Abstract
BACKGROUND AND PURPOSE
Emesis is a multi-system reflex, which is usually investigated using in vivo models. The aim of the study is to compare the response induced by emetic compounds across species and investigate whether dogs, ferrets and rats are all similarly predictive of humans.
EXPERIMENTAL APPROACH
A systematic review was carried out and relevant publications were identified from PubMed. The search was restricted to four species (human, dog, ferret, rat) and ten compounds representative of various mechanisms of emesis induction (apomorphine, cisplatin, cholecystokinin octapeptide, copper sulphate, cyclophosphamide, ipecacuanha, lithium chloride, morphine, nicotine, rolipram).
KEY RESULTS
1046 publications were reviewed, and 311 were included, the main reason for exclusion was the lack of quantitative data. Emetic or pica data were extracted as incidence, intensity or latency. All three animal species identified emetic liability but interspecies differences for dose sensitivity were detected.
CONCLUSIONS AND IMPLICATION
These results suggest that emetic liability can be reliably identified in a common laboratory species such as the rat. However, to evaluate the characteristics of the emetic response, no animal species is a universal predictor of emetic liability and the choice of species should be an informed decision based on the type of compound investigated. Limitations relating to the conduct and reporting of emesis studies were identified, the main ones being the lack of comparable outcome measures between human and animal data, and the limited availability of human data in the public domain.
Keywords: nausea, vomiting, emesis, dog, ferret, rat, human, pica, emetic liability
Introduction
Nausea (a feeling of sickness often associated with the urge to vomit) and emesis (forceful oral expulsion of the gastrointestinal contents) are components of a natural defence mechanism to protect the body against the absorption of ingested toxins (Davis et al., 1986). However, this defensive response is not always appropriately triggered, and nausea and emesis represent the most commonly encountered side effect of drugs marketed for clinical use. Over half of the drugs in the electronic Medicines Compendium list nausea as an adverse effect, and more than a third list both nausea and vomiting (Lee, 2007). These symptoms can limit the dose that can be tolerated, lead to poor quality of life, impair normal nutrition and reduce patient compliance. In drug discovery and development programmes, nausea and emesis may impede the development of a valuable new drug (Holmes et al., 2009).
Emesis is a reflex motor response with a complex mechanism, which requires the integration of multiple pathways and the coordination of respiratory, circulatory, neural and digestive systems. For this reason, animal models are used to study emetic mechanisms and identify emetic liability and anti-emetic efficacy of novel chemical entities (NCEs). Commonly used species include the ferret (Mustela putorius furo), which played a key role in the identification of 5-HT3 and NK1 receptor antagonists, two classes of anti-emetic drugs currently in clinical use (Christie and Tansey, 2007). Other species include the house musk shrew (Suncus murinus) and the dog (Canis lupus familiaris), which is typically included in pharmaceutical toxicology studies. In rats (Rattus norvegicus), which do not have the emetic reflex (Sanger et al., 2011), the ingestion of non-nutritive substances such as clay has been observed following the administration of compounds that are emetic in other species. This behaviour, termed pica, is believed to reflect gastrointestinal malaise and is argued to be an indication of ‘nausea’. Other potential models of nausea have been described in rats and include conditioned taste aversion, context aversion conditioning and conditioned gaping. Reduced food intake and delayed gastric emptying may also indicate nausea, but these symptoms are not specific (see Stern et al., 2011 for review).
To reduce reliance on animal models, we hypothesized that the quantity of in vivo emetic data collected by the broad scientific community (including academia, industry and contract research organisations) over the last century would be sufficient to develop an algorithm to predict the likelihood of a novel compound inducing emesis. Information on emetogenicity would be cross-referenced with the intrinsic attributes of a compound, including pharmacology and structure. This will ultimately enable estimation of the probability of the emetic liability of a novel compound at an earlier stage in the drug development process, and inform the decision to stop the development of a compound before in vivo toxicology studies. Such an approach would thus minimize animal use, reduce costs and improve the probability that a novel compound will be successfully developed.
The present study represents the first step towards this goal and was designed to appraise the emetic data in the public literature and assess the translational value of preclinical data. We have created a database compiled from systematic review and meta-analysis of the literature, an approach that has been used successfully to identify opportunities to reduce animal use and improve animal welfare in the ferret model of cisplatin-induced emesis (Percie du Sert et al., 2011). As a proof of concept, the current study was restricted to the four most commonly used species in emesis research: ferret, dog, rat and human. The aims of the study were to compare the response induced by emetic compounds across species and investigate whether dogs, ferrets and rats are all similarly predictive of humans. The results provide a better understanding of the translational value of species commonly used in emesis toxicology and provide an evidence base for species choice for assessing emetic liability of specific compound classes.
Methods
Search strategy and data extraction
Studies were identified from PubMed in different stages; all searches were performed between February and April 2010. Details of the PubMed searches are included as supplementary information Appendix S1. First, publications potentially containing emetic data in the ferret or pica data in the rat were identified using the searches ‘ferret AND emesis’ and ‘pica AND (rats OR rodents)’. Following a preliminary analysis, 10 compounds representative of different pharmacological classes and diverse mechanisms of emesis induction were researched further to identify data in dogs and humans.
These compounds were chosen based on the availability of ferret and rat data; being commonly used emetic inducers, it was assumed that data would be available across all four species. The 10 compounds were apomorphine, cholecystokinin octapeptide (CCK-8), cisplatin, copper sulphate, cyclophosphamide, ipecacuanha, lithium chloride, morphine, nicotine and rolipram. Publications on emesis in dogs and humans with nicotine, lithium chloride, CCK-8, copper sulphate and rolipram were identified with the search: ‘drug name AND emesis’. Due to the wide number of publications available for the remaining compounds, searches were carried out separately for dogs and humans. Publications of clinical data with ipecacuanha and morphine were identified using the searches ‘drug name AND emesis AND (volunteer AND volunteers)’. This restricted the search to healthy volunteers, a cohort potentially more homogeneous and readily comparable with preclinical studies using healthy animals. In addition, early non-clinical safety studies are usually designed to detect liabilities for NCEs in first-in-human trials, which for the most part are healthy volunteer studies. Therefore, understanding the translation from animals to healthy human volunteers was a key goal for this project. The search for apomorphine using the previous search term only identified one publication and was therefore extended to ‘apomorphine AND emesis AND (human OR humans)’. To identify studies with the chemotherapeutic agents cisplatin and cyclophosphamide, the search ‘drug name AND placebo AND antiemetic’ was used, as no studies using anti-cancer cytotoxic drugs in healthy volunteers were expected. Reports of cisplatin, apomorphine, ipecacuanha, morphine and cyclophosphamide in dogs were identified using the search ‘drug name AND emesis AND (dog OR dogs)’. Because of the extensive number of publications identified for apomorphine, the search was restricted to reports published from 1985 (time of the first publications on the ferret model) to present. For all other searches, all publications available on PubMed were reviewed. Hand searching of the authors' personal files was carried out if no publication was identified for a particular compound in a particular species.
Data relating to the emetic or pica response in the 24 h or less following administration of a single drug dose were extracted as at least one of the following:
-
Incidence:
○ Incidence of emesis (percentage of individuals developing an emetic response).
○ Incidence of nausea (humans only)
○ Incidence of pica behaviour (rats only)
-
Intensity:
○ Number of retches + vomits [defined as rhythmic abdominal contractions that are either associated with the oral expulsion of solid or liquid material from the gastrointestinal tract (i.e. vomiting), or not associated with the passage of material (i.e. retching)]
○ Number of vomits
○ Number of emetic episodes (series of retches and/or vomits separated by a maximum of 5 s)
○ Number of bouts of emesis (series of retches and/or vomits separated by more than 5 s – typically a few minutes). Note that episodes reported without a definition were included in that category.
○ Pica data were extracted as kaolin or clay consumption within the first 24 h following drug administration; the consumption in the control group was subtracted from the consumption in the drug-treated group.
Latency (delay between the administration of the drug and the onset of emesis)
Exclusion criteria:
No quantification of emetic or pica data as described above and/or mean or individual data not reported. In preclinical studies, data reported as emetic dose (e.g. ED50) were not extracted. For human data, however, owing to the scarcity of published data and variability of methods used, data reported as threshold doses were included in the incidence outcome.
Reviews, letters to the editor or comments with no original data
Case reports
Duplicate publications (if all datasets in the publication were duplicates, the entire publication was excluded, if only some duplicate datasets were identified, the remaining original datasets were included)
Animal not conscious during the emetic response (under anaesthesia or decerebrate)
Animal/subject given prophylactic anti-emetic drugs
No methods and/or results unclear or dose unknown (dose ranges were accepted and entered as the mean dose for the group if available, or the mid-range)
No vehicle-treated control group for pica
Individuals pre-screened to select high or low responders or excluded from study if developing emesis
Complex treatment administered and possibility that the emetic response is not primarily due to the drug of interest. NB: when a combination of anti-chemotherapeutic agents was used, data were collected for the most emetic agent (Roila et al., 2006).
Emetic data for another species (other than the four species of interest: ferret, rat, dog and human)
Emetic data for another emetogen (other than the 10 compounds of interest)
Full publication not located
Analysis
Incidence, intensity and latency of emesis are the minimum parameters necessary to characterize the emetic response adequately (Andrews and Davis, 1995). Incidence and latency data were used unchanged directly from the publication. Outcomes relating to the intensity of the response (i.e. retches + vomits, vomits, episodes, bouts, kaolin consumption) were combined in an emetic scale. To enable inter-species comparisons, for each species, an emetic unit was defined as the maximal emetic response to cisplatin. Cisplatin was chosen as it is one of the most emetic compounds across all four species and for the profusion of published data available, enabling characterization of the emetic/pica response in a reliable manner. One emetic unit is defined as the response to 1–5 mg·kg−1 cisplatin in humans, dogs and rats; a higher dose range was chosen in the ferret: 6–10 mg·kg−1, as a higher dose is required to induce maximal emetic response in this species. For all cisplatin data collected within the dose range of interest, weighted means for all measures of intensity were computed to establish the characteristics of one emetic unit for each species (see Table 1). For example, if cisplatin induced 10 emetic episodes in a species and another compound causes five episodes, the latter would be 0.5 units in that species. In ferrets, dogs and humans, if more than one intensity outcome had been collected, the emetic scale was calculated in priority from the retches + vomits if available, then vomits, then episodes and then bouts. Weighted means were calculated for incidence, emetic scale and latency using species, dose and mode of administration as variables (SPSS 16.0, SPSS Inc., Chicago, IL, USA). Plots were generated (GraphPad Prism 5.0, San Diego, CA, USA) and ED50 values (dose that induces emesis, nausea or pica in 50% of the individuals) were calculated for the incidence using a sigmoidal curve fit (variable slope, normalized). For the purpose of calculating ED50, if the response followed a bell-shaped curve, only the ascending part of the curve was considered. Second-order polynomial curves were fitted to examine data distribution (not shown). The emetic scale and the latency data were fitted with a second order polynomial curve (GraphPad Prism).
Table 1.
Characteristics of an emetic unit per species
| Species | Dose range (mg.kg−1) | R + V | Vomits | Episodes | Bouts | Kaolin consumption (g) |
|---|---|---|---|---|---|---|
| Human | 1–5 | n/a | n/a | n/a | 7 | n/a |
| Dog | 1–5 | 138 | 13 | n/a | 13 | n/a |
| Ferret | 6–10 | 134 | 16 | 37 | 17 | n/a |
| Rat | 1–5 | n/a | n/a | n/a | n/a | 6 |
Cisplatin was administered i.v. or i.p.. The characteristics of the response induced by cisplatin were computed as the weighted average for all doses within the dose range, with data from 180 human patients, 476 dogs, 635 ferrets and 106 rats.
Drugs
The 10 compounds investigated in the present study, along with their IUPAC name (PubChem), are apomorphine ((6aR)-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline-10,11-diol), a non-selective dopamine receptor agonist (Millan et al., 2002); the gastrointestinal hormone cholecystokinin octapeptide (CCK-8; (3S)-3-[[2-[[(2S)-2-[[2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-4-hydroxy-4-oxobutanoyl]amino]-3-(4-sulphooxyphenyl)propanoyl]amino]-4-methylsulphanylbutanoyl]amino]acetyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-4-methylsulphanylbutanoyl]amino]-4-[[(2S)-1-amino-1-oxo-3-phenylpropan-2-yl]amino]-4-oxobutanoic acid), a CCK1/CCK2 receptor agonist (Alexander et al., 2011); the chemotherapeutic agents cisplatin [dichloroplatinum(2+)] and cyclophosphamide [N-bis(2-chloroethyl)-1-oxo-6-oxa-2-aza-1λ5-phosphacyclohexan-1-amine]; copper sulphate (CuSO4), ipecacuanha (mix of emetine [(2S,3R,11bS)-2-[[(1R)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolin-1-yl]methyl]-3-ethyl-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinoline] and cephaline) (Manno and Manno, 1977); lithium chloride (LiCl); morphine (5α,6α)-7,8-didehydroo-4,5-epoxy-17-methylmorphinan-3,6-diol), a selective µ opioid receptor agonist (Alexander et al., 2011); nicotine (3-[(2S)-1-methylpyrrolidin-2-yl]pyridine), a non-selective nicotinic acetylcholine receptor agonist (Brioni et al., 1997); rolipram (4-[3-(cyclopentyloxy)-4-methoxyphenyl]-2-pyrrolidinone), a selective PDE4 inhibitor (Souness and Rao, 1997).
Results
Publications
A total number of 1046 publications were identified and reviewed. As described in Figure 1, the search for emetic data identified 266 publications using ferrets and 106 publications using rat pica, 670 publications using dogs and humans were subsequently identified for the 10 compounds of interest. No numerical data were found on LiCl-induced emesis in dogs and humans, rolipram-induced emesis in humans, nicotine-induced emesis in ferrets and ipecacuanha-induced pica in rats. Personal files were specifically searched for such publications, and a further four publications were identified. 735 publications were excluded (see Figure 1), the main reason (30% of the excluded studies) being that no numerical emetic data could be found. Another 30% of the studies were excluded because emesis/pica were investigated in a species other than ferret, dog, rat or human, and/or was induced by an emetic challenge other than the 10 compounds of interest. Reviews, case reports, letters or comments to the editor represented 20% of the excluded publications, and 15% were excluded because prophylactic anti-emetics were administered with the compound of interest. Finally, 311 publications relevant to the present study were included in the meta-analysis (see Table 2); 38%, 35% and 9% contained ferret, dog and rat data, respectively, and 25% contained human data.
Figure 1.
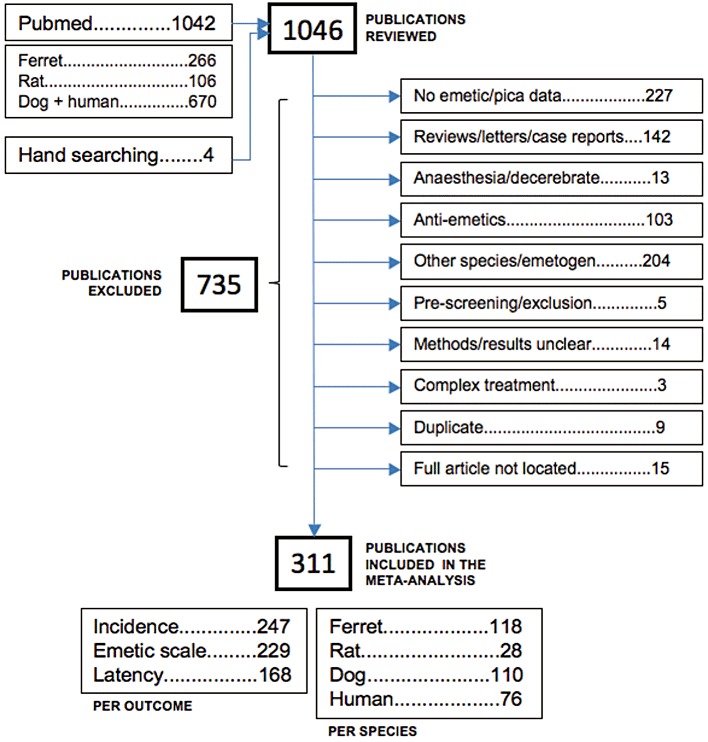
Flow chart of identified studies. Reproduced and adapted from the PRISMA statement diagram (Moher et al., 2009).
Table 2.
Included studies
| Study | Species | Study | Species | Study | Species | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human | Dog | Ferret | Rat | Human | Dog | Ferret | Rat | Human | Dog | Ferret | Rat | |||
| (Akahori et al., 1985) | ✓ | (Carpenter et al., 1986) | ✓ | (Eglen et al., 1993) | ✓ | ✓ | ||||||||
| (Alphin et al., 1986) | ✓ | (Carpenter et al., 1988) | ✓ | (Eglen et al., 1994) | ✓ | |||||||||
| (Andrews et al., 1990) | ✓ | (Castro et al., 2000) | ✓ | (Eglen et al., 1995) | ✓ | ✓ | ||||||||
| (Andrews et al., 1992) | ✓ | (Cerbo et al., 1997) | ✓ | (Endo et al., 1990) | ✓ | |||||||||
| (Andrews et al., 1993) | ✓ | (Chang et al., 1981) | ✓ | (Fernandez et al., 1992) | ✓ | |||||||||
| (Andrews et al., 2001) | ✓ | (Chey et al., 1988) | ✓ | (Fink-Jensen et al., 1992) | ✓ | |||||||||
| (Angel et al., 1993) | ✓ | (Chipkin et al., 1988) | ✓ | (Fitzpatrick et al., 1990) | ✓ | ✓ | ||||||||
| (Angrist et al., 1975) | ✓ | (Chu et al., 2010a) | ✓ | (Florczyk et al., 1982) | ✓ | |||||||||
| (Angrist et al., 1980) | ✓ | (Chu et al., 2010b) | ✓ | (Foss et al., 1993) | ✓ | |||||||||
| (Aoki et al., 2001) | ✓ | (Clark et al., 1993) | ✓ | ✓ | (Foss et al., 1998) | ✓ | ||||||||
| (Araya et al., 2001) | ✓ | (Cohen et al., 1989) | ✓ | (Foulds et al., 1998) | ✓ | |||||||||
| (Araya et al., 2003) | ✓ | (Cohen et al., 1990) | ✓ | (Freedman et al., 1987) | ✓ | |||||||||
| (Ariyoshi et al., 1992) | ✓ | (Cooper et al., 2002) | ✓ | (Fukuda et al., 1991) | ✓ | ✓ | ||||||||
| (Aung et al., 2003) | ✓ | (Corsini et al., 1976) | ✓ | (Fukui et al., 1992) | ✓ | |||||||||
| (Aung et al., 2004) | ✓ | (Costall et al., 1986) | ✓ | (Fukui et al., 1993a) | ✓ | |||||||||
| (Axelsson et al., 2004) | ✓ | (Costall et al., 1987) | ✓ | (Fukui et al., 1993b) | ✓ | |||||||||
| (Axelsson et al., 2006) | ✓ | (Costall et al., 1990a) | ✓ | (Fukui et al., 1994) | ✓ | |||||||||
| (Bailey et al., 1993) | ✓ | (Costall et al., 1990b) | ✓ | (Fukui et al., 1999) | ✓ | |||||||||
| (Barnes et al., 1988) | ✓ | (Cote et al., 2008) | ✓ | (Fukunaka et al., 1998) | ✓ | |||||||||
| (Barnes et al., 1991) | ✓ | (Cournot et al., 1987) | ✓ | (Gale et al., 2003) | ✓ | |||||||||
| (Bassi et al., 1979) | ✓ | (Cubeddu et al., 1990a) | ✓ | (Gardner et al., 1995) | ✓ | ✓ | ||||||||
| (Beck et al., 1993) | ✓ | (Cubeddu et al., 1990b) | ✓ | (Gardner et al., 1996) | ✓ | ✓ | ||||||||
| (Beck, 1992) | ✓ | (Cubeddu et al., 1994) | ✓ | (Gardocki et al., 1964) | ✓ | |||||||||
| (Bermudez et al., 1988) | ✓ | ✓ | (Dana et al., 1985) | ✓ | (Gidda et al., 1995) | ✓ | ||||||||
| (Bhandari et al., 1991) | ✓ | (Davis et al., 2009) | ✓ | (Goldenberg et al., 1976) | ✓ | ✓ | ||||||||
| (Bhargava et al., 1981) | ✓ | (Dax et al., 1979) | ✓ | (Gonsalves et al., 1996) | ✓ | |||||||||
| (Billig et al., 2001) | ✓ | (De Jonghe et al., 2008) | ✓ | (Gotteland et al., 2001) | ✓ | |||||||||
| (Blancquaert et al., 1985) | ✓ | (De Jonghe et al., 2009) | ✓ | (Groner et al., 1988) | ✓ | |||||||||
| (Blancquaert et al., 1986) | ✓ | (Decker et al., 1969) | ✓ | (Gupta et al., 1989) | ✓ | |||||||||
| (Blower, 1990) | ✓ | (Decker et al., 1994) | ✓ | (Gupta et al., 2002) | ✓ | |||||||||
| (Bromage et al., 1982) | ✓ | (Delagrange et al., 1996) | ✓ | (Gylys et al., 1979) | ✓ | |||||||||
| (Bromage et al., 1983) | ✓ | (Denaro et al., 1990) | ✓ | (Gylys et al., 1988) | ✓ | |||||||||
| (Buonamici et al., 1991) | ✓ | (Depoortere et al., 2008) | ✓ | (Haga et al., 1993) | ✓ | ✓ | ||||||||
| (Burnouf et al., 2000) | ✓ | (DiBenedetto et al., 1995) | ✓ | (Hammas et al., 1998) | ✓ | |||||||||
| (Buser et al., 1993) | ✓ | (D'Olimpio et al., 1985) | ✓ | (Hanson, 1967) | ✓ | |||||||||
| (Harada et al., 1995) | ✓ | (Klein et al., 1968) | ✓ | (McCutcheon et al., 1992) | ✓ | |||||||||
| (Harding et al., 1985) | ✓ | (Klein et al., 1970) | ✓ | (McNamara et al., 1989) | ✓ | |||||||||
| (Harding et al., 1987) | ✓ | (Knox et al., 1993) | ✓ | (Mehendale et al., 2004) | ✓ | |||||||||
| (Harrison et al., 2001) | ✓ | (Kolaric et al., 1983) | ✓ | (Mehendale et al., 2005) | ✓ | |||||||||
| (Hasegawa et al., 2002) | ✓ | (Koller et al., 1986) | ✓ | (Metz et al., 1987) | ✓ | |||||||||
| (Hatanaka et al., 1995) | ✓ | (Koller et al., 1987) | ✓ | (Miaskiewicz et al., 1989) | ✓ | |||||||||
| (Hawthorn et al., 1988) | ✓ | (Kondo et al., 1995) | ✓ | (Minami et al., 1997) | ✓ | |||||||||
| (Hawthorn et al., 1990) | ✓ | (Krenzelok et al., 1986) | ✓ | (Minami et al., 1998) | ✓ | |||||||||
| (Hayashi et al., 1993) | ✓ | (Kris et al., 1996) | ✓ | (Miner et al., 1986) | ✓ | |||||||||
| (Heaslip et al., 1995) | ✓ | (La Rossa et al., 1977) | ✓ | (Miner et al., 1987) | ✓ | |||||||||
| (Hebenstreit et al., 1989) | ✓ | (Laffan et al., 1957) | ✓ | (Minton et al., 1995) | ✓ | |||||||||
| (Higgins et al., 1989) | ✓ | (Lang et al., 1986) | ✓ | (Mitchell et al., 1976) | ✓ | |||||||||
| (Hikasa et al., 1986) | ✓ | (Lang et al., 1988) | ✓ | (Mitchell et al., 1977a) | ✓ | |||||||||
| (Hikasa et al., 1992) | ✓ | (Lang et al., 1992) | ✓ | (Mitchell et al., 1977b) | ✓ | |||||||||
| (Hollingworth et al., 2006) | ✓ | (Lao et al., 1995) | ✓ | (Money et al., 1983) | ✓ | |||||||||
| (Horn et al., 2009) | ✓ | (Lao et al., 2003) | ✓ | (Monkovic et al., 1988) | ✓ | ✓ | ||||||||
| (Hsu et al., 1986) | ✓ | (Lasheras et al., 1996) | ✓ | (Montastruc et al., 1994) | ✓ | |||||||||
| (Humphrey et al., 2006) | ✓ | (Lau et al., 2005a) | ✓ | (Monteiro et al., 2009) | ✓ | |||||||||
| (Huo et al., 1986) | ✓ | (Lau et al., 2005b) | ✓ | (Moore et al., 1994) | ✓ | |||||||||
| (Ikeda et al., 1992) | ✓ | (Lee et al., 1985) | ✓ | (Murphy et al., 1997) | ✓ | |||||||||
| (Ito et al., 1987) | ✓ | (Lefebvre et al., 1981) | ✓ | (Nagakura et al., 2007) | ✓ | |||||||||
| (Iwanaga et al., 1996) | ✓ | (Lehmann et al., 1996) | ✓ | (Nakajima et al., 1996) | ✓ | |||||||||
| (Jones, 1983) | ✓ | (Lelieveld et al., 1987) | ✓ | (Nakayama et al., 2004) | ✓ | ✓ | ||||||||
| (Jorenby et al., 1995) | ✓ | (Levine et al., 1984) | ✓ | (Nakayama et al., 2005) | ✓ | |||||||||
| (Kalfarentzos et al., 1992) | ✓ | (Liu et al., 2003) | ✓ | (Neuvonen et al., 1983) | ✓ | |||||||||
| (Kamato et al., 1991) | ✓ | ✓ | (Liu et al., 2005) | ✓ | (Niemegeers et al., 1980) | ✓ | ||||||||
| (Kamato et al., 1993) | ✓ | (Liu et al., 2008) | ✓ | (Noda et al., 2002) | ✓ | |||||||||
| (Kamerling et al., 1982) | ✓ | (Madden et al., 1999) | ✓ | (Nussey et al., 1988) | ✓ | |||||||||
| (Kan et al., 2002) | ✓ | (Malakhovskii et al., 1988) | ✓ | ✓ | (Ogilvie et al., 1989) | ✓ | ||||||||
| (Karniol et al., 1978) | ✓ | (Malik et al., 2007) | ✓ | (Ohta et al., 1992) | ✓ | |||||||||
| (Kase et al., 1997) | ✓ | (Malmlof et al., 2005) | ✓ | ✓ | (Olivares et al., 2001) | ✓ | ||||||||
| (Kayashima et al., 1978) | ✓ | (Marr et al., 1994a) | ✓ | (Osinski et al., 2003) | ✓ | |||||||||
| (Kim et al., 2005) | ✓ | (Marr et al., 1994b) | (Osinski et al., 2005) | ✓ | ||||||||||
| (King, 1988) | ✓ | (Matsui et al., 1992) | ✓ | ✓ | (Ozaki et al., 1999) | ✓ | ||||||||
| (King et al., 2005) | ✓ | (Matsushima et al., 1995) | ✓ | (Percie du Sert et al., 2009a) | ✓ | |||||||||
| (Percie du Sert et al., 2009b) | ✓ | (Seeley et al., 2000) | ✓ | (Wang et al., 2005b) | ✓ | |||||||||
| (Perry et al., 1994) | ✓ | (Self et al., 2009) | ✓ | (Warneck et al., 2008) | ✓ | |||||||||
| (Proctor et al., 1978) | ✓ | (Shapiro et al., 1988) | ✓ | (Watanabe et al., 2008a) | ✓ | |||||||||
| (Reynolds et al., 1991) | ✓ | (Sharma et al., 1997) | ✓ | (Watanabe et al., 2008b) | ✓ | |||||||||
| (Robichaud et al., 1999) | ✓ | (Shintani et al., 1982) | ✓ | (Watson et al., 1988) | ✓ | |||||||||
| (Robichaud et al., 2001) | ✓ | (Shiokawa et al., 2007) | ✓ | (Watson et al., 1995) | ✓ | ✓ | ||||||||
| (Roila et al., 1985) | ✓ | (Shiroshita et al., 1993) | ✓ | (Weaver et al., 1969) | ✓ | |||||||||
| (Rowe et al., 1979) | ✓ | (Simoneau et al., 2001) | ✓ | (Wilson et al., 2005) | ✓ | |||||||||
| (Rudd et al., 1994a) | ✓ | (Singh et al., 1997) | ✓ | (Wilson et al., 2006) | ✓ | |||||||||
| (Rudd et al., 1994b) | ✓ | (Smith et al., 1986) | ✓ | (Wilson et al., 2007) | ✓ | |||||||||
| (Rudd et al., 1996a) | ✓ | (Smith et al., 1989) | ✓ | (Wong et al., 1997) | ✓ | |||||||||
| (Rudd et al., 1996b) | ✓ | (Smith et al., 1996) | ✓ | (Wu et al., 1985) | ✓ | |||||||||
| (Rudd et al., 1996c) | ✓ | (Soderpalm et al., 2001) | ✓ | (Wynn et al., 1993) | ✓ | |||||||||
| (Rudd et al., 1997) | ✓ | (Srivastava et al., 1991) | ✓ | (Yahagi et al., 2000) | ✓ | |||||||||
| (Rudd et al., 1998) | ✓ | (Stables et al., 1987) | ✓ | (Yamakuni et al., 2000) | ✓ | |||||||||
| (Rudd et al., 2002) | ✓ | (Stuart-Harris et al., 1983) | ✓ | (Yamakuni et al., 2002) | ✓ | ✓ | ||||||||
| (Rudd et al., 2006) | ✓ | (Suminaga et al., 1993) | ✓ | (Yamakuni et al., 2006) | ✓ | |||||||||
| (Saeki et al., 2001) | ✓ | (Sun et al., 2007) | ✓ | (Yamamoto et al., 2004) | ✓ | |||||||||
| (Sagrada et al., 1991) | ✓ | (Suzuki et al., 2005) | ✓ | (Yamamoto et al., 2007) | ✓ | |||||||||
| (Saincher et al., 1997) | ✓ | (Takahashi et al., 2007) | ✓ | (Yamamura et al., 1989) | ✓ | |||||||||
| (Saito et al., 1998) | ✓ | (Tattersall et al., 1993) | ✓ | (Yamashita et al., 1997) | ✓ | ✓ | ||||||||
| (Saito et al., 1999) | ✓ | (Tattersall et al., 1994) | ✓ | (Yamashita et al., 2002) | ✓ | |||||||||
| (Saito et al., 2006) | ✓ | (Tattersall et al., 2000) | ✓ | (Yokochi et al., 1988) | ✓ | |||||||||
| (Salin-Pascual et al., 1996) | ✓ | (Thebault et al., 1981) | ✓ | (Yoshida et al., 1992a) | ✓ | ✓ | ||||||||
| (Sam et al., 2001) | ✓ | (Thompson et al., 1992) | ✓ | (Yoshida et al., 1992b) | ✓ | |||||||||
| (Sam et al., 2003) | ✓ | (Tsuchiya et al., 2002) | ✓ | (Yoshida et al., 1993) | ✓ | |||||||||
| (Sam et al., 2007) | ✓ | (Tsukamoto et al., 2007) | ✓ | (Yoshida et al., 1995) | ✓ | |||||||||
| (Schallier et al., 1985) | ✓ | (Tuor et al., 1988) | ✓ | (Yoshikawa et al., 1996) | ✓ | |||||||||
| (Scharman et al., 2000) | ✓ | (Turconi et al., 1991) | ✓ | (Yoshikawa et al., 2001a) | ✓ | ✓ | ||||||||
| (Scherkl et al., 1990) | ✓ | (Vail et al., 2007) | ✓ | (Yoshikawa et al., 2001b) | ✓ | |||||||||
| (Schofferman, 1976) | ✓ | (Villafane et al., 2007) | ✓ | (Yoshikawa et al., 2002) | ✓ | |||||||||
| (Schurig et al., 1982) | ✓ | ✓ | (Wang et al., 1952) | ✓ | (Yoshikawa et al., 2003) | ✓ | ||||||||
| (Schwartz et al., 1994) | ✓ | ✓ | (Wang et al., 1954) | ✓ | (Yu et al., 2009) | ✓ | ||||||||
| (Scott et al., 1991) | ✓ | (Wang et al., 2005a) | ✓ | |||||||||||
This table lists the studies from which data were extracted along with the species investigated. Full references are available as supplementary information Appendix S2.
The incidence of either emesis or pica was only available and extracted from 80% of the included publications, and data for the emetic scale and the latency outcomes were extracted from 74% and 54% of the publications, respectively. Data were extracted for a total of 6001 humans, 3670 dogs, 1887 ferrets and 480 rats.
Emetic liability
Three parameters were used to quantify the emetic response in dogs, ferrets and humans: incidence, intensity and latency. Data concerning the occurrence of nausea in humans were only collected as incidence, whereas the pica response in rats was exclusively reported as intensity, apart from one report of incidence for rolipram-induced pica.
Apomorphine. Apomorphine induced a response in all species. The sensitivity order for the incidence of emesis was dog > human > ferret (Table 3, Figure 2). Following s.c. and i.m. administration in humans, slightly higher doses were required for a 50% incidence of emesis compared with nausea (×1.5–2). The incidence of emesis in the ferret clearly followed a bell-shaped distribution after s.c and i.v. administration, whereas there was no indication of a bell-shaped distribution following s.c. administration in the dog. Not enough data were available in humans and for other modes of administration in the dog to determine the shape of the incidence distribution.
Table 3.
ED50 for the incidence of emesis, pica and nausea
| Ferret emesis | Dog emesis | Rat pica | Human emesis | Human nausea | |
|---|---|---|---|---|---|
| Apomorphine | |||||
| s.c. | 0.056 | 0.013 | n/a | 0.016 | 0.011 |
| i.v. | 0.620 | 0.003 | n/a | 0.070 | n/a |
| i.c.v. | n/c (<0.01) | 0.001 | n/a | n/a | n/a |
| i.m. | n/a | n/c (<0.034) | n/a | 0.029 | 0.014 |
| p.o. | n/a | 2.439 | n/a | n/a | n/a |
| CCK-8 | |||||
| i.v. | n/c (>0.05) | 0.001 | n/a | 0.0004 | n/c (<4.5×10−8) |
| Cisplatin | |||||
| i.v. | 2.447 | 0.316 | n/a | n/c (<0.8) | n/c (<1.9) |
| i.p. | 3.477 | n/a | n/a | n/a | n/a |
| i.a. | n/a | n/c (>3.5) | n/a | n/a | n/a |
| Copper sulphate | |||||
| p.o. | 2.874 | 2.598 | n/a | 0.168 | 0.143 |
| i.v. | n/a | 2.221 | n/a | n/a | n/a |
| Cyclophosphamide | |||||
| i.v. | n/c (<50.0) | 14.781 | n/a | 2.694 | n/c (<2.7) |
| i.p. | 100.000 | n/a | n/a | n/a | n/a |
| Ipecacuanha | |||||
| p.o. | 0.314 | n/c (<0.49) | n/a | 0.041 | 0.091 |
| LiCl | |||||
| i.v. | n/c (<86.0) | n/a | n/a | n/a | n/a |
| p.o. | n/a | n/a | n/a | n/c (>3.74) | n/a |
| Morphine | |||||
| s.c. | 0.014 | n/a | n/a | n/a | n/a |
| i.v. | n/c (<0.05) | 0.036 | n/a | n/c | n/c |
| i.c.v. | n/a | 0.0003 | n/a | n/a | n/a |
| i.m. | n/a | 0.506 | n/a | n/a | n/c |
| p.o. | n/a | 3.614 | n/a | n/a | n/a |
| i.t. | n/a | n/a | n/a | 0.005 | n/c |
| Nicotine | |||||
| s.c. | 3.368 | n/a | n/a | n/a | n/a |
| i.v. | n/a | n/c (<0.081) | n/a | n/a | n/a |
| i.m. | n/a | 3.972 | n/a | n/a | n/a |
| p.o. | n/a | n/c (>13.4) | n/a | 0.126 | n/c (>0.046) |
| t.d. | n/a | 2.235 | n/a | 1.169 | 0.0385 |
| inhalation | n/a | n/a | n/a | n/c (>0.034) | n/c (>0.034) |
| Rolipram | |||||
| i.v. | n/a | 0.189 | n/a | n/a | n/a |
| p.o. | 0.842 | 0.062 | 0.479 | n/a | 0.030 |
| s.c. | n/c | n/a | n/a | n/a | n/a |
| i.c.v. | n/c | n/a | n/a | n/a | n/a |
ED50 (dose at which 50% of the individuals developed emesis, pica or nausea) are expressed in mg·kg−1, expect for i.c.v. administration which is expressed in mg. ED50 were extrapolated following a sigmoidal curve fit (variable slope, normalized); values in parentheses are given as indications, when the data were too limited to fit a curve. n/c: not computed (the data available were not sufficient to enable ED50 calculation). n/a: not available (there were no data for that species at that mode of administration).
Figure 2.
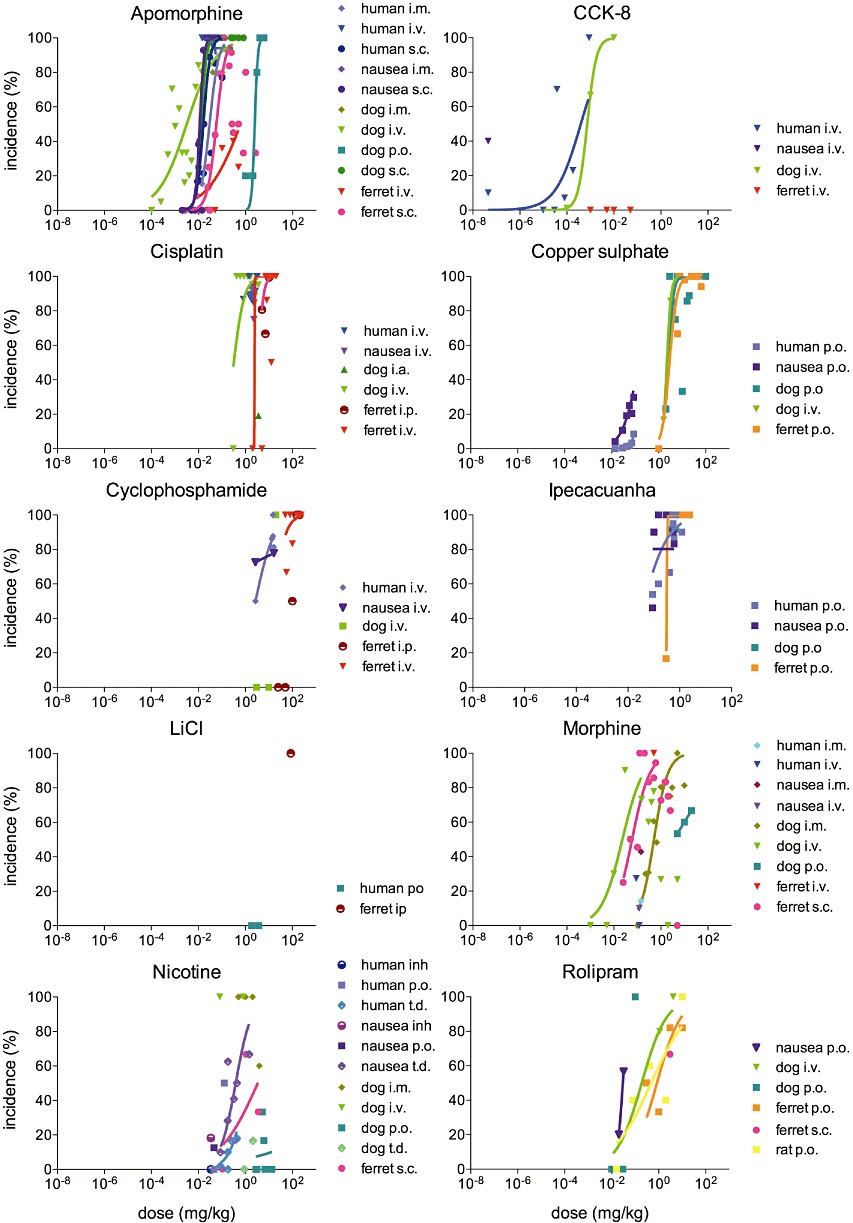
Incidence of emesis, pica and nausea for each drug in human, dog, ferret and rat. Incidence (percentage of individuals developing an emetic response) at each dose is plotted as weighted mean. The data were fitted using a sigmoidal curve (variable slope, normalized). Only the ascending part of the curve was considered for drugs following an inverted U distribution (apomorphine s.c. in the ferret, morphine s.c. in the ferret and morphine i.v. in the dog; inflexion points were determined with a second order polynomial curve; not shown). Note that for clarity, only peripheral modes of administration are shown on the graphs.
Regarding the intensity of the response, different modes of administration were used across species, and very limited data were found for humans and rats (one data point each, Figure 3), rendering direct comparison difficult. However, the relative intensity of human emesis following i.m. administration was consistent with i.m. administration in the dog. Irrespective of the mode of administration, higher intensities at lower doses were observed in dogs, compared with ferrets. Following s.c. administration, there was also evidence of a bell-shaped distribution in these two species, which peaked at 0.2 mg·kg−1. The relative intensity of pica was 0.5 units, which was comparable with the intensity observed in dogs and humans at doses two orders of magnitude lower.
Figure 3.
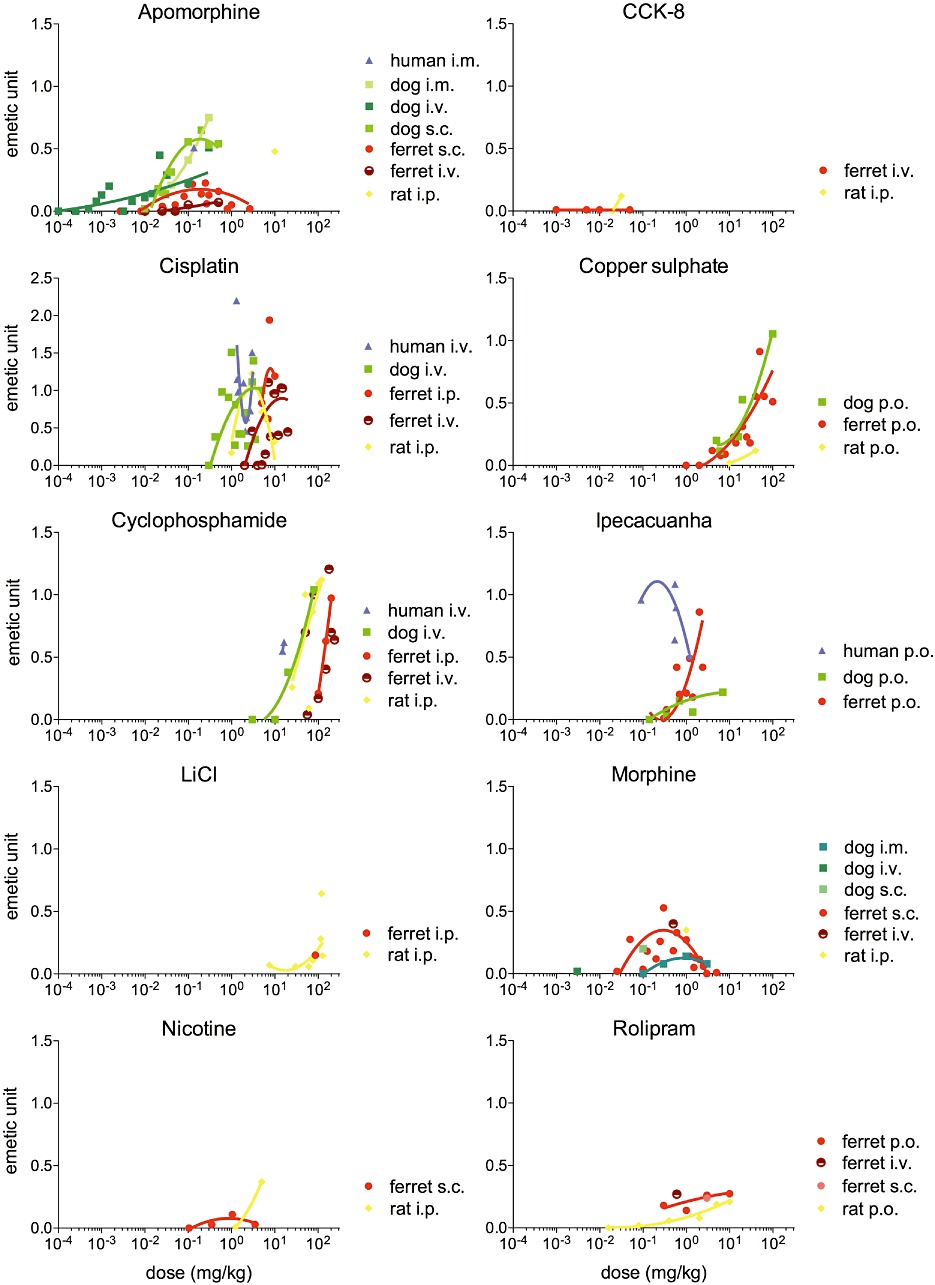
Emetic scale for each drug in human, dog, ferret and rat. The emetic scale at each dose is plotted as weighted mean. For each species, an emetic unit is defined as the maximal emetic response to cisplatin. The data were fitted using a second-order polynomial curve.
In all three emetic species, the latency to the onset of emesis was less than 30 min (see Figure 4). It was clearly dose-dependent following oral (p.o.) and s.c. administration in dogs, decreasing as the dose increased. Following i.v. administration in dogs and ferrets, the latency increased with the dose, and no clear relationship was observed following s.c. administration in ferrets. A bell-shaped distribution was observed following s.c. administration in humans, but this result should be taken with caution since it is based on limited data. Following i.c.v. administration, the latency of apomorphine-induced emesis in the dog was clearly dose-related with 1 µg inducing emesis in 6 min compared with less than 2 min for a dose of 20 µg. A shorter onset was observed in the ferret with 10 µg inducing emesis within 20 s.
Figure 4.
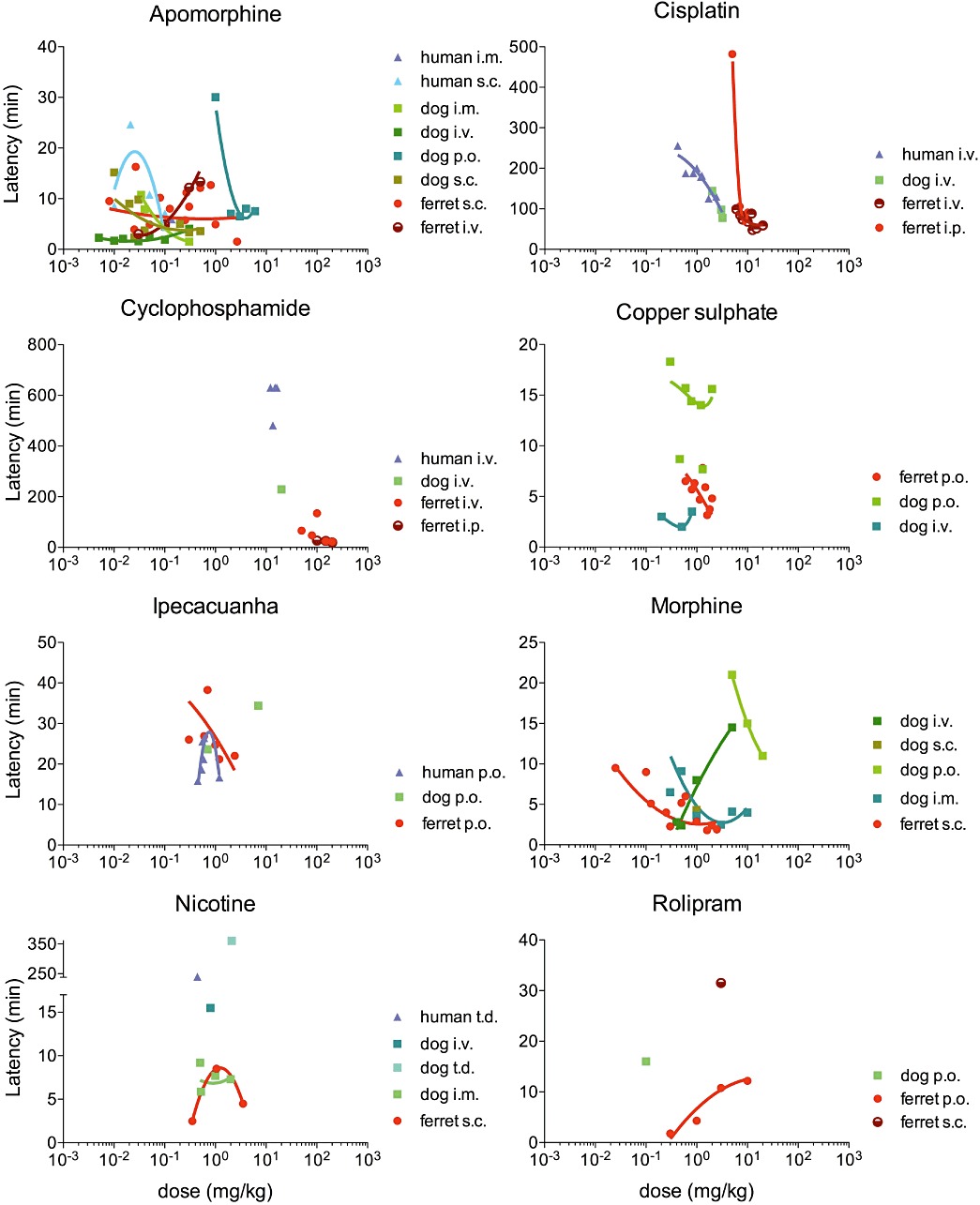
Latency to the onset of the emetic response for each drug, in human, dog and ferret. The latency at each dose is plotted as weighted mean. The data were fitted using a second-order polynomial curve.
CCK-8. When administered i.v., CCK-8 was found to induce emesis in dogs and humans with an incidence sensitivity order human > dog (Table 3, Figure 2). CCK-8 did not induce emesis in the ferret at any of the doses reported (0.001–0.05 mg·kg−1 i.v.). However, it did induce low intensity pica (0.1 units) in the rat following the i.p. administration of 0.03 mg·kg−1 (Figure 3). No latency data could be found in any of the species for CCK-8.
Cisplatin. ED50 value for the incidence of cisplatin-induced emesis was eight times lower in the dog compared with the ferret when administered i.v., and 11 times lower when administered i.p. ED50 values could not be calculated for the incidence of emesis and nausea in humans, as all doses tested in humans induced nausea and emesis in over 75% of the patients. The doses inducing nausea and emesis in over 75% of the human population were however lower than the ferret ED50 (Table 3, Figure 2).
The intensity of the response to cisplatin i.v. followed a similar distribution in dogs and ferrets, characterized by a dose-dependent increase and a plateau phase. In the ferret, however, the curve was shifted to the right by one order of magnitude. No data were found on i.v. administration in the rat, but following i.p. administration, the response followed a bell-shaped distribution culminating at 3 mg·kg−1, which was the dose at which maximal intensity was observed in the dog. No clear dose–response relationship was observed following i.v. administration in humans (Figure 3).
The latency of the response decreased as the dose increased in all species. However, a step-change was observed between 5 mg·kg−1 i.p and higher doses in the ferret, whereas the latency reduction appeared continuous following i.v. administration in the three species (Figure 4).
Copper sulphate. When administered p.o., copper sulphate induced emesis in dogs and ferrets, and nausea and emesis in humans with a sensitivity: human > > dog > ferret. The ED50 for dogs and ferrets were in the same order of magnitude, while a dose over 15 times lower was required to induce emesis in 50% of humans. The ED50 values were similar for the incidence of emesis and nausea in humans (Table 3, Figure 2).
No intensity data were available for copper sulphate in humans, and the response was dose-dependent in the three remaining species. Similar intensity at similar doses was observed in dogs and ferrets, whereas the rat responded with lower intensity. At 40 mg·kg−1 (the maximal dose used in rats), the intensity was 0.1 units in rats and over 0.5 units in ferrets and dogs (Figure 3).
The latency to the onset of emesis was less than 20 min in dogs and ferrets; it was dose-dependent in both species following p.o. administration, but shorter latencies were observed in the ferret (∼5 min) compared with the dog (∼15 min). In the dog, i.v. dosing resulted in a much lower latency than p.o. with the emetic response developing in under 4 min (Figure 4). No human data were available regarding the latency of the response to copper sulphate.
Cyclophosphamide. Cyclophosphamide induced a response in all species. When administered i.v., the ED50 in dogs was five times higher than the ED50 for emesis in humans. In ferrets, the ED50 for i.v. administration could not be determined as the lowest incidence reported in ferrets was 66%. The i.p. ED50 in ferrets was six times higher than the dog's i.v. ED50 (Table 3, Figure 2).
Cyclophosphamide displayed a sharp dose-dependent increase in intensity in all four species. The intensity of the response was similar following i.v. administration in dogs and i.p. administration in rats. Only two data points were found for i.v. administration in humans, but these values show that a similar intensity in humans was reached at approximately half the dose required in dogs and rats. It was not possible to fit a curve to the ferret i.v. data, but the intensity curve for i.p. administration in this species was shifted to the right compared with rat and dogs, and a similar intensity compared with humans was observed at doses two orders of magnitude higher (Figure 3).
The latency of emesis was between 8 and 10 h in humans, 4 h in dogs and between 20 min and 2 h in ferrets. The doses used were however different between species as doses between 12 and 16 mg·kg−1 i.v. were reported in humans, and 20 mg·kg−1 i.v and 50–100 mg·kg−1 in dogs and ferrets, respectively (Figure 4).
LiCl. The emetic incidence of LiCl was only investigated p.o. in humans and i.v. in ferrets; not enough data were available to examine interspecies sensitivity (Table 3, Figure 2).
Regarding the intensity of the response, only data on i.p. administration were found in rats and ferrets, with only one data point for ferrets. The intensity of the response was dose-dependent in rats and reached an intensity of 0.2 units at 100 mg·kg−1; in the ferret, the intensity was comparable with that observed in the rat (Figure 3). No intensity data were found for dogs and humans, and no latency data could be found in any of the four species.
Ipecacuanha. Ipecacuanha p.o. induced emesis in all three emetic species. The ED50 for dogs could not be extrapolated as all doses tested induced emesis in over 90% of the animals. The human ED50 was eight times lower than ferrets, indicating a greater sensitivity to ipecacuanha. The ED50 for the incidence of human nausea was two times higher than human emesis (Table 3, Figure 2).
Reports of the intensity of the response to ipecacuanha were only found in humans, dogs and ferrets. In humans, maximal intensity was greater than 1 emetic unit and the distribution followed a bell-shaped curve culminating at 0.2 mg·kg−1. A very different response was observed in the dog in which emetic intensity followed a shallow dose-dependent relationship. A maximal intensity of 0.25 units was reached after the dose was increased by two orders of magnitude to 7 mg·kg−1. In the ferret, a more rapid dose-response relationship was evident, with a maximal intensity of 0.8 emetic units observed at 2 mg·kg−1, as the dose increased over one order of magnitude (Figure 3).
The latency of ipecacuanha-induced emesis ranged between 15 and 40 min in all three species. It followed a negative dose-dependent relationship in the ferret, decreasing as the dose increased. The distribution appeared bell-shaped in humans, but this should be treated with caution as activated charcoal was reported to be given with the higher dose – which might have affected the latency – whereas the use of charcoal was not reported for any of the lower doses or with any of the other species. Data in the dog were too limited to examine the relationship between latency and dose (Figure 4).
Morphine. Seven different modes of administration [s.c., i.v., i.m., i.p., p.o., i.c.v., intrathecal (i.t.)] of morphine were used across the three species. Regarding the incidence of the response, morphine i.v. induced emesis across all three species and nausea in humans, but the ED50 could only be calculated in dogs (Table 3); thus, no interspecies sensitivity comparisons could be drawn. Following s.c. administration in ferrets, i.m and i.c.v administration in dogs, and i.t. administration in humans, the emetic incidence followed a bell-shaped distribution (Figure 2).
No data for the intensity of the response to morphine were available for humans, and only one data point was available for rats. The intensity of the response followed a bell-shaped distribution following i.m. administration in the dog and s.c. administration in ferret. A maximal intensity of 0.4 emetic units was observed at 0.3 mg·kg−1 s.c. in ferrets, and the response reached 0.15 emetic units at 1 mg·kg−1 i.m. in dogs. The intensity of the response following 1 mg·kg−1 i.p. in the rat was comparable with s.c. administration in the ferret (Figure 3).
No human data were available regarding the latency of the emetic response to the systemic administration of morphine; the latency was dose-dependent in dogs and ferrets, and emesis occurred within 20 min regardless of the mode of administration in both species. I.m. administration in the dog and s.c. administration in the ferret were comparable, and the latency decreased as the dose increased; a minimum latency of 2 min was reached in both species for doses between 1 and 3 mg·kg−1. The latency also decreased as the dose increased following p.o. administration in the dog, but higher doses were required compared with other modes of administration. Following i.v. dosing in the dog, the response was clearly dose-dependent, but the latency increased with the dose (Figure 4). Only limited data were available regarding the latency following central administration. The latency of emesis in the dog following morphine i.c.v. slowly increased with the dose; the response developing within 1.5 min with a dose of 0.25 µg, and increasing to 3.25 min as the dose increased to 25 µg.
Nicotine. Seven different modes of administration [s.c., i.v., i.m., i.p., p.o., transdermal (t.d.) and inhalation] of nicotine were used across species, preventing direct interspecies comparisons. When administered t.d., the ED50 for nicotine in dogs was twice the ED50 in humans. The ED50 for s.c. administration in the ferret was comparable with the ED50 for i.m and t.d. administration in the dog. The ED50 for the incidence of emesis was four times higher than for nausea in humans (Table 3, Figure 2).
The intensity of the response was only reported for rats and ferrets; no human or dog data were found. Administration of nicotine s.c. induced a very low intensity emetic response in ferrets, which culminated at 0.1 units for doses up to 3.5 mg·kg−1. In rats, following i.p. administration, the intensity of the response increased with the dose and reached 0.4 units at 5 mg·kg−1 (Figure 3).
Limited data were available regarding the latency of nicotine-induced emesis in all three species, and no clear dose-response relationship could be identified (Figure 4). Following i.v., i.m and s.c. administration in dogs and ferrets, emesis developed within 15 min, whereas much longer latencies were observed following t.d. administration in humans (4 h) and dogs (6 h).
Rolipram. Based on ED50 values, the species sensitivity for the incidence of the response to rolipram p.o. was: human nausea > dog emesis > rat pica > ferret emesis (Table 3, Figure 2). Emesis was not documented in any of the human studies and incidence in humans could not be compared with animal species.
Following oral administration in rats, the intensity of the response gradually increased over four orders of magnitude to reach 0.21 units at 10 mg·kg−1. The emetic response induced in the ferret was also dose-dependent. Similarly to the rat, an intensity of 0.27 units was reached at the highest dose tested (10 mg·kg−1). Only one data point was collected for i.v and s.c. administration in ferrets, preventing examination of the distribution (Figure 3).
No latency data were available in humans. In the ferret, the latency followed a positive dose-dependent relationship in the ferret, increasing as the dose increased following p.o. administration. The minimum latency observed was 2 min at 0.3 mg·kg−1 p.o., and it took 12 min for the emetic response to develop at 10 mg·kg−1 p.o. The latency following a 3 mg·kg−1 dose of rolipram was longer in the ferret following s.c. administration (32 min) than following p.o. dosing (11 min). Only one data point was available for the dog, in which a latency of 16 min was observed at 0.1 mg·kg−1 p.o. (Figure 4).
Discussion
Despite some limitations, the conclusions that can be drawn from the present study identify a more elaborate way of approaching emetic liability studies and inform the design of experiments for emetic research. This will prevent the replication of experiments in several species to no avail and will overall reduce the number of animals used. Additionally, a systematic approach identified novel, interesting findings (e.g. the latency to the onset of rolipram follows a positive dose-dependent relationship).
Early detection of emetic liability
Are the three laboratory species interchangeable to detect emetic liability? The broad answer is yes. When tested, emetic liability was consistently detected across species. Quantitative data for human nausea or emesis were collected for all of the 10 compounds investigated. All compounds were reported to induce some degree of emesis in humans with the exception of LiCl. Only one study was found to report quantitative data on LiCl in humans; LiCl did not induce emesis in this study, and nausea was observed but not quantified (Karniol et al., 1978). Several studies have however reported that nausea and vomiting are commonly observed side effects of lithium therapy (Yung, 1984; Masi et al., 2009), suggesting that the emetic and pica responses observed in the ferret and the rat, respectively, are predictive of the emetic liability in humans. All compounds were also reported to induce emesis in the dog, once again, with the exception of LiCl for which no emetic data could be found. Only one of the 10 compounds investigated, CCK-8, did not induce emesis in the ferret. Regarding the apparent discrepancy between the effects of CCK-8 in the ferret and other species, it is noteworthy that CCK-8 was associated with an increase in plasma vasopressin in the ferret (Billig et al., 2001), a physiological marker, which correlates with the occurrence of nausea in humans (Rowe et al., 1979; Koch et al., 1989) and is observed following emetogenic challenges in ferrets (Hawthorn et al., 1988; Wilkens and Yates, 2005). This suggests that rather than being completely resistant to CCK-8-induced emesis, the ferret is less sensitive than dogs and humans but would probably develop emesis following the administration of a higher dose. Data from the rat were scarce compared with the other three species and could only be extracted from less than 10% of the 311 included publications. Pica data were however collected for all but one (ipecacuanha) of the 10 compounds investigated.
Based on these data, since all three species can detect emetic liability in humans, the use of higher-order species capable of an emetic response such as ferrets or dogs is not necessary to make an initial broad assessment of whether an NCE has the potential for emetic liability or not. Using the rat will enable the detection of such adverse effects at an earlier stage of the drug development process, as an assessment of pica may be combined with other studies already being carried out as part of drug development (e.g. pharmacokinetics). This will reduce animal use and resources required. This also presents advantages in terms of the amount of background data available in rats and the availability of in-bred strains to reduce variability (Davis et al., 2009).
However, these findings should be considered carefully as only 10 compounds were examined across the four species. These drugs represent a wide diversity of emetic agents, inducing emesis via different mechanisms, from activation of dopamine or opioid receptors in the brainstem to activation of gastrointestinal vagal afferents as shown in Figure 5. However, the emetic or pica response to these specific compounds might not be representative of other compounds in the same pharmacological class. Additionally, the data collection was limited to publicly available data and restricted to one database: PubMed. Ideally, for a systematic review, more than one database should be searched, to ensure limited bias. Furthermore, the 10 drugs investigated were originally chosen for their potential to induce emesis, enabling an assessment of predictivity but not specificity. Negative control data, i.e. from non-emetic compounds, are needed to investigate the occurrence of false positives, i.e. drugs that would not induce nausea or emesis in humans but would induce an emetic response in dogs or ferrets, or a pica response in rats. The specificity of each model species needs to be determined to avoid discarding potentially valuable compounds (Pugsley et al., 2008). It is also noteworthy that whereas all three species predicted emetic liability, these results do not reflect the effect of anti-emetic drugs, which might reveal further interspecies differences.
Figure 5.
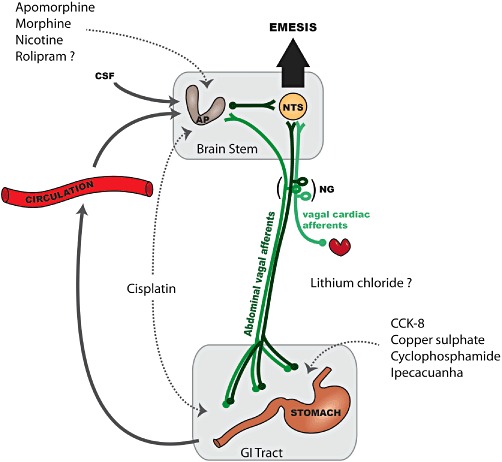
Site(s) of action implicated in the emetic or pica response induced by the ten compounds reviewed. Systemic or i.c.v. apomorphine, morphine and nicotine activate dopamine D2 and D3, µ (possibly µ1) opioid and nicotinic receptors, respectively, in the area postrema (AP) (Laffan and Borison, 1957; Osinski et al., 2005; Rudd and Naylor, 1995; Wang and Borison, 1952), although the possibility of an action on the subjacent nucleus tractus solitarius (NTS) cannot be excluded by such studies. The cytotoxic drugs cisplatin and cyclophosphamide induce the local release of 5-HT from enteroendocrine cells, which activate 5-HT3 receptors on the peripheral terminals of abdominal vagal afferents (Rudd and Andrews, 2005). Additionally, cisplatin-induced emesis (delayed phase) can be mediated by the area postrema, but the molecular mechanism is not known (Percie du Sert et al., 2009). Abdominal vagal afferents have been implicated in the mechanism by which intragastric ipecacuanha (Andrews and Davis, 1995), copper sulphate (Wang and Borison, 1951) and CCK-8 induce emesis (Lang et al., 1988), although in the case of the latter, a direct action on receptors located on the nodose ganglion (NG) or in the brain stem cannot be excluded. The site of emetic action of lithium chloride has not been investigated but the AP is required for it to induce conditioned taste aversion in the rat (Borison, 1989). A central site of action for the PDE4 inhibitor rolipram is supported by electrophysiological (Carpenter et al., 1988) and isoform distribution studies (Mori et al., 2010), but in contrast to other compounds, the effect of area postrema ablation and vagotomy have not been investigated.
Which species best characterize emetic liability in humans?
In terms of sensitivity to various stimuli, the ranking of compounds by intensity revealed common features between species. For example, cytotoxic drugs induced the most intense response in all four species (see Table 4). The ranking also revealed interspecies differences, such as the response to ipecacuanha, which was very intense in humans but only weak in dogs. It is worth noting that compounds were ranked by intensity rather than incidence in order to include the rat data. However, such a ranking using maximal incidence, regardless of the dose, would not be feasible as most compounds induced an emetic response in all individuals at a high enough dose. An analysis based on intensity thus revealed interspecies differences that would have been missed if only incidence data had been collected.
Table 4.
Emetic compounds ranked by the intensity of the response in humans, dogs, ferrets and rats
| Human | Dog | Ferret | Rat |
|---|---|---|---|
| Cisplatin | Cisplatin | Cisplatin | Cisplatin |
| Ipecacuanha | Copper sulphate | Cyclophosphamide | Cyclophosphamide |
| Cyclophosphamide | Cyclophosphamide | Copper sulphate | Lithium chloride |
| Apomorphine | Apomorphine | Ipecacuanha | Apomorphine |
| Morphine | Morphine | Nicotine | |
| Ipecacuanha | Rolipram | Morphine | |
| Apomorphine | Rolipram | ||
| Lithium Chloride | Copper sulphate | ||
| Nicotine | CCK-8 | ||
| CCK-8 |
Note that maximal intensity was considered, regardless of the dose or mode of administration. Compounds are listed with those inducing the most intense emetic or pica response at the top and the least intense at the bottom.
As regards the latency to the onset of emesis, the data were remarkably consistent for the same compound across humans, dogs and ferrets. There is no equivalent parameter for comparison in the rat data, although future studies using automated measurement of kaolin may eventually enable such comparisons (Yamamoto et al., 2011). The latency arguably reflects comparability between species in the emetic mechanism activated; it is also an indication of the rate and extent of absorption from the various routes of administration to achieve a given plasma exposure and, in the case of cyclophosphamide, hepatic metabolism (Seymour, 1993).
In terms of dose sensitivity, the dog was very predictive of humans for most of the compounds, and very similar ED50 values were observed in the two species. For gastric mucosal irritants, however, such as copper sulphate and ipecacuanha, the dog was much less sensitive than humans. For ipecacuanha, limited data were available regarding the incidence, precluding any solid conclusion for this parameter. Only a low intensity emetic response was observed at the highest dose tested in dogs. In contrast, humans displayed a response as intense as cisplatin-induced emesis following a dose two orders of magnitude lower than the highest dose used in the dog. In the case of copper sulphate, the emetic response was remarkably similar to the response observed in ferret in terms of incidence and severity, but the onset of emesis was consistently quicker in the ferret. Both species however required doses 20 times higher than humans for a comparable incidence of emesis. These findings contrast with the view that vomiting in dog studies is not relevant because ‘dogs vomit to anything’, a widespread dogma in the toxicology field. Collecting emetic data during existing toxicology studies using dogs would therefore represent a considerable advantage as the results of the present study indicate that emesis in the dog is predictive of human emesis. Humans actually displayed a higher emetic sensitivity than dogs for most of the compounds in this limited study. A careful account of the emetic response (incidence, latency and intensity) recorded at this stage would save resources and animals, possibly preventing the need for further emetic liability studies.
Despite accurately predicting emetic liability, the ferret displayed a lower dose sensitivity than humans. Typically higher doses (∼1 to 2 orders of magnitude higher) were required to induce emesis with a comparable relative severity.
Regarding pica data in the rat, for cytotoxic drugs (cisplatin and cyclophosphamide), the intensity of pica was comparable with the intensity of emesis in the dog, which suggests that pica in rats may be a good predictor of human emesis for this class of drug. Our findings would therefore indicate that it may not be necessary to conduct dog or ferret emesis studies for this class of compound, and human emesis can be characterized based on pica studies in rats. Note the inverted U shape of the response to cisplatin, which could be explained by deterioration of the animal's health as the dose of cisplatin increases. Certainly, high dose cisplatin is associated with a significant level of toxicity in the rat (Rudd et al., 2002), the LD50 of cisplatin being 7.4 mg·kg−1 (Wondergem et al., 1993). In rats, it is conceivable that an active behaviour such as pica would be reduced as the toxicity of the dose increases and the animal becomes too ill to eat kaolin (Andrews and Horn, 2006). For the remaining compounds, when data were available across several doses, the intensity of pica appeared to be dose-related, but relatively low levels were reached at the higher doses.
Therefore, in order to identify how severe the emetic response is expected to be, rather than just identifying it as a potential adverse effect, the question of species choice is more complicated. Further work would be useful to determine which species is most relevant for each class of drug to increase confidence in nonclinical data, enable the use of rats where possible and reduce overall animal use. Our data show that for many compounds – excluding gastric irritants – the dog and human emetic sensitivity are comparable; if further data confirm this result, then it will be important to consider how studies on emetic liability can be combined with pharmacology and toxicology studies to minimize animal use.
Problems encountered while attempting to assess the translational value of the preclinical data
Lack of data/outcomes measured in human studies. A surprising finding of the present study was the scarcity of emetic data publicly available for humans. We identified two reasons: firstly, although the number of reports identified for humans was comparable with those identified in other species such as dogs and ferrets, many did not investigate emesis as their primary purpose. Consequently, emesis was not quantified but merely mentioned as a side effect, which precluded the inclusion of these reports in the present study. Secondly, human studies were not as comprehensive as animal studies. Even though data were collected from over 6000 individuals, a number comparable with the cumulative number of animals in the three other species, the overall benefit of human studies was limited due to poor data collection. This appears to represent a wasted opportunity as few human data were available for comparison to animal species, especially regarding either the intensity or the timing of the emetic response. For example, whereas 48% of the studies from which ferret data were extracted reported the incidence of emesis, the latency and a measure of intensity, and nearly 80% reported at least two of these outcomes, only 8% of human studies reported three outcomes, and the majority (58%) only reported one. Altogether, the reporting of preclinical studies was nonetheless poor, with only one-third of the studies from which data were extracted reporting all three outcomes.
Human and animal studies measuring different outcomes. An issue deriving from the incomplete reporting of emetic data was the lack of common ground to draw interspecies comparisons, and while the incidence of emesis was the preferred outcome in human studies (reported in 86% of the publications), the equivalent outcome was only reported in 4% of the publications with rat data, the most reported outcome being the intensity (100% of the rat publication but only 23% of the human publications). The lack of common outcomes for many of the drugs therefore precluded direct comparison across species.
One can only speculate on the reason why human emetic data are not reported as consistently as in dogs and ferrets. Is it considered an additional burden to patients (e.g. cytotoxic chemotherapy)? Is the clinical research team too busy to monitor patient well-being and take detailed measurements of the emetic response? Is it assumed that one endpoint is sufficient? Are these data appropriately collected but not reported? The European Medicines Agency (EMEA) recommends a measure of intensity (number of episodes, vomits and/or retches) and a measure of incidence (percentage of patients with complete control) as primary endpoints to assess the efficacy of anti-emetic therapy, while measuring the latency to the onset of the emetic response (time to treatment failure) as a secondary efficacy parameter (European Medicines Agency, 2006). Additionally, the latest American Society for Clinical Oncology (ASCO) guidelines for anti-emetic drugs clearly state that ‘emesis, measured by counting the number of vomiting episodes after treatment, is the most important clinical trial end point for studies of antiemetic drugs’ (Kris et al., 2006), highlighting the importance of including a measure of intensity. Although these guidelines refer to the evaluation of anti-emetic efficacy, there is no reason to assess emetic liability differently. Even though a measure of incidence generally seems to be considered adequate to detect an adverse affect, the present study demonstrates that all three outcomes should be reported to maximize the use of clinical data and assess the translational value of preclinical data.
As regards the reporting of pica data in rats, kaolin intake is usually measured at the end of the 24 h period following the emetic challenge, and an average measurement for the group is reported. Such a design only allows the intensity of the response to be quantified, leaving out indications about the temporal profile (e.g. latency to the onset) or the proportion of individuals affected (i.e. incidence), which are the two outcomes most likely to be reported in human studies. It has been shown recently that precise time profiling of pica behaviour in individual animals is possible using kaolin containers equipped with weight sensors (Yamamoto et al., 2011); this technique would thus enable measurements of the incidence and latency of the pica response.
In preclinical studies, the intensity was the most commonly used outcome, reported in 80% of the studies; however, the incidence – being the outcome most likely to be reported in human studies (see above) – should be reported without exception. The latency provides an indication of the mechanisms underlying the emetic response (or pica response); this measurement is crucial for interspecies comparisons and provides an assessment of the translational value of animal models.
Discrepancy between measures of intensity. The intensity of the emetic response can be measured in several different ways; the most precise being the individual number of retches and vomits (Stables et al., 1987). Other intensity measures include the number of vomiting or emetic episodes and the number of bouts of emesis. In the present study, episodes were defined as series of retches and/or vomits separated by 5 s, which was the definition most commonly encountered (e.g. Sam et al., 2001). However, definitions varied between studies and were often left to the interpretation of the reader. The vast majority of the human intensity data were extracted as undefined bouts of emesis, which undermines its quality and greatly reduces the precision of the present meta-analysis, as potentially heterogeneous data were combined in the measure of emetic intensity. Non-standardized measures of intensity may be sufficient to investigate the efficacy of an anti-emetic drug (assuming internal consistency), but it does not enable adequate interspecies comparisons and hinders the use of meta-analyses. Experimental outcomes of in vivo experiments should be defined clearly, as recommended in the ARRIVE guidelines (Kilkenny et al., 2010), recently endorsed by many high quality journals including the British Journal of Pharmacology (McGrath et al., 2010).
In an attempt to combine the different measures of intensity, especially between emetic (retches and vomits, episodes, etc.) and non-emetic species (kaolin intake), we devised an ‘emetic unit’, based on the response to cisplatin, and standardized the intensity data against that unit. A drawback of this method is that it precludes inter-species comparisons regarding the intensity of the response to cisplatin itself; it does however enable valid conclusions to be drawn regarding the shape of the distribution. Another limitation is that this method is based on the assumption that the response to cisplatin is similar across species, which is questionable. For example, the intensity of the response to cytotoxic drugs in rats was much higher than the response to other drugs (Figure 3). Consequently, expressing the response in cisplatin units blunted the intensity of other drugs in comparison with other species. Whether the intensity of the response to non-cytotoxic drugs is genuinely lower in the rat, or the response intensity to cytotoxic drugs is higher in the rat compared with other species is unknown.
To reduce the number of variables, the analysis was carried out under the assumption that the entire response had been quantified. The duration of the observation period was limited to 24 h, which would include the acute but not the delayed emetic response to cytotoxic drugs (e.g. cisplatin, Percie du Sert et al., 2011). Depending on the drugs and the studies, the observation period varied from 30 min to 24 h, and some reports included in the present study may have used an observation period shorter than the entire duration of the response.
Furthermore, reports involving human patients (e.g. cisplatin, cyclophosphamide) systematically included anti-emetic medication. Groups of patients receiving prophylactic anti-emetics were not included in this study, but patients receiving rescue anti-emetic treatments were. While this should not have any influence on the incidence of emesis or nausea, and the latency to the emetic response, we should expect the intensity of emesis to be reduced. As cisplatin was used to standardize the response induced by other drugs, this may have created a bias in the quantification of the intensity in humans compared with other species, and the intensity of drugs other than cisplatin might be artificially higher in humans.
Mode of administration. Difficulties forming conclusions in the present study were also encountered due to the lack of consistency in the experimental methods used in the reviewed publications and due to the way they were reported. Particularly, the mode of administration varied greatly between drugs and was not consistent between species. Ten different modes of administration were found across the four species preventing direct comparison. For example, i.p. is commonly used as a mode of administration in small species such as rats and ferrets, but none of the studies included in the present review reported the use of this mode of administration in humans. Additionally, the range of doses used in clinical and preclinical studies was inherently different, which would be expected. The majority of human studies use low doses to avoid emesis, whereas the majority of animal studies used doses close to the ED100 to ensure that the animals developed emesis or pica, as the intent is to use the models to study anti-emetics.
Certainly, plasma drug levels would be useful to standardize doses and modes of administration and to compare the emetic responses between species based on plasma exposure rather than dose. Unfortunately, plasma exposure levels were hardly ever reported in the studies we reviewed, and owing to the time and resource constraint of the present study, we did not specifically search for plasma exposure data but only collected it when available in the emetic studies identified. This was not sufficient as less than 4% of the 311 included publications were found to report plasma concentration following drug administration. Undoubtedly, a broader and more targeted search would identify more plasma exposure data, but whether the data available in the public domain would include the variety of mode of administration used in each of the species remains unclear.
Conclusion
These data provide us with a basis to make preliminary recommendations on the use of non-clinical models to detect emetic liability of NCEs. All three animal species predicted emetic liability accurately, but interspecies differences emerged in terms of dose sensitivity. Therefore, we suggest that the rat may be used for detecting whether emetic liability exists. Rats are frequently the species of choice in early drug development, and therefore, this approach enables data on emetic liability to inform initial decision processes while reducing animal use, particularly avoiding the use of non-rodents.
For a more detailed evaluation of the emetic potential, studies should be tailored to the purpose of the experiment and the type of drug investigated. Species selection for such studies is more complicated, and a framework may be proposed where rats are the species of choice for some compounds, and dogs for others depending on the pharmacology of the compound or the type of emetic mechanism activated. For all species, emetic response evaluation should be combined with other studies already ongoing as part of drug development where possible.
This study identified several limitations in translating non-clinical information to humans, the main ones being the lack of comparable measurements between human and animal studies, the lack of plasma exposure data and the insufficiency of rat and human data in the public domain to conduct a quantitative analysis. Better reporting of human and animal data is needed to enable robust analysis of preclinical data and to improve the efficiency of drug development. The ultimate goal would be to use these data to select compounds with reduced emetic liability leading to improved patient compliance and reduced attrition.
Our findings indicate that an in silico method to detect emetic liability must be based on more data than is available in the public domain. However, it is essential to improve the reporting and increase the availability of data to enable interspecies sensitivity differences to be characterized on a broader selection of compounds. This will be the essential next step in building a reliable in silico model that accurately predicts emetic liability.
Acknowledgments
This project was funded by Pfizer Inc. and the UK National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs). We would also like to thank Dr Kathryn Chapman (NC3Rs) for helpful comments on the manuscript.
Conflict of interest
There is no conflict of interest for any of the authors.
Supporting information
Appendix S1 Pubmed search terms.
Appendix S2 List of included studies.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PLR, Davis CJ. Physiology of emesis induced by anti-cancer therapy. In: Reynolds J, Andrews PL, Davis CJ, editors. Serotonin and the Scientific Basis of Anti-Emetic Therapy. Oxford: Oxford Clinical Communications; 1995. pp. 25–49. [Google Scholar]
- Andrews PLR, Horn CC. Signals for nausea and emesis: implications for models of upper gastrointestinal diseases. Auton Neurosci. 2006;125:100–115. doi: 10.1016/j.autneu.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billig I, Yates BJ, Rinaman L. Plasma hormone levels and central c-Fos expression in ferrets after systemic administration of cholecystokinin. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1243–R1255. doi: 10.1152/ajpregu.2001.281.4.R1243. [DOI] [PubMed] [Google Scholar]
- Borison HL. Area postrema: chemoreceptor circumventricular organ of the medulla oblongata. Prog Neurobiol. 1989;32:351–390. doi: 10.1016/0301-0082(89)90028-2. [DOI] [PubMed] [Google Scholar]
- Brioni JD, Decker MW, Sullivan JP, Arneric SP. The pharmacology of (-)-nicotine and novel cholinergic channel modulators. Adv Pharmacol. 1997;37:153–214. doi: 10.1016/s1054-3589(08)60950-3. [DOI] [PubMed] [Google Scholar]
- Carpenter DO, Briggs DB, Knox AP, Strominger N. Excitation of area postrema neurons by transmitters, peptides, and cyclic nucleotides. J Neurophysiol. 1988;59:358–369. doi: 10.1152/jn.1988.59.2.358. [DOI] [PubMed] [Google Scholar]
- Christie DA, Tansey EM, editors. The Discovery, Use and Impact of Platinum Salts As Chemotherapy Agents for Cancer. London: Wellcome Witnesses to Twentieth Century Medicine; 2007. [Google Scholar]
- Davis CJ, Harding RK, Leslie RA, Andrews PLR. The organisation of vomiting as a protective reflex: a commentary on the first day's discussions. In: Davis CJ, Lake-Bakaar GV, Grahame-Smith DG, editors. Nausea and Vomiting: Mechanisms and Treatment. Berlin: Springer-Verlag; 1986. pp. 78–93. [Google Scholar]
- Davis TG, Peterson JJ, Kou JP, Capper-Spudich EA, Ball D, Nials AT, et al. The identification of a novel phosphodiesterase 4 inhibitor, 1-ethyl-5-{5-[(4-methyl-1-piperazinyl)methyl]-1,3,4-oxadiazol-2-yl}-N-(tet rahydro-2H-pyran-4-yl)-1H-pyrazolo[3,4-b]pyridin-4-amine (EPPA-1), with improved therapeutic index using pica feeding in rats as a measure of emetogenicity. J Pharmacol Exp Ther. 2009;330:922–931. doi: 10.1124/jpet.109.152454. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. Guideline on Non-Clinical and Clinical Development of Medicinal Products for the Treatment of Nausea and Vomiting Associated with Cancer Chemotherapy. CPMP/EWP/4937/03. London: EMEA; 2006. [Google Scholar]
- Hawthorn J, Andrews PLR, Ang VT, Jenkins JS. Differential release of vasopressin and oxytocin in response to abdominal vagal afferent stimulation or apomorphine in the ferret. Brain Res. 1988;438:193–198. doi: 10.1016/0006-8993(88)91338-8. [DOI] [PubMed] [Google Scholar]
- Holmes AM, Rudd JA, Tattersall FD, Aziz Q, Andrews PLR. Opportunities for the replacement of animals in the study of nausea and vomiting. Br J Pharmacol. 2009;157:865–880. doi: 10.1111/j.1476-5381.2009.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karniol IG, Dalton J, Lader MH. Acute and chronic effects of lithium chloride on physiological and psychological measures in normals. Psychopharmacology (Berl) 1978;57:289–294. doi: 10.1007/BF00426753. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KL, Stern RM, Summy-Long J, Bingaman S, Sperry N. Gastric dysrhythmias precede activation of vasopressinergic pathways during vection-induced nausea in man. J Gastrointest Mot. 1989;1:53. [Google Scholar]
- Kris MG, Hesketh PJ, Somerfield MR, Feyer P, Clark-Snow R, Koeller JM, et al. American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol. 2006;24:2932–2947. doi: 10.1200/JCO.2006.06.9591. [DOI] [PubMed] [Google Scholar]
- Laffan RJ, Borison HL. Emetic action of nicotine and lobeline. J Pharmacol Exp Ther. 1957;121:468–476. [PubMed] [Google Scholar]
- Lang IM, Marvig J, Sarna SK. Comparison of gastrointestinal responses to CCK-8 and associated with vomiting. Am J Physiol. 1988;254:G254–G263. doi: 10.1152/ajpgi.1988.254.2.G254. [DOI] [PubMed] [Google Scholar]
- Lee A. Adverse Drug Reactions. 2nd. London: Pharmaceutical Press; 2007. [Google Scholar]
- Manno BR, Manno JE. Toxicology of ipecac: a review. Clin Toxicol. 1977;10:221–242. doi: 10.3109/15563657708987968. [DOI] [PubMed] [Google Scholar]
- Masi G, Milone A, Manfredi A, Pari C, Paziente A, Millepiedi S. Effectiveness of lithium in children and adolescents with conduct disorder: a retrospective naturalistic study. CNS Drugs. 2009;23:59–69. doi: 10.2165/0023210-200923010-00004. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J Pharmacol Exp Ther. 2002;303:791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori F, Perez-Torres S, De Caro R, Porzionato A, Macchi V, Beleta J, et al. The human area postrema and other nuclei related to the emetic reflex express cAMP phosphodiesterases 4B and 4D. J Chem Neuroanat. 2010;40:36–42. doi: 10.1016/j.jchemneu.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Osinski MA, Uchic ME, Seifert T, Shaughnessy TK, Miller LN, Nakane M, et al. Dopamine D2, but not D4, receptor agonists are emetogenic in ferrets. Pharmacol Biochem Behav. 2005;81:211–219. doi: 10.1016/j.pbb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Percie du Sert N, Rudd JA, Moss R, Andrews PL. The delayed phase of cisplatin-induced emesis is mediated by the area postrema and not the abdominal visceral innervation in the ferret. Neurosci Lett. 2009;465:16–20. doi: 10.1016/j.neulet.2009.08.075. [DOI] [PubMed] [Google Scholar]
- Percie du Sert N, Rudd JA, Apfel CC, Andrews PL. Cisplatin-induced emesis: systematic review and meta-analysis of the ferret model and the effects of 5-HT(3) receptor antagonists. Cancer Chemother Pharmacol. 2011;67:667–686. doi: 10.1007/s00280-010-1339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley MK, Authier S, Curtis MJ. Principles of safety pharmacology. Br J Pharmacol. 2008;154:1382–1399. doi: 10.1038/bjp.2008.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roila F, Hesketh PJ, Herrstedt J. Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference. Ann Oncol. 2006;17:20–28. doi: 10.1093/annonc/mdj078. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Shelton RL, Helderman JH, Vestal RE, Robertson GL. Influence of the emetic reflex on vasopressin release in man. Kidney Int. 1979;16:729–735. doi: 10.1038/ki.1979.189. [DOI] [PubMed] [Google Scholar]
- Rudd JA, Andrews PLR. Mechanisms of acute and delayed nausea and vomiting induced by anticancer therapies. In: Hesketh P, editor. Management of Nausea and Vomiting in Cancer and Cancer Treatment. Sudbury, MA: Jones and Bartlett Publishers, Inc; 2005. pp. 15–66. [Google Scholar]
- Rudd JA, Naylor RJ. Opioid receptor involvement in emesis and anti-emesis. In: Reynolds DJ, Andrews PLR, Davis CJ, editors. Serotonin and the Scientific Basis of Anti-Emetic Therapy. Oxford: Oxford Clinical Communications; 1995. pp. 208–221. [Google Scholar]
- Rudd JA, Yamamoto K, Yamatodani A, Takeda N. Differential action of ondansetron and dexamethasone to modify cisplatin-induced acute and delayed kaolin consumption (‘pica’) in rats. Eur J Pharmacol. 2002;454:47–52. doi: 10.1016/s0014-2999(02)02472-x. [DOI] [PubMed] [Google Scholar]
- Sam TS, Chan SW, Rudd JA, Yeung JH. Action of glucocorticoids to antagonise cisplatin-induced acute and delayed emesis in the ferret. Eur J Pharmacol. 2001;417:231–237. doi: 10.1016/s0014-2999(01)00915-3. [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Holbrook JD, Andrews PL. The translational value of rodent gastrointestinal functions: a cautionary tale. Trends Pharmacol Sci. 2011;32:402–409. doi: 10.1016/j.tips.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Seymour MT. The pharmacokinetics and pharmacodynamics of chemotherapeutics agents. In: Andrews PLR, Sanger GJ, editors. Emesis in Anti-Cancer Therapy. London: Chapman & Hall; 1993. pp. 9–45. [Google Scholar]
- Souness JE, Rao S. Proposal for pharmacologically distinct conformers of PDE4 cyclic AMP phosphodiesterases. Cell Signal. 1997;9:227–236. doi: 10.1016/s0898-6568(96)00173-8. [DOI] [PubMed] [Google Scholar]
- Stables R, Andrews PLR, Bailey HE, Costall B, Gunning SJ, Hawthorn J, et al. Antiemetic properties of the 5HT3-receptor antagonist, GR38032F. Cancer Treat Rev. 1987;14:333–336. doi: 10.1016/0305-7372(87)90026-0. [DOI] [PubMed] [Google Scholar]
- Stern RM, Koch KL, Andrews PLR. Nausea, Mechanisms andManagement. New York: Oxford University Press; 2011. pp. 171–239. [Google Scholar]
- Wang SC, Borison HL. Copper sulphate emesis; a study of afferent pathways from the gastrointestinal tract. Am J Physiol. 1951;164:520–526. doi: 10.1152/ajplegacy.1951.164.2.520. [DOI] [PubMed] [Google Scholar]
- Wang SC, Borison HL. A new concept of organization of the central emetic mechanism: recent studies on the sites of action of apomorphine, copper sulfate and cardiac glycosides. Gastroenterology. 1952;22:1–12. [PubMed] [Google Scholar]
- Wilkens EP, Yates BJ. Pretreatment with ondansetron blunts plasma vasopressin increases associated with morphine administration in ferrets. Anesth Analg. 2005;101:1029–1033. doi: 10.1213/01.ane.0000166954.61498.2c. table of contents. [DOI] [PubMed] [Google Scholar]
- Wondergem J, Siddik ZH, Strebel FR, Bull JM. Effect of whole body hyperthermia on cis-diamminedichloroplatinum (II)-induced antitumour activity and tissue Pt-distribution: do anaesthetics influence the therapeutic ratio? Eur J Cancer. 1993;29A:549–554. doi: 10.1016/s0959-8049(05)80149-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Asano K, Matsukawa N, Imaizumi M, Yamatodani A. Time-course analysis of pica in rats using an automatic feeding monitoring system. J Pharmacol Toxicol Methods. 2011;63:30–34. doi: 10.1016/j.vascn.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Yung CY. A review of clinical trials of lithium in medicine. Pharmacol Biochem Behav. 1984;21(Suppl. 1):51–55. doi: 10.1016/0091-3057(84)90163-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


