Abstract
BACKGROUND AND PURPOSE
The transient receptor potential vanilloid 1 (TRPV1) plays a role in the activation of sensory neurons by various painful stimuli and is a therapeutic target. However, functional TRPV1 that affect microvascular diameter are also expressed in peripheral arteries and we attempted to characterize this receptor.
EXPERIMENTAL APPROACH
Sensory TRPV1 activation was measured in rats by use of an eye wiping assay. Arteriolar TRPV1-mediated smooth muscle specific responses (arteriolar diameter, changes in intracellular Ca2+) were determined in isolated, pressurized skeletal muscle arterioles obtained from the rat and wild-type or TRPV1−/− mice and in canine isolated smooth muscle cells. The vascular pharmacology of the TRPV1 agonists (potency, efficacy, kinetics of action and receptor desensitization) was determined in rat isolated skeletal muscle arteries.
KEY RESULTS
Capsaicin evoked a constrictor response in isolated arteries similar to that mediated by noradrenaline, this was absent in arteries from TRPV1 knockout mice and competitively inhibited by TRPV1 antagonist AMG9810. Capsaicin increased intracellular Ca2+ in the arteriolar wall and in isolated smooth muscle cells. The TRPV1 agonists evoked similar vascular constrictions (MSK-195 and JYL-79) or were without effect (resiniferatoxin and JYL-273), although all increased the number of responses (sensory activation) in the eye wiping assay. Maximal doses of all agonists induced complete desensitization (tachyphylaxis) of arteriolar TRPV1 (with the exception of capsaicin). Responses to the partial agonist JYL-1511 suggested 10% TRPV1 activation is sufficient to evoke vascular tachyphylaxis without sensory activation.
CONCLUSIONS AND IMPLICATIONS
Arteriolar TRPV1 have different pharmacological properties from those located on sensory neurons in the rat.
Keywords: vanilloid receptor (TRPV1), resistance artery, vascular autoregulation
Introduction
The transient receptor potential vanilloid 1 (TRPV1) is a non-selective cation channel, originally found in sensory C and Aδ fibres (Caterina et al., 1997). It functions as a ligand-, proton- and heat-activated molecular integrator of nociceptive stimuli (Szallasi and Blumberg, 1999; Di Marzo et al., 2002; Ross, 2003) and hence represents a promising drug target for analgesia (Szallasi et al., 2007; Gunthorpe and Szallasi, 2008).
However, TRPV1 expression has recently been identified in many cells in addition to sensory neurons. In particular, TRPV1 expression was detected in various cell types in the brain (Toth et al., 2005a), and in the periphery, including arteriolar receptors responsible for vasoconstriction (Kark et al., 2008). Moreover, while functional expression of TRPV1 in the CNS remained elusive, activation of vascular TRPV1 has been shown to result in substantial vasoconstriction both in vivo and in vitro (Kark et al., 2008). TRPV1 antagonists are in clinical trials for various conditions including dental pain, osteoarthritis, neuropathic pain, overactive bladder, chronic cough, rectal hypersensitivity, migraine, lower back pain and interstitial cystitis (Khairatkar-Joshi and Szallasi, 2009). Although some results of these trials are promising, they also revealed that TRPV1 antagonists can evoke serious hyperthermia (Gavva et al., 2008). This hyperthermia is probably related to the involvement of TRPV1 in temperature regulation in vivo (Gavva et al., 2007). However, the mechanism of this effect is not clear. Although some antagonists cause hyperthermia (Gavva et al., 2008), others are without thermoregulatory effects in humans (Khairatkar-Joshi and Szallasi, 2009). This suggests that the TRPV1 responsible for analgesia is pharmacologically different from that involved in thermoregulation. The nature and identity of these TRPV1-dependent responses have not been identified yet, but it is plausible that a separate pool of receptors exists (Steiner et al., 2007).
Capsaicin evokes vasoconstriction in skeletal muscle arteries presumably by activating TRPV1 located in smooth muscle (Kark et al., 2008). Here we attempted to characterize this receptor pharmacologically. To achieve this, we chose a series of commercially available TRPV1 agonists and tested them in assays that measured not only their potency and efficacy, but also their kinetics of action and ability to induce desensitization (Toth et al., 2005b). Our experiments revealed different pharmacological profiles for vascular TRPV1 when compared with that of TRPV1 responsible for sensory activation. These findings indicate that sensory neuronal and arterial receptor populations of TRPV1 can be selectively targeted.
Methods
The applied drug/molecular target nomenclature (e.g. receptors, ion channels) conforms to the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2011).
Animals, anaesthesia and general preparation in the in vivo experiments
The experiments were performed on male Wistar rats (n = 119 rats) weighing 250–450 g and on male mice (six control C57BL/6J and five TRPV1−/− knockout mice). Rats (WKY/NCrl) were obtained from Charles River (Isaszeg, Hungary), while mice was obtained from Jackson Laboratories (Bar Harbor, ME, USA) and maintained on a standard laboratory food (CRLT/N chow from Szinbad Kft, Godollo, Hungary) and water ad libitum. Anaesthesia was induced by administration of pentobarbital sodium (100 mg·kg−1 i.p.). All animal care and experimental procedures complied with NIH guidelines and were approved by the Ethical and Experimental Animal Research Committee of the University of Debrecen.
Isolation of arterioles and measurement of vascular diameter
The isolation of skeletal muscle (m. gracilis) arterioles of the rat and measurement of the diameter of arterioles were performed as described previously (Lizanecz et al., 2006). Briefly, arterioles were kept in a physiological saline solution (PSS; composition in mM: 110 NaCl, 5.0 KCl, 2.5 CaCl2, 1.0 MgSO4, 1.0 KH2PO4, 5.0 glucose and 24.0 NaHCO3 equilibrated with a gas mixture of 10% O2, 5% CO2 and 85% N2, at pH 7.4.) at an intraluminal pressure of 80 mmHg until the development of spontaneous myogenic response (constriction to intraluminal pressure). Changes in intraluminal arteriolar diameter were measured after the various treatments. First ACh was used to determine dilator capacity and endothelium function, and then noradrenaline (NA) was applied to measure maximal constrictor response and smooth muscle function. Changes in diameter to TRPV1 agonists were tested next with cumulative doses of the agonists (capsaicin, 0.1 nM–1 µM; resiniferatoxin, 1 pM–10 nM; JYL-273, 0.1 nM–1 µM; MSK-195, 0.1 nM–3 µM; JYL-79, 3 pM–10 µM; JYL-1511, 1 nM–1 µM). The specificity of the capsaicin responses was tested by the application of the TRPV1 antagonist AMG9810. Cumulative dose-response curves for capsaicin were obtained in the absence and presence of 100, 300 and 1000 nM AMG9810 (obtained from Tocris Bioscience, Ellisville, MO, USA). Desensitization of arteriolar TRPV1 was tested in separate experiments. Acute desensitization (decrease in response in the continuous presence of agonist) was determined by measurement of arteriolar diameter during 20 min incubations with a high concentration of the drugs. This was followed by 40 min regeneration (in PSS solution) and tachyphylaxis (decrease of response upon re-administration of the agonist) was assessed by measuring the response to 1 µM capsaicin. Arterioles were isolated from wild-type and TRPV1 knockout mice as detailed for the rat. Experiments were also performed similarly; ACh was used to determine endothelial function, and NA was applied to estimate smooth muscle function. Changes in diameter to TRPV1 agonists were tested by measuring responses to cumulative doses of capsaicin (0.1 nM–30 µM).
Determination of antagonist equilibrium dissociation constant
A conventional Schild plot (Arunlakshana and Schild, 1959) was constructed based on the measured values. EC50 of capsaicin was calculated in the absence (designated as A) or in the presence of AMG9810 (designated as A'), then log((A/A')-1) values were plotted as a function of the logarithm of AMG9810 concentration (Figure 2B). Data were fitted by linear regression, and the antagonist equilibrium dissociation constant was obtained from the x-intercept.
Figure 2.
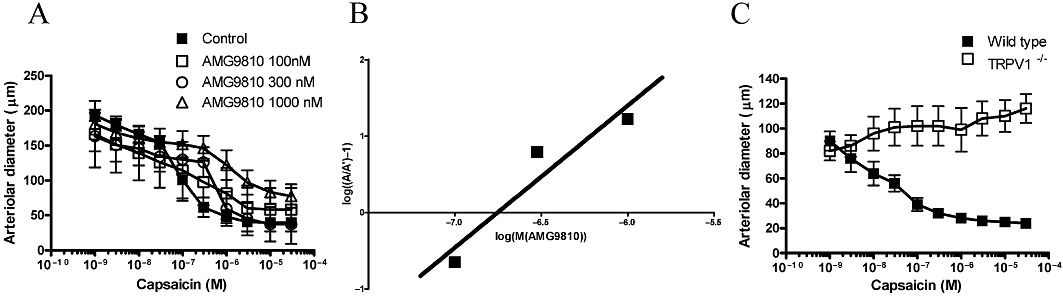
TRPV1 specificity of capsaicin-evoked vasoconstriction. Internal diameter of cannulated gracilis arteries was measured at 80 mmHg intraluminal pressure upon addition of different doses of capsaicin (cumulative dose-response curve) in the absence (control) and presence of the TRPV1 antagonist AMG9810 (100, 300 and 1000 nM, A). Symbols are means ± SEM of five to nine independent determinations. The equilibration dissociation constant of AMG9810 was determined by the conventional Schild plot (x-intercept, B). Finally, gracilis arterioles isolated from control (wild-type) and TRPV1 knockout (TRPV1−/−) mice were also tested for capsaicin-mediated vasoconstriction (C). Symbols are mean ± SEM of five to six independent determinations.
Parallel measurement of vascular diameter and intracellular Ca2+ concentrations
Skeletal muscle arterioles were isolated and cannulated from the gracilis muscle of the rat, as mentioned above. After the arteries had been mounted in the tissue chamber, the physiological buffer was supplemented with 1% BSA and 5 µM Fura-2AM fluorescent Ca2+ indicator dye for 60–120 min until a spontaneous myogenic tone developed. Then, the tissue chamber was placed on the stage of a Nikon TS100 (Tokyo, Japan) inverted microscope to measure intracellular Ca2+ concentrations by an IncyteIm2 instrument (Intracellular Imaging Inc, Cincinnati, OH, USA) by recording images (cut-off >510 nM) excited alternatively by 340 and 380 nm light. Images were recorded every 2–5 s and evaluated offline. Outer diameter of the arteries was determined on each recorded image and arteriolar Ca2+ concentrations were detected by calculating ratios between averaged signal intensity at 340 and 380 nm excitation in the whole arteriolar segment (representing a minimum of 200 pixels). A movie representative of the full experiment has been uploaded as a supplementary video file, and additional movies can also be seen at our website (http://www.debkard.hu/upload/file/klinfiz/kkk/Vascularsystem/Vascularsystem.html).
Isolation of smooth muscle cells from canine coronary arteries
Adult beagle dogs (10–14 kg) were anaesthetized with an i.v. injection containing 10 mg·kg−1 ketamine hydrochloride (Calypsol, Richter Gedeon, Hungary) and 1 mg·kg−1 xylazine hydrochloride (Sedaxylan, Eurovet Animal Health BV, Bladel, the Netherlands). After the chest had been opened, the heart was rapidly removed and the right coronary artery was perfused with Ca2+-free minimum essential Eagle's medium, Joklik modification solution, supplemented with taurine (2.5 g·L−1), pyruvic acid (175 mg·L−1), ribose (750 mg·L−1), allopurinol (13.5 mg·L−1) and NaH2PO4 (200 mg·L−1) equilibrated with a mixture of 95% O2 and 5% CO2 (similar to all further solutions) for 5 min to remove the blood. Then the solution was changed to Dulbecco's modified Eagle's medium (DMEM), and an approximately 2.5 cm long right coronary artery segment was isolated and cannulated at both ends. The cannulae were connected to a peristaltic pump, and the solution was pumped from the tissue chamber into the arteriolar lumen (from which it leaked back to the tissue chamber). Then DMEM was supplemented with 3 mg·mL−1 collagenase type I (Worthington, Lakewood, NJ, USA) for 30 min and with 1 mg·mL−1 elastase (Worthington) at 60 min. The vessel fell apart after about 90 min under these conditions; the cell-rich solution was then transferred into 24-well plates. After the adherence of the cells to the glass coverslips placed in the wells (about 10 min), the solution was replaced with DMEM to remove the digesting enzymes, and the cells were incubated for 60 min in a CO2 thermostate. Then, the media was changed to DMEM containing 1 mg·mL−1 BSA and 5 µM fura2-acetoxymethyl ester (Molecular Probes, Eugene, OR, USA) for 2 h at room temperature. The cover slips were then placed in a suitable chamber for intracellular Ca2+ concentration measurements. These measurements were started by washing the cells with Dulbecco's PBS (DPBS) three times, and the measurements were performed in DPBS. The fluorescence of individual cells was measured with an InCyt Im2 fluorescence imaging system (Intracellular Imaging Inc., Cincinnati, OH, USA). The cells within a field were illuminated alternately at 340 and 380 nm. Emitted light at >510 nm was measured. The cells were treated with 1 µM capsaicin and then with 100 mM KCl. Data were analysed with the InCyt 4.5 software and further processed with Excel (Microsoft Corp, Redmond, WA, USA) and Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA) software.
Measurement of eye wiping
The eye wiping assay was performed as described previously (Jakab et al., 2005). In short, one drop (10 µL) of agonists (capsaicin, 1 µM; resiniferatoxin, 10 nM; JYL-273, 1 µM; MSK-195, 1 µM; JYL-79, 1 µM; JYL-1511, 1 µM) was put into the right or left conjunctiva of the rat (single treatment for each rat). The number of eye wipes was counted for 60 s. In the control group, the same volume of solvent was administered in a similar manner.
Materials and solutions
Chemicals were from Sigma-Aldrich (St. Louis, MO, USA) if not stated otherwise. Resiniferatoxin, JYL-273, MSK-195, JYL-79 and JYL-1511 were from Alexis (Enzo Life Sciences AG, Lausen, Switzerland). TRPV1 agonists were dissolved in ethanol.
Statistical analysis
Arteriolar diameter was measured in µm, determined at 80 mmHg intraluminal pressure. Results are shown as mean ± SEM. Statistical differences were evaluated by Student's t-test by comparing values before and after treatments (paired) or comparing eye wipes of vehicle-treated rats with those of TRPV1 agonist-treated rats (unpaired).
Results
Application of the TRPV1-specific agonist capsaicin (1 µM) resulted in a substantial constriction (decrease of arteriolar diameter from 210 ± 11 µm to 91 ± 17 µm, n = 7, P < 0.01) of skeletal muscle (m. gracilis) arterioles (Figure 1) similar to NA (10 µM, decrease of arteriolar diameter to 68 ± 9 µm, n = 7, Figure 1). In contrast, the endothelium-dependent vasodilator ACh evoked dilatation (increase in arteriolar diameter to 240 ± 20 µm, n = 7, P = 0.028, Figure 1).
Figure 1.
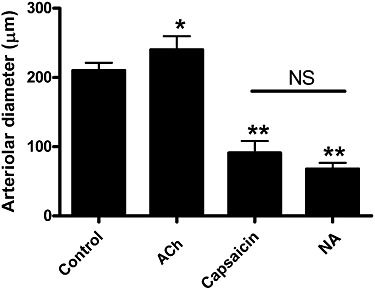
Functional effects of TRPV1 stimulation in skeletal muscle arteries. Internal diameter of cannulated gracilis arteries were measured at 80 mmHg intraluminal pressure before treatments (control). The existence of spontaneous myogenic tone and viability of endothelium was determined by ACh (10 µM)-evoked dilatations. The constrictor response to TRPV1 agonist capsaicin (1 µM) was compared with the effect of NA (10 µM). Experiments were performed on the same (n = 7) arteries. Values are mean ± SEM. Significant differences are represented by asterisks (*P < 0.05 or **P < 0.01).
The vast majority of published data suggest that vascular TRPV1 stimulation produces a dilatation. It was therefore necessary to test the TRPV1 specificity of these capsaicin-mediated contractile responses. First, a competitive antagonist of TRPV1 was applied. AMG9810 antagonized capsaicin-mediated contractions in a dose-dependent manner (Figure 2A). Moreover, the potency of AMG9810 determined in these assays (177 nM, Figure 2B) was in agreement with its potency determined in other TRPV1-specific systems (Gavva et al., 2005). Nonetheless, the TRPV1 selectivity of these capsaicin-mediated contractile responses was also tested in TRPV1 knockout (TRPV1−/−) mice. The potency of capsaicin (EC50) was 137 nM (Figure 2C) and efficacy was 73% (decrease in diameter from 69 ± 8 µm to 24 ± 3 µm, n = 6, Figure 2C) in arteries from wild-type mice, while the same capsaicin treatments were without effect in TRPV1−/− mice (Figure 2C, n = 5).
Next, the potential mechanism of TRPV1-mediated constrictions was evaluated. Activation of TRPV1 results in an increase in intracellular Ca2+ concentrations in many TRPV1-expressing cell types and this contributes to the physiological effects. To detect capsaicin-mediated changes in intracellular Ca2+ concentrations, a Ca2+ imaging system was applied. Simultaneous measurement of intracellular Ca2+ concentration and vascular diameter (outer diameter in this case) of cannulated rat arterioles isolated from the gracilis muscle of the rat was performed (Figure 3). The capsaicin-evoked vasoconstriction was parallelled by an increase in intracellular Ca2+ concentration (supplementary video file and Figure 3A). Moreover, both vascular diameter and intracellular Ca2+ concentration increased in a dose-dependent manner, with potency in the nanomolar range (note maximal responses at 1 µM, Figure 3B). To identify the TRPV1-expressing cell type, arteriolar smooth muscle cells were isolated from canine coronary arteries (these arteries also responded to capsaicin treatment with a dose-dependent constriction; data not shown) and changes in intracellular Ca2+ concentrations to capsaicin (1 µM) and KCl (100 mM) treatments were tested (Figure 4). The capsaicin-mediated increase in intracellular Ca2+ concentrations in the cells responding to capsaicin (10 out of 28 cells, representative data in Figure 4A and B) was similar (increase in 340/380 ratio from 0.69 ± 0.10 to 0.93 ± 0.17, Figure 4C) to the increase evoked by depolarization (100 mM KCl, 340/380 ratio was 1.04 ± 0.20, Figure 4C).
Figure 3.
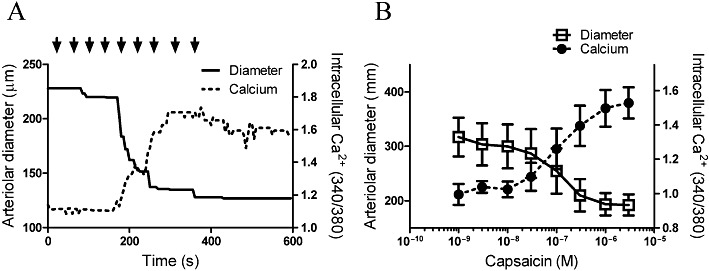
Mechanism of capsaicin-mediated vasoconstriction: skelatal muscle arteries. Capsaicin-evoked changes in arteriolar diameter were recorded in parallel with changes in intracellular Ca2+ concentrations of the vascular wall. An individual experiment is shown in (A) (the full recorded experiment also available in the supplementary movie). Solid line represents the arteriolar diameter (please note that in this specific case, the outer diameter is plotted), while dotted line shows intracellular Ca2+ concentrations expressed as 340/380 ratio. Capsaicin was administered in a cumulative fashion (indicated by the arrows, the applied capsaicin doses were: 3 × 10−10, 10−9, 3 × 10−9, 10−8, 3 × 10−8, 10−7, 3 × 10−7, 10−6, 3 × 10−6 M). (B)The mean responses ±SEM of n = 5 single determinations.
Figure 4.
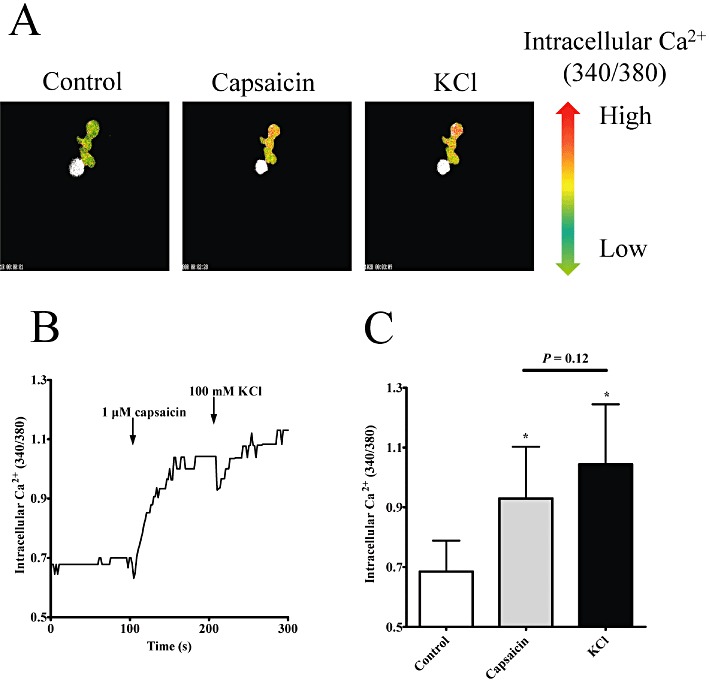
Mechanism of capsaicin-mediated vasoconstriction: isolated arteriolar smooth muscle cells. Canine, freshly isolated coronary arteriolar smooth muscle cells were loaded with fura-2 fluorescent Ca2+-sensitive dye and treated with capsaicin (1 µM) and KCl (100 mM). Changes in intracellular Ca2+ concentrations were detected as changes in the 340/380 fluorescence ratio (a representative experiment is shown in A, where green pixels represent low values and red represent high values). Capsaicin evoked a fast increase in the intracellular Ca2+ concentrations in some cells, which was not increased further upon the addition of KCl (B). These observations were confirmed when responses of the capsaicin-sensitive cells (10 out of 28 viable cells) were evaluated (C). Columns represent mean ± SEM.
Having established the TRPV1 specificity of capsaicin-evoked vasoconstriction, the pharmacological properties of these receptors on skeletal muscle arteries of the rat were characterized in detail. The potency of capsaicin on this receptor (EC50) was 221 nM (Figure 5A), efficacy was 58 ± 7% constriction (n = 7), which was not significantly different from the efficacy of NA (69 ± 3% constriction, n = 6, P < 0.01 vs. control, P = 0.08 vs. capsaicin). The kinetics of the vasoconstrictor response was determined by continuous application of capsaicin (1 µM) for 20 min. Maximal constriction (decrease of arteriolar diameter from 160 ± 11 µm to 76 ± 16 µm, n = 9) was achieved at 90 s (Figure 5B). After that, an acute desensitization (decrease of response in the presence of agonist) was observed. Arteriolar diameter was similar to the control at the end of the 20 min treatment (gradual increase to 150 ± 13 µm, n = 9, Figure 5B). Finally, tachyphylaxis (decrease of response upon repeated application of the agonist) was measured by the re-application of capsaicin (1 µM) after a 40 min regeneration period. Arteriolar diameter decreased from 161 ± 17 µm to 109 ± 18 µm (n = 6), suggesting significant resensitization of the receptor (Figure 5C).
Figure 5.
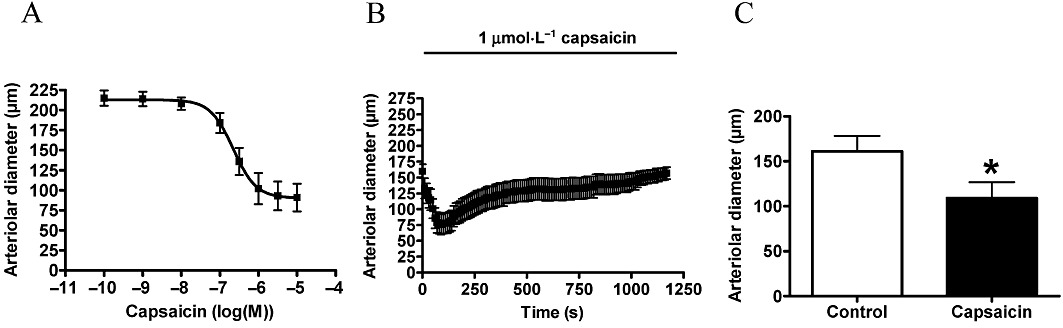
Pharmacological characterization of vascular responses to capsaicin. Experiments were performed on cannulated arteries as mentioned above. First, a cumulative dose-response curve was obtained (A, n = 7). Next on a separate set of arteries, the kinetics of response were measured by the application of 1 µM capsaicin for 20 min. Arteriolar diameter was measured at 10 s intervals (B, n = 9). After this 20 min treatment, the arteries were washed and were incubated in PSS solution for 40 min (regeneration). At the end of regeneration, vasoconstriction to the same dose of capsaicin (1 µM) was measured to determine tachyphylaxis (C, n = 7). Values are mean ± SEM, significant difference (P < 0.05) is represented by an asterisk.
Resiniferatoxin was tested under the same conditions (Figure 6). Surprisingly no vascular effects were detected upon application in a concentration range from 1 pM to 10 nM (Figure 6A). Moreover, no effects were detected upon application of 10 nM for 20 min (Figure 6B). However, capsaicin (1 µM) was without effect after 40 min regeneration (Figure 6C), suggesting complete desensitization of arterial TRPV1 upon the otherwise ineffective resiniferatoxin treatments.
Figure 6.
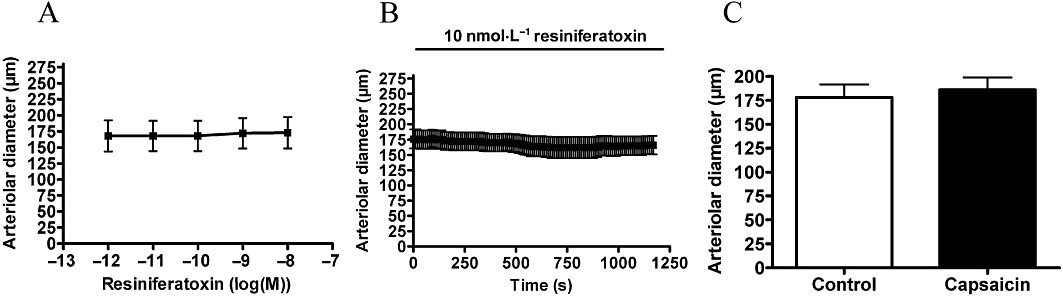
Arteriolar response to resiniferatoxin. Experiments were performed as mentioned in Figure 4 with resiniferatoxin. Responses to cumulative doses are shown in (A) (n = 3). No functional response was detected after 20 min of resiniferatoxin (B, 10 nM, n = 5). However, this treatment desensitized the receptors to capsaicin (1 µM) measured after regeneration (C, n = 5).
JYL-273 was ineffective at evoking arteriolar vasoconstriction in the concentration range 0.1 nM to 1 µM (n = 7, Figure 7A), nor did 1 µM JYL-273 applied for 20 min have any effect (n = 5, Figure 7B). However, similar to resiniferatoxin, this 20 min incubation resulted in complete desensitization of TRPV1 as evidenced by the lack of a response to capsaicin (n = 4, Figure 7C).
Figure 7.
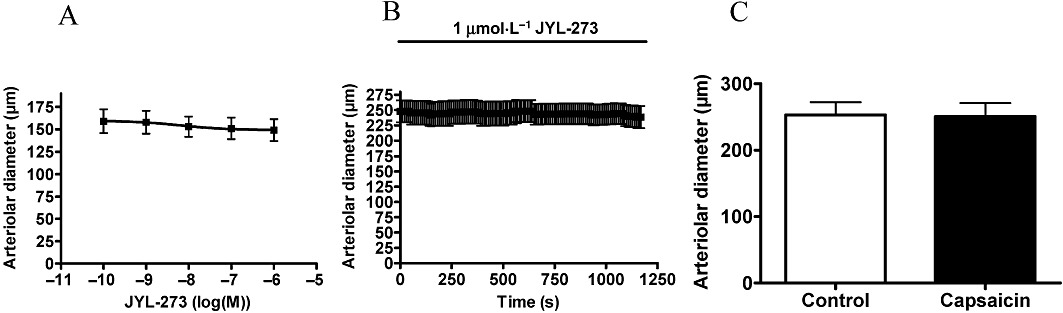
Arteriolar response to JYL-273. Experiments were performed as mentioned in Figure 4 with JYL-273. Responses to cumulative doses are shown in (A) (n = 7). No functional response was detected after 20 min of JYL-273 (B, 1 µM, n = 5). However, this treatment desensitized the receptors to capsaicin (1 µM) measured after regeneration (C, n = 4).
MSK-195 had a potency of 120 nM and an efficacy of 71 ± 11% (n = 5, Figure 8A). Application of 1 µM MSK-195 for 20 min resulted in a transient decrease in arterial diameter (decrease from 235 ± 19 µm to 155 ± 25 µm at 90 s, n = 6, Figure 8B). However, the kinetics of this acute desensitization were slower than that for capsaicin, since the original arteriolar diameter was not restored during the 20 min incubation (arterial diameter after 20 min incubation was 193 ± 25, P = 0.03 vs. before treatment, n = 6, Figure 8B). Similar to all the agonists mentioned above, MSK195 also evoked a complete desensitization of capsaicin-sensitive vascular TRPV1 (Figure 8C).
Figure 8.
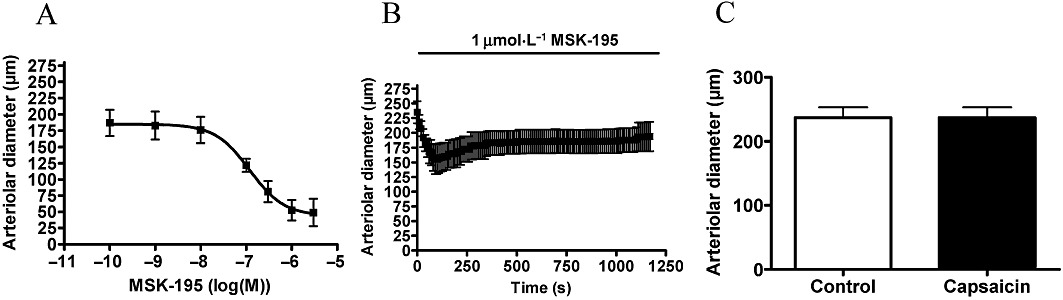
Arteriolar response to MSK-195. Experiments were performed as mentioned in Figure 4 with MSK-195. Responses to cumulative doses are shown in (A) (n = 5). A transient vasoconstriction was observed upon MSK-195 stimulation for 20 min (B, 1 µM, n = 6). In addition, this treatment desensitized the receptors to capsaicin (1 µM) measured after regeneration (C, n = 6).
JYL-79 was found to be more potent on vascular TRPV1 (EC50= 3.9 nM, n = 8, Figure 9A) than capsaicin. Its efficacy was 36 ± 8% (n = 8, Figure 9A). It also evoked a transient vasoconstriction when applied at a concentration of 1 µM (decrease of vascular diameter from 228 ± 13 µm to 127 ± 12 µm at 100 s, n = 5, Figure 9B). The desensitization of the receptor was not complete at the end of the 20 min incubation (vascular diameter at 20 min was 204 ± 13 µm, P = 0.046 vs. before treatment, n = 5, Figure 9B). Moreover, no response to capsaicin (1 µM) was observed after 40 min regeneration (n = 5, Figure 9C).
Figure 9.
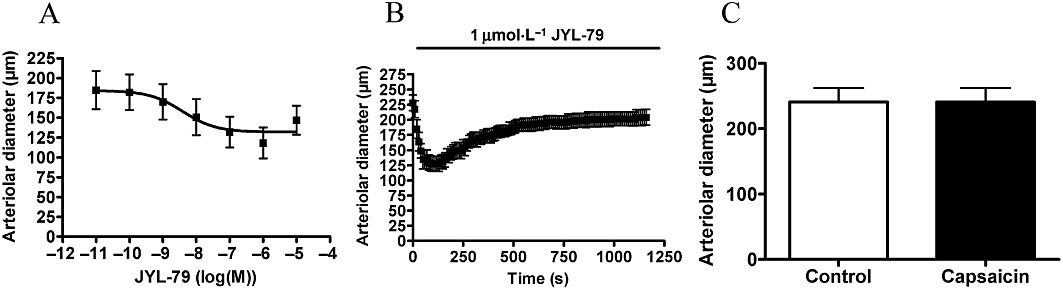
Arteriolar response to JYL-79. Experiments were performed as mentioned in Figure 4 with JYL-79. Responses to cumulative doses are shown in (A) (n = 8). A transient vasoconstriction was observed upon JYL-79 stimulation for 20 min (B, 1 µM, n = 5). In addition, this treatment desensitized the receptors to capsaicin (1 µM) measured after regeneration (C, n = 5).
To estimate the threshold of TRPV1 stimulation, which causes complete desensitization of vascular TRPV1, a partial agonist (JYL-1511) was applied. Its efficacy as an agonist was about 17%, and its potency was 3 nM in a CHO cells overexpressing rat TRPV1 (CHO-TRPV1) cell line (Wang et al., 2003). JYL-1511 was without effect on the vascular diameter in the concentration range 1 nM–1 µM (n = 6, Figure 10A). Application of 1 µM for 20 min was also without effect (Figure 10B). A partial inhibition (tachyphylaxis) of the capsaicin response (1 µM) was noted after 40 min regeneration (decrease of vascular diameter from 244 ± 14 µm to 209 ± 17 µm, P = 0.02, n = 6, Figure 10C). The level of partial agonism/antagonism was also determined (Figure 11). Application of JYL-1511 (1 µM) resulted in a decrease in the arteriolar diameter (arterial diameter decreased to 94 ± 3%, n = 6) and immediate capsaicin treatment (1 µM) resulted in a decrease in diameter to 83 ± 6% (P = 0.04, n = 6). In contrast, the same capsaicin treatment alone evoked a decrease in diameter to 43 ± 7% (P < 0.01, n = 7) in separate experiments. According to these data, the agonism of JYL-1511 is 10 ± 5% and antagonism is 70 ± 11% at the vascular TRPV1.
Figure 10.
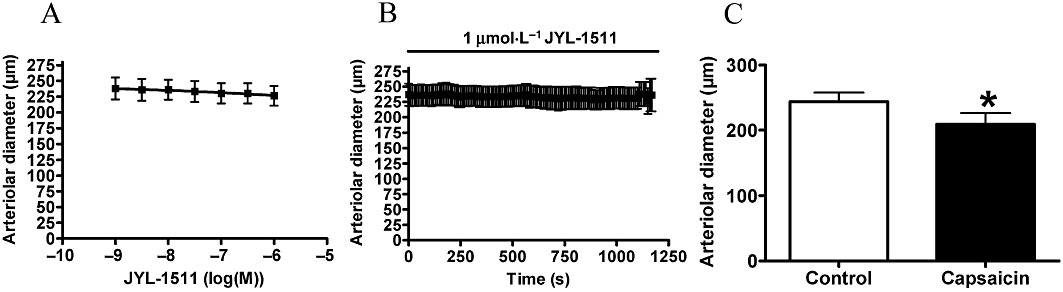
Arteriolar response to JYL-1511. Experiments were performed as mentioned in Figure 4 with JYL-1511. Responses to cumulative doses are shown in (A) (n = 6). No functional response was detected after 20 min of JYL-1511 (B, 1 µM, n = 6). However, this treatment desensitized the receptors to capsaicin (1 µM) measured after regeneration (C, n = 6).
Figure 11.
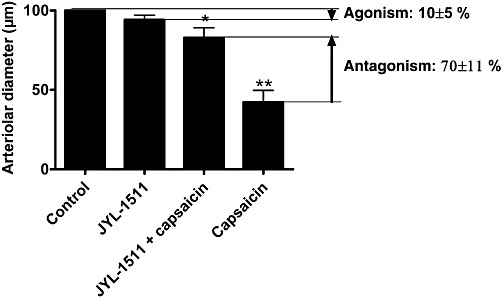
Partial agonism/antagonism of JYL-1511 on TRPV1 located in vascular smooth muscle. Changes in internal diameter of the arteries were measured before treatments (control, n = 6) and after addition of 1 µM JYL-1511 (JYL-1511, n = 6). Capsaicin responses were also determined in the presence of 1 µM JYL-1511 (JYL-1511 + capsaicin, n = 6). Finally, capsaicin responses alone (1 µM, without any pretreatment) were also measured on a different set of arteries (capsaicin, n = 7). The efficacy of JYL-1511 as a partial agonist was expressed as the percentage of decrease in arteriolar diameter evoked by the application of capsaicin alone (100%, capsaicin). Its efficacy as a partial antagonist was expressed as the percentage of decrease in capsaicin constriction (100%, capsaicin) in the presence of JYL-1511 (1 µM, JYL-1511 + capsaicin).
The aim of this study was to detect differences between TRPV1 populations responsible for sensory neuronal activation and vasoconstriction. A weakness in the previous characterization of the applied agonists is that their effects were tested only in vitro, many times only on TRPV1 receptors expressed exogenously (Table 1). Sensory neuronal activation was also tested here by use of the eye wiping assay. JYL-1511 did not evoke significant effects, while all of the other agonists increased the number of eye wipes (Figure 12). Note, that although these data were in complete agreement with previous in vitro results (Table 1), differences between sensory neuronal and vascular effects were also noted. In particular, resiniferatoxin and JYL-273 were both ineffective at evoking acute activation of vascular TRPV1 receptors (Figures 6 and 7, respectively).
Table 1.
Pharmacological properties of TRPV1 agonists
| Agonist | Dorsal root ganglion/CHO-TRPV1 | CHO-TRPV1 | Arteriolar TRPV1 (vasoconstriction) | |||||
|---|---|---|---|---|---|---|---|---|
| Ki (binding) | EC50 (45Ca2+ uptake) | EC50 (intracellular Ca2+) | Acute desensitization | Potency | Efficacy | Acute desensitization | Tachyphylaxis | |
| Capsaicin | 1.8 ± 0.3 µM (Wang et al., 2003) | 95 ± 8 nM (Pearce et al., 2008) | 35 ± 11 nM (Toth et al., 2005b) | + (Toth et al., 2005b) | 221 nM | 58 ± 7% | + | − |
| Resiniferatoxin | 23 pM (Lee et al., 2001) | 1.5 ± 0.3 nM (Pearce et al., 2008) | 81 ± 20 pM (Toth et al., 2005b) | − (Toth et al., 2005b) | >10 nM | No effect at 10 nM | N/A | + |
| JYL-273 | 11 ± 4 nM (Lee et al., 2001) | 361 ± 54 nM (Lee et al., 2002) | No data | No data | >3 µM | No effect at 1 µM | N/A | + |
| MSK-195 | No data | 162 ± 33 nM (Lee et al., 2002) | 52 ± 12 nM (Toth et al., 2005b) | − (Toth et al., 2005b) | 120 nM | 71 ± 11% | + | + |
| JYL-79 | 19 ± 4 nM (Lee et al., 2001) | 58 ± 8 nM (Lee et al., 2002) | 2.4 ± 1.0 nM (Toth et al., 2005b) | − (Toth et al., 2005b) | 3.9 nM | 36 ± 8% | + | + |
| JYL-1511 | 50 ± 17 nM (Wang et al., 2003) | 3.4 ± 0.5 µM (Wang et al., 2003) | No data | + (Wang et al., 2003) | N/A | No effect at 1 µM | N/A | +/− |
Figure 12.
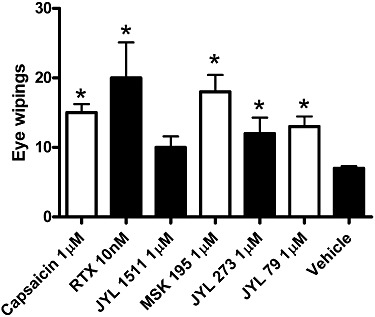
Sensory neuronal irritation evoked by application of the TRPV1 ligands. TRPV1 agonists or vehicle were applied in the eye of rats to determine their ability to evoke sensory neuronal irritation. Number of eye wipes was counted for 60 s after application of 10 µL of the drugs onto the conjunctiva of the rats. Concentrations of the drugs were chosen to represent the highest dose used in the vascular experiments (indicated in the figure). Columns represent mean ± SEM (n = 5), significant differences (P < 0.05) from the control (wipes upon administration of the vehicle alone) are represented by asterisks.
Discussion and conclusions
Here we report an analysis of the pharmacological properties of a vasoconstrictor population of TRPV1. Changes in vascular diameter in response to various agonists and partial agonist/antagonists of the receptor were measured. Our data suggest that significant differences in the pharmacological properties of endogenous TRPV1 pools exist. There are at least two important consequences of this observation. Firstly, TRPV1 antagonists being developed as analgesic agents should be tested for side effects on the circulation. Secondly, selective modulation of vascular TRPV1 may also be a therapeutic target. Pharmacological exploitation of vascular TRPV1 seems to be a reasonable aim with the substantial chemical libraries constructed to develop successful TRPV1 antagonists.
Vascular TRPV1 was characterized here by measuring the vasoconstriction upon TRPV1 stimulation. Previously, TRPV1 was shown to be expressed in vascular smooth muscle cells and it was suggested that activation of TRPV1 is directly linked to intracellular Ca2+ elevations in smooth muscle (Kark et al., 2008). Indeed, in the present study we found that a decrease in arteriolar diameter was paralleled by an increase in intracellular Ca2+ concentrations in the vascular wall (Figure 3); moreover, direct intracellular Ca2+ concentration measurements revealed, for the first time, the presence of functional TRPV1 in isolated arteriolar smooth muscle cells (Figure 4).
Vasoconstriction in response to TRPV1 stimulation was reported decades ago (Molnar and Gyorgy, 1967; Toda et al., 1972; Donnerer and Lembeck, 1982; Duckles, 1986; Edvinsson et al., 1990) and this effect was confirmed later (Szolcsanyi et al., 2001; Dux et al., 2003; Scotland et al., 2004; Keeble and Brain, 2006; Lizanecz et al., 2006). Nonetheless, these responses were not thought to be of interest. One of the reasons for this was that pharmaceutical research had concentrated on the exploitation of the obvious potential of sensory neuronal TRPV1 as an analgesic target. Another reason was that stimulation of sensory neuronal TRPV1 in the perivascular nerves evokes vasodilatation (Zygmunt et al., 1999), probably obscuring the vasoconstrictor response in many cases. In accordance with this latter mechanism, earlier reports showed concentration-dependent biphasic effects of TRPV1 stimulation; low dose capsaicin evoked dilatation, while higher concentrations resulted in constriction (Edvinsson et al., 1990; Dux et al., 2003). This suggested the involvement of different receptors or different pharmacology for TRPV1-mediating vascular dilatation and constriction.
Although the mechanism of the vasoconstrictor effects of TRPV1 agonists were generally not investigated in detail (Porszasz et al., 2002; Dux et al., 2003; Keeble and Brain, 2006), it was suggested that TRPV1-induced vasoconstriction is probably mediated by endothelin-1 (Szolcsanyi et al., 2001) or substance P (Scotland et al., 2004) release from sensory neurons.
We have recently shown that stimulation of TRPV1 in skeletal muscle arterioles results in a substantial vasoconstriction (Lizanecz et al., 2006). Moreover, intra-arterial injection of capsaicin into the hind limb evoked a dose-dependent increase in blood flow in the skin (probably representing vasodilatation in this organ) and simultaneously, a decrease of blood flow in skeletal muscle (representing vasoconstriction) (Kark et al., 2008). These data suggested that vascular TRPV1 have sensory neuron-independent physiological effects.
The TRPV1 specificity of capsaicin-mediated arteriolar vasoconstriction was ultimately proven here. Most importantly, capsaicin-mediated responses were absent in TRPV1 knockout mice (Figure 2C). Moreover, an effort was also made to investigate the potential mechanisms. The intracellular Ca2+ concentration measurements we obtained showed that capsaicin increased the diameter of the arterial wall of skeletal muscle arteries (Figure 3), as well as in isolated arteriolar smooth muscle cells (Figure 4). Although only 10 out of 28 isolated smooth muscle cells responded to capsaicin, these data strongly suggest that functional TRPV1 is expressed in arterial smooth muscle cells and that the activation of these receptors leads to an increase in smooth muscle intracellular Ca2+ concentrations and to vasoconstriction. Next, the effects of pharmacological TRPV1 inhibition on this response were tested. The TRPV1 antagonist AMG9810 (previously tested on exogenous and sensory neuronal TRPV1) inhibited this capsaicin-evoked arteriolar constriction in a competitive fashion. These findings suggest that TRPV1 antagonists developed as analgesic agents may also interfere with skeletal muscle blood perfusion by inhibiting vascular TRPV1.
Nonetheless, the major goal of this present work was to investigate the structure-activity relationship of TRPV1 agonists for the vascular TRPV1 in functional assays. Our results confirmed that TRPV1 stimulation by capsaicin evokes a substantial constriction in isolated cannulated skeletal muscle arteries (Lizanecz et al., 2006; Kark et al., 2008). Here, a series of commercially available agonists were also tested in addition to capsaicin. Significant differences in potency, efficacy and desensitization were found (Table 1). It was observed that some of the TRPV1 agonists (such as resiniferatoxin, JYL-273) were able to desensitize vascular TRPV1 without any apparent vascular effects. This behaviour of resiniferatoxin in the vascular diameter assay is not unprecedented; a very similar action (‘desensitization’ to capsaicin without prior activation) has been demonstrated for pulmonary chemoreflex (Szolcsanyi et al., 1990). One hypothesis for this desensitization is that low level activation of TRPV1 with certain structures may be sufficient to evoke complete tachyphylaxis, without increasing the intracellular Ca2+ concentrations to those levels needed to induce vasoconstriction. Alternatively, tachyphylaxis may be the reason for the irreversible activation of TRPV1 by resiniferatoxin (Jeffry et al., 2009) leading to a sustained Ca influx. To measure the level of activation needed to evoke tachyphylaxis a partial agonist (JYL-1511) was used. Its partial antagonism was confirmed on vascular receptors (about 10% agonism and 70% antagonism), and its application resulted in significant tachyphylaxis, suggesting a role for desensitization rather than sustained Ca2+ influx in this system. In addition, although the functional response to capsaicin was transient and the arteries were completely desensitized to capsaicin, after a 40 min regeneration period, capsaicin was able to evoke vasoconstriction, suggesting resensitization of the arteries and only a partial tachyphylaxis. Taken together, these results suggest the vascular smooth muscle-located receptor and also the TRPV1 responsible for eye irritation upon capsaicin treatment in vivo (Figure 12) seem to have different ligand selectivity for desensitization from that of the TRPV1 expressed in CHO cells (Table 1).
It was observed that the kinetics of acute desensitization were different in the case of agonists evoking vascular constriction. With capsaicin, complete acute desensitization was observed, while for other agonists, JYL-79, MSK-195, only a partial desensitization was observed. The fact that different agonists evoked responses with different durations suggests that TRPV1 agonists may be tailored to desired duration of vascular effects.
Several mechanisms have been suggested to regulate TRPV1 sensitivity and desensitization. These include PKC- (Bhave, 2003) or PKA- (Bhave et al., 2002) mediated phosphorylation and calcineurin-mediated dephosphorylation (Docherty et al., 1996). As regards TRPV1 mediating skeletal muscle vasoconstriction, phosphorylation seems to be the most likely candidate. It was found that anandamide (Lizanecz et al., 2006), similar to resiniferatoxin and JYL-273 (shown in this report), evokes complete tachyphylaxis on vascular TRPV1 without functional effects. However, it was also shown, that the anandamide-mediated tachyphylaxis was antagonized by a protein phosphatase 2B (calcineurin) inhibitor (Lizanecz et al., 2006). Moreover, in accordance with this hypothesis, TRPV1 responses to agonists were differently modulated by inhibition of calcineurin in a CHO-TRPV1 cell line (Pearce et al., 2008), suggesting ligand selectivity for phosphorylation-dependent TRPV1 sensitization/desensitization/tachyphylaxis.
Taken together, our results indicate that TRPV1 (a non-specific Ca2+ channel) activation leads to an increase in intracellular Ca2+ concentrations in isolated coronary smooth muscle cells and in the wall of isolated skeletal muscle arteries, resulting in vasoconstriction. The pharmacological profile of the vascular TRPV1 differs from that of the TRPV1 population responsible for sensory irritation. Arteriolar TRPV1 was inhibited by a competitive TRPV1 antagonist developed as an analgesic suggesting that vascular TRPV1 activation may represent a side effect of TRPV1 antagonists when used as analgesics in vivo. Moreover, vascular TRPV1 may be a new therapeutic target for the regulation of tissue blood distribution.
Acknowledgments
The study was supported by the Hungarian Academy of Sciences OTKA (K68077 and K84300 to AT and T046244 to RP) and by the Hungarian Ministry of Health (ETT 377/2009 to AT); the work is supported by the TÁMOP 4.2.1./B-09/1/KONV-2010-0007 project (to AT and ZP). The project is implemented through the New Hungary Development Plan, co-financed by the European Social Fund; and by the National Innovation Office of Hungary (Baross Gábor Project, ÉletMent). Finally, AT was supported by the Bolyai János Research Scholarship of the Hungarian Academy of Sciences.
Glossary
- AMG9810
(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide
- CHO-TRPV1
CHO cells overexpressing rat TRPV1
- DRG
dorsal root ganglion
- JYL-1511
N-(4-tert-butylbenzyl)-N'-[3-methoxy-4-(methyl-sulphonylamino)benzyl]thiourea
- JYL-273
2-(4-t-butylbenzyl)-3-{[(4-hydroxy-3-methoxybenzyl)ami- no]carbothioyl}propyl pivalate
- JYL-79
2-(3,4-dimethylbenzyl)-3-{[(4-hydroxy-3-methoxy-benzyl)amino]carbothioyl}propyl pivalate
- MSK-195
N-[2-(3,4-dimethylbenzyl)-3-(pivalyloxy)propyl]-2-[4-(2-aminoethoxy)-3 methoxyphenyl]acetamide
- TRPV1
transient receptor potential vanilloid 1
- TRPV1-/-
B6.129X1-Trpv1tm1Jul/J mice
Conflicts of interest
The authors report no conflicts of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G. From the cover: protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci USA. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau R. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Blumberg PM, Szallasi A. Endovanilloid signaling in pain. Curr Opin Neurobiol. 2002;12:372–379. doi: 10.1016/s0959-4388(02)00340-9. [DOI] [PubMed] [Google Scholar]
- Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- Donnerer J, Lembeck F. Analysis of the effects of intravenously injected capsaicin in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1982;320:54–57. doi: 10.1007/BF00499072. [DOI] [PubMed] [Google Scholar]
- Duckles SP. Effects of capsaicin on vascular smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1986;333:59–64. doi: 10.1007/BF00569661. [DOI] [PubMed] [Google Scholar]
- Dux M, Santha P, Jancso G. Capsaicin-sensitive neurogenic sensory vasodilatation in the dura mater of the rat. J Physiol. 2003;552:859–867. doi: 10.1113/jphysiol.2003.050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L, Jansen I, Kingman TA, McCulloch J. Cerebrovascular responses to capsaicin in vitro and in situ. Br J Pharmacol. 1990;100:312–318. doi: 10.1111/j.1476-5381.1990.tb15801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang TJ, Immke D, et al. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther. 2005;313:474–484. doi: 10.1124/jpet.104.079855. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Bannon AW, Surapaneni S, Hovland DN, Jr, Lehto SG, Gore A, et al. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136:202–210. doi: 10.1016/j.pain.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Szallasi A. Peripheral TRPV1 receptors as targets for drug development: new molecules and mechanisms. Curr Pharm Des. 2008;14:32–41. doi: 10.2174/138161208783330754. [DOI] [PubMed] [Google Scholar]
- Jakab B, Helyes Z, Varga A, Bolcskei K, Szabo A, Sandor K, et al. Pharmacological characterization of the TRPV1 receptor antagonist JYL1421 (SC0030) in vitro and in vivo in the rat. Eur J Pharmacol. 2005;517:35–44. doi: 10.1016/j.ejphar.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Jeffry JA, Yu SQ, Sikand P, Parihar A, Evans MS, Premkumar LS. Selective targeting of TRPV1 expressing sensory nerve terminals in the spinal cord for long lasting analgesia. PLoS ONE. 2009;4:e7021. doi: 10.1371/journal.pone.0007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kark T, Bagi Z, Lizanecz E, Pasztor ET, Erdei N, Czikora A, et al. Tissue-specific regulation of microvascular diameter: opposite functional roles of neuronal and smooth muscle located vanilloid receptor-1. Mol Pharmacol. 2008;73:1405–1412. doi: 10.1124/mol.107.043323. [DOI] [PubMed] [Google Scholar]
- Keeble JE, Brain SD. Capsaicin-induced vasoconstriction in the mouse knee joint: a study using TRPV1 knockout mice. Neurosci Lett. 2006;401:55–58. doi: 10.1016/j.neulet.2006.02.083. [DOI] [PubMed] [Google Scholar]
- Khairatkar-Joshi N, Szallasi A. TRPV1 antagonists: the challenges for therapeutic targeting. Trends Mol Med. 2009;15:14–22. doi: 10.1016/j.molmed.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Lee J, Lee J, Kim J, Kim SY, Chun MW, Cho H, et al. N-(3-Acyloxy-2-benzylpropyl)-N'-(4-hydroxy-3-methoxybenzyl) thiourea derivatives as potent vanilloid receptor agonists and analgesics. Bioorg Med Chem. 2001;9:19–32. doi: 10.1016/s0968-0896(00)00216-9. [DOI] [PubMed] [Google Scholar]
- Lee J, Lee J, Kang MS, Kim KP, Chung SJ, Blumberg PM, et al. Phenolic modification as an approach to improve the pharmacology of the 3-acyloxy-2-benzylpropyl homovanillic amides and thioureas, a promising class of vanilloid receptor agonists and analgesics. Bioorg Med Chem. 2002;10:1171–1179. doi: 10.1016/s0968-0896(01)00387-x. [DOI] [PubMed] [Google Scholar]
- Lizanecz E, Bagi Z, Pasztor ET, Papp Z, Edes I, Kedei N, et al. Phosphorylation-dependent desensitization by anandamide of vanilloid receptor-1 (TRPV1) function in rat skeletal muscle arterioles and in Chinese hamster ovary cells expressing TRPV1. Mol Pharmacol. 2006;69:1015–1023. doi: 10.1124/mol.105.015644. [DOI] [PubMed] [Google Scholar]
- Molnar J, Gyorgy L. Pulmonary hypertensive and other haemodynamic effects of capsaicin in the cat. Eur J Pharmacol. 1967;1:86–92. doi: 10.1016/0014-2999(67)90043-x. [DOI] [PubMed] [Google Scholar]
- Pearce LV, Toth A, Ryu H, Kang DW, Choi HK, Jin MK, et al. Differential modulation of agonist and antagonist structure activity relations for rat TRPV1 by cyclosporin A and other protein phosphatase inhibitors. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:149–157. doi: 10.1007/s00210-007-0258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porszasz R, Porkolab A, Ferencz A, Pataki T, Szilvassy Z, Szolcsanyi J. Capsaicin-induced nonneural vasoconstriction in canine mesenteric arteries. Eur J Pharmacol. 2002;441:173–175. doi: 10.1016/s0014-2999(01)01596-5. [DOI] [PubMed] [Google Scholar]
- Ross RA. Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland RS, Chauhan S, Davis C, De Felipe C, Hunt S, Kabir J, et al. Vanilloid receptor TRPV1, sensory C-fibers, and vascular autoregulation: a novel mechanism involved in myogenic constriction. Circ Res. 2004;95:1027–1034. doi: 10.1161/01.RES.0000148633.93110.24. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, et al. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci. 2007;27:7459–7468. doi: 10.1523/JNEUROSCI.1483-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- Szolcsanyi J, Szallasi A, Szallasi Z, Joo F, Blumberg PM. Resiniferatoxin: an ultrapotent selective modulator of capsaicin-sensitive primary afferent neurons. J Pharmacol Exp Ther. 1990;255:923–928. [PubMed] [Google Scholar]
- Szolcsanyi J, Oroszi G, Nemeth J, Szilvassy Z, Blasig IE, Tosaki A. Functional and biochemical evidence for capsaicin-induced neural endothelin release in isolated working rat heart. Eur J Pharmacol. 2001;419:215–221. doi: 10.1016/s0014-2999(01)00973-6. [DOI] [PubMed] [Google Scholar]
- Toda N, Usui H, Nishino N, Fujiwara M. Cardiovascular effects of capsaicin in dogs and rabbits. J Pharmacol Exp Ther. 1972;181:512–521. [PubMed] [Google Scholar]
- Toth A, Boczan J, Kedei N, Lizanecz E, Bagi Z, Papp Z, et al. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res Mol Brain Res. 2005a;135:162–168. doi: 10.1016/j.molbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Toth A, Wang Y, Kedei N, Tran R, Pearce LV, Kang SU, et al. Different vanilloid agonists cause different patterns of calcium response in CHO cells heterologously expressing rat TRPV1. Life Sci. 2005b;76:2921–2932. doi: 10.1016/j.lfs.2004.10.056. [DOI] [PubMed] [Google Scholar]
- Wang Y, Toth A, Tran R, Szabo T, Welter JD, Blumberg PM, et al. High-affinity partial agonists of the vanilloid receptor. Mol Pharmacol. 2003;64:325–333. doi: 10.1124/mol.64.2.325. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


