Abstract
Injecting mice with killed cells of non-capsulated strain RM200 adsorbed on Al(OH)3 (pneumococcal whole-cell vaccine; WCV) reduces nasopharyngeal colonization by capsular serotype 6B and prevents fatal aspiration pneumonia by serotype 3 or serotype 5 strains. To further examine the potential for omni-strain immunity, we here examined a panel of clinical isolates and a library of capsule-switch variants in the TIGR4 background. IgG binding to these bacteria in sera of rabbits injected with WCV or Al(OH)3 alone was assayed by ELISA without and with adsorption with cell-wall polysaccharide, a species-common antigen. The examined strains were 23 primary isolates including at least 10 different MLS types and 13 serotypes; 15 of these strains were invasive isolates, subsequently mouse-passed. Additionally, to investigate the effect of capsulation, TIGR4 strain constructs with the capsulation genes of 20 different serotypes was evaluated. In ELISA all strains showed a large difference in IgG binding due to the immunization, of which most of the antibody typically was not adsorbed and presumably directed to exposed protein antigens. Increased binding of IgG in the WCV-immunized serum to the 20 isogenic capsule-switch strains was shown also by flow cytometry. Further, all these 20 strains elicited IL-17A in T cells of WCV-vaccinated mice, a cytokine known to accelerate pneumococcal clearance. Thus WCV induced both humoral and TH17 cell-mediated immunity against all tested strains.
Keywords: Streptococcus pneumoniae, vaccine, colonization, sepsis, IL-17, serotype-independence, species-common antigen
Introduction
Prevention of disease due to Streptococcus pneumoniae continues to represent a global health priority, with pneumococcal infection accounting for approximately one million childhood deaths per year [1]. The majority of pneumococcus-associated mortality and morbidity occurs in the developing world. Pneumococcal conjugate vaccines, though highly effective at reducing vaccine-type carriage and disease, have several disadvantages, namely limited coverage against >90 known pneumococcal serotypes, replacement in carriage and disease prevalence by non-vaccine serotypes, and high cost of manufacture [2]. For these reasons, many laboratories have been investigating surface-expressed proteins common to all serotypes of the pneumococcal species, with the goal of inducing serotype-independent protection from pneumococcal disease and/or carriage and to be of lower cost. Over twenty such proteins with protective potential have been discovered [3].
An economical approach we have been investigating is immunization with killed cells of a capsule-negative strain, which would present many surface proteins in native configuration, un-occluded by capsule. We hypothesized that this whole-cell antigen (WCA), due to redundancy in protective antigen expression, would elicit immune responses to pneumococci of varying capsular type, isolation site, and genetic background. WCA strain RM200 was constructed with the added features of deletion of the lytA autolysin and expression of a pneumolysin variant with attenuated hemolytic activity [4, 5]. Immunization of mice with WCA, designated whole-cell vaccine (WCV) when administered with appropriate adjuvant, has demonstrated multi-serotype protection in the models tested thus far. For example, when adsorbed to Al(OH)3 and given subcutaneously, WCV protects C57BL/6 mice from fatal aspiration-sepsis with serotypes 3 and 5 and reduces nasopharyngeal colonization with serotype 6B [5].
We are currently testing the protective effect of active WCV immunization in several other mouse challenge models using different serotypes and routes of inoculation; however, the number of serotypes that can be used to infect mice is limited and will not allow for a comprehensive assessment the serotype coverage of WCV-induced immunity. Therefore to more broadly test the potential coverage, we examined the effect of WCV immunization against a panel of selected strains in several assays in vitro. Based on what is understood about immunity to pneumococcal disease and carriage, we chose several techniques to assess the cross-serotype immune responses elicited by WCV. In preclinical development studies, WCV-induced serum antibodies had been shown to be sufficient for protection from fatal pneumonia and sepsis, since anti-WCA IgG titers above a certain value correlated with protection, immunity could be transferred with serum, and WCV-immunized animals treated with anti-CD4+ antibodies were still protected [5]. Here we therefore evaluated the IgG titers in WCV-immunized sera against live clinical isolates varying in serotype, MLS type, and isolation site, and against a library of 20 isogenic capsule-switch strains generated from a TIGR4 parent strain.
However, reduction in pneumococcal carriage in WCV-immunized animals has been found to be antibody-independent and occurs in a CD4+ T cell-dependent and IL-17A-mediated manner [6, 7]. In light of this, we additionally measured the IL-17A production in vitro by WCV-primed splenocytes stimulated with the 20 capsule-switch variants.
MATERIALS AND METHODS
Whole-cell vaccine preparations
Pneumococcal strain RM200 derived from Rx1 is capsule-negative, autolysin-negative, and expresses a non-hemolytic pneumolysoid as described [4, 8]. Cells were centrifuged, washed and killed using beta-propiolactone (BPL) as described [5]. Protein concentrations were determined by bovine serum albumin standardized Total Protein Kit (Sigma) and the antigen, designated WCA, was frozen in aliquots at −80°C until further use. Three hours prior to immunization, aliquots were thawed, diluted in sterile saline (B. Braun Medical Inc., Bethlehem, PA) with Al(OH)3 (aluminum hydroxide) from Brenntag North America (2% Alhydrogel), and gently mixed at 4°C until use; these preparations were designated whole-cell vaccine (WCV).
Immunization of animals
C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were used. Animals were allowed to acclimate to our animal facility for 2–3 days prior to first immunization at age 4–6 weeks. Unanesthetized and gently restrained animals were injected 3 times at 2-week intervals in the lower back. Each injection contained 100 μg of WCA and 240 μg of Al(OH)3. Immunized mice were euthanized by CO2 inhalation 2–4 weeks following last immunizations, and spleens were harvested.
For antibody titers determined by enzyme-linked immunosorbent assay (ELISA), antiserum was generated at MPI (Mattawan, MI) by immunizing rabbits with Al(OH)3-adsorbed WCA (500 μg of WCV) as previously described [5] with Al(OH)3-immunized rabbit serum used as a control. For use in flow cytometry, hyper-immune rabbit serum was generated by Cocalico Biologicals Inc. (Reamstown, PA) by injecting animals subcutaneously with WCA, 1mg/dose, mixed with Freund’s adjuvant in 4 doses (days 0, 14, 21 and 49) according to Cocalico’s custom antibody protocol.
Preparation of bacteria for immunological assays
Isogenic capsule-switch variants were generated as described [9, 10]. For use as the capture coat for antibody ELISAs, bacteria were grown in Todd Hewitt Broth with 0.5% yeast extract (THY) (BD, Franklin Lakes, NJ) to mid-log phase (OD600 = 0.6) and used to coat plates as described below. For use as stimuli on splenocytes from WCV-immunized mice, bacteria were grown in THY (1ml thawed starter culture in 49ml THY) to OD600 = 1.0 at 37°C with 5% CO2 and then resuspended to 3×109/ml prior to BPL-killing as previously described [5].
IgG binding detected by flow cytometry
Isogenic capsule-switch strains grown in 5ml THY were aliquoted in 1-ml volumes, and cells were pelleted by centrifugation and resuspended in 1ml PBS. Given the requirement that only non-viable bacteria be used in the cytometer, cells were heat-killed in a 58°C heat block for one hour. The cells were pelleted and resuspended in 1ml PBS 1%BSA and rotated overnight at 4°C for blocking. Cells were again pelleted and resuspended in 1 ml of diluted serum from the same rabbit either pre- or post-4X-WCV immunization (1:1000 dilution in PBS/0.5%Tween/1%BSA (PBST/BSA)) and incubated on a rotator for one hour. After two washes in PBST/BSA, pelleted cells were resuspended in 250 μl of Alexa Fluor®488 goat anti-rabbit IgG (Invitrogen, Carlsbad, CA) at a 1:50 dilution and rotated in the dark for one hour. The cells were washed twice, resuspended in 500 μl sterile PBS, and kept in the dark until analysis. Flow cytometry was performed using a Cytomation MoFlo (Beckman Coulter). At least three separate samples of each capsular type were prepared and analyzed and at least 10,000 events were included in each mean fluorescence intensity measurement.
Enzyme-linked immunosorbent assay (ELISA)
Live clinical isolates and isogenic capsule-switch variants in the TIGR4 background were suspended at 2×108 cells/ml in sterile PBS. Immulon®2 96-well flat-bottom plates were coated with 50 μl cell suspension/well (approximately 1×107 cells per well) overnight at 4°C and then blocked with 100 μl/well PBS 1% bovine serum albumin (BSA; Sigma Aldrich, St. Louis, MO) for one hour at room temperature. Rabbit serum dilutions were prepared 2-fold in PBST; Al(OH)3-immunized sera were diluted from 1:25 to 1:800, and WCV-immunized sera from 1:200 to 1:6400.. Pneumococcal cell-wall polysaccharide (CWPS; Statens Serum Institut, Copenhagen, Denmark) was added, where indicated, to dilute sera to a final concentration of 0 or 10 μg/ml and incubated for one hour at room temperature. Fifty μl of non-adsorbed and CWPS-adsorbed sera dilutions were then added to the blocked cell-coated plates and incubated for 2 hours. Donkey anti-rabbit IgG HRP (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at 1:20,000 dilution was used to detect bound antibody, followed - after washing - with Sureblue TMB substrate (KPL, Inc., Gaithersburg, MD). Reactions were stopped with 2N sulfuric acid, and optical densities were determined at 450 nm.
Assay of IL-17A from stimulation of splenocytes of WCV-immunized mice
Spleens from WCV-immunized mice were harvested and processed as previously described [7]. BPL-killed isogenic capsule switch TIGR4 variants were used as stimuli. All strains were resuspended to 3×109/ml prior to BPL killing. All killed preparations were diluted in splenocyte stimulation medium (DMEM/F12 with L-glutamine supplemented with 10% fetal calf serum, 50 μM 2-mercaptoethanol, and 10 μg/ml ciprofloxacin) to a final concentration of 1×106 pneumococcal cells in 250μl total volume in the well containing 1×106 splenocytes. After 72 hours at 37°C in 5% CO2, IL-17A was measured from supernatants of cell culture after stimulation (R&D Systems ELISA kits, Minneapolis, MN).
Statistical analysis
Data analysis and graphing were performed using PRISM (version 4.0, GraphPad Software, Inc.). The methods to quantify the in vitro neutrophil killing efficiency of the isogenic capsule-switch variants were previously described [9]; non-parametric Spearman correlation was used to evaluate the association between these values and the median IL-17A elicited by these capsule-switch strains in WCV-immunized splenocytes.
RESULTS
Choice of strains and assays
The cross-strain immunogenicity of WCV was evaluated with a variety of strains described in Table 1 including a) 23 clinical isolates representing both carriage (isolated nasopharyngeally) and invasive (isolated from blood) strains and b) a library of TIGR4 strains isogenic except for the capsule loci expressing 20 different serotypes. All the strains were tested in ELISA with live bacteria as capture antigen, determining the IgG antibody titer in sera of rabbits immunized with WCV or co-housed rabbits injected with Al(OH)3 alone as control. While a constant concentration of live cells was used as the capture layer in the ELISA, consistent coating from strain to strain could not be assured. Thus while the titers are not strictly comparable among strains, the control versus immunized titers are comparable for a given strain. The titers were determined without and with adsorption with cell wall polysaccharide (CWPS), a species-common antigen, thus the titer in the adsorbed serum shows presumed protein-specific antibody. In addition, to avoid possible artifacts from the coating process, the TIGR4 capsule-switch panel was examined for WCV-elicited antibody binding by flow cytometry. Further, this panel was tested for the IL-17A produced in culture when the bacteria were used to stimulate splenocytes from WCV-immunized mice.
Table 1.
Description of clinical strains used to determine IgG titer from WCV-immunized sera.
| Serotype | Site of isolation | MLST |
|---|---|---|
| 1 | Nasopharynx (NP) | 227 |
| 4 | NP | 205 |
| 7C | NP | 1757 |
| 7F | Blood | 191 |
| 9V | NP | 156 |
| 12F | Blood | 218 |
| 15B | NP | 199 |
| 18C | NP | 113 |
| 22F | NP | 433 |
| 23F | NP | 33 |
| 3 | Blood | NA (not available) |
| 4 (two different isolates) | Blood | NA |
| 6B (four different isolates) | Blood | NA |
| 9V (two different isolates) | Blood | NA |
| 14 (three different isolates) | Blood | NA |
| 23F | Blood | NA |
Effect of WCV-immunization of rabbits on IgG titers to the various strains in capture ELISA
Against all strains tested, higher titers were measured in WCV-immunized serum over alum-control serum, both without and with adsorption by CWPS. With adsorption (the more important measurement; Table 2) the fold-rise against 22 of 23 of the clinical isolates ranged from 19–300, while the often sub-cultured serotype 3 strain WU2 (fold-rise = 10) was an outlier. The range against the 20 isogenic capsule-switch variants was less variable at 26–43. As a control for the effect of capsulation per se, an isogenic TIGR4 strain with the capsule locus deleted (Δcapsule) was included and showed a fold-rise of 43. In ELISA without CWPS adsorption somewhat higher titers were found in both control and WCV-immunized sera. With the clinical strains, non-CWPS IgG represented 42–72% of the total measured IgG in WCV-immunized sera; for isogenic TIGR4 capsule variants, non-CWPS IgG represented 68–94% of the total.
Table 2.
Titers of IgG antibody in alum control or WCV-immunized rabbit sera to live log- phase pneumococci of the indicated strain/serotype. Strains are described in Table 1 and represent a variety of clinical carriage and disease isolates as well as a library of capsule-switch variants constructed on the TIGR4 genetic background [9]. The assays were determined with and without adsorption with the cell wall polysaccharide to indicate presumed protein-specific and total antibodies, respectively. Titers are reported as reciprocal of the dilution at which OD450 0.3 was reached.
| Serotype | CWPS-adsorbed titer | Non-adsorbed titer | ||||
|---|---|---|---|---|---|---|
| Al(OH)3 control | WCV-immunized | Fold-difference | Al(OH)3 control | WCV-immunized | Fold-difference | |
| Clinical isolates | ||||||
| 3 | 65 | 670 | 10 | 55 | 1000 | 18 |
| 4 | 38 | 1700 | 45 | 45 | 2300 | 51 |
| 4 | 100 | 2000 | 20 | 120 | 2900 | 24 |
| 4 | 25 | 1500 | 60 | 88 | 3200 | 36 |
| 6B | 110 | 2500 | 23 | 125 | 3800 | 30 |
| 6B | 25 | 1500 | 60 | 90 | 2100 | 23 |
| 6B | 25 | 1600 | 64 | 92 | 3500 | 38 |
| 9V | 97 | 1800 | 19 | 120 | 3800 | 32 |
| 9V | 67 | 1300 | 19 | 110 | 3100 | 28 |
| 9V | 54 | 2200 | 41 | 46 | 3000 | 65 |
| 12F | 35 | 2100 | 60 | 39 | 3800 | 97 |
| 14 | 25 | 1400 | 56 | 90 | 2000 | 22 |
| 14 | 85 | 1800 | 21 | 90 | 2900 | 32 |
| 15B | 74 | 3600 | 49 | 110 | 6600 | 60 |
| 23F | 25 | 3400 | 140 | 100 | 5000 | 50 |
| 1 | 17 | 2200 | 130 | NA | NA | NA |
| 6B | 58 | 3000 | 52 | NA | NA | NA |
| 7C | 32 | 3300 | 100 | NA | NA | NA |
| 7F | 19 | 3000 | 160 | NA | NA | NA |
| 14 | 33 | 2300 | 70 | NA | NA | NA |
| 18C | 6 | 1800 | 300 | NA | NA | NA |
| 22F | 31 | 1600 | 52 | NA | NA | NA |
| 23F | 30 | 2300 | 77 | NA | NA | NA |
|
Isogenic TIGR4 strains TIGR4:Capsular Type | ||||||
| TIGR4:1 | 70 | 2400 | 34 | 80 | 2900 | 36 |
| TIGR4:2 | 88 | 2500 | 28 | 110 | 3300 | 30 |
| TIGR4:3 | 78 | 3400 | 43 | 80 | 4000 | 50 |
| TIGR4:4 | 85 | 3700 | 43 | 95 | 4200 | 44 |
| TIGR4:5 | 80 | 2900 | 36 | 80 | 3400 | 43 |
| TIGR4:6A | 105 | 2900 | 27 | 110 | 3100 | 28 |
| TIGR4:6B | 100 | 3200 | 32 | 110 | 3700 | 34 |
| TIGR4:7F | 80 | 2400 | 30 | 85 | 2800 | 33 |
| TIGR4:8 | 100 | 3000 | 30 | 120 | 3400 | 28 |
| TIGR4:9N | 100 | 3000 | 30 | 110 | 3400 | 31 |
| TIGR4:9V | 100 | 2800 | 28 | 120 | 3500 | 29 |
| TIGR4:10A | 100 | 2700 | 27 | 120 | 3800 | 32 |
| TIGR4:11A | 120 | 3100 | 26 | 130 | 3900 | 30 |
| TIGR4:12F | 70 | 2800 | 40 | 80 | 3200 | 40 |
| TIGR4:14 | 78 | 3200 | 41 | 80 | 3400 | 43 |
| TIGR4:18C | 90 | 2800 | 31 | 95 | 3100 | 33 |
| TIGR4:19A | 90 | 3000 | 33 | 130 | 4400 | 34 |
| TIGR4:19F | 110 | 3100 | 28 | 120 | 4200 | 35 |
| TIGR4:23F | 100 | 2900 | 29 | 120 | 3400 | 28 |
| TIGR4:35B | 80 | 3000 | 38 | 80 | 3200 | 40 |
| TIGR4: Δcapsule | 100 | 4400 | 43 | 110 | 5000 | 45 |
NA – not assayed
Assessing the effect of WCV-immunization by flow cytometry
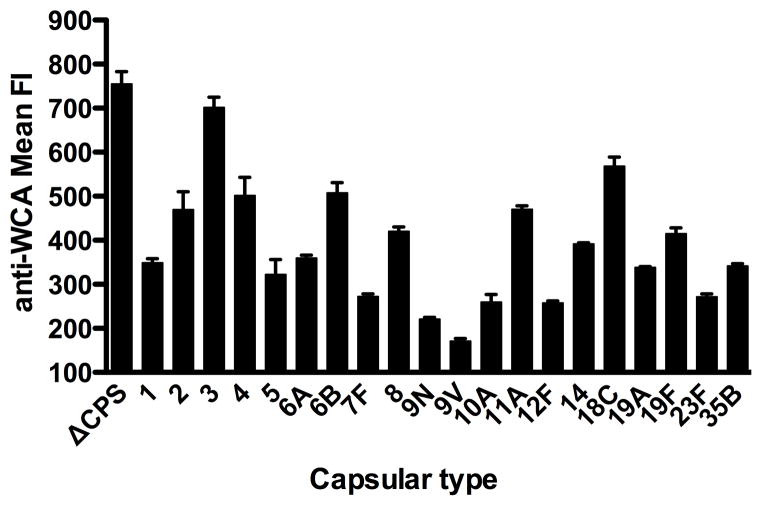
To assess IgG binding without possible complications from adherence of the pneumococcal cells to polystyrene ELISA wells, heat-killed isogenic capsule-switch variants were reacted with WCV-immunized rabbit or control sera, and binding was detected by flow cytometry. Isogenic TIGR4 strains of 20 different capsule types and an unencapsulated variant were each grown to mid-log phase and then heat-killed. After incubation with WCV-immunized rabbit sera, or separately with pre-immune sera from the same animal, the washed cells were reacted with secondary fluorophore and mean fluorescence intensity (MFI) of each preparation was measured. As shown in Figure 1, the MFI of bound IgG from the immune serum ranged from 171–702 and the MFI of the unencapsulated cells was 755. Pre-immune serum demonstrated minimal binding: the MFI ranged from 2.9–4.9.
Figure 1. WCV-elicited antibody reactivity against isogenic strains bearing different capsular types.
Heat-killed TIGR4 isogenic strains of varying capsular type were detected with sera from rabbits immunized with WCV then stained with an anti-rabbit IgG fluorophore and measured by flow cytometry. The same strains were detected with pre-immune sera from the same animal with range of mean fluorescence intensity 2.9 – 4.9. The data represent the median and interquartile range from at least three separately prepared samples.
Measuring the IL-17A immunogenicity of isogenic capsule-switch variants in WCV-primed splenocytes
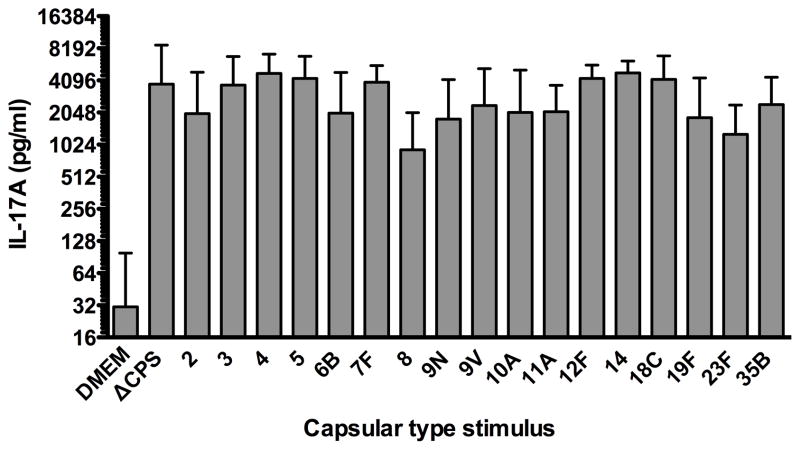
To investigate the IL-17A immunogenicity of the non-capsular antigens presented in pneumococci expressing varying capsular types, we studied the response of WCV-immunized splenocytes to stimulation with the isogenic capsule-switch strains. Spleens from 10 WCV-immunized mice were processed and stimulated separately; splenocytes were not pooled. IL-17A in each stimulation supernatant was measured; individual and median IL-17A responses for each capsular-type whole cell antigen are shown in Figure 2. The median response to medium alone was 30 pg/ml of IL-17A. Median IL-17A responses to capsule-switch whole cell antigens ranged from 900 (type 8) to 4800 (type 14) pg/ml. For comparison, the isogenic strain with capsule locus deleted (ΔCPS) was used as stimulus and the median IL-17A response was 3800 pg/ml.
Figure 2. WCV-induced IL-17A response to isogenic pneumococcal strains bearing differing capsular types from splenocytes obtained from mice immunized with WCV.
Splenocytes from WCB immunized C57Bl/6 mice (n=10) were stimulated with killed TIGR4 isogenic strains of the indicated capsular type. Stimulation was with 1×106 pneumococcal cells per 1×106 splenocytes. IL-17A was measured from the supernatants following a 72-hour incubation. Height of the columns represents median values with interquartile ranges indicated by bars.
Assessing the correlation between a serotype’s in vitro IL-17A immunogenicity and resistance to surface phagocytosis
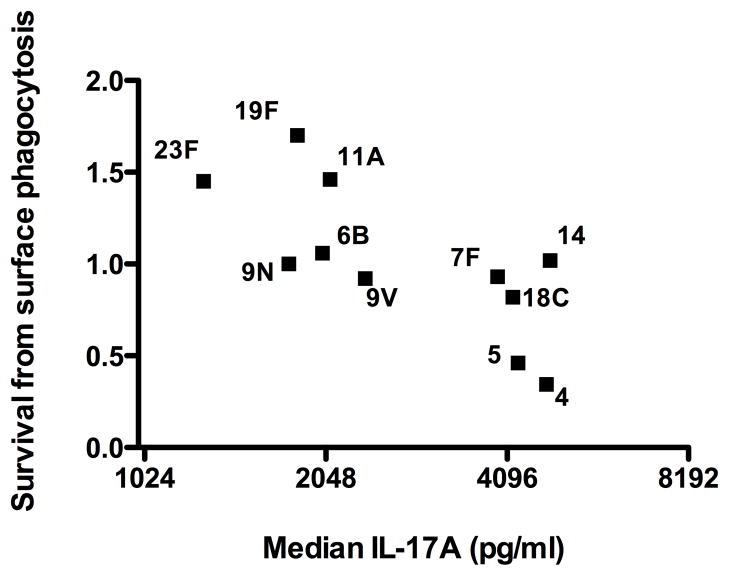
Previous work identified several biochemical and physical features of the polysaccharide capsule of pneumococcus that correlate with strain prevalence in carriage rates and with in vitro assessments of survival from surface phagocytosis [9]. Based on the demonstrated role of IL-17A in enhancing neutrophil-mediated clearance of mucosally colonizing pneumococci [7], we hypothesized that the capsular types that elicit the highest IL-17A levels systemically would be most efficiently killed by surface phagocytosis. Using the phagocytosis survival values obtained in the cited study, we found that median IL-17A levels elicited from WCV-immunized splenocytes by varying capsular type whole-cell antigens correlated inversely with survival from surface phagocytosis (Figure 3) with a Spearman ρ = −0.6545 (p= 0.03).
Figure 3. Correlation of IL-17A response and survival in a surface phagocytosis assay.
Correlation of median IL-17A responses stimulated by capsule-switch isogenic pneumococcal whole cell antigens in WCV-immunized splenocytes with relative survival from surface phagocytosis (normalized to survival by the serotype 14 strain, as measured in [9]). IL-17A responses correlate significantly and inversely with survival from neutrophil-mediated surface phagocytosis (Spearman ρ = −0.6545; p = 0.03). The isogenic capsule-switch variants eliciting the highest IL-17A demonstrate the lowest survival index in an in vitro assay of surface phagocytosis.
DISCUSSION
The effects of the 7-valent pneumococcal conjugate vaccine (PCV) on reduction of invasive disease rates in the US [11] and of a 9-valent PCV in developing nations [12, 13] demonstrate the possibility for global pneumococcal disease prevention if broad enough serotype coverage were provided. While expanded-valency PCVs represent one approach to extending this coverage, the cost and complexity of these vaccines to manufacture may limit their accessibility worldwide. In addition, serotype replacement and serotype-specific changes in pneumococcal ecology following implementation of PCV programs may impact the longstanding effect of PCV at reducing pneumococcal disease [2, 14, 15]. The various species-common protein projects under development represent an approach to address these problems, with the aim to either include protein antigens that confer protection against colonization and invasive disease, or incorporate these proteins in existing PCVs [3]. Our WCV approach might work more simply and economically by conferring immunity to a range of noncapsular pneumococcal species antigens that are well conserved across varying serotypes and sequence types.
Some of the typical correlates of immunity that are reliably assessed in PCV efficacy trials, such as vaccine-induced anticapsular IgG levels, will not apply to evaluation of WCV-induced (or for that matter, protein-induced) immunity, and this could pose a challenge for licensing strategies for this approach [16]. Additionally, pneumococcus is an essentially human pathogen; the paucity of pneumococcal carriage and invasive disease animal models limit comprehensive evaluation of serotype coverage via active immunization studies. For these reasons, we are evaluating other methods of assessing the serotype coverage of the immunity elicited by WCV administration. The in vitro assays described herein were chosen because they represent immune correlates that apply to what is known about acquired immunity to various phases of pneumococcal infection.
The role of anticapsular antibodies in acquired immunity to pneumococcal disease has been largely corroborated by the efficacy of PCVs in reduction of both carriage and invasive disease rates due to vaccine-types. There is less known about the role of noncapsular antibodies, but studies with several pneumococcal species proteins suggest that these entities can protect against invasive disease, albeit with higher titers needed than of capsular antibody; for this reason vaccination with mixtures of several such proteins is being tested (reviewed in [3]). Hypothetically, the WCV preparation should preserve these noncapsular components in native configuration as well as many other as of yet uncharacterized antigens that can serve as targets for protective antibody production. Empirically WCV: a) elicits antibodies to the known well studied proteins PspA, PsaA, and Ply; and to the CWPS, which perhaps has protective potential [17, 18] b) elicits antibodies to a large number of other proteins, not identified, as seen with Western blotting, and c) expresses protective antigens redundantly, e.g. is still protective even if the choline-binding class of proteins [19] or the pneumolysin protein (unpublished data) are eliminated. Therefore it is reasonable to expect that the humoral response elicited by WCV could contribute to protection regardless of serotype and other genetic variations.
As is shown by the titers in Table 2 and the flow cytometric binding in Figure 1, antibodies to WCV bound to pneumococcal cells across all tested MLST sequences and serotypes. The magnitude of the fold-rise varied, especially in the titers to clinical strains, for structural reasons unknown. This variation appears not due to the effect of capsule on antigen accessibility to antibody: the fold rises in the isogenic capsule switch variants in the TIGR4 background were less variable, and the noncapsulated variant reacted similarly to the capsulated strains. This may suggest that the role of capsule per se on IgG antibody access to noncapsular antigens is minimal, but the inherent differences across sequence types in representation of accessible antibody targets contribute to greater variability. The CWPS, which has only slight antigenic variation, is common to all pneumococci examined, but the protective capacity of CWPS antibody in invasive infection is controversial [17, 18, 20–23]. Although our ELISA showed a reduction in titer in all the strains following adsorption with CWPS, the majority of WCV-generated antibody appears to be directed at non-CWPS targets in most of the strains.
Interpretation of protective potential of these antibody titers and binding capacities is not clear; unlike anticapsular antibody for which threshold levels have been determined that correlate with protection from disease with encapsulated organisms such as S. pneumoniae and Haemophilus influenzae type b [24], such correlates for noncapsular pneumococcal antibodies do not exist and may be difficult to establish. In active immunization studies thus far, both 2- and 3-dose schedules of subcutaneous WCV administered at two-week intervals provided dose-dependent protection from sepsis via aspiration of WU2 serotype 3 to 100% of animals compared with 0–30% survival in animals injected with Al(OH)3 alone [5]. The challenge strain in this study is the same serotype 3 strain to which the titer rise (10-fold, Table 2) was lowest indicating that, at least for this strain in this model, a modest rise in titer is sufficient for protection from invasive disease. It may be possible to further evaluate the function of the WCV-elicited antibody in an opsonin-dependent killing assay using various strains in vitro with a human neutrophil source and WCV-immunized sera. Such an assay is in development and may further inform us about the serotype coverage of WCV immunization. It may also identify a correlate of protection against invasive disease by which WCV-generated immunity can be measured.
While the effect of PCV immunization programs on reduction of carriage of vaccine- types demonstrates that anticapsular antibody is sufficient to prevent colonization [25], it is not necessary. We have demonstrated antibody-independent mechanisms of WCV- elicited clearance of colonization [6, 7], and others have similarly shown that reduction in the duration of carriage following exposure to live pneumococcus is not antibody- mediated [26, 27]. Furthermore, the critical role of the CD4+ T cell effector cytokine IL- 17A in clearance of carriage has been confirmed in models of primary colonization [28] as well as in WCV-elicited mechanisms of immunity to colonization [7]. The capacity of T cells (in pre-challenge blood samples) of WCV-immunized animals to produce IL-17A in culture correlates inversely with the burden of pneumococci recovered from nasal washes following intranasal challenge with serotype 6B [5, 7, 8]: IL-17A levels of greater than 300 pg/ml predict a low to non-detectable pneumococcal count in post-challenge nasal washes. In these IL-17A assays, the WCA used to stimulate the T cells is noncapsulated. To assess the effect of capsule on the accessibility of antigens able to stimulate IL-17A secretion in WCV-immunized T cells, we used the isogenic capsule- switch panel of TIGR4 strains. As shown in Figure 2, all 20 strains elicited IL-17A responses well above the 300 pg/ml correlate; most elicited medians (among 10 mice) of 1000–4000 pg/ml. While it is difficult to directly apply the cited protective threshold (determined following intranasal immunization and produced in whole blood samples) to that determined here with subcutaneous vaccination and produced in splenocyte samples, the magnitude of the response is encouraging and does not suggest that capsule alone significantly impacts the accessibility of T cell antigens that trigger the IL- 17A responses generated by WCV immunization.
Studies have demonstrated that IL-17A facilitates clearance of pneumococci from mucosal surfaces by recruiting neutrophils that effectively kill the bacteria [7, 28]. Furthermore, using the same isogenic capsule-switch library as that used here to stimulate splenocytes, it had been shown that survival of killing by neutrophils in vitro correlated with the size of the capsule expressed in the TIGR4 background [9]. Here we found that the magnitude of the IL-17A response from WCV-immunized splenocytes correlated significantly and inversely with the resistance to killing by neutrophils that had been determined for that capsular type (Figure 3). For example, variants expressing capsular serotypes such as 23F and 19F that elicited the lowest IL-17A responses demonstrated higher mean relative survival rates in the neutrophil assay than serotypes such as 4 and 5 that elicited the highest IL-17A responses. This suggests the hypothesis that a capsular type that is both more susceptible to neutrophils and that primes for more robust IL-17A responses would be less fit for survival in carriage. As demonstrated in the cited study, the capsule size of these isogenic variants correlates with prevalence in carriage [9]. The effect of capsule on eliciting IL-17A responses may also be reflected in these differences in the ability of pneumococci of varying serotype to colonize at mucosal sites, differences represented by differing carriage prevalence rates.
In summary, the data presented here demonstrate robust WCV-elicited humoral and cellular immunogenicity to a comprehensive panel of pneumococci of varying MLST and serotypes. In light of this potential for omni-strain coverage from WCV immunization, as well as the economy of manufacture, plans for Phase I clinical trials of the WCV in the USA are underway.
Highlights.
We show the immunologic responses to a species-specific pneumococcal whole cell vaccine administered with aluminum hydroxide
Antibody elicited by the vaccine cross-reacts to a panel of clinical pneumococcal isolates of diverse MLST and serotypes
Immunization with this vaccine primes T cells to produce robust IL-17A responses to pneumococci regardless of serotype
This data suggest broad responses and provide further support for the clinical development of this vaccine
Acknowledgments
We gratefully acknowledge PATH for their support of this work. This work was also supported by NIH/NIAID grant AI066013. R.M. gratefully acknowledges support from the Translational Research Program at Children’s Hospital Boston. We would also like to thank Marc Lipsitch and Claudette Thompson for their assistance and providing us with various pneumococcal strains. Additionally, Elizabeth Boush is thanked for her assistance with flow cytometric analyses. RM is a member of the scientific advisory board of Genocea Biosciences, Cambridge, MA, USA and Arsanis Biosciences, Vienna, Austria.
Abbreviations
- WCV
whole cell vaccine
- Al(OH)3
aluminum hydroxide
- CWPS
cell wall polysaccharide
- MLST
multilocus sequence typing
- PCV
pneumococcal conjugate vaccine
- MFI
mean fluorescence intensity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009 Sep 12;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011 Apr 12; doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moffitt KL, Malley R. Next generation pneumococcal vaccines. Curr Opin Immunol. 2011 Apr 20; doi: 10.1016/j.coi.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liberman C, Takagi M, Cabrera-Crespo J, Sbrogio-Almeida ME, Dias WO, Leite LC, et al. Pneumococcal whole-cell vaccine: optimization of cell growth of unencapsulated Streptococcus pneumoniae in bioreactor using animal-free medium. J Ind Microbiol Biotechnol. 2008 Nov;35(11):1441–5. doi: 10.1007/s10295-008-0445-3. [DOI] [PubMed] [Google Scholar]
- 5.Lu YJ, Leite L, Goncalves VM, Dias Wde O, Liberman C, Fratelli F, et al. GMP- grade pneumococcal whole-cell vaccine injected subcutaneously protects mice from nasopharyngeal colonization and fatal aspiration-sepsis. Vaccine. 2010 Nov 3;28(47):7468–75. doi: 10.1016/j.vaccine.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci U S A. 2005 Mar 29;102(13):4848–53. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008 Sep;4(9):e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu YJ, Yadav P, Clements JD, Forte S, Srivastava A, Thompson CM, et al. Options for inactivation, adjuvant, and route of topical administration of a killed, unencapsulated pneumococcal whole-cell vaccine. Clin Vaccine Immunol. 2010 Jun;17(6):1005–12. doi: 10.1128/CVI.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinberger DM, Trzcinski K, Lu YJ, Bogaert D, Brandes A, Galagan J, et al. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathog. 2009 Jun;5(6):e1000476. doi: 10.1371/journal.ppat.1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trzcinski K, Thompson CM, Lipsitch M. Construction of otherwise isogenic serotype 6B, 7F, 14, and 19F capsular variants of Streptococcus pneumoniae strain TIGR4. Appl Environ Microbiol. 2003 Dec;69(12):7364–70. doi: 10.1128/AEM.69.12.7364-7370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al. Decline in invasive pneumococcal disease after the introduction of protein- polysaccharide conjugate vaccine. N Engl J Med. 2003 May 1;348(18):1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 12.Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, Okoko JB, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005 Mar-Apr;365(9465):1139–46. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 13.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003 Oct 2;349(14):1341–8. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 14.Croucher NJ, Harris SR, Fraser C, Quail MA, Burton J, van der Linden M, et al. Rapid pneumococcal evolution in response to clinical interventions. Science. 2011 Jan 28;331(6016):430–4. doi: 10.1126/science.1198545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanage WP. Serotype-specific problems associated with pneumococcal conjugate vaccination. Future Microbiol. 2008 Feb;3(1):23–30. doi: 10.2217/17460913.3.1.23. [DOI] [PubMed] [Google Scholar]
- 16.Feavers I, Knezevic I, Powell M, Griffiths E. Challenges in the evaluation and licensing of new pneumococcal vaccines, 7–8 July 2008, Ottawa, Canada. Vaccine. 2009 Jun 8;27(28):3681–8. doi: 10.1016/j.vaccine.2009.03.087. [DOI] [PubMed] [Google Scholar]
- 17.Lu YJ, Forte S, Thompson CM, Anderson PW, Malley R. Protection against Pneumococcal colonization and fatal pneumonia by a trivalent conjugate of a fusion protein with the cell wall polysaccharide. Infect Immun. 2009 May;77(5):2076–83. doi: 10.1128/IAI.01554-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDaniel LS, Waltman WD, 2nd, Gray B, Briles DE. A protective monoclonal antibody that reacts with a novel antigen of pneumococcal teichoic acid. Microb Pathog. 1987 Oct;3(4):249–60. doi: 10.1016/0882-4010(87)90058-1. [DOI] [PubMed] [Google Scholar]
- 19.Trzcinski K, Thompson CM, Srivastava A, Basset A, Malley R, Lipsitch M. Protection against Nasopharyngeal Colonization by Streptococcus pneumoniae is Mediated by Antigen-Specific CD4+ T Cells. Infect Immun. 2008 Apr 7; doi: 10.1128/IAI.00141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szu SC, Schneerson R, Robbins JB. Rabbit antibodies to the cell wall polysaccharide of Streptococcus pneumoniae fail to protect mice from lethal challenge with encapsulated pneumococci. Infect Immun. 1986 Nov;54(2):448–55. doi: 10.1128/iai.54.2.448-455.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musher DM, Watson DA, Baughn RE. Does naturally acquired IgG antibody to cell wall polysaccharide protect human subjects against pneumococcal infection? Journal of Infectious Diseases. 1990;161(4):736–40. doi: 10.1093/infdis/161.4.736. [DOI] [PubMed] [Google Scholar]
- 22.Briles DE, Forman C, Horowitz JC, Volanakis JE, Benjamin WH, Jr, McDaniel LS, et al. Antipneumococcal effects of C-reactive protein and monoclonal antibodies to pneumococcal cell wall and capsular antigens. Infect Immun. 1989 May;57(5):1457–64. doi: 10.1128/iai.57.5.1457-1464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skov Sorensen UB, Blom J, Birch-Andersen A, Henrichsen J. Ultrastructural localization of capsules, cell wall polysaccharide, cell wall proteins, and F antigen in pneumococci. Infect Immun. 1988 Aug;56(8):1890–6. doi: 10.1128/iai.56.8.1890-1896.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010 Jul;17(7):1055–65. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang SS, Hinrichsen VL, Stevenson AE, Rifas-Shiman SL, Kleinman K, Pelton SI, et al. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009 Jul;124(1):e1–11. doi: 10.1542/peds.2008-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCool TL, Weiser JN. Limited Role of Antibody in Clearance of Streptococcus pneumoniae in a Murine Model of Colonization. Infect Immun. 2004 Oct;72(10):5807–13. doi: 10.1128/IAI.72.10.5807-5813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richards L, Ferreira DM, Miyaji EN, Andrew PW, Kadioglu A. The immunising effect of pneumococcal nasopharyngeal colonisation; protection against future colonisation and fatal invasive disease. Immunobiology. Apr;215(4):251–63. doi: 10.1016/j.imbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009 Jun 8; doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]