Abstract
Background
Rates of smoking cessation have not changed in a decade, accentuating the need for novel approaches to prompt quit attempts.
Methods
Within a nationwide randomized clinical trial (N=849) to induce further quit attempts and cessation, smokers currently unmotivated to quit were randomized to a practice quit attempt (PQA) alone or to nicotine replacement therapy (hereafter referred to as nicotine therapy), sampling within the context of a PQA. Following a 6-week intervention period, participants were followed up for 6 months to assess outcomes. The PQA intervention was designed to increase motivation, confidence, and coping skills. The combination of a PQA plus nicotine therapy sampling added samples of nicotine lozenges to enhance attitudes toward pharmacotherapy and to promote the use of additional cessation resources. Primary outcomes included the incidence of any ever occurring self-defined quit attempt and 24-hour quit attempt. Secondary measures included 7-day point prevalence abstinence at any time during the study (ie, floating abstinence) and at the final follow-up assessment.
Results
Compared with PQA intervention, nicotine therapy sampling was associated with a significantly higher incidence of any quit attempt (49% vs 40%; relative risk [RR], 1.2; 95% CI, 1.1–1.4) and any 24-hour quit attempt (43% vs 34%; 1.3; 1.1–1.5). Nicotine therapy sampling was marginally more likely to promote floating abstinence (19% vs 15%; RR, 1.3; 95% CI, 1.0–1.7); 6-month point prevalence abstinence rates were no different between groups (16% vs 14%; 1.2; 0.9–1.6).
Conclusion
Nicotine therapy sampling during a PQA represents a novel strategy to motivate smokers to make a quit attempt.
Trial Registration
clinicaltrials.gov Identifier: NCT00706979
Despite advances in clinical care and policy, rates of smoking cessation have held constant in the past decade,1 indicating a need for novel approaches.2 Strategies to further prompt quitting must increase quit attempts or the likelihood that each quit attempt succeeds.3 Most strategies focus on the latter, leaving few options for smokers who have little interest in quitting in the near future. Many smokers say they want to quit eventually,4 but in any given year, approximately 60% do not try to quit5 and less than 10% of smokers plan to quit in the next month.6 Therefore, most smokers are ambivalent about or resistant to quitting in the near future. These unmotivated smokers are an important and understudied target for public health.
For smokers unmotivated to quit, the immediate goal is to promote quit attempts, a strategy referred to as cessation induction. Cessation induction studies2,7,8 differ from traditional cessation trials in at least the following 2 ways: (1) the target population is smokers without current intention to quit (smokers unmotivated to quit) and (2) the studies primarily focus on prompting quit attempts (abstinence is the eventual goal but is secondary).
In contrast to policy interventions,9–11 clinical interventions for promoting quit attempts have received less attention, and the options are narrow. The most common method of increasing motivation to quit is physician-delivered brief advice.3 A more time-intensive clinical option is motivational interviewing.12 Both physician advice and motivational interviewing rely on repeated counseling, which could overtime frustrate both smokers and clinicians. Furthermore, many physicians lack confidence in smoking counseling.13 New strategies that do not rely exclusively on verbal counseling could provide an alternative to smokers who have not responded to physician-delivered brief advice in the past.
Committing to quit is difficult for many smokers because they have failed in the past and fear embarrassment if unsuccessful.14 An alternative is to have smokers engage in less-threatening cessationlike activities, such as a practice quit attempt (PQA) (eg, attempting to not smoke for a few hours or days, without pressure to permanently quit). A successful PQA experience could increase self-efficacy and teach coping skills for quitting. Practice quit attempts have been recommended15,16 but have not been empirically tested. In addition, if smokers unmotivated to quit sample cessation medication such as nicotine replacement therapy (NT) (hereafter referred to as nicotine therapy) during a PQA, its therapeutic benefits could empower them to make a quit attempt. Despite strong evidence that NT is efficacious,3,17 52% to 78% of smokers never use these products.18–20 Underuse of cessation pharmacotherapy in part reflects misperception and doubt,21–24 which could be reversed through real-world NT sampling.
A 2008 study25 of one-time NT use demonstrated a positive shift in attitudes but did not test for effects on cessation behavior. Offering NT to motivated smokers (eg, callers to a quitline) has been shown to increase quit attempts and abstinence.26–28 This provides indirect support for the potential efficacy of NT sampling, but it is unknown whether a similar intervention would influence smokers unmotivated to quit. This article reports on a randomized trial of NT sampling during PQAs as a novel method to induce quit attempts among smokers unmotivated to quit. Primarily, we hypothesized that NT-aided PQAs would result in a higher incidence of subsequent attempts to permanently quit smoking compared with PQAs alone. Secondarily, we hypothesized that NT sampling would result in higher 7-day point prevalence abstinence at the 6-month final follow-up assessment.
METHODS
Trial methods and a detailed rationale for the study design have been reported elsewhere.29 In this randomized 2-group study design, smokers who were unmotivated to quit were assigned (1) to a PQA alone or (2) to NT sampling within the context of a PQA. The study was approved by the Institutional Review Board of the Medical University of South Carolina.
PARTICIPANTS
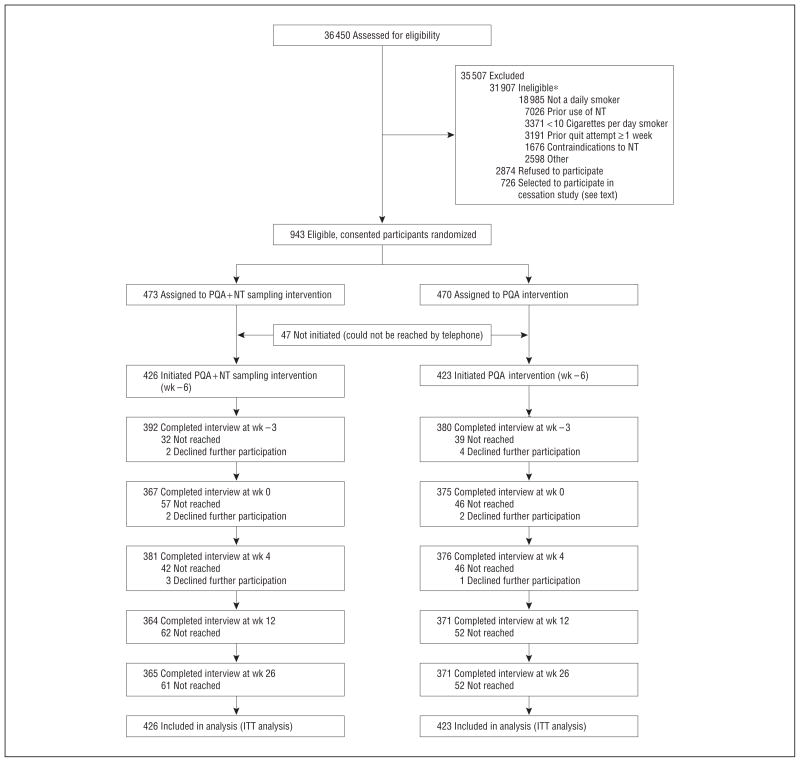
Participants were recruited from January 5, 2009, to February 10, 2010, via the Internet using an e-mail database of potential enrollees provided by a marketing research firm. Potential participants were e-mailed a study invitation and completed a brief online survey to establish study eligibility. Recruitment flow, including participant retention throughout the study, is shown in Figure 1. Study participants had to meet the following criteria: (1) be 18 years or older, (2) currently smoke at least 10 cigarettes per day, (3) be a nonuser of noncigarette tobacco, (4) be accessible by telephone for a 6-month study period, (5) have no Food and Drug Administration contraindications for nicotine lozenge use, (6) have no previous NT use, and (7) have no quit attempt longer than 1 week in the past year.
Figure 1.
Recruitment flow. ITT indicates intent to treat; NT, nicotine therapy; and PQA, practice quit attempt. *Exclusion criteria are not mutually exclusive.
To recruit smokers who were unmotivated to quit, eligible participants were offered the following 2 comparable study options: one for smokers wanting to quit in the next 30 days and another for smokers who wanted to quit at some later time but not in the next 30 days. Self-selection into either option denoted motivation to quit; only those who chose the latter study option were included in this study.
GENERAL PROCEDURES
Eligible participants were mailed an informed consent form and were asked to return a signed copy if they wished to participate. Participants were then randomized to treatment groups in a 1:1 ratio using a random number generator with block randomization. Research staff then made calls to initiate formal study enrollment. Of 943 who initially signed and returned consent forms, 94 (10.0%) could not be reached by telephone (Figure 1). The remaining 849 participants comprised the final study sample.
Blinding study staff and participants to study group was infeasible because the intervention was behaviorally based. The intervention consisted of 3 telephone calls during 6 weeks. Both treatment conditions were matched for duration. Approximately 5% of telephone calls were randomly recorded and monitored regularly for treatment fidelity against semiscripted protocols. At the last treatment telephone call, participants in both groups were given brief advice to quit smoking. Follow-up telephone contacts were made at weeks 4, 12, and 26 following the end of treatment to ascertain study outcomes. Across 5094 (849 × 6) scheduled telephone calls, 90% were completed; 73% of participants completed all 6 telephone calls, without significant group differences in retention.
INTERVENTIONS
The primary focus of the study was NT sampling. This was embedded within the behavioral exercise of a PQA.
Practice Quit Attempt
The PQA was designed with the intent to pause from smoking and to reflect on the process of quitting. We discussed with the smoker that quitting often requires practice before succeeding and that a PQA can be a good opportunity to practice and build confidence. The central theme of the PQA was to remove pressure of trying to permanently quit smoking. Telephone counselors worked collaboratively with each participant to establish a PQA plan that lasted a few hours or a few days or simply to see how long he or she could go without smoking. Participants were not required to engage in a PQA. At the second intervention telephone call (week –3), the success and barriers of the first PQA were reviewed, and a second PQA was solicited. After both intervention telephone calls, support materials were mailed, including further rationale for the PQA and coping strategies. The third and final treatment telephone call (week 0) reviewed overall progress and changed the focus to permanently quitting. Participants were not specifically asked to make a commitment to quit or to set a specific target quit date.
PQA-Based NT Sampling
Nicotine therapy sampling was provided within the context of a PQA to learn what it is and how it works. We hypothesized that NT sampling would familiarize NT-naive smokers with nicotine lozenges in particular and cessation aids in general and would dispel misperceptions about safety and adverse effects. Nicotine lozenges were used because (1) they are an over-the-counter product, (2) they can be used ad libitum (unlike nicotine patches), and (3) they seem to be more palatable than nicotine gum. Following the intervention telephone call, we mailed 1 box (72 Nicorette [previously Commit] lozenges; GlaxoSmithKline, Brentford, Middlesex, England]) of the preferred flavor (cherry or mint) with 2-mg or 4-mg doses, based on individual smoking level per the package instructions. The second intervention telephone call (week –3) built on the initial success. Counselors queried whether and how the lozenge was effective. A second PQA was established, and another NT sampling package was mailed. Similar support materials were mailed but with additional information (frequently asked questions) on nicotine lozenges. The third and final treatment telephone call was the same as that for the non–NT sampling condition. No lozenges were provided beyond the 6-week intervention period (2 NT sampling phases).
OUTCOMES
Outcomes were assessed via telephone. The primary outcome was an attempt to permanently quit smoking. To distinguish from a PQA, we specifically asked participants if they tried to quit smoking with the intent of permanently quitting. Quit attempts were self-defined as (1) any attempt to permanently quit and (2) any serious quit attempt of at least 24 hours. The latter fits the definition of a quit attempt by the Centers for Disease Control and Prevention,5 but it is unclear if this definition is more valid than the former.30,31 We also measured whether participants were ever abstinent for 7 days during the study, as short-term durations of abstinence predict eventual cessation.7
There is no method to biochemically verify our primary outcome of quit attempts, which are variable in time and transient in length. Verification of our secondary outcomes of 7-day point prevalence abstinence would have been ideal but was excluded, as it seems unnecessary with minimal interventions that incur few experimenter demand characteristics.8,32
Process measures of cessation readiness included the following: (1) the use of cessation pharmacotherapy (yes or no), (2) the use of behavioral support (eg, quitlines and classes [yes or no]), (3) motivation to quit in the next 30 days, (4) confidence to quit, and (5) knowledge and attitudes toward NT. Motivation and confidence were assessed on a scale of 0 to 10, adapted from prior items.33–35 Nicotine therapy knowledge was represented by the number of correct items out of 10. Using a Likert-type scale ranging from 1 to 4, positive (8 items) and negative (4 items) attitudes were evaluated. All measures were assessed at each contact except for NT knowledge and attitudes, which were measured at baseline, end of treatment, and the final follow-up assessment.
SAMPLE SIZE ESTIMATION
Based on the estimate that 41% of smokers make a 24-hour quit attempt annually,36 we projected that 21% would do so in 6 months. The PQA intervention effect was estimated based on outcomes from telephone-based cessation counseling,37 increasing the incidence of serious quit attempts by a factor of 1.4 and leading to an estimated incidence of quit attempts in the PQA group of 29% (0.21 × 1.4). We hypothesized that NT sampling would increase this incidence by 10% more. To detect a difference of 39% vs 29% required a targeted sample size of 750.
STATISTICAL ANALYSIS
A logistic regression model was used to examine the primary hypothesis that NT-enhanced PQAs would yield higher rates for the 2 primary outcomes of (1) any ever occurring quit attempt and (2) any 24-hour quit attempt, as well as the 2 secondary outcomes of (3) seven-day point prevalence abstinence at any time during the study (ie, floating abstinence) and (4) seven-day point prevalence abstinence at the 6-month final follow-up assessment. Generalized estimating equations examined group, time, and group × time interactions for each of the following additional process measures of cessation readiness: (1) intent to quit in the next month, (2) confidence in quitting, (3) attitudes toward NT, and (4) knowledge about NT. All analyses were based on an intent-to-treat approach; participants with missing data were assumed to have not quit smoking and to have made no quit attempts.
RESULTS
The behavioral filter effectively screened for smokers unmotivated to quit. On a 10-point scale, the mean (SD) intent to quit in the next month was 2.6 (2.9), and 92% of participants were in the stage of precontemplation (not wanting to quit in the next 6 months) or contemplation (wanting to quit in the next 2–6 months but not in the next 30 days). There were no statistically significant differences between the treatment groups on any baseline measure (Table 1).38
Table 1.
Baseline Characteristics of Participantsa
| Characteristic | PQA + NT Group (n=426) | PQA Group (n=423) |
|---|---|---|
| Age, mean (SD), y | 50.5 (11.8) | 50.7 (11.4) |
| Female sex, % | 62.4 | 66.0 |
| Race/ethnicity, % | ||
| White | 87.6 | 87.9 |
| Black | 7.8 | 9.3 |
| Other | 4.5 | 2.9 |
| Education, % | ||
| ≤High school | 26.8 | 21.0 |
| Some college | 47.4 | 50.6 |
| College graduate | 25.8 | 28.4 |
| Cigarettes per day, mean (SD) | 18.6 (8.8) | 19.2 (8.4) |
| Score on test for nicotine dependence by Fagerström,38 mean (SD) | 4.9 (2.3) | 5.1 (2.1) |
| >1 Prior quit attempt, % | 83.2 | 83.1 |
| Residence, % | ||
| Live alone | 23.7 | 23.0 |
| Live with others, none smoke | 32.9 | 30.4 |
| Live with others, ≥1 smoke | 43.4 | 46.6 |
| Intent to quit in the next month, mean (SD)b | 2.4 (2.8) | 2.7 (2.9) |
| Confidence in quitting, mean (SD)b | 4.0 (3.0) | 3.9 (3.0) |
| Stage of change, % | ||
| Preparation | 7.8 | 7.3 |
| Contemplation | 45.9 | 48.6 |
| Precontemplation | 46.4 | 44.1 |
| Attitudes toward NT, mean (SD)c | ||
| Positive | 3.0 (0.4) | 3.0 (0.4) |
| Negative | 2.6 (0.7) | 2.6 (0.7) |
| Knowledge about NT, mean (SD)b | 4.5 (2.5) | 4.4 (2.5) |
Abbreviations: NT, nicotine therapy; PQA, practice quit attempt.
There were no baseline differences between study groups.
On a scale of 0 to 10.
On a scale of 1 to 4.
PQA ENGAGEMENT AND NT SAMPLING
Across the intervention period, 82% of PQA+NT participants and 85% of PQA participants engaged in at least 1 PQA (P =.3). Within the PQA+NT group, 311 (73.0%) used nicotine lozenges during the first NT sampling period. The mean (SD) number of days of nicotine lozenge use was 6.8 (5.3), and the mean (SD) number of nicotine lozenges used per day of use was 3.8 (3.6). During the second NT sampling period, the proportion of users remained constant (73%), but the mean (SD) use increased slightly to 9.3 (8.8) days of use and to 4.0 (3.5) nicotine lozenges used per day of use.
QUIT ATTEMPTS
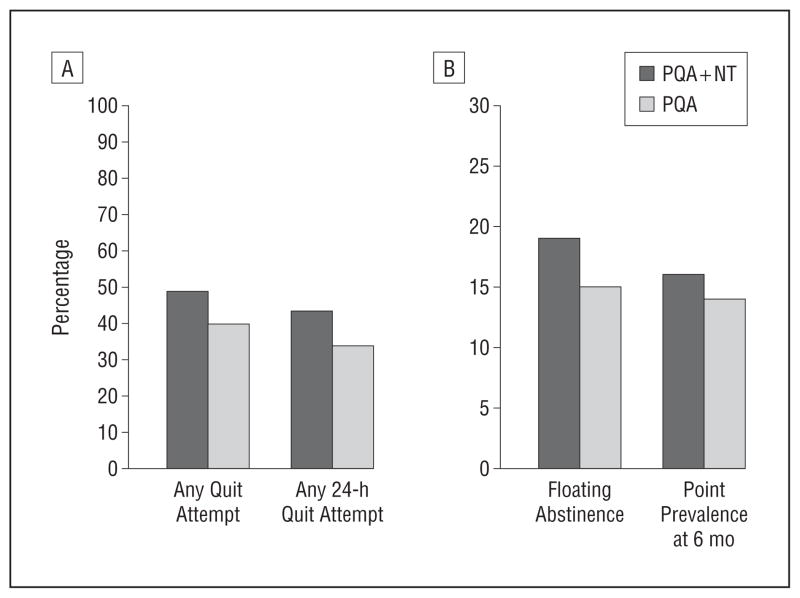
By 4 weeks following the end of treatment, 22% of PQA+NT participants and 13% of PQA participants had made a 24-hour quit attempt (P<.001); by 12 weeks following the end of treatment, these rates were 32% and 23%, respectively (P =.003). At the final follow-up assessment, the PQA+NT group had a significantly higher incidence than the PQA group of any quit attempt (49% vs 40%, P =.008) and any 24-hour quit attempt (43% vs 34%, P =.004) (Figure 2).
Figure 2.
Rates of any quit attempt (relative risk, 1.2; 95% CI, 1.1–1.4) and 24-hour quit attempt (1.3; 1.1–1.5) (A) and rates of floating abstinence (7-day point prevalence abstinence at any time during the study) (1.3; 1.0–1.7) and 7-day point prevalence abstinence at the final follow-up assessment (week 26) (1.2; 0.9–1.6) (B). NT indicates nicotine therapy; PQA, practice quit attempt.
CESSATION
By 4 weeks following the end of treatment, 7% of PQA + NT participants and 3% of PQA participants had quit smoking for at least 7 consecutive days (P =.004); by 12 weeks following the end of treatment, these rates were 13% and 8%, respectively (P =.03). At the final follow-up assessment, the PQA + NT group had a nonsignificantly higher incidence than the PQA group of floating abstinence (19% vs 15%, P = .09) (Figure 2). Seven-day point prevalence abstinence at 6 months was reported by 16% of PQA + NT participants vs 14% of PQA participants (P =.3).
MEASURES OF CESSATION READINESS
During the postintervention follow-up period, the PQA+NT group was significantly more likely to use cessation medication than the PQA group but was less likely to receive behavioral support services (Table 2). The most predominant medication used by the PQA+NT group was nicotine lozenge, with no significant between-group differences for other medications. Among the PQA+NT group, 137 participants (32.2%) were using cessation medication at the final follow-up assessment (week 26). Among these participants, 117 (85.4%) used nicotine lozenges, and 20 (17.1%) of them reported independent purchase of the product.
Table 2.
Use of Cessation Resources During the Postintervention Follow-up Period (Weeks 0–26)
| Resource Use | PQA + NT Group, % | PQA Group, % | RR (95% CI)a |
|---|---|---|---|
| Cessation medication | 69.0 | 12.5 | 5.5 (4.2–7.1) |
| Cessation medication other than nicotine lozenges | 9.9 | 11.3 | 0.9 (0.6–1.3) |
| Behavioral support servicesb | 35.7 | 43.7 | 0.8 (0.7–1.0) |
| Any of these | 75.1 | 47.5 | 1.6 (1.4–1.8) |
Abbreviations: NT, nicotine therapy; PQA, practice quit attempt; RR, relative risk.
Referent is PQA group.
Quitlines, classes, individual sessions, and speaking with a physician about quitting.
For intent to quit in the next month, confidence in quitting, negative attitudes toward NT, and knowledge about NT, there were significant group × time interactions during the intervention period but not during the follow-up period. That is, these outcomes improved for the PQA+NT group during the NT sampling period (Table 3) but remained stable for the PQA group (P < .001 for all group × time interactions). There were no group, time, or group × time interaction effects for positive attitudes toward NT.
Table 3.
Measures of Cessation Readiness
| Measure, Mean (SD) | Baseline
|
End of Treatment
|
Final Follow-up Assessment
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| PQA+NT Group | PQA Group | P Value | PQA+NT Group | PQA Group | P Value | PQA+NT Group | PQA Group | P Value | |
| Intent to quit in the next montha | 2.4 (2.8) | 2.7 (2.9) | .1 | 4.0 (3.4) | 3.2 (3.1) | .001 | 4.5 (3.9) | 4.3 (3.8) | .4 |
| Confidence in quittinga | 4.0 (3.0) | 3.9 (3.0) | .6 | 4.8 (3.2) | 4.0 (3.1) | .001 | 5.6 (3.4) | 5.1 (3.5) | .06 |
| Attitudes toward NTb | |||||||||
| Positive | 3.0 (0.4) | 3.0 (0.4) | .6 | 3.2 (0.4) | 2.9 (0.5) | <.001 | 3.1 (0.5) | 3.0 (0.5) | <.001 |
| Negative | 2.6 (0.7) | 2.6 (0.7) | .8 | 2.1 (0.7) | 2.7 (0.8) | <.001 | 2.3 (0.8) | 2.8 (0.9) | <.001 |
| Knowledge about NTa | 4.5 (2.5) | 4.4 (2.5) | .6 | 6.5 (2.0) | 4.6 (2.3) | <.001 | 6.0 (2.4) | 4.5 (2.4) | <.001 |
Abbreviations: NT, nicotine therapy; PQA, practice quit attempt.
On a scale of 0 to 10.
On a scale of 1 to 4.
COMMENT
This randomized trial tested a novel strategy for cessation induction among smokers unmotivated to quit (ie, whether brief periods of NT sampling during a PQA acts as a catalyst for quitting). This intervention increased the likelihood of quit attempts and the periods of abstinence during the trial but did not increase abstinence at the 6-month final follow-up assessment. Proxy measures of readiness to quit tended to favor the PQA+NT group, providing evidence that NT sampling operated through the hypothesized pathways. The rates of quit attempts and 7-day point prevalence abstinence with the PQA+NT strategy met or exceeded rates associated with existing options for cessation induction, such as motivational interviewing,12,39 physician-delivered brief advice,40 and smoking reduction.41,42 Our results suggest that brief NT sampling during a PQA is an efficacious intervention to motivate smokers who do not want to quit to successfully initiate the cessation process.
Many smokers are nonresponsive to motivational interventions such as physician-delivered brief advice.43 For smokers who do not respond to cognitively based interventions, an action-oriented strategy such as PQA+NT could be a viable treatment option that gradually introduces the smoker toward quitting. The use of NT for a PQA could occur in an over-the-counter setting without physician involvement; however, it is likely that explanation of the PQA concept and NT sampling by a physician would increase uptake and enhance outcomes.
The NT sampling intervention produced statistically and clinically significant increases in quit attempts, but replication of these findings should focus on abstinence. Methods to enhance abstinence outcomes could include longer duration of NT sampling, NT sampling from a menu of products, or instructions to increase the amount of product to use. As research in this area develops, further consideration should be given to additional mechanisms of change (eg, coping strategies), moderators of treatment effect (eg, prior quitting history), and direct comparison with existing cessation induction strategies.
The PQA intervention seemed to be beneficial, which likely led to the outcomes of the PQA+NT intervention being more modest than if no intervention control group had been used. The rates of quit attempts and cessation in the PQA group were substantially higher than would be expected based on national norms5 (ie, at 6 months, 34% vs 20% had made a quit attempt, and 14% vs 2% were abstinent). Although we know of no experimental test of the efficacy of PQAs to prompt subsequent quitting, evaluations of the Great American Smokeout44 and similar mass media campaigns9 suggest that PQAs could be effective. Future research on PQAs per se is indicated.
Study strengths include a novel treatment approach (tested among a group of proactively recruited smokers nationwide), a unique method of identifying cessation-resistant smokers, and strong rates of study retention. Study limitations include the absence of a control group without treatment, which prevented a test on PQAs per se. A nonintervention control group was not included because the research question focused on NT sampling in the context of a PQA, rendering PQA alone as the most apt control. Some might view the absence of a placebo control as another weakness. However, our primary aim was to determine if provision of NT sampling promotes quit attempts, not whether any such effect was pharmacologically mediated. An additional limitation was the potential lack of representativeness of the study sample, which was 88% non-Hispanic white, a likely consequence of the online recruitment strategy. However, although a potentially skewed sample recruited through online methods may affect external validity, this would not affect the internal validity of a randomized trial.45 Finally, the study would have been strengthened by inclusion of biochemical verification of the secondary end point of abstinence.
In summary, providing brief NT sampling to smokers who do not want to quit, when used within a behavioral exercise of a PQA, is efficacious to motivate unmotivated smokers toward quitting. Considering the stagnant incidence of quit attempts in the past decade, this novel and easy-to-use cessation induction strategy holds promise for translation to primary care settings.
Acknowledgments
Funding/Support: This study was supported by grants R01DA021619 and K23DA020482 (Dr Carpenter), K05DA000490 (Dr Hughes), and K12DA000357 (Dr Gray) from the National Institute on Drug Abuse. Discounted nicotine lozenges were provided by GlaxoSmithKline.
Footnotes
Financial Disclosure: Since January 1, 2008, Dr Hughes has received research grants from the National Institutes of Health and from Pfizer Pharmaceuticals; the latter develops and sells smoking cessation medications. During this time, he has accepted honoraria or consulting fees from the following nonprofit and for-profit organizations and companies that develop, sell, or promote smoking cessation products or services or educate or advocate about smoking cessation: Abbott Pharmaceuticals, Aradigm, American Academy of Addiction Psychiatry, American Psychiatric Association, American Psychiatric Institute for Research and Education, Cambridge Hospital, Dartmouth College, Dartmouth-Hitchcock, Dean Foundation, DLA Piper, EPI-Q, European Respiratory Society, Evotec, Free and Clear, GlaxoSmithKline, Golin Harris, Healthwise, Integrated Communication, Invivodata, Maine Health, McGill University Medical School, McNeil Pharmaceuticals, Novartis Pharmaceuticals, Oglivy Health PR, Ottawa Heart Institute, Pfizer Pharmaceuticals, Pinney Associates, Propagate Pharmaceuticals, Reckner Associates, Scientia, University of Arkansas for Medical Sciences, University of California–San Francisco, University of Wisconsin, National Institutes of Health, and Wolters Publishing.
Previous Presentation: Results of this study were presented at the 17th Annual Meeting of the Society for Research on Nicotine and Tobacco; February 17, 2011; Toronto, Ontario, Canada.
Author Contributions: Study concept and design: Carpenter, Hughes, Saladin, and Alberg. Acquisition of data: Carpenter and Gray. Analysis and interpretation of data: Carpenter, Hughes, Gray, and Wahlquist. Drafting of the manuscript: Carpenter, Hughes, Gray, Wahlquist, Saladin, and Alberg. Critical revision of the manuscript for important intellectual content: Carpenter, Hughes, Gray, Wahlquist, Saladin, and Alberg. Statistical analysis: Carpenter, Hughes, and Wahlquist. Obtained funding: Carpenter and Hughes. Administrative, technical, and material support: Carpenter and Gray. Study supervision: Carpenter, Gray, Saladin, and Alberg.
Additional Contributions: Amy Boatright, BA; Nicola Thornley, BA; Elizabeth Byrd, BS; Diana Rivera, BA; and Michelle Byczkiewicz, BS, contributed to the trial. We thank the study participants for their time and effort.
References
- 1.Centers for Disease Control and Prevention (CDC) State-specific prevalence and trends in adult cigarette smoking—United States, 1998–2007. MMWR Morb Mortal Wkly Rep. 2009;58(9):221–226. [PubMed] [Google Scholar]
- 2.Baker TB, Mermelstein R, Collins LM, et al. New methods for tobacco dependence treatment research. Ann Behav Med. 2011;41(2):192–207. doi: 10.1007/s12160-010-9252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiore MC, Jaen CR, Baker TB, et al. 2008 PHS Guideline Update Panel, Liaisons, and Staff. Treating tobacco use and dependence: 2008 update U.S. Public Health Service Clinical Practice Guideline executive summary. Respir Care. 2008;53(9):1217–1222. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults—United States, 2000. MMWR Morb Mortal Wkly Rep. 2002;51(29):642–645. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults and trends in smoking cessation—United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58(44):1227–1232. [PubMed] [Google Scholar]
- 6.Wewers ME, Stillman FA, Hartman AM, Shopland DR. Distribution of daily smokers by stage of change: Current Population Survey results. Prev Med. 2003;36(6):710–720. doi: 10.1016/s0091-7435(03)00044-6. [DOI] [PubMed] [Google Scholar]
- 7.Aveyard P, Wang D, Connock M, Fry-Smith A, Barton P, Moore D. Assessing the outcomes of prolonged cessation-induction and aid-to-cessation trials: floating prolonged abstinence. Nicotine Tob Res. 2009;11(5):475–480. doi: 10.1093/ntr/ntp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- 9.Bala M, Strzeszynski L, Cahill K. Mass media interventions for smoking cessation in adults. Cochrane Database Syst Rev. 2008;(1):CD004704. doi: 10.1002/14651858.CD004704.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Fichtenberg CM, Glantz SA. Effect of smoke-free workplaces on smoking behaviour: systematic review. BMJ. 2002;325(7357):188–194. doi: 10.1136/bmj.325.7357.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martire KA, Mattick RP, Doran CM, Hall WD. Cigarette tax and public health: what are the implications of financially stressed smokers for the effects of price increases on smoking prevalence? Addiction. 2011;106(3):622–630. doi: 10.1111/j.1360-0443.2010.03174.x. [DOI] [PubMed] [Google Scholar]
- 12.Heckman CJ, Egleston BL, Hofmann MT. Efficacy of motivational interviewing for smoking cessation: a systematic review and meta-analysis. Tob Control. 2010;19(5):410–416. doi: 10.1136/tc.2009.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garg A, Serwint JR, Higman S, et al. Self-efficacy for smoking cessation counseling parents in primary care: an office-based intervention for pediatricians and family physicians. Clin Pediatr (Phila) 2007;46(3):252–257. doi: 10.1177/0009922806290694. [DOI] [PubMed] [Google Scholar]
- 14.Hughes JR. Motivating and helping smokers to stop smoking. J Gen Intern Med. 2003;18(12):1053–1057. doi: 10.1111/j.1525-1497.2003.20640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkins KA, Stitzer M, Lerman C. Medication screening for smoking cessation: a proposal for new methodologies. Psychopharmacology (Berl) 2006;184(3–4):628–636. doi: 10.1007/s00213-005-0105-5. [DOI] [PubMed] [Google Scholar]
- 16.Brown RA, Lejuez CW, Kahler CW, Strong DR, Zvolensky MJ. Distress tolerance and early smoking lapse. Clin Psychol Rev. 2005;25(6):713–733. doi: 10.1016/j.cpr.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes JR. How confident should we be that smoking cessation treatments work? Addiction. 2009;104(10):1637–1640. doi: 10.1111/j.1360-0443.2009.02645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Individual differences in adoption of treatment for smoking cessation: demographic and smoking history characteristics. Drug Alcohol Depend. 2008;93(1–2):121–131. doi: 10.1016/j.drugalcdep.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. Am J Prev Med. 2008;34(2):102–111. doi: 10.1016/j.amepre.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 20.Fix BV, Hyland A, Rivard C, et al. Usage patterns of stop smoking medications in Australia, Canada, the United Kingdom, and the United States: findings from the 2006–2008 International Tobacco Control (ITC) Four Country Survey. Int J Environ Res Public Health. 2011;8(1):222–233. doi: 10.3390/ijerph8010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiffman S, Ferguson SG, Rohay JM, Gitchell JG. Perceived safety and efficacy of nicotine replacement therapies among US smokers and ex-smokers: relationship with use and compliance. Addiction. 2008;103(8):1371–1378. doi: 10.1111/j.1360-0443.2008.02268.x. [DOI] [PubMed] [Google Scholar]
- 22.Ryan KK, Garrett-Mayer E, Alberg AJ, Cartmell KB, Carpenter MJ. Predictors of cessation pharmacotherapy use among black and non-Hispanic white smokers. Nicotine Tob Res. 2011;13(8):646–652. doi: 10.1093/ntr/ntr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes JR, Marcy TW, Naud S. Interest in treatments to stop smoking. J Subst Abuse Treat. 2009;36(1):18–24. doi: 10.1016/j.jsat.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carpenter MJ, Ford ME, Cartmell KB, Alberg AJ. Misperceptions and misconceptions of nicotine replacement therapy within racially and ethnically diverse smokers. J Natl Med Assoc. doi: 10.1016/s0027-9684(15)30444-2. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=13690967&dopt=Abstract. In press. [DOI] [PMC free article] [PubMed]
- 25.Schneider NG, Cortner C, Justice M, et al. Preferences among five nicotine treatments based on information versus sampling. Nicotine Tob Res. 2008;10(1):179–186. doi: 10.1080/14622200701767837. [DOI] [PubMed] [Google Scholar]
- 26.Bush TM, McAfee T, Deprey M, et al. The impact of a free nicotine patch starter kit on quit rates in a state quit line. Nicotine Tob Res. 2008;10(9):1511–1516. doi: 10.1080/14622200802323167. [DOI] [PubMed] [Google Scholar]
- 27.Miller N, Frieden TR, Liu SY, et al. Effectiveness of a large-scale distribution programme of free nicotine patches: a prospective evaluation. Lancet. 2005;365 (9474):1849–1854. doi: 10.1016/S0140-6736(05)66615-9. [DOI] [PubMed] [Google Scholar]
- 28.Walker N, Howe C, Bullen C, et al. Does improved access and greater choice of nicotine replacement therapy affect smoking cessation success? findings from a randomized controlled trial. Addiction. 2011;106(6):1176–1185. doi: 10.1111/j.1360-0443.2011.03419.x. [DOI] [PubMed] [Google Scholar]
- 29.Carpenter MJ, Alberg AJ, Gray KM, Saladin ME. Motivating the unmotivated for health behavior change: a randomized trial of cessation induction for smokers. Clin Trials. 2010;7(2):157–166. doi: 10.1177/1740774510361533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carpenter MJ, Hughes JR. Defining quit attempts: what difference does a day make? Addiction. 2005;100(2):257–258. doi: 10.1111/j.1360-0443.2004.00952.x. [DOI] [PubMed] [Google Scholar]
- 31.Hughes JR, Callas PW. Definition of a quit attempt: a replication test. Nicotine Tob Res. 2010;12(11):1176–1179. doi: 10.1093/ntr/ntq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benowitz N, Jacob P, Ahijevych K, et al. SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 33.Bansal MA, Cummings KM, Hyland A, Giovino GA. Stop-smoking medications: who uses them, who misuses them, and who is misinformed about them? Nicotine Tob Res. 2004;6(suppl 3):S303–S310. doi: 10.1080/14622200412331320707. [DOI] [PubMed] [Google Scholar]
- 34.Cummings KM, Hyland A, Giovino GA, Hastrup JL, Bauer JE, Bansal MA. Are smokers adequately informed about the health risks of smoking and medicinal nicotine? Nicotine Tob Res. 2004;6(suppl 3):S333–S340. doi: 10.1080/14622200412331320734. [DOI] [PubMed] [Google Scholar]
- 35.Etter JF, Perneger TV. Attitudes toward nicotine replacement therapy in smokers and ex-smokers in the general public. Clin Pharmacol Ther. 2001;69(3):175–183. doi: 10.1067/mcp.2001.113722. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults—United States, 2003. MMWR Morb Mortal Wkly Rep. 2005;54(20):509–513. [PubMed] [Google Scholar]
- 37.Stead LF, Lancaster T. Telephone counselling for smoking cessation [update in Cochrane Database Syst Rev. 2003;(1):CD002850] Cochrane Database Syst Rev. 2001;(2):CD002850. doi: 10.1002/14651858.CD002850. [DOI] [PubMed] [Google Scholar]
- 38.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström K-O. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 39.Lai DTC, Cahill K, Qin Y, Tang JL. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. 2010;(1):CD006936. doi: 10.1002/14651858.CD006936.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Stead LF, Bergson G, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD000165. doi: 10.1002/14651858.CD000165.pub3. [DOI] [PubMed] [Google Scholar]
- 41.Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. J Consult Clin Psychol. 2004;72(3):371–381. doi: 10.1037/0022-006X.72.3.371. [DOI] [PubMed] [Google Scholar]
- 42.Hughes JR, Carpenter MJ. Does smoking reduction increase future cessation and decrease disease risk? a qualitative review. Nicotine Tob Res. 2006;8(6):739–749. doi: 10.1080/14622200600789726. [DOI] [PubMed] [Google Scholar]
- 43.Kreuter MW, Chheda SG, Bull FC. How does physician advice influence patient behavior? evidence for a priming effect. Arch Fam Med. 2000;9(5):426–433. doi: 10.1001/archfami.9.5.426. [DOI] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention (CDC) Impact of promotion of the Great American Smokeout and availability of over-the-counter nicotine medications, 1996. MMWR Morb Mortal Wkly Rep. 1997;46(37):867–871. [PubMed] [Google Scholar]
- 45.Etter JF. The Internet and the Industrial Revolution in smoking cessation counselling. Drug Alcohol Rev. 2006;25(1):79–84. doi: 10.1080/09595230500459545. [DOI] [PubMed] [Google Scholar]