Abstract
Preliminary findings suggest that transcranial direct current stimulation (tDCS) can have antidepressant effects. We sought to test this further in a parallel-group, double-blind clinical trial with 40 patients with major depression, medication-free randomized into three groups of treatment: anodal tDCS of the left dorsolateral prefrontal cortex (active group – ‘DLPFC’); anodal tDCS of the occipital cortex (active control group – ‘occipital’) and sham tDCS (placebo control group – ‘sham’). tDCS was applied for 10 sessions during a 2-wk period. Mood was evaluated by a blinded rater using the Hamilton Depression Rating Scale (HDRS) and Beck Depression Inventory (BDI). The treatment was well tolerated with minimal side-effects that were distributed equally across all treatment groups. We found significantly larger reductions in depression scores after DLPFC tDCS [HDRS reduction of 40.4% (±25.8%)] compared to occipital [HDRS reduction of 21.3% (±12.9%)] and sham tDCS [HDRS reduction of 10.4% (±36.6%)]. The beneficial effects of tDCS in the DLPFC group persisted for 1 month after the end of treatment. Our findings support further investigation on the effects of this novel potential therapeutic approach – tDCS – for the treatment of major depression.
Keywords: Brain stimulation, electrical stimulation, major depression, prefrontal cortex, transcranial direct current stimulation
Introduction
Different neurostimulation techniques are considered for treatment of depression: electroconvulsive therapy (UK ECT Review Group, 2003), vagus nerve stimulation (Sackeim et al., 2001), deep brain stimulation (Mayberg et al., 2005), repetitive transcranial magnetic stimulation (rTMS) (Gershon et al., 2003; Herrmann and Ebmeier, 2006; Martin et al., 2003); or magnetic seizure therapy (Kosel et al., 2003). Despite mixed findings in the past (Lolas, 1977), recent preliminary data suggest that transcranial direct current stimulation (tDCS), another non-invasive method of brain stimulation, might be useful in depression if appropriate currents, electrode sizes, and montages are employed (Fregni et al., 2006b).
In tDCS, low-amplitude direct currents are injected into the brain via scalp electrodes (Nitsche and Paulus, 2000). As shown by a recent modelling study, a significant amount of electric current can reach the brain using appropriately large electrodes and suitable placements (Miranda et al., 2006). Recent studies with up-to-date parameters of stimulation have shown that tDCS is a powerful neuromodulation technique (Nitsche et al., 2003b). Compared with other brain stimulation techniques tDCS has the advantage of being non-invasive, simple and inexpensive, offers reliable sham conditions, and provides a true neuromodulatory intervention, whereby neuronal membrane resting potentials are shifted (Bindman et al., 1964). Thus responsivity of the targeted brain regions to afferent input or efferent demand is modified.
We report the findings of a phase II, parallel-group, sham controlled, double-blind clinical trial with 40 patients. We hypothesized that active stimulation of the anodal prefrontal cortex would result in a mood improvement superior to sham stimulation and active stimulation of the occipital cortex.
Methods
Study population
Forty patients with major depression participated in the study [28 females, mean age 49.4±7.4 yr (mean±s.d.)]. Diagnosis of unipolar major depressive disorder was confirmed by a licensed, senior clinical psychiatrist (S.P.R.) using the Structured Clinical Interview for DSM-IV Axis I Disorders. All patients were right-handed as assessed by the Edinburgh Handedness Inventory. In order to exclude the confounding effect of medications, patients were required to be off medications (antidepressants) for 2 months prior to the trial – indeed patients were untreated (we did not request antidepressant wash-out). Use of other psychotropic medications were allowed; however, only four patients were using benzodiazepines [two in the left dorsolateral prefrontal cortex (DLPFC), one in the occipital and one in the sham tDCS group]. Exclusion criteria were neurological disorders, any comorbid Axis I disorders, or substance abuse within 3 months of study participation. Furthermore, patients with major depression with psychotic features, bipolar disorder or Axis II disorders were excluded.
The study was performed in accordance with the Declaration of Helsinki (1964). Written, informed consent was obtained from all participants before inclusion in the study, which was approved by the local ethics committee and registered in the national ethics committee [SISNEP (National System of Ethics in Research) 0900.0.015.000-04]. The study was performed at the Psychiatric Institute of the University of Sao Paulo.
Study design
Following initial evaluation, patients were randomly assigned to receive active tDCS of the DLPFC, active tDCS of occipital cortex, or sham tDCS treatment in a 2:1:1 ratio. This randomization strategy (2:1:1) was chosen in order to have the same number of patients receiving active and control stimulation. Randomization was performed using the order of entrance in the study and a previous randomization list generated by computer. To determine the sample size, we used the results of our previous pilot study (Fregni et al., 2006b). In this study, after treatment, a mean reduction of Beck Depression Inventory (BDI) scores of 69.4% (±25.7) in the active group and 29.9% (±28.4) in the sham group was reported (we used the BDI as this scale is more effective in capturing the placebo effect, therefore it is a more conservative choice for sample size calculation). Considering a power of 90% and a critical alpha of 5%, 20 patients (10 in each group) were needed to detect group differences. As we decided to have another control condition, we added 20 additional patients (10 for active stimulation and 10 for active control). Therefore 40 patients were entered into the study. Finally the blind was broken at the end of the follow-up period and patients were then offered a standard treatment with antidepressants.
Outcome measures
Subjects were assessed at baseline and immediately, 15 d, and 30 d after the end of treatment. Rating scales included the 21-item Hamilton Depression Rating Scale (HDRS), which was the primary outcome measure, and the BDI that was the secondary outcome measure. Rating was performed by a trained experienced psychologist (M.L.M.) blinded to the patients’ treatment group assignment. Clinical response was defined as a ≥50% decrease in HDRS score from baseline, and remission was defined as an HDRS score of ≤7 (Frank et al., 1991).
Transcranial direct current stimulation
Direct current was transferred by a saline-soaked pair of surface sponge electrodes and delivered by a specially developed, battery-driven, constant current stimulator with a maximum output of 2 mA (for more technical details, please contact S. A. Boggio at: sboggio@colband.com.br). This device has a special feature that makes it particularly reliable for double-blind trials and was developed by our group as we had noted in our previous trials that patients attempted to look at the tDCS display during the stimulation and encountered situations in which we had to hide the device from patients receiving sham treatment. We therefore incorporated a switch in the back of the tDCS device that could be activated by the researcher to interrupt the electrical current while maintaining the display ‘on ’ and displaying the parameters of stimulation throughout the procedure. Electrode montage varied across treatment groups:
Active group – anodal tDCS over the left DLPFC (cathode electrode over the right supraorbital area – 21 patients).
Control groups (9 and 10 patients, respectively) – we decided to have two different types of control: active control – in which anodal tDCS was applied over the occipital cortex; and placebo control – in which sham tDCS was applied over the left DLPFC. We included an active control condition (occipital tDCS) in order to control for non-specific effects of tDCS (as occipital activity is not changed in patients with depression compared to healthy subjects) and also to test whether the reference electrode (right supraorbital area) was responsible for the effects of anodal stimulation of the left DLPFC.
For these three conditions, the reference electrode (in this case, the cathode electrode) was placed over the contralateral supraorbital area.
Electrode position was determined by the International 10/20 System for EEG Electrodes, such as that F3 corresponded to the DLPFC. For occipital stimulation, the anode electrode was placed in the midline and 2 cm above the inion. Patients were comfortably seated and awake during the stimulation period. Electrodes were placed on the scalp and fixed with two rubber bands. At the beginning of the session, the impedance was tested and if detected as excessively large, adjustment of the electrodes in respect to the contact and the degree of wetness was performed.
For the active conditions, patients received 2 mA tDCS for 20 min for 10 d (Monday–Friday on two consecutive weeks). These parameters of stimulation were chosen based on recent studies showing that 2 mA of stimulation induces a larger behavioural effect compared to 1 mA tDCS (Boggio et al., 2006; Iyer et al., 2005) and a study suggesting that effects of tDCS are cumulative (Fregni et al., 2006a). For sham stimulation, the stimulator was turned on for 30 s only (Gandiga et al., 2006) and current intensity was gradually increased (at the beginning of the session – ‘ramp up’) and decreased (at the end of the session – ‘ramp down’) to diminish its perception.
Data analysis
Analyses were performed with SAS statistical software (version 9.1, Cary, NC, USA). We used a mixed linear model to analyse mood changes throughout the trial. We modelled mood change (as indexed by HDRS) using the covariates of time, group and interaction term between group and time. In this model, we accounted for the repeated measures of time, modelling the covariance using the compound symmetry covariance structure that appeared to be the best fit to our data according to the log-likelihood test. In addition we used a model with two-part linear spline functions – thus allowing us to have different slopes over different time-points – as we predicted a-priori that patients would improve after 10 d of treatment and then maintain these effects; thus changing the slope significantly after 10 d of treatment. For post-hoc comparisons, a Bonferroni correction for multiple comparisons was performed. We also calculated the effect size of Cohen’s d between the active and sham tDCS treatment groups.
We repeated this analysis for our secondary outcome measure – the BDI. We also compared the number of responders (immediately after treatment) across the groups of treatment using the χ2 test. There were no dropouts, and the few missing data (<3% of the data) were considered at random.
We then performed correlation tests – using Pearson’s correlation – to evaluate whether there was a correlation between mood changes after stimulation of the left DLPFC vs. demographic and baseline clinical characteristics. Data are reported as mean and standard deviation (s.d.). Statistical significance refers to a two-tailed p value <0.05.
Results
The tDCS treatment was well tolerated by the patients. There were few, mild adverse events that were equally distributed across the three treatment groups (p= 0.95): mild transient headache (lasting <1 h) (in 14.2%, 11% and 10% of subjects in the DLPFC, occipital and sham tDCS groups, respectively), itching sensation on the site of stimulation (19.1%, 33.3% and 20%, respectively) and mild transient redness of skin at the site of stimulation (5%, 11.1% and 10%, respectively) – that lasted <40 min after the end of stimulation. There were no adverse effects related to application of tDCS during the follow-up period. Demographic characteristics were not significantly different across the treatment groups as detailed in Table 1. As previously mentioned, patients were not taking antidepressants.
Table 1.
Demographic and clinical characteristics
| Active DLPFC tDCS | Active occipital tDCS | Sham tDCS | p valueb | |
|---|---|---|---|---|
| n | 21 | 9 | 10 | |
| Age [yr (s.d.)] | 51.6 (7.7) | 46.3 (5.8) | 46.5 (7.1) | 0.25 |
| Gender (F/M) | 14/7 | 6/3 | 7/3 | 0.98 |
| Duration of disease [yr (s.d.)] | 7.1 (6.2) | 8.3 (7.6) | 6.4 (7.4) | 0.88 |
| Refractorinessa (s.d.) | 1.6 (1.2) | 1.7 (1.2) | 1.5 (1.5) | 0.89 |
| Baseline HDRS (s.d.) | 21.1 (4.4) | 21.6 (4.9) | 21.9 (4.8) | 0.89 |
tDCS, Transcranial direct current stimulation; DLPFC, dorsolateral prefrontal cortex; HDRS, Hamilton Depression Rating Scale; s.d., standard deviation.
As indexed by the number of failed antidepressants.
p value: one-way ANOVA for continuous variables and Fisher’s exact test for categorical variables.
For our primary outcome (HDRS), the mixed model revealed a significant main effect of group (F2,111=5.2, p=0.01), time (F3,111=12.0, p<0.0001) and significant interaction term group vs. time (F6,111=2.6, p=0.02). When comparing the effects of tDCS on mood immediately after the end of treatment, there was a significant difference between DLPFC and sham groups (p=0.0018), and between DLPFC and occipital groups (p=0.009), but not between occipital and sham groups (p=0.6). In addition the effects of this treatment lasted for 30 d after the end of treatment as shown by a significant difference between sham and DLPFC at the follow-up assessment (p=0.04) (Figure 1; see also Supplementary Table 1 – available in the online version of the paper).
Figure 1.
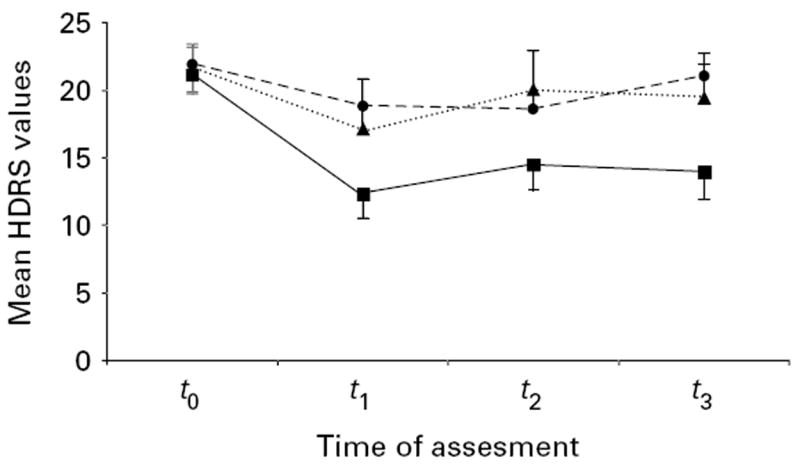
Plots showing mood scores changes [as indexed by the Hamilton Depression Rating Scale (HDRS)] over time (t0, baseline; t1, immediately after treatment; t2, 15 d after end of treatment; t3, 30 d after the end of treatment). Each data-point represents HDRS mean score; error bars indicate s.e.m. –■–, DLPFC tDCS; ····▲····, occipital tDCS; - -●- -, sham tDCS.
Our model using spline transformation showed a significant interaction term (F2,114=4.87, p=0.009) up to the first evaluation (immediately after treatment) but not for the follow-up – 15 d and 30 d after treatment (F2,114=0.04, p=0.95); suggesting that after the 10 d treatment, the three groups had a similar performance (i.e. no further improvement or worsening over time. Indeed this model explained the data better than the linear model that used time as a continuous variable [Akaike Information Criterion (AIC) for the model with spline function=951.0 and for the linear model=998.3].
The BDI scores revealed similar results: a significant interaction term (F6,111=4.2, p=0.0007) and significant difference between DLPFC and sham groups immediately and 30 d after treatment (p=0.0045 and p=0.03, respectively) (Figure 2) [see Supplementary Table 2 (available online) for further details]. The effect size of Cohen’s d when comparing the DLPFC and sham groups immediately after treatment, as indexed by HDRS, was 1.11.
Figure 2.
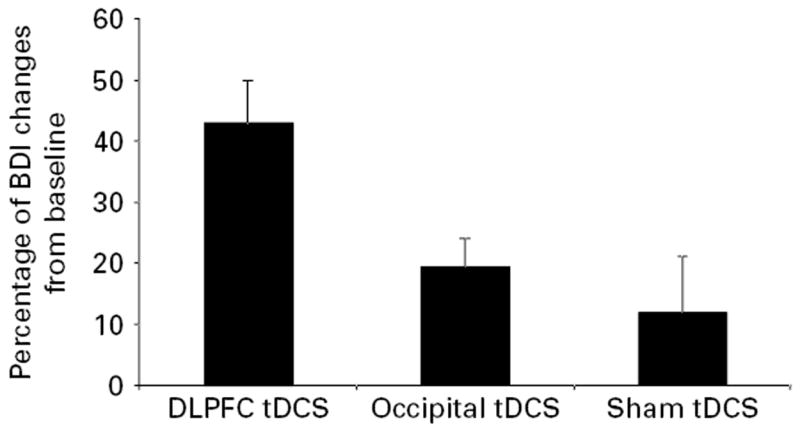
Depression scores changes immediately after 10 d of stimulation compared with baseline in the three groups of treatment. Each column represents mean Hamilton Depression Rating Scale (HDRS) score reduction; error bars indicate s.e.m.
There was a significantly greater number of responders after DLPFC tDCS (8 responders) compared with sham and occipital tDCS (2 and 0 responders, respectively) (χ21=5.43, p=0.019). In addition, there were five remissions in the DLPFC group and no remissions in the other two controls group (χ21=5.17, p=0.02).
There were no significant correlations between mood improvement as indexed by HDRS and clinical variables (baseline HDRS and duration of disease) or demographic characteristics (age and, gender).
Discussion
The results of this study show that cortical stimulation with tDCS is associated with a significant reduction in depression scores that is specific to the site of stimulation and lasts for at least 30 d after the end of treatment. Furthermore, 10 d of treatment induced only minor adverse effects during the treatment period and follow-up similarly distributed in the three groups of stimulation.
tDCS might offer some advantages compared with rTMS as a relatively safe, non-expensive and easy-to-administer technique of non-invasive brain stimulation (for review see Nitsche et al., 2003b). Besides the fact that we observed only mild adverse effects; in other studies, we showed that tDCS of the prefrontal cortex is not associated with cognitive impairment (Fregni et al., 2006c) but, on the contrary, leads to an improvement of performance in a go/no-go task (Boggio et al., In Press). In addition, stimulation of the occipital cortex does not result in performance worsening of the go/no-go task (Boggio et al., In Press). The intrinsic limitations in focality of tDCS (given the stimulation area roughly corresponds to the size of the electrode that has 35 cm2) make the precise placement of the electrodes less critical than the demands on targeting with a focal TMS coil. The portability of the device and the absence of associated sensations such as pain and scalp sensations, make it particularly well suited for ambulatory treatment in combination with behavioural or other therapies that might amplify the effects of the stimulation.
The mechanisms of tDCS in alleviating depression might be related to a modulation of the activity in the DLPFC. It has been shown that tDCS changes cortical excitability by modulating the membrane resting potential especially during the period of stimulation – and also by modifying synaptic transmission (long-term potentiation and depression-like mechanisms) according to pharmacological studies (Liebetanz et al., 2002; Nitsche et al., 2003a). Therefore, it is reasonable to assume that DLPFC tDCS might have induced a change in the DLPFC activity, a critical area in the cortico-subcortical, mood-related neural network.
Because tDCS is a technique that modulates brain activity in two areas (under the anode and cathode electrode), one may argue that cathodal stimulation of the right supraorbital area might be responsible for the effects of anodal tDCS of the left DLPFC. However, this alternative explanation is unlikely as anodal stimulation of the occipital cortex, which used the same electrode reference (cathodal over the supraorbital area), did not result in a significant change in depression scores compared with sham tDCS. Although a decreased placebo effect and increased response in the active treatment group due to a blind break is possible, this alternative explanation is unlikely for several reasons. First, tDCS with a stimulation protocol comparable with the one used in the current study is a reliable tool for sham-controlled experiments as shown by a recent study (Gandiga et al., 2006). Second, we included an active control; therefore any potential blind break would have been captured by this group. Third, because subjects were naive to tDCS and patients received only active or sham tDCS, any potential differences in the sensation associated with stimulation would not be detected by patients. Finally, although we consider that a blind break is improbable, we did not assess it directly and therefore this should be viewed as a limitation of the present study.
The positive results of this initial study have some implications for the prospect of this technique as a potential treatment for major depression. First, future studies need to explore different parameters of stimulation in order to optimize the clinical effects of this treatment; including also the combination of this treatment with cognitive behavioural therapy and, perhaps, antidepressants. Second, larger studies and studies in different populations are needed to confirm the results of our initial findings. Third, further studies investigating higher dosages of tDCS are warranted; however, safety, in this case, still needs to be assessed.
Acknowledgments
This work was supported by a research grant from FAPESP. F.F. is supported by grants from NIH (DK071851-01) and the Harvard University David Rockefeller Center – Jorge Paulo Lemann Fellowship. A.P.-L. is supported by NIH grant K24 RR018875. The authors are grateful to Marco Antonio Marcolin for administrative support and Barbara Bonetti for invaluable help in the coordination of this study.
Footnotes
Note Supplementary information accompanies this paper on the Journal’s website (http://journals.cambridge.org).
Statement of Interest
None.
References
- Bindman LJ, Lippold OC, Redfearn JW. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. Journal of Physiology. 1964;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Bermpohl F, Vergara AO, Muniz AL, Nahas FH, Leme PB, Rigonatti SP, Fregni F. Go-no-go task performance improvement after anodal transcranial DC stimulation of the left dorsolateral prefrontal cortex in major depression. Journal of Affective Disorders. doi: 10.1016/j.jad.2006.10.026. In Press. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual-Leone A, Fregni F. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. Journal of Neurological Science. 2006;249:31–38. doi: 10.1016/j.jns.2006.05.062. [DOI] [PubMed] [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Archives of General Psychiatry. 1991;48:851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Lima MC, Ferreira MJ, Wagner T, Rigonatti SP, Castro AW, Souza DR, Riberto M, Freedman SD, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006a;122:197–209. doi: 10.1016/j.pain.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Nitsche MA, Marcolin MA, Rigonatti SP, Pascual-Leone A. Treatment of major depression with transcranial direct current stimulation. Bipolar Disorders. 2006b;8:203–204. doi: 10.1111/j.1399-5618.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Nitsche MA, Rigonatti SP, Pascual-Leone A. Cognitive effects of repeated sessions of transcranial direct current stimulation in patients with depression. Depression & Anxiety. 2006c;23:482–484. doi: 10.1002/da.20201. [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clinical Neurophysiology. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. American Journal of Psychiatry. 2003;160:835–845. doi: 10.1176/appi.ajp.160.5.835. [DOI] [PubMed] [Google Scholar]
- Herrmann LL, Ebmeier KP. Factors modifying the efficacy of transcranial magnetic stimulation in the treatment of depression: a review. Journal of Clinical Psychiatry. 2006;67:1870–1876. doi: 10.4088/jcp.v67n1206. [DOI] [PubMed] [Google Scholar]
- Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64:872–875. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- Kosel M, Frick C, Lisanby SH, Fisch HU, Schlaepfer TE. Magnetic seizure therapy improves mood in refractory major depression. Neuropsychopharmacology. 2003;28:2045–2048. doi: 10.1038/sj.npp.1300293. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- Lolas F. Brain polarization: behavioral and therapeutic effects. Biological Psychiatry. 1977;12:37–47. [PubMed] [Google Scholar]
- Martin JL, Barbanoj MJ, Schlaepfer TE, Thompson E, Perez V, Kulisevsky J. Repetitive transcranial magnetic stimulation for the treatment of depression. Systematic review and meta-analysis British Journal of Psychiatry. 2003;182:480–491. doi: 10.1192/bjp.182.6.480. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Miranda PC, Lomarev M, Hallett M. Modeling the current distribution during transcranial direct current stimulation. Clinical Neurophysiology. 2006;117:1623–1629. doi: 10.1016/j.clinph.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. Journal of Physiology. 2003a;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W. Modulation of cortical excitability by weak direct current stimulation – technical, safety and functional aspects. Clinical Neurophysiology. 2003b;56(Suppl):255–276. doi: 10.1016/s1567-424x(09)70230-2. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackeim HA, Rush AJ, George MS, Marangell LB, Husain MM, Nahas Z, Johnson CR, Seidman S, Giller C, Haines S, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25:713–728. doi: 10.1016/S0893-133X(01)00271-8. [DOI] [PubMed] [Google Scholar]
- UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361:799–808. doi: 10.1016/S0140-6736(03)12705-5. [DOI] [PubMed] [Google Scholar]


