Abstract
SUMMARY
The AAA+-ATPase Rea1 removes the ribosome biogenesis factor Rsa4 from pre-60S ribosomal subunits in the nucleoplasm to drive nuclear export of the subunit. To do this, Rea1 utilizes a MIDAS domain to bind a conserved motif in Rsa4. Here, we show that the Rea1 MIDAS domain binds another pre-60S factor, Ytm1, via a related motif. In vivo Rea1 contacts Ytm1 before it contacts Rsa4, and its interaction with Ytm1 coincides with the exit of early pre-60S particles from the nucleolus to the nucleoplasm. In vitro, Rea1’s ATPase activity triggers removal of the conserved nucleolar Ytm1-Erb1-Nop7 subcomplex from isolated early pre-60S particle. We suggest that the Rea1 AAA+-ATPase functions at successive maturation steps to remove ribosomal factors at critical transition points, first driving the exit of early pre-60S particles from the nucleolus and then driving late pre-60S particles from the nucleus.
INTRODUCTION
Ribosomes consisting of ribosomal RNA (rRNA) and ribosomal proteins (r proteins) are the machines that synthesize all cellular proteins. In eukaryotes, the two ribosomal subunits (60S and 40S) are first assembled in the nucleolus, a territory of the nucleus specialized for ribosome production, before export to the cytoplasm. During ribosome synthesis ~200 nonribosomal factors and ~100 small nucleolar RNAs (snoRNAs) transiently work on the evolving ribosomal subunits to facilitate their assembly and maturation (Fromont-Racine et al., 2003; Henras et al., 2008; Tschochner and Hurt, 2003). Ribosome biogenesis requires extensive regulation and coordination to meet the cellular demands for continuous ribosome production, which is essential for all actively dividing cells. Accordingly, the misregulation of signaling pathways in cancer cells stimulates ribosome biogenesis, and, conversely, defects in ribosome assembly can cause inherited human diseases collectively called ribosomopathies (Freed et al., 2010; Ganapathi and Shimamura, 2008; Narla and Ebert, 2010). This all indicates that a detailed mechanistic understanding of ribosome biogenesis could aid in designing new strategies for cancer therapy or curing ribosomopathies.
In the past, ribosome biogenesis was extensively studied in yeast by numerous integrative approaches (reviewed in Fromont-Racine et al., 2003; Granneman and Baserga, 2004; Henras et al., 2008; Kressler et al., 2009; Strunk and Karbstein, 2009; Tschochner and Hurt, 2003). To date, there are fewer investigations of ribosome assembly in higher eukaryotes, but nevertheless they suggest that the mechanism of ribosome formation and the participating biogenesis factors have been conserved during evolution (Grimm et al., 2006; Hölzel et al., 2005, 2007; Rohrmoser et al., 2007; Thomas and Kutay, 2003; Trotta et al., 2003; Zemp et al., 2009). The emerging consensus from all of these studies is that nonribosomal factors act sequentially with distinct recruitment and displacement during the interdependent steps of ribosome formation (reviewed in Fromont-Racine et al., 2003; Henras et al., 2008; Tschochner and Hurt, 2003).
Among the myriad of trans-acting factors are energyconsuming enzymes like GTPases, DExD/H-box ATPases, kinases, and AAA+-ATPases. In particular, the AAA+-ATPases Rix7, Rea1, and Drg1 were shown to be involved in 60S subunit biogenesis by providing the energy for ATP-hydrolysis-driven removal of biogenesis factors (Kressler et al., 2009). In their cases, putative substrate proteins associated with different pre-60S particles could be identified (Kressler et al., 2008; Pertschy et al., 2007; Ulbrich et al., 2009).
The first mechanistic insight for the Rea1 AAA+-ATPase was obtained by demonstrating how this enzyme stimulates the removal of ribosome biogenesis factors from the evolving 60S subunit (Ulbrich et al., 2009). Rea1 consists of a hexameric AAA-motor head domain and a long flexible tail that carries a MIDAS domain (metal ion-dependent adhesion site) at the carboxy-terminal end (Garbarino and Gibbons, 2002; Ulbrich et al., 2009). Electron microscopy revealed that Rea1 contacts the pre-60S particle at two discrete sites on the pre-60S particle. Its AAA-motor head is fixed in close vicinity to the adaptor Rix1-Ipi3-Ipi1 subcomplex, whereas the MIDAS domain at the tip of the tail contacts the pre-ribosome at a second site where the pre-60S factor Rsa4 is located (Ulbrich et al., 2009). It is possible that such a constellation of biogenesis factors on the pre-60S particle could generate a pulling force upon ATP hydrolysis to release Rsa4, the Rix1-Ipi3-Ipi1 subcomplex, and Rea1 from the pre-60S particle, preparing it for nuclear export (Ulbrich et al., 2009).
Bioinformatic analysis indicated that another pre-60S factor in yeast, Ytm1, carries an N-terminal domain that is homologous to the Rsa4 N-terminal domain (Nal et al., 2002), which was previously shown to bind the Rea1 MIDAS (Ulbrich et al., 2009). Here, we demonstrate that Ytm1 is capable of binding to the Rea1 MIDAS and that this contact is essential for an earlier step during 60S subunit biogenesis. In vitro studies indicated that the Ytm1-Rea1 interaction is required for removal of several nucleolar factors from the early pre-60S particle. Thus, Rea1 induces the release of the Ytm1-Erb1-Nop7 subcomplex from the nascent 60S subunit and consequently enables this early pre-60S intermediate to exit from the nucleolus. Later on during 60S biogenesis, Rea1 functions again on the Rix1 particle to mediate removal of Rsa4 and the Rix1-Ipi3-Ipi1 subcomplex (Ulbrich et al., 2009).
RESULTS
MIDO Domains in Ytm1 and Rsa4 Bind to the Rea1 MIDAS
The conserved yeast pre-60S biogenesis factors Rsa4 and Ytm1 (in higher eukaryotes, termed Nle and WDR12, respectively) both consist of an amino-terminal extension (~90–150 residues) with significant homology (Figure S1 available~online) (Nal et al., 2002) and a C-terminal β propeller (WD40) domain. Because the Rsa4N domain binds to the Rea1 MIDAS (Ulbrich et al., 2009), we tested for an analogous interaction between the Ytm1 N domain and the Rea1 MIDAS. Yeast two-hybrid and in vitro binding assays demonstrated that amino acids 1–92 of Ytm1 (Figure S1) are sufficient for binding the intact, but not mutated, Rea1 MIDAS, which is predicted to be impaired in MIDAS ion coordination (Figures 1A and 1B).
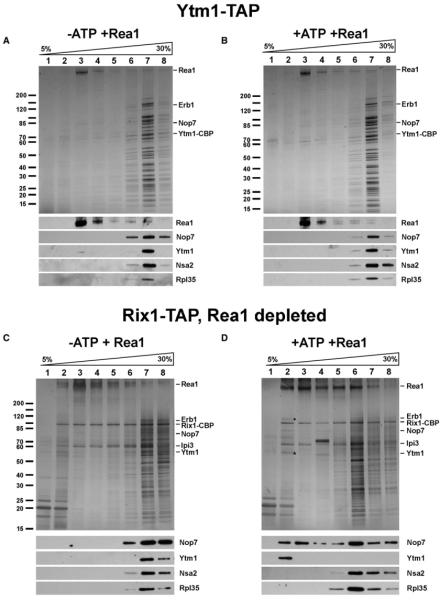
Figure 1. The MIDO of Ytm1 Interacts with Rea1’s MIDAS In Vivo and In Vitro.
(A) Yeast two-hybrid interaction between wild-type and mutant alleles of Rea1-MIDAS and Ytm1-MIDO. Yeast two-hybrid plasmids expressing the indicated GAL4-BD (GAL4 DNA-binding domain) and GAL4-AD (GAL4 activation domain) fusion proteins were transformed into the yeast reporter strain PJ69-4A. Transformants were spotted in 10-fold serial dilutions onto SDC-Trp-Leu (SDC) or SDC-Trp-Leu-His (SDC-His) and incubated at 30 °C for 4 days. The Rea1-MIDAS comprises residues 4622–4910; the MIDO of Rsa4, residues 1–154; and the MIDO of Ytm1, residues 1–92. Growth on SDC-His plate indicates a two-hybrid interaction. (B) Rea1-MIDAS and Ytm1-MIDO bind directly to each other. The GST-TEV-tagged MIDAS of Rea1 (wild-type or DAA mutant; residues 4608–4910) was coexpressed with His6-Ytm1-MIDO (1–92 aa) or the His6-Ytm1-MIDO E80A mutant in E. coli in the indicated combinations. The GST-MIDAS fusion proteins were affinity purified on GSH beads and eluted by TEV cleavage. Supernatants and eluates were analyzed by SDS-PAGE and Coomassie staining (top) and western blotting (bottom) using anti-HIS antibodies to detect Ytm1 in eluates and total cell lysates. Protein bands above GST-MIDAS are E. coli contaminants. (C) The ytm1-MIDO mutant S78L is genetically linked to the rea1-DTS mutant. The YTM1Δ/REA1Δ double-shuffle strain was transformed with wild-type and the indicated mutant alleles of YTM1 and REA1, respectively. Transformants were spotted in 10-fold serial dilutions onto SDC-Trp-Leu (SDC) or SDC+FOA to test for synthetic lethality. Plates were incubated at 30 °C for 2 (SDC) or 4 days (SDC+FOA).
A sequence analysis of Ytm1 MIDO is shown in Figure S1.
Previously, we identified a conserved glutamate in the Rsa4 N domain (E114) to be essential for the interaction with the Rea1 MIDAS (Ulbrich et al., 2009). Sequence alignment indicated that this essential E114 in Rsa4 corresponds to the highly conserved E80 in the N-terminal extension of Ytm1 (Figure S1). Consistently, mutation of E80 > A in Ytm1 (ytm1 E80A) rendered the protein nonfunctional because it could not complement the lethal phenotype of the ytm1Δ strain (Figure S2A). Moreover, Ytm1 E80A could not bind to the Rea1 MIDAS in the two-hybrid and in the in vitro reconstitution assay (Figures 1A and 1B). Thus, Ytm1 and Rsa4 bind to the Rea1 MIDAS by a similar mechanism involving a conserved glutamate within the MIDAS interacting domain (MIDO) to coordinate the MIDAS ion. Altogether, these findings suggest that Rea1 in concert with Ytm1 could play a so far uncharacterized role in 60S biogenesis.
Genetic Interaction between YTM1 MIDO and REA1 MIDAS
To determine whether Ytm1 and Rea1 are functionally linked during ribosome biogenesis, we tested for genetic interactions between these two factors. However, the ytm1-1 allele, which carries two point mutations in the C-terminal β propeller known to impair binding of Ytm1 to the nascent 60S subunit (via Erb1 and Nop7) (Miles et al., 2005; Tang et al., 2008), showed no genetic linkage to the rea1-DTS (MIDAS) mutation (Figure 1C). Hence, we developed a genetic screen to identify ytm1 mutations, which exhibit a genetic (i.e., synthetic lethal) interaction with rea1-DTS (see Experimental Procedures). The ytm1 S78L mutant, isolated from this screen, was viable in the presence of the intact REA1 but synthetic lethal when combined with the rea1-DTS allele (Figure 1C). Of note, the S78L mutation lies close to the critical E80 residue in Ytm1 that is essential for the interaction with the Rea1 MIDAS (Figure S1). In contrast, ytm1 S78L was not genetically linked to the rsa4-1 mutation (data not shown), which was also synthetic lethal with rea1-DTS (Ulbrich et al., 2009). Thus, Ytm1 and Rsa4 are physically and functionally linked to the MIDAS domain of Rea1 via their amino-terminal MIDO motifs, but the functions of Rsa4 and Ytm1 appear to be independent from each other. Consistent with this notion, Ytm1 is associated with early nucleolar pre-60S particles (e.g., the Nsa1-TAP particle) (Miles et al., 2005; Ulbrich et al., 2009), whereas Rsa4 is part of a later pre-60S (Rix1) particle in the nucleoplasm (de la Cruz et al., 2005; Ulbrich et al., 2009).
GAL::ytm1 E80A Exerts a Dominant-Lethal Phenotype with Defects in Early 60S Subunit Biogenesis
To clarify the in vivo requirement for the Ytm1-Rea1 interaction for 60S biogenesis, we placed the ytm1 E80A allele under the control of the inducible GAL promoter for overexpression studies in yeast. Like in the case of GAL::rsa4 E114D (Ulbrich et al., 2009), overproduction of GAL::ytm1 E80A in galactose-containing medium was toxic to the cells (Figure 2A). Induction of the dominant-negative Ytm1 E80A caused a robust 60S biogenesis defect with a reduction of free 60S subunits (Figure S2D) and accumulation of the 60S reporter Rpl25-GFP within the nucleolus (Figure 2C). In contrast, the localization of the 40S reporter Rps3-GFP was not affected (data not shown). Northern blot analysis of the rRNA processing in the Ytm1 E80A mutant revealed increased levels of early 27S pre-rRNA processing intermediates (Figure 2B, lanes 6–10), consistent with the existence of stalled nucleolar particles. In contrast,overproduction of thedominant Rsa4 E114D mutant induced a later pre-rRNA processing defect, as displayed by the accumulation of 7S pre-rRNA (Figure 2B, lanes 16-20), and pre-60S particles were shown to accumulate in the nucleoplasm (Ulbrich et al., 2009). The appearance of 27S pre-rRNAs in the Rsa4 E114D mutant could be due to trapping of Rea1 in the nucleoplasm (e.g., on Rix1 particles), which, in a feedback mechanism, could affect earlier 60S biogenesis steps that also require Rea1 (see below). Moreover, both Ytm1 E80A and Rsa4 E114D showed a temporal later increase of 23S and 35S rRNAs, which is likely due to secondary effects. As Ytm1 copurifies with pre-60S particles containing 27S pre-rRNAs (Miles et al., 2005), whereas Rsa4 is associated with pre-60S particles containing 7S and 25S rRNA (Ulbrich et al., 2009; see below), we conclude that an interaction between Ytm1 and Rea1 is required for a nucleolar step during 60S subunit formation, which occurs prior to the nucleoplasmic Rsa4-Rea1 interaction.
Figure 2. Dominant-Lethal Ytm1 E80A Inhibits Formation of 60S Subunits.

(A) The ytm1 E80A mutant causes a dominant-negative growth phenotype. Wild-type YTM1 and the indicated ytm1 E80A mutant allele were overexpressed under the control of the inducible GAL1 promoter in a wild-type yeast strain. Transformants were spotted in 10-fold serial dilutions on SDC-Leu (glucose) and SGC-Leu (galactose) plates. Glucose plates were incubated for 2 days and galactose plates for 7 days at 30 °C. (B) Overexpression of the Ytm1 E80A protein blocks 27S to 7S pre-rRNA processing. Cells were grown in SRC-Leu (time point 0) before expression of YTM1, ytm1 E80A, RSA4, rsa4 E114D was induced by transferring cells into SGC-Leu. Total RNA was isolated after 0, 2, 4, 6, and 8 hr of induction and analyzed by northern blot. Probes used for the shown autoradiographs are indicated aside. Panels for 35S, 27SA2, and 23S rRNA show detection from the same exposure time. (C) Analysis of 60S ribosomal export in the dominant-negative GAL::ytm1 E80A mutant. Wild-type yeast strain, harboring plasmids pRS314-RFP-NOP1-RPL25-GFP and YCplac111-GAL::YTM1 wild-type or ytm1 E80A, was grown (30 °C) in raffinose-containing medium before cells were shifted to galactose medium to induce overexpression of Ytm1 and Ytm1 E80A, respectively. The subcellular location of the Rpl25-GFP (reporter for 60S export) and mRFP-Nop1 (nucleolar marker) were analyzed by fluorescence microscopy after 5 hr shift. Single and merged pictures of Rpl25-GFP and mRFP-Nop1 as well as Nomarski (DIC) pictures, are shown. Scale bar, 2 μm.
For further characterization of the ytm1 E80A mutant, see Figure S2.
Nucleolar Relocation of the Rix1-Defined Pre-60S Particle upon Ytm1 E80A Overexpression
To identify biogenesis factors whose dynamic association with nascent pre-60S subunits depends on a productive Ytm1-Rea1 interaction, we sought to test the intracellular location of early, intermediate, and late pre-60S factors upon GAL::ytm1 E80A induction. Wild-type Ytm1-GFP and mutant Ytm1 E80A-GFP did not differ in their subcellular localization (Figure S2C). However, GFP-tagged Rea1, Rix1, Ipi1, and Ipi3 were significantly shifted from a nucleoplasmic to a nucleolar location upon overexpression of Ytm1 E80A (Figures 3 and S4). Other factors tested (e.g., nucleolar Erb1, Nop7, and Has1 or nucleoplasmic Rsa4 and Arx1) remained unaffected in their steady-state localizations (Figure S3). Thus, overexpression of dominant Ytm1 E80A caused the redistribution of Rea1 and the Rix1-Ipi3-Ipi1 subcomplex from the nucleoplasm to the nucleolus.
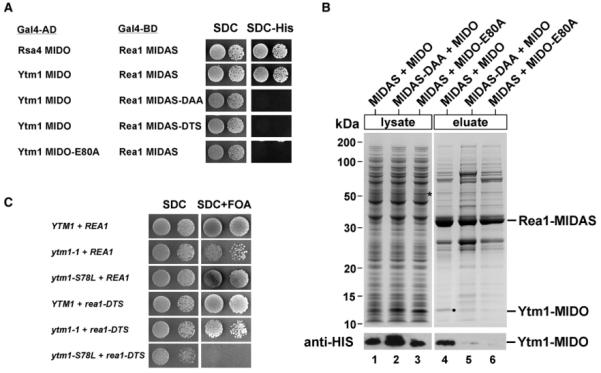
Figure 3. GFP-Rea1 and the Rix1-Ipi3-Ipi1 Subcomplex Become Mislocalized upon Overexpression of Dominant-Negative Ytm1 E80A.
Yeast strains carrying chromosomal GFP fusion proteins (PRea1::GFP-Rea1, Rix1-GFP, Ipi3-GFP, and Ipi1-GFP) were transformed with pRS314 mRFP-Nop1 (nucleolar marker) and YCplac111-GA-L::YTM1 or YCplac111-GAL::ytm1-E80A. Transformants were grown in SRC-Leu-Trp medium (raffinose) and shifted to SGC-Leu-Trp (galactose) medium for 6 hr to induce overexpression of Ytm1 or Ytm1-E80A. Subcellular location of GFP fusion proteins was determined by fluorescence microscopy. Nomarski (DIC), GFP, RFP, and merge pictures are shown. Scale bar, 2μm. Figure S3 shows the localization of additional 60S biogenesis factors upon Ytm1 E80A overexpression.
Dominant-Negative Ytm1 E80A or Rea1 Depletion Shift the Nucleoplasmic Rix1 Particle to an Early Nucleolar Pre-60S Intermediate
Next, we investigated the protein and the RNA composition of the pre-60S (Rix1) particle carrying Rea1 and the Rix1 subcomplex upon overexpression of toxic Ytm1 E80A. Rix1-TAP particles, affinity purified from GAL::ytm1 E80A cells, co-enriched the dominant Ytm1 E80A mutant protein and numerous early pre-60S factors (e.g., Rrp5, Noc1, Noc2, Noc3, Spb1, Erb1, Nop4, Nop2, Spb4, Nop12, Has1, and Fpr4) as well as 27S pre-rRNAs. Typical Rix1 particle protein factors (e.g., Rea1, Rsa4, Nog2, Arx1, and Alb1), as well as Rix1-associated rRNA intermediates like 25S, 7S, and 5.8S rRNAs, were significantly depleted (Figures 4A, 4B, and S4A). On the other hand, 27S rRNAs were significantly increased in the Rix1-TAP particle derived from the dominant ytm1 E80A mutant. Affinity purification of dominant-negative Ytm1 E80A-TAP itself yielded a similar set of co-enriched early pre-60S factors when compared to affinity-purified wild-type Ytm1-TAP, but some differences were observed (e.g., Dbp10 and Drs1 were reduced, but Spb4 was increased in the Ytm1 E80A-TAP purification; Figure S2B). These data show that impairment of the Ytm1-Rea1 interaction shifts the Rix1 pre-60S particle toward an earlier intermediate. This earlier intermediate largely resembles the nucleolar Ytm1-TAP particle in protein composition but, in addition, carries the Rix1-Ipi3-Ipi1 subcomplex and a residual pool of Rea1 that is normally absent from the wild-type Ytm1-TAP particle (Figure S2B).
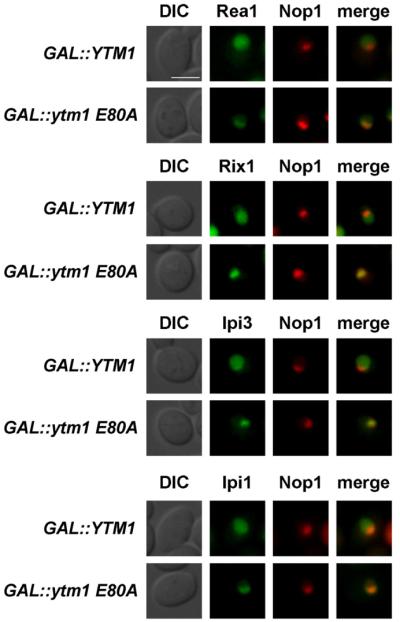
Figure 4. Rix1-TAP Co-Enriches a Nucleolar Pre-60S Particle upon Overexpression of Ytm1 E80A or Rea1 Depletion.
(A and B) Yeast strain Rix1-TAP was transformed with YCplac111-GAL::YTM1 or YCplac111-GAL::ytm1 E80A. Transformants were grown in SRC-Leu medium (raffinose) and shifted to YPG (galactose) medium for 6 hr to induce overexpression of Ytm1 or Ytm1 E80A. Subsequently, TAP purifications were performed, and final EGTA eluates were analyzed by 4%–12% gradient SDS-PAGE and Coomassie staining (A) and western blotting (B) using the indicated antibodies. Molecular weight marker (M) is indicated. Labeled protein bands were identified by mass spectrometry (see D for band assignation). (C) Yeast strain Rix1-TAP with GAL::HA-REA1 was grown in YPG (galactose) and shifted to YPD (glucose) for 16 hr to deplete for Rea1. Subsequently, Rix1-TAP purification was performed, and the final EGTA eluate were analyzed by 4%–12% gradient SDS-PAGE and Coomassie staining (Rea1 depl., lane 2). This eluate was compared to a Rix1-TAP purification when Rea1 was overexpressed (grown in YPG, “wt” lane 1). Molecular weight marker (M) is indicated, and labeled protein bands were identified by mass spectrometry (see D for band assignation). (D) Band assignation.
The rRNA content of the purified particles, as well as the rRNA processing defect of a Gal::REA1 strain, is shown in Figure S4.
Because these data indicated that Rea1 collaborates with Ytm1 in early pre-60S biogenesis, we next depleted Rea1 to test whether 60S formation becomes arrested at a similar step as in the Ytm1 E80A mutant, upstream of the Rea1-Rsa4 contact. Rix1-TAP affinity purifications were performed using extracts from GAL::REA1 cells grown in either galactose (Rea1 expression) or glucose-containing medium (Rea1 depletion). In the presence of Rea1, the Rix1 particle exhibited the typical composition of associated factors, including Rea1, Rsa4, and the Rix1-Ipi3-Ipi1 subcomplex. When REA1 expression was repressed, the Rix1 particle no longer contained Rea1 or Rsa4 but was co-enriched in Ytm1 and other early pre-60S factors (e.g., Erb1, Spb1, Nop2, Noc2, Noc3, and Spb4), as well as early 27S pre-rRNAs (Figures 4C and S4B). Moreover, pre-60S factors and pre-rRNAs typically found in late nucleoplasmic or cytoplasmic nascent 60S subunits (e.g., Arx1, Tif6, and 7S rRNA) were depleted from this unusual Rix1 particle. However, unlike in the Ytm1 E80A mutant, Noc1, Rrp5, and 27SA2 were not co-enriched when Rix1-TAP was affinity purified from Rea1-depleted cells (Figures 4 and S4B). Consistent with Rea1 playing a dual role together with either Ytm1 or Rsa4, respectively, its depletion leads to the accumulation of 27S and 7S pre-rRNAs (Figure S4C). Thus, both the expression of toxic Ytm1 E80A and the depletion of Rea1 shift the Rix1 pre-60S particle to an earlier stage containing nucleolar but lacking nucleoplasmic biogenesis factors.
In Vitro Assay for Rea1-Dependent Removal of the Ytm1-Erb1-Nop7 Subcomplex from the Pre-60S Particle
In vitro Rea1 can mediate ATP-dependent release of Rsa4 and the Rix1-Ipi3-Ipi1 subcomplex from Rix1-purified pre-60S particles (Ulbrich et al., 2009). To test in vitro whether Rea1 can induce the release of Ytm1 from pre-60S subunits, we incubated the Ytm1-TAP particle (Figure S2B) with purified Rea1 and ATP. Subsequently, the reaction was separated on a sucrose gradient and analyzed by SDS-PAGE and Coomassie staining or western blotting to monitor the release of biogenesis factors from the pre-60S subunit. However, neither Ytm1 nor other biogenesis factors were found to dissociate from this pre-60S particle (Figures 5A and 5B).
Figure 5. Rea1 Extracts Ytm1-Erb1-Nop7 from the Purified Pre-60S Ribosomal Particle In Vitro.
(A and B) The Ytm1-TAP particle was affinity purified, mixed with purified Rea1, and incubated for 45 min at 23 °C with ± 4 mM ATP. Subsequently, the mixture was loaded on a 5%–30% sucrose gradient and centrifuged for 16 hr at 27.000 rpm. Gradient fractions 1–8 were analyzed by SDS-PAGE and Coomassie staining (top) or western blotting using the indicated antibodies (bottom).
(C and D) The Rix1-TAP particle was affinity purified from Rea1-depleted cells (see Figure 4C), mixed with purified Rea1, and incubated for 45 min at 23 °C with ± 4 mM ATP. Subsequently, the mixture was loaded on a 5%-30% sucrose gradient and centrifuged for 16 hr at 27.000 rpm as described in (A) and (B). Gradient fractions 1–8 were analyzed by SDS-PAGE and silver staining (top) or western blotting using the indicated antibodies (bottom). Bands released by ATP treatment were Erb1 (•) and Ytm1 (*), which were identified by mass spectroscopy.
We speculated that the purified Ytm1 particle is not suitable for releasing Ytm1 in vitro. Therefore, we tested a Rix1-TAP particle, isolated from the Rea1-depleted cells, that yielded a pre-60S particle harboring the Rix1 subcomplex, Ytm1, and the other early pre-60S factors but devoid of Rea1 (see Figure 4C, lane 2). Strikingly, when this pre-60S particle was incubated with purified Rea1, an ATP-dependent release of Ytm1, Erb1, and, to a lesser extent, Nop7 from the pre-60S particle was observed (Figures 5C and 5D). In contrast, other biogenesis factors, including Rlp24 and Nsa2, remained bound to this in vitro matured pre-60S particle. Further in vitro tests showed that ATP or Rea1 alone, or addition of Rea1 with a nonhydrolyzable ATP analog (AMP-PNP), did not induce the release of Ytm1 from the pre-60S particle (Figure 6B and data not shown). Moreover, the mutant Ytm1 E80A protein, when assembled onto the pre-60S particle, was not removed in vitro from this particle even in the presence of Rea1 and ATP (Figure 6C). These data indicate that the interaction between Rea1 and the Ytm1-MIDO is required for an ATP-hydrolysis-dependent removal of the Ytm1-Erb1-Nop7 subcomplex from an early pre-60S particle.
Figure 6. The Release of Ytm1 Depends on the Ytm1-Rea1 Interaction and ATP Hydrolysis.
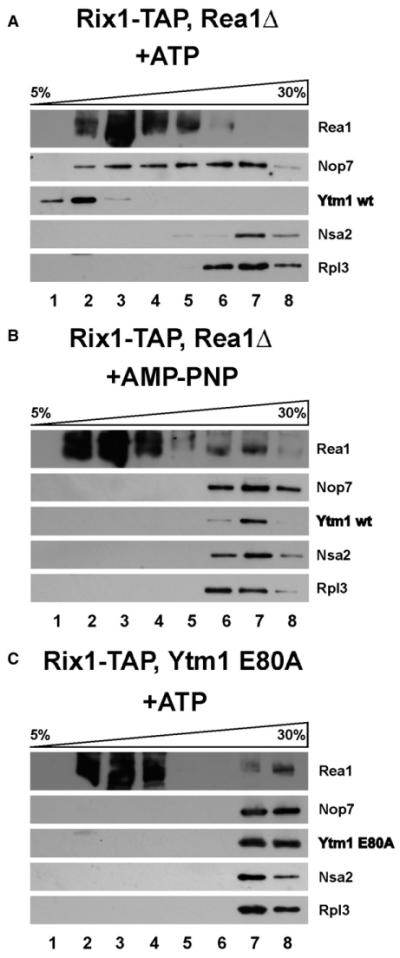
(A–C) The Rix1-TAP particle was affinity purified from Rea1-depleted cells (A and B) (see Figure 4C) or from Ytm1-E80A-induced cells (C) (see Figure 4A), mixed with purified Rea1, and incubated for 45 min at 23 °C with 4 mM ATP or AMP-PNP, respectively. Subsequently, the mixture was loaded on a 5%–30% sucrose gradient and centrifuged for 16 hr at 27.000 rpm. Gradient fractions 1–8 were analyzed by western blotting using the indicated antibodies.
DISCUSSION
This study has revealed that the conserved Ytm1-Erb1-Nop7 subcomplex associated with a nucleolar pre-60S particle is a target of the Rea1 AAA+-ATPase. Thus, Rea1 performs a sofar unrecognized role during early pre-60S subunit biogenesis in the nucleolus. Taken together, the data from this and previous work (Ulbrich et al., 2009) demonstrate that the MIDO of Ytm1 and Rsa4 interact directly but sequentially with the Rea1 MIDAS during 60S subunit biogenesis. Abrogation of the Rea1-Ytm1 interaction by the dominant-lethal Ytm1 E80A mutation causes an early block during 60S biogenesis at the transition from the nucleolus to the nucleoplasm. In contrast, impairing the Rea1-Rsa4 interaction by the dominant Rsa4 E114D mutation inhibited export of the pre-60S particle from the nucleoplasm to the cytoplasm (Ulbrich et al., 2009). Because Ytm1 is recruited first to pre-60S particles in the nucleolus, it is possible that the binding site occupied by Ytm1 overlaps with the binding site to which Rsa4 is subsequently recruited. Hence, the Ytm1-Erb1-Nop7 subcomplex may block premature recruitment of Rsa4 to the nucleolar pre-60S particle. Alternatively, Rsa4 binding to pre-60S particles may require a distinct pre-rRNA processing step (e.g., 27S→25S, 7S) that depends directly or indirectly on the Ytm1-Erb1-Nop7 subcomplex. How Rea1 promotes the release of its substrates from the pre-60S particles remains to be determined. As suggested previously for the Rea1-dependent Rsa4 release, Rea1 may act as a mechanochemical enzyme and, due to the presence of its long and flexible tail domain, could exert a tensile force affecting distantly located factors on the surface of the pre-60S particle (for discussion, see Ulbrich et al., 2009). However, other mechanisms are also imaginable, such as nucleotide-dependent conformational changes within Rea1 that may apply tension to bound proteins, thereby allowing Rea1 to dissociate protein-protein interactions (Vale, 2000). In addition, the Rea1 ATPase activity may not only be used to release factors, but also to remodel the structure of the pre-60S particle, such as the restructuring of pre-rRNA for subsequent processing steps (e.g., 27S→7S, 25S and 7S→5.8S).
A pre-60S particle carrying both Ytm1 and the Rix1 subcomplex has not been reported. However, it is possible that this hypothetical biogenesis intermediate is short lived but could be significantly accumulated upon Rea1 repression or induction of the dominant Ytm1 E80A mutant. Of note, only this mutant intermediate, but not the wild-type Ytm1-TAP particle, was competent for in vitro release of Ytm1-Erb1-Nop7. This finding indicates that the wild-type Ytm1-TAP particle may not yet be “ready” for this step, perhaps because it still requires a structural rearrangement (e.g., rRNA processing) or recruitment of additional trans-acting factors (e.g., the Rix1-Ipi3-Ipi1 subcomplex). Of interest, the Rix1 pre-60S particle purified from the Ytm1 E80A background is largely depleted of Rea1. This could indicate that a functional Ytm1 is required to recruit Rea1 to nucleolar pre-60S particles.
Our in vitro data further indicated that both a productive Rea1-Ytm1 interaction and ATP hydrolysis are necessary to release the Ytm1-Erb1-Nop7 subcomplex from the pre-ribosomal particle. Whereas Ytm1 and Erb1 were efficiently dissociated, Nop7 was only partially released. This finding is consistent with other biochemical data, which indicate that a pool of Nop7 remains associated with late nucleoplasmic pre-60S particles that are devoid of Ytm1 and Erb1 (Kressler et al., 2008; Nissan et al., 2002). Therefore, this residual pool of Nop7 may be removed later from the evolving pre-60S subunit by other biogenesis factors. In vivo removal of Ytm1-Erb1-Nop7 may also be affected by additional mechanisms, as it was reported that addition of phosphatase inhibitors to cell lysates caused a dissociation of the Ytm1-Erb1-Nop7 subcomplex from pre-60S particles (Miles et al., 2005). This observation suggests that phosphorylation and/or dephosphorylation may be also involved in factor removal from the early pre-60S particle.
In vivo, the transition of the pre-60S particle from the nucleolus to the nucleoplasm is not only accompanied with the release of Ytm1 and Erb1, but also with the release of additional nucleolar factors, including Spb1, Nop2, Noc2, Noc3, Nop4, Puf6, Spb4, Nop12, Has1, and Fpr4. It remains to be determined whether the removal of the Ytm1-Erb1-Nop7 subcomplex by Rea1 could pave the way for the subsequent dissociation of other early pre-60S factors in vivo, an event concomitant with the release of the nascent pre-60S particle from the nucleolus to the nucleoplasm. However, additional mechanisms may exist for the removal of factors, such as displacement by “downstream” factors such as Arx1, Rsa4, or Nog2/Nug2 upon transition of the particle into the nucleoplasm or removal of Nsa1 from an early pre-60S particle by the Rix7 AAA+ ATPase (Gadal et al., 2001; Kressler et al., 2008).
Because Rea1 and its targets, Ytm1 and Rsa4, are conserved from yeast to human, it is likely that the Rea1-driven 60S biogenesis steps occur in all eukaryotes. Consistent with this notion, the human nucleolar PeBoW complex composed of Pes1 (Nop7), Bop1 (Erb1), and WDR12 (Ytm1) is essential for cell proliferation and processing of rRNA in mammalian cells (Davies et al., 2008; DeLaBarre and Brunger, 2005; Grimm et al., 2006; Hölzel et al., 2007; Hölzel et al., 2005; Rohrmoser et al., 2007). Thus, future studies regarding the structure and function of the conserved Rea1 and its binding partners could contribute to general understanding of eukaryotic ribosome biogenesis and its link to other cellular processes and diseases.
EXPERIMENTAL PROCEDURES
Strains, Media, and Plasmids
Plasmids and yeast Saccharomyces cerevisiae strains used in this study are listed in Tables S1 and S2. Yeast double-shuffle strains were generated as described (Strässer et al., 2000). Yeast genetic methods such as gene deletion or GFP tagging of genes at the genomic locus (Longtine et al., 1998; Puig et al., 1998), transformation, mating, and tetrad analysis were performed according to published procedures.
Screen for ytm1 Mutants that Are Synthetic Lethal with rea1-DTS
A library of ytm1 mutant alleles was generated by incubating plasmid pRS314-YTM1 in hydroxylamine buffer (1 M hydroxylamine, 400 mM NaOH, 20 mM Na3PO4, and 5 mM EDTA) for 20 hr at 50°C. The DNA was precipitated with 80% ethanol and transformed into the screening strain, ytm1::natNT2, rea1::kanMX6, his3, trp1, leu2, ura3, ade2, ade3 harboring plasmids pCEN6*-ADE3-HIS3-YTM1, pRS416-REA1 (URA3), and YC-plac111-TAP-rea1-DTS (LEU2). Transformants were selected on SDC-TrpLeu-Ura. Colonies that carry a lethal ytm1 mutant depend on the presence of the plasmid pCEN6*-ADE3-HIS3-YTM1. In contrast, transformants with viable ytm1 alleles can lose this plasmid, which can be identified by a red-white sectoring phenotype (yeast cells that are ade2 exhibit a red color, whereas ade2 ade3 cells are white). Importantly, plasmid pCEN6*-ADE3-HIS3-YTM1 carries a mutated cen6* that enables enhanced plasmid loss under nonselective conditions. White colonies were picked and further grown on SDC-Trp-Leu plates to allow for loss of pRS416-REA1 before plating the cells on SDC+5-FOA. No growth on 5-FOA-containing plates indicated synthetic lethality of the ytm1 allele in combination with the rea1-DTS allele. Plasmids carrying this type of mutated ytm1 alleles were re-isolated and retransformed into the corresponding tester strains to confirm the synthetic lethal phenotype.
Rix1-TAP Purification from Cell Lysates Containing Overexpressed Ytm1 E80A or Lacking Rea1
The Rix1-TAP strain was transformed with the plasmid encoding GAL::ytm1-E80A or GAL::YTM1. Pre-cultures were grown in 2l SRC-Leu to prevent plasmid loss to an OD of 2.0, followed by shifting cells to galactose medium (YPG) for 6 hr.
To deplete Rea1 from Rix1-TAP cells, the endogenous REA1 promoter was replaced by the inducible GAL promoter. Rix1-TAP GAL1::REA1 cells were grown in YPG medium before shifting cells into 2l YPD medium for 16 hr to deplete for Rea1. Finally, cells were harvested and lysed, and TAP purification was performed (Nissan et al., 2002) to isolate the Rix1-containing particle.
In Vitro Release of the Ytm1-Erb1-Nop7 Subcomplex from the Pre-60S Particle
The nucleolar pre-60S particle was affinity purified via Rix1-TAP from Rea1-depleted cells (see above). For isolation of Rea1, Rea1 was overexpressed in yeast from plasmid pGAL::ProtA-TEV-CBP-FLAG-REA1 by shifting a 1 l pre-culture (SRC-Leu) grown to OD 1.0 into 2l YPG medium for 6 hr at 30 °C and subsequent tandem affinity purification of TAP-Rea1. The eluted pre-60S particle (10 mM Tris [pH 8.0], 5 mM EGTA, and 15 mM MgCl2) was mixed with or without purified Rea1 before addition of 4 mM ATP and incubation for 45 min at 23 °C. This 600 μl reaction mixture was loaded on a 5%-30% sucrose gradient and centrifuged using an SW40 rotor for 16 hr at 27.000 rpm and 4 °C. Gradient fractions were collected, precipitated by TCA, and analyzed by SDS-PAGE and Coomassie staining or western blotting using the indicated antibodies according to Ulbrich et al. (2009).
Miscellaneous
Additional methods used in this study and described earlier include TAP purification of pre-60S particles (Bassler et al., 2001; Nissan et al., 2002), purification of TAP-Rea1 (Ulbrich et al., 2009), sucrose gradient analysis to obtain ribosomal and polysomal profiles (Bassler et al., 2001), ribosomal export assays using the large subunit reporter Rpl25-GFP (Gadal et al., 2002) monitored by fluorescence microscopy according to Bassler et al. (2006), and yeast twohybrid analysis (Kressler et al., 2008). Preparation of rRNA, northern blot analysis (including sequences of probes D-A2, A2-A3, E-C2, 18S, 25S, and 5.8S), western blot analysis, and expression of recombinant proteins in E. coli and their subsequent affinity purification were performed as described (Ulbrich et al., 2009). The sequence of the 5S northern blotting probe is GGTCACCCA CTACACTACTCGG.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. J. de la Cruz, H. Tschochner, M. Fromont-Racine, A.W. Johnson, M. Seedorf, B. Stillman, and J.L. Woolford for antibodies. We are grateful to Dr. E. Thomson and Dr. D. Kressler for fruitful discussion on the project and yeast strains Nog1-GFP and Erb1-GFP; Dr. K. Galani for strain Rix1-TAP, GAL1::Rea1; Dr. T. Nissan for Nop7-GFP and Has1-GFP strains; and W. Chang for Rea1Δ, Ytm1Δ double deletion strain. E.H. and J.B. are recipients of grants from the Deutsche Forschungsgemeinschaft (Hu363/9-2 and Gottfried Wilhelm Leibniz Program) and Fonds der Chemischen Industrie. B.P. was supported by the Austrian Science Fund (FWF; Hertha Firnberg grant T404-B12).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes four figures and two tables and can be found with this article online at doi:10.1016/j.molcel.2010.05.024.
REFERENCES
- Bassler J, Grandi P, Gadal O, Lessmann T, Petfalski E, Tollervey D, Lechner J, Hurt E. Identification of a 60S preribosomal particle that is closely linked to nuclear export. Mol. Cell. 2001;8:517–529. doi: 10.1016/s1097-2765(01)00342-2. [DOI] [PubMed] [Google Scholar]
- Bassler J, Kallas M, Hurt E. The NUG1 GTPase reveals and N-terminal RNA-binding domain that is essential for association with 60 S pre-ribosomal particles. J. Biol. Chem. 2006;281:24737–24744. doi: 10.1074/jbc.M604261200. [DOI] [PubMed] [Google Scholar]
- Davies JM, Brunger AT, Weis WI. Improved structures of full-length p97, an AAA ATPase: implications for mechanisms of nucleotide-dependent conformational change. Structure. 2008;16:715–726. doi: 10.1016/j.str.2008.02.010. [DOI] [PubMed] [Google Scholar]
- de la Cruz J, Sanz-Martínez E, Remacha M. The essential WD-repeat protein Rsa4p is required for rRNA processing and intranuclear transport of 60S ribosomal subunits. Nucleic Acids Res. 2005;33:5728–5739. doi: 10.1093/nar/gki887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLaBarre B, Brunger AT. Nucleotide dependent motion and mechanism of action of p97/VCP. J. Mol. Biol. 2005;347:437–452. doi: 10.1016/j.jmb.2005.01.060. [DOI] [PubMed] [Google Scholar]
- Freed EF, Bleichert F, Dutca LM, Baserga SJ. When ribosomes go bad: diseases of ribosome biogenesis. Mol. Biosyst. 2010;6:481–493. doi: 10.1039/b919670f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromont-Racine M, Senger B, Saveanu C, Fasiolo F. Ribosome assembly in eukaryotes. Gene. 2003;313:17–42. doi: 10.1016/s0378-1119(03)00629-2. [DOI] [PubMed] [Google Scholar]
- Gadal O, Strauss D, Braspenning J, Hoepfner D, Petfalski E, Philippsen P, Tollervey D, Hurt EC. A nuclear AAA-type ATPase (Rix7p) is required for biogenesis and nuclear export of 60S ribosomal subunits. EMBO J. 2001;20:3695–3704. doi: 10.1093/emboj/20.14.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadal O, Strauss D, Petfalski E, Gleizes PE, Gas N, Tollervey D, Hurt E. Rlp7p is associated with 60S preribosomes, restricted to the granular component of the nucleolus, and required for pre-rRNA processing. J. Cell Biol. 2002;157:941–951. doi: 10.1083/jcb.200111039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathi KA, Shimamura A. Ribosomal dysfunction and inherited marrow failure. Br. J. Haematol. 2008;141:376–387. doi: 10.1111/j.1365-2141.2008.07095.x. [DOI] [PubMed] [Google Scholar]
- Garbarino JE, Gibbons IR. Expression and genomic analysis of midasin, a novel and highly conserved AAA protein distantly related to dynein. BMC Genomics. 2002;3:18–28. doi: 10.1186/1471-2164-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman S, Baserga SJ. Ribosome biogenesis: of knobs and RNA processing. Exp. Cell Res. 2004;296:43–50. doi: 10.1016/j.yexcr.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Grimm T, Hölzel M, Rohrmoser M, Harasim T, Malamoussi A, Gruber-Eber A, Kremmer E, Eick D. Dominant-negative Pes1 mutants inhibit ribosomal RNA processing and cell proliferation via incorporation into the PeBoW-complex. Nucleic Acids Res. 2006;34:3030–3043. doi: 10.1093/nar/gkl378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henras AK, Soudet J, Gérus M, Lebaron S, Caizergues-Ferrer M, Mougin A, Henry Y. The post-transcriptional steps of eukaryotic ribosome biogenesis. Cell. Mol. Life Sci. 2008;65:2334–2359. doi: 10.1007/s00018-008-8027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel M, Rohrmoser M, Schlee M, Grimm T, Harasim T, Malamoussi A, Gruber-Eber A, Kremmer E, Hiddemann W, Bornkamm GW, Eick D. Mammalian WDR12 is a novel member of the Pes1-Bop1 complex and is required for ribosome biogenesis and cell proliferation. J. Cell Biol. 2005;170:367–378. doi: 10.1083/jcb.200501141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel M, Grimm T, Rohrmoser M, Malamoussi A, Harasim T, Gruber-Eber A, Kremmer E, Eick D. The BRCT domain of mammalian Pes1 is crucial for nucleolar localization and rRNA processing. Nucleic Acids Res. 2007;35:789–800. doi: 10.1093/nar/gkl1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressler D, Roser D, Pertschy B, Hurt E. The AAA ATPase Rix7 powers progression of ribosome biogenesis by stripping Nsa1 from pre-60S particles. J. Cell Biol. 2008;181:935–944. doi: 10.1083/jcb.200801181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressler D, Hurt E, Bassler J. Driving ribosome assembly. Biochim. Biophys. Acta. 2009;1803:673–683. doi: 10.1016/j.bbamcr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A, III, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;10:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Miles TD, Jakovljevic J, Horsey EW, Harnpicharnchai P, Tang L, Woolford JL., Jr. Ytm1, Nop7, and Erb1 form a complex necessary for maturation of yeast 66S preribosomes. Mol. Cell. Biol. 2005;25:10419–10432. doi: 10.1128/MCB.25.23.10419-10432.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nal B, Mohr E, Silva MI, Tagett R, Navarro C, Carroll P, Depetris D, Verthuy C, Jordan BR, Ferrier P. Wdr12, a mouse gene encoding a novel WD-Repeat Protein with a notchless-like amino-terminal domain. Genomics. 2002;79:77–86. doi: 10.1006/geno.2001.6682. [DOI] [PubMed] [Google Scholar]
- Narla A, Ebert BL. Ribosomopathies: human disorders of ribosome dysfunction. Blood. 2010;115:3196–3205. doi: 10.1182/blood-2009-10-178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissan TA, Bassler J, Petfalski E, Tollervey D, Hurt EC. 60S pre-ribosome formation viewed from assembly in the nucleolus until export to the cytoplasm. EMBO J. 2002;21:5539–5547. doi: 10.1093/emboj/cdf547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertschy B, Saveanu C, Zisser G, Lebreton A, Tengg M, Jacquier A, Liebminger E, Nobis B, Kappel L, van der Klei I, et al. Cytoplasmic recycling of 60S preribosomal factors depends on the AAA protein Drg1. Mol. Cell. Biol. 2007;27:6581–6592. doi: 10.1128/MCB.00668-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig O, Rutz B, Luukkonen BG, Kandels-Lewis S, Bragado-Nilsson E, Séraphin B. New constructs and strategies for efficient PCR-based gene manipulations in yeast. Yeast. 1998;14:1139–1146. doi: 10.1002/(SICI)1097-0061(19980915)14:12<1139::AID-YEA306>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Rohrmoser M, Hölzel M, Grimm T, Malamoussi A, Harasim T, Orban M, Pfisterer I, Gruber-Eber A, Kremmer E, Eick D. Interdependence of Pes1, Bop1, and WDR12 controls nucleolar localization and assembly of the PeBoW complex required for maturation of the 60S ribosomal subunit. Mol. Cell. Biol. 2007;27:3682–3694. doi: 10.1128/MCB.00172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strässer K, Bassler J, Hurt EC. Binding of the Mex67p/Mtr2p heterodimer to FXFG, GLFG, and FG repeat nucleoporins is essential for nuclear mRNA export. J. Cell Biol. 2000;150:695–706. doi: 10.1083/jcb.150.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunk BS, Karbstein K. Powering through ribosome assembly. RNA. 2009;15:2083–2104. doi: 10.1261/rna.1792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Sahasranaman A, Jakovljevic J, Schleifman E, Woolford JL., Jr. Interactions among Ytm1, Erb1, and Nop7 required for assembly of the Nop7-subcomplex in yeast preribosomes. Mol. Biol. Cell. 2008;19:2844–2856. doi: 10.1091/mbc.E07-12-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas F, Kutay U. Biogenesis and nuclear export of ribosomal subunits in higher eukaryotes depend on the CRM1 export pathway. J. Cell Sci. 2003;116:2409–2419. doi: 10.1242/jcs.00464. [DOI] [PubMed] [Google Scholar]
- Trotta CR, Lund E, Kahan L, Johnson AW, Dahlberg JE. Coordinated nuclear export of 60S ribosomal subunits and NMD3 in vertebrates. EMBO J. 2003;22:2841–2851. doi: 10.1093/emboj/cdg249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschochner H, Hurt E. Pre-ribosomes on the road from the nucleolus to the cytoplasm. Trends Cell Biol. 2003;13:255–263. doi: 10.1016/s0962-8924(03)00054-0. [DOI] [PubMed] [Google Scholar]
- Ulbrich C, Diepholz M, Bassler J, Kressler D, Pertschy B, Galani K, Böttcher B, Hurt E. Mechanochemical removal of ribosome biogenesis factors from nascent 60S ribosomal subunits. Cell. 2009;138:911–922. doi: 10.1016/j.cell.2009.06.045. [DOI] [PubMed] [Google Scholar]
- Vale RD. AAA proteins: Lords of the ring. J. Cell Biol. 2000;150:F13–F19. doi: 10.1083/jcb.150.1.f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemp I, Wild T, O’Donohue MF, Wandrey F, Widmann B, Gleizes PE, Kutay U. Distinct cytoplasmic maturation steps of 40S ribosomal subunit precursors require hRio2. J. Cell Biol. 2009;185:1167–1180. doi: 10.1083/jcb.200904048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.