Abstract
Protein kinase D (PKD) binds to diacylglycerol (DAG) in the trans-Golgi network (TGN) and is activated by trimeric G-protein subunits βγ. This complex then regulates the formation of transport carriers in the TGN that traffic to the plasma membrane in non-polarized cells. Here we report specificity of different PKD isoforms in regulating protein trafficking from the TGN. Kinase-inactive forms of PKD1, PKD2 and PKD3 localize to the TGN in polarized and non-polarized cells. PKD activity is required only for the transport of proteins containing basolateral sorting information, and seems to be cargo specific.
Protein kinase D1 (PKD1) is a serine/threonine kinase that binds to the TGN through its first cysteine-rich domain in a DAG-dependent process1,2. Kinase-inactive PKD1 (PKD-KD) induces the formation of TGN tubules containing TGN 46 and furin, proteins that cycle between the TGN and the plasma membrane3. Resident enzymes of the TGN, such as sialyltransferase or coat proteins of other transport carriers (COPI or clathrin), are not found in these tubules3. Furthermore, PKD1 is specific for the transport of proteins from the TGN to the cell surface in non-polarized cells3. PKD2 (ref. 4) and PKD3 (ref. 5) have been identified, but it is unknown whether different PKD isoforms have specificity for different classes of cargo proteins or are functionally redundant.
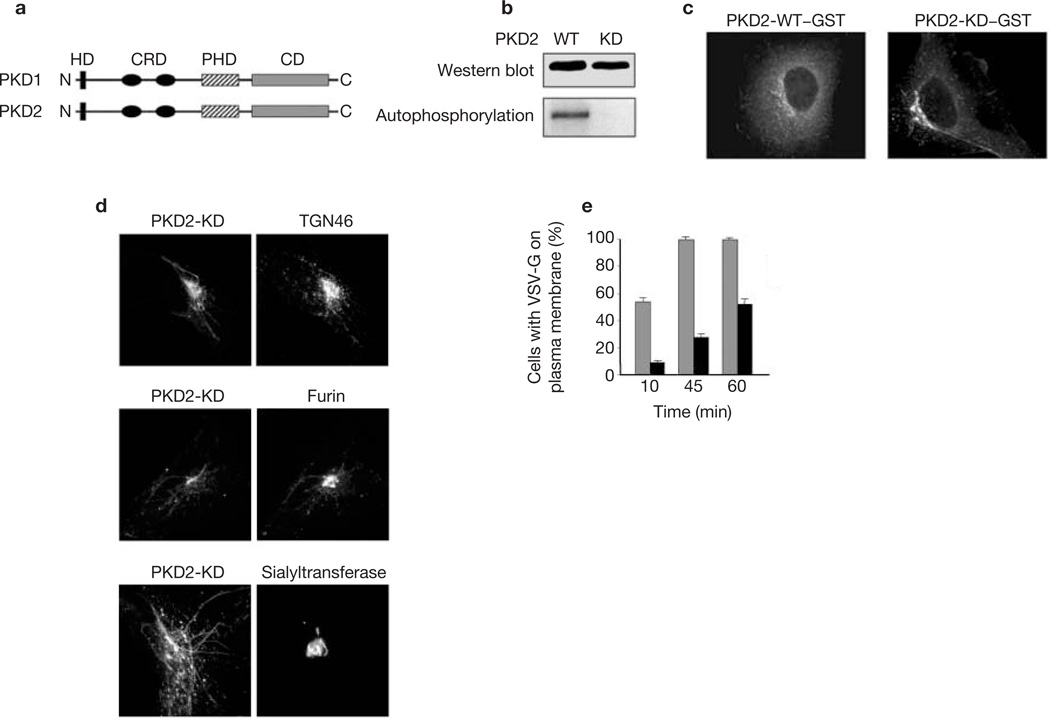
The intracellular distribution and function of PKD2 were examined with glutathione S-transferase (GST)-tagged wild-type (WT) and kinase-dead (KD) proteins expressed in HeLa cells (Fig. 1). The TGN of PKD2-KD transfected cells was tubulated and contained TGN 46 and furin (Fig. 1c, d). Neither resident enzymes of the TGN such as sialyltransferase (Fig. 1d) nor coat proteins (COPI and clathrin; data not shown) were present in these tubules. We examined the effects of PKD2-KD expression on post-Golgi transport of ts-G–GFP (green fluorescent protein-tagged ts045 mutant vesicular stomatitis virus-G protein (VSV-G)), a well-characterized exocytic marker6. HeLa cells were co-transfected with cDNAs for ts-G–GFP and GST–PKD2-KD at a ratio of 1:5 (ts-G–GFP to PKD2). After incubation overnight at 4 °C, cells were shifted to 20 °C for 3 h in the presence of cycloheximide to accumulate ts-G–GFP in the TGN, and then transferred to 32 °C to allow the protein to leave the TGN. The percentage of cells (n = 500) with ts-G–GFP on the plasma membrane was quantified at different times after the shift to 32 °C. In cells expressing PKD2-WT, about 50% of the cells expressed ts-G–GFP on the plasma membrane within 10 min of the shift to 32 °C, and by 45 min all cells examined had cell-surface ts-G–GFP (Fig. 1e). In contrast, fewer than 10% of cells expressing PKD2-KD had ts-G–GFP protein in the plasma membrane at 10 min, and after 60 min of incubation at 32 °C only about 50% of cells had detectable surface ts-G–GFP (Fig. 1e). In these cells ts-G–GFP accumulated within TGN tubules that contained PKD2-KD (data not shown). Thus, like PKD1-KD (ref. 3), PKD2-KD affected TGN-to-plasma membrane trafficking in HeLa cells. The effect of PKD2-KD expression was specific for this transport step, because other transport pathways were not affected, including endocytosis, transport between endoplasmic reticulum (ER) and Golgi complex (GC), or from the GC to endosomes (data not shown). In summary, the intracellular localization and phenotypic consequences of expressing PKD2-KD in non-polarized HeLa cells are identical to those we reported previously for PKD1-KD (refs 1–3).
Figure 1.
PKD2-KD localized to the TGN causes extensive tubulation and blocks transport to the cell surface. (a) Schematic representation of the domains of PKD1 and PKD2. HD, hydrophobic domain; CRD, cysteine-rich domain; PHD, PH domain; CD, catalytic domain. (b) PKD2-WT–GST and PKD2-KD–GST were expressed in 293T cells. The proteins were isolated by adsorption on glutathione–Sepharose from cell lysates and incubated with [32P]ATP for 10 min at 32 °C. The kinase reaction and measurement of the radioactivity associated with the respective kinases were performed as described previously3. The top panel shows the amounts of respective proteins used in the experiment; the bottom panel shows the autophosphorylation of PKD2-WT in contrast to PKD2-KD. (c) HeLa cells were transfected with PKD2-WT–GST and PKD2-KD–GST and detected by staining with anti-GST antibodies. The WT form is found in the pericentriolar region as well as diffusely dispersed in the cytoplasm. In contrast, the KD form is found predominantly in the pericentriolar region and the GC-associated tubules. (d) HeLa cells were transfected with PKD2-KD–GST and stained for endogenous TGN 46 (top panels), or were co-transfected with PKD2-KD-GST and either furin–EGFP (middle panels) or sialyltransferase–EGFP (bottom panels). PKD2-KD was revealed by staining with anti-GST. Note that PKD2-KD tubules contain TGN 46 and furin but not the TGN resident enzyme sialyltransferase. (e) HeLa cells were co-transfected with PKD2-KD–GST and VSV-G–GFP (black bars). As a control, cells were co-transfected with PKD2-WT–GST and VSV-G–GFP (grey bars). Results are means ± s.e.m. for three independent experiments.
Are PKD1, PKD2 and PKD3 functionally redundant? Reverse transcriptase polymerase chain reaction (RT–PCR) shows that HeLa cells express PKD2 and PKD3, but not PKD1 (data not shown). To address the possibility that PKD2 and PKD3 have similar functions in exocytic trafficking, we expressed PKD3-KD in HeLa cells and monitored the transport of ts-G–GFP. When expressed at high concentrations, PKD3-KD caused a significant delay in transport of ts-G–GFP from TGN to the cell surface, similar to that observed after the expression of PKD2-KD (data not shown). These data suggest that PKD2 and PKD3 have similar functions in HeLa cells and that the formation of exocytic transport carriers from the TGN in these non-polarized cells is most probably mediated by PKD2 and PKD3, rather than PKD1, as originally proposed3. Why, then, does PKD1-KD expression alter TGN morphology and decrease ts-G–GFP transport in HeLa cells? Although we cannot exclude the possibility that minor amounts of PKD1 are expressed and are the target of inhibition, it is perhaps more likely that PKD1-KD acts by binding irreversibly to the TGN through DAG, and that, because PKD auto-phosphorylation is required for its release from the membrane1, this binding competitively inhibits the recruitment of endogenous PKD2 and PKD3 to the TGN. Although these results show that the expression of PKD-KD mutants has similar consequences for TGN function in non-polarized HeLa cells, analysis of their effects in polarized epithelial (MDCK) cells reveals differences that indicate that PKD1, PKD2 and PKD3 are not functionally redundant.
In polarized epithelial cells, protein transport from the TGN is directed towards two structurally and functionally different plasma-membrane domains, apical and basolateral. Intrinsic signals for sorting apical and basolateral membrane proteins have been identified7,8. For basolateral proteins, adaptor protein complexes AP-1B9 and AP-4 (ref. 10) might be involved in sorting proteins in the TGN and/or endosomes11–13. Transport from the TGN to the apical plasma membrane is dependent on dynamin and a kinesin-like motor protein14. Cdc42 seems to regulate trafficking to both apical and basolateral membranes15–17, and assays in vitro for factors involved in vesicle production have revealed a requirement for an Arf-like GTPase, and activities of PKC-like and phospholipase D-like enzymes18,19.We examined roles of PKD isoforms in protein trafficking in Madin-Darby canine kidney (MDCK) cells.
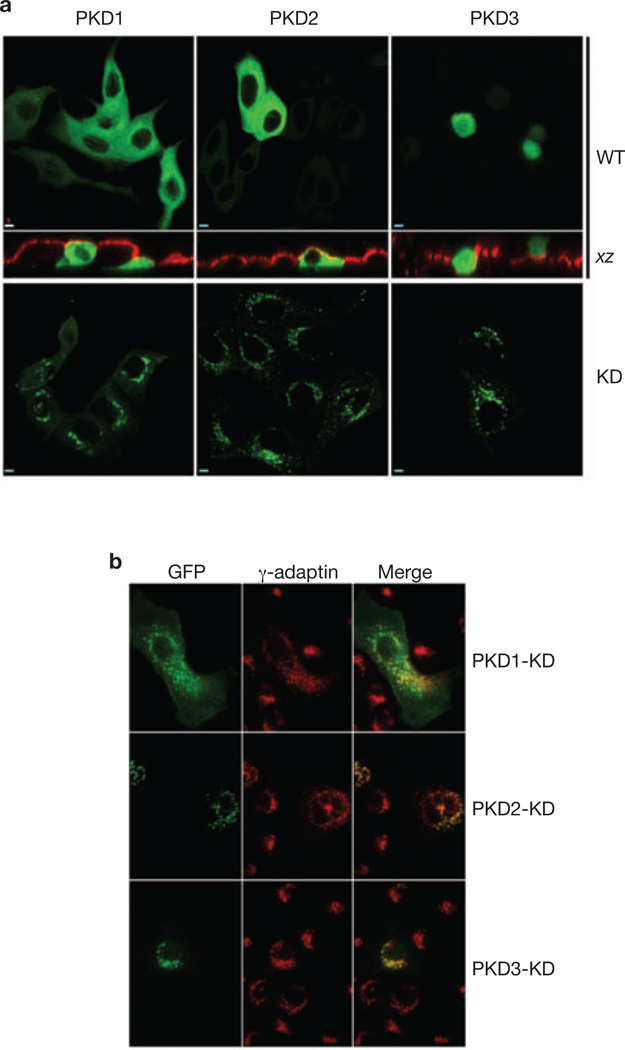
To examine the role of different PKD isoforms in MDCK cells, we first used RT–PCR to show that all three PKD isoforms are expressed (data not shown). Distributions of PKD1, PKD2 and PKD3, as well as their kinase-inactive mutants, in non-polarized MDCK cells grown on glass coverslips were similar to those in HeLa cells: PKD1-WT, PKD2-WT were distributed diffusely throughout the cytosol (Fig. 2a), and PKD3-WT was found in both the cytoplasm and the nucleus (Fig. 2a). PKD1-KD, PKD2-KD and PKD3-KD mutants were localized predominantly to a perinuclear compartment, and each co-localized there with a subset of membranes that contained γ-adaptin, a marker of the TGN (Fig. 2b).
Figure 2.
KD isoforms of PKD localize to the TGN of MDCK cells. (a) MDCK cells were transiently transfected with WT or KD PKD1, PKD2 and PKD3, and cultured on Transwell filters (upper panels) or coverslips (lower panels). Cells expressing wtPKDs were co-stained with antibodies against gp135 to define the borders of the apical plasma membrane. Note that PKD1-WT and PKD2-WT were diffusely distributed throughout the cells, whereas PKD3-WT was present in the cytoplasm and the nucleus. The KD forms of these proteins were highly enriched on a perinuclear compartment. Scale bar, 5 µm. (b) Sub-confluent MDCK cells transiently expressing PKD1-KD, PKD2-KD or PKD3-KD were co-stained with antibodies against γ-adaptin, a TGN marker. Note that each kinase co-localized with a subset of membranes that were positive for γ-adaptin. Scale bar, 5 µm.
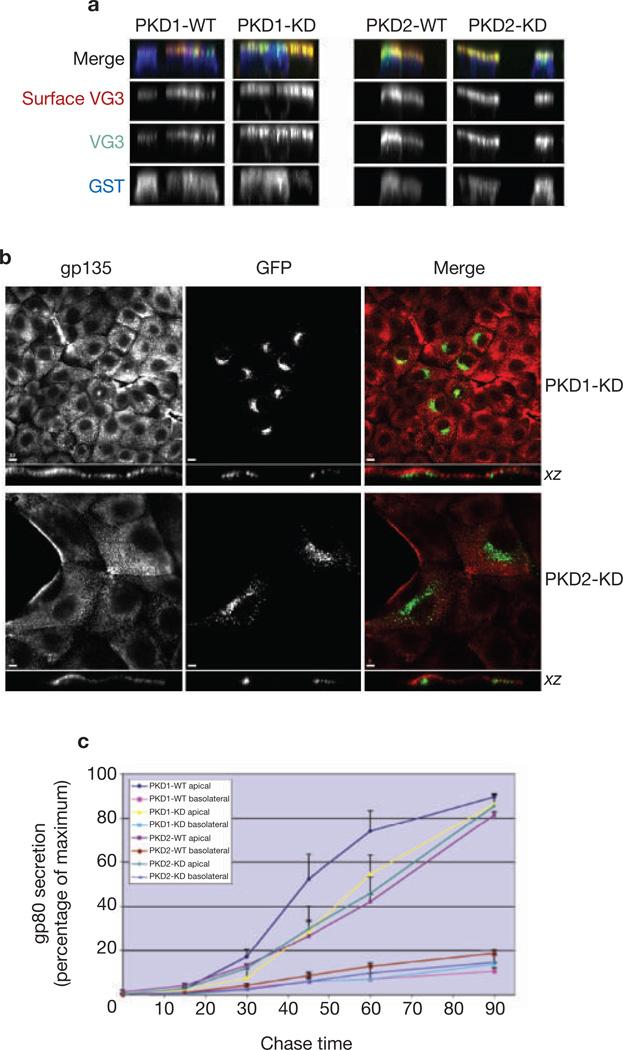
To determine whether PKD1 or PKD2 functions in the transport of transmembrane proteins to the apical plasma membrane, we first examined the trafficking of a mutant form of VSV-G protein, VSV-G3 (ref. 20), which accumulates on the apical membrane (see below). Polarized MDCK cells grown on filters were co-injected with VSV-G3 cDNA and PKD1-WT, PKD2-WT, PKD1-KD or PKD2-KD. Surface VSV-G3 was revealed by confocal microscopy in non-permeabilized cells by the use of an anti-ectodomain antibody.VSV-G3 was expressed primarily on the apical plasma membrane regardless of whether cells expressed WT or KD forms of PKD1 or PKD2 (Fig. 3a). In addition, neither PKD1-KD nor PKD2-KD altered the steady-state distribution of an endogenous apical membrane protein, gp135 (Fig. 3b). Therefore the overexpression of PKD1-KD or PKD2-KD does not alter the polarized expression of either of these proteins on the apical plasma membrane, or result in accumulation of either one in the TGN.
Figure 3.
Expression of mutant PKD1 or PKD2 does not affect transport to the apical domain in polarized MDCK cells. (a) VSV-G3 is a mutant form of VSV-G that is transported exclusively to the apical surface. MDCK cells were injected with the cDNA in combination with either the WT or the KD form of PKD1 and PKD2. The VSV-G3 expressed on the plasma membrane was revealed by fluorescence microscopy with antibodies against the ectodomain of VSV-G3. Quantification of the amount of VSV-G3 at the apical cell surface revealed no significant differences in cells expressing the WT or the KD form of either PKD1 or PKD2. (b) MDCK cells were transfected with PKD1-KD or PKD2-KD. After 48 h, cells were fixed, permeabilized and labelled with antibody specific for the endogenous apical plasma membrane protein gp135. Images were obtained by laser scanning confocal microscopy (see Methods). Vertical xz images of cells are shown to indicate that PKD1-KD and PKD2-KD are expressed in a compartment that is just beneath the apical plasma membrane in MDCK cells. Note that gp135 does not accumulate in these intracellular compartments but is correctly expressed at the apical plasma membrane. What seems to be lateral staining in two cells in the lower panels is actually the free, apical surface of these cells, which are not engaged in cell–cell contacts on this side. Scale bars, 10 µm (PKD1-KD) and 5 µm (PKD2-KD). (c) MDCK cells stably expressing WT or KD isoforms of PKD1 or PKD2 were pulsed-labelled with [35S]methionine/cysteine and chased for various times in complete medium. Aliquots of chase medium from the apical and basolateral compartments were collected at each time point and analysed by SDS–PAGE/autoradiography to quantify gp80 concentrations. Secretion of gp80 to the apical surface was found to be insensitive to PKD kinase activity.
As an additional test to determine whether PKD1 or PKD2 is required for apical trafficking, we examined the secretion of gp80, an endogenous apical protein, in clonal MDCK cell lines expressing WT or KD forms of PKD1 or PKD2. In all cases, gp80 was efficiently secreted into the apical medium (Fig. 3c). Overexpression of PKD1-WT, but not PKD2-WT, caused a slight increase in the rate of gp80 secretion to the apical medium, but neither PKD1-KD nor PKD2-KD reduced exocytic efficiency or altered the polarity of gp80 secretion. We conclude that neither PKD1 nor PKD2 is required for exocytic trafficking of soluble (gp80) or transmembrane proteins (VSV-G3 and gp135) from the TGN to the apical surface of polarized MDCK cells.
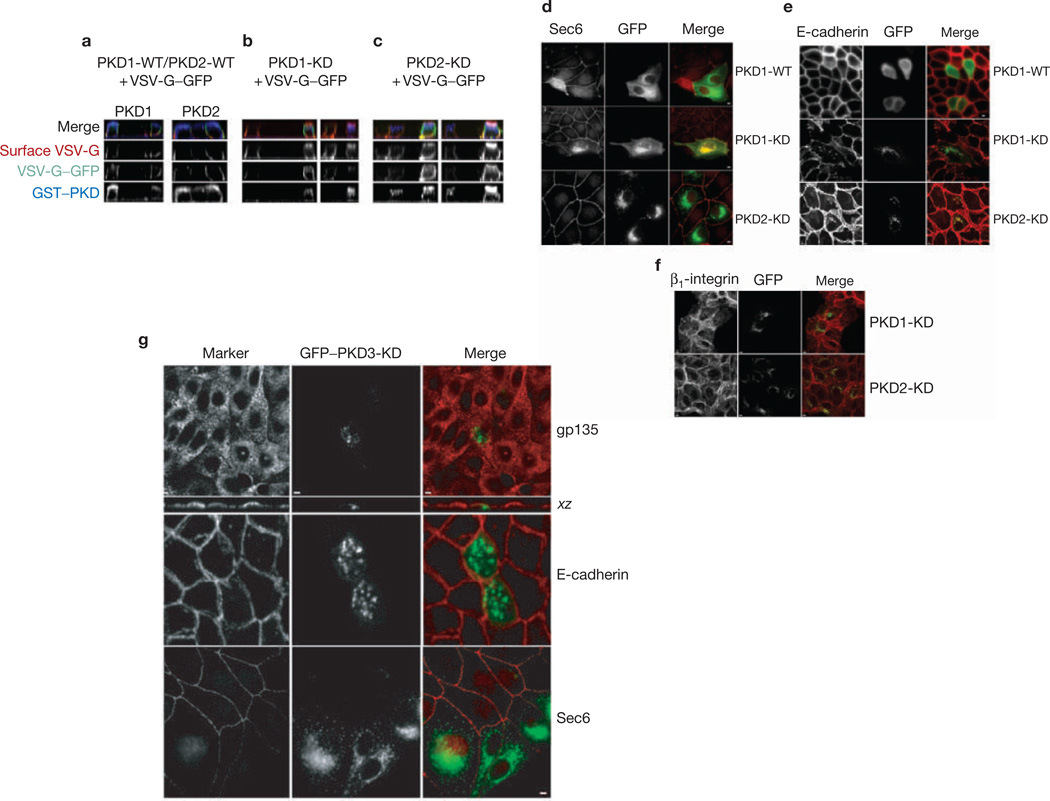
Because neither PKD1 nor PKD2 seems to be involved in apical exocytosis, we examined whether they regulate the formation and trafficking of vesicles containing basolateral cargo. Complementary DNAs encoding ts-G–GFP, a marker of basolateral exocytosis, and the KD form of either PKD1 or PKD2 were microinjected into polarized MDCK cells grown on filters. After incubation at 20 °C to accumulate ts-G–GFP in the TGN, the temperature was shifted to 32 °C to allow transport to the basolateral membrane. Cells were stained for PKD (blue) and surface ts-G–GFP (red), and total ts-G–GFP was imaged as green fluorescence. Pixel intensities of apical and basolateral surface ts-G–GFP were measured for about 30–35 cells for each condition. In cells expressing PKD1-WT and PKD2-WT, ts-G–GFP was expressed primarily on the basolateral surface (Ap:Bl expression ratios 0.46 ± 0.17 and 0.41 ± 0.16, respectively). Even when expressed at high concentrations, neither PKD1-WT nor PKD2-WT had a measurable effect on the steady-state accumulation of ts-G–GFP on the basolateral membrane (Fig. 4a–c). In addition, note that little or no ts-G–GFP was mis-sorted to the apical plasma membrane under these conditions. In contrast, cells expressing high concentrations of either PKD1-KD or PKD2-KD had approximately double the VSV-G in the apical membrane compared with that in cells expressing WT kinases (Ap:Bl expression ratios 1.01 ± 0.42 and 0.80 ± 0.42, respectively). The accumulation of VSV-G on apical membranes in cells expressing either PKD1-KD or PKD2-KD mutants might be due to the inhibition of basolateral transport vesicle formation, resulting in a passive entry of basolateral cargo into apical transport carriers15,21.
Figure 4.
Expression of mutant PKD1 or PKD2 blocks transport to the basolateral surface. (a–c) Filter-grown polarized MDCK cells were microinjected with WT or KD forms of PKD1 or PKD2 together with VSVG–GFP. After accumulation at 20 °C for 2.5 h, the filters were shifted to 31.5 °C for 2.5 h in the presence of cycloheximide. (a) VSV-G–GFP (green) was correctly localized to the basolateral surface in the presence of WT forms of PKD (blue). Surface labelling of VSV-G (red) was performed with antibodies against the ectodomain of VSV-G in non-permeabilized cells. Subsequently, cells were permeabilized with saponin and stained against GST-tagged PKDs with anti-GST antibodies (blue). (b, c) The same experiment as in a was performed except that PKD1-KD (b) or PKD2-KD (c) was microinjected. Note that cells expressing high concentrations of either mutant exhibited some mis-sorting of VSV G to the apical surface (red). (d–f) MDCK cells were transfected with PKD1-KD or PKD2-KD. After 48 h, cells were fixed, permeabilized, labelled with specific antibodies and revealed by fluorescence microscopy. Images were obtained by laser scanning confocal microscopy (see Methods). Scale bar, 5 µm. (d) Sec6 staining is normally absent from the TGN but accumulates there only upon expression of PKD1-KD. Note that overexpression of either PKD1-WT or PKD2-KD does not promote the accumulation of Sec6 on the TGN. (e, f) Distributions of basolateral proteins E-cadherin (e) and β1-integrin (f) were revealed in cells expressing PKD1-KD or PKD2-KD. Note that each of these proteins accumulates with PKD2-KD in a perinuclear compartment but accumulates in distinct cytoplasmic vesicles in cells expressing PKD1-KD. No such accumulation was observed in non-transfected cells or cells expressing PKD1-WT. (g) Distributions of gp135, Sec6 and E-cadherin in cells expressing PKD3-KD. No cytoplasmic accumulation of any of these proteins was observed in these cells.
The mammalian exocyst (also termed the Sec6/8 complex) is an evolutionarily conserved octameric protein complex peripherally associated with the plasma membrane, and contributes to the final stages in the delivery of post-TGN transport vesicles to the basolateral membrane of polarized MDCK cells22. It was reported previously that in cells expressing PKD1-KD, Sec6 is found concentrated on the TGN23, presumably because transmembrane proteins that bind Sec6 are retained in the TGN under these conditions. Overexpression of PKD1-WT does not promote the accumulation of Sec6 in the TGN (Fig. 4d–f). Because PKD1 and PKD2 seem to be functionally redundant in non-polarized HeLa cells, we expected that overexpression of PKD2-KD would also result in the accumulation of Sec6 in the TGN of MDCK cells. However, we never observed an accumulation of Sec6 in the TGN of cells expressing PKD2-KD (Fig. 4d–f), indicating that in polarized epithelial cells PKD1 and PKD2 might have different functions.
To extend our analysis of PKD1 and PKD2 functions in basolateral transport we examined the effect of overexpressing KD mutants on the trafficking of two endogenous basolateral membrane proteins, E-cadherin and β1-integrin. MDCK cells transiently expressing PKD2-KD co-accumulated E-cadherin, β1-integrin and the KD mutant in the TGN (Fig. 4g); the endogenous proteins also localized to the basolateral plasma membrane (cell–cell contacts), presumably as a consequence of their transport to the plasma membrane before accumulation of the transiently expressed PKD2-KD. Note that there is little or no intracellular accumulation of either E-cadherin or β1-integrin in neighbouring cells that do not express PKD2-KD (Fig. 4g). In cells expressing PKD1-KD there was also an intracellular accumulation of both E-cadherin and β1-integrin compared with neighbouring, non-expressing cells, but neither cargo protein co-localized with PKD1-KD (Fig. 4d–g). It is possible that PKD1 activity is not required in the TGN for the formation of vesicles containing these cargo molecules. On the basis of the observations on the effects of mutant PKD1 on Sec6 localization, it is perhaps more likely that post-TGN transport vesicles carrying E-cadherin and β1-integrin fail to dock with the basolateral plasma membrane because Sec6 is retained in the TGN in these cells.
Expression of PKD3-KD in MDCK cells had no effect on transport of the apical cargo gp135 or basolateral Sec6, β1-integrin or E-cadherin (Fig. 4g). In summary, results from experiments in MDCK cells show, first, that PKD1-KD, PKD2-KD and PKD3-KD each promote distinct effects on cargo transport from the TGN, probably representing different activities of endogenous kinase counterparts, and, second, that none of these kinases seems to be required for apical transport, at least of the markers examined here.
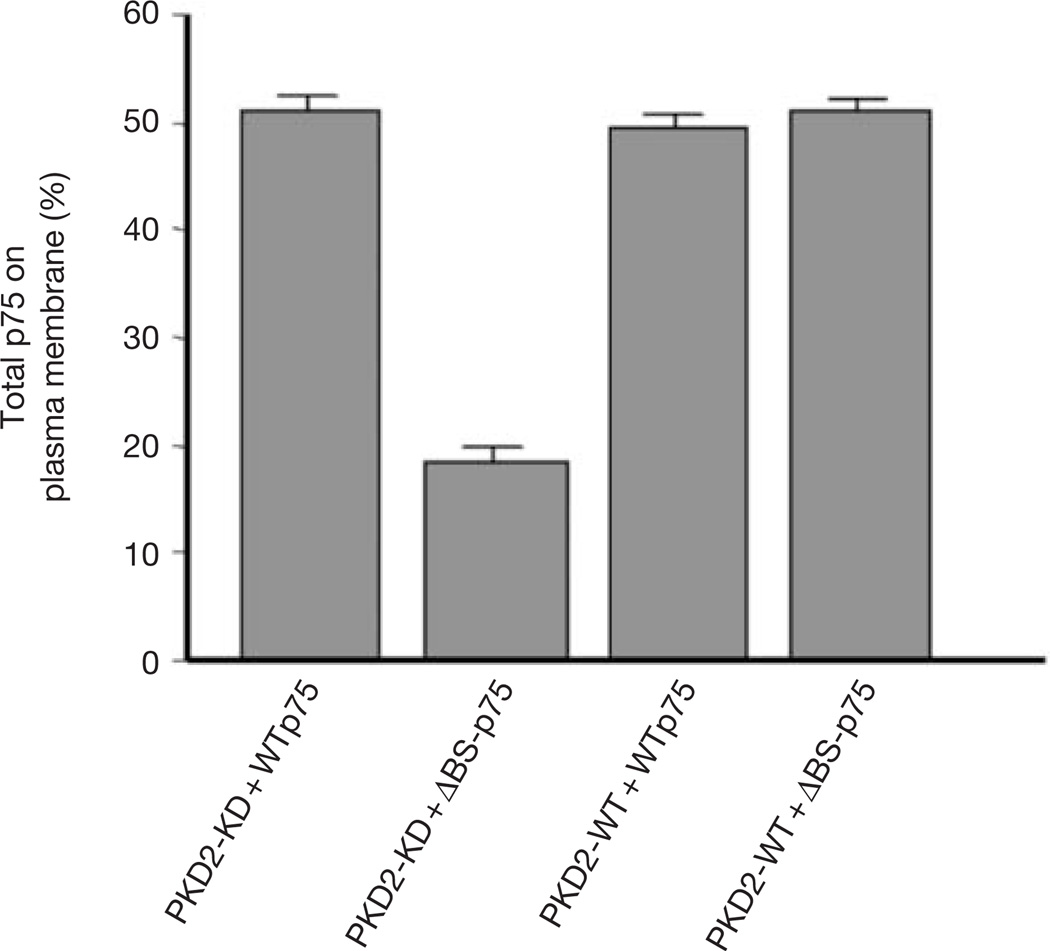
The finding that PKD2 is involved in the exit from the TGN of proteins destined for the basolateral, but not the apical, membrane of polarized MDCK cells raises an interesting question: would PKD2-KD expression in non-polarized cells differentially affect the transport of proteins normally delivered to apical and basolateral surfaces of polarized cells? To answer this question, non-polarized HeLa cells were transiently co-transfected with PKD2-KD and either WTp75 or ΔBS-p75, transmembrane proteins targeted to the apical and basolateral plasma membranes of polarized MDCK cells, respectively8. Transport of WTp75 to the plasma membrane of HeLa cells was unaffected by the expression of PKD2-KD.However, expression of PKD2-KD decreased the expression of ΔBS-p75 on the plasma membrane to less than onethird (Fig. 5). Thus, expression of PKD2-KD inhibits proteins carrying basolateral sorting signals from leaving the TGN, regardless of whether the cells have the capacity to develop apical and basolateral domains of the plasma membrane.
Figure 5.
Non-polarized HeLa cells sort proteins into apical-like (PKD-independent) and basolateral-like (PKD-dependent) pathways. HeLa cells were co-transfected with PKD2-WT or PKD2-KD and either WTp75 (apical surface protein) or ΔBS-p75 (basolateral). Amounts of WTp75 or ΔBS-p75 on the cell surface were determined by surface biotinylation (see Methods). Expression of PKD2-KD selectively decreased the efficiency of transport of ΔBS-p75 to the plasma membrane but had no effect on the exocytosis of WTp75.
In conclusion, our findings support a model in which non-polarized cells generate TGN-derived transport carriers through activity of PKD. HeLa cells express PKD2 and PKD3, and both might be involved in the transport of proteins that contain basolateral sorting signals. Polarized cells such as MDCK cells express PKD1, PKD2 and PKD3. In these cells, PKD1 and PKD2 are also involved in the transport of basolateral, but not apical, cargo. On the basis of our finding that a mutant PKD3-KD blocks VSV-G transport to the cell surface in non-polarized (HeLa) cells, we speculate that in polarized epithelial cells PKD3 also regulates the exit of cargo from the TGN, although we do not yet know the identity of the cargo. On the basis of our findings it is tempting to propose that, analogously to polarized cells that have distinct apical and basolateral targeting pathways, non-polarized cells use a PKD-dependent pathway to deliver basolateral proteins to the plasma membrane and a PKD-independent mechanism to deliver apical proteins. This interpretation is consistent with previous studies on protein sorting in nonpolarized cells, which concluded that certain aspects of ‘apical’ and ‘basolateral’ protein sorting in the TGN are constitutive events common to polarized and non-polarized cells24,25.
METHODS
Construction of plasmids
pME-Py-GST–PKD-WT (GST-tagged PKD1 expression plasmid) and pME-Py-GST–PKD-KD have been described previously1,3. To construct pME-Py-GST–PKD2, the EcoRI–XhoI fragment containing the human PKD2 coding region from pcDNA3-Flag–PKD2 (ref. 4) was subcloned into the same site of the pME-Py-GST vector1. For PKD2-KD (pMEPy-GST–PKD2-KD), lysine 580 (codon AAG) was mutated to asparagine (AAC), resulting in the creation of an HpaI restriction site. pEGFP-PKD2-KD was constructed by subcloning the PKD2-KD fragment from pME-Py-GST–PKD2-KD into the pEGFP-C1 vector (Clontech, Palo Alto, CA). To construct pME-Py-GST–PKD3, a SalI–SpeI fragment containing the human PKD3 coding region was subcloned into the pME-Py-GST vector1. For PKD3-KD (pME-Py-GST–PKD3-KD), lysine 605 (codon AAG) was mutated to asparagine (AAC), as described above for PKD1-KD and PKD2-KD. Plasmid encoding VSV-G–GFP ts045 (‘ts-G–GFP’) was kindly provided by S. J. Scales and R. H. Scheller (Stanford University, Stanford, CA). Plasmids encoding native human WTp75 and ΔBS-p75(Δ168–218) were described previously8.
Cell culture and transfections
HeLa, 293T and MDCK strain II cells were cultured in DMEM medium (Invitrogen, Carlsbad, CA) supplemented with 10% FBS, 2 mM glutamine, 100 U ml−1 penicillin and 100 µg ml−1 streptomycin at 37 °C in a humidified atmosphere of 5% CO2. Transient transfections of HeLa, 293T and MDCK cells were performed with the calcium phosphate method as described previously3. MDCK cells were transfected with 20 µg plasmid, seeded onto collagen-coated coverslips or 12 mm Transwell filters and incubated for 48 h to accumulate expressed protein.MDCK cells stably expressing PKD1-WT, PKD1-KD, PKD2-WT or PKD2-KD were generated by co-transfecting cells with the respective pME-Py-GST vectors together with pMV6 (NeoR) by calcium phosphate precipitation. G418-resistant colonies were screened by immunoblotting and immunofluorescence with anti-GST antibodies (Amersham Pharmacia, Piscataway, NJ). Microinjected MDCK cells were plated onto Transwells at a density of 105 cells cm−2. Cells were grown for 4 days and then used for microinjection experiments after excision from the filter holders9. The expression plasmids encoding the various GST–PKD and either VSV-G or VSV-G3 cDNAs (0.2 mg ml−1) were co-injected into the nuclei of ~400 cells over a period of up to 30 min with an Eppendorf Transjector 5246 microinjection system mounted on a Zeiss Axiovert S100TV inverted microscope with a 40 °C heated stage. The filters were maintained in medium with 10 mM Hepes buffer. After injection, cells were incubated at 37 °C for 1 h to initiate transcription, then at 20 °C for 3.5 h and finally shifted to 32 °C in medium containing 0.1 mg ml−1 cycloheximide.
Quantification of membrane-associated pool of PKD
Cells expressing PKD2-WT and PKD2-KD were homogenized, and the amount and location of cytosolic (soluble pool) and membrane-associated (insoluble pool) kinase were determined by western blotting and immunofluorescence, respectively. In cells expressing PKD2-WT, 70% of the total is cytosolic and the remainder is associated with GC membranes and plasma membrane (data not shown). However, in cells expressing PKD2-KD, more than 70% (data not shown) of the expressed protein is associated with membranes (Fig. 1c).
Immunofluorescence microscopy
Transfected HeLa cells were grown on coverslips, washed with PBS, fixed in 4% paraformaldehyde at room temperature for 10 min, washed, blocked and permeabilized with blocking buffer (0.1% Triton X-100 and 2.5% FBS in PBS) for 20 min. The coverslips were incubated with primary antibodies diluted in blocking buffer for 30 min, washed, incubated with secondary antibodies diluted in blocking buffer for 20 min, washed, mounted in Mowiol and examined with a Nikon Microphot FXA. The anti-(TGN 46) (rabbit; diluted 1:200) was a gift from Vas Ponnambalam (University of Leeds, UK). The GFP-tagged sialyltransferase (pCMV3-ST–GFP) and furin (pEGFP–furin) expression plasmids were provided by I. Trowbridge (Salk Institute, La Jolla, CA) and G. Thomas (Vollum Institute, Portland, OR), respectively. MDCK cells were fixed in 2% paraformaldehyde for 20 min, quenched by washing in Ringer’s saline containing 50 mM NH4Cl, and permeabilized/blocked by incubation in Ringer’s saline containing 0.5% BSA, 50 mM NH4Cl and 0.1% saponin. Monoclonal antibodies against Sec6 (mAb 9H5, as hybridoma supernatant diluted 1:10), γ-adaptin (courtesy of E. Ungewickell; diluted 1:100), and gp135 (as hybridoma supernatant; diluted 1:10). Rabbit polyclonal antibodies against E-cadherin (E2; diluted 1:100), β1-integrin (AIIBII; diluted 1:100), or goat anti-GST (Pharmacia; diluted 1:500) were diluted in blocking buffer and applied to cells for 2 h at 4 °C. After five washes in blocking buffer, secondary antibodies were applied for 1 h at 4 °C. Coverslips and filters were washed five times and mounted in VectaShield (Vector Laboratories, Burlingame, CA). Samples were viewed with either a Nikon Microphot-FX microscope (63× or 100× objectives) or a Molecular Dynamics MultiProbe 2010 confocal laser scanning microscope (63× objective). Primary and secondary antibodies were diluted in blocking buffer and applied to cells for 2 h and 1 h at 4 °C, respectively, washed and mounted in VectaShield (Vector Laboratories). Confocal immunofluorescence microscopy of microinjected MDCK cells was performed with a Zeiss LSM 510 laser scanning microscope. Images were processed with Adobe Photoshop (Adobe Systems, Inc., version 7.0 software).
Kinase assay for PKD
This was performed as described previously3.
Biotinylation of cell surface proteins
To monitor the transport of WTp75 (apical) and ΔBSp75 (basolateral), HeLa cells were co-transfected with cDNA encoding either WT or KD GST–PKD2 together with p75 apical or basolateral mutant, at a ratio of 20:1. At 24 h after transfection, cells were treated with trypsin to remove p75 on the cell surface, then replated onto two plates for duplicate experiments. After incubation overnight, cells were biotinylated with sulphosuccinimidyl-6-(biotinamido) hexanoate (0.5 mg ml−1 in PBS) for 20 min on ice. Cells were then washed twice in complete medium and twice in PBS, then lysed with radioimmunoprecipitation assay (RIPA) buffer containing a cocktail of protease inhibitors for 30 min on ice. WTp75 and ΔBSp75 were immunoprecipitated with anti-p75 antibodies and Protein A/G–Sepharose. The samples were analysed by SDS–polyacrylamide-gel electrophoresis (SDS–PAGE)26. To reveal biotinylated p75 (plasma membrane) the gels were western blotted with streptavidin–horseradish peroxidase. To reveal total apical or basolateral p75 the gels were immunoblotted with anti-p75. For quantification the films were scanned and analysed with Kodak Digital Science 1D Image Analysis Software (Kodak, Rochester, NY). The biotinylated WTp75 or ΔBSp-75 was determined as a percentage of the total protein.
Quantification of VSV-G and VSV-G3 at the surface of MDCK cells
Volocity Classification software was used (Improvision Inc., Lexington, MA). MDCK cells expressing qualitatively similar concentrations of PKD (WT or KD) with VSV-G were chosen. Cells were immunolabelled for PKD by using anti-GST antibodies, and surface VSV-G was monitored with the TKG antibody. Regions delimiting the apical and basolateral domains were drawn with the line tool and selected for measuring the number of pixels within those areas labelled by the antibodies against the ectodomain of VSV-G. Ratios of apical to basolateral pixels were calculated.
Quantification of cell-surface gp80 in MDCK cells
MDCK cells stably expressing either WT or KD PKD1 or PKD2 were seeded at confluent densities on 12 mm Transwell 0.45 µm polycarbonate filters, that were refed daily for 5 days to allow development of polarity. For metabolic radiolabelling, cells were preincubated for 60 min in DMEM/FBS in the absence of methionine and cysteine, and then for 15 min in the presence of medium containing 2 mCi ml−1 (25 µCi per filter) [35S]methionine/cysteine (Amersham). Cells were then rinsed three times in prewarmed DMEM/FBS containing 5× methionine/cysteine, then incubated in that medium for different time points, when an aliquot was removed from both the apical and basolateral compartments of triplicate filters to assess gp80 secretion. Protein samples were incubated in SDS sample buffer for 10 min at 65 °C before separation by SDS–PAGE. gp80 is the predominant labelled secretory protein observed in MDCK cells; under reducing conditions it migrates as two bands, at 35 and 45 kDa (ref. 27). For fluorography, gels were soaked for 30 min in 1 M sodium salicylate (ref. 28), dried under vacuum and exposed to PhosphorImager screens (Molecular Dynamics). The amount of labelled protein in both the 35 and 45 kDa bands was determined directly with a laser-scanning PhosphorImager model 820 (Molecular Dynamics).
ACKNOWLEDGEMENTS
Work in the Malhotra laboratory is funded by grants (GM 53747 and GM 46224) from the NIH and the Human Frontier Science Program, in the Nelson laboratory from the NIH (GM35227), and by a Howard Hughes Medical Institute Biomedical Research Support Program Faculty Startup Package to C.Y. I.A. is supported by a fellowship from the Spanish Ministry of Education, Culture and Sport.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
References
- 1.Maeda Y, Beznoussenko GV, Van Lint J, Mironov AA, Malhotra V. Recruitment of protein kinase D to the trans-Golgi network via the first cysteine-rich domain. EMBO J. 2001;20:5982–5990. doi: 10.1093/emboj/20.21.5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron CL, Malhotra V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science. 2002;295:325–328. doi: 10.1126/science.1066759. [DOI] [PubMed] [Google Scholar]
- 3.Liljedahl M, et al. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 2001;104:409–420. doi: 10.1016/s0092-8674(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 4.Sturany S, et al. Molecular cloning and characterization of the human protein kinase D2. A novel member of the protein kinase D family of serine threonine kinases. J. Biol. Chem. 2001;276:3310–3318. doi: 10.1074/jbc.M008719200. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi A, Seki N, Hattori A, Kozuma S, Saito T. PKCnu, a new member of the protein kinase C family, composes a fourth subfamily with PKCmu. Biochim. Biophys. Acta. 1999;1450:99–106. doi: 10.1016/s0167-4889(99)00040-3. [DOI] [PubMed] [Google Scholar]
- 6.Mostov KE, Bergmann JE. Using temperature-sensitive mutants of VSV to study membrane protein biogenesis. Methods Cell. Biol. 1989;32:85–110. doi: 10.1016/s0091-679x(08)61168-1. [DOI] [PubMed] [Google Scholar]
- 7.Mostov KE, Verges M, Altschuler Y. Membrane traffic in polarized epithelial cells. Curr. Opin. Cell Biol. 2000;12:483–490. doi: 10.1016/s0955-0674(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 8.Yeaman C, et al. The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells. J. Cell Biol. 1997;139:929–940. doi: 10.1083/jcb.139.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folsch H, Pypaert M, Schu P, Mellman I. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J. Cell Biol. 2001;152:595–606. doi: 10.1083/jcb.152.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simmen T, Honing S, Icking A, Tikkanen R, Hunziker W. AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nature Cell Biol. 2002;4:154–159. doi: 10.1038/ncb745. [DOI] [PubMed] [Google Scholar]
- 11.Hunziker W, Harter C, Matter K, Mellman I. Basolateral sorting in MDCK cells requires a distinct cytoplasmic domain determinant. Cell. 1991;66:907–920. doi: 10.1016/0092-8674(91)90437-4. [DOI] [PubMed] [Google Scholar]
- 12.Aroeti B, Kosen PA, Kuntz ID, Cohen FE, Mostov KE. Mutational and secondary structural analysis of the basolateral sorting signal of the polymeric immunoglobulin receptor. J. Cell Biol. 1993;123:1149–1160. doi: 10.1083/jcb.123.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gan Y, McGraw TE, Rodriguez-Boulan E. The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nature Cell Biol. 2002;4:605–609. doi: 10.1038/ncb827. [DOI] [PubMed] [Google Scholar]
- 14.Kreitzer G, Marmorstein A, Okamoto P, Vallee R, Rodriguez-Boulan E. Kinesin and dynamin are required for post-Golgi transport of a plasma-membrane protein. Nature Cell Biol. 2000;2:125–127. doi: 10.1038/35000081. [DOI] [PubMed] [Google Scholar]
- 15.Kroschewski R, Hall A, Mellman I. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nature Cell Biol. 1999;1:8–13. doi: 10.1038/8977. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, et al. Cdc42 interacts with the exocyst and regulates polarized secretion. J. Biol. Chem. 2001;276:46745–46750. doi: 10.1074/jbc.M107464200. [DOI] [PubMed] [Google Scholar]
- 17.Musch A, Cohen D, Kreitzer G, Rodriguez-Boulan E. cdc42 regulates the exit of apical and basolateral proteins from the trans-Golgi network. EMBO J. 2001;20:2171–2179. doi: 10.1093/emboj/20.9.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon JP, et al. The in vitro generation of post-Golgi vesicles carrying viral envelope glycoproteins requires an ARF-like GTP-binding protein and a protein kinase C associated with the Golgi apparatus. J. Biol. Chem. 1996;271:16952–16961. doi: 10.1074/jbc.271.28.16952. [DOI] [PubMed] [Google Scholar]
- 19.Simon JP, et al. The production of post-Golgi vesicles requires a protein kinase C-like molecule, but not its phosphorylating activity. J. Cell Biol. 1996;135:355–370. doi: 10.1083/jcb.135.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toomre D, Keller P, White J, Olivo JC, Simons K. Dual-color visualization of trans-Golgi network to plasma membrane traffic along microtubules in living cells. J. Cell Sci. 1999;112:21–33. doi: 10.1242/jcs.112.1.21. [DOI] [PubMed] [Google Scholar]
- 21.Matter K, Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr. Opin. Cell Biol. 1994;6:545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 22.Grindstaff KK, et al. Sec6/8 complex is recruited to cell–cell contacts and specifies transport vesicle delivery to the basal–lateral membrane in epithelial cells. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- 23.Yeaman C, Grindstaff KK, Wright JR, Nelson WJ. Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells. J. Cell Biol. 2001;155:593–604. doi: 10.1083/jcb.200107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musch A, Xu H, Shields D, Rodriguez-Boulan E. Transport of vesicular stomatitis virus G protein to the cell surface is signal mediated in polarized and nonpolarized cells. J. Cell Biol. 1996;133:543–558. doi: 10.1083/jcb.133.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshimori T, Keller P, Roth MG, Simons K. Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J. Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Urban J, Parczyk K, Leutz A, Kayne M, Kondor-Koch C. Constitutive apical secretion of an 80-kD sulfated glycoprotein complex in the polarized epithelial Madin–Darby canine kidney cell line. J. Cell Biol. 1987;105:2735–2743. doi: 10.1083/jcb.105.6.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chamberlain JP. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal. Biochem. 1979;98:132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]