Abstract
An exciting aspect of the heptahelical orexin receptor 1 (OX1R) has emerged recently, when it was shown that it drives apoptosis in human colon cancer cell lines. Here we review recent findings related to the role of OX1R in colorectal cancers and the unexpected mechanism whereby this G protein-coupled receptor works. The OX1R is aberrantly expressed at all steps of primary colorectal tumour progression and after local (lymph node) or distant (liver, lung) metastasis. No OX1R is detected in normal colonic epithelial cells. Treatment of human colon cancer cells in culture with orexins promotes robust apoptosis and subsequent reduction of growth including in cells that are resistant to 5-fluorouracil, the most commonly used drug in chemotherapy. When human colon cancer cells are xenografted in nude mice, treatment with orexins dramatically slows tumour growth and even reverses the development of established tumours. Thus, OX1R agonists might be novel candidates for colon cancer therapy. Activation of OX1R drives apoptosis through Gq protein but independently of classical Gαq activation of phospholipase C. In fact, it is the freed βγ dimer of Gq that plays a pivotal role by stimulating Src-tyrosine kinase. This results in phosphorylation of two immunoreceptor tyrosine-based inhibitory motifs (ITIM) in OX1R and subsequent recruitment by OX1R of the phosphotyrosine phosphatase SHP-2, which is activated thereby. Downstream events include release of cytochrome c from mitochondria and activation of caspase-3 and caspase-7. The role of ITIMs in OX1R-driven apoptosis represents a new paradigm of G protein-coupled receptor signalling.
LINKED ARTICLES
This article is part of a themed section on the Molecular Pharmacology of G Protein-Coupled Receptors (GPCRs). To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-6. To view the 2010 themed section on the same topic visit http://onlinelibrary.wiley.com/doi/10.1111/bph.2010.159.issue-5/issuetoc
Keywords: apoptosis, cancer therapy, caspase, colon cancer, G protein-coupled receptor, hypocretin, immunoreceptor tyrosine-based inhibitory motif, metastases, orexin, protein phosphatase SHP2, tyrosine-based motif, tumour
Introduction
Colon cancer is a common malignancy worldwide and causes considerable morbidity and mortality (Segal and Saltz, 2009). Molecular genetics has identified key genes, including tumour-suppressor genes and oncogenes, whose mutations or altered expression are associated with colorectal cancer (Markowitz and Bertagnolli, 2009). Colon cancer initiation and progression are dependent on these genes but are also under the control of many growth factors or hormones present in primary tumour environment and acting at tyrosine kinase receptors or G protein-coupled receptors (GPCRs). Colon cancers do express a variety of GPCRs that fall into three categories. Many receptors are already present at similar levels in normal colon epithelial cells, others are simply overexpressed and some of them are aberrantly expressed in cancer cells, which means that they are not present in normal colonic epithelium (Laburthe et al., 1978; Maoret et al., 1994; Singh et al., 2000; Darmoul et al., 2003; Gratio et al., 2009). The growth-promoting effects of peptide hormones such as gastrin (Singh et al., 2000) or neurotensin (Maoret et al., 1999), serine proteases such as thrombin (Darmoul et al., 2003) or trypsin (Darmoul et al., 2004) or lipids such as lysophosphatidic acid (Yang et al., 2005) or prostaglandin E2 (Chell et al., 2006) are mediated by GPCRs. Activation of these GPCRs promotes tumour cell growth through G protein transduction pathways and/or by transactivating the tyrosine kinase epidermal growth factor receptor (EGFR) for epidermal growth factor (Darmoul et al., 2004; Lappano and Maggiolini, 2011). Due to its autoactivity and because it can be transactivated by GPCRs, EGFR is prominent in the growth of colon cancer and is already a therapeutic target for antibodies directed against its extracellular domain (Segal and Saltz, 2009).
While a large body of evidence shows that the environment of primary colon tumours is rich in growth factors (see earlier discussion), almost nothing was known until recently regarding the existence of growth inhibitory factors for colon cancer. In order to try to identify such inhibitory factors, we developed a very simple strategy consisting of screening the ability of a large series of peptide hormones and neuropeptides to inhibit colon cancer growth (Rouet-Benzineb et al., 2004). We found that the neuropeptides orexins acting at the seven-transmembrane domain receptor orexin receptor 1 (OX1R) are robust stimulants of apoptosis in colon cancer cells (Rouet-Benzineb et al., 2004).
In this review, we aim to summarize current knowledge and recent findings on orexin receptors in colon cancer. Specifically, we will discuss two aspects: (i) the expression and pro-apoptotic role of OX1R in primary colorectal tumours and metastases and in colon tumour cell lines. The recent data support the view that OX1R represents an Achilles heel of colon cancer and is a new promising therapeutic target; (ii) the entirely novel mechanism by which the seven-pass transmembrane GPCR OX1R triggers apoptosis. It involves phosphorylation of two immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in OX1R resulting in the recruitment of the phosphotyrosine phosphatase SHP-2, the activation of which is responsible for mitochondrial apoptosis. This mechanism clearly represents a new paradigm in GPCR signalling.
Orexins and orexin receptors: a short survey of general features
The two orexin neuropeptides were discovered in 1998 by two independent laboratories using subtractive cDNA cloning (de Lecea et al., 1998) or as endogenous ligands for two orphan GPCRs (Sakurai et al., 1998). They were referred to as orexin-A and orexin-B (Sakurai et al., 1998) or hypocretin-1 and hypocretin-2 (de Lecea et al., 1998). Similarly, the receptors were named OX1R and OX2R (Sakurai et al., 1998) or Hctr1 and Hctr2 (de Lecea et al., 1998). Both names are problematic because the regulation of feeding and appetite is probably not the major action of the neuropeptides, and they have no clear sequence homology with secretin. In the absence of recommendation by the International Union of Pharmacology and for the sake of clarity, we have used the names orexin and orexin receptor OXR in this review.
Several reviews on central or peripheral actions of orexins as well as on orexin receptors have been published during the last decade (Voisin et al., 2003; Heinonen et al., 2008; de Lecea, 2010; Kodadek and Cai, 2010; Laburthe et al., 2010; Sakurai et al., 2010; Scammell and Winrow, 2011). The main features are summarized in the following discussion. Orexin-A and orexin-B are encoded by the same gene and originate from a single precursor synthesized by hypothalamic neurons that project throughout the brain. They regulate sleep, wakefulness, breathing, reward system and drug addiction. Their strong impact on sleep–wakefulness is emphasized in human pathology, because orexin deficiency results in narcolepsy and cataplexy (Nishino et al., 2000). Functions of orexins have been also described in peripheral tissues including digestive tract, pancreas, gonads and adrenal glands. The expression of orexins at the periphery (Johren et al., 2001) needs to be clarified, and their presence in blood is still debatable (Voisin et al., 2003; Heinonen et al., 2008). The actions of orexins are mediated by two seven-pass transmembrane GPCRs OX1R and OX2R that recognize with poor selectivity the two closely related orexins that share 46% amino acid identity in humans. Classically, the activation of both orexin receptors induces cellular calcium transients through increase of intracellular inositol triphosphate (InsP3), and the OX1R has been also shown to be linked to calcium influx through transient receptor potential cation 3 channel (Peltonen et al., 2009). Orexin receptors belong to the class A of GPCRs.
Discovery of orexins as pro-apoptotic peptides in colon cancer
The discovery of orexins as pro-apototic peptides came from the screening of peptide receptor agonists for their ability to inhibit colon cancer cell growth. We tested 26 peptide hormones and neuropeptides claimed to be expressed in the gut, and the screen was performed with the human colon cancer cell line Human tumour (HT)-29 grown in standard conditions in the presence of the robust growth-promoting effect of 10% fetal calf serum. Only two closely related peptides orexin-A and orexin-B were shown to inhibit HT29 cell growth (Rouet-Benzineb et al., 2004). Orexins do not alter cell cycle and cell proliferation but promote cell apoptosis with typical externalization of plasma membrane phosphatidylserine, chromatin condensation and DNA fragmentation of nuclei (Rouet-Benzineb et al., 2004; Voisin et al., 2008). It appeared shortly that: (i) among the two orexin receptors OX1R and OX2R, only OX1R is expressed by HT29 cells and is responsible for orexin-induced apoptosis; and (ii) orexins induce mitochondrial apoptosis with cytochrome c release from mitochondria to cytosol and activation of caspase-3 and caspase-7 (Rouet-Benzineb et al., 2004).
Aberrant expression of OX1R in colorectal tumours and metastases
In the early report on orexin receptors in colon cancer cells, it was already shown that OX1R is expressed in the human colon cancer cell lines HT29, Caco-2, SW480 and LoVo but not in explant cultures of dissected human normal colonic mucosa (Rouet-Benzineb et al., 2004). It is now known that the OX1R is present in 100% of primary colorectal tumours tested by reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR) and/or immunohistochemistry, including 21 tumours of the proximal colon and 17 tumours of the distal colon whatever their Dukes' stages (Voisin et al., 2011). In sharp contrast, normal colonocytes adjacent to tumours as well as normal proximal and distal mucosae of patients with irritable bowel syndrome, taken as controls, are always negative for OX1R expression. Thus, OX1R is aberrantly expressed in epithelial cells during colon carcinogenesis (Voisin et al., 2011). The molecular mechanisms whereby OX1R is ectopically expressed during colon cancer progression are still unknown. The gene encoding OX1R in humans maps to chromosomal region 1p33 (Sakurai et al., 1998) within the 1p32-36 loci, which are known to undergo genetic changes in human colorectal tumourigenesis (Hanash, 1996). These genetic changes may hinder the repression of OX1R gene that occurs in a normal colon. However, many other hypotheses could be raised including epigenetic alterations in colon cancer (Grady and Carethers, 2008) of the OX1R gene.
The possibility to use OX1R-targeted agonists in order to induce apoptosis of colon cancer cells (see further discussion) is more relevant in metastases than in the primary tumour, which can be resected. In this context, it is important to consider the fact that the status of a given receptor may be different in primary tumours and metastases. For example, the EGFR is expressed in most primary colorectal tumours, but a loss of EGFR expression is observed in a significant number of lymph node and liver metastases, an event that has evident implication for the treatment with EGFR-targeted antibodies (Scartozzi et al., 2004; Bralet et al., 2005). A large body of evidence indicates that OX1R is present in colon tumour metastases. The receptor is expressed in all hepatic metastases tested as well as in human colon cancer cell lines established from lymph nodes, ascite and lung metastases (Voisin et al., 2011). Figure 1 illustrates the expression of OX1R in primary colorectal tumours, local metastases in lymph modes and distant metastases in liver and lung.
Figure 1.
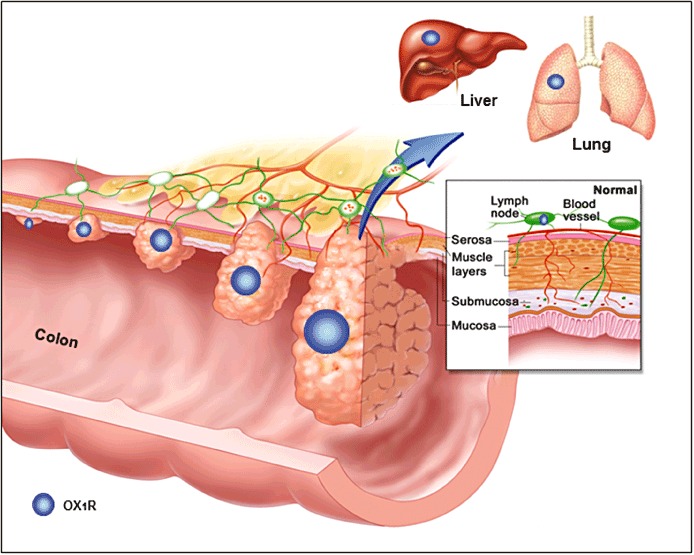
Expression of the orexin receptor OX1R during colon cancer progression and metastasis. OX1R (blue circles) is aberrantly expressed in primary tumours and metastases. The expression of OX1R was detected very early during carcinogenesis and whatever the Dukes' stage of the primary tumours. After metastasis in the colon (lymph node) and in distant organs (liver, lung), OX1R is still expressed. No OX1R is present in normal colonic epithelial cells from which cancer cells derive. Adapted with permission from © 2005 Terese Winslow, US Government has certain rights.
The orexin receptor OX1R mediates apoptosis in colon cancer cells in vitro and in vivo
The expression of OX1R by primary colorectal tumours as well as in metastases allows to induce apoptosis of cancer cells upon treatment with orexins. In vitro, the extent of orexin-induced apoptosis in various human colon cancer cell lines is clearly correlated to the level of OX1R mRNA expression determined by RT-qPCR (Voisin et al., 2011). Moreover, apoptosis can be induced in cell lines established from primary tumours and metastases as well (Voisin et al., 2011). In view of the fact that failure of tumour cells to undergo apoptosis translates into tumour progression and chemotherapeutic resistance and given apoptosis emerges as a potential target for cancer treatment at various stages of tumour progression (Watson, 2004; 2006; Huerta et al., 2006), the OX1R becomes a new promising therapeutic target in colon cancer. However, two important issues had to be resolved for progress in this direction.
The first issue is related to colon cancer resistance to chemotherapy (Wolpin and Mayer, 2008; Segal and Saltz, 2009). The most commonly used drug, 5-fluorouracil (5-FU), is able to induce apoptosis in colon cancer, but development of drug resistance is a primary cause of failure of chemotherapy. In this context, whether OX1R is expressed and is able to mediate orexin-induced apoptosis in 5-FU-resistant colon cancer cells are crucial questions. They have been addressed using the HT29-FU colon cancer cell line model in which a long-term 5-FU exposure selected cells resistant to the drug (Lesuffleur et al., 1991). The OX1R expression is similar in HT-29-FU cells and parental HT-29 cells (Voisin et al., 2011). Moreover, treatment of HT-29-FU cells with orexins induces apoptosis and subsequent growth inhibition. This observation has important implications for colon cancer therapy and suggests that orexins promote apoptosis through a mechanism that is different from that of 5-FU and that persists in 5-FU-resistant cells (Voisin et al., 2011).
The second issue is related to the efficacy of orexin treatment in vivo. This has been addressed using human colon cancer cells xenografted in nude mice. Upon subcutaneous inoculation of LoVo cells established from left supraclavicular metastases of colon cancer, a tumour develops rapidly at the site of inoculation. Daily i.p. injection of orexin-A beginning the day cancer cells are xenografted, dose-dependently reduces tumour volume. After a 15 day treatment, the tumour volume is decreased by ∼80% with a dose as low as 1 µmol orexin-A/Kg (Voisin et al., 2011). Similar data were reported with xenografts of HT-29 cells established from a primary colon tumour (Voisin et al., 2011). Much more relevant in terms of therapy is the ability of orexins to rapidly and strongly reverse the development of established tumours as demonstrated by treatment of LoVo tumours in nude mice that is initiated 7 days after inoculation of cancer cells (Voisin et al., 2011). It has been shown that orexins inhibit cultured colon cancer cell growth by inducing apoptosis (Rouet-Benzineb et al., 2004). It is likely that orexins also reduce tumour growth in vivo by promoting apoptosis, because activation of caspase-3 occurs in tumours upon orexin treatment (Voisin et al., 2011). The direct action of orexin injected in nude mice on OX1R-bearing cancer cells in the tumour is supported by experiments with the only colon cancer cell line HCT116, which does not express OX1R. Indeed, xenografts of HCT116 cells in nude mice result in development of tumours that are insensitive to orexin treatments (Voisin et al., 2011). In conclusion, activation by exogenous orexins results in strong decrease of tumour development in mice xenografted with colon cancer cells in vivo without any adverse effect of orexins during treatment. Another interesting point is that long-term treatment of nude mice with orexins does not down-regulate OX1R mRNA levels in tumours (Voisin et al., 2011). It is thus unlikely that down-regulation of OX1R in tumours upon orexin treatment may become a cause of resistance.
OX1R: an Achilles heel of colon cancers?
The widespread expression of OX1R in primary colorectal cancers and metastases, as well as the ability of exogenous orexins to promote colon cancer cell apoptosis and inhibition of tumour growth in vivo, raise several comments:
OX1R is expressed in primary colon tumours but not in normal colonic epithelial cells. Likewise, OX1R is still expressed after migration of colon cancer cells in their main site of metastasis, for example, the liver, but not in normal liver cells (Voisin et al., 2011). This represents an important feature in view of the possible use of OX1R as a therapeutic target. In this context, it is interesting to consider the fact that besides OX1R, which induces apoptosis through the intrinsic or mitochondrial pathway (Rouet-Benzineb et al., 2004), colon cancer cells also express Fas receptors (O'Connell et al., 2000), which, as death receptors, induce apoptosis through the extrinsic apoptosis pathway (Watson, 2004; 2006; Huerta et al., 2006). Unfortunately, most colon cancer cell lines are somewhat resistant to Fas ligand-mediated apoptosis even if they are positive to Fas receptors (Huerta et al., 2006). Moreover, normal colonic epithelial cells and hepatocytes are exquisitely sensitive to Fas-mediated apoptosis (Huerta et al., 2006). This strongly limits the potential use of FasR agonists as possible candidates for chemotherapeutic intervention, because patients' cancer cells would remain relatively resistant to apoptosis, whereas normal colon and liver cells would be destined to commit suicide (Huerta et al., 2006). Similarly, tumour necrosis factor (TNF)-related apoptosis ligand (TRAIL)-mediated resistance to apoptosis in colon cancer has been noted at multiple steps in the extrinsic pathway of apoptosis (van Geelen et al., 2004) limiting the potential therapeutic use of TRAIL as an inductor of apoptosis in tumour cells. Attempts to use TNF and Fas ligand have also been thwarted by induction of NFκB-mediated inflammation and fulminant hepatic failure respectively (Watson, 2004). In the present state of our knowledge, OX1R clearly does not suffer from any of the limits encountered with death receptors. Another remarkable property of OX1R-mediated apoptosis in colon cancer cells is that it works in 5-FU-resistant cells (Voisin et al., 2011) making OX1R a potential therapeutic target for OX1R agonists that would be able to act in combination with classical chemotherapies in colon cancer (Wolpin and Mayer, 2008; Segal and Saltz, 2009).
OX1R in colon cancer might be considered as a new type of gene in cancer, because it is aberrantly expressed as a functional protein whose function, when activated by an agonist, is to promote apoptosis of the cancer cell. The colon cancer cells unexpectedly provide a new gate to promote their death, which was not present in the normal colonocytes from which they derive. In this context, an important question arises: is OX1R activated in colorectal primary tumours or metastases in vivo by endogenous orexins? The answer is probably no for two main reasons: (i) colon tumours do not express the orexin precursor mRNA (Voisin et al., 2011), ruling out the possibility that OX1R in colon cancer cells might be activated by an intracrine pathway or by an autocrine loop; (ii) the major source of orexins is the brain where the orexinergic neurons are restricted to the hypothalamus (see earlier discussion). The sites of synthesis of orexins in the periphery are still debatable. Though early immunohistochemical studies detected orexins in the small intestine, stomach and pancreas in rodents (Kirchgessner and Liu, 1999), further RT-qPCR experiments identified orexin precursor mRNA in rat testis but not in most other peripheral tissues including the gut and liver (Johren et al., 2001). This is in line with the absence of orexin precursor mRNA in normal human colonic mucosa and liver tissues. In this context, the OX1R aberrantly expressed in colon cancer cells is most probably not activated by endogenous orexins in patients. On the other hand, there is no evidence suggesting that OX1R exhibits constitutive activity in the absence of ligand, because transfection of OX1R in CHO cells or HEK cells does not enhance basal apoptosis, whereas it confers the ability of orexins to promote apoptosis in those cells (Voisin et al., 2008 and M. Laburthe and T. Voisin, unpubl. data). Therefore, it may be suggested that OX1R in colorectal cancer constitutes a gate to apoptosis that probably remains unopened in vivo but could be openable by therapeutic administration of exogenous orexins or OX1R agonists. In that respect, OX1R might be considered as an Achilles heel of colon cancer, because targetting OX1R with agonists leads to cancer cell death by apoptosis. The development of long-lived peptide agonists or non-peptide agonists of orexin receptors will represent thereby an important advance not only in neuroscience (Boss et al., 2009) but also in colon cancer research. The OX1R, orexins and forthcoming OX1R agonists might be novel candidates for colorectal cancer therapy.
OX1R-driven apoptosis: a novel mechanism for a GPCR involving ITIMs
At first sight, the OX1R appears to be a classical Gq-coupled receptor the activation of which induces calcium transients (Voisin et al., 2003). This classical Gq-mediated calcium response is certainly not sufficient to explain the OX1R-driven apoptosis even though calcium participates in the onset of apoptosis (Rizzuto et al., 2003). Indeed, several GPCRs in colon cancer cells do promote an increase in intracellular calcium but not only do not trigger apoptosis and rather stimulate cell proliferation, that is, muscarinic receptors (Medina and Rivera, 2010), neurotensin NT1 receptor (Maoret et al., 1999), protease-activated receptor-2 (Darmoul et al., 2001) or protease-activated receptor-1 (Darmoul et al., 2004). Moreover, inhibition of intracellular InsP3 increase abolishes OX1R-mediated calcium transients but does nothing to OX1R-driven apoptosis (Voisin et al., 2008). Finally, promotion of apoptosis by orexins is an intrinsic property of OX1R, because transfection of the receptor cDNA in cells devoid of endogenous OX1R is sufficient to confer the ability of orexins to promote apoptosis as shown in Chinese hamster ovary CHO cells (Rouet-Benzineb et al., 2004; Ammoun et al., 2006; Voisin et al., 2008; El Firar et al., 2009) and mouse embryonic fibroblast (MEF) cells (Voisin et al., 2008). Altogether, these observations prompted us to analyse the sequence of OX1R for identification of new motifs that might be associated with its ability to trigger apoptosis. We identified two tyrosine-based motifs in OX1R and demonstrated their crucial role in OX1R-driven apoptosis (Voisin et al., 2008; El Firar et al., 2009). The first motif to be characterized (Voisin et al., 2008) is a canonical ITIM present in the intracellular domain connecting the seventh transmembrane helix to the C-terminal tail of the OX1R (Figure 2). The consensus ITIM sequence (Ile/Val/Leu/Ser)-X-Tyr-X-X-(Ile-Leu-Val) is not considered to be a signature of GPCRs but represents a hallmark of immune inhibitory receptors on lymphoid and myeloid cells, the immunoglobulin G Fc-receptor FcγRIIB being prototypical of such receptors (Ravetch and Lanier, 2000; Daeron et al., 2008). The second motif to be characterized (El Firar et al., 2009) is an immunoreceptor tyrosine-based switch motif (ITSM) present in the intracellular domain connecting the first intracellular loop to the second transmembrane helix of the OX1R (Figure 2). The consensus sequence of ITSM, Thr-X-Tyr-X-X-(Val,Ile) had never been identified previously in any GPCRs but was previously characterized in the signalling lymphocyte-activating molecule family of immunoreceptors (Ostrakhovitch and Li, 2006). This sequence is ITIM-like, and it may be suggested that ITIM and ITSM are very similar with a common permissive sequence (Ile/Leu/Val/Ser/thr)-X-Tyr-X-X-(Ile,Leu,Val). Indeed, ITIMs and ITSMs appear to function following the same paradigm because both motifs contain tyrosine residues that can be phosphorylated on activation of the corresponding immunoreceptors (see Sidorenko and Clark, 2003; Daeron et al., 2008 for reviews).
Figure 2.
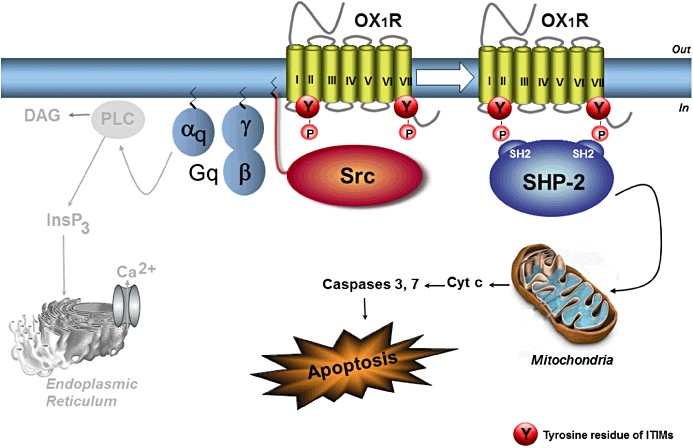
Mechanism of OX1R-driven apoptosis. Activation of OX1R by orexins promotes the dissociation of Gq protein into αq and βγ dimers. The classical pathway resulting in phospholipase C activation by αq is not involved in OX1R-mediated apoptosis and is shown in grey. Freed βγ dimers stimulate the Src-tyrosine kinase resulting in the phosphorylation of the two ITIMs of OX1R. The phosphotyrosine phosphatase SHP-2 is then recruited by OX1R and activated thereby. Activated SHP-2 is mandatory for subsequent cytochrome c-mediated mitochondrial apoptosis. PLC, phospholipase C; Cyt c, cytochrome c; DAG, diacylglycerol; SHP-2, SH2 domain-containing phosphotyrosine phosphatase-2. See text for details.
The mechanism of OX1R-driven apoptosis is schematized in Figure 2 and is the following. On activation of OX1R by orexins, the tyrosine-based motifs ITIM and ITSM are tyrosine phosphorylated (Voisin et al., 2008; El Firar et al., 2009). This is a Gq-mediated event even though classical activation of phospholipase C is not involved (see earlier discussion). Indeed, transfection of OX1R cDNA in Gq-deficient MEF cells does not confer the ability of orexins to promote apoptosis, whereas it does in Gq-bearing MEF cells (Voisin et al., 2008). The activation of OX1R allows the dissociation of the Gq protein into αq and βγ dimers. The freed βγ dimers are known to activate Src-like tyrosine kinases (Gentili et al., 2006), and experimental sequestration of βγ dimers in cells (Koch et al., 1994) abolishes OX1R-driven apoptosis (M. Laburthe and T. Voisin, unpubl. data). Next, activated Src kinases phosphorylate tyrosine within ITIM and ITSM of OX1R as demonstrated using inhibitors of Src (Voisin et al., 2008) and transfection of a dominant negative mutant of Src (M. Laburthe and T. Voisin, unpubl. data). The phosphorylation of the two motifs ITIM and ITSM is crucial, because mutation of tyrosine in either motif totally abolishes OX1R-driven apoptosis (El Firar et al., 2009). On tyrosine phosphorylation of ITIM and ITSM, the receptor recruits the phosphotyrosine phosphatase SHP-2 and thereby activates it (Voisin et al., 2008; El Firar et al., 2009). The activation of SHP-2 represents an early event in the initiation of apoptosis, and SHP-2 activation is mandatory in the process of orexin-induced apoptosis. This mechanism accounts for the dual participation of Gq and ITIMs in OX1R-driven apoptosis and explains why the classical Gq-mediated pathway resulting in the activation of phospholipase C is not involved in the apoptotic response (Figure 2). This mechanism is novel in GPCRs, although it follows the general paradigm for ITIM function in immunoreceptors (Ravetch and Lanier, 2000; Daeron et al., 2008). In that respect, it is interesting to consider the fact that ITIMs function in tandem in immunoreceptors, and the phosphoprotein phosphatases require their two SH2 domains to bind to adjacent ITIMs separated by a short connecting peptide within the receptor (Bruhns et al., 1999). In OX1R, the two sites ITIM and ITSM are far from each other in the primary sequence of the protein (Figure 2). A structural model of the human OX1R has been developed showing that the distance between the two phosphorus atoms of phosphotyrosines in ITSM and ITIM compares well with the phosphopeptide binding sites in the SH2 domain of the protein phosphatase SHP-2 (El Firar et al., 2009). It is thus suggested that ITIMs function in a spatial tandem in OX1R (Laburthe et al., 2010) contrasting with the linear tandems of two adjacent sites described in immunoreceptors (Daeron et al., 2008).
The mechanism of OX1R-driven apoptosis schematized in Figure 2 implies that the βγ dimers of Gq are freed upon receptor activation and stimulate Src-tyrosine kinase, which in turn phosphorylates ITIMs initiating the apoptotic response thereby. Quite evidently, free βγ dimers are also released during the activation of all GPCRs, and nevertheless, this does not result in apoptosis. The mechanisms that create selectivity in tyrosine phosphorylation of OX1R ITIMs by Src are currently unknown. Two nonexclusive mechanisms can be suggested: (i) compartmentalization of the signalling complex may occur. Indeed, the Gβγ dimer, which is membrane associated due to the isoprenylation of the Gγ subunit (Marrari et al., 2007), is likely to remain tethered to the activated receptor. Because Src can associate to the plasma membrane owing to its myristoylation and the presence of six basic residues at its amino terminus (Bjorge et al., 2000), it could be activated at the vicinity of the activated OX1R by the Gβγ dimer (Figure 3); (ii) a multi-step mechanism of activation of OX1R may also occur (Figure 3). Binding of orexins to OX1R leads to the Gq protein dissociation from the receptor to yield a Gαq-GTP monomer (see Figure 2) and a Gβγ dimer, which activates Src-tyrosine kinase. A speculative further step of OX1R activation would lead to another change in receptor conformation allowing exposure of tyrosine in ITIM and ITSM of OX1R. This step would be necessary for phosphorylation of ITIM and ITSM in the receptor. We synthesized orexin analogs, which are able to promote intracellular calcium transients but are totally unable to promote apoptosis (M. Laburthe and T. Voisin, unpubl. data). These data may fit with the sequential conformational changes of OX1R suggested in Figure 3, although selection of distinct conformers consistent with ligand-directed signalling may also contribute. Whatever the mechanism(s), after tyrosine phosphorylation of ITIM and ITSM, the OX1R recruits SHP-2 initiating the apoptotic response (see Figure 2).
Figure 3.
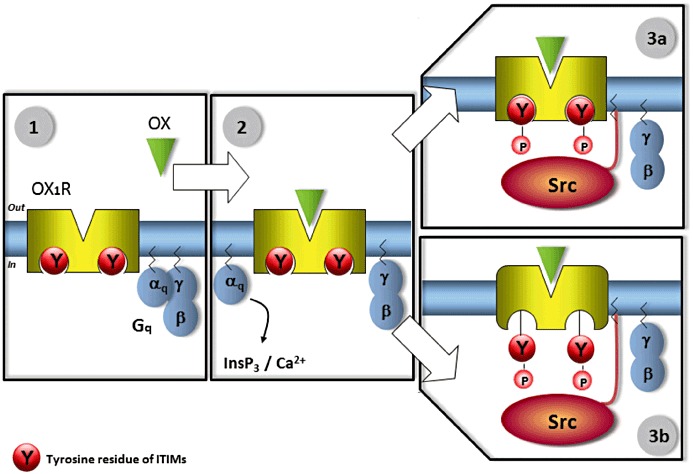
Proposed multi-step mechanisms of activation of OX1R to account for the high specificity of the OX1R-driven apoptosis. In step 1, OX1R is not activated by orexins and the tyrosine residues of ITIMs are not phosphorylated (resting step). During step 2, binding of orexins to OX1R leads to the Gq protein dissociation from the receptor to yield a Gαq-GTP monomer and a Gβγ dimer, which are now free to modulate the activity of other intracellular protein. The Gαq-GTP monomer activates phospholipase C leading to an increase in intracellular Ca++, a process that is not involved in the apoptotic response. In Step 3a, the mechanism that could create selectivity in tyrosine phosphorylation of ITIMs by Src is compartmentalization of the signalling complex in which the Gβγ dimer activates Src at the vicinity of the activated OX1R. Alternatively or additionally, a speculative Step 3b would lead to another ligand-induced change in receptor conformation allowing exposure of tyrosine in the two ITIMs of OX1R. This step would be necessary for phosphorylation of ITIMs in the receptor. This scheme including Steps 3a and/or 3b would explain why all other GPCRs that are also able to free Gβγ dimers are not able to phosphorylate ITIMs in OX1R and to promote apoptosis. After tyrosine phosphorylation of ITIMs, the OX1R processes to further steps by recruiting SHP-2 resulting in the activation of the tyrosine phosphatase and the apoptotic response (see Figure 2).
Recruitment and activation of SHP-2 by OX1R is such a proximal point of apoptosis regulation that many subsequent apoptosis-related events ending in release of cytochrome c from mitochondria (Rouet-Benzineb et al., 2004) are currently under investigation in our group. At this stage, the key targets of SHP-2 leading to apoptosis remain elusive, as well as the targets of the tyrosine phosphatases SHPs in general (Neel et al., 2003).
Conclusions and perspectives
The characterization of orexins as pro-apoptotic peptides in colon cancers and the entirely novel mechanism whereby the OX1 receptor triggers apoptosis through phosphorylation of ITIMs open many questions or speculations and pave the way for promising perspectives. We would like to discuss four points:
There is considerable interest in the development of small-molecule orexin receptor antagonists as a novel therapy for the treatment of insomnia and more generally of those medical and psychiatric conditions associated with disturbed vigilance (Boss et al., 2009; Coleman and Renger, 2010; Kodadek and Cai, 2010; Scammell and Winrow, 2011). It is to be hoped that all these studies may also lead to the discovery of small-molecule agonists of orexin receptors, which may prove therapeutically useful in the treatment of colon cancer. This should be possible because orexin receptors are peptide receptors belonging to class A or rhodopsin-like GPCRs for which many nonpeptide agonists are now available. In this context, we do encourage pharmaceutical companies to screen chemical libraries not only for orexin receptor antagonist but also for agonists.
The possibility of the expression of orexin receptors by other solid tumours in humans is an important issue and is currently under investigation. We have already shown that OX1R is expressed in a neuroblastoma cell line that undergoes apoptosis upon orexin treatment (Rouet-Benzineb et al., 2004). The OX2R is present in a pancreatic carcinoma cell line in which orexin-induced apoptosis has been characterized (Voisin et al., 2006). Orexin receptors have been also characterized in adrenocortical adenomas (Spinazzi et al., 2005) but relation to apoptosis is not documented.
Orexin neurons originating from the hypothalamus project throughout the brain where orexin receptors are widespread (see earlier discussion). The question thus arises of whether brain orexin receptors drive apoptosis in health and diseases. Normal adult brain neurons, which express orexin receptors and are stimulated by endogenous orexins, do not undergo apoptosis. This may be related to the high resistance of differentiated neurons to apoptosis induced by cytochrome c (Wright et al., 2004), the mechanism whereby orexins induce apoptosis. In fact, mature neurons do not express detectable levels of Apaf-1 to which cytochrome c binds leading to the formation of the apoptosome (Johnson et al., 2007). Because aberrant neuronal cell death is an outstanding feature of neurodegenerative diseases (Bredesen et al., 2006), we previously made the hypothesis that orexin receptors may participate in the onset of apoptosis during neurodegeneration (Laburthe et al., 2010). In this context, it is interesting to notice that a dual orexin receptor antagonist decreases amyloid-β plaque formation in a transgenic mice model suggesting that orexins may play a role in the pathogenesis of Alzheimer's disease (Kang et al., 2009).
A functional role of ITIMs in GPCRs has been described in very rare cases (see El Firar et al., 2009). It is estimated that the human genome contains # 1000 genes that code for proteins of the GPCR structure with seven-transmembrane spanning domains. We previously carried out a non-exhaustive manual search for the presence of the permissive ITIM sequence, which revealed that ITIMs are much more frequent in GPCRs than initially thought (Laburthe et al., 2010). We are currently mining automatically the GPCR databases with a new algorithm that is suitable to identify the small and degenerate sequence of permissive ITIM. These studies should provide new insights into the presence of ITIMs in all classes of GPCRs and during phylogenesis.
Acknowledgments
This work was supported by INSERM, CNRS and Université Paris-Diderot.
Glossary
- 5-FU
5-fluorouracil
- EGFR
epidermal growth factor receptor
- GPCR
G protein-coupled receptor
- InsP3
inositol triphosphate
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- ITSM
immunoreceptor tyrosine-based switch motif
- OX1R
orexin receptor 1
- OX2R
orexin receptor 2
- SHP-2
Src homology domain 2-containing protein tyrosine phosphatase 2
Conflict of interest
There is no conflict of interest.
References
- Ammoun S, Lindholm D, Woortz H, Akerman KE, Kukkonen JP. G-protein-coupled OX1 orexin/hctr-1 hypocretin receptors induce caspase-dependent and -independent cell death through p38 mitogen-/stress-activated protein kinase. J Biol Chem. 2006;281:834–842. doi: 10.1074/jbc.M508603200. [DOI] [PubMed] [Google Scholar]
- Bjorge JD, Jakymiv A, Fujita DJ. Selected glimpses into the activation and function of Src kinase. Oncogene. 2000;19:5620–5635. doi: 10.1038/sj.onc.1203923. [DOI] [PubMed] [Google Scholar]
- Boss C, Brisbare-Roch C, Jenck F. Biomedical application of orexin/hypocretin receptor ligands in neuroscience. J Med Chem. 2009;52:891–903. doi: 10.1021/jm801296d. [DOI] [PubMed] [Google Scholar]
- Bralet MP, Paule B, Adam R, Guettier C. Loss of epidermal growth factor receptor expression in lymph node and liver metastases of colon carcinoma. J Clin Oncol. 2005;23:5844–5845. doi: 10.1200/JCO.2005.01.6436. [DOI] [PubMed] [Google Scholar]
- Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature. 2006;443:796–802. doi: 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhns P, Marchetti P, Fridman WH, Vivier E, Daeron M. Differential roles of N- and C-terminal immunoreceptor tyrosine-based inhibition motifs during inhibition of cell activation by killer cell inhibitory receptors. J Immunol. 1999;162:3168–3175. [PubMed] [Google Scholar]
- Chell SD, Witherden IR, Dobson RR, Moorghen M, Herman AA, Qualtrough D, et al. Increased EP4 receptor expression in colorectal cancer progression promotes cell growth and anchorage independence. Cancer Res. 2006;66:3106–3113. doi: 10.1158/0008-5472.CAN-05-3702. [DOI] [PubMed] [Google Scholar]
- Coleman PJ, Renger JJ. Orexin receptor antagonists: a review of promising compounds patented since 2006. Expert Opin Ther Pat. 2010;20:307–324. doi: 10.1517/13543770903567085. [DOI] [PubMed] [Google Scholar]
- Daeron M, Jaeger S, Du PL, Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev. 2008;224:11–43. doi: 10.1111/j.1600-065X.2008.00666.x. [DOI] [PubMed] [Google Scholar]
- Darmoul D, Marie JC, Devaud H, Gratio V, Laburthe M. Initiation of human colon cancer cell proliferation by trypsin acting at protease-activated receptor-2. Br J Cancer. 2001;85:772–779. doi: 10.1054/bjoc.2001.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmoul D, Gratio V, Devaud H, Lehy T, Laburthe M. Aberrant expression and activation of the thrombin receptor protease-activated receptor-1 induces cell proliferation and motility in human colon cancer cells. Am J Pathol. 2003;162:1503–1513. doi: 10.1016/S0002-9440(10)64283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmoul D, Gratio V, Devaud H, Laburthe M. Protease-activated receptor 2 in colon cancer: trypsin-induced MAPK phosphorylation and cell proliferation are mediated by epidermal growth factor receptor transactivation. J Biol Chem. 2004;279:20927–20934. doi: 10.1074/jbc.M401430200. [DOI] [PubMed] [Google Scholar]
- El Firar A, Voisin T, Rouyer-Fessard C, Ostuni MA, Couvineau A, Laburthe M. Discovery of a functional immunoreceptor tyrosine-based switch motif in a 7-transmembrane-spanning receptor: role in the orexin receptor OX1R-driven apoptosis. FASEB J. 2009;23:4069–4080. doi: 10.1096/fj.09-131367. [DOI] [PubMed] [Google Scholar]
- van Geelen CM, de Vries EG, de Jong S. Lessons from TRAIL-resistance mechanisms in colorectal cancer cells: paving the road to patient-tailored therapy. Drug Resist Updat. 2004;7:345–358. doi: 10.1016/j.drup.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Gentili C, Boland R, Russo de Boland A. Implication of G beta gamma proteins and c-SRC tyrosine kinase in parathyroid hormone-induced signal transduction in rat enterocytes. J Endocrinol. 2006;188:69–78. doi: 10.1677/joe.1.06397. [DOI] [PubMed] [Google Scholar]
- Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135:1079–1099. doi: 10.1053/j.gastro.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratio V, Walker F, Lehy T, Laburthe M, Darmoul D. Aberrant expression of proteinase-activated receptor 4 promotes colon cancer cell proliferation through a persistent signaling that involves Src and Erb-2 kinase. Int J Cancer. 2009;124:1517–1525. doi: 10.1002/ijc.24070. [DOI] [PubMed] [Google Scholar]
- Hanash SM. A role for chromosome 1 in colorectal cancer. Gastroenterology. 1996;111:250–252. doi: 10.1053/gast.1996.v111.agast961110250. [DOI] [PubMed] [Google Scholar]
- Heinonen MV, Purhonen AK, Mäkelä KA, Herzig KH. Functions of orexins in peripheral tissues. Acta Physiol (Oxf) 2008;192:471–485. doi: 10.1111/j.1748-1716.2008.01836.x. [DOI] [PubMed] [Google Scholar]
- Huerta S, Goulet EJ, Livingston EH. Colon cancer and apoptosis. Am J Surg. 2006;191:517–526. doi: 10.1016/j.amjsurg.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Johnson CE, Huang YY, Parrish AB, Smith MI, Vaughn AE, Zhang Q, et al. Differential Apaf-1 levels allow cytochrome c to induce apoptosis in brain tumors but not in normal neural tissues. Proc Natl Acad Sci U S A. 2007;104:20820–20825. doi: 10.1073/pnas.0709101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johren O, Neidert SJ, Kummer M, Dendorfer A, Dominiak P. Prepro-orexin and orexin receptor mRNA are differentially expressed in peripheral tissues of male and female rats. Endocrinology. 2001;142:3324–3331. doi: 10.1210/endo.142.8.8299. [DOI] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-β dynamics are regulated by orexin and the sleep-wake. Cycle Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchgessner AL, Liu M. Orexin synthesis and response in the gut. Neuron. 1999;24:941–951. doi: 10.1016/s0896-6273(00)81041-7. [DOI] [PubMed] [Google Scholar]
- Koch WJ, Hawes BE, Inglese J, Luttrell LM, Lefkowitz RJ. Cellular expression of carboxyl terminus of a G protein-coupled receptor kinase attenuates G beta gamma-mediated signaling. J Biol Chem. 1994;269:6193–6197. [PubMed] [Google Scholar]
- Kodadek T, Cai D. Chemistry and biology of orexin signaling. Mol Biosyst. 2010;6:1366–1375. doi: 10.1039/c003468a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laburthe M, Rousset M, Boissard C, Chevalier G, Zweibaum A, Rosselin G. Vasoactive intestinal peptide: a potent stimulator of adenosine 3′:5′-cyclic monophosphate accumulation in gut carcinoma cell lines in culture. Proc Natl Acad Sci U S A. 1978;75:2772–2775. doi: 10.1073/pnas.75.6.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laburthe M, Voisin T, El Firar A. Orexins/hypocretins and orexin receptors in apoptosis: a mini-review. Acta Physiol (Oxf) 2010;198:393–402. doi: 10.1111/j.1748-1716.2009.02035.x. [DOI] [PubMed] [Google Scholar]
- Lappano R, Maggiolini M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10:47–60. doi: 10.1038/nrd3320. [DOI] [PubMed] [Google Scholar]
- de Lecea L. A decade of hypocretins: past, present and future of the neurobiology of arousal. Acta Physiol (Oxf) 2010;198:203–208. doi: 10.1111/j.1748-1716.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesuffleur T, Kornowski A, Luccioni C, Muleris M, Barbat A, Beaumartin J, et al. Adaptation to 5-fluorouracil of the heterogeneous human colon cancer tumor cell line HT-29 results in the selection of cells committed to differentiation. Int J Cancer. 1991;49:721–730. doi: 10.1002/ijc.2910490516. [DOI] [PubMed] [Google Scholar]
- Maoret JJ, Pospai D, Rouyer-Fessard C, Couvineau A, Voisin T, Laburthe M. Neurotensin receptor and its mRNA are expressed in many human colon cancer cell lines but not in normal colonic epithelium: binding studies and RT-PCR experiments. Biochem Biophys Res Commun. 1994;203:465–471. doi: 10.1006/bbrc.1994.2205. [DOI] [PubMed] [Google Scholar]
- Maoret JJ, Anini Y, Rouyer-Fessard C, Gully D, Laburthe M. Neurotensin and a non-peptide neurotensin receptor antagonist control human colon cancer cell growth in cell culture and in cells xenografted into nude mice. Int J Cancer. 1999;80:448–454. doi: 10.1002/(sici)1097-0215(19990129)80:3<448::aid-ijc19>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Markowitz SD, Bertagnolli MM. Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrari Y, Crouthamel M, Irannejad R, Wedegaertner PB. Assembly and trafficking of heterotrimeric G proteins. Biochemistry. 2007;46:7665–7677. doi: 10.1021/bi700338m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina VA, Rivera ES. Histamine receptors and cancer pharmacology. Br J Pharmacol. 2010;161:755–767. doi: 10.1111/j.1476-5381.2010.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel BG, Gu H, Pao L. The ‘Shp'ing news: SH2 domain-containing tyrosine phosphatase in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- O'Connell J, Bennett MW, Nally K, Houston A, O'Sullivan GC, Shanahan F. Altered mechanisms of apoptosis in colon cancer: Fas resistance and counterattack in the tumor-immune conflict. Ann N Y Acad Sci. 2000;910:178–192. doi: 10.1111/j.1749-6632.2000.tb06708.x. [DOI] [PubMed] [Google Scholar]
- Ostrakhovitch EA, Li SS. The role of SLAM family of receptors in immune cell signaling. Biochem Cell Biol. 2006;84:832–843. doi: 10.1139/o06-191. [DOI] [PubMed] [Google Scholar]
- Peltonen HM, Magga JM, Bart G, Turunen PM, Antikainen MS, Kukkonen JP, et al. Involvement of TRPC3 channels in calcium oscillations mediated by OX(1) orexin receptor. Biochem Biphys Res Commun. 2009;385:408–412. doi: 10.1016/j.bbrc.2009.05.077. [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton R, Ferrari D, Chami M, Szabadkai G, Magalhaes PJ, et al. Calcium and apoptosis: facts and hypotheses. Oncogene. 2003;22:8619–8627. doi: 10.1038/sj.onc.1207105. [DOI] [PubMed] [Google Scholar]
- Rouet-Benzineb P, Rouyer-Fessard C, Jarry A, Avondo V, Pouzet C, Yanagisawa M, et al. Orexins acting at native OX(1) receptor in colon cancer and neuroblastoma cells or at recomninant OX(1) receptor suppress cell growth by inducing apoptosis. J Biol Chem. 2004;279:45875–45886. doi: 10.1074/jbc.M404136200. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Mieda M, Tsujino N. The orexin system: roles in sleep/wake regulation. Ann N Y Acad Sci. 2010;1200:149–161. doi: 10.1111/j.1749-6632.2010.05513.x. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Winrow CJ. Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2011;51:243–266. doi: 10.1146/annurev-pharmtox-010510-100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scartozzi M, Bearzi I, Berardi R, Mandelosi A, Fabris G, Cascinu S. Epidermal growth factor receptor (EGFR) status in primary colorectal tumors does not correlate with EGFR expression in related metastatic sites: implications for treatment with EGFR-targeted monoclonal antibodies. J Clin Oncol. 2004;22:4772–4778. doi: 10.1200/JCO.2004.00.117. [DOI] [PubMed] [Google Scholar]
- Segal NH, Saltz LB. Evolving treatment of advanced colon cancer. Annu Rev Med. 2009;60:207–219. doi: 10.1146/annurev.med.60.041807.132435. [DOI] [PubMed] [Google Scholar]
- Sidorenko SP, Clark EA. The dual-function CD150 receptor subfamily: the viral attraction. Nat Immunol. 2003;4:19–24. doi: 10.1038/ni0103-19. [DOI] [PubMed] [Google Scholar]
- Singh P, Dai B, Wu H, Owlia A. Role of autocrine and endocrine gastrin-like peptides in colonic carcinogenesis. Curr Opin Gastroenterol. 2000;16:68–77. doi: 10.1097/00001574-200001000-00013. [DOI] [PubMed] [Google Scholar]
- Spinazzi R, Rucinski M, Neri G, Malendowicz LK, Nussdorfer GG. Preproorexin and orexin receptors are expressed in cortisol-secreting adrenocortical adenomas, and orexins stimulate in vitro cortisol secretion and growth of tumor cells. J Clin Endocrinol Metab. 2005;90:3544–3549. doi: 10.1210/jc.2004-2385. [DOI] [PubMed] [Google Scholar]
- Voisin T, Rouet-Benzineb P, Reuter N, Laburthe M. Orexin and their receptors: structural aspects and role in peripheral tissues. Cell Mol Life Sci. 2003;60:72–87. doi: 10.1007/s000180300005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin T, El Firar A, Avondo V, Laburthe M. Orexin-induced apoptosis: the key role of the seven-transmembrane domain orexin type 2 receptor. Endocrinology. 2006;147:4977–4984. doi: 10.1210/en.2006-0201. [DOI] [PubMed] [Google Scholar]
- Voisin T, El Firar A, Rouyer-Fessard C, Gratio V, Laburthe M. A hallmark of immunoreceptor, the tyrosine-based inhibitory motif ITIM, is present in the G protein-coupled receptor OX1R for orexins and drives apoptosis: a novel mechanism. FASEB J. 2008;22:1993–2002. doi: 10.1096/fj.07-098723. [DOI] [PubMed] [Google Scholar]
- Voisin T, El Firar A, Fasseu M, Rouyer-Fessard C, Descatoire V, Walker F, et al. Aberrant expression of OX1 receptors for orexins in colon cancers and liver metastases: an openable gate to apoptosis. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-10-3473. Published OnlineFirst March 17, 2011; doi:10.1158/0008-5472.CAN-10-3473. [DOI] [PubMed] [Google Scholar]
- Watson AJ. Apoptosis and colorectal cancer. Gut. 2004;53:1701–1709. doi: 10.1136/gut.2004.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AJ. An overview of apoptosis and the prevention of colorectal cancer. Crit Rev Oncol Hematol. 2006;57:107–121. doi: 10.1016/j.critrevonc.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Wolpin BM, Mayer RJ. Systemic treatment of colorectal cancer. Gastroenterology. 2008;134:1296–1310. doi: 10.1053/j.gastro.2008.02.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KM, Linhoff MW, Potts PR, Desmukh M. Decreased apoptosome activity with neuronal differentiation sets the threshold for strict IAP regulation of apoptosis. J Cell Biol. 2004;167:303–313. doi: 10.1083/jcb.200406073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhong WW, Srivastava N, Yang J, Hoey T, An S. G protein-coupled lysophosphatidic acid receptors stimulate proliferation of colon cancer cells through the β-catenin pathway. Proc Natl Acad Sci U S A. 2005;102:6027–6032. doi: 10.1073/pnas.0501535102. [DOI] [PMC free article] [PubMed] [Google Scholar]


