Abstract
Trifluoroacetic acid is a metabolite of the inhaled anesthetics halothane, desflurane and isoflurane as well as a major contaminant in HPLC-purified peptides. Ligand-gated ion channels, including cys-loop receptors such as the glycine receptor, have been the targets of peptide-based drug design and are considered to be likely candidates for mediating the effects of anesthetics in vivo, but the possible secondary contributions of contaminants and metabolites to these effects have not been studied. We used two-electrode voltage-clamp electrophysiology to test glycine, GABAA and 5-HT3 receptors expressed in Xenopus oocytes for their sensitivities to sodium trifluoroacetate. Trifluoroacetate (100 μM–3 mM) enhanced the currents elicited by low concentrations of glycine applied to α1 homomeric and α1β heteromeric glycine receptors, but it had no effects when co-applied with a maximally-effective glycine concentration. Trifluoroacetate had no effects on α1β2γ2S GABAA or 5-HT3A receptors at any GABA or serotonin concentration tested. The results demonstrate that trifluoroacetate acts as an allosteric modulator at the glycine receptor with greater specificity than other known modulators. These results have important implications for both the secondary effects of volatile anesthetics and the presence of contaminating trifluoroacetate in HPLC-purified peptides, which is potentially an important source of experimental variability or error that requires control.
Keywords: Anesthetic metabolism, Allosteric modulation, Glycine receptor, GABAA receptor, 5-HT3 receptor, Trifluoroacetate
1. Introduction
Trifluoroacetic acid is widely used in organic chemistry, particularly in peptide synthesis, where it functions as an ion-pairing agent during the HPLC purification step of peptide synthesis. The addition of trifluoroacetic acid increases the hydrophobicities of peptides by forming ionic pairs with their charged groups, favoring interactions between peptides and a hydrophobic stationary phase, thus enabling separation (Garcia, 2005). It binds to the free amino termini of peptides as well as the side chains of positively charged lysine, histidine and arginine residues (Cornish et al., 1999), forming trifluoroacetate (TFA) salts. This ion pairing is extremely strong and requires an additional ion replacement step during purification to remove TFA from the purified peptides. Thus, HPLC-purified peptides are often prepared and used as TFA salts, resulting in purified peptides with varying levels of TFA contamination.
Trifluoroacetic acid is also a major metabolite of the volatile anesthetics halothane, isoflurane and desflurane (Cohen, 1971; Hitt et al., 1974). It is thought to be responsible for the development of halothane-induced hepatitis and neurotoxicity (Gut et al., 1995; Ma et al., 1990) and may play a role in the cardioprotective effects of isofluorane (Han et al., 2001).
A large number of protein targets of inhaled anesthetics have been identified, among them members of the cys-loop receptor family such as the glycine (GlyR) and γ-aminobutyric acid (GABAA-R) receptors (Franks, 2006). Both the GlyR (Harrison et al., 1993; Mascia et al., 1996) and GABAA-R (Wakamori et al., 1991; Nishikawa et al., 2002) are sensitive to clinically-relevant concentrations of a wide variety of volatile anesthetics that are hypothesized to interact with these receptors at defined molecular sites (Mascia et al., 2000). However, whether a metabolite of some of these anesthetics could also affect the functioning of these cys-loop receptors, and possibly contribute to anesthetic actions, has thus far not been investigated.
Based on multiple published reports of inhaled anesthetic modulation of ion channels and our previous work that identified novel peptides that act as allosteric modulators at the GlyR (Tipps et al., 2010), we tested TFA for its effects on the functioning of several cys-loop receptors. We found that TFA reversibly modulates GlyR responses and that these modulatory effects do not extend to other members of the cys-loop receptor family. These results are relevant to the development and testing of future peptide-based drugs, as well as highlighting possible secondary central nervous system effects following the administration and metabolism of some inhaled anesthetics.
2. Materials and Methods
2.1 - Oocyte Isolation and DNA Microinjection
Xenopus laevis were obtained from Nasco (Fort Atkinson, WI) and treated in accordance with an approved institutional animal care and use protocol at the University of Texas. Stage V and VI oocytes were surgically isolated, and receptor subunit cDNAs injected blindly into oocyte nuclei, as described previously (Welsh et al., 2010). The human glycine receptor α1 subunit cDNA was injected on its own to form homomeric receptors or with the β subunit cDNA in a 1:20 α1:β v/v ratio to form heteromeric receptors. Human GABAA α1, β2 and γ2S subunit cDNAs were combined in a 1:1:3 v/v ratio before being injected, while the mouse 5-HT3A subunit cDNA was injected alone. In each case, a total of 1.5 ng of cDNA (in 30 nL) was injected per oocyte. All chemicals were obtained from Sigma- Aldrich (St. Louis, MO). Mutation of serine-267 to glutamine (S267Q) in the GlyR α1 subunit was described previously (Findlay et al., 2002).
2.2 - Electrophysiology
Oocytes were assayed for receptor expression one to four days after cDNA injection. Two high-resistance (0.5–10 MΩ) glass electrodes filled with 3 M KCl were used to impale the animal poles of isolated oocytes for electrophysiological recording. Cells were voltage-clamped at −70 mV using a Warner Instruments OC-725C oocyte clamp (Hamden, CT) and perfused with Modified Barth’s Saline [MBS; 88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 10 mM HEPES, 0.82 mM MgSO4·7H2O, 0.33 mM Ca(NO3)2 , 0.91 mM CaCl2; pH 7.5] at a rate of 2 ml/min using a Masterflex USA peristaltic pump (Cole Parmer Instrument Co, Vernon Hills, IL) through 18-gauge polyethylene tubing. Perfusion tubing was washed with 100% ethanol for 60 sec and rinsed with MBS for approximately five minutes before each oocyte was placed in the recording chamber. All drug solutions were prepared in MBS. Trifluoroacetate (100 μM–3 mM) was pre-applied to oocytes for 30 sec before being co-applied with agonist for a further 10 or 45 sec for the GABAA and glycine receptors or 10 or 90 sec for 5-HT3A receptors. The 10 sec applications were used when maximally-effective concentrations of agonists were applied. Clamping currents were acquired at 100 Hz using a PowerLab 4/30 digitizer and digitally filtered at 50 Hz using LabChart 7 software (ADInstruments, Bella Vista NSW, Australia). Peak current responses were visually determined from LabChart files, and TFA effects are expressed as percent changes in peak currents compared with the effects produced by agonists applied alone. Significant differences between experimental conditions were determined using one- or two-way ANOVAs or the Mann-Whitney Rank Sum test.
3. Results
3.1–Lack of non-specific membrane effects of TFA in oocytes
TFA is a chaotropic compound, and other compounds of this class are known to alter membrane integrity and function. Thus, we first tested TFA for its effects on holding currents in uninjected oocytes. When applied alone, 100 μM–3 mM TFA had no effects on the holding currents of uninjected oocytes across a range of command voltages (0 to −70 mV), nor did it elicit currents in oocytes expressing the tested receptors in the absence of the respective receptor agonists. These results suggest that TFA cannot directly gate channels and that it does not significantly alter the function of any of the other endogenous oocyte proteins responsible for the holding current (data not shown).
3.2–TFA acts as an allosteric modulator of the GlyR
TFA was tested for its effects on glycine, GABAA and serotonin (5-HT) 3A receptor function. To control for variability across occytes, the concentration of agonist producing 5–10% of a maximal response (EC5-10) was identified for each oocyte and used throughout the experiment. TFA (100 μM–3 mM) was pre-applied alone for 30 sec before being co-applied with the EC5-10 agonist.
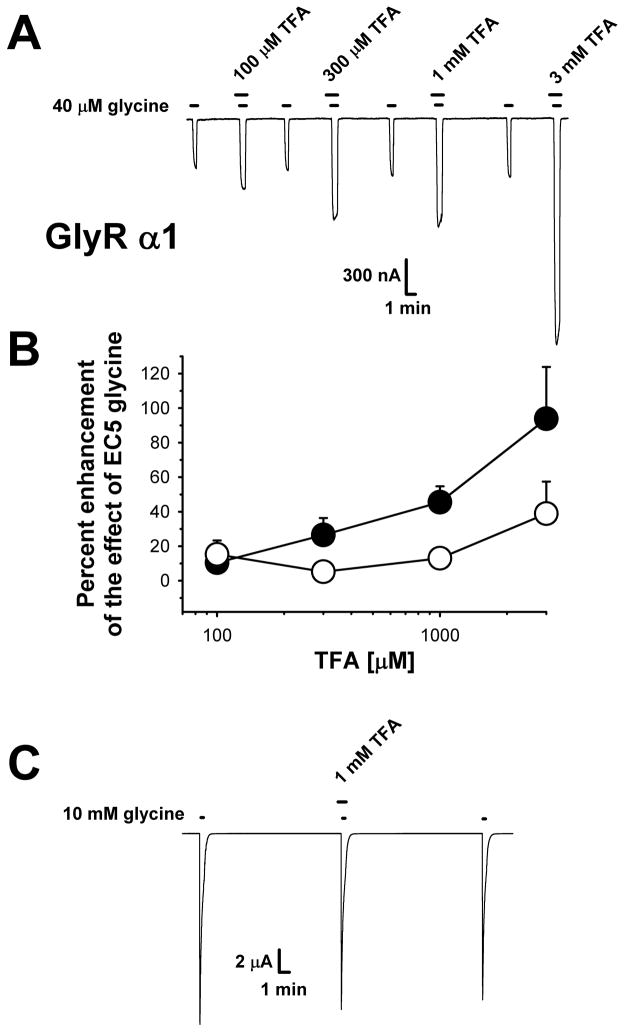
TFA acted as an allosteric modulator of the GlyR by enhancing the currents elicited by EC5-10 glycine in wild-type homomeric α1 GlyRs in a concentration-dependent and reversible manner [F(3,31)=4.73, p<0.01] (Figs. 1A,B). When co-applied with a saturating glycine concentration of 10 mM, TFA had no effect on wild-type GlyR (1.3 ± 6.7% potentiation, Fig 1C), further supporting the conclusion that TFA is acting as an allosteric modulator of this channel, that acts by leftshifting the glycine concentration-response curve . TFA had no effects on glycine receptor desensitization. The currents measured five sec after observing the peak current response were compared to those seen at the peak. When 10 mM glycine was applied alone currents decreased to 68% ± 4% (n=6) of peak within 5 sec and 68% ± 5% (n=6) when glycine was co-applied with TFA [t(10) = 0.05, p>0.96].
Figure 1.
Concentration-dependent enhancement of homomeric α1 GlyR function by TFA. (A) Sample tracings demonstrating the enhancing effects of TFA on glycine-mediated responses in oocytes voltage-clamped at −70 mV. For each oocyte the EC5-10 concentration of glycine (43 ± 5.1 μM glycine resulting in 5.9 ± 0.5% of maximal current) was first applied alone for 45 sec. A 30 sec pre-incubation of TFA preceded the co-application of TFA plus EC5-10 glycine for a further 45 sec. TFA did not alter the holding current when applied alone. Reversibility of the effects of TFA is shown by intervening glycine-alone applications following 5–10 min washouts. (B) Summary of data showing the effects of 100 μM–3 mM TFA on wild-type α1 GlyR (solid symbols) as well as the S267Q α1 mutant (hollow symbols), which was almost completely resistant to TFA. Data are presented as the mean ± S.E.M. of 7–8 oocytes obtained from at least two batches of frogs. (C) Although TFA significantly enhanced responses to EC5-10 glycine in wild-type α1 GlyR, it had no enhancing effects on the responses elicited by a maximally-effective glycine concentration. A 30 sec pre-incubation with 1 mM TFA preceded glycine application with TFA for a further 10 sec.
3.2 – TFA modulation involves α1 GlyR residue S267
Given that TFA is a metabolite of several volatile anesthetics that have been shown to modulate the GlyR at specific residues, we next assessed the importance of one of these residues to the modulatory effects of TFA. The mutation of residue serine-267 to glutamine (S267Q) was previously shown to result in a loss of ethanol enhancement of GlyR α1 function (Mihic et al., 1997). The occupation of this site with thiol reagents following the mutation of this residue to a cystine (S267C) prevents the functional enhancement of GlyRs by the volatile anesthetic isoflurane (Mascia et al., 2000). To test whether this site is involved in TFA-mediated GlyR potentiation, we evaluated the modulatory effects of TFA on the GlyR S267Q mutant. TFA produced significantly less enhancement of the glycine effect in the S267Q mutant (Fig. 1B, hollow symbols), compared to the wild-type α1 GlyR [F(1,59) = 6.56, p<0.01], suggesting that this residue is also important for mediating the effects of TFA. This raises the interesting possibility that TFA modulation may share some of its underlying mechanism with the anesthetics from which it is metabolized.
3.3 – TFA shows differential modulation of GlyR subtypes
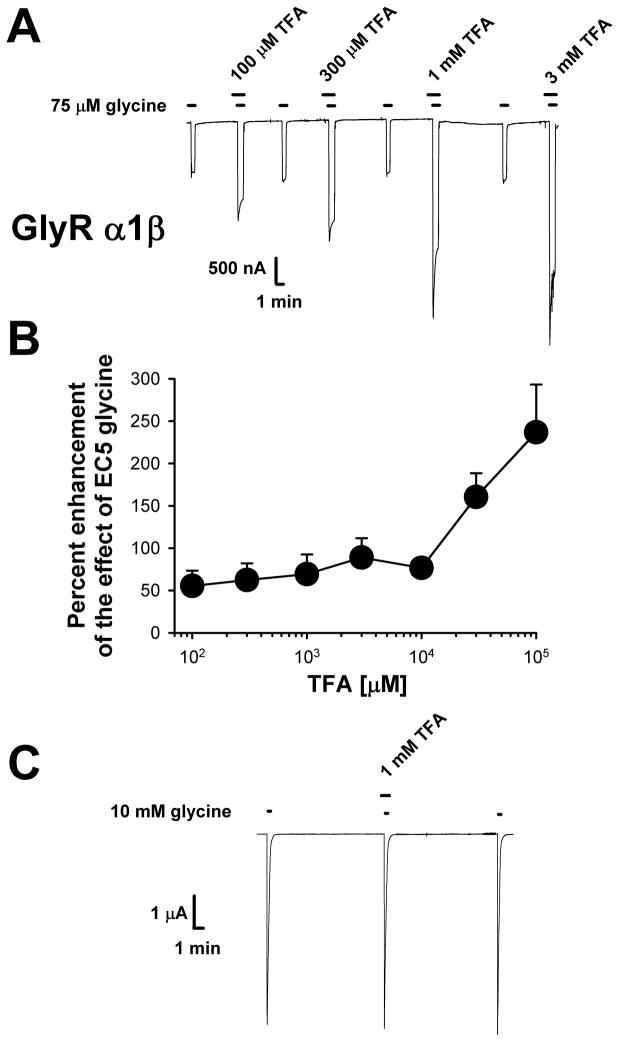
As most GlyR receptors in the mammalian nervous system are composed of α and β subunits (Lynch, 2004), TFA was also tested on heteromeric α1β glycine receptors and had effects similar to those seen in the homomeric α1 GlyR (Fig. 2). Interestingly, it appears as though the lowest concentration of TFA tested (100 μM) had a greater effect on α1β receptors than α1 receptors [Mann– Whitney U = 14, n1 = 8, n2 = 10, p=0.02, two-tailed], although considerably greater variation was seen in the α1β responses compared to those of the homomeric α1 GlyR. In the case of heteromeric α1β GlyR, TFA was tested up to a concentration of 100 mM (Fig 2B), with no sign of saturation and this might reflect more than a single site of action. As expected, there was no effect of TFA on α1β GlyR when it was co-applied with a maximally-effective concentration of glycine (0.3 ± 3.4% potentiation, n = 6) (Fig. 2C). TFA also had no effects on glycine receptor desensitization in α1β GlyR. When 10 mM glycine was applied alone currents decreased to 62% ± 3% (n=6) of the peak response after 5 sec had elapsed and 65% ± 3% (n=6) of peak when glycine was co-applied with TFA [t(10) = 0.53, p>0.6].
Figure 2.
Role of the β GlyR subunit in TFA effects on the GlyR. (A) Sample tracings demonstrating the enhancing effects of TFA on α1β heteromeric GlyR. For each oocyte the EC5-10 concentration of glycine (80 ± 4.8 μM resulting in currents that were 7.4 ± 1.8% of maximal) was first applied alone for 45 sec. A 30 sec pre-incubation of TFA preceded co-application of TFA plus EC5-10 glycine for a further 45 sec. TFA was washed out for 5-10 minutes, depending on the concentration. TFA did not alter the holding current when applied alone. (B) Summary of data showing the effects of TFA on wild-type α1β GlyR. Data are presented as the mean ± S.E.M. of 5-15 oocytes obtained from at least two batches of frogs. (C) TFA had no effects on wild-type α1β GlyR when tested using a saturating (10 mM) glycine concentration applied for 10 sec. A 30 sec pre-incubation with 1 mM TFA preceded glycine application.
3.4 – TFA modulation does not extend to other cys-loop receptors
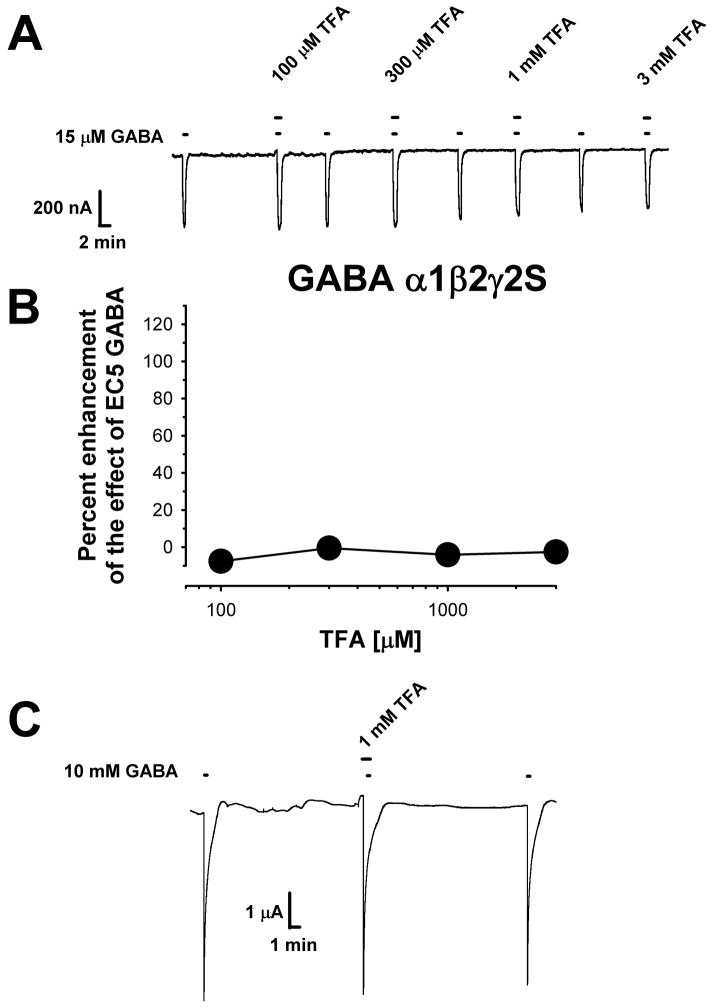
Anesthetics, and indeed most modulators of the GlyR, also have effects on other members of the cys-loop receptor family. Thus, we next tested another anionic cys-loop receptor, the GABAA receptor, for its sensitivity to TFA. The α1, β2 and γ2S subunits were chosen because they are widely expressed throughout the mammalian CNS, and many GABA receptors are thought to be composed of these subunits. TFA did not significantly enhance the effects of EC5-10 GABA concentrations (Figs. 3A and 3B), and at a concentration of 3 mM it also could not antagonize the ~150% enhancement of GABA receptor function produced by 0.2 mM isoflurane (data not shown). In addition, TFA had no effect when co-applied with a 10 mM concentration of GABA (3.8 ± 4.8% potentiation, n = 3) (Fig. 3C).
Figure 3.
Heteromeric α1β2γ2S GABAA receptors are insensitive to TFA. (A) Sample tracings showing that TFA had no effects on GABA-mediated responses in oocytes voltage-clamped at −70 mV. For each oocyte, the EC5-10 concentration of GABA (8.3 ± 2.1 μM resulting in currents that were 8.3 ± 1.6% of maximal) was first applied alone for 45 sec. A 30 sec pre-incubation of TFA preceded co-application of TFA plus EC5-10 GABA for a further 45 sec. TFA did not alter the holding current when applied alone. (B) Summary of data demonstrating a lack of effect of 100 μM –3 mM TFA on wild-type α1β2γ2S GABAA receptors. Data are presented as the mean ± S.E.M. of 6 oocytes obtained from at least two batches of frogs. (C) TFA had no effects on wild-type α1β2γ2S GABAA receptors when tested using a maximally-effective (10 mM) GABA concentration that was applied for 10 sec. A 30 sec pre-incubation with 1 mM TFA preceded the GABA application.
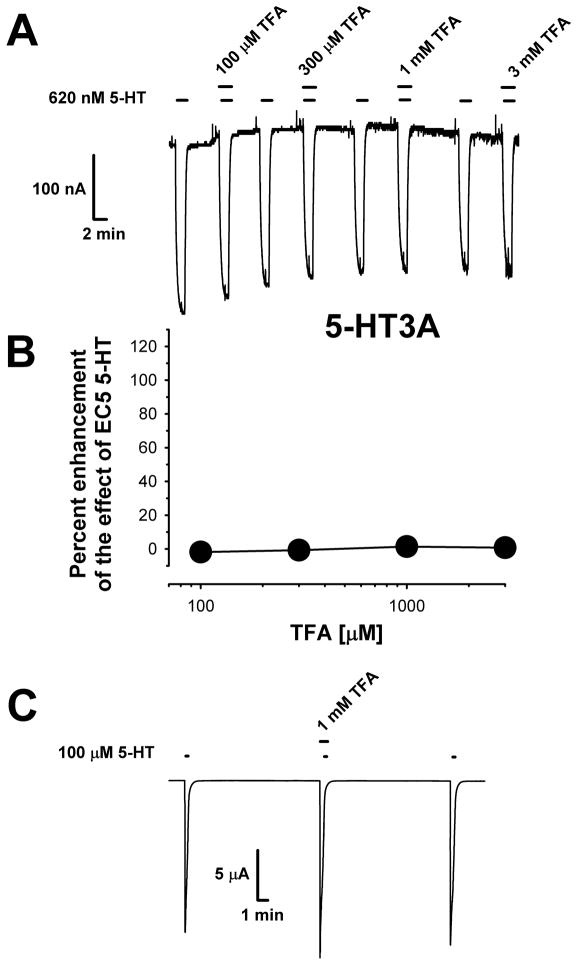
We also tested the effects of TFA on the 5-HT3A receptor, which is the cation-conducting cys-loop receptor that is most closely related to the GlyR (Fig. 4). Because it takes longer for the 5-HT3A currents to plateau in response to the application of low concentrations of serotonin than GABAA-R or GlyR, 5-HT incubations lasted for 90 sec. TFA was without effect when co-applied with either low concentrations of 5-HT (Figs 4A,B) or with a saturating concentration of 5-HT (12.3 ± 7.2% enhancement) (Fig. 4C).
Figure 4.
TFA does not affect homomeric 5-HT3A receptor function. (A) Sample tracings showing that TFA had no effects on serotonin-mediated responses in oocytes voltage-clamped at −70 mV. For each oocyte the EC5-10 concentration of serotonin (798 ± 82 nM resulting in currents that were 3.6 ± 0.5% of maximal) was first applied alone for 90 sec. A 30 sec pre-incubation of TFA preceded co-application of TFA plus EC5-10 serotonin for a further 90 sec. TFA did not alter the holding current when applied alone. (B) Summary of data demonstrating a lack of effect of 100 μM – 3 mM TFA on wild-type 5-HT3A receptors. Data are presented as the mean ± S.E.M. of 4 oocytes obtained from two batches of frogs. (C) TFA also had no effects on 5-HT3A receptors when tested using a maximally-effective (100 μM) serotonin concentration applied for 10 sec. A 30 sec pre-incubation with 1 mM TFA preceded serotonin application.
4. Discussion
Our findings demonstrate that TFA, a common contaminant in synthesized peptides and a major metabolite of halothane, isofluorane and desflurane, acts to enhance GlyR function. TFA enhancement of the effects of low concentrations of glycine was reversible, and no TFA effect was seen when it was co-applied with a maximally-effective glycine concentration, suggesting that it acts as a transient allosteric modulator. These effects are similar to those seen when ethanol or inhaled anesthetics enhance GlyR function. The lack of a TFA effect at a saturating glycine concentration indicates that TFA is not exerting its effects by increasing channel conductance. This leaves the possibility that TFA increases channel mean open times, decreases closed channel lifetimes or increases burst durations, similar to ethanol (Welsh et al., 2009), but differentiating among these possibilities will require obtaining single channel recordings.
A considerable body of evidence suggests that alcohols, volatile anesthetics and inhaled drugs of abuse act at discrete sites on glycine and GABAA receptors, specifically within circumscribed protein pockets (Mascia et al., 2000; Mihic et al., 1997; Beckstead et al., 2000). An amino acid residue (serine-267) in the second transmembrane segment of the α1 GlyR is thought to play an important role in the enhancing effects of ethanol and volatile anesthetics and, upon mutation, this enhancement is diminished or abolished. Mutation of the S267 residue to glutamine (S267Q), which results in the loss of ethanol enhancement of the α1 GlyR (Findlay et al., 2002), led to a decreased TFA effect (Fig. 1B), suggesting that TFA may act on this receptor in a manner similar to that of ethanol and volatile anesthetics. However, this conclusion is at odds with the finding that TFA modulation of the GlyR does not extend to other members of the cys-loop receptor family. Considering that a similar anesthetic binding pocket has been characterized in the closely-related GABAA receptors (Jung and Harris, 2006), we were surprised to find that the α1β2γ2S GABAA receptor was completely resistant to the effects of TFA. TFA thus displays rather remarkable selectivity as an allosteric modulator at glycine but not GABAA or 5-HT3 receptors. This of particular interest given that other GlyR modulators, such as inhalants, anesthetics, zinc, picrotoxin and tropisetron (Lynch, 2004), are far less specific, acting on multiple members of the cys-loop receptor family. Thus, TFA is one of the only known compounds to show GlyR-specific modulation, suggesting that this compound, or derivatives of this compound, may be useful for characterizing GlyR-specific effects in complex systems that contain other cys-loop receptors.
However, the GlyR is not the only ion channel that displays sensitivity to TFA. A study by Han et al. (2001) showed that TFA reversibly activated ATP-sensitive potassium (KATP) channels in ventricular myocytes. Analysis of single channel recordings suggested that TFA acts on this channel by increasing the durations of bursts of channel openings and decreasing the sensitivity of KATP channels to ATP. The authors hypothesized that this action of TFA may be responsible for the cardioprotective effects of isofluorane in vivo (Han et al, 2001). However, our report is the first to identify a target within the central nervous system for TFA, expanding the possible secondary effects of this compound.
TFA may also act at other biochemical sites in vivo. For example, it alters the proliferation rates of certain cell types, increasing the growth rates of some, while reducing the growth of others (Cornish et al., 1999). The exact mechanisms underlying these effects on cell division are unknown; however, as a chaotropic anion, TFA and other compounds of this class could conceivably affect membrane function, enzymatic catalysis, secondary protein structure, and protein stability. It is important to note that in our study, no such non-specific membrane effects were seen.
The most common source of TFA in vivo is the metabolism of volatile anesthetics. Post-surgical blood TFA levels were monitored by Gauntlett et al. (1989) in two patients pretreated with the enzyme-inducing agent isoniazid and then anesthetized with isoflurane. TFA concentrations as high as 40 μM were observed two days after surgery and remained elevated for at least a week. TFA is thought to bind to peptides and proteins in the body, thus remaining the system long after the anesthetic has been cleared, leading to unwanted side effects such as halothane-induced hepatitis following a second administration of volatile anesthetics (Holaday, 1977; Gut et al., 1995).
This concern becomes more prevalent with the increased use of synthesized peptides as potential therapeutic drugs. Following HPLC purification, peptides carry an unknown, and often variable, amount of TFA. This can lead to wide variability in effects across different batches of the same synthesized peptide. Reports comparing the effects of peptides with and without TFA contamination have been mixed. While some groups find that the presence of TFA alters the behavior or conformation of the tested peptide (Roux et al., 2007), others have found that the in vitro efficacy and toxicity are the same for TFA and other counter ions (Pini et al., 2011). However, these peptides often have very different toxicity profiles in vivo, with the TFA-bound peptides showing higher toxicity (Cornish et al., 1999; Pini et al., 2011). Even for peptides that show no difference in the presence or absence of TFA, the secondary effects of TFA, such as the GlyR modulation reported here, and the extended lifetime of TFA in the body could drastically alter the effect of the peptide in vivo. Thus, our results have important implications for the growing field of peptide-based drug design.
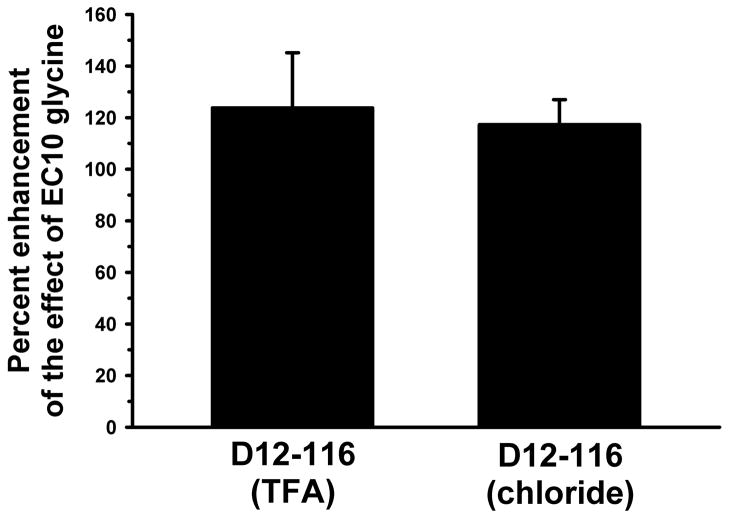
Based on our finding that TFA modulates GlyR function and reports that TFA-bound peptides can differ markedly from those synthesized without TFA, we retested a previously published dodecapeptide (D12-116) that also enhanced GlyR function as a chloride salt (Tipps et al., 2010). No differences were seen in the abilities of the TFA-bound peptide or the chloride salt-bound peptide to enhance α1 GlyR function (Fig. 5), suggesting that the unknown amount of TFA present at a peptide concentration of 30 μM was, in this case, too low to affect the glycine receptor. However, future studies will be required to evaluate the impact of these two peptide formulations in vivo.
Figure 5.
The chloride salt of peptide D12-116 produces the same enhancement of GlyR function as does the TFA salt. In both cases 30 μM of the D12-116 peptide (amino acid sequence YESIRIGVAPSQ) was applied with EC10 glycine.
5. Conclusions
The results of this study demonstrate that TFA, a major metabolite of fluorinated volatile anesthetics and a contaminant in synthesized peptides, acts as an allosteric modulator at the GlyR, but not at the closely-related GABAA or 5- HT3A receptors. The specificity of this effect is highly surprising, given that other GlyR modulators, such as ethanol, inhaled anesthetics and metals such as zinc, do not show this degree of specificity. Our studies also suggest that TFA contamination in HPLC-purified peptides is potentially an important source of experimental variability or error that requires control in vitro and may have unexpected effects when peptide drugs are administered in vivo.
Highlights.
Trifluoroacetate is an allosteric modulator of the glycine receptor.
Serine-267 of the α1 glycine receptor plays a role in trifluoroacetate modulation.
Trifluoroacetate does not affect GABAA or 5-HT3 receptor function.
Acknowledgments
This research was supported by National Institute on Alcohol Abuse & Alcoholism grant R03 AA018197.
Abbreviations
- GlyR
glycine receptor
- MBS
Modified Barth’s Saline
- TFA
trifluoroacetate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beckstead MJ, Weiner JL, Eger EI, 2nd, Gong DH, Mihic SJ. Glycine and gamma-aminobutyric acidA receptor function is enhanced by inhaled drugs of abuse. Mol Pharmacol. 2000;57:1199–205. [PubMed] [Google Scholar]
- Cohen EN. Metabolism of the volatile anesthetics. Anesthesiology. 1971;35:193–202. doi: 10.1097/00000542-197108000-00019. [DOI] [PubMed] [Google Scholar]
- Cornish J, Callon KE, Lin CQ, Xiao CL, Mulvey TB, Cooper GJ, Reid IR. Trifluoroacetate, a contaminant in purified proteins, inhibits proliferation of osteoblasts and chondrocytes. Am J Physiol. 1999;277:E779–83. doi: 10.1152/ajpendo.1999.277.5.E779. [DOI] [PubMed] [Google Scholar]
- Findlay GS, Wick MJ, Mascia MP, Wallace D, Miller GW, Harris RA, Blednov YA. Transgenic expression of a mutant glycine receptor decreases alcohol sensitivity of mice. J Pharmacol Exp Ther. 2002;300:526–34. doi: 10.1124/jpet.300.2.526. [DOI] [PubMed] [Google Scholar]
- Franks NP. Molecular targets underlying general anaesthesia. Br J Pharmacol. 2006;147(Suppl 1):S72–81. doi: 10.1038/sj.bjp.0706441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García MC. The effect of the mobile phase additives on sensitivity in the analysis of peptides and proteins by high-performance liquid chromatography-electrospray mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;825:111–23. doi: 10.1016/j.jchromb.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Gauntlett IS, Koblin DD, Fahey MR, Konopka K, Gruenke LD, Waskell L, Eger EI., 2nd Metabolism of isoflurane in patients receiving isoniazid. Anesth Analg. 1989;69:245–9. [PubMed] [Google Scholar]
- Gut J, Christen U, Frey N, Koch V, Stoffler D. Molecular mimicry in halothane hepatitis: biochemical and structural characterization of lipoylated autoantigens. Toxicology. 1995;97:199–224. doi: 10.1016/0300-483x(94)03010-y. [DOI] [PubMed] [Google Scholar]
- Han J, Kim N, Kim E. Trifluoroacetic acid activates ATP-sensitive K+ channels in rabbit ventricular myocytes. Biochem Biophys Res Commun. 2001;285:1136–42. doi: 10.1006/bbrc.2001.5291. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Kugler JL, Jones MV, Greenblatt EP, Pritchett DB. Positive modulation of human GABAA and glycine receptors by the inhalation anesthetic isoflurane. Mol Pharmacol. 1993;44:628–32. [PubMed] [Google Scholar]
- Hitt BA, Mazze RI, Cousins MJ, Edmunds HN, Barr GA, Trudell JR. Metabolism of isoflurane in Fischer 344 rats and man. Anesthesiology. 1974;40:62–7. doi: 10.1097/00000542-197401000-00015. [DOI] [PubMed] [Google Scholar]
- Holaday DA. Absorption, biotransformation, and storage of halothane. Environ Health Perspect. 1977;21:165–169. doi: 10.1289/ehp.7721165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Harris RA. Sites in TM2 and 3 are critical for alcohol-induced conformational changes in GABA receptors. J Neurochem. 2006;96:885–92. doi: 10.1111/j.1471-4159.2005.03617.x. [DOI] [PubMed] [Google Scholar]
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–95. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- Ma TG, Ling YH, McClure GD, Tseng MT. Effects of trifluoroacetic acid, a halothane metabolite, on C6 glioma cells. J Toxicol Environ Health. 1990;31:147–58. doi: 10.1080/15287399009531444. [DOI] [PubMed] [Google Scholar]
- Mascia MP, Machu TK, Harris RA. Enhancement of homomeric glycine receptor function by long-chain alcohols and anaesthetics. Br J Pharmacol. 1996;119:1331–6. doi: 10.1111/j.1476-5381.1996.tb16042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia MP, Trudell JR, Harris RA. Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc Natl Acad Sci USA. 2000;97:9305–10. doi: 10.1073/pnas.160128797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature. 1997;389:385–9. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Nishikawa K, Jenkins A, Paraskevakis I, Harrison NL. Volatile anesthetic actions on the GABAA receptors: contrasting effects of alpha1(S270) and beta2(N265) point mutations. Neuropharmacol. 2002;42:337–45. doi: 10.1016/s0028-3908(01)00189-7. [DOI] [PubMed] [Google Scholar]
- Tipps ME, Lawshe JE, Ellington AD, Mihic SJ. Identification of novel specific allosteric modulators of the glycine receptor using phage display. J Biol Chem. 2010;285:22840–5. doi: 10.1074/jbc.M110.130815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamori M, Ikemoto Y, Akaike N. Effects of two volatile anesthetics and a volatile convulsant on the excitatory and inhibitory amino acid responses in dissociated CNS neurons of the rat. J Neurophysiol. 1991;66:2014–21. doi: 10.1152/jn.1991.66.6.2014. [DOI] [PubMed] [Google Scholar]
- Welsh BT, Goldstein BE, Mihic SJ. Single-channel analysis of ethanol enhancement of glycine receptor function. J Pharmacol Exp Ther. 2009;330:198–205. doi: 10.1124/jpet.109.154344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh BT, Kirson D, Allen HM, Mihic SJ. Ethanol enhances taurine-activated glycine receptor function. Alcohol Clin Exp Res. 2010;34:1634–9. doi: 10.1111/j.1530-0277.2010.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]