophage activation27–31 and macrophages are the main leucocyte population found in granulomas.32–34 The results showed that unusually large amounts of IFN-γ (but not TNF-α) were produced by liver mononuclear cells in NKT-less mice treated with zymosan A. This was true in both the in vivo and in vitro experiments. We next examined the production of IL-10 – an immunosuppressive cytokine that is often produced by T cells or activated NKT cells.35–39 The results showed that WT mice produced large amounts of IL-10, especially within 1–2 hr of zymosan A administration. This suggests that NKT cells play an important role in suppressing zymosan A-mediated granuloma formation in the liver by modulating the production of IFN-γ and IL-10.
There was also evidence that NKT cells were directly activated by zymosan A. In addition to down-regulated expression of the CD1-tetramer by NKT cells, the results showed elevated expression of TLR2 (from 2·1% to 10·0% of cells by 2 hr after zymosan A administration). In the case of macrophages, a large proportion (59·3%) of resting macrophages was TLR2+, and this was increased by treatment with zymosan A.
To determine the source of IFN-γ, the cytoplasmic expression of IFN-γ was examined in various leucocyte populations in both WT and NKT-less mice. The results showed that NKT cells, NK cells, CD4+ T cells and macrophages produced IFN-γ. Production of IFN-γ by CD8+ T cells was minimal. The importance of IFN-γ in granuloma formation was also examined in IFN-γ−/− mice. Few granulomas were seen in IFN-γ−/− mice treated with zymosan A.
One of the functions of NKT cells was cytotoxicity against autologous thymocytes and hepatocytes. Cytotoxicity against thymocytes did not require specific activation,40,41 but cytotoxicity against hepatocytes required activation with α-GalCer.42–44 However, other immunosuppressive functions of NKT cells were also reported.45–48 For example, NKT cells suppress autoimmune uveitis by inhibiting T helper type 17 differentiation,49 and play a protective role in acute and chronic arthritis by ameliorating antigen-specific T helper type 1 responses.50
Granuloma formation in the liver and other organs is important for sealing microbes within the tissue and preventing their dissemination into the systemic circulation. It is thought that TLR2-mediated or other TLR-mediated granuloma formation is one such response.16,17 However, excessive granuloma formation is sometimes dangerous and can lead to tissue damage (e.g. hepatic failure). In this regard, positive and negative regulation of granuloma formation in the liver might be critical for the protection of the whole body. The present results suggest that NKT cells play an important role in regulating such responses. The key factor modulating macrophage activation appears to be IFN-γ, with IL-10 acting to provide negative feedback.
Acknowledgments
This work was supported by the grants-in-aid for scientific research from the Ministry of Education, Science and Culture, Japan and Tsukada Medical Foundation. The authors thank Mrs Yuko Kaneko for preparation of the manuscript.
Glossary
- NKT
natural killer T
- TLR2
toll-like receptor 2
- WT
wild-type
Disclosures
None of the authors have any conflict of interest with any of the content of this manuscript.
References
- 1.Bendelac A. Mouse NK1+ T cells. Curr Opin Immunol. 1995;7:367–74. doi: 10.1016/0952-7915(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald HR. NK1.1+ T cells receptor-α/β+ cells: new clues to their origin, specificity, and function. J Exp Med. 1995;182:633–8. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bix M, Locksley RM. Natural T cells: cells that co-express NKRP-1 and TCR. J Immunol. 1995;155:1020–2. [PubMed] [Google Scholar]
- 4.Vicari AP, Zlotnik A. Mouse NK1.1+ T cells: a new family of T cells. Immunol Today. 1996;17:71–6. doi: 10.1016/0167-5699(96)80582-2. [DOI] [PubMed] [Google Scholar]
- 5.Abo T, Kawamura T, Watanabe H. Physiological responses of extrathymic T cells in the liver. Immunol Rev. 2000;174:135–49. doi: 10.1034/j.1600-0528.2002.017415.x. [DOI] [PubMed] [Google Scholar]
- 6.Sato K, Ohtsuka K, Hasegawa K, Yamagiwa S, Watanabe H, Asakura H, Abo T. Evidence for extrathymic generation of intermediate T cell receptor cells in the liver revealed in thymectomized, irradiated mice subjected to bone marrow transplantation. J Exp Med. 1995;182:759–67. doi: 10.1084/jem.182.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe H, Miyaji C, Kawachi Y, Iiai T, Ohtsuka K, Iwanaga T, Takahashi-Iwanaga H, Abo T. Relationships between intermediate TCR cells and NK1.1+ T cells in various immune organs. NK1.1+ T cells are present within a population of intermediate TCR cells. J Immunol. 1995;155:2972–83. [PubMed] [Google Scholar]
- 8.Jomantaitė I, Dikopoulos N, Kröger A, Leithäuser F, Hauser H, Schirmbeck R, Reimann J. Hepatic dendritic cell subsets in the mouse. Eur J Immunol. 2004;34:355–65. doi: 10.1002/eji.200324336. [DOI] [PubMed] [Google Scholar]
- 9.Lau AH, Thomson AW. Dendritic cells and immune regulation in the liver. Gut. 2003;52:307–14. doi: 10.1136/gut.52.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kingham TP, Chaudhry UI, Plitas G, Katz SC, Raab J, DeMatteo RP. Murine liver plasmacytoid dendritic cells become potent immunostimulatory cells after Flt-3 ligand expansion. Hepatology. 2007;45:445–54. doi: 10.1002/hep.21457. [DOI] [PubMed] [Google Scholar]
- 11.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–17. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 12.Castellaneta A, Sumpter TL, Chen L, Tokita D, Thomson AW. NOD2 ligation subverts IFN-α production by liver plasmacytoid dendritic cells and inhibits their T cell allostimulatory activity via B7-H1 up-regulation. J Immunol. 2009;183:6922–32. doi: 10.4049/jimmunol.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connolly MK, Bedrosian AS, Mallen-St Clair J, et al. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-α. J Clin Invest. 2009;119:3213–25. doi: 10.1172/JCI37581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowdish DM, Sakamoto K, Kim MJ, et al. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000474. e1000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivas-Santiago B, Hernandez-Pando R, Carranza C, Juarez E, Contreras JL, Aguilar-Leon D, Torres M, Sada E. Expression of cathelicidin LL-37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect Immun. 2008;76:935–41. doi: 10.1128/IAI.01218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenhalls G, Squires GR, Stevens-Muller L, Bezuidenhout J, Amphlett G, Duncan K, Lukey PT. Associations between toll-like receptors and interleukin-4 in the lungs of patients with tuberculosis. Am J Respir Cell Mol Biol. 2003;29:28–38. doi: 10.1165/rcmb.2002-0163OC. [DOI] [PubMed] [Google Scholar]
- 17.Reiling N, Hölscher C, Fehrenbach A, Kröger S, Kirschning CJ, Goyert S, Ehlers S. Cutting edge: toll-like receptor (TLR) 2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J Immunol. 2003;169:3480–4. doi: 10.4049/jimmunol.169.7.3480. [DOI] [PubMed] [Google Scholar]
- 18.Mendiratta SK, Martin WD, Hong S, Boesteanu A, Joyce S, van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–77. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 19.Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–42. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 20.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and toll-like receptor 2. J Exp Med. 2003;197:1107–17. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dillon S, Agrawal S, Banerjee K, et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest. 2006;116:916–28. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moriyama H, Yamamoto T, Takatsuka H, Umezu H, Tokunaga K, Nagano T, Arakawa M, Naito M. Expression of macrophage colony-stimulating factor and its receptor in hepatic granulomas of Kupffer-cell-depleted mice. Am J Pathol. 1997;150:2047–60. [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka K, Morimoto J, Kon S, et al. Effect of osteopontin alleles on β-glucan-induced granuloma formation on the mouse liver. Am J Pathol. 2004;164:567–75. doi: 10.1016/s0002-9440(10)63146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikeda Y, Adachi Y, Ishii T, Miura N, Tamura H, Ohno N. Dissociation of toll-like receptor 2-mediated innate immune response to zymosan by organic solvent-treatment without loss of dectin-1 reactivity. Biol Pharm Bull. 2008;31:13–8. doi: 10.1248/bpb.31.13. [DOI] [PubMed] [Google Scholar]
- 25.Kindler V, Sappini AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–40. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 26.Senaldi G, Yin S, Shaklee CL, Piguet PF, Mak TW, Ulich TR. Corynebacterium parvum- and Mycobacterium bovis bacillus Calmette–Guérin-induced granuloma formation is inhibited in TNF receptor I (TNF-RI) knockout mice and by treatment with soluble TNF-RI. J Immunol. 1996;157:5022–6. [PubMed] [Google Scholar]
- 27.Moreira AL, Tsenova L, Murray PJ, Freeman S, Bergtold A, Chiriboga L, Kaplan G. Aerosol infection of mice with recombinant BCG secreting murine IFN-γ partially reconstitutes local protective immunity. Microb Pathog. 2000;29:175–85. doi: 10.1006/mpat.2000.0382. [DOI] [PubMed] [Google Scholar]
- 28.Boros DL, Whitfield JR. Endogenous IL-10 regulates IFN-γ and IL-5 cytokine production and the granulomatous response in Schistosoma mansoni-infected mice. Immunology. 1998;94:481–7. doi: 10.1046/j.1365-2567.1998.00544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinstock JV, Elliott D. The substance P and somatostatin interferon-γ immunoregulatory circuit. Ann NY Acad Sci. 1998;840:532–9. doi: 10.1111/j.1749-6632.1998.tb09592.x. [DOI] [PubMed] [Google Scholar]
- 30.Smith D, Hänsch H, Bancroft G, Ehlers S. T-cell-independent granuloma formation in response to Mycobacterium avium: role of tumour necrosis factor-α and interferon-γ. Immunology. 1997;92:413–21. doi: 10.1046/j.1365-2567.1997.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chensue SW, Warmington KS, Ruth JH, Lincoln P, Kunkel SL. Cytokine function during mycobacterial and schistosomal antigen-induced pulmonary granuloma formation. Local and regional participation of IFN-γ, IL-10, and TNF. J Immunol. 1995;154:5969–76. [PubMed] [Google Scholar]
- 32.Joshi AD, Raymond T, Coelho AL, Kunkel SL, Hogaboam CM. A systemic granulomatous response to Schistosoma mansoni eggs alters responsiveness of bone-marrow-derived macrophages to Toll-like receptor agonists. J Leukoc Biol. 2008;83:314–24. doi: 10.1189/jlb.1007689. [DOI] [PubMed] [Google Scholar]
- 33.Ordway D, Harton M, Henao-Tamayo M, Montoya R, Orme IM, Gonzalez-Juarrero M. Enhanced macrophage activity in granulomatous lesions of immune mice challenged with Mycobacterium tuberculosis. J Immunol. 2006;176:4931–9. doi: 10.4049/jimmunol.176.8.4931. [DOI] [PubMed] [Google Scholar]
- 34.Ma J, Chen T, Mandelin J, Ceponis A, Miller NE, Hukkanen M, Ma GF, Konttinen YT. Regulation of macrophage activation. Cell Mol Life Sci. 2003;60:2334–46. doi: 10.1007/s00018-003-3020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imler TJ, Jr, Petro TM. Decreased severity of experimental autoimmune encephalomyelitis during resveratrol administration is associated with increased IL-17+ IL-10+ T cells, CD4– IFN-γ+ cells, and decreased macrophage IL-6 expression. Int Immunopharmacol. 2009;9:134–43. doi: 10.1016/j.intimp.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 36.Zhou L, Wang H, Zhong X, et al. The IL-10 and IFN-γ pathways are essential to the potent immunosuppressive activity of cultured CD8+ NKT-like cells. Genome Biol. 2008;119:1–18. doi: 10.1186/gb-2008-9-7-r119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh UP, Singh S, Singh R, Cong Y, Taub DD, Lillard JW., Jr CXCL10-producing mucosal CD4+ T cells, NK cells, and NKT cells are associated with chronic colitis in IL-10–/– mice, which can be abrogated by anti-CXCL10 antibody inhibition. J Interferon Cytokine Res. 2008;28:31–43. doi: 10.1089/jir.2007.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miellot A, Zhu R, Diem S, Boissier MC, Herbelin A, Bessis N. Activation of invariant NK T cells protects against experimental rheumatoid arthritis by an IL-10-dependent pathway. Eur J Immunol. 2005;35:3704–13. doi: 10.1002/eji.200535235. [DOI] [PubMed] [Google Scholar]
- 39.Wei H, Zheng X, Lou D, Zhang L, Zhang R, Sun R, Tian Z. Tumor-induced suppression of interferon-γ production and enhancement of inteleukin-10 production by natural killer (NK) cells: paralleled to CD4+ T cells. Mol Immunol. 2005;42:1023–31. doi: 10.1016/j.molimm.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 40.Kawamura T, Takeda K, Mendiratta SK, Kawamura H, van Kaer L, Yagita H, Abo T, Okumura K. Critical role of NK1+ T cells in IL-12-induced immune responses in vivo. J Immunol. 1998;160:16–9. [PubMed] [Google Scholar]
- 41.Bannai M, Oya H, Kawamura T, et al. Disparate effect of beige mutation on cytotoxic function between natural killer and natural killer T cells. Immunology. 2000;100:165–9. doi: 10.1046/j.1365-2567.2000.00040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osman Y, Kawamura T, Naito T, Takeda K, van Kaer L, Okumura K, Abo T. Activation of hepatic NKT cells and subsequent liver injury following administration of α-galactosylceramide. Eur J Immunol. 2000;30:1919–28. doi: 10.1002/1521-4141(200007)30:7<1919::AID-IMMU1919>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 43.Kaneko Y, Harada M, Kawano T, Yamashita M, Shibata Y, Gejyo F, Nakayama T, Taniguchi M. Augmentation of Vα14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J Exp Med. 2000;191:105–14. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeda K, Hayakawa Y, van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci U S A. 2000;97:5498–503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dieli F, Taniguchi M, Kronenberg M, et al. An anti-inflammatory role for Vα14 NK T cells in Mycobacterium bovis bacillus Calmette–Guérin-infected mice. J Immunol. 2003;171:1961–8. doi: 10.4049/jimmunol.171.4.1961. [DOI] [PubMed] [Google Scholar]
- 46.Kuwata K, Watanabe H, Jiang S-Y, Yamamoto T, Miyaji C, Abo T, Miyazaki T, Naito M. AIM inhibits apoptosis of T cells and NKT cells in Corynebacterium-induced granuloma formation in mice. Am J Pathol. 2003;162:837–47. doi: 10.1016/S0002-9440(10)63880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Komocsi A, Lamprecht P, Csernok E, et al. Peripheral blood and granuloma CD4+ CD28– T cells are a major source of interferon-γ and tumor necrosis factor-α in Wegener's granulomatosis. Am J Pathol. 2002;160:1717–24. doi: 10.1016/s0002-9440(10)61118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabata A, Kaneda K, Watanabe H, Abo T, Yano I. Kinetics of organ-associated natural killer cells and intermediate CD3 cells during pulmonary and hepatic granulomatous inflammation induced by mycobacterial cord factor. Microbiol Immunol. 1996;40:651–8. doi: 10.1111/j.1348-0421.1996.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 49.Oh K, Byoun OJ, Ham DI, Kim YS, Lee DS. Invariant NKT cells regulate experimental autoimmune uveitis through inhibition of Th17 differentiation. Eur J Immunol. 2011;41:392–402. doi: 10.1002/eji.201040569. [DOI] [PubMed] [Google Scholar]
- 50.Teige A, Bockermann R, Hasan M, Olofsson KE, Liu Y, Issazadeh-Navikas S. CD1d-dependent NKT cells play a protective role in acute and chronic arthritis models by ameliorating antigen-specific Th1 responses. J Immunol. 2010;185:345–56. doi: 10.4049/jimmunol.0901693. [DOI] [PubMed] [Google Scholar]


