INSULIN-LIKE GROWTH FACTORS
Overview and Discovery of Insulin-Like Growth Factors
The IGF family includes three structurally related ligands: insulin, IGF-I, and IGF-II and two high-affinity cell-surface receptors: the IGF-I receptor (IGF-IR) and the IGF-II receptor (IGF-IIR). Although the insulin receptor (IR) shares significant, ~70%, sequence homology with the IGF-I receptor it possesses a distinct ligand affinity profile. The IGF binding proteins (IGFBP1–6) are important physiologic regulators of the interaction of IGFs with their receptors within the gastrointestinal tract and liver. Growth hormone (GH) is structurally unrelated to IGFs, but its actions are mediated primarily through regulation of IGF-I synthesis and secretion by the liver; hence, GH and its biology are intimately intertwined with IGF-I and are referenced in this chapter.
The IGF system functions as a leading endocrine, paracrine, and autocrine regulatory axis for cellular proliferation, survival, and apoptosis in the gastrointestinal tract. In addition, it has general activities relating to energy metabolism, body size, carcinogenesis, and various organ specific functions. A number of comprehensive reviews have been written (1–9).
Insulin-Like Growth Factor Genes and Proteins in the Gastrointestinal Tract and Liver
IGF-I and IGF-II are two closely related members of the insulin superfamily of peptide hormones. IGF-I and IGF-II are 67% identical, single-polypeptide chains that share ~40% amino acid identity with insulin. Unlike insulin, IGFs are not produced and stored solely in β-cells of the pancreatic islets as is insulin. They are synthesized and secreted by many cells in the body, including cells of the gastrointestinal tract and liver, in a highly regulated manner.
The IGF-I gene is located on human chromosome 12q22–q23. The genomic sequence is large, spanning more than 80 kB DNA, including 6 exons (10). At least 4 transcriptional start sites have been identified, and IGF-I mRNA species range from about 1.0 to 8.0 kB. Complex, tissue-specific alternate splicing patterns have been observed, but the biological significance of these variants has not been clearly delineated for gastrointestinal tissues (11). For human pro-IGF-I, two primary translation products exist, IGF-IEa and IGF-IEb. The most commonly recognized mature IGF-I. IGF-IEa, is a 70-amino-acid, secreted protein. The existance of a putative peptide derived from alternative splicing which yields the IGF-IEc variant, mechanogrowth factor (MGF), remains speculative (12). However, evidence suggests MGF plays a role in pathophysiologic response of the gastrointestinal smooth muscle to inflammation and injury (13).
The majority of IGF-I in the peripheral circulation is synthesized in the liver under the control of GH and circulates bound to IGFBPs, primarily IGFBP-3 and the acid-labile subunit (ALS) as a ternary complex. In this context, IGF-I is an endocrine hormone growth factor that stimulates somatic growth and exerts feedback to the pituitary to down-regulate GH synthesis. Most growth-stimulating activities of GH can be mimicked by IGF-I. Peripheral tissues in the gastrointestinal tract are also abundant sources of IGF-I, particularly intestinal smooth muscle, that acts in an autocrine and paracrine fashion to regulate cell growth and survival. While GH regulates IGF production in the liver and in other tissues as well under normal circumstances (see later), in disease states, e.g. malnutrition and Crohn’s disease, a relative GH-insensitive state exists whereby the stimulatory effect of GH on IGF-I expression and secretion are markedly reduced and IGF-I expression is regulated by other factors. IGF-I binds to the IGF-IR with high affinity and to the IGF-IIR with lower affinity.
The human IGF-II gene is located on chromosome 11p15.5. Like the IGF-I precursor, the IGF-II precursor is large, and multiple splice variants have been described, many of which are developmentally regulated and contain gut-specific promoters (14). A pro-IGF-II–containing carboxy-terminal precursor sequence is secreted from cells before processing to the mature 67-amino-acid protein (5). The IGF-II gene is relatively unique in that it is “imprinted” in 90% of humans, meaning that normally one allele is silenced on the basis of parental origin. When maternal IGF-II silencing is lost (LOI), biallelic IGF-II expression correlates strongly with the hypomethylation of a differentially methylated region (DMR) near its promoter and results in increased IGF-II levels. IGF-II levels resulting from LOI are increased and are associated with an increased risk for a variety of cancers including colorectal cancer (15) More recently other mechanisms responsible for elevated levels of IGF-II in colorectal cancer have been identified including microsatellite instability (16). IGF-II binds to both IGF-1R and IGF-IIR with high affinity.
Insulin-Like Growth Factor Receptor Genes and Proteins
The IGF-I receptor (IGF-IR) gene is located on human chromosome 15q26.3 and is 70% identical to the insulin receptor gene (17). The protein is synthesized as a single polypeptide chain that is cleaved by proteolysis at a tetrabasic amino acid site resulting in α and β subunits. These subunits associate by disulfide bridging into α and β heterodimers that further associate by disulfide bridging forming the mature heterotetrameric α2β2 receptors. Structurally, this overall configuration is identical to the insulin receptor; in fact, hybrid IGF-IR and insulin receptors are well recognized (18). Most biological activities of IGF-I and IGF-II in the gastrointestinal tract and liver are mediated by the IGF-IR.
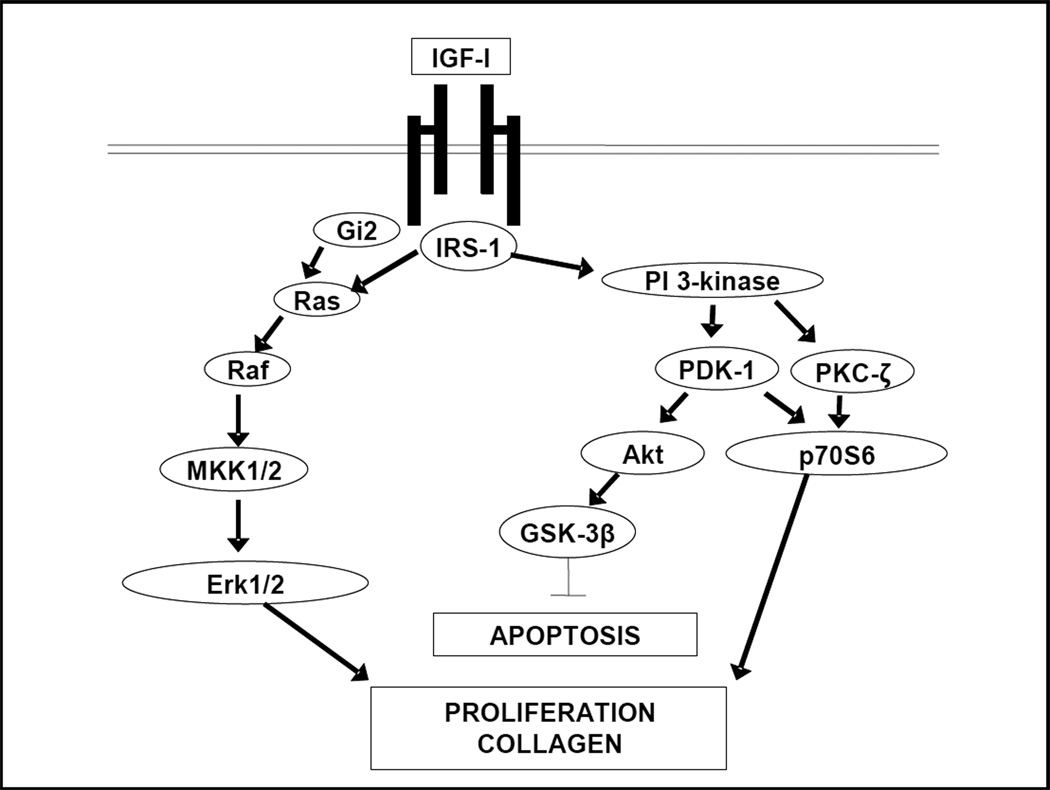
IGF-I binding results in autophosphorylation of specific cytosolic tyrosine residues within the IGF-IR receptor, activation of its intrinsic tyrosine kinase activity, and phosphorylation of intracellular substrates (Fig. 1). Insulin receptor substrate 1 (IRS-1), a 185-kDa intracellular signaling protein with multiple phosphorylation sites and SH domains permitting docking of multiple intracellular signaling molecules is a key early mediator of IGF-IR function (19). The result of IRS-1 phosphorylation is activation of a variety of signaling cascades, including the Ras-Erk1/2 and phosphoinosotide-3 kinase (PI-3K) pathways. The IGF-IR also activates the heterotrimeric G-protein, Gi2, that is coupled to activation of the Erk1/2 pathway (20). In intestinal smooth muscle, the intensity and duration of IGF-I stimulated IGF-IR activity is positively regulated by ligand occupancy of αVβ3 integrin by the resultant temporally regulated translocation of SH2 domain-containing tyrosine phosphatase-2 from αVβ3 integrin to the IGF-I receptor (21). Activation of IGF-IR regulates cellular proliferation and survival, key regulatory activities of IGFs in the gastrointestinal tract.
Figure 1.
Signaling cascades activated by the activated IGF-I receptor. The IGF-I receptor is located on the basolateral membrane of intestinal epithelial cells and on smooth muscle cells. Ligand binding and activation of the IGF-I receptor elicts phosphrylation of cytoplasmic tyrosine residues in this receptor tyrosine kinase. Subsequently binding of scaffolding and docking proteins results in activation of distinct intracellular signaling cascades that regulate proliferation and survival and collagen IαI expression.
The IGF-IIR, also referred to as the cation-independent mannose-6 phosphate receptor, bears no structural homology with the IR or IGF-IR (9). It is located on human chromosome 6q26. The IGF-IIR receptor is a single, transmembrane polypeptide that binds IGF-II with a greatly reduced affinity for IGF-I and insulin. It has 15 cysteine-laden, contiguous, extracellular repeats and a short intracellular sequence with no recognizable signaling motifs (4). While the function of IGF-II signaling is controversial, and most experts believe it down-modulates IGF-II activity by regulating its endocytosis and intracellular degradation by targeting the proteins to the lysosome. This is relevant to the gastrointestinal tract and is consistent with data showing that IGF-IIR is a tumor-suppressor gene, acting as a “sink” or reservoir for IGF-II and consistent with the effects of LOI of IGF-II in colorectal cancer(6, 15, 16, 22).
Insulin-Like Growth Factor Binding Protein Gene and Protein Family in the Gastrointestinal Tract
IGFBPs are a well-characterized family of six secreted proteins, designated IGFBP1–6, that bind IGFs with high affinity and display a broad spectrum of biological activity. Extensive information about the gene organization, protein structure, and molecular biology and physiology of IGFBPs can be found in the reviews referenced at the beginning of this section. The IGFBPs are potent modulators of IGF activity in the gastrointestinal tract, exerting both positive and negative effects, because their generally 10- to 100-fold greater affinity for IGFs than for the IGF receptors. Both IGF-I and IGF-II bind to all six IGFBPs, albeit with different affinity.
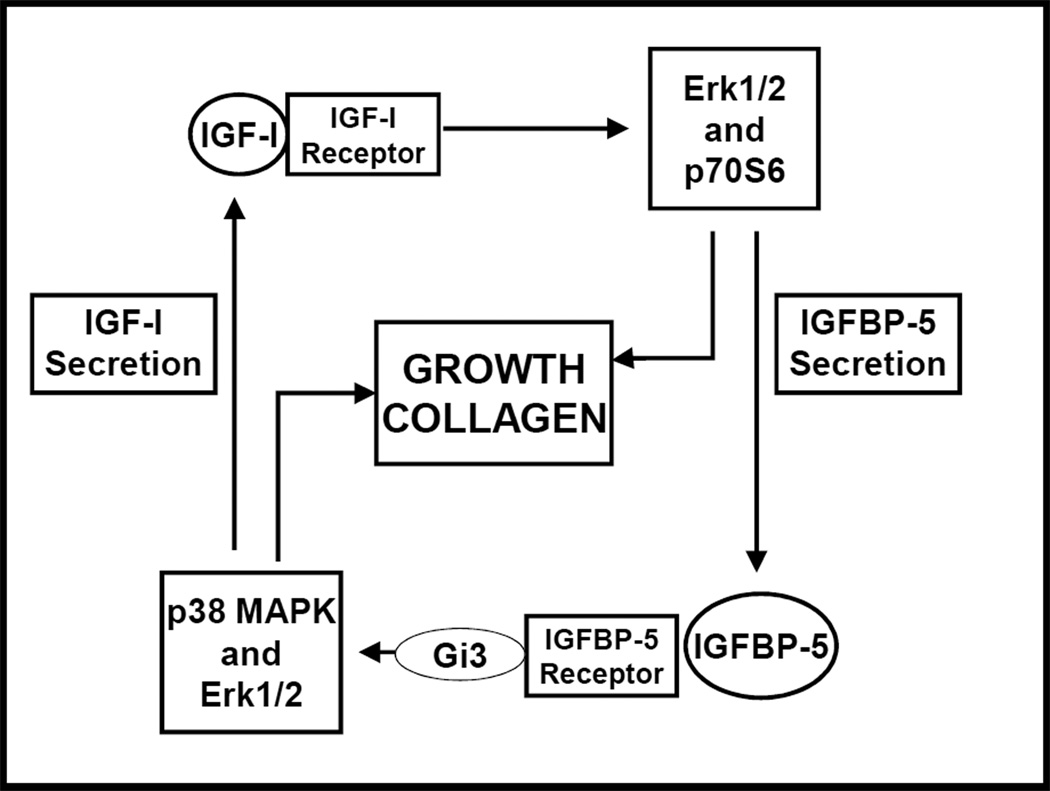
The structure of all six IGFBPs are similar due to their conserved evolution and can be divided into three domains: highly conserved C- and N-terminal domains, and a central domain that is unique among IGFBP family members. The C- and N-terminal domains bind IGFs. It is the central domain that is modified by posttranslational modifications and confers functional diversity among IGFBPs (7). Depending on the experimental setting and IGF function being assessed, IGFBPs may potentiate or inhibit IGF activity in the gastrointestinal tract primarily by altering the interaction of IGF-I or IGF-II with the IGF-IR. IGFBPs exert inhibitory effects by binding IGFs into a biologically inaccessible pool. Whereas stimulatory or potentiation of IGF action occurs by the facilitiation of IGFs binding with the IGF-IR. Regulated proteolysis of IGFBPs is a key mechanism for release of IGF and regulating its bioavailability to cells of the gastrointestinal tract (7). For example, addition of IGFBP-3 to colon cancer cell culture medium decreases bioactivity of IGF-I. Addition of MMP-7 cleaves IGFBP-3 into four fragments, releases IGF-I, and potentiates IGF-I biological activity (7). An important function of hepatic-derived IGFBP function is to transport IGFs made in the liver in the circulation and in extracellular fluids. IGFBPs are detectable in plasma and extracellular fluids and are expressed during GI tract development and into adult life. The primary hepatic derived IGFBP is IGFBP-3, which carries approximately 75% of circulating IGF-I and IGF-II bound to it and the co-carrier, ALS (7). Activities of IGFBPs (IGFBP-1, IGFBP-3, IGFBP-4 and IGFBP-5) in the gastrointestinal tract that are IGF-independent are also recognized. These play a role both in GI tract physiology and in pathophysiologic events. IGFBP-3 directly activates the TGF-βRI/II receptor complex and initiates Smad signaling in human intestinal smooth muscle cells (23, 24). In intestinal muscle cells, while IGFBP-5 facilitiates interaction of IGF-I with the IGF-IR, it also acts independently of IGF-I to stimulate proliferation and further increase expression of IGF-I (Fig 2.) (25). Both processes involving IGFBP-3 and IGFBP-5 play a role in muscle hyperplasia in stricturing Crohn’s disease but also the comcomitant excess collagen production.
Figure 2.
Positive feedback between IGFBP-5 and IGF-I. The expression and effects of IGF-I and IGFBP-5 are linked whereby IGF-I stimulates IGFBP-5 expression and IGFBP-5, independent of IGF-I, stimulates IGF-I expression each reinforcing the expression and effects of the other.
IGFBP-3 and IGFBP-5 also possess COOH-terminal consensus nuclear localization sequences that allow cell entry and the direct nuclear activity of these binding proteins via a β-importin-dependent nuclear translocation mechanism. By this mechanism IGFBP-3 regulates apoptosis in prostate cancer cells, and IGFBP-5 regulates heterodimerization of RXR and vitamin D receptors and modulates vitamin-D-dependent differentiation (26, 27). A NH2-terminal sequence is a consensus transactivator domain that has been shown to possess strong IGF-I-independent transactivation activity (28). The participation of these mechanisms in gastrointestinal tract function has not yet been examined.
Biology of the Insulin-Like Growth Factor Family in the Gastrointestinal Tract
The gastrointestinal tract is a major target organ of IGF action (29). One of the most prominent effects is stimulation of intestinal epithelial cell and muscle cell proliferation and maintenance of cell survival by reduction of apoptosis. Other activities relate to the diverse effects of this ligand/receptor family on somatic growth, energy balance, and metabolism of glucose, carbohydrate, and proteins.
Insulin-Like Growth Factor Family Distribution in the Gastrointestinal Tract
The presence and distribution of IGFs and IGF receptors in the gastrointestinal tract has been extensively characterized (11,14,30–40). IGF-I, IGF-II, and IGFBPs are also present in human breast milk and in gastrointestinal tract secretions (41–43). A portion of enterally administered 125I-IGF-I and 125I-IGF-II can be recovered intact from gastrointestinal tissues of suckling rats, which indicates that the peptide is stable in the milieu of the neonatal stomach and small intestine (44, 45). While the expression of this ligand receptor system in the gastrointestinal epithelium is clear, a clear understanding of its distribution along the crypt-villus axis and in the epithelial-mesenchymal compartments has not emerged. It is clear that both IGF-I and IGF-II bind to intestinal epithelial cells (35,37,39,46, 47) and that IGF-IR and IGF-IIR are targeted to the basolateral membrane domain of the enterocyte (48,49). Multiple components of the IGF system, including IGFBPs, are expressed in subepithelial myofibroblasts and lamina propria in the gastrointestinal tract, implying an important role in regulation of epithelial-mesenchymal interactions (50,51). The intestinotrophic effects of IGF-I are mediated by GLP2-dependent regulation of myofibroblast IGF-I expression. In support of this, transgenic mice in which IGF-I is overexpressed in the intestinal lamina propria under the direction of an α-smooth muscle actin promoter show increased proliferation of the ileal epithelium (52).
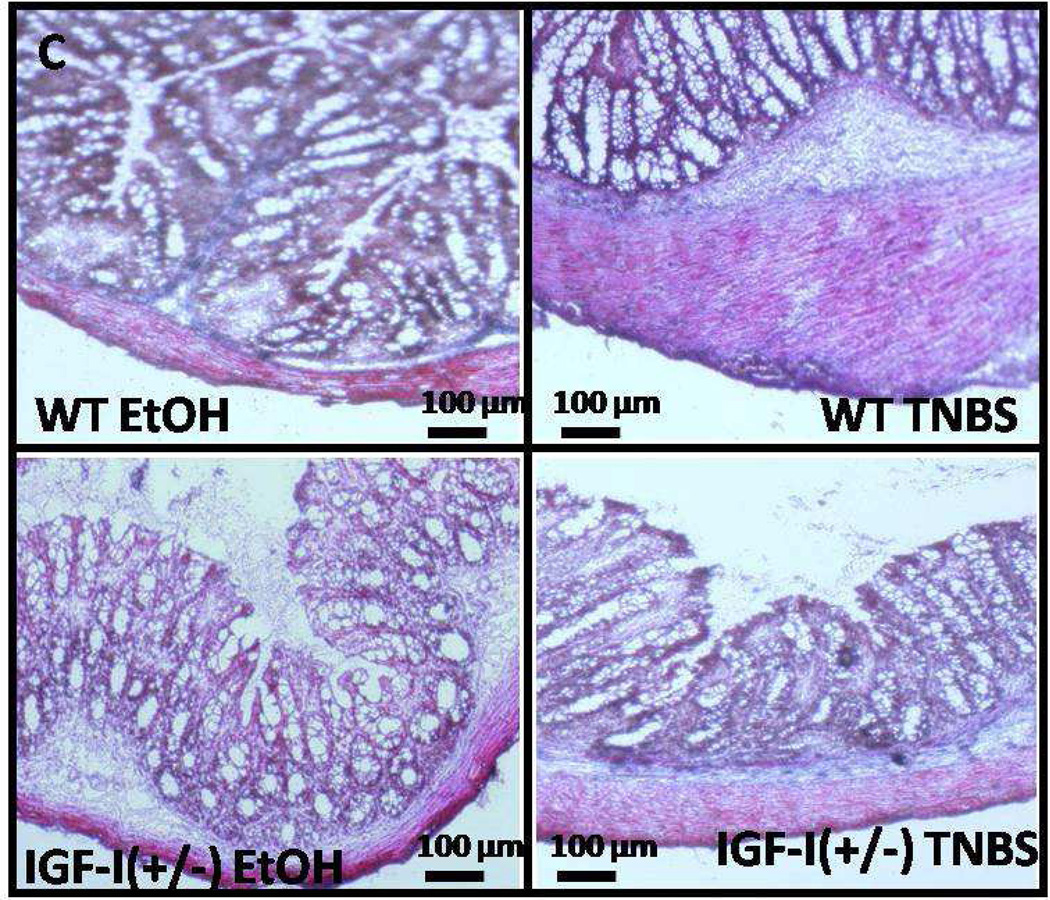
Several other observations in transgenic mice are worth noting. In mice with an hepatic deletion of IGF-I, the gastrointestinal tract, including the muscularis propria, develops normally. However, while mice overexpressing IGF-I have a normal gastrointestinal epithelium but expanded submucosa, and the muscularis propria is hyperplastic and hypertrophic (53). C57BL/6J mice heterozygous for IGF-I [IGF-I(+/−)], have normal gastrointestinal development, but with a thinner submucosal compartment compared wildtype mice in the neonatal period (54). The hyperplasia and stricturing that occurs during the course of TNBS-induced colitis is markedly attenuated in IGF-I(+/−) mice (Fig 3) (55). In aggregate, these observations highlight the autocrine role of IGF-I produced in the gastrointestinal tract, particularly by smooth muscle cells of the gastrointestinal tract, in its growth, development and response to inflammation.
Figure 3.
Inflammation-induced fibrosis is decreased in IGF-I(+/−) mice. Collagen deposition in smooth muscle layer of vehicle-treated IGF-I(+/−) mice and its increase in response to TNBS-induced colitis are lower than in wildtype C57BL/6J mice.
As noted earlier, most circulating IGF-I is synthesized in the liver under the regulation of GH. Consequently, hepatocyte levels of IGF-I are very high. IGFBPs are also synthesized in the liver. Interestingly, however, the normal hepatocyte is not considered a major target for IGF action, because of the extremely low level of IGF-IR expression (56). Hepatic stellate cells and myofibroblasts also express IGFBPs and IGF-I (57). It is these cells that are believed to play a pivotal role in the fibrogenic response to IGFs in the liver (58).
The expression of IGF-II and IGF-IIR are highly developmentally regulated. IGF-II RNA transcripts are readily detectable in the intestine during gestation, but are much less apparent in the adult rat (11,32). Similar observations have been made in human stomach and intestine (40). IGF-IIR levels similarly are developmentally regulated during rat and human intestinal development (11,59). IGFBP-2 has high affinity for IGF-II and tightly regulates IGF-II availability during fetal and early neonatal growhth (60). Overall these studies indicate that the IGF-II/IGF-IIR axis plays an important role in fetal intestinal development (40,59). A clear pattern of developmental expression of IGF-I/IGFIR has not emerged, however, IGF-I is generally recognized to be the predomant IGF-I in adult. Fluctuations in expression are observed and the degree of change is less apparent than that described for IGF-II (11,39,49,60).
Insulin-like Growth Factor Stimulates Cellular Proliferation
IGFs are mitogenic for intestinal epithelial and smooth muscle cells and for hepatic stellate cells in vitro and in vivo. In vitro, IGFs stimulate intestinal epithelial proliferation, but, in most instances, less so than other growth factors such as EGF (60,61,62–65). When EGF is provided with IGF-I or insulin, a synergistic effect on intestinal epithelial proliferation often is observed. Isolated intestinal smooth muscle cells and isolated hepatic stellate cells in vitro also proliferate in response to IGF-I (66,67).
In vivo evidence for a the proliferative effects of IGF on the intestinal epithelium are robust and derive from experimental models using both enteral and parenteral IGF-I. Oral feeding of IGF-I to neonatal pigs increases indices of small intestinal weight, DNA content, protein content, and villus height in the small intestine (68). In utero ligation of the esophagus in fetal sheep deprives the intestine from growth regulatory peptides in amniotic fluid. A 10-day infusion of IGF-I distal to the ligation results in increased small intestinal growth (69). A small increase in intestinal crypt labeling was seen even after a brief treatment of mice with intraperitoneal administration of IGF-I (70). A 14-day parenteral administration of IGF-I to adult rats increased crypt depth and villus height by 30% (71). In multiple models, infusion of LR3IGF-I, an N-terminal–extended analogue of IGF-I that has a reduced affinity for IGFBPs, better stimulates proliferation in the epithelium and muscularis of the small intestine than the parent peptide (72,73). The effects of IGF-I in these studies are most prominent in the proximal intestine and are not seen not in the pancreas or stomach.
IGF-I also has important regulatory effects on intestinal growth in pathophysiologic conditions as well. Adaptive mucosal proliferation in the small intestine of rats that have undergone a partial small intestinal resection is increased by IGF-I (74–76). Atrophy of the jejunal mucosa in parenterally fed rats, is blunted by administration of inclusion of IGF-I in the parenteral nutrition (TPN) solution (77). Small intestinal atrophy occuring in the setting of chronic liver disease or sepsis is also reduced by administration of IGF-I (78,79). Fibrosis and stricture formation in patients with Crohn’s disease and in animal models of ileocolitis, including TNBS-induced colitis, are associated with increased IGF-I expression and increased smooth muscle proliferation (21). Smooth muscle proliferation and fibrosis from TNBS-induced colitis are significantly diminshed in IGF-I(+/−) heterozygous mice (55).
Studies in transgenic mice also demonstrate the proliferative effect of GH or IGFs on the intestinal mucosa. Mice overexpressing GH have increased plasma and intestinal mucosal IGF-I levels, increased bowel length and mass, but normal intestinal crypt cell proliferation rate, indicative of GH-stimulated survival of intestinal epithelial cells (80). In mice overexpressing IGF-I under an MT-I promoter, circulating GH was undetectable, because of negative feedback from IGF-I, allowing determination of the specific effects of GH and IGF-I on the intestine. MT-IGF-I mice exhibit a significantly greater small intestinal length and mass, an increased villus height, greater crypt depth, and a higher crypt cell mitotic index compared with wildtype mice while differentiation was not altered (81). In contrast, transgenic overexpression of IGF-II has variable effects on mass of the gastrointestinal tract (82,83). Transgenic overexpression of IGFBP-3 was associated with increased liver mass (84). Trangenic overexpression of IGFBP-4 coupled to a smooth muscle α-actin promoter (SMP4/SMP8-IGFBP-4) induced hypoplasia of intestinal smooth muscle suggesting that IGFBP-4 acts as an endogenous inhibitor of IGF-I actions in intestinal smooth muscle. This was confirmed in vitro in human intestinal smooth muscle cells (85). A detailed analysis of the gastrointestinal effects of IGFBP deletion or overexpression has not been reported.
These studies suggest that IGF-I and GH, both of which are used extensively in the clinical arena for other indications, may be useful therapeutic agents in patients with short bowel syndrome and some studies suggest that GH improves intestinal function in patients with short bowel syndrome (86,87). In animal models and in humans studies of short bowel syndrome, intestinal atrophy, or inflammatory bowel disease, IGF-I is more potent in stimulating intestinal growth than GH. This may be because of induction of suppressor of cytokine signaling-2 (SOCS-2) by GH, but not IGF-I (88). More recently the role of GLP-2 in this respect has been examined. GLP-2 directly and indirectly, via induction of IGF-I expression, increases intestinal growth. In IGF-I null mice, the ability of GLP-2 to increase intestinal growth is lost (89). New clinical trials using IGF-I and in combination with GLP-2 may offer more encouraging results in subjects with decreased gastrointestinal mucosal function (90,91).
Insulin-like Growth Factor Is Pro-Survival
The studies discussed earlier indicate that IGFs increase intestinal growth, in part, by inhibition of apoptosis. MT-IGF-I transgenic mice have a lower basal level of apoptosis in small intestine crypts and a lower level of apoptosis in response to irradiation (92). This is consistent with studies showing that IGF-I inhibits apoptosis in cultured cells (93).
In vitro and in vivo studies demostrate that autocrine IGF-I in addition to stimulating proliferation inhibits apoptotis (promotes survival) in smooth muscle cells of the muscularis propria of humans and mice (55,94).
Insulin-like Growth Factor Is Profibrogenic
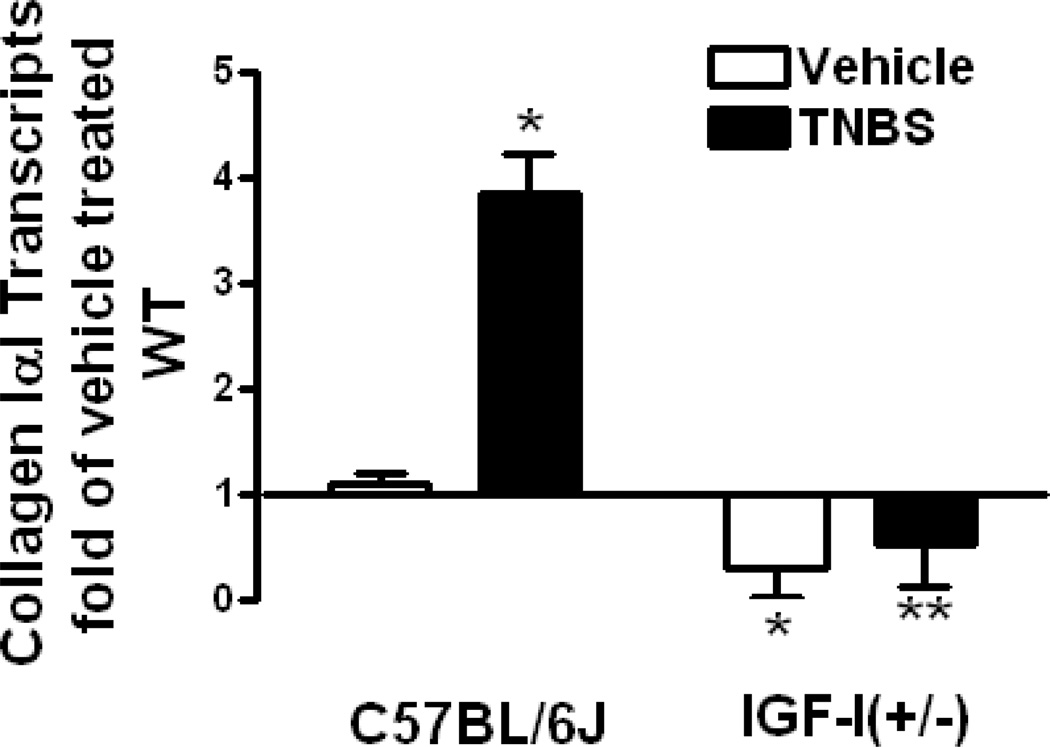
Several lines of investigation demostrate the profibrogenic actions of IGFs in the intestinal tract. IGF-I not only stimulates proliferation and inhibits apoptosis of fibroblasts, myofibroblasts, and smooth muscle cells, it also increases collagen expression and production in each of these cells (95,96). In SMP8-IGF-I mice, transgenic expression of IGF-I increases the mass of the muscularis propria and length of the intestine (53,55). The adaptive response to surgical resection of the small intestine in this same SMP8-IGF-I mouse is characterized by a marked lengthening of the residual bowel, suggesting IGF-I autocrine activity increases the mesenchymal elements in the intestine (97). In Crohn’s disease, a human disorder characterized by fibrosis and stricture formation, mucosal IGF-I and IGF-IR levels are increased relative to normal intestine as are muscularis propria IGF-I, IGFBP-3 and IGFBP-5 levels. In human intestinal muscle the predominant collagen isotype is collagen IαI. In these cells and in animal models of Crohn’s disease (eg TNBS-induced colitis), the increased IGF-I, IGFBP-3 and IGFBP-5 levels (and increased TGF-β1 levels) that are present in the inflamed intestine, individually and in concert, stimulate collagen IαI expression leading to increased collagen secretion and fibrosis (Fig 4.) (24,25, 55,98). It is noteworthy that a profibrogenic response to IGF-I in all regions of the gastrointestinal tract is not seen. In the liver, carbon tetrachloride injury in the SMP8-IGF-I mouse, results in reduced collagen synthesis and amelioration of the extent of liver injury (99 ).
Figure 4.
Inflammation-induced collagen IαI expression is decreased in IGF-I(+/−) mice. Collagen IαI transcripts in smooth muscle cells of vehicle-treated IGF-I(+/−) mice and its increase in response to TNBS-induced colitis are lower than in wildtype C57BL/6J mice. Transcript levels were measured by real-time PCR using the 2−ΔΔCt method.
Insulin-like Growth Factor in Gastrointestinal Cancers
The prominent effects of IGF ligands mediated via IGF receptors on cellular proliferation and survival suggest that the IGF axis may play an important role in the development of dysplasia and neoplasia. Multiple lines of investigation have supported this view (6,8). While circulating IGF-I levels vary considerably among healthy individuals, population-based studies suggest an overall trend toward increasing cancer risk, including cancers of the gastrointestinal tract, in persons at the high end of the normal range of IGF-I blood levels. Loss of IGF-II imprinting results in a modest increase in IGF-II levels, and is now widely accepted as a marker for colorectal cancer risk (15). Increased expression of IGF-I, IGF-II, and IGF-IR are observed in colorectal cancers (100). IGF-II overexpressing mice treated with 1,2-dimethylhydrazine to induce neoplastic alteration promoted the growth of colonic aberrant crypt foci and increased colonic tumor volume without affecting tumor numbers compared to wildtype mice (101). Transgenic overexpression of IGFBP-2, which has high affinity for IGF-II, reduced the appearance of dysplastic aberant crypt foci and inhibited tumor growth in the same model (102).
IGFBP-3 has been shown to promote TGF-β1-mediated epithelial to mesenchymal transition (EMT) and tumor cell invasion in esophageal cancer (103). Interestingly, these effects appeared to be mediated independent of IGF-I.
SUMMARY
The liver is a major source of IGF’s and IGFBP’s that are present in the circulation and have important endocrine activities relating to energy metabolism, body size, carcinogenesis, and various organ specific functions. While IGFs have only minor effects on the normal liver itself, production of IGF’s and IGFBP’s in a tissue specific fashion in the gastrointestinal tract exert important regulatory effects, via autocine and paracrine mechanisms, on cellular proliferation, survival, and apoptosis. IGF’s and IGFBP’s play important regulatory roles in the response of both the liver and the gastrointestinal tract to inflammation and in the development of neoplasia.
Acknowledgments
Supported by DK-49691 from the National Institutes of Diabetes, Digestive and Kidney Diseases
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The author has no relevant disclosures to make.
REFERENCES
- 1.LeRoith D. Insulin-like growth factor receptors and binding proteins. Baillieres Clin Endocrinol Metab. 1996;10:49–73. doi: 10.1016/s0950-351x(96)80298-9. [DOI] [PubMed] [Google Scholar]
- 2.Rubin R, Baserga R. Insulin-like growth factor-I receptor. Its role in cell proliferation, apoptosis, tumorigenicity. Lab Invest. 1995;73:311–331. [PubMed] [Google Scholar]
- 3.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen FC. The molecular and cellular biology of insulin-like growth factor II. Prog Growth Factor Res. 1992;4:257–290. doi: 10.1016/0955-2235(92)90023-b. [DOI] [PubMed] [Google Scholar]
- 5.Sussenbach JS. The gene structure of the insulin-like growth factor family. Prog Growth Factor Res. 1989;1:33–48. doi: 10.1016/0955-2235(89)90040-9. [DOI] [PubMed] [Google Scholar]
- 6.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 7.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 8.Foulstone E, Prince S, Zaccheo O, Burns JL, Harper J, Jacobs C, Church D, Hassan AB. Insulin-like growth factor ligands, receptors, and binding proteins in cancer. J Pathol. 2005;205:145–153. doi: 10.1002/path.1712. [DOI] [PubMed] [Google Scholar]
- 9.Dupont J, Holzenberger M. Biology of insulin-like growth factors in development. Birth Defects Res C Embryo Today. 2003;69:257–271. doi: 10.1002/bdrc.10022. [DOI] [PubMed] [Google Scholar]
- 10.Rotwein P, Pollock KM, Didier DK, Krivi GG. Organization and sequence of the human insulin-like growth factor I gene. Alternative RNA processing produces two insulin-like growth factor I precursor peptides. J Biol Chem. 1986;261:4828–4832. [PubMed] [Google Scholar]
- 11.Lund PK, Moats-Staats BM, Hynes MA, Simmons JG, Jansen M, D’Ercole AJ, Van Wyk JJ. Somatomedin-C/insulin-like growth factor-I and insulin-like growth factor-II mRNAs in rat fetal and adult tissues. J Biol Chem. 1986;261:14539–14544. [PubMed] [Google Scholar]
- 12.Matheny RW, Nindl BC, Adamo ML. Minireview: Mechano-growth factor: A putative product of the IGF-I gene expression involved in tissue repair and regeneration. Endocrinology. 2010;151:865–875. doi: 10.1210/en.2009-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg KM, Bowers JG, Kuemmerle JF. The IGF-IEa (IGF-I) and IGF-IEc (MGF) splice variants of the IGF-I gene mediate hypertrophy and hyperplasia of human intestinal smooth muscle. Gastroenterology. 2007;132 A-234. [Google Scholar]
- 14.Han VK, Lund PK, Lee DC, D’Ercole AJ. Expression of somatomedin/insulin-like growth factor messenger ribonucleic acids in the human fetus: identification, characterization, and tissue distribution. J Clin Endocrinol Metab. 1988;66:422–429. doi: 10.1210/jcem-66-2-422. [DOI] [PubMed] [Google Scholar]
- 15.Cui H, Cruz-Correa M, Giardiello FM, Hutcheon DF, Kafonek DR, Brandenburg S, Wu Y, He X, Powe NR, Feinberg AP. Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science. 2003;299:1753–1755. doi: 10.1126/science.1080902. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Y-W, Idrees K, Shattock R, Khan SA, Zeng Z, Brennan CW, Paty P, Barany F. Loss of imprinting and marked gene elevation are 2 forms of aberrant IGF2 expression in colorectal cancer. Int J Cancer. 2009;127:568–577. doi: 10.1002/ijc.25086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbott AM, Bueno R, Pedrini MT, Murray JM, Smith RJ. Insulin-like growth factor I receptor gene structure. J Biol Chem. 1992;267:10759–10763. [PubMed] [Google Scholar]
- 18.Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, Belfiore A. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem. 2002;277:39684–39695. doi: 10.1074/jbc.M202766200. [DOI] [PubMed] [Google Scholar]
- 19.Izumi T, White MF, Kadowaki T, Takaku F, Akanuma Y, Kasuga M. Insulin-like growth factor I rapidly stimulates tyrosine phosphorylation of a Mr 185,000 protein in intact cells. J Biol Chem. 1987;262:1282–1287. [PubMed] [Google Scholar]
- 20.Kuemmerle JF, Murthy KS. Coupling of the insulin-like growth factor-I receptor tyrosine kinase to Gi2 in human intestinal smooth muscle: Gαγ-dependent mitogen-activated protein kinase acitvation and growth. J Biol Chem. 2001;276:71787–77194. doi: 10.1074/jbc.M011145200. [DOI] [PubMed] [Google Scholar]
- 21.Flynn RS, Murthy KS, Grider JR, Kellum JM, Kuemmerle JF. Endogenous IGF-I and alphaVbeta3 integrin ligands regulate increased smooth muscle hyperplasia in stricturing Crohn’s disease. 2010;138:285–293. doi: 10.1053/j.gastro.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Sahagian GG. Demonstration of tumor suppression by mannose 6-phosphate/insulin-like growth factor 2 receptor. Oncogene. 2004;23:9359–9368. doi: 10.1038/sj.onc.1208039. [DOI] [PubMed] [Google Scholar]
- 23.Kuemmerle JF, Murthy KS, Bowers JG. IGFBP-3 activates TGF-beta receptors and directly inhibits growth in human intestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G795–G802. doi: 10.1152/ajpgi.00009.2004. [DOI] [PubMed] [Google Scholar]
- 24.Flynn RS, Mahavadi S, Murthy KS, Grider JR, Kellum JM, Akbari H, Kuemmerle JF. Endogenous IGFBP-3 regulates excess collagen expression in intestinal smooth muscle cells of Crohn’s disease strictures. Inflamm Bowel Dis. 2011;17:193–201. doi: 10.1002/ibd.21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flynn RS, Madavadi S, Murthy KS, Kellum JM, Kuemmerle JF. Insulin-like growth factor-binding protein-5 stimulates growth of human intestinal muscle cells by activation of Gαi3. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1232–G1238. doi: 10.1152/ajpgi.00323.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schedlich LJ, Le Page SL, Firth SM, Briggs LJ, Jans DA, Baxter RC. Nuclear import of insulin-like growth factor-binding protein-3 and -5 is mediated by the importin beta subunit. J Biol Chem. 2000;275:23462–23470. doi: 10.1074/jbc.M002208200. [DOI] [PubMed] [Google Scholar]
- 27.Schedlich LJ, Marthukaruppan A, O’Han MK, Baxter RC. Insulin-lole growth factor binding protein-5 interacts with the vitamin D receptor and modulates the vitamin D response in osteoblasts. Mol Endocrinol. 2007;21:2378–2380. doi: 10.1210/me.2006-0558. [DOI] [PubMed] [Google Scholar]
- 28.Schedlich LJ, Graham LD, O’Han MK, Murthukaruppan A, Wan X, Firth SM, Baxter RC. Molecular basis of the interaction between IGFBP-3 and retinoid X receptor: role in modulation of RAR-signaling. Arch Biochem Biophys. 2007;465:359–369. doi: 10.1016/j.abb.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Howarth GS. Insulin-like growth factor-I and the gastrointestinal system: therapeutic indications and safety implications. J Nutr. 2003;133:2109–2112. doi: 10.1093/jn/133.7.2109. [DOI] [PubMed] [Google Scholar]
- 30.Laburthe M, Rouyer-Fessard C, Gammeltoft S. Receptors for insulin-like growth factors I and II in rat gastrointestinal epithelium. Am J Physiol. 1988;254:G457–G462. doi: 10.1152/ajpgi.1988.254.3.G457. [DOI] [PubMed] [Google Scholar]
- 31.Ryan J, Costigan DC. Determination of the histological distribution of insulin like growth factor 1 receptors in the rat gut. Gut. 1993;34:1693–1697. doi: 10.1136/gut.34.12.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown AL, Graham DE, Nissley SP, Hill DJ, Strain AJ, Rechler MM. Developmental regulation of insulin-like growth factor II mRNA in different rat tissues. J Biol Chem. 1986;261:13144–13150. [PubMed] [Google Scholar]
- 33.Hoyt EC, Van Wyk JJ, Lund PK. Tissue and development specific regulation of a complex family of rat insulin-like growth factor I messenger ribonucleic acids. Mol Endocrinol. 1988;2:1077–1086. doi: 10.1210/mend-2-11-1077. [DOI] [PubMed] [Google Scholar]
- 34.Heinz-Erian P, Kessler U, Funk B, Gais P, Kiess W. Identification and in situ localization of the insulin-like growth factor-II/mannose-6-phosphate (IGF-II/M6P) receptor in the rat gastrointestinal tract: comparison with the IGF-I receptor. Endocrinology. 1991;129:1769–1778. doi: 10.1210/endo-129-4-1769. [DOI] [PubMed] [Google Scholar]
- 35.Adamo M, Lowe WL, Jr, LeRoith D, Roberts CT., Jr Insulin-like growth factor I messenger ribonucleic acids with alternative 5′-untranslated regions are differentially expressed during development of the rat. Endocrinology. 1989;124:2737–2744. doi: 10.1210/endo-124-6-2737. [DOI] [PubMed] [Google Scholar]
- 36.Pillion DJ, Grizzle WE, Yang M, Meezan E, Stockard CR, Ganapathy V, Leibach FH, Myers RB, Haskell JF. Expression of IGF-II/Man-6-P receptors on rat, rabbit, and human colon epithelial cells. Am J Physiol. 1993;264:R1101–R1110. doi: 10.1152/ajpregu.1993.264.6.R1101. [DOI] [PubMed] [Google Scholar]
- 37.Pillion DJ, Haskell JF, Atchison JA, Ganapathy V, Leibach FH. Receptors for IGF-I, but not for IGF-II, on proximal colon epithelial cell apical membranes. Am J Physiol. 1989;257:E27–E34. doi: 10.1152/ajpendo.1989.257.1.E27. [DOI] [PubMed] [Google Scholar]
- 38.Termanini B, Nardi RV, Finan TM, Parikh I, Korman LY. Insulinlike growth factor I receptors in rabbit gastrointestinal tract. Characterization and autoradiographic localization. Gastroenterology. 1990;99:51–60. doi: 10.1016/0016-5085(90)91228-x. [DOI] [PubMed] [Google Scholar]
- 39.Schober DA, Simmen FA, Hadsell DL, Baumrucker CR. Perinatal expression of type I IGF receptors in porcine small intestine. Endocrinology. 1990;126:1125–1132. doi: 10.1210/endo-126-2-1125. [DOI] [PubMed] [Google Scholar]
- 40.Freier S, Eran M, Reinus C, Ariel I, Faber J, Wilschanski M, Braverman D. Relative expression and localization of the insulin-like growth factor system components in the fetal, child and adult intestine. J Pediatr Gastroenterol Nutr. 2005;40:202–209. doi: 10.1097/00005176-200502000-00023. [DOI] [PubMed] [Google Scholar]
- 41.Eriksson U, Duc G, Froesch ER, Zapf J. Insulin-like growth factors (IGF) I and II and IGF binding proteins (IGFBPs) in human colostrum/transitory milk during the first week postpartum: comparison with neonatal and maternal serum. Biochem Biophys Res Commun. 1993;196:267–273. doi: 10.1006/bbrc.1993.2244. [DOI] [PubMed] [Google Scholar]
- 42.Donovan SM, Hintz RL, Rosenfeld RG. Insulin-like growth factors I and II and their binding proteins in human milk: effect of heat treatment on IGF and IGF binding protein stability. J Pediatr Gastroenterol Nutr. 1991;13:242–253. doi: 10.1097/00005176-199110000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Chaurasia OP, Marcuard SP, Seidel ER. Insulin-like growth factor I in human gastrointestinal exocrine secretions. Regul Pept. 1994;50:113–119. doi: 10.1016/0167-0115(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 44.Philipps AF, Rao R, Anderson GG, McCracken DM, Lake M, Koldovsky O. Fate of insulin-like growth factors I and II administered orogastrically to suckling rats. Pediatr Res. 1995;37:586–592. doi: 10.1203/00006450-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Philipps AF, Kling PJ, Grille JG, Dvorak B. Intestinal transport of insulin-like growth factor-I (igf-I) in the suckling rat. J Pediatr Gastroenterol Nutr. 2002;35:539–544. doi: 10.1097/00005176-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 46.Rouyer-Fessard C, Gammeltoft S, Laburthe M. Expression of two types of receptor for insulinlike growth factors in human colonic epithelium. Gastroenterology. 1990;98:703–707. doi: 10.1016/0016-5085(90)90291-8. [DOI] [PubMed] [Google Scholar]
- 47.Park JH, Vanderhoof JA, Blackwood D, Macdonald RG. Characterization of type I and type II insulin-like growth factor receptors in an intestinal epithelial cell line. Endocrinology. 1990;126:2998–3005. doi: 10.1210/endo-126-6-2998. [DOI] [PubMed] [Google Scholar]
- 48.Dahms NM, Seetharam B, Wick DA. Expression of insulin-like growth factor (IGF)-I receptors, IGF-II/cation-independent mannose 6-phosphate receptors (CI-MPRs), and cation-dependent MPRs in polarized human intestinal Caco-2 cells. Biochim Biophys Acta. 1996;1279:84–92. doi: 10.1016/0005-2736(95)00234-0. [DOI] [PubMed] [Google Scholar]
- 49.Wick DA, Seetharam B, Dahms NM. Basolateral sorting signal of the 300-kDa mannose 6-phosphate receptor. Am J Physiol Gastrointest Liver Physiol. 2002;282:G51–G60. doi: 10.1152/ajpgi.00028.2001. [DOI] [PubMed] [Google Scholar]
- 50.Shoubridge CA, Steeb CB, Read LC. IGFBP mRNA expression in small intestine of rat during postnatal development. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1378–G1384. doi: 10.1152/ajpgi.2001.281.6.G1378. [DOI] [PubMed] [Google Scholar]
- 51.Winesett DE, Ulshen MH, Hoyt EC, Mohapatra NK, Fuller CR, Lund PK. Regulation and localization of the insulin-like growth factor system in small bowel during altered nutrient status. Am J Physiol. 1995;268:G631–G640. doi: 10.1152/ajpgi.1995.268.4.G631. [DOI] [PubMed] [Google Scholar]
- 52.Williams KL, Fuller CR, Fagin J, Lund PK. Mesenchymal IGF-I overexpression: paracrine effects in the intestine, distinct from endocrine actions. Am J Physiol Gastrointest Liver Physiol. 2002;283:G875–G885. doi: 10.1152/ajpgi.00089.2002. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Niu W, Nikoforov Y, Naito S, Chernausek S, Witte D, LeRoith D, Strauch A, Fagin JA. Targeted overexpression of IGF-I evokes distinct patterns of organ remodeling in smooth muscle cell tissue beds of transgenic mice. J Clin Invest. 1997;100:1425–1439. doi: 10.1172/JCI119663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herman AC, Carlisle EM, Paxton JB, Gordon PV. Insulin-like growth factor-I governs submucosal growth and thickness in the newborn mouse ileum. Pediatr Res. 2004;55:507–513. doi: 10.1203/01.PDR.0000110525.30786.50. [DOI] [PubMed] [Google Scholar]
- 55.Mahavadi S, Flynn RS, Grider JR, Qiao LY, Murthy KS, Hazelgrove KB, Kuemmerle JF. Ameloriation of excess collagen IαI, fibrosis, and smooth muscle growth in TNBS-induced coitis in IGF-I(+/−) mice. Inflamm Bowel Dis. 2010 Aug 18; doi: 10.1002/ibd.21437. (Epub ahead of time). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zimmermann EM, Li L, Hoyt EC, Pucilowska JB, Lichtman S, Lund PK. Cell-specific localization of insulin-like growth factor binding protein mRNAs in rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;278:G447–G457. doi: 10.1152/ajpgi.2000.278.3.G447. [DOI] [PubMed] [Google Scholar]
- 57.Novosyadlyy R, Tron K, Dudas J, Ramadori G, Scharf JG. Expression and regulation of the insulin-like growth factor axis components in rat liver myofibroblasts. J Cell Physiol. 2004;199:388–398. doi: 10.1002/jcp.10437. [DOI] [PubMed] [Google Scholar]
- 58.Scharf JG, Dombrowski F, Novosyadlyy R, Eisenbach C, Demori I, Kubler B, Braulke T. Insulin-like growth factor (IGF)-binding protein-1 is highly induced during acute carbon tetrachloride liver injury and potentiates the IGF-I-stimulated activation of rat hepatic stellate cells. Endocrinology. 2004;145:3463–3472. doi: 10.1210/en.2003-1541. [DOI] [PubMed] [Google Scholar]
- 59.Sklar MM, Kiess W, Thomas CL, Nissley SP. Developmental expression of the tissue insulin-like growth factor II/mannose 6-phosphate receptor in the rat. Measurement by quantitative immunoblotting. J Biol Chem. 1989;264:16733–16738. [PubMed] [Google Scholar]
- 60.Young GP, Taranto TM, Jonas HA, Cox AJ, Hogg A, Werther GA. Insulin-like growth factors and the developing and mature rat small intestine: receptors and biological actions. Digestion. 1990;46(suppl 2):240–252. doi: 10.1159/000200392. [DOI] [PubMed] [Google Scholar]
- 61.Kurokowa M, Lynch K, Podolsky DK. Effects of growth factors on an intestinal epithelial cell line: transforming growth factor b inhibits proliferation and stimulates differentiation. Biochem Biophys Res Comm. 1987;142:775–782. doi: 10.1016/0006-291x(87)91481-1. [DOI] [PubMed] [Google Scholar]
- 62.Conteas CN, McMorrow B, Luk GD. Modulation of epidermal growth factor-induced cell proliferation and receptor binding by insulin in cultured intestinal epithelial cells. Biochem Biophys Res Commun. 1989;161:414–419. doi: 10.1016/0006-291x(89)92614-4. [DOI] [PubMed] [Google Scholar]
- 63.Duncan MD, Korman LY, Bass BL. Epidermal growth factor primes intestinal epithelial cells for proliferative effect of insulin-like growth factor I. Dig Dis Sci. 1994;39:2197–2201. doi: 10.1007/BF02090371. [DOI] [PubMed] [Google Scholar]
- 64.Jonas CR, Ziegler TR, Gu LH, Jones DP. Extracellular thiol/disulfide redox state affects proliferation rate in a human colon carcinoma (Caco2) cell line. Free Radic Biol Med. 2002;33:1499–1506. doi: 10.1016/s0891-5849(02)01081-x. [DOI] [PubMed] [Google Scholar]
- 65.Lahm H, Suardet L, Laurent PL, Fischer JR, Ceyhan A, Givel JC, Odartchenko N. Growth regulation and co-stimulation of human colorectal cancer cell lines by insulin-like growth factor I, II and transforming growth factor alpha. Br J Cancer. 1992;65:341–346. doi: 10.1038/bjc.1992.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuemmerle JF, Zhou H, Bowers JG. IGF-I stimulates human intestinal smooth muscle cell growth by regulation of G1 phase cell cycle proteins. Am J Physiol Gastrointest Liver Physiol. 2004;286:G412–G419. doi: 10.1152/ajpgi.00403.2003. [DOI] [PubMed] [Google Scholar]
- 67.Skrtic S, Wallenius K, Gressner AM, Jansson JO. Insulin-like growth factor signaling pathways in rat hepatic stellate cells: importance for deoxyribonucleic acid synthesis and hepatocyte growth factor production. Endocrinology. 1999;140:5729–5735. doi: 10.1210/endo.140.12.7166. [DOI] [PubMed] [Google Scholar]
- 68.Burrin DG, Wester TJ, Davis TA, Amick S, Heath JP. Orally administered IGF-I increases intestinal mucosal growth in formula-fed neonatal pigs. Am J Physiol. 1996;270:R1085–R1091. doi: 10.1152/ajpregu.1996.270.5.R1085. [DOI] [PubMed] [Google Scholar]
- 69.Trahair JF, Wing SJ, Quinn KJ, Owens PC. Regulation of gastrointestinal growth in fetal sheep by luminally administered insulin-like growth factor-I. J Endocrinol. 1997;152:29–38. doi: 10.1677/joe.0.1520029. [DOI] [PubMed] [Google Scholar]
- 70.Potten CS, Owen G, Hewitt D, Chadwick CA, Hendry H, Lord BI, Woolford LB. Stimulation and inhibition of proliferation in the small intestinal crypts of the mouse after in vivo administration of growth factors. Gut. 1995;36:864–873. doi: 10.1136/gut.36.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steeb CB, Trahair JF, Tomas FM, Read LC. Prolonged administration of IGF peptides enhances growth of gastrointestinal tissues in normal rats. Am J Physiol. 1994;266:G1090–G1098. doi: 10.1152/ajpgi.1994.266.6.G1090. [DOI] [PubMed] [Google Scholar]
- 72.Steeb CB, Shoubridge CA, Tivey DR, Read LC. Systemic infusion of IGF-I or LR3)IGF-I stimulates visceral organ growth and proliferation of gut tissues in suckling rats. Am J Physiol. 1997;272:G522–G533. doi: 10.1152/ajpgi.1997.272.3.G522. [DOI] [PubMed] [Google Scholar]
- 73.Steeb CB, Trahair JF, Read LC. Administration of insulin-like growth factor-I (IGF-I) peptides for three days stimulates proliferation of the small intestinal epithelium in rats. Gut. 1995;37:630–638. doi: 10.1136/gut.37.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mantell MP, Ziegler TR, Adamson WT, Roth JA, Zhang W, Frankel W, Bain A, Chow JC, Smith RJ, Rombeau JL. Resection-induced colonic adaptation is augmented by IGF-I and associated with upregulation of colonic IGF-I mRNA. Am J Physiol. 1995;269:G974–G980. doi: 10.1152/ajpgi.1995.269.6.G974. [DOI] [PubMed] [Google Scholar]
- 75.Vanderhoof JA, McCusker RH, Clark R, Mohammadpour H, Blackwood DJ, Harty RF, Park JH. Truncated and native insulinlike growth factor I enhance mucosal adaptation after jejunoileal resection. Gastroenterology. 1992;102:1949–1956. doi: 10.1016/0016-5085(92)90318-s. [DOI] [PubMed] [Google Scholar]
- 76.Dahly EM, Guo Z, Ney DM. IGF-I augments resection-induced mucosal hyperplasia by altering enterocyte kinetics. Am J Physiol Regul Integr Comp Physiol. 2003;285:R800–R808. doi: 10.1152/ajpregu.00014.2003. [DOI] [PubMed] [Google Scholar]
- 77.Peterson CA, Ney DM, Hinton PS, Carey HV. Beneficial effects of insulin-like growth factor I on epithelial structure and function in parenterally fed rat jejunum. Gastroenterology. 1996;111:1501–1508. doi: 10.1016/s0016-5085(96)70011-2. [DOI] [PubMed] [Google Scholar]
- 78.Inaba T, Saito H, Fukushima R, Hashiguchi Y, Lin MT, Inoue T, Fukatsu K, Muto T, Oka T, Takenaka A, Takahashi S, Noguchi T. Insulin-like growth factor 1 has beneficial effects, whereas growth hormone has limited effects on postoperative protein metabolism, gut integrity, and splenic weight in rats with chronic mild liver injury. JPEN J Parenter Enteral Nutr. 1997;21:55–62. doi: 10.1177/014860719702100255. [DOI] [PubMed] [Google Scholar]
- 79.Chen K, Okuma T, Okamura K, Tabira Y, Kaneko H, Miyauchi Y. Insulin-like growth factor-I prevents gut atrophy and maintains intestinal integrity in septic rats. JPEN J Parenter Enteral Nutr. 1995;19:119–124. doi: 10.1177/0148607195019002119. [DOI] [PubMed] [Google Scholar]
- 80.Ulshen MH, Dowling RH, Fuller CR, Zimmermann EM, Lund PK. Enhanced growth of small bowel in transgenic mice overexpressing bovine growth hormone. Gastroenterology. 1993;104:973–980. doi: 10.1016/0016-5085(93)90263-c. [DOI] [PubMed] [Google Scholar]
- 81.Ohneda K, Ulshen MH, Fuller CR, D’Ercole AJ, Lund PK. Enhanced growth of small bowel in transgenic mice expressing human insulin-like growth factor I. Gastroenterology. 1997;112:444–454. doi: 10.1053/gast.1997.v112.pm9024298. [DOI] [PubMed] [Google Scholar]
- 82.Ward A, Bates P, Fisher R, Richardson L, Graham CF. Disproportionate growth in mice with Igf-2 transgenes. Proc Natl Acad Sci U S A. 1994;91:10365–10369. doi: 10.1073/pnas.91.22.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blackburn A, Schmitt A, Schmidt P, Wanke R, Hermanns W, Brem G, Wolf E. Actions and interactions of growth hormone and insulin-like growth factor-II: body and organ growth of transgenic mice. Transgenic Res. 1997;6:213–222. doi: 10.1023/a:1018494108654. [DOI] [PubMed] [Google Scholar]
- 84.Murphy LJ, Rajkumar K, Molner P. Phenotypic manifestations of insulin-like growth factor binding protein-1 (IGFBP-1) and IGFBP-3 overexpression in transgenic mice. Prog Growth Factor Res. 1995;6:425–432. doi: 10.1016/0955-2235(95)00026-7. [DOI] [PubMed] [Google Scholar]
- 85.Kuemmerle JF, Teng B-Q. Regulation of IGFBP-4 levels in human intestinal smooth muscle cells: Confluence-dependent production of an endogenous IGF-I-activated IGFBP-4 protease. Am J Physiol Gastrointest Liver Physiol. 2000;279:G975–G982. doi: 10.1152/ajpgi.2000.279.5.G975. [DOI] [PubMed] [Google Scholar]
- 86.Seguy D, Vahedi K, Kapel N, Souberbielle JC, Messing B. Low-dose growth hormone in adult home parenteral nutrition-dependent short bowel syndrome patients: a positive study. Gastroenterology. 2003;124:293–302. doi: 10.1053/gast.2003.50057. [DOI] [PubMed] [Google Scholar]
- 87.Scolapio JS. Current update of short-bowel syndrome. Curr Opin Gastroenterol. 2004;20:143–145. doi: 10.1097/00001574-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 88.Miller ME, Michaylira CZ, Simmons JG, Ney DM, Dahly EM, Heath JK, Lund PK. Suppressor of cytokine signaling-2: a growth hormone-inducible inhibitor of intestinal epithelial cell proliferation. Gastroenterology. 2004;127:570–581. doi: 10.1053/j.gastro.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 89.Dube PE, Forse CL, Bahrami J, Brubaker PL. The essential role of Insulin-like growth factor-1 in the intestinal tropic effects of glucagon-like peptide-2 in mice. Gastroenterology. 2006;131:589–605. doi: 10.1053/j.gastro.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 90.Yazbeck R, Howarth GS, Abbott CA. Grwoth factor based therapies and intestinal disease: is glucagon-like peptide-2 the new way forward? Cytokine Growth Factor Res. 2009;20:175–184. doi: 10.1016/j.cytogfr.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 91.Yazbeck R, Abbott CA, Howarth GS. The use of GLP-2 and related growth factors in intestinal diseases. Curr Opin Investig Drugs. 2010;11:440–446. [PubMed] [Google Scholar]
- 92.Wilkins HR, Ohneda K, Keku TO, D’Ercole AJ, Fuller CR, Williams KL, Lund PK. Reduction of spontaneous and irradiation-induced apoptosis in small intestine of IGF-I transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2002;283:G457–G464. doi: 10.1152/ajpgi.00019.2002. [DOI] [PubMed] [Google Scholar]
- 93.Parrizas M, Saltiel AR, LeRoith D. Insulin-like growth factor 1 inhibits apoptosis using the phosphatidylinositol 3′-kinase and mitogen-activated protein kinase pathways. J Biol Chem. 1997;272:154–161. doi: 10.1074/jbc.272.1.154. [DOI] [PubMed] [Google Scholar]
- 94.Flynn RS, Murthy KS, Grider JR, Kellum JM, Kuemmerle JF. Endogenous IGF-I and alphaVbeta3 integrin ligands regulate increased smooth muscle hyperplasia in strictuting Crohn’s disease. Gastroenterology. 2010;138:285–293. doi: 10.1053/j.gastro.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xin X, Hou YT, Li L, Schmiedlin-Ren P, Christman GM, Cheng HL, Bitar KN, Zimmermann EM. IGF-I increases IGFBP-5 and collagen alpha1(I) mRNAs by the MAPK pathway in rat intestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G777–G783. doi: 10.1152/ajpgi.00293.2003. [DOI] [PubMed] [Google Scholar]
- 96.Kuemmerle JF. Endogenous IGF-I protects human intestinal smooth muscle cells from apoptosis by regulation of GSK-3 beta activity. Am J Physiol Gastrointest Liver Physiol. 2005;288:G101–G110. doi: 10.1152/ajpgi.00032.2004. [DOI] [PubMed] [Google Scholar]
- 97.Knott AW, Juno RJ, Jarboe MD, Profitt SA, Erwin CR, Smith EP, Fagin JA, Warner BW. Smooth muscle overexpression of IGF-I induces a novel adaptive response to small bowel resection. Am J Physiol Gastrointest Liver Physiol. 2004;287:G562–G570. doi: 10.1152/ajpgi.00438.2003. [DOI] [PubMed] [Google Scholar]
- 98.Theiss AL, Fruchtman S, Lund PK. Growth factors in inflammatory bowel disease: the actions and interactions of growth hormone and insulin-like growth factor-I. Inflamm Bowel Dis. 2004;10:871–880. doi: 10.1097/00054725-200411000-00021. [DOI] [PubMed] [Google Scholar]
- 99.Sanz S, Pucilowska JB, Liu S, Rodriguez-Ortigosa CM, Lund PK, Brenner DA, Fuller CR, Simmons JG, Pardo A, Martinez-Chantar ML, Fagin JA, Prieto J. Expression of insulin-like growth factor I by activated hepatic stellate cells reduces fibrogenesis and enhances regeneration after liver injury. Gut. 2005;54:134–141. doi: 10.1136/gut.2003.024505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nosho K, Yamamoto H, Taniguchi H, Adachi Y, Yoshida Y, Arimura Y, Endo T, Hinoda Y, Imai K. Interplay of insulin-like growth factor-II, insulin-like growth factor-I, insulin-like growth factor-I receptor, COX-2, and matrix metalloproteinase-7, play key roles in the early stage of colorectal carcinogenesis. Clin Cancer Res. 2004;10:7950–7957. doi: 10.1158/1078-0432.CCR-04-0875. [DOI] [PubMed] [Google Scholar]
- 101.Diehl D, Oesterle D, Eimlinger MW, Hoeflich A, Wolf E, Lahm H. IGF-II transgenic mice display increased aberrant colon crypt multiplicity and tumor volume after 1,2-dimethylhydrazine treatment. J Carcinog. 2006;5:24. doi: 10.1186/1477-3163-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diehl D, Hessel E, Oesterile D, Rener-Muller I, Elmlinger M, Langhammer M, Gottlicher M, Wolf E, Lahm H, Hoeflich A. IGFBP-2 overexpression reduces the appearance of dysplastic aberrant crypt foci and inhibits growth of adenomas in chemically induced colorectal carcinogenesis. Int J Cancer. 2009;124:2220–2225. doi: 10.1002/ijc.24193. [DOI] [PubMed] [Google Scholar]
- 103.Natasuizaka M, Ohashi S, Wong GS, Ahmadi A, Kalman RA, Budo D, Klein-Szanto AJ, Herlyn M, Diehl JA, Nakagawa H. Insulin-like growth factor-binding protein-3 promotes transforming growth factor-β1-mediated epithelial-to-mesenchymal transition and motility in transformed human esophageal cells. Carcinogenesis. 2010;31:1344–1353. doi: 10.1093/carcin/bgq108. [DOI] [PMC free article] [PubMed] [Google Scholar]