SUMMARY
The steroid, 17β-estradiol (E2), is well known to influence hippocampal functions such as memory, affective behaviors, and epilepsy. There is growing awareness that in addition to responding to ovarian E2, the hippocampus of both males and females synthesizes E2 as a neurosteroid that could acutely modulate synaptic function. Previous work on acute E2 actions in hippocampus has focused on excitatory synapses. Here, we show that E2 rapidly suppresses inhibitory synaptic transmission in hippocampal CA1. E2 acts through the α form of the estrogen receptor to stimulate postsynaptic mGluR1-dependent mobilization of the endocannabinoid, anandamide, which then retrogradely suppresses GABA release from CB1 receptor-containing inhibitory presynaptic boutons. Remarkably, this effect of E2 is sex-specific, occurring in females but not males. Acute E2 modulation of endocannabinoid tone and consequent suppression of inhibition provides a new mechanism by which neurosteroid E2 could modulate hippocampus-dependent behaviors in a sex-specific manner.
Estrogens influence hippocampal function through multiple mechanisms with time courses ranging from minutes to days. Recent recognition that a key estrogen, 17β-estradiol (E2), is produced as a neurosteroid in the brains of both males and females has fueled a resurgence of interest in acute non-genomic estrogen signaling (Woolley, 2007). Many hippocampal neurons express the E2 synthesizing enzyme, P450 aromatase (Hojo et al., 2004), which could provide a source of locally generated E2 to acutely modulate synaptic function in vivo.
E2 applied to hippocampal slices rapidly potentiates synaptically evoked field EPSPs in the CA1 region (Teyler et al., 1980), as well as intracellularly recorded EPSPs (Wong and Moss, 1992) and EPSCs (Smejkalova and Woolley, 2010) in CA1 pyramidal cells. On the one hand, E2 appears to act on excitatory synapses through the β form of the classical estrogen receptor (ERβ). ERβ agonists rapidly increase AMPAR-mediated field EPSPs (Kramar et al., 2009) and EPSCs (Smejkalova and Woolley, 2010), whereas ERα agonists do not affect AMPAR-mediated responses. On the other hand, E2-induced potentiation of field EPSPs is reduced in ERα knockout compared to wildtype mice (Fugger et al., 2001), suggesting a more complex action of E2. One possibility is that E2 acutely potentiates excitatory synapses via ERβ, and simultaneously suppresses inhibitory synapses via ERα.
To investigate acute modulation of inhibitory synapses, we recorded GABAA receptor-mediated IPSCs in CA1 pyramidal cells with application of E2 to hippocampal slices from adult female rats. We found that, in a subset of cells, E2 rapidly suppresses IPSCs. Subsequent studies indicated that E2-induced IPSC suppression depends on ERα and mGluR1-dependent mobilization of endocannabinoids to decrease the probability of GABA release from CB1R-containing inhibitory synaptic inputs. Additionally, E2-induced suppression of IPSCs occurs in females but not in males. These results show that sex steroids can rapidly regulate inhibitory synaptic transmission in the hippocampus through a previously unknown and sex-specific mechanism.
RESULTS
Estradiol acutely suppresses a subset of inhibitory inputs through an ERα-dependent decrease in the probability of GABA release
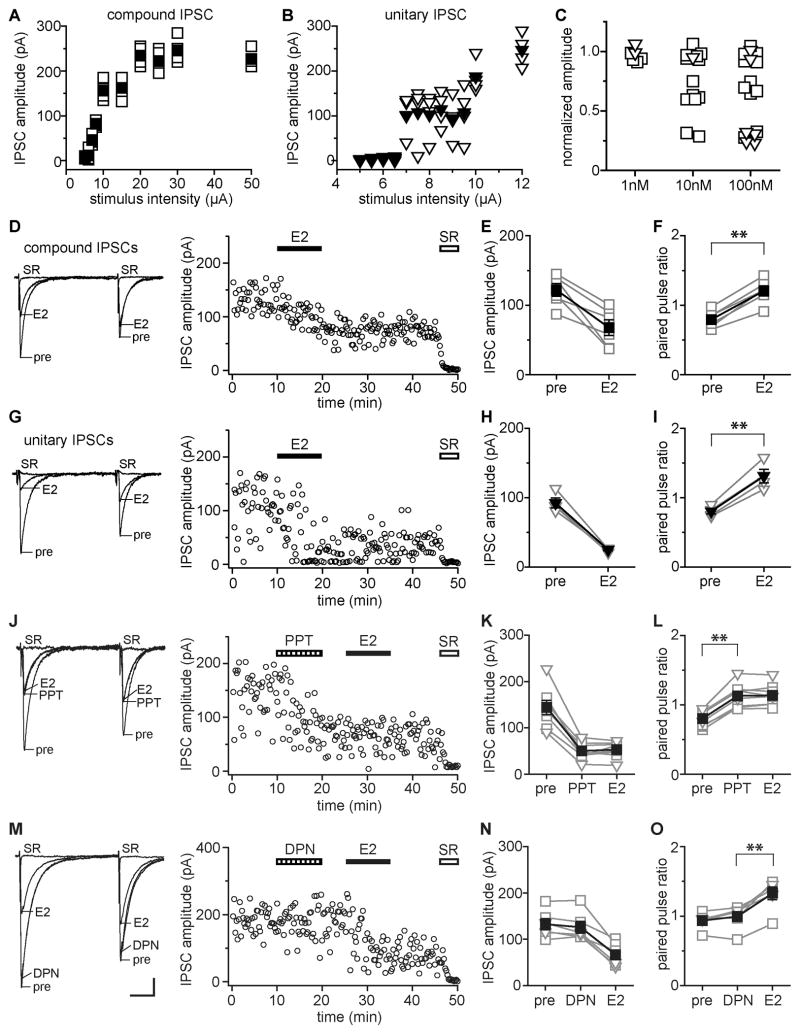
We investigated whether E2 acutely affects perisomatic IPSCs in hippocampal CA1 pyramidal cells of adult female rats. Based on stimulus-response curves (Fig. 1A, B), recordings were classified as unitary IPSCs or as compound IPSCs arising from activation of multiple inhibitory afferents. Pairs of IPSCs were recorded before, during, and after 10 min application of 1, 10, or 100 nM E2 to each slice. In 17 of 31 cells (55%), 10 or 100 nM E2 rapidly suppressed inhibitory synaptic transmission, evidenced by decreased IPSC amplitude and increased paired-pulse ratio (PPR). The remaining 14 cells showed no response to 10 or 100 nM E2, and none of 6 cells tested with 1 nM E2 showed any response. As evident in Fig. 1C, there were two distinct classes of E2 response: moderate or robust suppression of IPSCs. E2 moderately suppressed IPSCs (range 25–43%) in 9 of 17 E2-responsive cells whereas in the other 8, E2 robustly suppressed IPSCs (range 71–77%). Cells classified as showing no response to E2 ranged from a 6% decrease to a 9% increase in IPSC amplitude. Based on this distribution, we used a 25% decrease in amplitude as the threshold for identifying E2-responsive IPSCs.
Figure 1. E2 acts through ERα to acutely suppress GABA release at a subset of inhibitory synapses.
(A, B) Stimulus-response curves were used to identify compound (A) vs. unitary (B) IPSCs. Open symbols are individual sweeps; filled symbols are average of 4 sweeps at each stimulus intensity.
(C) Normalized IPSC amplitude changes with 1 nM (n=6), 10 nM (n=14), or 100 nM (n=17) E2 showing that E2 suppresses IPSCs robustly, moderately, or not at all. Square symbols are data for compound IPSCs; triangles are data for unitary IPSCs (also in E, F, H, I, K, L, N, O).
(D) Recording of E2-sensitive compound IPSCs showing individual traces and time course of the E2-induced decease in IPSC amplitude (100 nM E2). Each point is an individual sweep and SR 95531 (SR, 2 μM) applied at the end of the experiment blocked IPSCs (also in G, J, M).
(E) Group compound IPSC amplitude data for all experiments as in D (n=6). Connected open symbols are individual cells; filled symbols are mean ± SEM for all cells (also in F, H, I, K, L, N, O).
(F) Group PPR data for the same cells as in E. ** indicates p < 0.01, paired t test (also in I, L, O).
(G) Recording of E2-sensitive unitary IPSCs showing individual traces and time course (100 nM E2).
(H) Group unitary IPSC amplitude data for all experiments as in G (n=4).
(I) Group PPR data for the same cells as in H.
(J) Recording of PPT (ERα agonist, 200 nM)-sensitive IPSCs showing individual traces and time course. PPT mimicked and occluded E2-induced IPSC suppression.
(K) Group amplitude data for all experiments as in J (n=8).
(L) Group PPR data for the same cells as in K.
(M) Recording of E2-sensitive IPSCs tested with DPN (ERβ agonist, 500 nM). DPN did not affect E2-sensitive IPSCs. Scale indicates 25 pA, 25 msec and also applies to D, G, J.
(N) Group IPSC amplitude data for all experiments as in M (n=6).
(O) Group PPR data for the same cells as in N.
Most recordings were of compound IPSCs (Fig. 1D–F). In 6 of 11 cells, 100 nM E2 decreased compound IPSC amplitude by 44 ± 8% (range 25–72%; Fig. 1E), which was paralleled by an increase in PPR from 0.79 ± 0.05 to 1.21 ± 0.07 (paired t test, p < 0.001; Fig. 1F). Thus in addition to decreasing IPSC amplitude, E2 converted inhibitory synapses from depressing (PPR < 1.0) to facilitating (PPR > 1.0). Results were similar for unitary IPSCs, except that an E2 response, when it occurred, was consistently robust (Fig. 1G). In 4 E2-responsive unitary IPSC recordings, E2 decreased IPSC amplitude by 73 ± 2% (range 68–77%; Fig. 1H) and increased PPR from 0.80 ± 0.06 to 1.31 ± 0.12 (paired t test, p < 0.01; Fig. 1I). Results with 10 nM E2 were similar. In 7 of 14 cells, 10 nM E2 decreased IPSC amplitude by 47 ± 7% (range 29–74%). In cells classified as showing no IPSC amplitude response to 10 nM or 100 nM E2, PPR also was unaffected (0.71 ± 0.05 vs. 0.70 ± 0.07). There was no apparent relationship between initial release probability and the likelihood of a response to E2. Initial PPR of E2-sensitive IPSCs was 0.76 ± 0.03, not different from the 0.75 ± 0.03 initial PPR in E2-insensitive IPSCs. Likewise, among E2-sensitive IPSCs, there was no relationship initial PPR and the magnitude of response to E2. For all E2-sensitive IPSCs, the E2-induced changes in IPSC amplitude and PPR occurred rapidly, beginning within 2–3 min, and were not readily reversible. Application of the GABAA receptor antagonist SR 95531 at the end of each experiment confirmed that IPSCs were GABAA receptor-mediated.
Together, these results demonstrated that E2 rapidly suppresses inhibitory synaptic transmission at a subset of perisomatic inputs to CA1 pyramidal cells and acts, at least in part, by decreasing the probability of GABA release. Unitary IPSCs showed either robust suppression or none at all, indicating that only a subset of inhibitory inputs is sensitive to E2 and that E2 can profoundly suppress synaptic transmission at individual connections. Moderate suppression of compound IPSCs likely arises from a mixture of robust suppression at some synapses contributing to the IPSC and no effect at other synapses in the same IPSC. Compound IPSCs that showed robust suppression likely contain mostly E2-sensitive synapses and few E2-insensitive ones.
Experiments with ER subtype selective agonists, PPT for ERα and DPN for ERβ, showed that ERα mediates E2-induced suppression of inhibition. Concentrations of PPT and DPN were chosen to match the relative binding affinities of 100 nM E2. The ERα agonist PPT (200 nM) mimicked and occluded E2-induced IPSC suppression and increased PPR (Fig. 1J). In 8 of 13 cells (62%), PPT rapidly decreased IPSC amplitude, by 65 ± 3%, and E2 application after PPT produced no further suppression (Fig. 1K). PPT also increased PPR, from 0.80 ± 0.04 to 1.13 ± 0.06 (paired t test, p < 0.01; Fig. 1L). In 5 cells in which IPSC amplitude was not affected by PPT (5 ± 2%), PPR also was unchanged. Two of 8 PPT-responsive recordings were of unitary IPSCs, in which PPT decreased IPSC amplitude by 65% and 77%. In contrast to PPT, the ERβ agonist DPN (500 nM) failed to affect IPSCs in any of 12 cells. In 6 recordings with DPN, E2 applied after DPN suppressed IPSCs, confirming their E2-sensitivity. DPN alone produced negligible changes in IPSC amplitude (5 ± 3%) and PPR, whereas E2 applied after DPN decreased IPSC amplitude by 50 ± 6% and increased PPR from 0.94 ± 0.05 to 1.33 ± 0.09 (paired t test, p < 0.01; Fig. 1M-O). Two of 6 E2-responsive DPN recordings were of unitary IPSCs, both of which showed a 64% E2-induced decrease in amplitude.
Estradiol-induced suppression of inhibition requires CB1 receptor activation
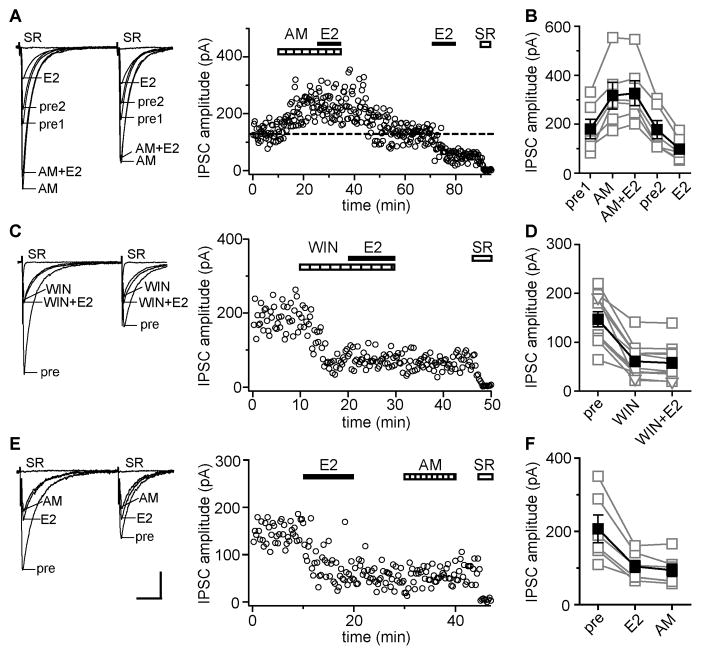
A subset of perisomatic inhibitory axonal boutons in CA1 contain CB1Rs that mediate suppression of GABA release by retrograde endocannabinoid signaling (Katona et al., 1999), as occurs in depolarization-induced suppression of inhibition (DSI; Wilson and Nicoll, 2001) and long-term depression of inhibition (I-LTD; Chevalreyre and Castillo, 2003). We found that E2-induced IPSC suppression also requires CB1Rs. Blocking CB1Rs with AM251 (AM, 10 μM) increased IPSC amplitude in 10 of 27 cells (37%), indicating tonic endocannabinoid-mediated suppression of inhibition. The effect of AM was reversible within ~20 min. In 7 experiments, we applied E2 twice, first after establishing a new stable (higher) baseline in AM and then again after reestablishing the original baseline following AM washout (Fig. 2A). E2 (100 nM) had no effect on IPSC amplitude (5 ± 3%) or PPR in the presence of AM. In 6 cells (86%), the second application of E2 after AM washout decreased IPSC amplitude by 43 ± 4% (Fig. 2B), confirming E2-sensitivity of IPSCs. E2-induced IPSC suppression after AM washout was paralleled by increased PPR, from 0.90 ± 0.03 to 1.04 ± 0.04 (paired t test, p < 0.05). In the one cell classified as not responding to E2 after AM washout, E2 tended to decrease IPSC amplitude but only by ~15%. In the other 3 recordings of AM-sensitive IPSCs, we applied E2 after the AM-induced increase was established and continued AM throughout the experiment. As before, E2 failed to affect IPSC amplitude (3 ± 4%) or PPR in the presence of AM.
Figure 2. Estradiol-induced IPSC suppression requires CB1Rs for induction, but not maintenance.
(A) Recording of AM251 (CB1R antagonist, AM, 10 μM)-sensitive IPSCs in which E2 was applied in the presence then absence of AM; individual traces and time course are shown. Each point is an individual sweep and SR 95531 (SR, 2 μM) applied at the end of the experiment blocked IPSCs (also in C, E). Dotted line shows average IPSC amplitude during 2 min before the second E2 application. AM blocked E2-induced IPSC suppression.
(B) Group IPSC amplitude data for all experiments as in A (n=6). Connected open symbols are individual cells; filled symbols are mean ± SEM in each condition for all cells (also in D, F)
(C) Recording of WIN 55,212-2 (CB1R agonist, WIN, 5 μM)-sensitive IPSCs; individual traces and time course are shown. WIN occluded E2-induced IPSC suppression.
(D) Group IPSC amplitude data for all experiments as in C (n=11).
(E) Recording of E2-sensitive IPSCs in which AM was applied after the E2-induced decrease in IPSC amplitude was established; individual traces and time course are shown. AM applied after E2 washout had no effect on IPSCs. Scale indicates 50 pA, 25 msec and also applies to A, C.
(F) Group IPSC amplitude data for all experiments as in E (n=6).
In 17 cells in which AM did not affect IPSCs, we applied E2 in the presence of AM and either washed both from the slice simultaneously (6 cells) or continued E2 for 10 additional min after AM washout (11 cells). As before, E2 never affected IPSC amplitude (1 ± 2%) or PPR in the presence of AM. In 9 of 11 cells in which we continued E2 after AM washout, IPSC amplitude remained unchanged in E2 (1 ± 3%) indicating that these were not E2-sensitive IPSCs. In the other 2 cells (18%), E2 decreased IPSC amplitude by 56% and 38% once AM was washed out. Together, these experiments demonstrated that inhibiting CB1Rs with AM blocks E2-induced IPSC suppression, and that while E2 can affect both AM-sensitive and insensitive IPSCs, AM-sensitive IPSCs are more likely to respond to E2 (86% vs. 18%).
To corroborate results with AM, we applied the CB1R agonist WIN 55,212-2 (WIN, 5 μM; Fig. 2C). WIN rapidly suppressed IPSCs and increased PPR in 11 of 12 (92%) cells, indicating that most recordings involved at least some CB1R-containing synapses. The WIN-induced decrease in IPSC amplitude was 59 ± 5% and WIN increased PPR from 0.76 ± 0.02 to 1.02 ± 0.07. Importantly, E2 applied in the presence of WIN induced no further suppression of IPSCs (3 ± 1%; Fig. 2D) or change in PPR (also 1.02 ± 0.07). Thus CB1R activation by WIN fully occluded E2’s effects on IPSCs confirming that E2-induced suppression of inhibition requires CB1Rs. To test whether CB1Rs are necessary for maintenance of E2-induced IPSC suppression, we applied AM following E2 washout, after IPSC suppression was established (Fig. 2E). In 6 of 6 cells, E2 decreased IPSC amplitude by 48 ± 4% and AM applied after E2 had no further effect on IPSC amplitude (8 ± 4%; Fig. 2F) or PPR. Thus acute suppression of inhibition by E2 requires CB1Rs for induction, but not maintenance.
Roles of 2-arachidonoylglycerol vs. anandamide in estradiol-induced IPSC suppression
Results with AM and WIN suggested that E2 suppresses inhibition by mobilizing endocannabinoids. There are two predominant endocannabinoids that act at GABAergic synapses in CA1 to suppress inhibitory synaptic transmission, 2-arachidonoylglycerol (2-AG) and anandamide (aka N-arachidonoylethanolamide or AEA). 2-AG and AEA are synthesized either tonically or on demand, and their levels are tightly regulated by distinct enzymatic pathways, which provides a way to investigate the roles of each in modulating synaptic transmission.
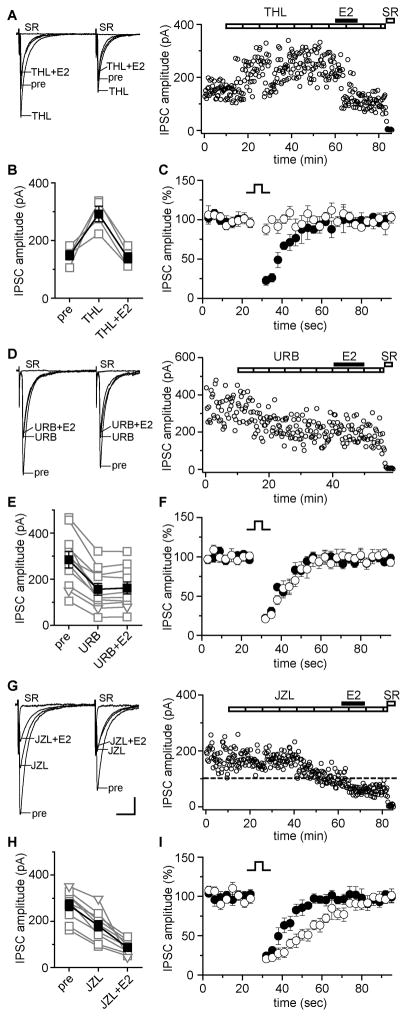
First we tested whether E2 could suppress IPSCs in the presence of the diacylglycerol lipase (DGL) inhibitor, tetrahydrolipstatin (THL, 10 μM), which inhibits synthesis of 2-AG and consequently blocks DSI when bath applied (Hashimotodani et al., 2008). For each cell, we tested DSI before and after applying THL, to confirm inhibition of 2-AG synthesis. In 4 of 9 cells (44%), THL increased IPSC amplitude, consistent with relief from tonic 2-AG-mediated suppression; in the remaining 5 cells, THL alone had no effect. In 6 of 9 (67%) cells, both THL-sensitive (4 cells, Fig. 3A, B) and THL-insensitive (2 cells, not shown), E2 (100 nM) decreased IPSC amplitude in the presence of THL, by 59 ± 7% (Fig. 3B). We confirmed that DSI was blocked by THL, indicating inhibition of 2-AG synthesis, before E2 was applied (Fig. 3C). Thus inhibiting 2-AG synthesis failed to block E2-induced IPSC suppression, a first indication that 2-AG is not required for E2-induced suppression of IPSCs.
Figure 3. Estradiol-induced IPSC suppression is mediated by AEA, not 2-AG.
(A) Recording of THL (DGL inhibitor, 10 μM)-sensitive IPSCs showing individual traces and time course. Each point is an individual sweep and SR 95531 (SR, 2 μM) applied at the end of the experiment blocked IPSCs (also in D, G). THL did not block E2-induced IPSC suppression.
(B) Group IPSC amplitude data for experiments as in A (n=4). Connected open symbols are individual cells; filled symbols are mean ± SEM for all cells (also in E, H).
(C) DSI tested before (filled symbols) and in the presence of (open symbols) THL confirmed that DSI was blocked before E2 application. Points are mean ± SEM for all E2-responsive THL experiments.
(D) Recording of URB 597 (FAAH inhibitor, URB, 1 μM)-sensitive IPSCs showing individual traces and time course. URB occluded E2-induced suppression of IPSCs.
(E) Group IPSC amplitude data for all experiments as in D (n=11). Compound IPSCs (squares) and unitary IPSCs (triangles) are plotted together (also in H).
(F) DSI tested before (filled symbols) and in the presence (open symbols) of URB confirmed that DSI was not affected by URB. Points are mean ± SEM for all experiments in E.
(G) Recording of JZL 184 (MGL inhibitor, JZL, 100 nM)-sensitive IPSCs showing individual traces and time course. Dotted line shows average IPSC amplitude during 2 min before E2 application. JZL did not occlude E2-induced IPSC suppression. Scale indicates 25 pA, 25 msec (50 pA, 25 msec in A, D).
(H) Group IPSC amplitude data for all experiments as in G (n=9).
(I) DSI tested before (filled symbols) and in the presence (open symbols) of JZL before E2 application showed that the decay of DSI was prolonged in JZL. Points are mean ± SEM for all experiments in H.
There is no selective inhibitor of AEA synthesis available. As an alternative, we compared the effect of blocking breakdown of AEA vs. 2-AG using selective inhibitors of fatty acid amide hydrolase (FAAH, for AEA) or monoacylglycerol lipase (MGL, for 2-AG). Because such inhibitors increase levels of their respective endocannabinoids, we reasoned that inhibition of endocannabinoid degradation might occlude E2’s ability to suppress IPSCs. The FAAH inhibitor URB 597 (URB, 1 μM) decreased IPSC amplitude in 11 of 14 (79%) cells, by 47 ± 4% (Fig. 3D, E), indicating tonic accumulation of AEA, whereas in the remaining 3 cells URB had no effect. Importantly, E2 (100 nM) applied in the presence of URB induced no further decrease in IPSC amplitude (4 ± 2%; Fig. 3E), indicating that inhibition of FAAH completely occluded E2-induced IPSC suppression. Similarly, E2 had no effect on IPSC amplitude in the 3 URB-insensitive cells (1 ± 4%). Consistent with the role of 2-AG rather than AEA in mediating DSI in the hippocampus (Kim and Alger, 2004; Pan et al., 2009), DSI was unaffected by URB (Fig. 3F). These findings suggested that AEA mediates E2-induced IPSC suppression.
To corroborate interpretation of results with URB, we performed analogous experiments with the MGL inhibitor, JZL 184 (JZL, 100 nM), which blocks breakdown of 2-AG (Pan et al., 2009). Because inhibition of 2-AG synthesis by THL failed to block E2-induced IPSC suppression, we hypothesized that inhibiting 2-AG breakdown with JZL would fail to occlude E2-induced IPSC suppression. In 13 of 16 cells (81%), JZL decreased IPSC amplitude, by 37 ± 3% (Fig. 3G, H). Once a stable baseline in JZL was established (~40 min), we applied E2 (100 nM) to determine whether further IPSC suppression was possible. In contrast to results with URB, E2 applied in the presence of JZL decreased IPSC amplitude by 49 ± 5% in 9 of 16 cells (Fig. 3H), almost identical to the effect of E2 alone. The remaining 7 cells, 4 JZL-sensitive and 3 JZL-insensitive, showed no effect of E2 (1 ± 3%). Consistent with accumulation of 2-AG (Pan et al., 2009), JZL prolonged the time course of DSI (Fig. 3I). Thus together with results using the 2-AG synthesis inhibitor THL, JZL experiments confirmed that 2-AG plays little to no role in E2-induced suppression of IPSCs. Comparing the complete occlusion of E2-induced IPSC suppression by inhibition of AEA breakdown with URB to the lack of occlusion by inhibition of 2-AG breakdown with JZL, these results strongly suggest that AEA mediates E2-induced IPSC suppression.
Estradiol-induced IPSC suppression requires mGluR1 and is sex-specific
E2-induced IPSC suppression resembles I-LTD more than DSI in that brief E2 exposure produces a lasting decrease in IPSC amplitude that depends on CB1Rs for induction but not maintenance. I-LTD is typically induced by trains of stimuli delivered to the str. radiatum; glutamate released during the train activates postsynaptic mGluRs that are coupled to endocannabinoid synthesis (Chevalreye and Castillo, 2003). Our experiments, however, involved neither trains nor stimulation in the str. radiatum. How could E2 produce a similar effect in the absence of released glutamate? Mermelstein and colleagues have shown in cultured hippocampal neurons that E2 can bind a membrane form of ERα to acutely activate mGluR1 in the absence of released glutamate (Boulware et al., 2005). To investigate whether a similar mechanism is involved in E2-induced suppression of inhibition, we tested whether mGluR1 and mGluR5 antagonists can inhibit E2-induced IPSC suppression.
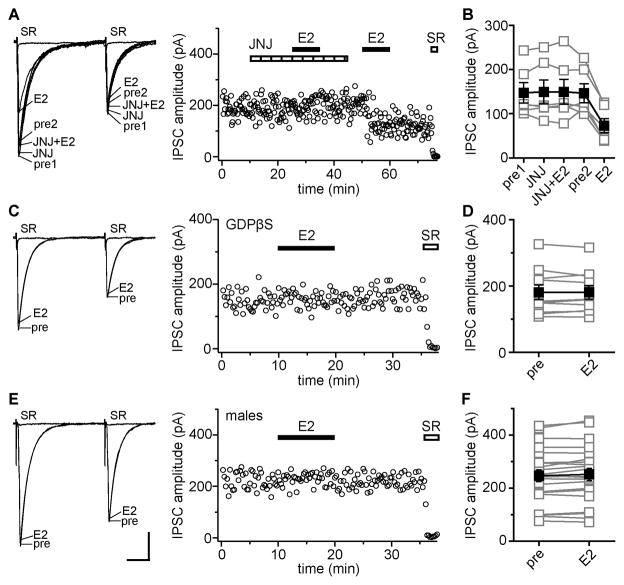
The mGluR1 antagonist JNJ 16259685 (JNJ, 0.2 μM) completely blocked E2-induced IPSC suppression (Fig. 4A). In 6 of 11 cells (55%), E2 had no effect on IPSCs in the presence of JNJ (2 ± 2%) but then decreased IPSC amplitude by 52 ± 5% after JNJ washout (Fig. 4B). The remaining 5 cells recorded with JNJ were not E2-responsive (7 ± 2%). The combination of JNJ and the mGluR5 inhibitor MPEP (40 μM), or the mGluR1/5 inhibitor CPCCOEt alone (100 μM), also blocked E2-induced IPSC suppression. In 6 cells, E2 had no effect on IPSC amplitude in JNJ + MPEP (2 ± 1%), but decreased IPSC amplitude by 52 ± 7% after washout. Similarly, E2 had no effect on IPSC amplitude in 4 cells recorded in CPCCOEt (3 ± 3%), but decreased IPSC amplitude by 47 ± 7% after washout. In contrast to JNJ, MPEP alone did not block E2-induced IPSC suppression. In 3 cells, E2 decreased IPSC amplitude by 65 ± 4% in the presence of MPEP. Thus inhibiting mGluR1, but not mGluR5, blocks E2-induced IPSC suppression. To investigate whether E2-induced IPSC suppression depends on pre- or postsynaptic mGluR1, we tested whether E2 could suppress IPSCs with postsynaptic G protein signaling blocked by GDPβS in the recording pipette (Fig. 4C). E2 (100 nM) had no effect on IPSC amplitude in any of 10 GDPβS-loaded cells (0.7 ± 1.7%, Fig. 4D), strongly suggesting that the mGluR1 required to induce IPSC suppression is postsynaptic.
Figure 4. E2-induced IPSC suppression requires postsynaptic mGluR 1 and is sex-specific.
(A) Recording of E2-sensitive IPSCs in which E2 was applied in the presence and then absence of JNJ 16259685 (mGluR1 antagonist, JNJ, 0.2 μM); individual traces and time course are shown. Each point is an individual sweep and SR 95531 (SR, 2 μM) applied at the end of the experiment blocked IPSCs (also in C, E). JNJ blocked E2-induced IPSC suppression.
(B) Group amplitude data for all experiments as in A (n=6). Connected open symbols are individual cells; filled symbols are mean ± SEM for all cells (also in D, F).
(C) Recording of IPSCs with GDPβS in the recording pipette showing individual traces and time course. E2 had no effect on IPSCs with postsynaptic G protein signaling blocked by GDPβS.
(D) Group IPSC amplitude data for all experimetns as in C (n=10).
(E) Recording of IPSCs in a male tested with 100 nM E2 showing individual traces and time course. E2 did not affect IPSCs. Scale indicates 25 msec, 50 pA (also in A, C).
(F) Group IPSC amplitude data for all but one recording in males. E2 (10 nM, n=7 or 100 nM, n=15) had no effect on IPSC amplitude in 22 of 23 cells. In one recording not represented here, E2 produced a rapidly reversible decrease in IPSCs amplitude.
One unusual feature of the E2-ERα-mGluR1 interaction demonstrated by Boulware et al. (2005) is that it occurs only in cultures derived from female rat pups and not from males. Because E2-induced IPSC suppression appeared to depend on a similar mechanism, we asked whether this effect of E2 is also sex-specific (Fig. 4E). In 23 cells recorded from males, E2 had no effect on IPSC amplitude (Fig. 4F) or PPR in any of 15 cells tested with 100 nM E2 or in 7 of 8 cells with 10 nM E2. Whether males were gonadally intact (17 cells, 4 ± 1%) or castrated (5 cells, 1 ± 3%) did not affect the results. By contrast, the same concentrations of E2 decreased IPSC amplitude and increased PPR in 55% of cells in females (Fig. 1). In 1 male cell, 10 nM E2 did decrease IPSC amplitude, by 26%, but the effect reversed quickly upon E2 washout; in females, IPSC amplitude always remained low after E2 washout. These results show that acute E2-induced IPSC suppression occurs much more often in females than males. In the rare instances in which E2 does affect IPSCs in males, this occurs through a distinct mechanism.
DISCUSSION
We show here that E2 acutely suppresses synaptic inhibition in the hippocampus through a previously unknown and sex-specific mechanism. E2 activates ERα-mGluR1-dependent mobilization of AEA, which decreases the probability of GABA release at a subset of CB1R-containing presynaptic inputs. More cells were responsive to the CB1R agonist WIN (92%) than to E2 (55%), indicating that the presence of ERα, mGluR1, and the appropriate coupling between them are likely to be limiting factors that determine which CB1R-containing inputs respond to E2. The effect of E2 could be robust, up to a 77% reduction in unitary IPSC amplitude, was initiated within a few minutes, and was not readily reversible. Our findings are the first to demonstrate that E2 acutely regulates synaptic inhibition in the hippocampus, and show that endogenous AEA can be mobilized in the hippocampus to activate CB1R-dependent plasticity of inhibitory synapses. Acute modulation of inhibition may be an important mediator of neurosteroid E2 actions.
That E2 acts though AEA and not 2-AG to modulate inhibition was surprising because other types of acute CB1R-dependent signaling in the hippocampus, such as in DSI and I-LTD, are mediated by 2-AG (Chevaleyre and Castillo, 2003; Kim and Alger, 2004; Pan et al., 2009). The main distinction between our experiments and previous studies is that we used females. That we studied females may also explain why other studies with males have not seen evidence of tonic AEA mobilization (Kim and Alger, 2004), which we did observe, or an effect of E2 on field IPSPs (Kramar et al, 2009). These contrasts point to substantial differences between males and females in modulation of synaptic function in the hippocampus, which has not been considered previously.
The dependence of E2-induced IPSC suppression on postsynaptic G protein signaling strongly suggests that E2 activates a postsynaptic ERα-mGluR interaction to stimulate AEA mobilization. This does not rule out presynaptic E2 action as well, however. Extranuclear ERα is found in perisomatic axonal boutons in CA1, particularly of CCK-containing inhibitory interneurons (Hart et al., 2007), which are also those that contain CB1Rs (Katona et al., 1999). Whether sex differences in pre- and/or postsynaptic extranuclear ERα signaling (Romeo et al., 2005) contribute to the lack of E2 effect in males remains to be determined. Comparing the levels, distribution, and function of each step in the pathways(s) leading from E2 activation of extranuclear ERα to modulation of GABA release in both males and females may point to which of the many signaling pathways acutely activated by E2 are relevant to acute suppression of inhibitory synaptic transmission.
E2 is well known to influence hippocampal functions such as memory and affective behaviors that differ between the sexes (Gillies and McArthur 2010), as well as neurological disorders that involve the hippocampus such as temporal lobe epilepsy (Guille et al., 2008). Most behavioral studies have examined effects of E2 in females and on a time scale corresponding to ovarian E2 fluctuations, which is much slower than the acute suppression of inhibition we report here. In addition, the concentrations of E2 required for acute suppression of IPSCs, 10–100 nM, are higher than peak circulating levels (~100 pM) indicating that inhibitory synapses are likely to be protected from acute modulation by relatively slow and low amplitude fluctuations in ovarian E2. In contrast, neurosteroid E2 is reportedly 5–10 nM on average (Hojo et al., 2009), likely higher near sites of aromatase activity, and its synthesis may be activity-dependent (Hojo et al., 2004). Thus neurosteroid E2 could provide a localized source of E2 to acutely modulate synaptic inhibition in vivo. A better understanding of sex-specific synaptic modulation in the hippocampus and how E2 acutely regulates endocannabinoid tone in females may point to targets for novel therapies to combat neurological or mental health disorders that differ between the sexes.
EXPERIMENTAL PROCEDURES
Animals were adult female (ovariectomized) or male (castrated or gonadally intact) rats. Using standard methods, hippocampal slices were prepared and whole-cell voltage-clamp recordings were made at 34–35°C with a K-gluconate-based internal solution. See Supplemental Experimental Procedures. Of note, E2 modulation of IPSCs was never observed when recording with a CsCl-based internal solution, possibly owing to interference with postsynaptic G protein-coupled signaling. All drugs used are noted in the text, including concentrations. Data are reported as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Indira Raman for many helpful discussions. This work was supported by R01 NS037324 (C.S.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25(20):5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Fugger HN, Kumar A, Lubahn DB, Korach KS, Foster TC. Examination of estradiol effects on the rapid estradiol mediated increase in hippocampal synaptic transmission in estrogen receptor α knockout mice. Neurosci Lett. 2001;309:207–209. doi: 10.1016/s0304-3940(01)02083-3. [DOI] [PubMed] [Google Scholar]
- Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010;62:155–198. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guille C, Spencer S, Cavus I, Epperson CN. The role of sex steroids in catamenial epilepsy and premenstrual dysphoric disorder: implications for diagnosis and treatment. Epilepsy Behav. 2008;13(1):12–24. doi: 10.1016/j.yebeh.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-alpha-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J Neurosci. 2007;27(8):2102–2111. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Maejima T, Fukami K, Kano M. Pharmacological evidence for the involvement of diacylglycerol lipase in depolarization-induced endocannabinoid release. Neuropharmacol. 2008;54(1):58–67. doi: 10.1016/j.neuropharm.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori T, Enami T, Furukawa A, Suzuki K, Ishii H, Mukai H, Morrison JH, Janssen WGM, Kominami S, et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc Natl Acad Sci USA. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Higo S, Ishii H, Ooishi Y, Mukai H, Murakami G, Kominami T, Kimoto T, Honma S, Poirier D, Kawato S. Comparison between hippocampus-synthesized and circulation-derived sex steroids in the hippocampus. Endocrinol. 2009;150(11):5106–5012. doi: 10.1210/en.2009-0305. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axonal terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J Neurosci. 2009;29:12982–12993. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Wang W, Long JZ, Sun D, Hillard CJ, Cravatt BF, Liu Q. Blockade of 2-arachidonoylglycerol hydrolysis by selelctive monoacylglycerol lipase inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxyl)methyl)piperidine-1-carboxylate (JZL 184) enhances retrograde endocannabinoid signaling. J Pharmacol Exp Ther. 2009;331:591–597. doi: 10.1124/jpet.109.158162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, McCarthy JB, Wang A, Milner TA, McEwen BS. Sex differences in hippocampal estradiol-induced N-methyl-D-aspartic acid binding and ultrastructural localization of estrogen receptor-alpha. Neuroendocrinol. 2005;81:391–399. doi: 10.1159/000089557. [DOI] [PubMed] [Google Scholar]
- Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 2010;30:16137–16148. doi: 10.1523/JNEUROSCI.4161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyler TJ, Vardaris RM, Lewis D, Rawitch AB. Gonadal steroids: effects on excitability of hippocampal pyramidal cells. Science. 1980;209:1017–1019. doi: 10.1126/science.7190730. [DOI] [PubMed] [Google Scholar]
- Wong M, Moss RL. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J Neurosci. 1992;12:3217–3225. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Ann Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.