Abstract
BACKGROUND AND PURPOSE
Growing evidence suggests that long-term abuse of ketamine does harm the heart and increases the risk of sudden death. The present study was performed to explore the cardiotoxicity of ketamine and the protective effects of metoprolol.
EXPERIMENTAL APPROACH
Rats and rabbits were divided into control, ketamine, metoprolol alone and ketamine plus metoprolol groups. Ketamine (40 mg·kg−1·day−1, i.p.) and metoprolol (20 mg·kg−1·day−1, p.o.) were administered continuously for 12 weeks in rats and 8 weeks in rabbits. Cardiac function, electrophysiological disturbances, cardiac collagen, cardiomyocte apoptosis and the remodelling-related proteins were evaluated.
KEY RESULTS
Rabbits treated with ketamine showed decreased left ventricular ejection fraction, slowed ventricular conduction velocity and increased susceptibility to ventricular arrhythmia. Metoprolol prevented these pathophysiological alterations. In ketamine-treated rats, cardiac collagen volume fraction and apoptotic cell number were higher than those of control animals; these effects were prevented by co-administration of metoprolol. Consistently, the expressions of poly (ADP-ribose) polymerases-1, apoptosis-inducing factor and NF-κB-light-chain-enhancer of activated B cells were all increased after ketamine treatment and sharply reduced after metoprolol administration. Moreover, ketamine enhanced sympathetic sprouting, manifested as increased growth-associated protein 43 and tyrosine TH expression. These effects of ketamine were prevented by metoprolol.
CONCLUSIONS AND IMPLICATIONS
Chronic treatment with ketamine caused significant ventricular myocardial apoptosis, fibrosis and sympathetic sprouting, which altered the electrophysiological properties of the heart and increased its susceptibility to malignant arrhythmia that may lead to sudden cardiac death. Metoprolol prevented the cardiotoxicity of ketamine, indicating a promising new therapeutic strategy.
Keywords: ketamine, cardiac remodelling, malignant arrhythmia, sudden cardiac death, metoprolol
Introduction
Ketamine, an N-methyl-D-aspartate receptor antagonist, has been found to exert analgesic effects in humans and is widely used as a dissociative anaesthetic. However, in the past decade, the application of ketamine has been considered as a double-edged sword. On the one hand, it is used as an anaesthetic agent, especially in paediatric and geriatric short-lasting surgeries. On the other hand, there are more and more concerns regarding the increasing abuse of ketamine, particularly by young people in social settings. Reports have indicated that ketamine, or ‘Special K’ as it is also known, is being used recreationally in the UK, Sweden, Australia, USA and many other parts of the world (Dillon et al., 2003). This rapidly spreading misuse could result in perceptual distortions, thought disorders, emotional withdrawal and ‘melting into the surrounding’. Severe addictive practices induced by ketamine abuse are difficult to control and incite abusers to progressively increase ketamine doses. More importantly, long-term use of ketamine may damage the cardiovascular system and increase the risk of sudden death (Weiner et al., 2000). Even though there was no information on ketamine-related morbidity and mortality upon routine data collections, recently, two reports have shown unusual cases of death caused by acute or chronic ketamine poisoning, and myocardial fibrosis and hyaline degeneration of small arteries were presented in a chronic ketamine poison victim's heart (Licata et al., 1994; Tao et al., 2005). Echocardiography under ketamine–xylazine anaesthesia revealed an increased left ventricular (LV) wall thickness and a decreased LV lumen diameter (Kamphoven et al., 2001). So ketamine misapplication is not only a drug abuse problem, but could also cause long-term disruption of the cardiovascular system. However, there have been few experimental studies performed to investigate ketamine-induced toxic effects on the cardiovascular system and a corresponding pharmacological therapeutic strategy. The underlying molecular mechanisms remain to be elucidated. Therefore, we performed this study, firstly, to determine the toxic effects of chronic ketamine use on the heart, such as ventricular myocardial apoptosis, fibrosis, sympathetic and electrophysiological remodelling and increased susceptibility to malignant arrhythmia, and explore the potential molecular mechanisms. Secondly, we assessed the protective effects of metoprolol against ketamine-induced cardiac toxicity.
Methods
All of the animal care and experimental procedures complied with the international guidelines for the care of laboratory and experimental animals, together with the investigation of pain in laboratory animals, and were approved by the Institutional Animal Care Committee (The first Hospital of Harbin Medical University, Heilongjiang, China). Drug and molecular target nomenclature conforms to the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2011).
Preparation of ketamine-induced toxicity models
Male Sprague-Dawley rats (180∼200 g) and Japanese white rabbits (2.0–2.5 kg) (Experimental Animal Center of the First Affiliated Hospital, Harbin Medical University, Harbin, China) were divided into four groups randomly: control, ketamine, metoprolol alone and ketamine plus metoprolol. There are 10 rats and six rabbits in each group. Ketamine hydrochloride (Gutian pharmaceutical company, Fujian, China) was administered i.p. at the dose of 40 mg·kg−1·day−1. Metoprolol tartrate (AstraZeneca Pharmaceuticals Company, Shanghai, China) was given orally to the animals (20 mg·kg−1·day−1) immediately after ketamine injection. Control animals received a matching saline injection and/or metoprolol tartrate orally. Rats were treated for 12 weeks and rabbits for 8 weeks. Then, the animals were killed, and tissues were harvested from LV free wall.
Echocardiographic examination
After the rabbits were sedated with 10% chloral hydrate (3 mg·kg−1, i.p.), ultrasound images were obtained with a 2–4 MHz phased-array probe by using Acuson Sequoia 512 Ultrasound System (Siemens Medical Solutions USA, Inc., Mountain View, CA, USA). A parasternal short-axis view at mid-LV level was performed to measure the following parameters: LV end-systolic diameter, LV end-diastolic diameter (LVED), ventricular septum thickness and LV posterior wall thickness. Additionally, LV mass was measured by M-mode echocardiography. The total examination time was less than 10 min. After the procedure, the rabbits were allowed to recover.
Electrophysiological measurements
All the rabbits were anaesthetized with 25 mg·kg−1 pentobarbital sodium i.v. After endotracheal intubation and mechanical ventilation, the rabbits underwent open-heart procedures through a midline sternotomy, and the heart was exposed and suspended in a pericardial cradle. Two pairs of epicardial electrodes were placed on LV free wall from high to low (electrode diameter, 1 mm; interelectrode distance, 6 mm; distance between electrode pairs, 3 mm), and then connected to a Prucka 32 lead electrophysiological recorder (GE Medical Systems Information Technology, Milwaukee, WI, USA). Ventricular effective refractory period (VERP), VERP dispersion (VERPd) and conduction velocity (CV) were determined as reported previously (Yang et al., 2008).
Histological and immunohistochemical determination
After being fixed, tissues specimens were embedded in paraffin, cut about 5 µm in thickness and stained with haematoxylin–eosin. Paraffin sections were incubated with polyclonal rabbit anti-NF-κB-light-chain-enhancer of activated B cells antibody (NF-κB, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-poly ADP-ribose polymerases (PARP, Abcam Cambridge, MA, USA), anti-apoptosis-inducing factor (AIF, Abcam), anti-TH (Abcam), and anti-growth-associated protein 43 (GAP-43, Abcam) overnight at 4°C. After being rinsed, the tissue sections were reacted with peroxidase conjugated goat anti-rabbit IgG (Zhongshan, Beijing, China) at 37°C for 20 min. Lastly, they were visualized with a DAB-based colorimetric method. Positive cell density, calculated as positive cell area to total area of statistical fields, was used to determine the expression of target proteins.
Terminal deoxynucleootidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining
According to the manufacturer's instructions (Roche, Indianapolis, IN, USA), TUNEL staining was performed to detect apoptotic myocytes. TUNEL-positive cells showed dark buffy nuclei staining under an Olympus BX-60 microscope (Olympus, Tokyo, Japan). TUNEL-negative cells exhibited blue nuclei. The samples were analysed under five high power fields randomly selected under a light microscope (20×). The total number of TUNEL-positive cells per field was calculated by digital medical image analysis system.
Masson's trichrome staining assessment
Masson's trichrome staining was performed exactly according to the manufacturer's protocols (Maxin Biotechnology, Fuzhou, China). The collagen fibres were stained blue and the myocytes red. A slice was chosen from each specimen at random. Five horizontal fields randomly selected in each slice were analysed under a light microscope, excluding blood vessels and perivascular interstitial cells. Collagen volume fraction (CVF) in each field was assessed by the digital medical image analysis system.
Western blot detection
Proteins were extracted from tissues by using RIPA Lysis Buffer (Higene, Shanghai, China). Protein sample (50 µg) was fractionated by SDS-PAGE (12% polyacrylamide gels) and transferred to PVDF membrane (Millipore, Bedford, MA, USA). The membranes were incubated overnight at 4°C with different primary antibodies to PARP (1:200), AIF (1:500), NF-κB (1:500), TH (1:200), GAP-43 (1:500), β-nerve growth factor (β-NGF; 1:400) (Abcam). After being rinsed in PBS-Tween for 5 min, the membranes were reacted with horseradish peroxidase-conjugated secondary antibody (1:5000, Higene). The films were visualized by an enhanced chemiluminescence buffer 50 s in a dark room.
Statistical analysis
Quantitative data were presented as mean ± SEM. Multiple comparisons were carried out among the animal groups using single-factor anova followed by Dunnet's t-test. Qualitative data were analysed with the chi-squared test. P < 0.05 was considered statistically significant.
Results
General characteristics of animals
Three rats (30%) in the ketamine group died at day 45, 50 and 52 of drug administration, while all rats survived through the entire procedure in the other three groups. Rats in the control and metoprolol alone groups gained weight steadily, while the ketamine-treated rats appeared to have thin, brittle and dull fur. In addition, they were emotionally unstable, more aggressive and easily became anxious. Ketamine-treated rats co-administered metoprolol were in a better state of nutrition and more stable emotionally than those treated only with ketamine.
After the i.p. injection of ketamine, the rats became overexcited and dysphoretic for about 1–3 min. They suffered nystagmus, clonus, hind limb stand followed by immediate falling down. After 15–25 min, they recovered completely without any treatment, but looked tired. The abnormal behaviours of rabbits were similar to those of rats. In addition, they nodded their heads rapidly and screamed occasionally. These reactions lasted for 1–3 min after injection, and full recovery required 15–25 min.
The hearts were enlarged in the ketamine-treated group and heart weight/body weight (HW/BW) ratio was significantly increased compared with control group (P < 0.05). In comparison with the ketamine only treated group, those co-administered metoprolol had a lower HW/BW ratio (P < 0.05, vs. ketamine-treated rats). Animals treated with metoprolol alone showed similar results to the control group (Figure 1).
Figure 1.
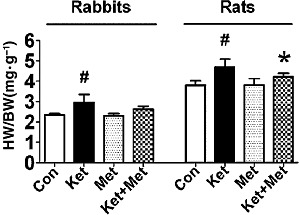
Effects of long-term application of ketamine (Ket) on HW/BW ratio in rabbits and rats. Con, control; Met, metoprolol. Data are expressed as mean ± SEM. n = 6 for rabbits, n = 10 for rats. #P < 0.05 versus control group; *P < 0.05 versus ketamine-treated group.
Structural and functional alterations after ketamine treatment in rabbits
The echocardiographic evaluation suggested the systolic function of the LV was impaired in the ketamine group. The ejection fraction (EF) value was markedly reduced and LVED notably increased (P < 0.05, vs. control group). Metoprolol treatment effectively prevented these structural and functional alterations. Metoprolol administration alone showed no significant difference from the control group (Figure 2).
Figure 2.
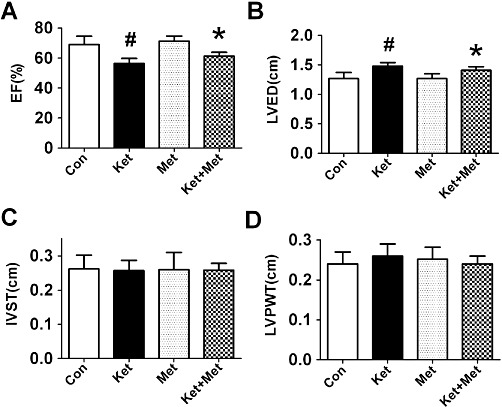
Effects of administration of ketamine (Ket) for 8 weeks on LV function evaluated by echocardiography in rabbits. Con, control; Met, metoprolol; IVST, interventricular septal thickness; LVPWT, left ventricular posterior wall thickness. Data are expressed as mean ± SEM. n = 6. #P < 0.05 versus control group; *P < 0.05 versus Ket group.
Alterations in cardiac electrophysiological properties after ketamine treatment in rabbits
During the course of thoracic surgery and the placing of epicardial multielectrode plaques, no tachycardia was observed in any of the rabbits. Ketamine slowed ventricular CV (0.26 ± 0.02 m·s−1 in ketamine group vs. 0.33 ± 0.02 m·s−1 in control group, P < 0.05) (Figure 3A), prolonged VERP (154.8 ± 6.3 ms in ketamine group vs. 116.8 ± 2.0 ms in control group, P < 0.05) (Figure 3B) and VERPd (47.9 ± 6.0 ms in ketamine group vs. 26.3 ± 5.8 ms in control group, P < 0.05) (Figure 3C). Metoprolol treatment inhibited the toxic effects of ketamine; the VERPd was shortened to 35.6 ± 5.9 ms (P < 0.05, vs. ketamine group) and there was a tendency for the CV to be increased, although this was not statistically significant (Figure 3A, C). More strikingly, ketamine treatment increased the susceptibility of rabbit hearts to ventricular arrhythmia (VA), with the VA inducibility rate was 0/6 in control rabbits, 5/6 in ketamine-treated rabbits and 1/6 in rabbits treated with ketamine plus metoprolol (Figure 3D). Metoprolol administration alone had no significant effects on the cardiac electrophysiological properties above (Figure 3).
Figure 3.
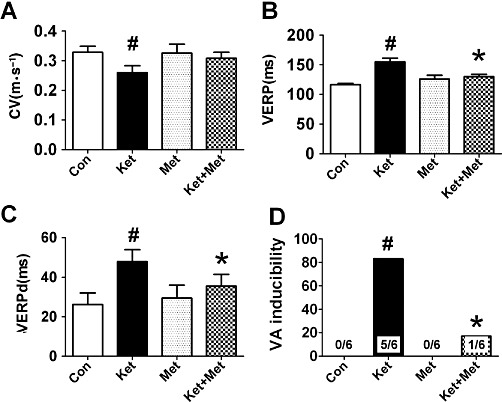
Alterations in electrophysiological parameters and increased susceptibility of rabbit hearts to VA after administration of ketamine (Ket) for 8 weeks. Con, control; Met, metoprolol. Data are expressed as mean ± SEM. n = 6. #P < 0.05 versus control group; *P < 0.05 versus Ket group.
Cardiac morphological changes, apoptosis and interstitial fibrosis of rats associated with ketamine administration
A rough epicardium and notable grey areas were found on the gross view of the hearts in ketamine-treated rats and rabbits. The epicardium looked like a ‘regional trichocardia’. In comparison, the epicardia of the control animals were smooth and pink. The epicardium in the ketamine plus metoprolol-treated animals showed no obvious grey and rough areas. As demonstrated in Figure 4A–C, the normal cardiomyocytes contained compactly arranged fibres with no intercellular space under the light microscopy in the control group, while in the ketamine group, cardiomyocytes were hypertrophic, oedematous and severely degenerated. The nuclei appeared distorted and varied in size. The above cardiac pathological changes were identical in both rats and rabbits. The situation was improved after metoprolol treatment.
Figure 4.
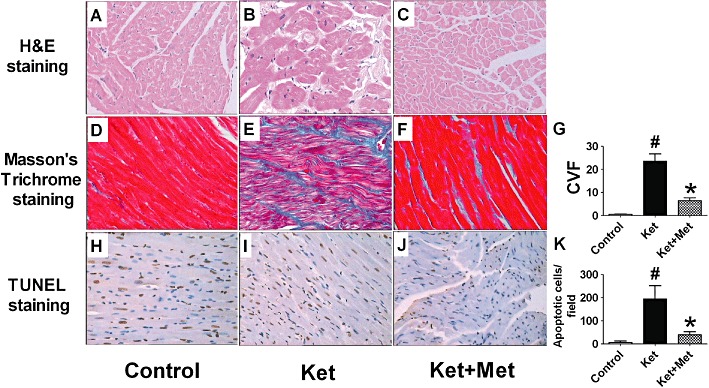
Morphological alterations [haematoxylin and eosin (H&E) staining, A–C], interstitial collagen deposition (Masson's trichrome staining, D–F) and apoptotic cell death (TUNEL staining, H–J) of rat hearts after administration of ketamine (Ket) for 12 weeks. Met, metoprolol. (G) Statistical analysis of CVF. (I) Statistical analysis of apoptotic cells. Data are expressed as mean ± SEM. n = 6. #P < 0.05 versus control group; *P < 0.05 versus Ket group. The magnification is ×20.
Ketamine exacerbated any cardiac interstitial fibrosis in rats. CVF in the heart of normal rats was 0.51 ± 0.12%, and this increased to 23.48 ± 3.21% (P < 0.01, vs. control group) in the heart of ketamine-treated rats. Metoprolol treatment reduced CVF to 6.46 ± 1.23% (P < 0.01, vs. ketamine-treated group) (Figure 4D–G).
Ketamine increased the apoptosis of cardiac myocytes. In the heart of control rats, few apoptotic cells were found (6.50 ± 6.57 per high power field), while in the heart of ketamine-treated rats, apoptotic cells sharply increased to 193.90 ± 57.41 per field (P < 0.01, vs. control group). Metoprolol treatment reduced TUNEL-positive cells to 39.30 ± 13.19 per field (P < 0.01, vs. ketamine-treated group) (Figure 4H–K).
Expression of apoptosis related proteins PARP-1, AIF and NF-κB after ketamine administration in rats
PARP-1, AIF and NF-κB are apoptosis-related proteins. In an immunohistochemical study, we found PARP-1, AIF and NF-κB-positive cells were scattered in the heart of control rats (Figure 5A, E, I), with positive cell densities of 0.41 ± 0.02%, 0.32 ± 0.09% and 0.39 ± 0.17%, respectively (Figure 5D, H, L). In ketamine-treated rats, PARP-1, AIF and NF-κB-positive cells all increased significantly (Figure 5B, F, J). The positive cell densities were 2.38 ± 0.56% for PARP-1, 5.58 ± 1.51% for AIF and 5.39 ± 1.67% for NF-κB (P < 0.01, vs. control group) (Figure 5D, H, L). Treatment with metoprolol significantly inhibited ketamine-induced up-regulation of PARP-1, AIF and NF-κB (Figure 5C, G, K), with the positive cell densities of 1.08 ± 0.29% for PARP-1, 2.62 ± 0.50% for AIF and 1.22 ± 0.39% for NF-κB (P < 0.01, vs. ketamine-treated group) (Figure 5D, H, Ls). Similar results were observed for quantitative analysis of PARP-1, AIF and NF-κB protein levels (Figure 6A–C).
Figure 5.
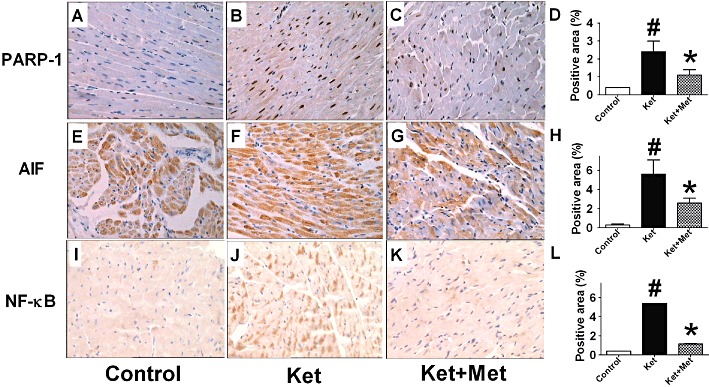
Effects of administration of ketamine (Ket) for 12 weeks on the expressions of PARP-1 (A–C), AIF (D–F) and NF-κB (G–I) in the rat hearts, assessed by immunohistochemistry. The magnification is 20×. D, H, L, Statistical analysis of the positive areas of PARP-1, AIF and NF-κB. Data are expressed as mean ± SEM. n = 6. #P < 0.05 versus control group; *P < 0.05 versus Ket group.
Figure 6.
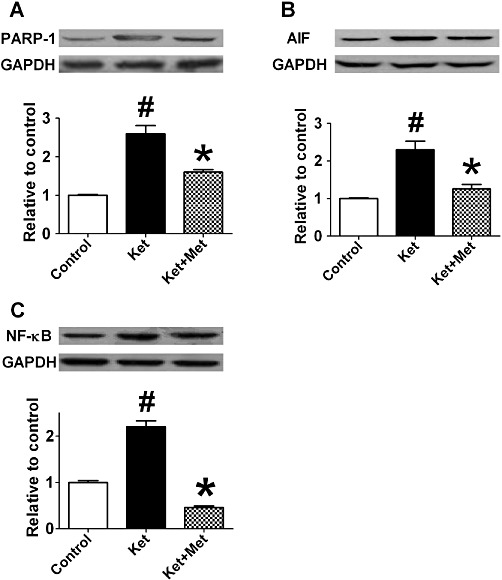
Effects of administration of ketamine (Ket) for12 weeks on the expression of PARP-1 (A), AIF (B) and NF-κB (C) in the rat hearts as assessed by Western blot. Met, metoprolol. Data are expressed as mean ± SEM. n = 5. #P < 0.05 versus control group; *P < 0.05 versus Ket group.
Cardiac sympathetic remodelling and β-NGF expression after ketamine treatment in rats
Ketamine induced extensive sympathetic sprouting and altered the distribution of nerve fibres. TH and GAP-43 are two markers expressed on sympathetic nerves. In the ventricle of ketamine-treated rats, TH- and GAP-43-positive fibre density was higher than that in control rats (P < 0.05) (Figure 7A, B, D, E). Moreover, in the ketamine group, the distribution of TH- and GAP-43-positive fibres was disrupted, and parts of the nerve fibres were enlarged. Consistently, the protein expressions of GAP-43 and TH were also increased after ketamine treatment (Figure 8A, B). Co-administration of metoprolol for 3 months decreased the ketamine-induced increase in TH and GAP-43 positive fibre density (Figure 7C, F) and the up-regulation of GAP-43 and TH (Figure 8A, B); further the morphology and distribution of nerve fibres were similar to those of the control group rats. Furthermore, β-NGF protein was observed to be up-regulated in the ketamine group and co-application of metoprolol significantly suppressed this increase in β-NGF protein expression (Figure 8C).
Figure 7.
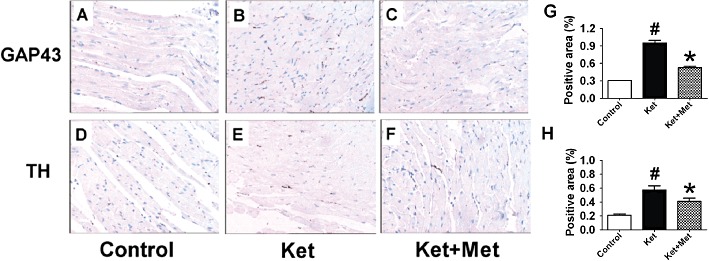
Effects of administration of ketamine (Ket) for 12-weeks on sympathetic sprouting and distribution of nerve fibres. A–C, GAP-43 expression; D–F, TH expression; Met, metoprolol. The magnification is ×20.
Figure 8.
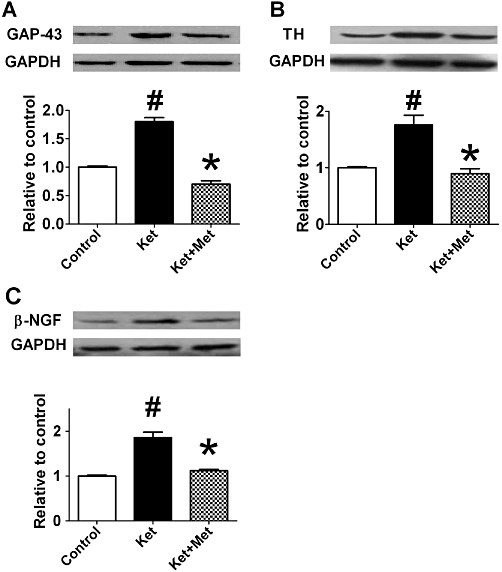
Effects of administration of ketamine (Ket) for 12 weeks on the protein expressions of GAP-43 (A), TH (B) and β-NGF (C) in the rat hearts by Western blotting. Met, metoprolol. Data are expressed as mean ± SEM. n = 5. #P < 0.05 versus control group; *P < 0.05 versus ketamine-treated group.
Discussion and conclusions
The analgesic effects of ketamine occur at low doses and amnestic effects appear at high doses (Smith et al., 2002). Recently, two reports showed unusual cases of death caused by acute or chronic ketamine poisoning (Licata et al., 1994; Tao et al., 2005). In our study, three rats died during the course of chronic ketamine administration. Currently, a complex VA has been pointed out as one of the aetiologies of sudden cardiac death, and high risk of sudden death is mostly due to VA (Sun et al., 2010). Large doses of ketamine used alone for an extensive period can cause palpitations, chest pain and tachycardia, indicating its potential to trigger arrhythmia (Ng et al., 2010). Consistently, we found that ketamine treatment increased the susceptibility of animals to the induction of VA; VA happened in five out of six rabbits after ketamine treatment, but in none of the normal rabbits. VA is associated with electrophysiological remodelling such as a decrease in ventricular CV, as well as VERP, and an increase in VERP dispersion. In this study, we found that after treament with ketamine, the CV was markedly reduced and VERP dispersion increased significantly, suggesting an increased susceptibility to VA.
Generally, it is believed that cardiac structural and sympathetic remodelling are the basis of electrophysiological disturbances. In the process of structural remodelling, myocardial interstitial fibrosis and redistribution induce a decrease in ventricular CV and an increase in VERPd, which contribute to the development of VA. Myocardial apoptosis and fibrosis are hallmarks of arrhythmogenic structural remodelling. In a previous animal study with Dutch belted rabbits, myocardial necrosis and interstitial fibrosis were observed after anaesthetizing them with ketamine three times within 30 days (Marini et al., 1999). Yang J (unpubl. obs.). showed that different doses of ketamine can induce cardiomyocyte apoptosis (including 4 mg·kg−1). In this study, an abundance of apoptotic cells and elevated CVF were found in the ketamine group and EF values were impaired, indicating structural changes, which led to loss of cardiac contractility and affected heart function. However, low doses of ketamine have been found to decrease the levels of Fas and bcl-2 and effectively reduce apoptosis caused by the acute myocardial infarction reperfusion (Wu et al., 2007). This discrepancy may be caused by the different models (normal myocardium vs. myocardial ischaemia-reperfusion injury) and doses employed.
PARP is a protein involved with a series of cellular processes including DNA repair and programmed cell death, cardiac hypertrophy and fibrosis. Inhibition of PARP decreased AP1-driven transcription (including collagen Iα1 and IIIα1, MMP-9 and tissue inhibitor of metalloproteinases-1) and effectively prevents cardiac fibrosis and cardiomyocytes from hypertrophy (Pillai et al., 2006; Huang et al., 2009). We observed that after chronic treatment with ketamine, PARP-1 expression was significantly elevated, suggesting its role in ketamine-induced apoptosis and fibrosis. AIF is involved in a caspase-independent pathway of apoptosis by translocating to the nucleus and causing large-scale DNA fragmentation and chromatin condensation. Translocation of AIF from mitochondria to the nucleus is required for PARP-1-mediated cell death (Kang et al., 2004). In our study, AIF was up-regulated after ketamine, indicating that PARP-1–AIF pathway may play an important role in this process. NF-κB is a family of inducible transcription factors that plays an anti-apoptotic role in cell cycling by regulating the expression of genes involved in apoptosis and cell proliferation. NF-κB induces the synthesis of important anti-apoptotic proteins that regulate caspase-8 activation and also limit the duration of JNK activity via several mechanisms (Salaun et al., 2010). Recent evidence supports a role for PARP-1 as a transcriptional co-regulator in the control of NF-κB (Kraus and Lis, 2003). In this study, changes in NF-κB expression showed a similar trend to those of PARP-1. Thus, PARP-1–NF-κB pathway may also contribute to ketamine-induced apoptosis.
Sympathetic stimulation increases cardiac susceptibility to VF. The level of sympathetic activation could be directly correlated with prognosis in congestive heart failure and other arrhythmic complications such as VA (Meredith et al., 1991; Kaye et al., 1995). In this study, we found ketamine promoted sympathetic remodelling. GAP-43 is a presynaptic protein and plays a vital role in axonal growth, neuronal differentiation and regeneration (Chakravarthy et al., 2008). As a marker of newly formed nerve fibres, GAP-43 expression is induced on nerve sprouting (Vento et al., 2001). In this study, GAP-43-positive nerve fibre density was significantly increased, and partial nerve fibres were distributed together after ketamine treatment, indicating the development of neuronal remodelling. TH is a highly specific enzyme responsible for catalysing the conversion of the amino acid L-tyrosine to dihydroxyphenylalanine, and accepted as a sympathetic neuron-specific marker. Previous studies have shown an increased density of nerve fibres with positive immunostaining for GAP-43 and TH after myocardial infarction and heart failure (Yuan et al., 2009). After ketamine treatment, TH-positive nerve fibre density also increased, which further confirmed sympathetic sprouting. NGF has been termed a neurotrophin and is essential for enhanced postinfarct sympathetic sprouting (Wernli et al., 2009). In myocardial infarction, VA and many other cardiovascular diseases, sympathetic sprouting and hyperinnervation may be associated with NGF signalling (Yuan et al., 2009). Gong et al. (2009) showed β-NGF levels closely correlated with atrial sympathetic nerve terminal profiles in intact animals with atrial fibrillation (AF) and confirmed atrial overexpression of β-NGF in AF. In addition to the morphological long-term effects of NGF on nerve sprouting, NGF modulates the function of sympathoadrenergic neurotransmission in co-cultures of cardiomyocytes with neurons (Lockhart et al., 1997). NGF infusion to the left stellate ganglion induced significant cardiac nerve sprouting in normal dogs (Cao et al., 2000). Consistently, our study showed β-NGF expression was up-regulated in ketamine-treated rats. Thus, β-NGF may play an important role in ketamine-induced sympathetic remodelling.
Metoprolol is a selective β1-adrenoceptor antagonist that has been demonstrated to reverse cardiac remodelling. Metoprolol inhibits sympathetic remodelling and electrical remodelling at the infarcted border zone after myocardial infarction (Jiang et al., 2007). Dogs treated with metoprolol showed an increase in LV EF and a reversal of LV global, structural and biochemical remodelling (Imai et al., 2007). Ablad et al. (2007) found metoprolol reduced the inducibility of ventricular fibrillation. In addition, remodelling of non-infarcted myocardium is associated with myofilament function, which may contribute to depressed LV function (van der Velden et al., 2004). Duncker et al. (2009) showed that β-blockade can improve the function of pathologically remodelled hearts by reversing myofilament dysfunction. We found metoprolol administration prevented rats from sudden death and alleviated ventricular myocardial apoptosis, fibrosis, sympathetic sprouting and electrophysiological remodelling after ketamine treatment, indicating a therapeutic role of metoprolol in ameliorating ketamine-induced cardiac toxicity.
Because abuse of ketamine is irregular, it is hard to determine the doses used by individuals committing ketamine-abuse. However, the effects of ketamine in animals have been studied. Trujillo et al. (2008) used 20 mg·kg−1 and 50 mg·kg−1 in rats. Hurley et al. (1994) used 35 mg·kg−1 ketamine in rabbits . Therefore, we chose an intermediate dose level (40 mg·kg−1), and with this dose, the animals showed abnormal behaviours, such as hyperactivity, dysphoria, body rolling, head weaving and screaming, similar to the manifestations in humans. The aim of the present study was to investigate the protective effects of metoprolol against ketamine-induced cardiac toxicity. In the present study metoprolol was co-administered with ketamine; for the results to have clinical implications the effects of metoprolol administered several weeks after starting ketamine administration need to be investigated.
In conclusion, our results suggest that long-term abuse of ketamine could trigger ventricular myocardial apoptosis, fibrosis and increased sympathetic sprouting, which alter cardiac electrophysiological properties and increase the potential of malignant arrhythmias that finally lead to sudden cardiac death. In this ketamine-induced model of cardiac toxicity, the PARP–AIF/NF-κB pathways may contribute to structural remodelling, while β-NGF may play an important role in sympathetic remodelling. Metoprolol was effective in preventing the ketamine-induced cardiac toxicity in our experiments.
Acknowledgments
This work was supported by a grant of Natural Science Foundation of China (No. 30971251, 81070160), the Program for New Century Excellent Talents in University from the Department of Education of Heilongjiang Province (No. 1152-NCET-011), the Youth Foundation of Heilongjiang Province (No. QC2010004), the Special Research Fund for Science & Technology Innovation Talents of Harbin (2009RFXXS209), and the Scientific Research Foundation of the First Clinical Hospital of Harbin Medical University (No. 2009B09).
Conflicts of interest
No conflict of interest was declared.
References
- Ablad B, Bjurö T, Björkman JA, Edström T. Prevention of ventricular fibrillation requires central beta-adrenoceptor blockade in rabbits. Scand Cardiovasc J. 2007;41:221–229. doi: 10.1080/14017430701383748. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, et al. Nerve sprouting and sudden cardiac death. Circ Res. 2000;86:816–821. doi: 10.1161/01.res.86.7.816. [DOI] [PubMed] [Google Scholar]
- Chakravarthy B, Rashid A, Brown L, Tessier L, Kelly J, Ménard M. Association of Gap-43 (neuromodulin) with microtubule-associated protein MAP-2 in neuronal cells. Biochem Biophys Res Commun. 2008;371:679–683. doi: 10.1016/j.bbrc.2008.04.119. [DOI] [PubMed] [Google Scholar]
- Dillon P, Copeland J, Jansen K. Patterns of use and harms associated with non-medical ketamine use. Drug Alcohol Depend. 2003;69:23–28. doi: 10.1016/s0376-8716(02)00243-0. [DOI] [PubMed] [Google Scholar]
- Duncker DJ, Boontje NM, Merkus D, Versteilen A, Krysiak J, Mearini G, et al. Prevention of myofilament dysfunction by beta-blocker therapy in postinfarct remodeling. Circ Heart Fail. 2009;2:233–242. doi: 10.1161/CIRCHEARTFAILURE.108.806125. [DOI] [PubMed] [Google Scholar]
- Gong YT, Li WM, Li Y, Yang SS, Sheng L, Yang N, et al. Probucol attenuates atrial autonomic remodeling in a canine model of atrial fibrillation produced by prolonged atrial pacing. Chin Med J (Engl) 2009;122:74–82. [PubMed] [Google Scholar]
- Huang D, Wang Y, Yang C, Liao Y, Huang K. Angiotensin ? promotes poly(ADP-ribosyl)ation of c-Jun/c-Fos in cardiac fibroblasts. J Mol Cell Cardiol. 2009;46:25–32. doi: 10.1016/j.yjmcc.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Hurley RJ, Marini RP, Avison DL, Murphy JC, Olin JM, Lipman NS. Evaluation of detomidine anesthetic combinations in the rabbit. Lab Anim Sci. 1994;44:472–478. [PubMed] [Google Scholar]
- Imai M, Rastogi S, Gupta RC, Mishra S, Sharov VG, Stanley WC, et al. Therapy with cardiac contractility modulation electrical signals improves left ventricular function and remodeling in dogs with chronic heart failure. J Am Coll Cardiol. 2007;49:2120–2128. doi: 10.1016/j.jacc.2006.10.082. [DOI] [PubMed] [Google Scholar]
- Jiang H, Lu Z, Yu Y, Zhao D, Jian X, Yang B, et al. Effects of metoprolol on sympathetic remodeling and electrical remodeling at infarcted border zone after myocardial infarction in rabbits. Cardiology. 2007;108:176–182. doi: 10.1159/000096647. [DOI] [PubMed] [Google Scholar]
- Kamphoven JH, Stubenitsky R, Reuser AJ, Van Der Ploeg AT, Verdouw PD, Duncker DJ. Cardiac remodeling and contractile function in acid alpha-glucosidase knockout mice. Physiol Genomics. 2001;5:171–179. doi: 10.1152/physiolgenomics.2001.5.4.171. [DOI] [PubMed] [Google Scholar]
- Kang YH, Yi MJ, Kim MJ, Park MT, Bae S, Kang CM, et al. Caspase-independent cell death by arsenic trioxide in human cervical cancer cells: reactive oxygen species-mediated poly(ADP-ribose) polymerase-1 activation signals apoptosis-inducing factor release from mitochondria. Cancer Res. 2004;64:8960–8967. doi: 10.1158/0008-5472.CAN-04-1830. [DOI] [PubMed] [Google Scholar]
- Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26:1257–1263. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- Kraus WL, Lis JT. PARP goes transcription. Cell. 2003;113:677–683. doi: 10.1016/s0092-8674(03)00433-1. [DOI] [PubMed] [Google Scholar]
- Licata M, Pierini G, Popoli G. A fatal keatmine poisoning. J Forensic Sci. 1994;39:1314–1320. [PubMed] [Google Scholar]
- Lockhart ST, Turrigiano GG, Birren SJ. Nerve growth factor modulates synaptic transmission between sympathetic neurons and cardiac myocytes. J Neurosci. 1997;17:9573–9582. doi: 10.1523/JNEUROSCI.17-24-09573.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini RP, Li X, Harpster NK, Dangler C. Cardiovascular pathology possibly associated with ketamine/xylazine anesthesia in Dutch belted rabbits. Lab Anim Sci. 1999;49:153–160. [PubMed] [Google Scholar]
- Meredith IT, Broughton A, Jennings GL, Esler MD. Evidence of a selective increase in cardiac sympathetic activity in patients with sustained ventricular arrhythmias. N Engl J Med. 1991;325:618–624. doi: 10.1056/NEJM199108293250905. [DOI] [PubMed] [Google Scholar]
- Ng SH, Tse ML, Ng HW, Lau FL. Emergency department presentation of ketamine abusers in Hong Kong: a review of 233 cases. Hong Kong Med J. 2010;16:6–11. [PubMed] [Google Scholar]
- Pillai JB, Gupta M, Rajamohan SB, Lang R, Raman J, Gupta MP. Poly(ADP-ribose) polymerase-1-deficient mice are protected from angiotensin II-induced cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2006;291:H1545–H1553. doi: 10.1152/ajpheart.01124.2005. [DOI] [PubMed] [Google Scholar]
- Salaun C, Leroy C, Rousseau A, Boitez V, Beck L, Friedlander G. Identification of a novel transport-independent function of PiT1/SLC20A1 in the regulation of TNF-induced apoptosis. J Biol Chem. 2010;285:34408–34418. doi: 10.1074/jbc.M110.130989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Larive LL, Romanelli F. Club drugs: methylenedioxymethamphetamine, flunitrazepam, ketamine hydrochloride, and gamma-hydroxybutyrate. Am J Health Syst Pharm. 2002;59:1067–1076. doi: 10.1093/ajhp/59.11.1067. [DOI] [PubMed] [Google Scholar]
- Sun LP, Wang L, Wang H, Zhang YH, Pu JL. Connexin 43 remodeling induced by LMNA gene mutation Glu82Lys in familial dilated cardiomyopathy with atrial ventricular block. Chin Med J (Engl) 2010;123:1058–1062. [PubMed] [Google Scholar]
- Tao Y, Chen XP, Qin ZH. A fatal chronic ketamine poisoning. J Forensic Sci. 2005;50:173–176. [PubMed] [Google Scholar]
- Trujillo KA, Zamora JJ, Warmoth KP. Increased response to ketamine following treatment at long intervals: implications for intermittent use. Biol Psychiatry. 2008;63:178–183. doi: 10.1016/j.biopsych.2007.02.014. [DOI] [PubMed] [Google Scholar]
- van der Velden J, Merkus D, Klarenbeek BR, James AT, Boontje NM, Dekkers DH, et al. Alterations in myofilament function contribute to left ventricular dysfunction in pigs early after myocardial infarction. Circ Res. 2004;95:e85–e95. doi: 10.1161/01.RES.0000149531.02904.09. [DOI] [PubMed] [Google Scholar]
- Vento P, Kiviluoto T, Keränen U, Järvinen HJ, Kivilaakso E, Soinila S. Quantitative comparison of growth-associated protein-43 and substance P in ulcerative colitis. J Histochem Cytochem. 2001;49:749–758. doi: 10.1177/002215540104900608. [DOI] [PubMed] [Google Scholar]
- Weiner AL, Vieira L, McKay CA, Bayer MJ. Ketamine abusers presenting to the emergency department: a case series. J Emerg Med. 2000;18:447–451. doi: 10.1016/s0736-4679(00)00162-1. [DOI] [PubMed] [Google Scholar]
- Wernli G, Hasan W, Bhattacherjee A, van Rooijen N, Smith PG. Macrophage depletion suppresses sympathetic hyperinnervation following myocardial infarction. Basic Res Cardiol. 2009;104:681–693. doi: 10.1007/s00395-009-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wu YS, Zhao CY. Effects of ketamine on apoptosis and expression of Fas and Bcl-2 protein in rats with experimental myocardial ischemia/reperfusion injury. Shu Li Yi Yao Xue Za Zhi. 2007;20:18–20. [Google Scholar]
- Yang SS, Han W, Zhou HY, Dong G, Wang BC, Huo H, et al. Effects of spironolactone on electrical and structural remodeling of atrium in congestive heart failure dogs. Chin Med J (Engl) 2008;121:38–42. [PubMed] [Google Scholar]
- Yuan MJ, Huang CX, Tang YH, Wang X, Huang H, Chen YJ, et al. A novel peptide ghrelin inhibits neural remodeling after myocardial infarction in rats. Eur J Pharmacol. 2009;618:52–57. doi: 10.1016/j.ejphar.2009.07.015. [DOI] [PubMed] [Google Scholar]


