Abstract
BACKGROUND AND PURPOSE
Pathological angiogenesis is associated with various human diseases, such as cancer, autoimmune diseases and retinopathy. The angiopoietin (Ang)–Tie2 system plays critical roles in several steps of angiogenic remodelling. Here, we have investigated the anti-angiogenic effect of a novel angiopoietin-derived peptide.
EXPERIMENTAL APPROACH
Using computational methods, we identified peptides from helical segments within angiopoietins, which were predicted to inhibit their activity. These peptides were tested using biochemical methods and models of angiogenesis. The peptide with best efficacy, A11, was selected for further characterization as an anti-angiogenic compound.
KEY RESULTS
The potent anti-angiogenic activity of A11 was demonstrated in a multicellular assay of angiogenesis and in the chorioallantoic membrane model. A11 bound to angiopoietins and reduced the binding of Ang-2 to Tie2. A11 was also significantly reduced vascular density in a model of tumour-induced angiogenesis. Its ability to inhibit Ang-2 but not Ang-1-induced endothelial cell migration, and to down-regulate Tie2 levels in tumour microvessels, suggests that A11 targets the Ang–Tie2 pathway. In a rat model of oxygen-induced retinopathy, A11 strongly inhibited retinal angiogenesis. Moreover, combination of A11 with an anti-VEGF antibody showed a trend for further inhibition of angiogenesis, suggesting an additive effect.
CONCLUSIONS AND IMPLICATIONS
Our results indicate that A11 is a potent anti-angiogenic compound, through modulation of the Ang–Tie2 system, underlining its potential as a therapeutic agent for the treatment of ocular and tumour neovascularization, as well as other pathological conditions that are dependent on angiogenesis.
Keywords: therapeutic peptide, angiogenesis, angiopoietin, Tie2, retinopathy, apoptosis, tumour, xenograft
Introduction
Angiogenesis, the growth of new capillary blood vessels, plays essential roles in development, reproduction and wound healing (Liekens et al., 2001). However, pathological angiogenesis is involved in a range of ‘angiogenesis-dependent diseases’, such as cancer, autoimmune diseases and retinopathy (Folkman, 2007).
Among the various angiogenic factors, VEGF and angiopoietins (Angs) are crucial for the formation of new blood vessels (Yancopoulos et al., 2000). Angiopoietins, Ang-1, Ang-2 and Ang-4, are ligands of Tie2, a tyrosine kinase receptor that is almost exclusively expressed by endothelial cells and haematopoietic stem cells (Thomas and Augustin, 2009). The Ang molecules are soluble secreted proteins that exist as dimers or higher order multimers that are determined by the small superclustering region and coiled-coil domains at the amino terminus, whereas the carboxyl terminal fibrinogen-like domains facilitate receptor binding (see Huang et al., 2010). The Ang–Tie2 system is involved in several steps of the angiogenic remodelling process, including destabilization of existing vessels, endothelial cell migration, tube formation and subsequent stabilization of newly formed blood vessels (Eklund and Olsen, 2006).
Ang-1 and Ang-2 are the best-characterized ligands of Tie2 and were originally thought to be agonist and antagonist respectively (Maisonpierre et al., 1997; Davis and Yancopoulos, 1999). Ang-1 was shown to support endothelial cell survival and promote endothelial integrity and vascular maturation (Papapetropoulos et al., 2000; Brindle et al., 2006), whereas Ang-2 was shown to inhibit the pro-angiogenic actions of Ang-1 and to promote endothelium destabilization. However, accumulating evidence indicates a more complex situation, whereby Ang-2 functions as a context-dependent antagonist, agonist or partial-agonist (Eklund and Olsen, 2006; Huang et al., 2010), and the outcome of its effect on Tie2 signalling depends on various parameters, such as type of vascular bed (Oshima et al., 2005), concomitant expression of Ang-1 (Yuan et al., 2009) or other angiogenic factors (Asahara et al., 1998). The functions of Ang-4 (or of its mouse orthologue, Ang-3) are far less characterized than those of Ang-1 and Ang-2. Ang-4 acts as an agonist of Tie2, inducing autophosphorylation in human endothelial cells and stimulating corneal angiogenesis (Lee et al., 2006). On the other hand, Ang-4 inhibited human endothelial cell migration and angiogenesis (Olsen et al., 2006).
Ang-1 is constitutively expressed by mural cells in many adult tissues, whereas Ang-2 is mainly secreted by endothelial cells and is predominantly expressed in tissues undergoing vascular remodelling, as occurs in tumours, functioning in an autocrine manner (Huang et al., 2010). When functioning as an Ang-1 antagonist, Ang-2 promotes the dissociation of pericytes from preexisting vessels and increase vascular permeability. Ang-1 and Ang-2 work in opposing manner to recruit mural cells and maintain vessel integrity (Huang et al., 2010). VEGF and Ang-2 modulate angiogenesis in a cooperative manner (reviewed in Augustin et al., 2009 and Saharinen et al., 2010). Ang-2 provides a key role in destabilizing existing vasculature, in a manner that is necessary for subsequent remodelling, while VEGF promotes differentiation and proliferation of ECs and the formation of immature vessels. Ang-2 can lead to robust angiogenesis in the presence of VEGF or to vessel regression when VEGF is absent or inhibited (Lobov et al., 2002). Recently, it has been shown that inhibition of Ang-2 reduced tumour growth and the number of blood vessels, but induced normalization of the newly formed tumour vessels, with increased pericyte coverage, reduced endothelial sprouting and remodelling into more uniform vessels (Falcon et al., 2009).
Here we report the in silico design, using the methods described by Kliger et al. (2009), synthesis and subsequent experimental evaluation of novel anti-angiogenic peptides, corresponding to helical structures within angiopoietins. One of these peptides, A11, chosen for further characterization, revealed substantial anti-angiogenic activity in several models, including the chorioallantoic membrane (CAM) assay and tumour-induced angiogenesis. A11 also showed robust inhibition of retinal neovascularization in an animal model of retinopathy, and its combination with an anti-VEGF antibody in this model resulted in an additive effect.
Methods
Design and synthesis of peptides
Peptides, with structures derived mostly from the coiled-coil domains of angiopoietins, were predicted in silico to form helical structures and to interfere with helix–helix interactions important for the activity of their parent proteins (Kliger et al., 2009). Using this approach, we have designed peptides that were predicted to bind and block human angiopoietins (see Table 1). All the peptides were synthesized by standard Fmoc solid-phase synthesis at Mimotopes or Pepscan (Lelystad, The Netherlands). All peptides were amidated at their C-terminus and acetylated at their N-terminus. Purity was determined by analytical RP-HPLC and their identity was confirmed by mass spectrometry.
Table 1.
Sequence and derivation of peptides tested
| Peptide | Derived from | Residues | Sequence |
|---|---|---|---|
| H2 | Ang-1 | 212–241 | LKEEKENLQGLVTRQTYIIQELEKQLNRAT |
| H3 | Ang-1 | 242–252 | TNNSVLQKQQL |
| A8 | Ang-1 | 254–264 | LMDTVHNLVNL |
| H7 | Ang-1 | 182–206 | NEILKIHEKNSLLEHKILEMEGKHK |
| G4 | Ang-2 | 215–230 | QLQVLVSKQNSIIEEL |
| G6 | Ang-2 | 250–270 | DLMETVNNLLTMMSTSNSAKD |
| F9 | Ang-4 | 210–245 | QEELASILSKKAKLLNTLSRQSAALTNIERGLRGVR |
| F12 | Ang-4 | 255–277 | QHSLRQLLVLLRHLVQERANASA |
| A11 | Ang-4 | 169–180 | ETFLSTNKLENQ |
| G2 | Ang-4 | 84–112 | TQQVKQLEQALQNNTQWLKKLERAIKTIL |
| C6 | Ang-4 | 150–167 | TDMEAQLLNQTSRMDAQM |
Functional tubule formation assay
Peptides were assessed for their effects on angiogenesis in vitro in a human multicellular model (AngioKitTM, TCS CellWorks, Buckingham, UK). Briefly, 24 well plates were seeded with cells on day 0, and medium was changed on days 3, 4, 7, 10 and 12 in accordance with the standard AngioKit procedure. Peptides and control compounds at the appropriate dilutions were included in the medium replaced on days 4, 7, 10 and 12. Peptides were dissolved in either 20% DMSO or in 1% NH4HCO3, to a stock concentration of 1 mg·mL−1, and were subsequently diluted in medium and assayed at 1 µg·mL−1 in duplicates. Wells treated with medium alone (‘untreated’) were used as negative controls. DMSO and NH4HCO3 were used as vehicle controls, and suramin (20 µM) and anti-Tie2 neutralizing antibody (5 µg·mL−1; R&D Systems, Inc., Minneapolis, MN, USA) were used as anti-angiogenic controls. On day 14, cultures were fixed and stained using the CD31 Staining Kits, according to the standard AngioKit procedure. Comparison of tubule development was conducted using the ‘AngioSys’ (TCS CellWorks) image analysis system. Four images were taken from predetermined positions within each duplicate well. Each test compound therefore yielded eight images for analysis.
Surface plasmon resonance (SPR) analysis
Peptide binding to angiopoietins. SPR analysis was performed by a BIAcore 3000 biosensor (BIAcore, Uppsala, Sweden). The chip design included Flow cell 1 (FC-1), which served as control (empty channel), FC-2 with immobilized Ang-1, FC-3 with immobilized Ang-2 and FC-4 with immobilized Ang-4. Briefly, recombinant human Ang-1 (rhAng-1; R&D Systems) at 10 µg·mL−1 in acetate buffer pH 4.5 was immobilized onto a research grade CM5 sensor chip (BIAcore) to a level of ≍7500 Resonance Units (RUs), using the BIAcore standard amine coupling procedure. Similarly, 50 µg·mL−1 rhAng-2 and 10 µg·mL−1 rhAng-4 (R&D Systems) were immobilized onto the same sensor chip to a level of ≍3600 and ≍8500RUs respectively. The chip was activated by amine coupling reagent (BIAcore) and suppressed by 70 µL of 1 M ethanolamine (at a rate of 10 µL·min−1), for the detection of non-specific binding to the chip surface. Peptides were dissolved in either 1% NH4HCO3 or 5% DMSO to a concentration of 1 mg·mL−1. Peptides were then diluted to 10–50 µM in PBS and injected at a rate of 20 µL·min−1 to determine binding to the angiopoietins on the chip. Regeneration was carried out between each sample with 1 mM NaOH. Binding kinetics were measured by serial dilution (to 25–1.56 µM or 1.25–0.039 µM) and injecting at a rate of 30 µL·min−1. Binding affinities were determined using a 1:1 Langmuir model (BIA Evaluation software version 4.1, BIAcore).
Inhibition of Ang binding to Tie2. SPR analysis was performed as described above. sTie2-Fc (a soluble recombinant protein corresponding to the extracellular domain of human Tie2 fused to Fc; rhTie-2/Fc; R&D Systems) at 100 µg·mL−1 in 10 mM acetate buffer pH 4.5 was immobilized to a level of 6375–10,000RUs. Peptides were diluted to 10 µM in PBS and injected at a rate of 20 µL·min−1. Ang-1 (100 nM), Ang-2 (500 nM), and Ang-4 (500 nM) were incubated alone or with peptides at a ratio of 1:100 (10–50 µM) in a total volume of 50 µL of PBS for 10–30 min at room temperature, before being injected at a rate of 20 µL·min−1. Regeneration was carried out with 50 mM NaOH.
In ovo avian chorioallantoic membrane (CAM) assay
Leghorn fertilized eggs were placed in an incubator and kept under constant humidity at 37°C. On day 4, a window was opened on the shell exposing the CAM, sealed to prevent dehydration, and the eggs were re-incubated. On day 9 of embryo development, compounds were applied in a 40 µL volume, on an area of the CAM restricted by a plastic ring (1 cm2). Peptides were tested at two different doses (0.5 and 5 nmol per egg). As positive controls we used a Tie2 neutralizing antibody (at 0.4 µg per egg; R&D Systems) and fumagillin (at 5 µg per egg; Tocris Bioscience, Ellisville, MO, USA). The appropriate vehicle controls were also tested. Eggs were resealed and re-incubated for additional 48 h. CAMs were fixed in situ, excised from the eggs, placed on slides and left to air-dry. Pictures were taken through a stereoscope equipped with a digital camera, and the total length of the vessels was measured using image analysis software (NIH Image). For each group, a total of 17–24 eggs from three separate experiments were analysed.
Migration assay
Human umbilical vein endothelial cells (HUVECs) were isolated using standard methodology (Papapetropoulos et al., 1997) and grown in M199 medium supplemented with 15% bovine calf serum, 50 U·mL−1 penicillin, 50 µg·mL−1 streptomycin, 50 µg·mL−1 gentamycin, 2.5 µg·mL−1 amphotericin B, 5 U·mL−1sodium heparin and 150 to 200 µg·mL−1 endothelial cell growth supplement. HUVEC between passages 1 and 3 were used for all experiments. Prior to the migration experiments, cells were serum-starved for 5 h in growth medium containing 0.25% BSA (Sigma, St. Louis, MO, USA). After trypsinization, 1 × 105 cells were added to Transwell inserts (8 µm pore size; Corning-Costar Inc. Corning, NY, USA) in 600 µL starvation medium. Migration of cells was induced by adding 250 ng·mL−1 of Ang-1 or Ang-2 (R&D Systems) at the lower compartment of the Transwell setup. When the peptide A11 was tested, cells were pre-incubated with 5 µg·mL−1 A11 for 30 min, placed in both the upper and lower compartments. HUVEC were allowed to migrate for 4 h at 37°C, after which non-migrated cells remaining at the upper compartment of the Transwell filter were removed with a cotton swab. The migrated cells were fixed in Carson's solution for 30 min and then stained in toluidine blue (Sigma) for 20 min, both at room temperature. Migrated cells were scored in eight random fields and the fold-change was determined compared with the number of migrated cells in control (vehicle) wells.
Colorectal cancer model
All animal care and experimental procedures complied with the US Animal Welfare Act and in accordance with the principles set forth in the ‘Guide for the Care and Use of Laboratory Animals’, Institute of Laboratory Animals Resources, National Research Council, National Academy Press (1996), and were approved by Duke University Institutional Animal Care and Use Committee. Athymic nude mice (NCr-nu-nu) were obtained from NCI-Frederick through the Duke Division of Laboratory Animal Resources (DLAR). HCT116 (CCL-247) human colorectal cancer cells were obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were injected in the flank of 6–8 week-old female athymic nude mice weighing 16.3–25.3 g (median 20.6 g). Forty-nine mice were enrolled sequentially for 2 weeks when tumour volume reached 100–150 mm3. Animals were treated with A11 for 14 days with either twice daily i.p. injections of 33.3 µg (low dose) or 100 µg (high dose) in 200 µL, or with continuous infusion of 48 µg of A11 per day using osmotic pumps (model 2002, 0.5 µL·h−1; ALZET Osmotic Pumps, Cupertino, CA, USA) implanted s.c. Bevacizumab (Avastin; Genentech, South San Francisco, CA, USA), used as a positive control, was administered by i.p. injections of 200 µg in 100 µL every other day for 14 days. Vehicle control (PBS) was administered by i.p. injections twice daily, or by osmotic pumps, similarly to A11. Tumour volume and body weight were measured every other day. Mice (n = 8–9) were killed 14 days after beginning of treatment. The hypoxia marker drug, EF5 (Koch et al., 1995) was obtained from Cameron Koch (University of Pennsylvania, Philadelphia, PA, USA), and was injected i.p. at 26.4 µL/g 3 h before tumour removal. For each mouse, the tumour and one kidney were collected and snap-frozen.
Immunohistochemistry and imaging
Frozen tumours were cryosectioned at 10 µm thickness using a Leica CM 1850 cryotome (Meyer Instruments, Houston, TX, USA). Tumour microvessels were visualized by staining with an anti-CD31 mAb (BD Bioscience, San Jose, CA, USA) at a dilution of 1:100. Cleaved Caspase-3 antibody (Cell Signaling Technology, Danvers, MA, USA), a marker of apoptosis, was used at a dilution of 1:400. EF5 was used as a marker of hypoxia, and staining was done with the anti-EF5 antibody ELK3-51 conjugated with Cy5 dye at a dilution of 1:1 (75 µg·mL−1, obtained from Cameron Koch, University of Pennsylvania). Mouse anti-Tie2 was stained at a dilution of 1:500 using diaminobenzidine (DAB) detection system (Thermo Scientific, Fremont, CA, USA). For fluorescence visualization of antibody reaction, primary antibodies were detected using secondary antibodies labelled with the Alexa Fluor 488 anti-rat IgG (Invitrogen, Carlsbad, CA, USA), DyLight 594 anti-rabbit IgG (Jackson Immunoresearch, West Grove, PA, USA) and FITC anti-rabbit IgG (Jackson Immunoresearch).
Whole tumour fluorescent images for CD31 and cleaved caspase-3 staining were acquired using a fluorescence microscope (Axioskop 2plus; Zeiss, Göttingen, Germany) equipped with a cooled charge-coupled device digital camera (Retiga 1300R; Q-Imaging) at ×50 or ×100 magnification. Images acquired from stage scanning were stitched and overlaid for direct comparison of dual fluorescent images. Image acquisition and control was accomplished using Adobe Photoshop CS2 v9.0.2 (Adobe Systems, San Jose, CA, USA) and ImageJ software (US National Institutes of Health, Bethesda, MD, USA). To avoid subjective selection of the threshold, an automatic threshold scheme, the Otsu thresholding method (Otsu, 1979), was used to threshold images. The average microvessel density for CD31 was acquired from five random fields (1 mm × 1.3 mm). The stained area for Tie2 was measured by Photoshop from two random fields (3.3 mm × 4 mm) using ×100 magnification. To evaluate normal tissue toxicity, kidney sections were stained for CD31. Regions of interest were drawn around the viable tumour area. Necrotic areas were identified using EF5 immunofluorescent image as a mask. Unstained regions surrounded by brightly stained hypoxic tissue were considered necrotic. EF5 binding intensity is inversely proportional to pO2; thus, the brightest staining is seen in the most hypoxic cells (Koch, 2002). However, dead cells are not able to retain the drug, which requires enzymatic bioreduction to become activated and bind to cellular proteins. Therefore, regions that do not stain, that are immediately adjacent to brightly staining regions, by definition, are beyond the oxygen diffusion distance and necrotic. Necrotic areas were measured in whole tumour images, and the percentage of the necrotic areas within the analysed areas (viable tumour plus necrotic area) was calculated.
Oxygen-induced retinopathy (OIR) model
All animal care and experimental procedures complied with the ‘Guide for the Care and Use of Laboratory Animals' and the ‘Statement for the Use of Animals in Ophthalmic and Visual Research', The association for Research in Vision and Ophthalmology. Sprague Dawley rat dams were obtained from Charles River (Raliegh, NC, USA) sprague–Dawley rats (n = 10–12) were raised from birth through post-natal day 14 (P14) in a variable oxygen atmosphere consisting of 24 h alternating cycles of 50% and 10% oxygen. Upon removal from the oxygen exposure chamber (on P14), and again 3 days later (on P17), rats received an intravitreal injection of 5 µL containing 75 or 375 ng of peptide A11, or 500 ng sTie2-Fc (rhTie-2/Fc; R&D Systems) as positive control. Vehicle (PBS) served as negative control. All pups were killed on P20 (i.e. 6 days after removal to room air) by cervical dislocation under deep anaesthesia (sodium pentobarbital (Nembutal; Ovation Pharmaceuticals, Inc., Deerfield, IL, USA, 50 mg/kg i.p). Abnormal pre-retinal neovascular growth was assessed in ADPase-stained retinal flat-mounts, using published methods (Yanni et al., 2009). In a separate study, we tested the combination of the A11 peptide (at 375 ng) with an anti-rat VEGF antibody (at 500 ng; AF564; R&D Systems), and compared with each compound alone (n = 7–11). All assessments were performed by a single, highly trained observer, unaware of the treatments. Areas of normal and abnormal vascular growth were measured via computer-assisted image analysis (Image J by NIH; Bethesda, MD, USA) using high-resolution digital images of the stained retinal flat-mounts.
Data analysis
Data in the text and Figures are shown as means ± SEM. Statistical analysis (one way anova followed by Dunnett's or Bonferroni post hoc test) was performed using Graph Pad Prism 4 or JMP Software (SAS Institute, Inc.; Cary, NC, USA). Where only two groups were compared Student's t –test was used. Differences were considered to be statistically significant if P < 0.05.
Results
Inhibition of in vitro tubule formation
The peptides, synthesized on the basis of the in silico predictions (Kliger et al., 2009), were first evaluated for anti-angiogenic activity using the AngioKit, an in vitro assay of angiogenesis that reproduces the known phases of the angiogenic process by using co-cultures of early passage primary human endothelial and interstitial cells (Bishop et al., 1999). The spontaneous development of a branching network of capillary-like tubules was assessed in the presence of the tested compounds. Suramin, a known inhibitor of angiogenesis (Gagliardi et al., 1992), served as an anti-angiogenic control. As a specific inhibitor of the Ang–Tie2 pathway, we used a neutralizing anti-Tie2 antibody that was previously shown to inhibit in vitro angiogenesis in a similar assay (Giuliani et al., 2003). Out of the 26 peptides tested, 11 peptides (listed in Table 1) reduced total tubule length compared with vehicle control (Figure 1). These peptides also reduced two other parameters of in vitro angiogenesis, the number of tubules and of branching points (data not shown). Peptides A8, A11, G4, G6 and C6 displayed the highest anti-angiogenic activity in this assay, reaching up to 36% inhibition of total tubule length. This level of inhibition was similar or somewhat greater than the effect obtained with the anti-Tie2 neutralizing antibody, which displayed 27% inhibition.
Figure 1.
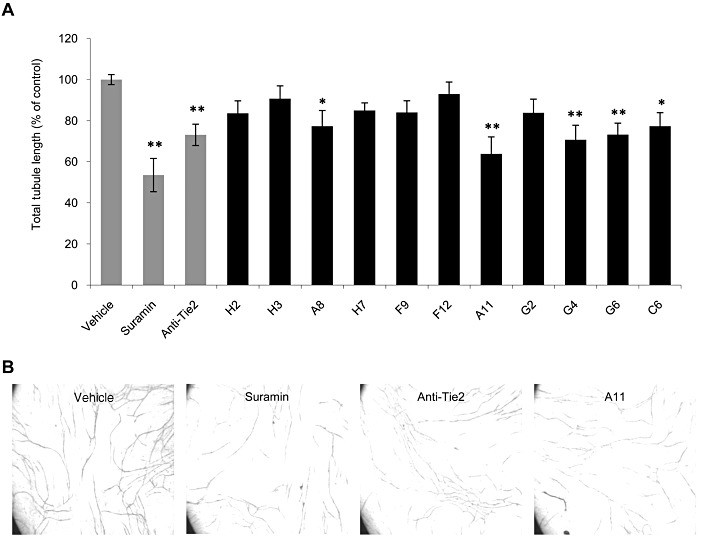
Peptides H2, H3, A8, H7, G4, G6, F9, F12, C6, A11 and G2 inhibit in vitro tubule formation. Co-cultures of human endothelial and interstitial cells (Angiokit) were treated in duplicates with 20 µM suramin, 5 µg·mL−1 anti-Tie2 neutralizing antibody or 1 µg·mL−1 of peptides. On day 14, cultures were fixed and stained for CD31, and tubule length was quantified. (A) Results are displayed as mean ± SEM of total tubule length relative to vehicle-treated cells (defined as 100%) and reflect the average obtained from four images in duplicate (total of eight measurements). *P < 0.05, **P < 0.01 significantly different from vehicle control; one-way ANOVA followed by Dunnett's post hoc test. (B) Representative images of cultures treated with vehicle control, suramin, anti-Tie2 mAb and peptide A11.
Binding to angiopoietins and inhibition of Ang binding to Tie2
As the peptides were derived from angiopoietins and were predicted to interfere with helix–helix interactions important for the activity of their parent proteins, the 11 peptides displaying inhibition of tubule formation were further examined for their ability to bind angiopoietins, by SPR using the BIAcore system. The calculated binding affinities for each peptide are summarized in Table 2. Several peptides (H2, H3, A8, H7 and G4) did not bind any of the angiopoietins, while others (F9, A11, and G6) revealed significant binding affinities to some or all of the Angs with a KD in the range of 20 nM–20 µM. Peptides F12 and G2, which demonstrated particularly high binding affinities in the range of 100 pM–10 nM, were later found to be ‘sticky’, showing high affinity binding also to non-relevant proteins (data not shown). Peptides G6 and C6 also demonstrated binding to Ang-2, but KD was not determined due to a ‘noisy’ signal.
Table 2.
Binding affinities of synthetic peptides to angiopoietins
| Binding affinity KD(nM) | |||
|---|---|---|---|
| Peptide | Ang-1 | Ang-2 | Ang-4 |
| H2 | – | – | – |
| H3 | – | – | – |
| A8 | – | – | – |
| H7 | – | – | – |
| G4 | – | – | – |
| G6 | 13.3 | N.D. | 22.1 |
| F9 | 21400 | 3070 | 2820 |
| F12 | 1.88 | 5840 | 0.243 |
| A11 | 6740 | 2360 | 3690 |
| G2 | 17.4 | 56.6 | 25 |
| C6 | – | N.D. | – |
–, no binding; N.D., KD not determined.
Peptides F9, A11, C6 and G6, which demonstrated binding to angiopoietins, were selected for further analysis. As these peptides are predicted to interfere with the active conformation of angiopoietins, their ability to inhibit the binding of angiopoietins to their Tie2 receptor was also examined by SPR. The binding of angiopoietins or peptides to an immobilized soluble Tie2 recombinant fusion protein (sTie2-Fc) was measured alone or in combination. Results, summarized in Figure 2A–C, indicate that all four peptides affected primarily the binding of Ang-2 to Tie2, reaching 40–100% inhibition, and also the binding of Ang-1 and Ang-4 to Tie2, albeit to a lower extent (22–34% and 20–67% inhibition respectively). Binding of peptides alone to Tie2 was negligible in most cases. These findings suggest that the peptides may exert their anti-angiogenic activity by interfering with the interaction between Angs (primarily Ang-2) and their receptor Tie2.
Figure 2.
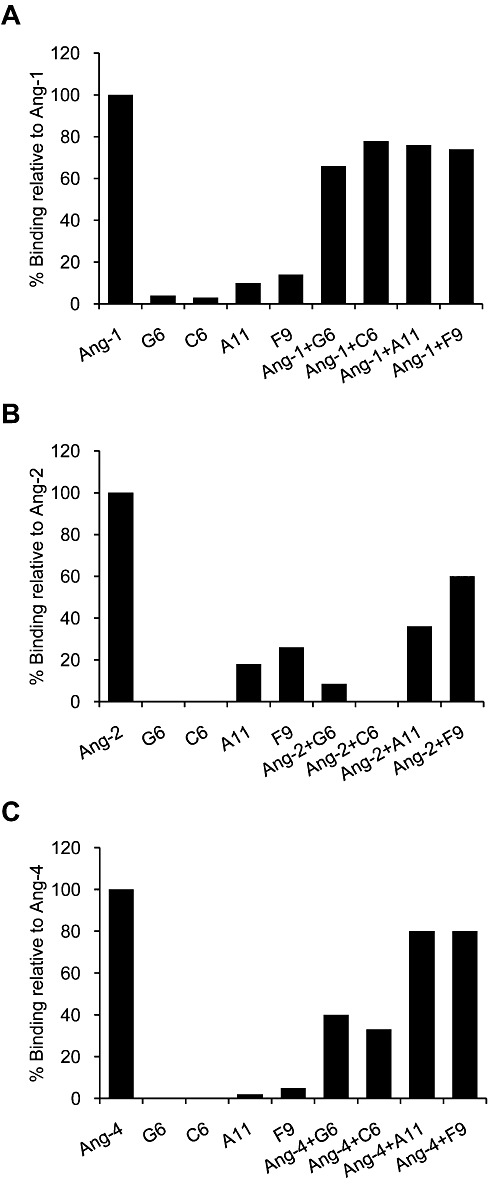
Inhibition of angiopoietin binding to Tie2. SPR analysis of angiopoietins binding to immobilized Tie2, in the presence or absence of peptides. The angiopoietin ligands and the four peptides tested, G6, C6, A11 and F9, were pre-incubated alone or together, and their ability to bind to immobilized Tie2 was measured. Results are shown for Ang-1 (A), Ang-2 (B) and Ang-4 (C) and are expressed relative to the binding to Tie2 of the respective ligand alone (defined as 100%).
Inhibition of in ovo angiogenesis
The four peptides mentioned above, F9, A11, C6 and G6, which showed the highest binding affinities to angiopoietins and interfered with Ang–Tie2 interaction, were further evaluated using the CAM assay (Tufan and Satiroglu-Tufan, 2005). The chicken forms of Ang-1 and Ang-2 share high homology with their human counterparts (Jones et al., 1998) and are both expressed in the CAM during angiogenesis. As human Ang-1 has been shown to have biological activity in chicken tissues (Kim et al., 2006), the CAM is a suitable model to examine the activity of peptides derived from human angiopoietins. As positive controls, we used fumagillin, a known angiogenesis inhibitor (Ingber et al., 1990), and an anti-Tie2 neutralizing mAb, a specific inhibitor of the Ang–Tie2 system. As shown in Figure 3, peptides F9, A11, and C6 displayed substantial anti-angiogenic activity at both doses, reaching a significant decrease of up to 40% in vessel length, which was similar to the extent of inhibition obtained with the positive controls, fumagillin and anti-Tie2 mAb (32% and 36% respectively). Peptide G6 had no effect on vessel growth. Although the inhibition of vessel formation by C6 was substantial and statistically significant, a large number of clots were noted in most of the eggs treated with this peptide; thus, results were interpreted with caution. Clot formation, which might be a result of peptide aggregates, was also observed after G6 treatment, although this peptide did not exhibit inhibitory activity in the CAM assay.
Figure 3.
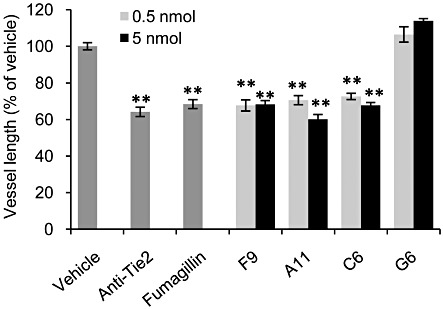
Inhibition of in ovo angiogenesis in the CAM model. Tested compounds were applied on a restricted area of the CAM of fertilized eggs (n = 17–25). Peptides were tested at 0.5 and 5 nmol per egg. Fumagillin and anti-Tie2 neutralizing antibody were used as positive controls, at 5 and 0.4 µg per egg respectively. Forty-eight hours after treatment, CAMs were fixed, and total length of vessels was measured with image analysis software. Data are presented as mean ± SEM and expressed as % of vehicle control. **P < 0.01 significantly different from vehicle control; one-way ANOVA followed by Dunnett's post hoc test.
Inhibition of endothelial cell migration
Peptide A11, which displayed the strongest inhibitory activity in the tubule formation assay (Figure 1), was chosen for further assessment based on its consistent and stronger inhibitory activity in the subsequent studies: Ang-2 binding to Tie2 and CAM assay (Figures 2 and 3 respectively). The ability of A11 to affect endothelial cell migration was measured on serum-starved HUVECs, which were stimulated with Ang-1 or Ang-2. Both Ang-1 and Ang-2 induced HUVEC migration by threefold compared with unstimulated cells (Figure 4). Treatment with A11 significantly inhibited Ang-2-induced HUVEC migration by 47% but did not affect the migration induced by Ang-1. This is further supported by the binding competition results showing that A11 inhibits primarily Ang-2 binding to Tie2 but has only a weak effect on Ang-1 binding (Figure 2). In addition, signalling experiments in HUVECs did not provide clear evidence for A11 inhibition of Ang-1-induced Tie2, Akt, or ERK1/2 phosphorylation (data not shown).
Figure 4.
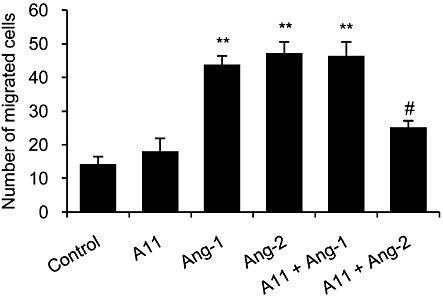
Inhibition of endothelial cell migration. HUVECs were serum-starved for 5 h, after which migration was induced by 250 ng·mL−1 of Ang-1 or Ang-2 in the presence or absence of A11. Cells were pre-treated with A11 (5 µg·mL−1) for 30 min. HUVECs were allowed to migrate for 4 h at 37°C, after which migrated cells were fixed, stained and scored. Data are presented as mean ± SEM and expressed as % of vehicle control. **P < 0.01 significantly different from vehicle control; one-way ANOVA followed by Dunnett's post hoc test. #P < 0.01 significantly different from Ang-2 alone; one-way ANOVA followed by Bonferroni's post hoc test.
Inhibition of tumour-induced angiogenesis in vivo
To study the anti-angiogenic activity of A11 and the mechanisms through which it is exerted in vivo, we evaluated this peptide in a xenograft tumour model, using HCT116 human colorectal cancer cells in nude mice. A11 was given by twice daily i.p. injections at two doses, or by continuous infusion using osmotic minipumps. During treatment, there was no significant weight loss in any of the groups. Treatment with A11, at the high dose and by continuous infusion, decreased blood vessel density (evaluated by anti-CD31 staining), and this anti-angiogenic effect was similar to that of bevacizumab, an anti-VEGF mAb inhibitor, which was used as positive control (Figure 5A,D). Staining with anti-Tie2 antibody also revealed reduced Tie2 levels in tumours treated with A11 (Figure 5B,D). Analysis of the ratio of Tie2 levels to microvessel density revealed that the reduction in the Tie2 levels could not be attributed solely to the decrease in microvessel density, as this ratio was reduced in tumours treated with A11 (Figure 5C). Importantly, the levels of Tie2 were not affected by treatment with bevacizumab, demonstrating a specific effect of A11 on the Ang–Tie2 pathway. These results suggest that down-regulation of Tie2 levels might underlie the anti-angiogenic effect of A11.
Figure 5.
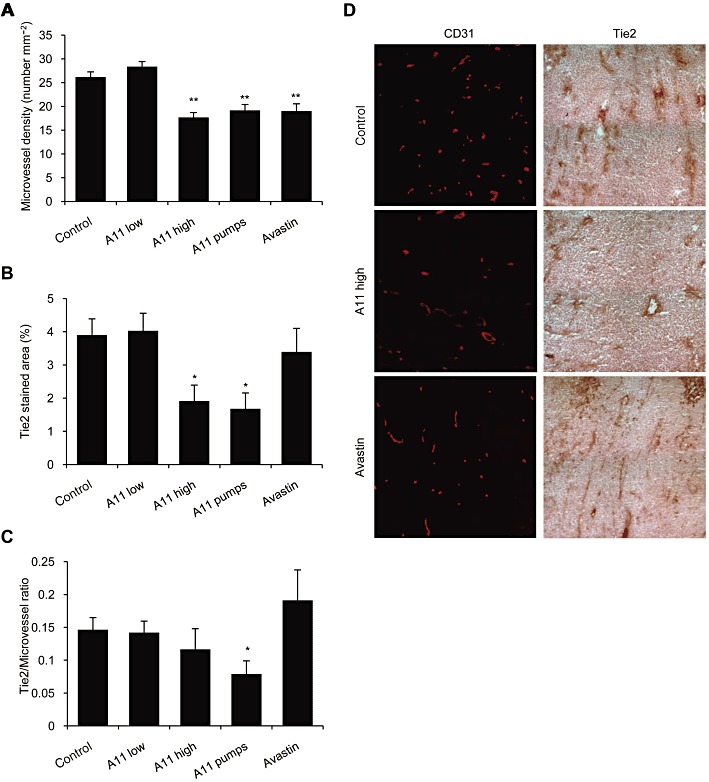
Effects of systemic treatment with A11 on tumour angiogenesis and Tie2 levels. HCT116 human colorectal cancer cells were injected to the flank of nude mice. Animals were treated with A11 using twice daily i.p. injections of 100 µg (high) or 33 µg (low) or by continuous infusion (pumps) of 48 µg per day for 14 days. Bevacizumab (Avastin) given i.p. every other day for 14 days was used as a positive control. Frozen tumours were cryosectioned and stained. (A) Tumour microvessels were visualized by staining with anti-CD31 antibodies. Microvessel density is presented as means ± SEM number of vessels per area. **P < 0.01 significantly different from vehicle control; one-way ANOVA followed by Dunnett's post hoc test. (B) Tie2 protein levels were evaluated using anti-Tie2 antibodies and are presented as mean ± SEM percent of stained area. *P < 0.05 significantly different from vehicle control; one-way ANOVA followed by Dunnett's post hoc test. (C) The ratio between Tie2 levels and microvessel number is presented as means ± SEM. *P < 0.05 significantly different from vehicle control; Student's t-test. (D) Representative images of immunohistochemical staining of angiogenesis (anti-CD31) and Tie2 levels (anti-Tie2).
Apoptosis in the sections of tumours, taken from animals treated with high dose of A11, were significantly increased, by as much as 2.9-fold, compared with control group (Figure 6A,C). Apoptosis was also increased in animals treated by continuous infusion of A11 (by 2.7-fold), although results did not reach statistical significance. The necrotic areas of A11 treated tumours were more prominent than in the control group (Figure 6B,C), but did not reach statistical significance, possibly since tumours were analysed rather early (14 days) after initiation of treatment, when tumour size did not exceed 1000 mm3. Notably, the effect of A11 on necrosis was significantly higher than that observed in the bevacizumab-treated tumours. A small effect on tumour growth delay was evident in animals treated with A11 towards the end of treatment period, but was not statistically significant (data not shown). Most likely, this effect on tumour growth would become more prominent as further necrosis develops with time.
Figure 6.
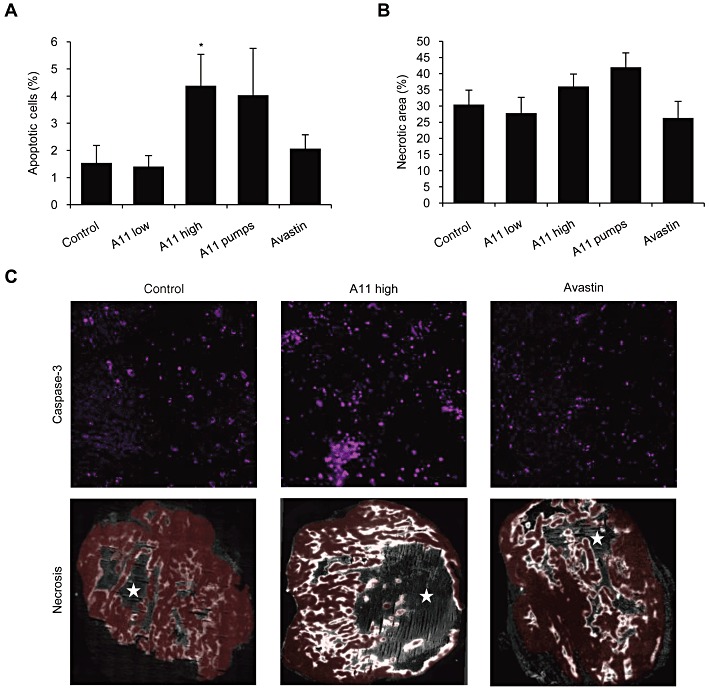
Effects of systemic treatment with A11 on tumour apoptosis and necrosis. HCT116 human colorectal cancer cells were injected to the flank of nude mice. Animals were treated with A11 using twice daily i.p. injections of 100 µg (high) or 33 µg (low) or by continuous infusion (pumps) of 48 µg per day for 14 days. Bevacizumab (Avastin) given i.p. every other day for 14 days was used as a positive control. Frozen tumours were cryosectioned and stained. (A) Apoptosis was followed using anti-cleaved caspase-3 antibodies and is presented as mean ± SEM percent of stained area. *P < 0.05 significantly different from vehicle control (t-test). (B) Necrosis was evaluated using EF5 immunofluorescent image (unstained necrotic regions were surrounded by brightly stained hypoxic regions). Results are presented as mean ± SEM percent of necrotic area within the analysed section (viable tumour plus necrotic area). (C) Representative images of immunohistochemical staining of apoptosis (cleaved caspase-3) and necrosis (EF5). Red colour marks the viable tumour tissue. Stars mark the necrotic areas.
In order to evaluate normal tissue toxicity, the vascularity of kidneys in animals treated with high dose of A11 or A11 pumps were compared with those of the control group. Both treated and untreated kidney sections remained highly vascularized (data not shown). This and the lack of weight loss suggest minimal acute toxicity of A11 at the doses used.
Inhibition of retinal neovascularization
A11 was also evaluated in another animal disease model of angiogenesis – oxygen-induced retinopathy (OIR). This model is often used to study the gross aspects of vasculogenesis and angiogenesis in vivo and is a well accepted model of human retinopathies, such as retinopathy of prematurity (ROP) and diabetic retinopathy (Barnett et al., 2010). In this model, neonatal animals with oxygen-induced regression of retinal blood vessels develop severe retinal hypoxia and consequent retinal neovascularization when removed from hyperoxia and placed back into room air (Barnett et al., 2010). sTie2-Fc, which was previously shown to inhibit retinal neovascularization in a similar model (Takagi et al., 2003), was used as a positive control. As shown in Figure 7A, intravitreal administration of peptide A11 demonstrated profound dose-dependent inhibition of retinal neovascularization, reaching 76% inhibition at the highest dose. The significant decrease in the pathological effects of oxygen-induced retinopathy displayed by A11 appears more pronounced than that obtained with sTie2-Fc (62% inhibition).
Figure 7.
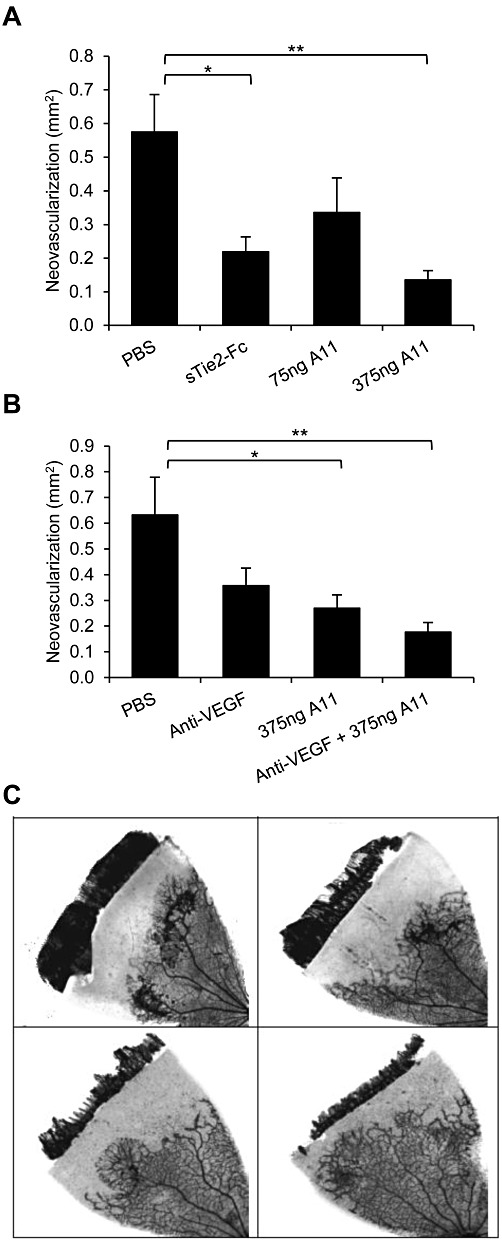
Effect of peptide A11 on retinal neovascular growth. Retinal neovascularization was induced and assessed in Sprague–Dawley rats, using the OIR model. Data represent areas of abnormal vascular growth and are presented as mean ± SEM. (A) Rats received intravitreal injections of vehicle (PBS), peptide A11 at 75 or 375 ng per eye, or sTie2/Fc at 500 ng per eye as positive control. Both A11 at the higher dose and sTie-2/Fc yielded inhibition relative to PBS-injected eyes; **P < 0.01, *P < 0.05, significantly different as indicated; one-way ANOVA followed by Dunnett's post hoc test. (B). Rats received intravitreal injections of vehicle (PBS), anti-VEGF 500 ng per eye, peptide A11 at 375 ng per eye, or a combination of anti-VEGF and peptide A11 at 500 ng per eye and 375 ng per eye, respectively. Both peptide A11 and the combination of peptide A11 and anti-VEGF yielded inhibition relative to PBS-injected eyes; *P < 0.05, **P < 0.01, significantly different as indicated; one-way ANOVA followed by Dunnett's post hoc test. (C) Representative images of the degree of retinal neovascularization in the OIR model, following intravitreal injection of PBS (upper left), anti-VEGF (upper right), A11 (lower left) and A11 plus anti-VEGF (lower right).
In a separate study, the efficacy of peptide A11 was compared with that of an anti-VEGF antibody, and both anti-angiogenic compounds were also tested in combination. As shown in Figure 7B,C, and in agreement with the results shown in Figure 7A, peptide A11 demonstrated a robust reduction of retinal neovascular growth, reaching 57% inhibition (p < 0.05) which was more effective than the anti-VEGF antibody (43% inhibition), which did not reach statistical significance. The combination of A11 with the anti-VEGF antibody resulted in in a stronger effect, reaching 72% inhibition (p < 0.01). The difference between the inhibitory effect achieved by the combination compared to anti-VEGF treatment alone was close to statistical significance with p = 0.06 (t-test), suggesting an additive effect. The marked inhibitory activity of peptide A11 in this animal model of angiogenesis further demonstrates the potent anti-angiogenic properties of this peptide.
Discussion and conclusions
In this study, we describe the in silico discovery and experimental evaluation of novel anti-angiogenic peptides derived from angiopoietins (Kliger et al., 2009). One of these peptides, A11, was chosen for further characterization based on its robust and consistent inhibitory activity. A11 showed substantial inhibition of in vitro tubule formation in a human multicellular system and of in ovo angiogenesis in the CAM model. Furthermore, A11 exhibited a robust reduction of tumour-induced angiogenesis and of retinal neovascularization in vivo. In all these studies, A11 displayed potent anti-angiogenic activity, which was similar or greater than that of the positive controls.
Previous studies have reported the identification of anti-angiogenic peptide inhibitors of the Ang–Tie2 system. Such peptides, however, were not derived from the sequence of angiopoietins, but were rather identified by screening phage display peptide libraries (Oliner et al., 2004; Tournaire et al., 2004; Wu et al., 2008; Doppalapudi et al., 2010).
The sequence of peptide A11 is derived from the coil-coiled domain of Ang-4, which is highly conserved among angiopoietins. Indeed, this peptide also shows high homology to the corresponding paralog segments of Ang-1 and Ang-2. The coiled-coil domain is involved in ligand homo-dimerization (Davis et al., 2003), and proper oligomerization/multimerization is a prerequisite for Tie2 binding and activation (Kim et al., 2005; 2009). Oligomers of Ang-1, having at least four subunits, are required for binding and activation of Tie2 (Davis et al., 2003; Cho et al., 2004; Kim et al., 2005) and are secreted by smooth muscle cells and pericytes. Dimeric Ang-2 is stored in endothelial cell Weibel-Palade bodies and is released upon activation of the quiescent endothelial cells (see Fiedler and Augustin, 2006). It has also been shown that a dominant-interfering form of Ang-1, called Ang1cc, which contains only the coiled-coil domain and lacks the fibrinogen like domain, can multimerize with Ang1 and inhibit its function in vitro and in vivo (Ward et al., 2004).
The Ang–Tie2 system is crucial for the angiogenic switch in tumours (Huang et al., 2010). Ang-2 expression is often found to exceed that of Ang-1, leading to vessel regression and hypoxia, and, together with VEGF, promotes the initiation of angiogenesis and maturation of new vessels (Saharinen et al., 2010). As recently reviewed in Huang et al. (2010), drug development targeting the Ang–Tie2 system to inhibit angiogenesis has been difficult given the complex and often confounding roles of angiopoietins. Ang-1 and Ang-2 may be viewed as pro- or anti-angiogenic, depending on context (e.g. vascular bed), and the ideal strategy to target the Ang–Tie2 pathway for therapeutic development remains ambiguous. Inhibition of Ang-2 is essential for targeting this pathway; however, dual inhibition of Ang-1 and Ang-2 might provide improved therapeutic benefit, as shown in a tumour xenograft model, whereby the antagonism of Ang-1 and Ang-2 mediated greater tumour suppression, than was achieved by inhibiting Ang-1 and Ang-2 individually (Falcon et al., 2009; Coxon et al., 2010). Indeed, both a dual Ang-1/Ang-2 antagonist peptide-Fc fusion protein, AMG-386 (Oliner et al., 2004; Herbst et al., 2009), and a specific Ang-2-binding CovX-Body, CVX-060 (Doppalapudi et al., 2010), are currently in clinical development for solid tumours. However, in other postnatal settings, such as oxygen-induced retinal angiogenesis, Ang-1 inhibition failed to augment the suppressive effect of Ang-2 inhibition (Coxon et al., 2010).
The Ang–Tie2 pathway is also involved in angiogenic ocular conditions. These include ROP, proliferative diabetic retinopathy (PDR) and age-related macular degeneration (AMD), which are the leading cause of irreversible vision loss in developed countries (Penn et al., 2008; Tolentino, 2009). Chronic hyperglycaemia in diabetic retinopathy induces cell damage and up-regulation of Ang-2, leading to retinal pericyte detachment, migration, apoptosis and progressive vasoregression (Hammes et al., 2011). Diabetic pericyte loss is the result of pericyte migration, a process that is modulated by the Ang–Tie2 system (Hammes et al., 2004; 2011; Ohashi et al., 2004; Pfister et al., 2008). Inhibitors of the Ang–Tie2 system, such as soluble Tie2 receptor (sTie2-Fc), indeed showed beneficial effects in various models of ocular angiogenesis (Lin et al., 1997; Asahara et al., 1998; Hangai et al., 2001; Takagi et al., 2003; White et al., 2003; Oliner et al., 2004; Singh et al., 2005).
Both VEGF and Ang-2 are induced by hypoxia and cooperate to induce angiogenesis (Hammes et al., 2011). Emerging evidence suggests that VEGFs and angiopoietins play complementary roles and that they modulate angiogenesis in a cooperative manner (Augustin et al., 2009; Saharinen et al., 2010), leading to the hypothesis that simultaneous interference of both pathways should result in additive anti-angiogenic effects. Combined inhibition of both VEGF and Tie2 indeed showed enhanced efficacy in models of retinal neovascularization (Takagi et al., 2003) and cancer angiogenesis (Jendreyko et al., 2005; Brown et al., 2010; Hashizume et al., 2010), and several compounds targeting both pathways simultaneously are currently under development (Doppalapudi et al., 2010; Koh et al., 2010).
The A11 peptide was tested in the rat OIR model, in which ischaemic proliferative retinopathy develops as a result of tissue hypoxia, and is widely accepted as a model for retinal disorders, such as ROP, diabetic retinopathy and retinal vein occlusion (Pang and Clark, 2010). The OIR model has served as the testing ground for numerous anti-angiogenic agents and delivery methods. A11 demonstrated marked dose-dependent inhibition of retinal neovascularization in this model, outperforming the effect of sTie2-Fc, which was used as control. Furthermore, combination of the A11 peptide with an anti-VEGF antibody showed a trend towards enhanced reduction in neovascularization, compared with each treatment used alone.
The exact mode of action of A11 is not clear. Our binding studies revealed that A11 binds to all three human angiopoietins with similar affinities, and that it inhibits the binding of Ang-2 to the Tie2 receptor to a higher extent than the binding of Ang-1 and Ang-4, suggesting that it may exert its inhibitory activity primarily by preventing the interaction of Ang-2 with Tie2. In agreement with these results, A11 was shown to significantly inhibit Ang-2-induced HUVEC migration, but not migration stimulated by Ang-1. Signalling experiments in HUVECs did not provide a clear indication of an inhibitory effect by A11 on Ang-1-induced Tie2 phosphorylation or downstream signalling. Our findings, showing reduction of Tie2 levels on tumour microvessels following treatment with A11, suggest that the in vivo anti-angiogenic activity of this peptide may involve down-regulation of the Tie2 receptor on endothelial cells. The reduction in neovascularization and increased apoptosis observed with A11 is consistent with the anti-apoptotic effect of the Ang–Tie2 pathway (Kwak et al., 1999; Papapetropoulos et al., 1999).
Taken together, our findings indicate a therapeutic potential for the A11 peptide in diseases involving pathological neovascularization, such as retinopathy and tumour angiogenesis, and suggest that its mechanism of action involves targeting of the Ang–Tie2 pathway. Furthermore, the benefit of this compound might be even greater if used in combination with anti-VEGF or other anti-angiogenic agents. The therapeutic activity of A11 in additional animal models, including cancer and autoimmune diseases, should be further evaluated.
Conflicts of interest
Zohar Tiran, Yossef Kliger, Ofer Levy, Itamar Borukhov, and Galit Rotman are employees of Compugen Ltd. The studies carried out by Greg Palmer, Won Park and Mark Dewhirst, Megan E. Capozzi and John S. Penn and by Anastasia Pyriochou, Zongmin Zhou, Ciro Coletta and Andreas Papapetropoulos, were conducted under a contractual agreement with Compugen Ltd.
Glossary
- AMD
age-related macular degeneration
- Ang
angiopoietin
- CAM
chorioallantoic membrane
- HUVEC
human umbilical vein endothelial cells
- OIR
oxygen-induced retinopathy
- PDR
proliferative diabetic retinopathy
- ROP
retinopathy of prematurity
- SPR
surface plasmon resonance
References
- Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, et al. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233–240. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- Barnett JM, Yanni SE, Penn JS. The development of the rat model of retinopathy of prematurity. Doc Ophthalmol. 2010;120:3–12. doi: 10.1007/s10633-009-9180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop ET, Bell GT, Bloor S, Broom IJ, Hendry NF, Wheatley DN. An in vitro model of angiogenesis: basic features. Angiogenesis. 1999;3:335–344. doi: 10.1023/a:1026546219962. [DOI] [PubMed] [Google Scholar]
- Brindle NP, Saharinen P, Alitalo K. Signaling and functions of angiopoietin-1 in vascular protection. Circ Res. 2006;98:1014–1023. doi: 10.1161/01.RES.0000218275.54089.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JL, Cao ZA, Pinzon-Ortiz M, Kendrew J, Reimer C, Wen S, et al. A human monoclonal anti-ANG2 antibody leads to broad antitumor activity in combination with VEGF inhibitors and chemotherapy agents in preclinical models. Mol Cancer Ther. 2010;9:145–156. doi: 10.1158/1535-7163.MCT-09-0554. [DOI] [PubMed] [Google Scholar]
- Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, et al. COMP-Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci USA. 2004;101:5547–5552. doi: 10.1073/pnas.0307574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxon A, Bready J, Min H, Kaufman S, Leal J, Yu D, et al. Context-dependent role of angiopoietin-1 inhibition in the suppression of angiogenesis and tumor growth: implications for AMG 386, an angiopoietin-1/2-neutralizing peptibody. Mol Cancer Ther. 2010;9:2641–2651. doi: 10.1158/1535-7163.MCT-10-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Yancopoulos GD. The angiopoietins: Yin and Yang in angiogenesis. Curr Top Microbiol Immunol. 1999;237:173–185. doi: 10.1007/978-3-642-59953-8_9. [DOI] [PubMed] [Google Scholar]
- Davis S, Papadopoulos N, Aldrich TH, Maisonpierre PC, Huang T, Kovac L, et al. Angiopoietins have distinct modular domains essential for receptor binding, dimerization and superclustering. Nat Struct Biol. 2003;10:38–44. doi: 10.1038/nsb880. [DOI] [PubMed] [Google Scholar]
- Doppalapudi VR, Huang J, Liu D, Jin P, Liu B, Li L, et al. Chemical generation of bispecific antibodies. Proc Natl Acad Sci USA. 2010;107:22611–22616. doi: 10.1073/pnas.1016478108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp Cell Res. 2006;312:630–641. doi: 10.1016/j.yexcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Falcon BL, Hashizume H, Koumoutsakos P, Chou J, Bready JV, Coxon A, et al. Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am J Pathol. 2009;175:2159–2170. doi: 10.2353/ajpath.2009.090391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27:552–558. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- Gagliardi A, Hadd H, Collins DC. Inhibition of Angiogenesis by Suramin. Cancer Res. 1992;52:5073–5075. [PubMed] [Google Scholar]
- Giuliani N, Colla S, Lazzaretti M, Sala R, Roti G, Mancini C, et al. Proangiogenic properties of human myeloma cells: production of angiopoietin-1 and its potential relationship to myeloma-induced angiogenesis. Blood. 2003;102:638–645. doi: 10.1182/blood-2002-10-3257. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Lin J, Wagner P, Feng Y, Vom Hagen F, Krzizok T, et al. Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes. 2004;53:1104–1110. doi: 10.2337/diabetes.53.4.1104. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Feng Y, Pfister F, Brownlee M. Diabetic retinopathy: targeting vasoregression. Diabetes. 2011;60:9–16. doi: 10.2337/db10-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangai M, Moon YS, Kitaya N, Chan CK, Wu DY, Peters KG, et al. Systemically expressed soluble Tie2 inhibits intraocular neovascularization. Hum Gene Ther. 2001;12:1311–1321. doi: 10.1089/104303401750270968. [DOI] [PubMed] [Google Scholar]
- Hashizume H, Falcon BL, Kuroda T, Baluk P, Coxon A, Yu D, et al. Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer Res. 2010;70:2213–2223. doi: 10.1158/0008-5472.CAN-09-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst RS, Hong D, Chap L, Kurzrock R, Jackson E, Silverman JM, et al. Safety, pharmacokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. J Clin Oncol. 2009;27:3557–3565. doi: 10.1200/JCO.2008.19.6683. [DOI] [PubMed] [Google Scholar]
- Huang H, Bhat A, Woodnutt G, Lappe R. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer. 2010;10:575–585. doi: 10.1038/nrc2894. [DOI] [PubMed] [Google Scholar]
- Ingber D, Fujita T, Kishimoto S, Sudo K, Kanamaru T, Brem H, et al. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature. 1990;348:555–557. doi: 10.1038/348555a0. [DOI] [PubMed] [Google Scholar]
- Jendreyko N, Popkov M, Rader C, Barbas CF., 3rd Phenotypic knockout of VEGF-R2 and Tie-2 with an intradiabody reduces tumor growth and angiogenesis in vivo. Proc Natl Acad Sci USA. 2005;102:8293–8298. doi: 10.1073/pnas.0503168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PF, McClain J, Robinson DM, Sato TN, Yancopoulos GD. Identification and characterisation of chicken cDNAs encoding the endothelial cell-specific receptor tyrosine kinase Tie2 and its ligands, the angiopoietins. Angiogenesis. 1998;2:357–364. doi: 10.1023/a:1009251004253. [DOI] [PubMed] [Google Scholar]
- Kim KT, Choi HH, Steinmetz MO, Maco B, Kammerer RA, Ahn SY, et al. Oligomerization and multimerization are critical for angiopoietin-1 to bind and phosphorylate Tie2. J Biol Chem. 2005;280:20126–20131. doi: 10.1074/jbc.M500292200. [DOI] [PubMed] [Google Scholar]
- Kim YM, Kim KE, Koh GY, Ho YS, Lee KJ. Hydrogen peroxide produced by angiopoietin-1 mediates angiogenesis. Cancer Res. 2006;66:6167–6174. doi: 10.1158/0008-5472.CAN-05-3640. [DOI] [PubMed] [Google Scholar]
- Kim HZ, Jung K, Kim HM, Cheng Y, Koh GY. A designed angiopoietin-2 variant, pentameric COMP-Ang2, strongly activates Tie2 receptor and stimulates angiogenesis. Biochim Biophys Acta. 2009;1793:772–780. doi: 10.1016/j.bbamcr.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Kliger Y, Levy O, Oren A, Ashkenazy H, Tiran Z, Novik A, et al. Peptides modulating conformational changes in secreted chaperones: from in silico design to preclinical proof of concept. Proc Natl Acad Sci USA. 2009;106:13797–13801. doi: 10.1073/pnas.0906514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch CJ. Measurement of absolute oxygen levels in cells and tissues using oxygen sensors and 2-nitroimidazole EF5. Methods Enzymol. 2002;352:3–31. doi: 10.1016/s0076-6879(02)52003-6. [DOI] [PubMed] [Google Scholar]
- Koch CJ, Evans SM, Lord EM. Oxygen dependence of cellular uptake of EF5 [2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl)acetamide] Br J Cancer. 1995;72:869–874. doi: 10.1038/bjc.1995.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh YJ, Kim HZ, Hwang SI, Lee JE, Oh N, Jung K, et al. Double antiangiogenic protein, DAAP, targeting VEGF-A and angiopoietins in tumor angiogenesis, metastasis, and vascular leakage. Cancer Cell. 2010;18:171–184. doi: 10.1016/j.ccr.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Kwak HJ, So JN, Lee SJ, Kim I, Koh GY. Angiopoietin-1 is an apoptosis survival factor for endothelial cells. FEBS Lett. 1999;448:249–253. doi: 10.1016/s0014-5793(99)00378-6. [DOI] [PubMed] [Google Scholar]
- Lee OH, Fueyo J, Xu J, Yung WK, Lemoine MG, Lang FF, et al. Sustained angiopoietin-2 expression disrupts vessel formation and inhibits glioma growth. Neoplasia. 2006;8:419–428. doi: 10.1593/neo.06109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liekens S, De Clercq E, Neyts J. Angiogenesis: regulators and clinical applications. Biochem Pharmacol. 2001;61:253–270. doi: 10.1016/s0006-2952(00)00529-3. [DOI] [PubMed] [Google Scholar]
- Lin P, Polverini P, Dewhirst M, Shan S, Rao PS, Peters K. Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie2 in pathologic vascular growth. J Clin Invest. 1997;100:2072–2078. doi: 10.1172/JCI119740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci USA. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Ohashi H, Takagi H, Koyama S, Oh H, Watanabe D, Antonetti DA, et al. Alterations in expression of angiopoietins and the Tie-2 receptor in the retina of streptozotocin induced diabetic rats. Mol Vis. 2004;10:608–617. [PubMed] [Google Scholar]
- Oliner J, Min H, Leal J, Yu D, Rao S, You E, et al. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell. 2004;6:507–516. doi: 10.1016/j.ccr.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Olsen MW, Ley CD, Junker N, Hansen AJ, Lund EL, Kristjansen PE. Angiopoietin-4 inhibits angiogenesis and reduces interstitial fluid pressure. Neoplasia. 2006;8:364–372. doi: 10.1593/neo.06127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima Y, Oshima S, Nambu H, Kachi S, Takahashi K, Umeda N, et al. Different effects of angiopoietin-2 in different vascular beds: new vessels are most sensitive. FASEB J. 2005;19:963–965. doi: 10.1096/fj.04-2209fje. [DOI] [PubMed] [Google Scholar]
- Otsu N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans Syst Man Cybern. 1979;9:62–66. [Google Scholar]
- Pang I, Clark A. Animal Models for Retinal Diseases. New York, NY: Humana Press; 2010. [Google Scholar]
- Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetropoulos A, Garcia-Cardena G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab Invest. 1999;79:213–223. [PubMed] [Google Scholar]
- Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O'Connor DS, Li F, et al. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275:9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27:331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister F, Feng Y, vom Hagen F, Hoffmann S, Molema G, Hillebrands JL, et al. Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes. 2008;57:2495–2502. doi: 10.2337/db08-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharinen P, Bry M, Alitalo K. How do angiopoietins Tie in with vascular endothelial growth factors? Curr Opin Hematol. 2010;17:198–205. doi: 10.1097/MOH.0b013e3283386673. [DOI] [PubMed] [Google Scholar]
- Singh N, Macnamara E, Rashid S, Ambati J, Kontos CD, Higgins E, et al. Systemic soluble Tie2 expression inhibits and regresses corneal neovascularization. Biochem Biophys Res Commun. 2005;332:194–199. doi: 10.1016/j.bbrc.2005.04.108. [DOI] [PubMed] [Google Scholar]
- Takagi H, Koyama S, Seike H, Oh H, Otani A, Matsumura M, et al. Potential role of the angiopoietin/tie2 system in ischemia-induced retinal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:393–402. doi: 10.1167/iovs.02-0276. [DOI] [PubMed] [Google Scholar]
- Thomas M, Augustin HG. The role of the Angiopoietins in vascular morphogenesis. Angiogenesis. 2009;12:125–137. doi: 10.1007/s10456-009-9147-3. [DOI] [PubMed] [Google Scholar]
- Tolentino MJ. Current molecular understanding and future treatment strategies for pathologic ocular neovascularization. Curr Mol Med. 2009;9:973–981. doi: 10.2174/156652409789712783. [DOI] [PubMed] [Google Scholar]
- Tournaire R, Simon MP, le Noble F, Eichmann A, England P, Pouyssegur J. A short synthetic peptide inhibits signal transduction, migration and angiogenesis mediated by Tie2 receptor. EMBO Rep. 2004;5:262–267. doi: 10.1038/sj.embor.7400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufan AC, Satiroglu-Tufan NL. The chick embryo chorioallantoic membrane as a model system for the study of tumor angiogenesis, invasion and development of anti-angiogenic agents. Curr Cancer Drug Targets. 2005;5:249–266. doi: 10.2174/1568009054064624. [DOI] [PubMed] [Google Scholar]
- Ward NL, Van Slyke P, Dumont DJ. Functional inhibition of secreted angiopoietin: a novel role for angiopoietin 1 in coronary vessel patterning. Biochem Biophys Res Commun. 2004;323:937–946. doi: 10.1016/j.bbrc.2004.08.185. [DOI] [PubMed] [Google Scholar]
- White RR, Shan S, Rusconi CP, Shetty G, Dewhirst MW, Kontos CD, et al. Inhibition of rat corneal angiogenesis by a nuclease-resistant RNA aptamer specific for angiopoietin-2. Proc Natl Acad Sci USA. 2003;100:5028–5033. doi: 10.1073/pnas.0831159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Li Z, Yao M, Wang H, Qu S, Chen X, et al. Identification and characterization of a novel peptide ligand of Tie2 for targeting gene therapy. Acta Biochim Biophys Sin (Shanghai) 2008;40:217–225. doi: 10.1111/j.1745-7270.2008.00389.x. [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Yanni SE, Barnett JM, Clark ML, Penn JS. The role of PGE2 receptor EP4 in pathologic ocular angiogenesis. Invest Ophthalmol Vis Sci. 2009;50:5479–5486. doi: 10.1167/iovs.09-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan HT, Khankin EV, Karumanchi SA, Parikh SM. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol. 2009;29:2011–2022. doi: 10.1128/MCB.01472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


