Abstract
Background/Aims
Frequent pathogens of nosocomial meningitis were investigated and the adequacy of empiric antibiotic therapy was assessed. Outcomes of nosocomial meningitis were also evaluated.
Methods
Ninety-one patients, who were diagnosed and treated for nosocomial meningitis at a single tertiary hospital in Daegu, Korea for 10 years, were included. Medical record and electronic laboratory data on the causative pathogens, antibiotics used, and outcomes were retrospectively investigated.
Results
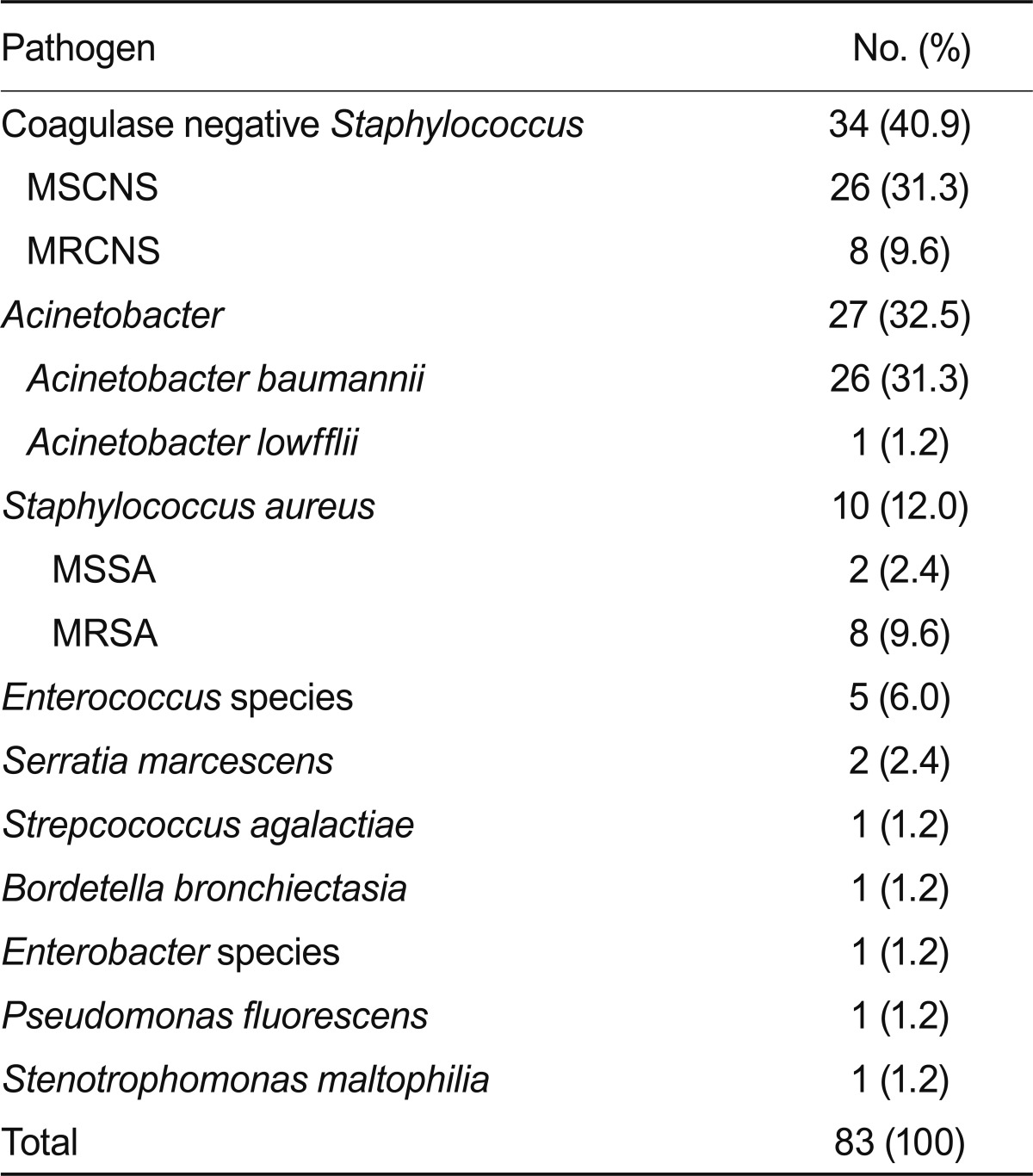
Coagulase-negative Staphylococcus (40.9%) was the most common pathogen, followed by Acinetobacter (32.5%). Both were cultured as a single organism in cerebrospinal fluid (CSF). Seventy-eight patients (85.7%) had infections related to external ventricular drains (EVD). The most common empirical antibiotics were extended-spectrum beta-lactam antibiotics plus vancomycin (35/91, 38.6%). Of the 27 patients who had cultured Acinetobacter in CSF, 10 (37%) were given the wrong empirical antibiotic treatment. Seven of the 27 patients (26.9%) with cultured Acinetobacter died, and overall mortality of the 91 patients was 16.5%. In the multivariate analysis, the presence of combined septic shock (p < 0.001) and a persistent EVD state (p = 0.021) were associated with a poor prognosis.
Conclusions
Acinetobacter is one of the leading pathogens of nosocomial meningitis and may lead to inadequate coverage of empiric antibiotic therapy due to increasing resistance. An EVD should be removed early in cases of suspected nosocomial meningitis, and carbapenem might be required for the poor treatment response.
Keywords: Meningitis, Acinetobacter, Drug resistance, Prognosis
INTRODUCTION
Nosocomial bacterial meningitis can be caused by invasive procedures (e.g., craniotomy, placement of internal or external ventricular catheters, lumbar puncture, intrathecal infusion of medications, or spinal anesthesia) and complicated head trauma [1]. Gram-positive pathogens such as methicillin-resistant coagulase-negative Staphylococcus (MRCNS) are the main target organisms to be controlled with empiric antibiotic therapy [2-11]. Gram-negative pathogens have increased as nosocomial meningitis pathogens and should be considered in the choice of empirical antibiotic treatments [12]. The frequency of neurosurgical procedures is increasing and the wide use of broad spectrum antibiotics may have changed the causative pathogens of nosocomial meningitis. Recently, Acinetobacter have been frequently reported as the nosocomial meningitis causative pathogen [12]. Additionally, multidrug-resistant Acinetobacter baumannii infections are increasing [13]. The aim of this study was to investigate the frequency of the pathogens and to assess the adequacy of empiric antibiotic therapy for nosocomial meningitis. We also evaluated outcomes and prognoses for patients with nosocomial meningitis.
METHODS
Patients
Ninety-one patients, who were diagnosed with nosocomial meningitis from December 27, 2000 to October 29, 2010, were enrolled. We gained institutional review board approval (KNUH 2011-06-016) for this study. Patients were screened by positive results of cerebrospinal fluid (CSF) cultures using electronic hospital laboratory data. Nosocomial meningitis was defined as meningitis acquired after 48 hours of admission. The diagnosis of meningitis was made if all of the following criteria were fulfilled: 1) at least one pathogen was cultured in CSF, 2) increased white blood cell (WBC) count (> 10/high power field) with dominant polymorphonuclear leukocytes (> 50%), and 3) body temperature > 38℃ that was not from other causes [14,15].
Methods
The admission site was defined as the site (the ward or intensive care unit) of the meningitis from the clinical symptom presentation. Septic shock was identified if there was severe sepsis plus one or both of the following systemic mean blood pressures: < 60 mmHg or < 80 mmHg if the patient had baseline hypertension, despite adequate fluid resuscitation [16]. WBC counts and levels of protein and glucose in CSF were investigated. Patients who had a continuous external ventricular drain (EVD) catheter after they were diagnosed with meningitis were defined as in a persistent EVD state. A negative CSF culture was defined as a negatively converted CSF culture after at least three follow-up cultures following the first positive result in CSF [17,18]. Empirical antibiotics were defined as antibiotics used immediately after the onset of the suspected or established clinical diagnosis to the time of the culture report, and specific antibiotics that changed or continued after the meningitis pathogens were discovered. The outcomes and duration of follow-up from admission date to the last visit or death date were also determined.
Statistical analyses
Descriptive statistics were performed, and univariate analyses with variables related to death were tested by the chi-square method for non-continuous variables and the t test for continuous variables. A multivariate logistic regression test was performed to identify risk factors for death. Variables with a p value < 0.2 in the univariate analysis were included in the multivariate analysis. A p value < 0.05 was considered significant.
RESULTS
Demographic and clinical characteristics
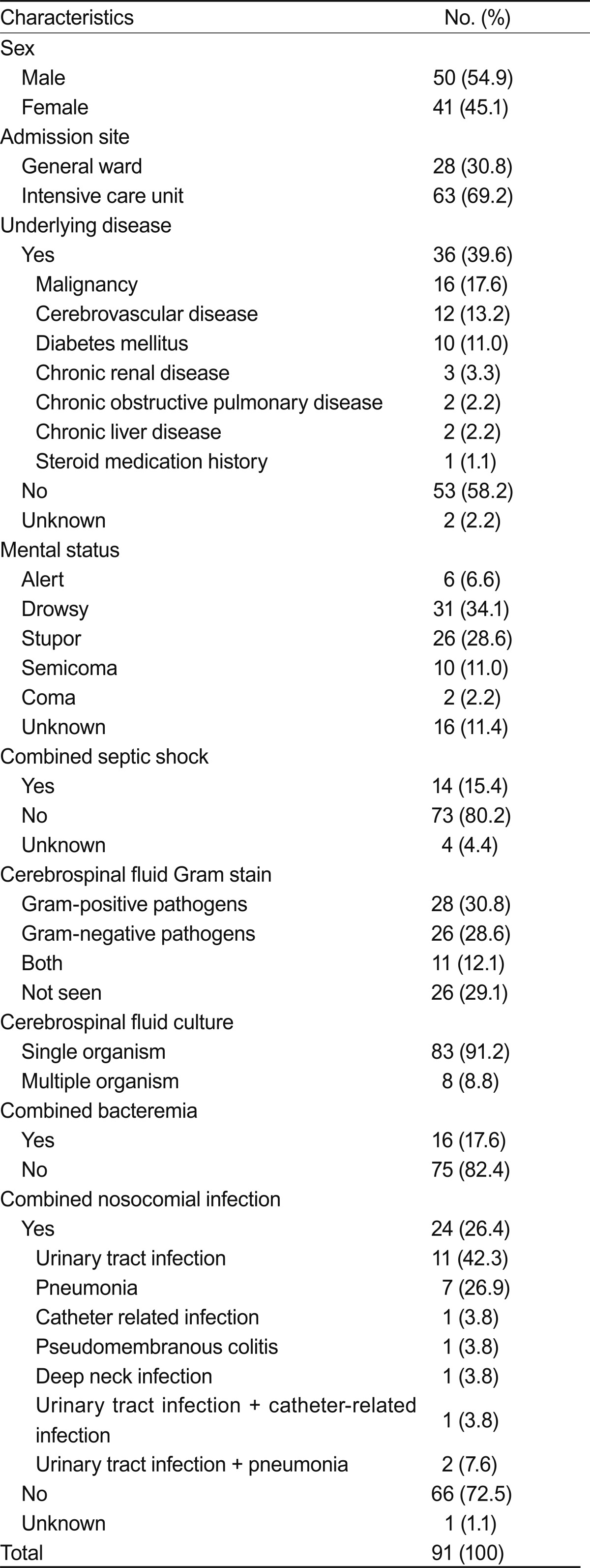
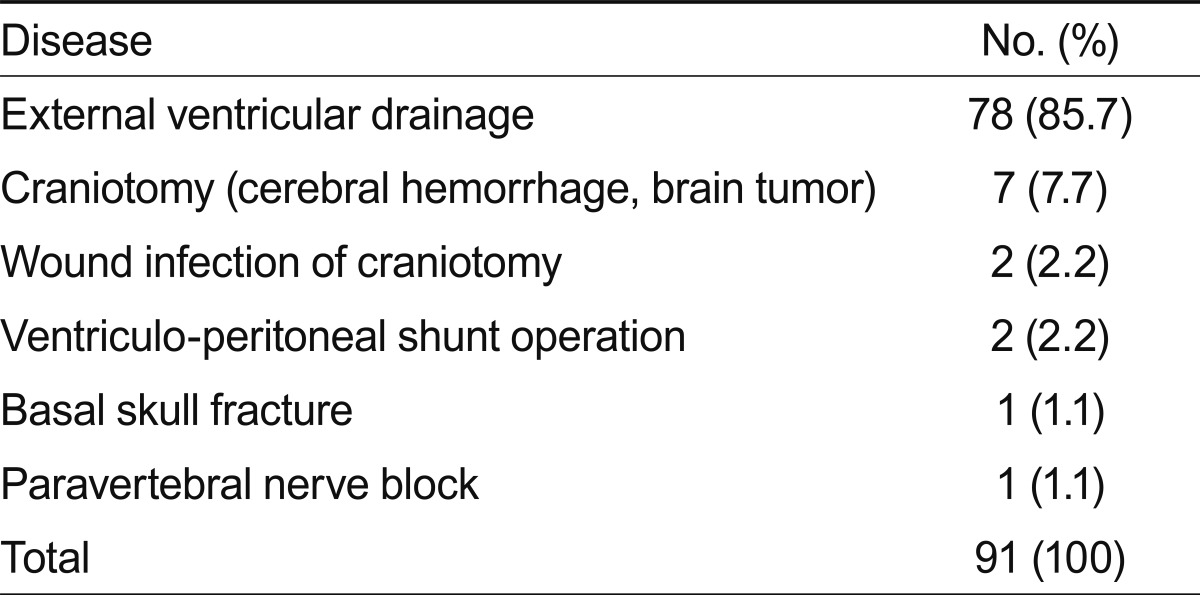
Of the 91 patients, ages ranged from 19 to 83 years (average age, 52.6). The duration from admission was 8-1,519 days (average duration, 90.3). Ten patients (11%) had diabetes mellitus as an underlying disease. Table 1 shows the patient demographic characteristics and underlying diseases. All patients had neurological problems. The most frequent causes of admission were: 41 patients (52.7%) had cerebral hemorrhage, 17 (18.7%) had a brain tumor, and six (6.6%) suffered from a traumatic head injury. Seventy-eight (85.7%) patients developed EVD-related infections. Thirteen patients (14.3%) had other predisposing factors for meningitis. The predisposing nosocomial meningitis factors are summarized in Table 2, and the CSF cultured organisms are shown in Table 3.
Table 1.
Clinical characteristics of the patients with nosocomial meningitis
Table 2.
Predisposing conditions of the 91 patients with nosocomial meningitis
Table 3.
Microbiology of the cerebrospinal fluid culture in the 91 patients with monoorganism culture-positive nosocomial meningitisa
MSCNS, methicillin sensitive coagulase negative Staphylococcus; MRCNS, methicillin resistant coagulase negative Staphylococcus; MSSA, methicillin-sensitive Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus.
aEight patients showed multiple organisms in the cerebrospinal fluid culture as follows: A. baumannii, E. coli and MSCNS (1), Enterococcus and S. maltophilia (1), Enterococcus and MRCNS (1), Enterococcus and MRSA (1), Enterococcus, MRCNS and S. maltophilia (1), Enterococcus, A. baumannii, P. aerusinosa, Enterobacter, E. coli, S. maltophilia and Providencia rettgeri (1), Enterococcus, Klebsiella pneumoniae and α-hemolytic Streptococcus (1), Acinetobacter, MRCNS (1).
Antibiotic regimens
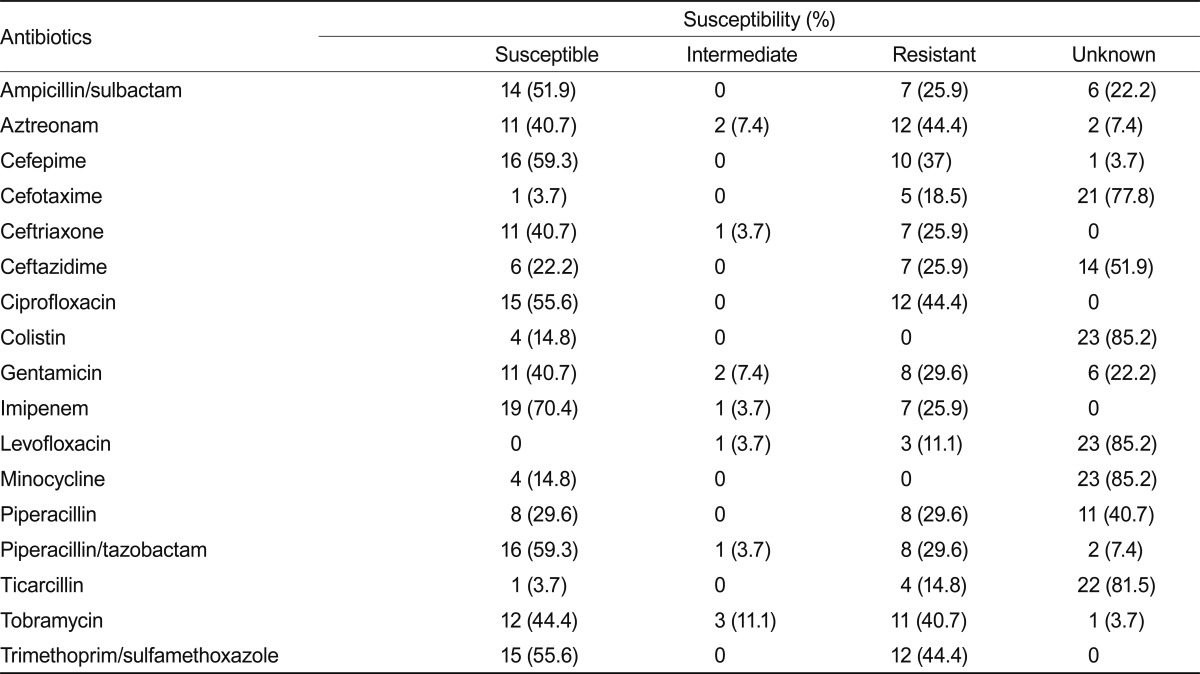
All 91 patients were treated with empirical antibiotics. Extended spectrum beta-lactam antibiotics plus vancomycin was the most favorable empirical regimen (35/91, 38.6%). Among them, ceftazidime plus vancomycin was administered to 24/35 patients (68.6%) and ceftriaxone plus vancomycin to 8/35 patients (22.9%). The resistance and sensitivity of each antibiotic to Acinetobacter were as follows: ceftriaxone, 40.7/3.7/25.9/0% (S/I/R/unknown), respectively; ceftazidime, 22.2/3.7/25.9/51.9% (S/I/R/unknown); cefepime, 59.3/0/37/3.7% (S/I/R/unknown); imipenem, 70.4/3.7/25.9/0% (S/I/R/unknown); and colistin, 14.8/85.2% (S/unknown). Table 4 shows a summary of the antibiotic susceptibilities for Acinetobacter spp. in CSF culture.
Table 4.
Antibiotic susceptibility of Acinetobacter discovered in cerebrospinal fluid cultures from patients with nosocomial meningitis
Clinical courses and prognoses
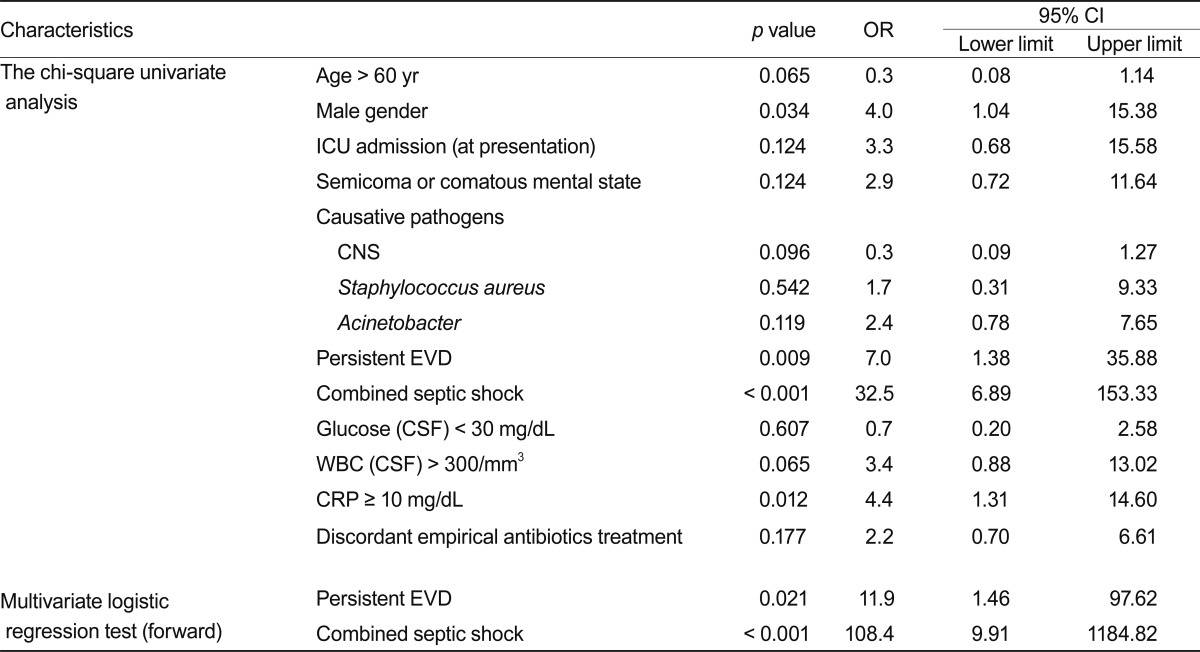
Seventy-three patients (93.6%) with EVD-related meningitis had their EVD catheters removed, and five (6.4%) had a persistent EVD catheter. We had difficulty removing the EVD from all patients due to difficulties controlling the increased intracranial pressure. Seventy patients (76.9%) showed a negative conversion on CSF culture with therapy, 10 patients (11%) showed a persistent positive culture after therapy, and 11 patients (12.1%) were unknown. The duration from the onset of diagnosis to a negative CSF culture was 1-146 days (average duration, 11.75 days). Of the 91 patients, 15 (16.5%) died, 72 (79.1%) survived, and four (4.4%) had unknown outcomes. The follow-up duration varied from 1-3,024 days (average follow-up, 683.8 days). In the chi-square analysis, male gender (p = 0.034; odds ratio [OR], 4.0), persistent EVD state after diagnosis of meningitis (p = 0.009; OR, 7.0), combined septic shock (p < 0.001; OR, 32.5), and C-reactive protein > 10 mg/dL (p = 0.012; OR, 4.4) were associated with a poor prognosis (Table 5). Acinetobacter in the CSF culture (p = 0.119; OR, 2.4) and discordant empirical antibiotic treatment (p = 0.177; OR, 2.2) were not statistically significant in the univariate analysis. Combined septic shock (p < 0.001; OR, 108.4) and persistent EVD state (p = 0.021; OR, 11.9) were related to higher mortality in the multivariate logistic regression analysis (Table 5).
Table 5.
Chi-square, univariate, and multivariate logistic regression analyses for the characteristics between the 91 dead and surviving patients with nosocomial meningitis
OR, odds ratio; CI, confidence interval; ICU, intensive care unit; CNS, coagulase negative staphylococcus; EVD, extraventricular drain; CSF, cerebrospinal fluid; WBC, white blood cells; CRP, C-reactive protein.
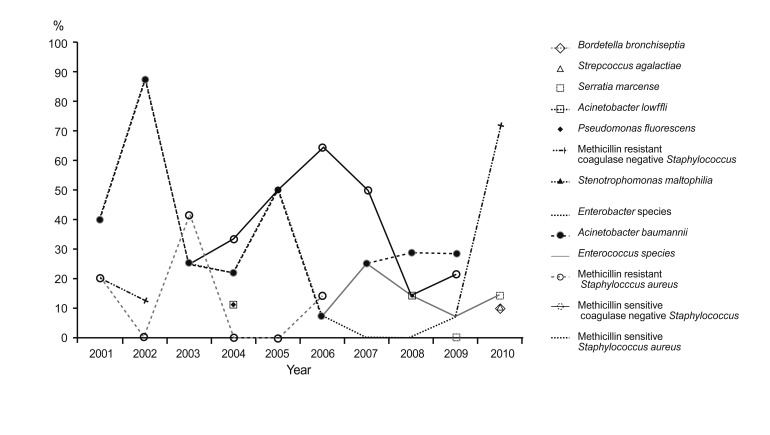
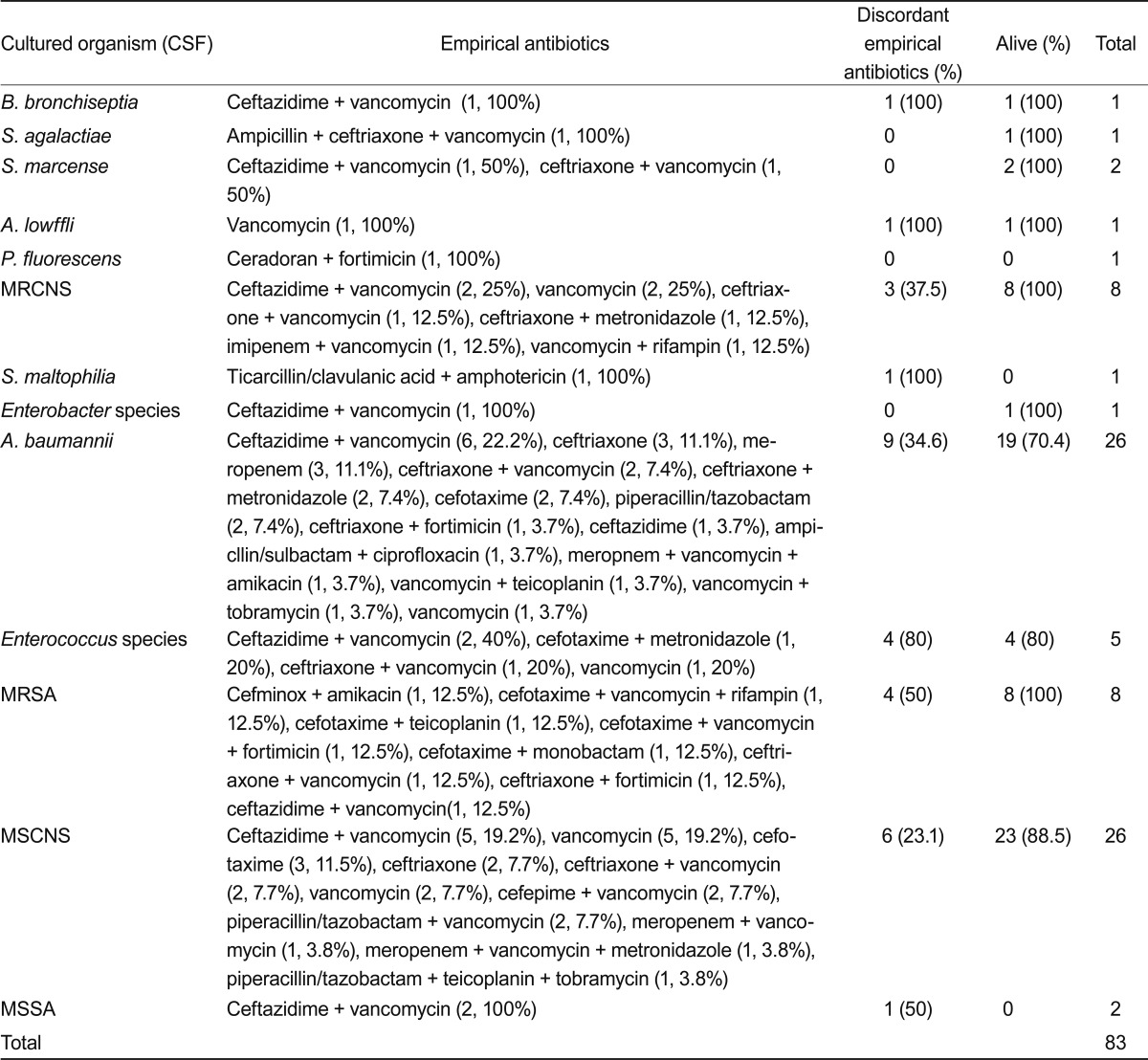
Organisms that were cultured for 10 years are shown in Fig. 1. Acinetobacter meningitis did not show a changing incidence pattern and had an endemic pattern. Table 6 summarizes the adequacy and outcomes of the empirical antibiotic treatments against a single pathogen in CSF culture. Thirty-five patients (38.5%) were treated with inappropriate empirical antibiotics, and their specific antibiotics were changed based on the culture report. MRCNS revealed 23.1% discordant empirical antibiotics, Acinetobacter revealed 37%, and methicillin-resistant S. aureus showed 37.5%. The mortality from Acinetobacter meningitis was high at 26.9%.
Figure 1.
Organisms in the cerebrospinal fluid culture report summarized for the last several years (single pathogens).
Table 6.
Empirical antibiotic treatment regimens against a single pathogen in cerebrospinal fluid (CSF) culture, treatment success rate, and outcomes of patients
MRCNS, methicillin resistant coagulase negative Staphylococcus; MRSA, methicillin-resistant Staphylococcus aureus; MSCNS, methicillin-sensitive coagulase negative Staphylococcus; MSSA, methicillin-sensitive Staphylococcus aureus.
DISCUSSION
Beer et al. [19] showed that Staphylococcus epidermidis (70%) and Staphylococcus aureus (10%) are common causative pathogens in patients with EVD-related meningitis. Coagulase-negative Staphylococcus was the most common pathogen of EVD-related meningitis in previous studies [20-22]. In our study, coagulase-negative Staphylococcus was also the most frequent pathogen (34/83, 40.9%), focusing on single-organism meningitis in CSF. Palabiyikoglu et al. [23] reported CSF cultures of 49 patients who were diagnosed with adult nosocomial meningitis; 61% of the patients had proven Gram-negative pathogens in a CSF study, and 34% had Gram-positive pathogens.
Acinetobacter spp. seem to be increasingly important pathogens associated with postneurosurgical meningitis [18]. In our study, Acinetobacter meningitis did not show increased prevalence in the last several years; however, it was proved in 27 patients (32.5%) as the second most common cause. These findings remind of us that recent significant increases in Gram-negative pathogens such as Acinetobacter should be considered when managing patients with nosocomial meningitis.
Our results and those of previous studies [18,23] suggest that a long intensive care unit stay and invasive procedures such as EVD, craniotomy, wound infections, shunts, basal skull fractures, and spinal anesthesia may be important risk factors for Acinetobacter meningitis. Many patients with meningitis have EVD after neurosurgery. Erdem et al. [12] reported that 63% of patients develop EVD difficulties. Katragkou and Roilides [24] reported nine patients diagnosed with A. baumannii meningitis, and five had an EVD. EVD-related meningitis was also the most common type of nosocomial meningitis in our study with 78 patients infected (85.7%). Therefore, these findings reconfirm that invasive procedures such as EVD are strongly related to the incidence of nosocomial meningitis. Additionally, physicians should consider that early removal of an EVD is important for a favorable outcome in cases of suspicious meningitis in hospitalized patients.
Rodriguez Guardado et al. [13] reported 51 patients with EVD-related meningitis after neurosurgery. All patients were treated with empirical antibiotics, and the therapy was appropriate in 42% of patients. Treatment was changed in five patients because of inappropriate antibiotics, 27 patients were treated with single intravenous antibiotics, and carbapenem was the most common specific antibiotic regimen (21 patients) [13]. In contrast, vancomycin plus ceftazidime was the most frequent empirical combination regimen used in our study. This regimen was used considering Gram-positive pathogens such as MRCNS and Gram-negative pathogens such as Pseudomonas. However, Gram-negative pathogens such as ceftazidime-resistant Acinetobacter must not be ignored. A recent study conducted with 2,621 collected Acinetobacter isolates showed that 44.6% were susceptible to ceftazidime, and 47.7% were susceptible to cefepime [25]. Acinetobacter shows frequent resistance to third- and fourth-generation cephalosporins and even to carbapenems [18]. Thus, intravenous meropenem, with or without an aminoglycoside administered via the intraventricular or intrathecal route, has been recommended for empirically treating Acinetobacter meningitis [18]. If the organism is subsequently found to be resistant to carbapenems, then colistin or polymyxin B should be substituted for meropenem and may need to be administered via the intraventricular or intrathecal route [26]. In this study, resistance of invasive Acinetobacter isolated in the CSF to ceftazidime and cefepime was significant. The resistance of carbapenem was lower than that of other antibiotics. The percentage of total misused empirical antibiotics (38.5%) was lower than that in a previous study [13]; however, 10 of the 27 inappropriate treatments (37%) were significant for Acinetobacter in this study. Carbapenem must be considered a therapeutic antibiotic when poor treatment outcome in Acinetobacter meningitis.
Erdem et al. [12] reported that nine (52.9%) of 17 patients died from Acinetobacter meningitis. Rodriguez Guardado et al. [13] reported on 51 patients with EVD-related Acinetobacter meningitis and 17 patients died from the infection. In a multivariate analysis, mortality shows a relationship with age, WBC counts, and removal of EVD alone [13]. In our study, mortality from Acinetobacter meningitis was high and the reported poor prognostic factors [13] such as CSF, WBC count, glucose level, age, and mental status were not associated with higher mortality; additionally, we failed to identify the relationship between duration from admission and diagnosis or treatment and antibiotic regimens, laboratory factors, and mortality from nosocomial meningitis. Removing the EVD is an important factor for increasing the cure rate of nosocomial meningitis [18]. Additionally, the relationship between the persistent EVD state, combined with septic shock and poor outcomes, was statistically significant. Early removal of the EVD and aggressive antibiotic treatment for suspected nosocomial meningitis would lead to better outcomes. This study involved a single-center analysis, so generalization is difficult, and a multicenter study is needed.
Nosocomial meningitis is rare, but treatment is difficult because of the increasing number of multidrug resistant Gram-negative pathogens. Acinetobacter is an increasingly prevalent causative pathogen, antibiotic resistance occurs frequently, and mortality was high. We think that vancomycin plus ceftazidime as a traditional empirical treatment regimen may not completely cover all current nosocomial meningitis pathogens. We suggest an immediate change to antibiotics with high activity against resistant Acinetobacter to adequately treat nosocomial meningitis.
Acknowledgments
This work was supported by a BioMedical Research Institute grant from Kyungpook National University Hospital (2003).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.van de Beek D, Drake JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med. 2010;362:146–154. doi: 10.1056/NEJMra0804573. [DOI] [PubMed] [Google Scholar]
- 2.Streharova A, Benca J, Holeckova K, et al. Comparison of postsurgical and community acquired bacterial meningitis: analysis of 372 cases within a nationwide survey. Neuro Endocrinol Lett. 2007;28(Suppl 3):7–9. [PubMed] [Google Scholar]
- 3.Weisfelt M, van de Beek D, Spanjaard L, de Gans J. Nosocomial bacterial meningitis in adults: a prospective series of 50 cases. J Hosp Infect. 2007;66:71–78. doi: 10.1016/j.jhin.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Ross D, Rosegay H, Pons V. Differentiation of aseptic and bacterial meningitis in postoperative neurosurgical patients. J Neurosurg. 1988;69:669–674. doi: 10.3171/jns.1988.69.5.0669. [DOI] [PubMed] [Google Scholar]
- 5.Wang KW, Chang WN, Huang CR, et al. Post-neurosurgical nosocomial bacterial meningitis in adults: microbiology, clinical features, and outcomes. J Clin Neurosci. 2005;12:647–650. doi: 10.1016/j.jocn.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Benca J, Ondrusova A, Kisac P, Krcmery V. Postoperative meningitis: shift in etiology? Neuro Endocrinol Lett. 2007;28(Suppl 3):22. [PubMed] [Google Scholar]
- 7.Pintado V, Meseguer MA, Fortun J, et al. Clinical study of 44 cases of Staphylococcus aureus meningitis. Eur J Clin Microbiol Infect Dis. 2002;21:864–868. doi: 10.1007/s10096-002-0814-1. [DOI] [PubMed] [Google Scholar]
- 8.Hodges GR, Perkins RL. Hospital-associated bacterial meningitis. Am J Med Sci. 1976;271:335–341. doi: 10.1097/00000441-197605000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267–1284. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 10.Durand ML, Calderwood SB, Weber DJ, et al. Acute bacterial meningitis in adults: a review of 493 episodes. N Engl J Med. 1993;328:21–28. doi: 10.1056/NEJM199301073280104. [DOI] [PubMed] [Google Scholar]
- 11.O'Neill E, Humphreys H, Phillips J, Smyth EG. Third-generation cephalosporin resistance among Gram-negative bacilli causing meningitis in neurosurgical patients: significant challenges in ensuring effective antibiotic therapy. J Antimicrob Chemother. 2006;57:356–359. doi: 10.1093/jac/dki462. [DOI] [PubMed] [Google Scholar]
- 12.Erdem I, Hakan T, Ceran N, et al. Clinical features, laboratory data, management and the risk factors that affect the mortality in patients with postoperative meningitis. Neurol India. 2008;56:433–437. doi: 10.4103/0028-3886.44629. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez Guardado A, Blanco A, Asensi V, et al. Multidrug-resistant Acinetobacter meningitis in neurosurgical patients with intraventricular catheters: assessment of different treatments. J Antimicrob Chemother. 2008;61:908–913. doi: 10.1093/jac/dkn018. [DOI] [PubMed] [Google Scholar]
- 14.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the cute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Yoo JH. Infectious complications after neurosurgery: mainly focusing on ventriculitis and meningitis. Infect Chemother. 2011;43:229–233. [Google Scholar]
- 16.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 17.Wen DY, Bottini AG, Hall WA, Haines SJ. Infections in neurologic surgery: the intraventricular use of antibiotics. Neurosurg Clin N Am. 1992;3:343–354. [PubMed] [Google Scholar]
- 18.Kim BN, Peleg AY, Lodise TP, et al. Management of meningitis due to antibiotic-resistant Acinetobacter species. Lancet Infect Dis. 2009;9:245–255. doi: 10.1016/S1473-3099(09)70055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beer R, Lackner P, Pfausler B, Schmutzhard E. Nosocomial ventriculitis and meningitis in neurocritical care patients. J Neurol. 2008;255:1617–1624. doi: 10.1007/s00415-008-0059-8. [DOI] [PubMed] [Google Scholar]
- 20.Jarvis WR, Martone WJ. Predominant pathogens in hospital infections. J Antimicrob Chemother. 1992;29(Suppl A):19–24. doi: 10.1093/jac/29.suppl_a.19. [DOI] [PubMed] [Google Scholar]
- 21.Vincent JL, Bihari DJ, Suter PM, et al. EPIC International Advisory Committee. The prevalence of nosocomial infection in intensive care units in Europe: results of the European Prevalence of Infection in Intensive Care (EPIC) Study. JAMA. 1995;274:639–644. [PubMed] [Google Scholar]
- 22.Gantz NM. Nosocomial central nervous system infections. In: Mayhall CG, editor. Hospital epidemiology and infection control. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 1999. pp. 301–322. [Google Scholar]
- 23.Palabiyikoglu I, Tekeli E, Cokca F, et al. Nosocomial meningitis in a university hospital between 1993 and 2002. J Hosp Infect. 2006;62:94–97. doi: 10.1016/j.jhin.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Katragkou A, Roilides E. Successful treatment of multidrug-resistant Acinetobacter baumannii central nervous system infections with colistin. J Clin Microbiol. 2005;43:4916–4917. doi: 10.1128/JCM.43.9.4916-4917.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gales AC, Jones RN, Sader HS. Global assessment of the antimicrobial activity of polymyxin B against 54 731 clinical isolates of Gram-negative bacilli: report from the SENTRY antimicrobial surveillance programme (2001-2004) Clin Microbiol Infect. 2006;12:315–321. doi: 10.1111/j.1469-0691.2005.01351.x. [DOI] [PubMed] [Google Scholar]
- 26.Falagas ME, Bliziotis IA, Tam VH. Intraventricular or intrathecal use of polymyxins in patients with Gram-negative meningitis: a systematic review of the available evidence. Int J Antimicrob Agents. 2007;29:9–25. doi: 10.1016/j.ijantimicag.2006.08.024. [DOI] [PubMed] [Google Scholar]