Abstract
Background
Helicobacter pylori infection induces a biased T helper type 1 (Th1) response that produces IFN-γ and Fas ligand (FasL). Th1 cytokines are associated with apoptosis in the gastric epithelial cells.
Aim
We aimed to define the role of the recently cloned IL-18, a IFN-γ inducing factor, in gastric mucosal injury induced by H. pylori infection.
Methods
Twenty-seven gastric ulcer (GU) patients and 20 functional dyspepsia (FD) patients were enrolled in this study. Mucosal biopsy samples were obtained from the gastric antrum and GU site during endoscopy. Samples were used for histological examination, H. pylori culture and in-situ stimulation for 48 h in the presence of 10 µg/ml phytohemagglutinin-P. IL-18, IFN-γ, and soluble FasL (sFasL) levels in culture supernatants were assayed by the enzyme-linked immunosorbent assay method. IL-18, IL-1β-converting enzyme (ICE) and caspase-3 were evaluated by western blotting in gastric cancer cell lines (MKN45) cocultured with H. pylori.
Results
All 27 GU patients and ten out of 20 FD patients were found to be H. pylori-positive, whereas ten FD patients were H. pylori-negative. Antral mucosal tissues from H. pylori-positive FD patients contained (P < 0.01) higher levels of IL-18, IFN-γ, and sFasL than those from uninfected FD patients. IL-18, IFN-γ, and sFasL levels at the ulcer site were significantly (P < 0.01) higher than those at distant sites in the antrum. A significant relationship was seen between IL-18 and IFN-γ levels at the ulcer site (r = 0.7, P < 0.01). H. pylori eradication led to a significant decrease in the levels of IL-18, IFN-γ, and sFasL at the ulcer site. Western blotting showed that IL-18, ICE, and caspase-3 were activated in gastric cancer cell lines cocultured with H. pylori.
Conclusion
This study suggests that H. pylori infection enhanced mucosal injury by stimulating a Th1 response, which was mediated by IL-18 upregulation as well as activation of ICE and caspase-3.
Keywords: caspase, cytotoxicity, gastric ulcer, Helicobacter pylori, IL-18
Introduction
Helicobacter pylori, a microaerophilic, gram-negative, spiral-shaped bacterium that colonizes the stomach, was first reported by Warren and Marshall in 1984 [1]. H. pylori is involved in the pathogenesis of chronic active gastritis as well as duodenal ulceration, gastric ulceration (GU), and gastric cancer [2]. The gastric mucosal inflammatory response induced by this bacterium is usually chronic and lifelong [3]. H. pylori induces release of chemoattractants from gastric epithelial cells in vitro [4], and persistent colonization results in the release of mucosal chemotactic factors that attract neutrophils and mononuclear cells in the stomach [5]. Recent evidence indicates that T cells are increased in H. pylori-infected gastric mucosa and these gastric mucosal T cells phenotypically resemble T helper type 1 (Th1) cells [6]. The presence of Th1 cells mediates subsequent tissue damage through direct cytotoxic activity or by producing Th1 cytokines, such as IFN-γ and TNF-α [7,8].
IL-18 was first described in 1989 as IFN-γ-inducing factor, which was produced during endotoxemia in mice preconditioned with an earlier infection of Propioni-bacterium acnes and subsequently challenged with lipopolysaccharide [9]. IL-18, which cooperates with IL-12 in the regulation of Th1 cytokine responses is known to be expressed in intestinal epithelial cells and thus may be important in the differentiation of effector Tcells toward a Th1 biased cytokine response pattern [10–12]. IL-18 has structural similarities with the IL-1 family of proteins [10]. Gene expression and/or protein secretion of IL-18 have been observed in macrophages, dendritic cells, mononuclear cells, keratinocytes, osteoblast cells, pituitary gland and adrenal cortical cells, astrocytes and microglia, and intestinal epithelial cells [13–17]. It has also been demonstrated that both precursor and mature forms of IL-18 exist [14]. IL-1β-converting enzyme (ICE), which is also called caspase-1, was originally identified as the protease responsible for the production of active IL-1b, a multifunctional cytokine that affects nearly every cell type and plays a central role in inflammation and associated pathological conditions [18,19]. ICE is an intracellular cysteine protease that is synthesized as a precursor protein of 45 kDa and which is cleaved to an intermediate and then to p20 and p10 forms, which are the biologically active forms of the enzyme [20]. Activated ICE also cleaves the IL-18 precursor (pro-IL-18) into the mature form, and only mature IL-18 is bioactive [20].
The major activity associated with IL-18 is induction of IFN-γ production from Th1 cells and natural killer cells, especially in the presence of IL-12, and enhancement of their cytotoxicity through the Fas ligand-mediated mechanism [21]. It has been suggested that IL-18 may promote a Th1 type response and play a role in the induction of an ineffective immune response against H. pylori. We reported earlier that Th1 cytokines associated with apoptosis in gastric epithelial cells, and Fas Ligand (FasL) and IFN-γ are involved in ulcerogenesis in patients with H. pylori-positive GU [22]. We hypothesized that IL-18 secreted at the ulcer site might play a role in ulcerogenesis through promotion of a Th1-type response. In this study, we examined the secretion of IL-18 and IFN-γ using biopsy specimens from ulcer and nonulcer sites in 27 GU patients with H. pylori infection.
Materials and methods
Study groups and mucosal biopsy samples
Mucosal biopsy samples were obtained from the gastric antrum and ulcer site in 27 patients with GU (17 male and 10 female, 53.1 ± 10.2 years old) and 20 with functional dyspepsia (FD) (11 male and nine female, 52.2 ± 7.3 years old), whose conditions were diagnosed during upper gastroduodenal endoscopic examination at Nagoya University Hospital from October 2000 to January 2002 (Table 1). Among the 27 GU patients, 14 had an active open ulcer and 13 had ulcer scarring. None of the patients took proton pump inhibitors, NSAIDs, antibiotics or bismuth compounds during the preceding 3 months. Six antral specimens were taken from adjacent areas of endoscopically intact mucosa in the GU and FD patients, with one for bacterial culture, and rapid urease test (CLO test; Delta West, Bentley, Australia) and histological examination, and three for in-vitro organ cultures for IL-18, soluble FasL (sFasL), and IFN-γ measurement. Four specimens were taken from the margin of the open ulcer or the center of the ulcer scar in GU patients, one for histological examination and three for IL-18, sFasL, and IFN-γ quantification. All samples were obtained with informed consent in accordance with the Helsinki Declaration and the study was approved by our institution’s ethics committee. H. pylori infection was confirmed by positive results for at least two out of four diagnostic methods: bacterial culture, rapid urease test, [13C]-urea breath test or identification of the organism on tissue sections (Giemsa stain). The absence of infection was defined by a negative result in all four tests. Eight GU patients with H. pylori infection were treated with a triple therapy regimen of omeprazole 20mg, amoxicillin 750 mg, and clarithromycin 200 mg (all b.i.d.), and H. pylori eradication was evaluated at 1 year after treatment.
Table 1.
Mucosal cytokine activities at gastric antrum of 20 FD patients according to H. pylori status
| H. pylori status | |||
|---|---|---|---|
| Cytokine (pg/mg protein) |
H. pylori-negative (n = 10) (median, range) |
H. pylori-positive (n = 10) (median, range) |
P value |
| IL-18 (median, range) | 2.5; 0.2–8.8 | 12.3; 2.7–29.5 | P< 0.01 |
| IFN-γ (median, range) | 4.2; 0.1–19.1 | 7.2; 0.2–292.2 | P< 0.01 |
| sFasL (median, range) | 11.8; 0.2–43.1 | 54.9; 8.2–318.5 | P< 0.01 |
FD, functional dyspepsia; H. pylori, Helicobacter pylori; sFasL, soluble Fas ligand.
Organ culture
Mucosal biopsy tissues were weighed and cultured in a 5% CO2 incubator for 48 h on a culture insert (Falcon, Oxnard, California, USA) placed over polystyrene plates (Falcon) containing RPMI 1640 (Life Technologies, Inc., Rockville, Maryland, USA) medium with 5% heat-inactivated fetal calf serum, 15mmol/l N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer, 100 U/ml of penicillin-G, and 100 µg/ml of streptomycin (culture medium) under the conditions of 10 µg/ml of phytohemagglutinin-P (Sigma Chemical Co., St. Louis, Missouri, USA). At the end of the culture period, the supernatants were collected and kept at −70°C until assayed for IL-18, sFasL, and IFN-γ levels. Total protein in biopsy homogenates was assayed by a modification of the Lowry method.
Production of IL-18 by MKN45 cells treated with IFN-γ or Helicobacter pylori
MKN45 human gastric epithelial cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and gentamicin 20 µg/ml in an atmosphere of 5% CO2 at 37°C. For coculture experiments, H. pylori was grown in Brucella broth with 5% FBS for 48 h. MKN45 cells, 1 × 106, were seeded in 7 ml volumes of medium without antibiotics in tissue culture dish (Falcon 3003) and IFN-γ (Boehringer Manheim, Germany) was added (500 units/ml) and cells were then incubated for another 16 h. H. pylori was added to MKN45 cells at a bacteria/cell ratio of 1000 : 1, and after 24 h, the supernatants were harvested from the well and kept at −70°C until the assay. Experiments were performed in antibiotic-free medium with 10% FBS using T-150 flasks (Corning Coster, Cambridge, Mississippi, USA.) or six-well polypropylene tissue culture plates (Becton Dickinson, Franklin Lakes, New Jersey, USA).
IL-18, soluble Fas ligand, and IFN-γ assay
Levels of IL-18, sFasL, and IFN-γ in the samples were measured in duplicate using the enzyme-linked immunosorbent assay kits specific for IL-18 (Medical Biology Laboratories Co., Nagoya, Japan), sFasL, and IFN-γ (Toray-Fuji Bionics Inc., Tokyo, Japan). The amount of IL-18, sFasL, and IFN-γ in the organ cultures was expressed relative to protein content in the homogenate of biopsy tissues (picogram per milligram biopsy protein).
Western blot analysis
Cell extracts were prepared by lysing 1 × 107 MKN45 cells in 500 00B5l of 1 × cell culture lysis reagent (Promega, Madison, Wisconsin, USA). Lysates were centrifuged for 10 min at 1000 ×g to remove cellular debris, and supernatants were harvested. After the amount of protein was quantified by the Bradford assay, the samples were fractionated (25–100 µg of protein/lane) by 15% SDS-polyacrylamide gel electrophoresis, transferred to poly-vinylidene difluoride membrane (Millipore, Bedford, Massachusetts, USA), and incubated for 1 h in blocking buffer (phosphate-buffered saline containing 5% milk powder and 0.05% tween 20). Blots were incubated for 1 h with anti-IL-18 antibody (MBL), anti-ICE (Upstate biotechnology, Lake Placid, New York, USA), or anti-caspase-3 (Pharmingen) in blocking buffer followed by a 1 h-incubation with anti-mouse or anti-rabbit immunoglobulin-peroxidase conjugate (Biosource international, Camarillo, California, USA) (1 : 2000) and then developed using the ECL method (Amersham Pharmacia Biotech, Buckinghamshire, UK).
Assessment of Helicobacter pylori cagA and vacA status
A chloroform-phenol extraction method was used to obtain DNA from the H. pylori isolates as described earlier [23]. cagA and vacA status was assessed by reverse hybridization using a line probe assay [24].
Statistical analyses
Analysis of paired observations was performed using a paired t test for normally distributed data. Linear regression analysis was done to examine the correlation using the Spearman rank correlation coefficient. A P value of less than 0.05 was considered statistically significant.
Results
Cytokine levels in organ culture
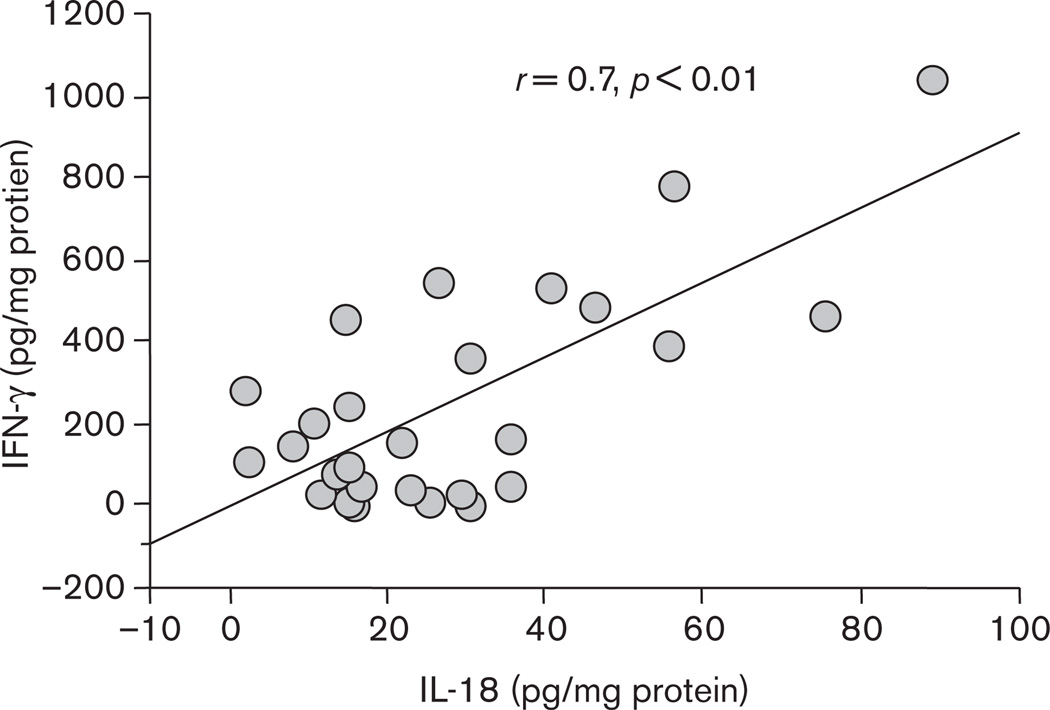
All 27 GU patients and 10 out of 20 FD patients were found to be H. pylori-positive, whereas 10 FD patients were H. pylori-negative. All 37 H. pylori strains from both GU and FD patients were cagA-positive and vacAs1/m1. Antral mucosal tissues from H. pylori-positive FD patients contained (P < 0.01) higher levels of IL-18, IFN-γ, and sFasL than those from uninfected FD patients, respectively (Table 1). The supernatants of organ cultures of gastric mucosal tissues from the edge of ulceration or ulcer scar site showed significantly (P < 0.01) higher levels of IL-18, IFN-γ, and sFasL compared with those from the uninvolved antral mucosa, respectively (Table 2). No significant difference in IL-18, IFN-γ, and sFasL levels in the supernatants of antral gastric tissue between GU and H. pylori-positive FD patients was observed. With regard to ulcer stage in GU patients, there was no significant difference in IL-18, IFN-γ, and sFasL levels between samples from open ulcers and ulcer scars, respectively (data not shown). A positive correlation between levels of IL-18 and IFN-γ levels (r = 0.7, P < 0.01) at the edge of ulceration or ulcer scar site (Fig. 1) was present. Eight patients were treated with triple therapy for H. pylori infection, and all were confirmed to be successfully eradicated 1 year after treatment. H. pylori eradication resulted in a significant decrease in the levels of IL-18, IFN-γ, and sFasL at the ulcer site, respectively (Table 3).
Table 2.
Mucosal cytokine activities of 27 GU patients according to the biopsy sites
| Biopsy site | |||
|---|---|---|---|
| Cytokine (pg/mg protein) |
Ulcer (median, range) |
Antrum (median, range) |
P value |
| IL-18 (median, range) | 22.4; 2.4–88.9 | 12.4; 0.8–41.5 | P< 0.01 |
| IFN-γ (median, range) | 49.1; 0.5–1049.1 | 5.8; 0.7–377.4 | P< 0.01 |
| sFasL (median, range) | 258.3; 3.5–1173.1 | 53.7; 2.1–508.7 | P< 0.01 |
GU, gastric ulcer; sFasL, soluble Fas ligand.
Fig. 1.
Relationship between IL-18 and IFN-γ levels in biopsy culture supernatants from the ulcer site. A significant positive correlation was seen between IL-18 and IFN-γ amounts at ulcer site (r = 0.7, P<0.01).
Table 3.
The influence of H. pylori eradication on mucosal cytokine levels of eight GU patients
| Before or after eradication therapy | |||
|---|---|---|---|
| Cytokine (pg/mg protein) |
Before (median, range) |
1 year after (median, range) |
P value |
| IL-18 (median, range) | 32.5; 2.8–78.9 | 2.5; 0.1–11.2 | P< 0.01 |
| IFN-γ (median, range) | 52.1; 1.5–998.5 | 3.9; 0.1–27.5 | P< 0.01 |
| sFasL (median, range) | 275.7; 10.8–1058.2 | 13.2; 0.3–59.2 | P< 0.01 |
GU, gastric ulcer; H. pylori, Helicobacter pylori; sFasL, soluble Fas ligand.
IL-18 protein expression levels in MKN45 cells
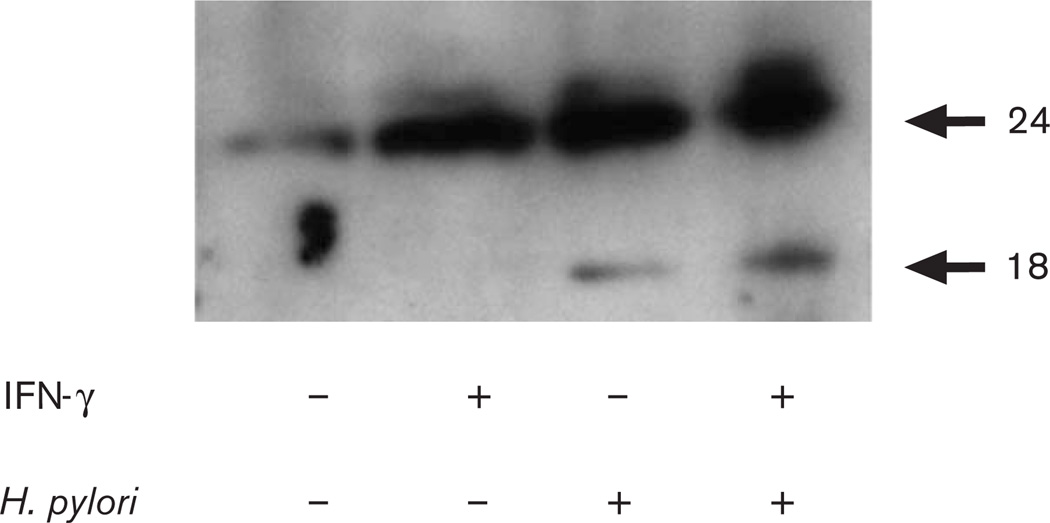
We next evaluated IL-18 protein expression levels in MKN45 cells after H. pylori infection to study mechanisms by which H. pylori induces IL-18 release. MKN45 cells produced IL-18 protein after H. pylori infection but this was augmented in cells pretreated with IFN-γ, and the 18.3-kDa mature form of IL-18 appeared only in H. pylori infected epithelial cells (Fig. 2).
Fig. 2.
Western blot analysis for IL-18 production from MKN45 cells cocultured with IFN-γ, H. pylori strain 26695, or both. IFN-γ: −, not pretreated; +, pretreated H. pylori: −, without H. pylori; +, with H. pylori.
Expression of IL-18, IL-1β-converting enzyme, and caspase-3
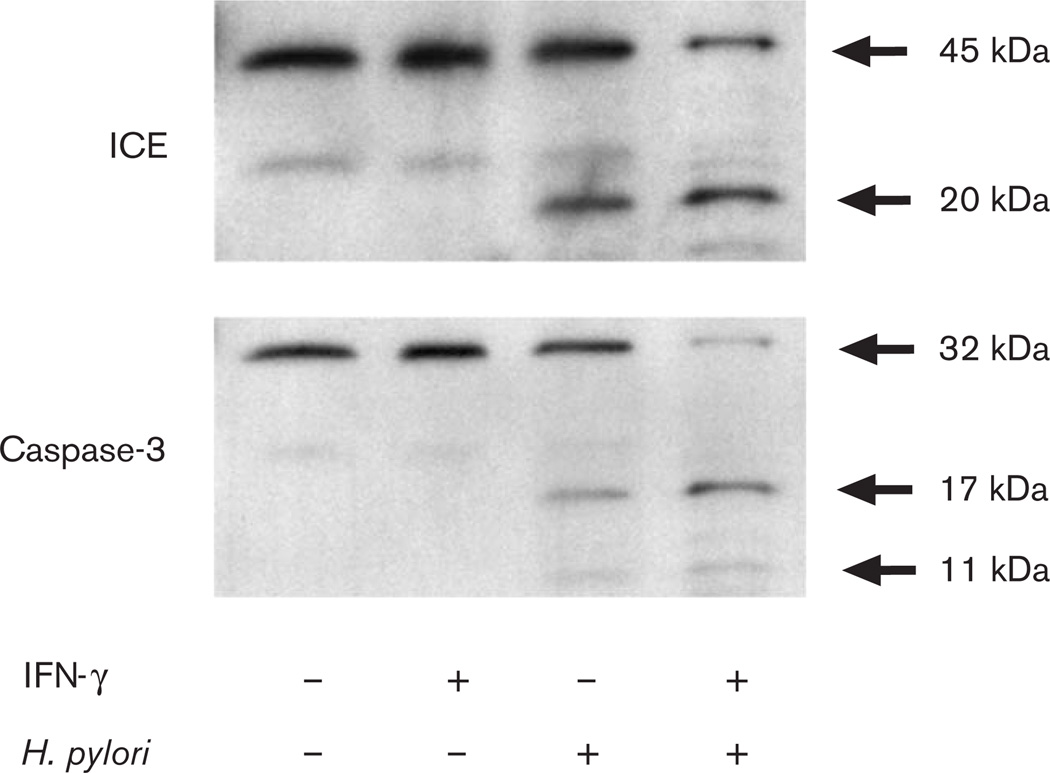
Earlier studies have shown that the active form of IL-18 is processed from the precursor protein through the proteolytic enzymatic cleavage by ICE. The activation of distal caspase-3 also executes cell death by proteolysis of important intracellular proteins, leading to DNA fragmentation. Therefore, we next examined the expression of IL-18 and caspases in MKN45 cells by western blotting. The active forms of IL-18, ICE, and caspase-3 were each detected after stimulation with H. pylori alone or with a combination of IFN-γ and H. pylori (Fig. 3).
Fig. 3.
Western blot analysis for IL-1β-converting enzyme (ICE) and caspase-3 production from MKN45 cells cocultured with IFN-γ, H pylori strain 26695, or both. IFN-γ: −, not pretreated; +, pretreated H. pylori: −, without H. pylori; +, with H. pylori.
IL-18 secretion from gastric epithelial cells
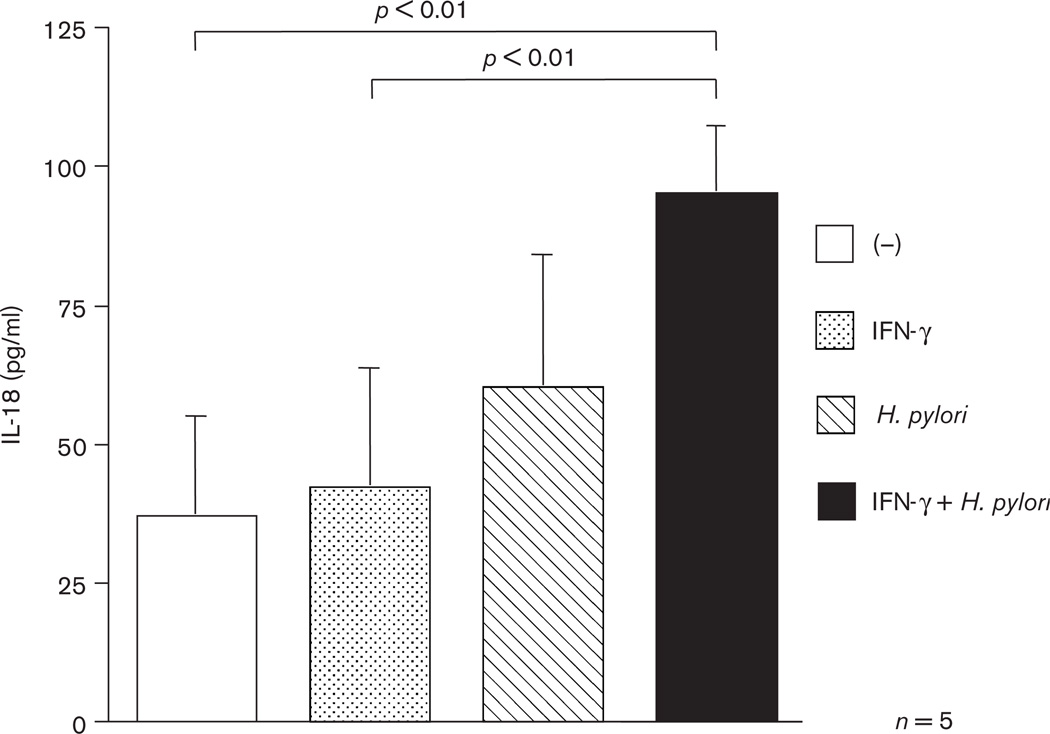
We further investigated whether H. pylori infection induces the secretion of IL-18 from epithelial cells by enzyme-linked immunosorbent assay. MKN45 cells with or without IFN-γ were infected with H. pylori and were cultured for 24 h. The levels of IL-18 secretion from MKN45 cells which were treated with both IFN-γ and H. pylori were significantly higher than nontreated or cells only treated with IFN-γ alone (P < 0.01) (Fig. 4).
Fig. 4.
IL-18 levels in culture supernatants of MKN45 cells cocultured with IFN-γ, H pylori strain 26695, or both. IFN-γ: −, not pretreated; +, pretreated H. pylori: −, without H. pylori; +, with H. pylori.
Induction of epithelial cell death
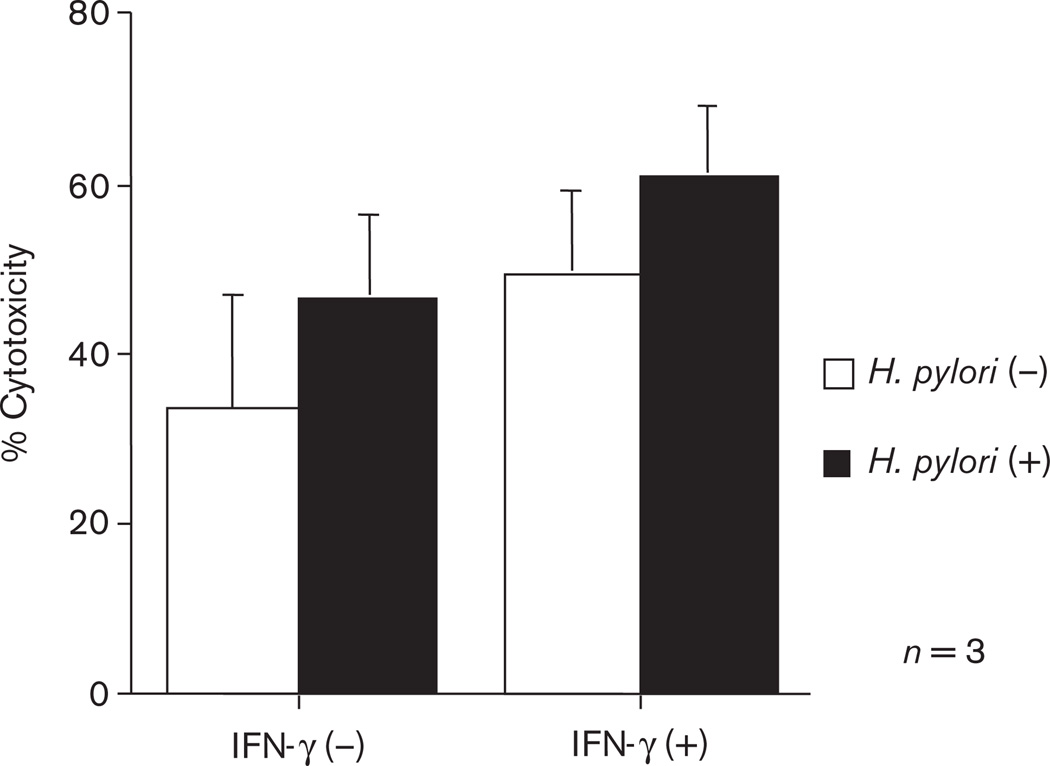
To investigate whether IL-18 could induce apoptosis in epithelial cells in vitro, we stimulated MKN45 with IFN-γ and/or H. pylori. The degree of apoptosis of MKN45 cells has significantly increased in the H. pylori-primed gastric epithelial cells, most notably with a combination of IFN-γ and H. pylori (Fig. 5).
Fig. 5.
Cytotoxicity of MKN45 cells cocultured with IFN-γ, H. pylori strain 26695, or both. IFN-γ: −, not pretreated; +, pretreated H. pylori: −, without H. pylori; +, with H. pylori.
Discussion
IL-18, IFN-γ, and sFasL levels were higher in H. pylori-positive patients than in H. pylori-negative patients, and were particularly increased at the ulcer site compared with those at distant sites in the antrum.
In H. pylori-positive GU patients, inflammatory cells infiltrate the gastric mucosa, particularly at sites of ulcer [25,26]. We took mucosal biopsies from the antral mucosa as well as the gastric site and compared them with IL-18, IFN-γ, and sFasL production. Given that GU is mainly associated with a corpus-predominant atrophic gastritis, a better understanding of the pathogenesis of atrophic gastritis and GU will require the conduct of studies of inflammatory phenomena in the corpus mucosa. Gastric mucosal epithelial cells secrete several kinds of cytokines in association with H. pylori infection, which may form cytokine networks between immunocompetent cells within the gastric mucosa [5]. Recently, the role of Tcells in the pathophysiology of gastric epithelial injury has gained attention, in which Th1 have been shown to dominate among T cells infiltrating in the gastric mucosa infected with H. pylori. Fan et al. [8] reported that H. pylori infection induces apoptosis of gastric epithelial cells, in which IFN-γ, a representative Th1 cytokine, is associated with cell death. In addition, there is accumulating evidence that IFN-γ enhances the expression of Fas on the epithelial cells, and mucosal T cells positive for FasL play a major role in the apoptosis of gastrointestinal epithelial cells [22]. Fas is a cell surface receptor molecule, and binding of which to FasL induces cellular apoptosis [27], suggesting that the Fas/FasL system may participate in the gastric mucosal injury associated with H. pylori infection.
IL-18, which was cloned by Okamura et al. in 1995 [28], has been shown to induce IFN-γ production by either T cells or natural killer cells, and to enhance either the expression or the function of FasL on lymphocytes. Tomita et al. [29] reported that IL-18 activity increased in gastric mucosa infected with H. pylori. We evaluated levels of local expressions of IL-18 and IFN-γ in the gastric mucosa in the patients with gastric ulcer, and found increased levels at the site of the ulcer, especially in the patients positive for H. pylori, and a decrease in cytokine levels after eradication of H. pylori. In our study, IFN-γ stimulates IL-18 protein production from MKN45 cells, which suggests that IFN-γ not only is stimulated by IL-18, but also stimulates IL-18 indicating that both IL-18 and IFN-γ produced by infiltrating T cells may participate in ulcerogenesis. Earlier studies have shown that there are the precursor and activated forms of IL-18, and that the 24 kD precursor form is processed by proteolytic cleavage by ICE to become biologically active 18 kD peptide [30]. We thus investigated the levels of IL-18 by western blotting, which revealed an increase in the levels of both precursor and activated forms of IL-18 in the cultured gastric epithelial cell line by the addition of H. pylori, the effect of which was augmented by pretreatment with IFN-γ. The active forms of IL-18, ICE, and caspase-3 were detected after stimulation with H. pylori alone or a combination of IFN-γ and H. pylori, suggesting that ICE is involved in the processing of the active form of IL-18 in these experimental conditions and that induction of apoptosis in gastric epithelial cells occurs through the enhancement of the expression of caspase-3.
Recently, caspase has been shown to be the key executing molecule of apoptosis [27]. We thus investigated the association of caspase in the cellular injury of cultured gastric mucosal cell line, and found that the expression of caspase-3 increased by the addition of H. pylori in the gastric epithelial cells, the effect of which was enhanced by the pretreatment with IFN-γ.
In this study, all 37 H. pylori strains from both GU and FD patients were cagA-positive and vacAs1/m1. Colonization by cagA-positive strains induces more intense cellular infiltration in the gastric mucosa and increases the risk of development of upper gastrointestinal diseases [30]. Here, we were unable to compare IL-18 production between the patients infected with cagA-positive H. pylori strains and those infected with cagA-negative strains. Further study should be carried out whether or not H. pylori virulence factor influences the production of IL-18 from gastric mucosa.
In summary, the results of this study suggest that gastric epithelial cells in association with H. pylori infection produce IL-18, and mucosal T cells, which are activated by activated form of IL-18, may be induced by IFN-γ production. In addition, our results suggest that apoptosis is induced because of the activation of caspase in the epithelial cells, which in turn associates with either the formation or recurrence of the gastric ulcer. Further studies focused on the interaction between gastric epithelial cells and immune cells are warranted to clarify the mechanism of mucosal injury associated with H. pylori infection.
Footnotes
Conflict of interest: none declared.
References
- 1.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Blaser MJ. Hypothesis: the changing relationships of Helicobacter pylori and humans: implications for health and disease. J Infect Dis. 1999;179:1523–1530. doi: 10.1086/314785. [DOI] [PubMed] [Google Scholar]
- 3.Israel DA, Peek RM. Pathogenesis of Helicobacter pylori-induced gastric inflammation. Aliment Pharmacol Ther. 2001;15:1271–1290. doi: 10.1046/j.1365-2036.2001.01052.x. [DOI] [PubMed] [Google Scholar]
- 4.Crabtree JE, Farmery SM, Lindley IJ, Figura N, Peichl P, Tompkins DS. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol. 1994;47:945–950. doi: 10.1136/jcp.47.10.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ando T, Kusugami K, Ohsuga M, Shinoda M, Sakakibara M, Saito H, et al. Interleukin-8 activity correlates with histological severity in Helicobacter pylori-associated antral gastritis. Am J Gastroenterol. 1996;91:1150–1156. [PubMed] [Google Scholar]
- 6.Bamford KB, Fan X, Crowe SE, Leary JF, Gourley WK, Luthra GK, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 7.Yasumoto K, Okamoto S, Mukaida N, Murakami S, Mai M, Matsushima K. Tumor necrosis factor alpha and interferon gamma synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-kB-like binding sites of the interleukin 8 gene. J Biol Chem. 1992;267:22506–22511. [PubMed] [Google Scholar]
- 8.Fan X, Crowe SE, Behar S, Gunasena H, Ye G, Haeberle H, et al. The effect of class II major histocompatibility complex expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for T helper cell type 1-mediated damage. J Exp Med. 1998;187:1659–1669. doi: 10.1084/jem.187.10.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura K, Okamura H, Wada M, Nagata K, Tamura T. Endotoxin-induced serum factor that stimulates gamma interferon production. Infect Immun. 1989;57:590–595. doi: 10.1128/iai.57.2.590-595.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinarello CA. IL-18: a TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol. 1999;103:11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- 11.Kanakaraj P, Ngo K, Wu Y, Angulo A, Ghazal P, Harris CA, et al. Defective interleukin (IL)-18-mediated natural killer and T helper cell type 1 responses in IL-1 receptor-associated kinase (IRAK)-deficient mice. J Exp Med. 1999;189:1129–1138. doi: 10.1084/jem.189.7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okamuto I, Kohno K, Tanimoto T, Ikegami H, Kurimoto M. Development of CD8+ effector T cells is differentially regulated by IL-18 and IL-12. J Immunol. 1999;162:3202–3211. [PubMed] [Google Scholar]
- 13.Stoll S, Muller G, Kurimoto M, Saloga J, Tanimoto T, Yamauchi H, et al. Production of IL-18 (IFN-gamma-inducing factor) messenger RNA and functional protein by murine keratinocytes. J Immunol. 1997;159:298–302. [PubMed] [Google Scholar]
- 14.Tone M, Thompson SA, Tone Y, Fairchild PJ, Waldmann H. Regulation of IL-18 (IFN-gamma-inducing factor) gene expression. J Immunol. 1997;159:6156–6163. [PubMed] [Google Scholar]
- 15.Puren AJ, Fantuzzi G, Gu Y, Su MS, Dinarello CA. Interleukin-18 (IFN gamma-inducing factor) induces IL-8 and IL-1beta via TNF alpha production from non-CD14 + human blood mononuclear cells. J Clin Invest. 1998;101:711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshimoto T, Nagai N, Ohkusu K, Ueda H, Okamura H, Nakanishi K. LPS-stimulated SJL macrophages produce IL-12 and IL-18 that inhibit IgE production in vitro by induction of IFN-gamma production from CD3intIL-2R beta + T cells. J Immunol. 1998;161:1483–1492. [PubMed] [Google Scholar]
- 17.Monteleone G, Trapasso F, Parrello T, Biancone L, Stella A, Iuliano R, et al. Bioactive IL-18 expression is up-regulated in Crohn’s disease. J Immunol. 1999;163:143–147. [PubMed] [Google Scholar]
- 18.Zuuner A, Eramo A, Peschle C, De Maria R. Caspase activation without death. Cell Death Differ. 1999;6:1075–1080. doi: 10.1038/sj.cdd.4400596. [DOI] [PubMed] [Google Scholar]
- 19.Fiorucci S, Santucci L, Antonelli E, Distrutti E, Del Sero G, Morelli O, et al. NO-aspirin protects from T cell-mediated liver injury by inhibiting caspase-dependent processing of Th1-like cytokines. Gastroenterology. 2000;118:404–421. doi: 10.1016/s0016-5085(00)70223-x. [DOI] [PubMed] [Google Scholar]
- 20.Lu H, Shen C, Brunham RC. Chlamydia trachomatis infection of epithelial cells induces the activation of caspase-1 and release of mature IL-18. J Immunol. 2000;165:1463–1469. doi: 10.4049/jimmunol.165.3.1463. [DOI] [PubMed] [Google Scholar]
- 21.Tsutsui H, Kayagaki N, Kuida K, Nakano H, Hayashi N, Takeda K, et al. Caspase-1-independent, Fas/Fas ligand-mediated IL-18 secretion from macrophages causes acute liver injury in mice. Immunity. 1999;11:359–367. doi: 10.1016/s1074-7613(00)80111-9. [DOI] [PubMed] [Google Scholar]
- 22.Shimada M, Ina K, Kyokane K, Imada A, Yamaguchi H, Nishio Y, et al. Upregulation of mucosal soluble fas ligand and interferon-gamma may be involved in ulcerogenesis in patients with Helicobacter pylori-positive gastric ulcer. Scand J Gastroenterol. 2002;37:501–511. doi: 10.1080/00365520252903026. [DOI] [PubMed] [Google Scholar]
- 23.Wilson K. Preparation of genomic DNA from bacteria. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current protocols in molecular biology 1. New York, USA: John Wiley; 1995. pp. 2.4.1–2.4.5. [Google Scholar]
- 24.Van Doorn LJ, Figueiredo C, Rossau R, Jannes G, van Asbroek M, Sousa JC, et al. Typing of Helicobacter pylori vacA gene and detection of cagA gene by PCR and reverse hybridization. J Clin Microbiol. 1998;36:1271–1276. doi: 10.1128/jcm.36.5.1271-1276.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ernst PB, Crowe SE, Reyes VE. How does Helicobacter pylori cause mucosal damage? The inflammatory response. Gastroenterology. 1997;113:S35–S42. doi: 10.1016/s0016-5085(97)80009-1. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu T, Kusugami K, Ina K, Imada A, Nishio Y, Hosokawa T, et al. Helicobacter pylori-associated gastric ulcer exhibits enhanced mucosal chemokine activity at the ulcer site. Digestion. 2000;62:87–94. doi: 10.1159/000007800. [DOI] [PubMed] [Google Scholar]
- 27.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 28.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 29.Tomita T, Jackson AM, Hida N, Hayat M, Dixon MF, Shimoyama T, et al. Expression of Interleukin-18, a Th1 cytokine, in human gastric mucosa is increased in Helicobacter pylori infection. J Infect Dis. 2001;183:620–627. doi: 10.1086/318541. [DOI] [PubMed] [Google Scholar]
- 30.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, et al. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]