Abstract
BACKGROUND
Observational studies have shown improvement in patients with type 2 diabetes mellitus after bariatric surgery.
METHODS
In this randomized, nonblinded, single-center trial, we evaluated the efficacy of intensive medical therapy alone versus medical therapy plus Roux-en-Y gastric bypass or sleeve gastrectomy in 150 obese patients with uncontrolled type 2 diabetes. The mean (±SD) age of the patients was 49 ± 8 years, and 66% were women. The average glycated hemoglobin level was 9.2 ± 1.5%. The primary end point was the proportion of patients with a glycated hemoglobin level of 6.0% or less 12 months after treatment.
RESULTS
Of the 150 patients, 93% completed 12 months of follow-up. The proportion of patients with the primary end point was 12% (5 of 41 patients) in the medical-therapy group versus 42% (21 of 50 patients) in the gastric-bypass group (P = 0.002) and 37% (18 of 49 patients) in the sleeve-gastrectomy group (P = 0.008). Glycemic control improved in all three groups, with a mean glycated hemoglobin level of 7.5 ± 1.8% in the medical-therapy group, 6.4 ± 0.9% in the gastric-bypass group (P<0.001), and 6.6 ± 1.0% in the sleeve-gastrectomy group (P = 0.003). Weight loss was greater in the gastric-bypass group and sleeve-gastrectomy group (−29.4 ± 9.0 kg and −25.1 ± 8.5 kg, respectively) than in the medical-therapy group (−5.4 ± 8.0 kg) (P<0.001 for both comparisons). The use of drugs to lower glucose, lipid, and blood-pressure levels decreased significantly after both surgical procedures but increased in patients receiving medical therapy only. The index for homeostasis model assessment of insulin resistance (HOMA-IR) improved significantly after bariatric surgery. Four patients underwent reoperation. There were no deaths or life-threatening complications.
CONCLUSIONS
In obese patients with uncontrolled type 2 diabetes, 12 months of medical therapy plus bariatric surgery achieved glycemic control in significantly more patients than medical therapy alone. Further study will be necessary to assess the durability of these results. (Funded by Ethicon Endo-Surgery and others; ClinicalTrials.gov number, NCT00432809.)
The growing incidence of obesity and type 2 diabetes mellitus globally is widely recognized as one of the most challenging contemporary threats to public health.1 Uncontrolled diabetes leads to macrovascular and microvascular complications, including myocardial infarction, stroke, blindness, neuropathy, and renal failure in many patients. The current goal of medical treatment is to halt disease progression by reducing hyperglycemia, hypertension, dyslipidemia, and other cardiovascular risk factors.2,3 Despite improvements in pharmacotherapy, fewer than 50% of patients with moderate-to-severe type 2 diabetes actually achieve and maintain therapeutic thresholds, particularly for glycemic control.4 Observational studies have suggested that bariatric or metabolic surgery can rapidly improve glycemic control and cardiovascular risk factors in severely obese patients with type 2 diabetes.5–9 Few randomized, controlled trials have compared bariatric surgery with intensive medical therapy, particularly in moderately obese patients (defined as those having a body-mass index [BMI, the weight in kilograms divided by the square of the height in meters] of 30 to 35) with type 2 diabetes.10 Accordingly, many unanswered questions remain regarding the relative efficacy of bariatric surgery in patients with uncontrolled diabetes.
This randomized, controlled, single-center study, called the Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently (STAMPEDE) trial, was designed to compare intensive medical therapy with surgical treatment (gastric bypass or sleeve gastrectomy) as a means of improving glycemic control in obese patients with type 2 diabetes.
METHODS
STUDY DESIGN
The study rationale and nonblinded design have been reported previously.11 From March 2007 through January 2011, we screened 218 patients at the Cleveland Clinic. Using a block-randomization method with a 1:1:1 ratio, we assigned 150 eligible patients to undergo intensive medical therapy alone or intensive medical therapy plus either Roux-en-Y gastric bypass or sleeve gastrectomy, with stratification according to the patients’ use of insulin at baseline. The study protocol is available with the full text of this article at NEJM.org.
Eligibility criteria were an age of 20 to 60 years, a diagnosis of type 2 diabetes (glycated hemoglobin level, >7.0%), and a BMI of 27 to 43. Patients were excluded if they had undergone previous bariatric surgery or other complex abdominal surgery or had poorly controlled medical or psychiatric disorders.
Recruitment strategies included the use of electronic medical records and advertisements in local media. Patients providing written informed consent entered a 12-week screening period and underwent repeated physical and laboratory evaluations to confirm eligibility.
STUDY TREATMENTS
All patients received intensive medical therapy, as defined by American Diabetes Association (ADA) guidelines, including lifestyle counseling, weight management, frequent home glucose monitoring, and the use of newer drug therapies (e.g., incretin analogues) approved by the Food and Drug Administration (FDA).2,12 Every 3 months for the first 12 months, patients returned for study visits with a diabetes specialist at the Cleveland Clinic. Patients were counseled by a diabetes educator and evaluated for bariatric surgery by a psychologist and encouraged to participate in the Weight Watchers program. The goal of medical management was modification of diabetes medications until the patient reached the therapeutic goal of a glycated hemoglobin level of 6.0% or less or became intolerant to the medical treatment (Fig. S1 and S2 in the Supplementary Appendix, available at NEJM.org). All patients were treated with lipid-lowering and antihypertensive medications, according to ADA guidelines, with the following targets: systolic blood pressure, 130 mm Hg or less; diastolic blood pressure, 80 mm Hg or less; and low-density lipoprotein (LDL) cholesterol, 100 mg per deciliter (2.6 mmol per liter) or less.
Bariatric procedures were performed laparoscopically by a single surgeon with the use of instruments provided by Ethicon Endo-Surgery. Gastric bypass consisted of the creation of a 15-to-20-ml gastric pouch, a 150-cm Roux limb, and a 50-cm biliopancreatic limb (Fig. S3 in the Supplementary Appendix).13 Sleeve gastrectomy involved a gastric-volume reduction of 75 to 80% by resecting the stomach alongside a 30-French endoscope beginning 3 cm from the pylorus and ending at the angle of His (Fig. S3 in the Supplementary Appendix). Patients who were assigned to undergo bariatric surgery were evaluated by surgical, nutrition, and psychology services as necessary.14 Vitamin and nutrient supplementation after gastric bypass included a multivitamin, iron, vitamin B12, and calcium citrate with vitamin D; after sleeve gastrectomy, such supplementation included a multivitamin and vitamin B12. Patients were assessed for nutritional deficiencies within 12 months after surgery.
DATA COLLECTION AND ASSESSMENT
At baseline, we collected data on demographic information, coexisting illnesses, rates of diabetes complications, anthropometric values, use of medications, and laboratory values; we also performed physical examinations.
We assessed body weight, waist and hip circumference, blood pressure, and levels of glycated hemoglobin and fasting plasma glucose at baseline and at months 3, 6, 9, and 12. Patients were scheduled for follow-up in a 4-year extension study to assess the durability of glycemic control and diabetes-related complications.
STUDY END POINTS
The primary end point was the proportion of patients with a glycated hemoglobin level of 6% or less (with or without diabetes medications) 12 months after randomization. Secondary end points included levels of fasting plasma glucose, fasting insulin, lipids, and high-sensitivity C-reactive protein (CRP); the homeostasis model assessment of insulin resistance (HOMA-IR) index; weight loss; blood pressure; adverse events; coexisting illnesses; and changes in medications.
STUDY OVERSIGHT
This investigator-initiated trial was sponsored by Ethicon Endo-Surgery, with support from LifeScan, the Cleveland Clinic, and the National Institutes of Health. The first author wrote the first draft of the manuscript and made the decision to submit the manuscript for publication. All authors had full and independent access to all the data and vouch for the integrity and the accuracy of the analysis and its fidelity to the protocol. Complete study governance is outlined in the Supplementary Appendix.
STATISTICAL ANALYSIS
The sample-size estimate was based on the primary end point of the proportion of patients with a glycated hemoglobin level of 6% or less at 12 months. On the basis of previous studies, we estimated that 20% of patients would achieve the primary outcome with intensive medical therapy,10 83% with gastric bypass,5,6,9 and 50% with sleeve gastrectomy.15,16 We determined that 150 patients (50 per group) would provide a power of 80% to detect a difference between the two surgical groups (on the basis of a two-sided alpha level of 0.05) and a power of more than 99% to detect any differences among the three groups.
Continuous variables with a normal distribution are reported as means ±SD. Variables with a non-normal distribution are reported as medians and interquartile ranges. Categorical variables are summarized with the use of frequencies. We used the chi-square test to analyze the primary end point and used analysis of variance to analyze continuous laboratory measurements and compare study groups. For glycemic measures and body weight, a mixed model for repeated measures was used to analyze the change from baseline and least-square means with corresponding standard errors plotted graphically. All analyses were performed with the use of SAS software, version 9.2 (SAS Institute).
RESULTS
PATIENTS
During the first year of follow-up, 8 patients withdrew from the study (1 who did not undergo sleeve gastrectomy and 7 in the medical-therapy group who did not have any follow-up visits). Another 2 patients in the medical-therapy group missed follow-up visits at 9 and 12 months, leaving 140 patients (93%) who completed all analyses. There were no significant differences in patients’ characteristics in the three study groups at baseline (Table 1). The mean BMI was 36, with 51 of 150 patients (34%) with a BMI of less than 35.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Medical Therapy (N = 50) | Gastric Bypass (N = 50) | Sleeve Gastrectomy (N = 50) | P Value |
|---|---|---|---|---|
| Duration of diabetes — yr | 8.9±5.8 | 8.2±5.5 | 8.5±4.8 | 0.72 |
| Use of insulin — no. (%) | 22 (44) | 22 (44) | 22 (44) | 1.00 |
| Age — yr | 49.7±7.4 | 48.3±8.4 | 47.9±8.0 | 0.46 |
| Female sex — no. (%) | 31 (62) | 29 (58) | 39 (78) | 0.08 |
| Body-mass index† | ||||
| Value | 36.8±3.0 | 37.0±3.3 | 36.2±3.9 | 0.42 |
| <35 — no. (%) | 19 (38) | 14 (28) | 18 (36) | 0.54 |
| Body weight — kg | 106.5±14.7 | 106.7±14.8 | 100.8±16.4 | 0.10 |
| Waist circumference — cm | 114.5±9.4 | 116.4±9.2 | 114.0±10.4 | 0.43 |
| Waist-to-hip ratio | 0.95±0.09 | 0.96±0.07 | 0.96±0.09 | 0.88 |
| White race — no. (%)‡ | 37 (74) | 37 (74) | 36 (72) | 0.97 |
| Smoker — no./total no. (%) | 15/42 (36) | 20/50 (40) | 11/50 (22) | 0.14 |
| Metabolic syndrome — no. (%) | 46 (92) | 45 (90) | 47 (94) | 1.00 |
| History of dyslipidemia — no./total no. (%) | 36/43 (84) | 44/50 (88) | 40/50 (80) | 0.55 |
| History of hypertension — no./total no. (%) | 26/43 (60) | 35/50 (70) | 30/50 (60) | 0.51 |
Plus–minus values are means ±SD. P values are for the overall comparisons.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Race was self-reported.
PRIMARY END POINT
The target glycated hemoglobin level of 6.0% or less at 12 months occurred in 5 of 41 patients (12%) in the medical-therapy group, as compared with 21 of 50 (42%) in the gastric-bypass group (P = 0.002) and 18 of 49 (37%) in the sleeve-gastrectomy group (P = 0.008). There were no significant differences in the primary end point between the two surgical groups (P = 0.59). However, all patients in the gastric-bypass group who achieved the target glycated hemoglobin level did so without medications, whereas 5 of 18 patients (28%) in the sleeve-gastrectomy group required the use of one or more glucose-lowering drugs. There was no significant heterogeneity among the subgroups stratified according to median age, BMI, use of insulin, or duration of diabetes (Table S5 in the Supplementary Appendix).
GLYCEMIC CONTROL
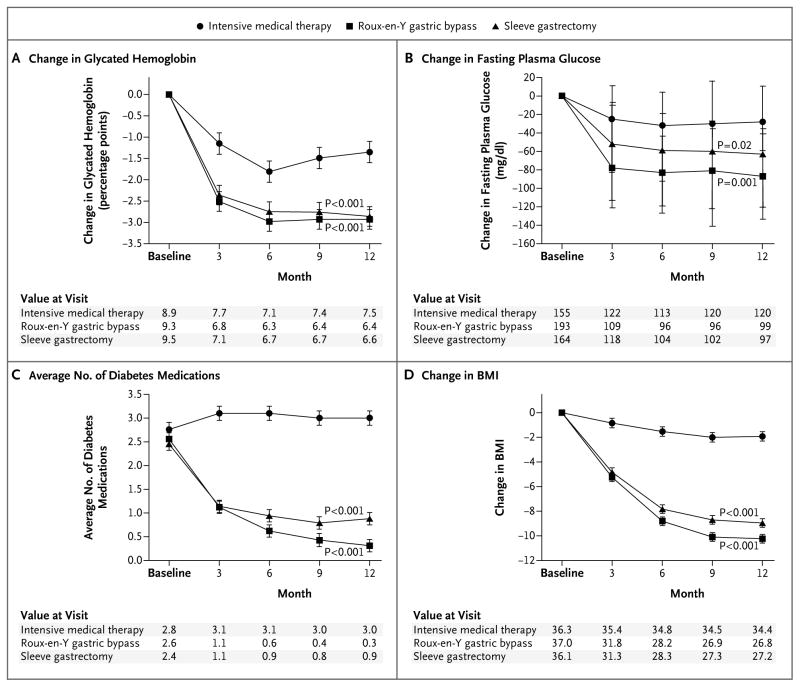
At 12 months, mean levels of glycated hemoglobin and fasting plasma glucose were significantly lower in the two surgical groups than in the medical-therapy group (P<0.01 for both comparisons) (Table 2). There was a large and rapid improvement (by 3 months) in levels of glycated hemoglobin and fasting plasma glucose after each of the surgical procedures, an improvement that was sustained over the year of observation with reduced hypoglycemic medication use (Fig. 1A and 1B). In contrast, patients receiving medical therapy alone had a smaller and more gradual improvement in glycemic control with some attenuation observed over the final 6 months, despite an increase in the use of hypoglycemic medications (Fig. 1A).
Table 2.
Primary and Secondary End Points at 12 Months.*
| End Point | Medical Therapy (N = 41) | Gastric Bypass (N = 50) | Sleeve Gastrectomy (N = 49) | P Value | ||
|---|---|---|---|---|---|---|
| Gastric Bypass vs. Medical Therapy | Sleeve Gastrectomy vs. Medical Therapy | Gastric Bypass vs. Sleeve Gastrectomy | ||||
| Glycated hemoglobin
| ||||||
| ≤6% — no. (%) | 5 (12) | 21 (42) | 18 (37) | 0.002 | 0.008 | 0.59 |
| ≤6% with no diabetes medications — no. (%) | 0 | 21 (42) | 13 (27) | <0.001 | <0.001 | 0.10 |
| Baseline — % | 8.9±1.4 | 9.3±1.4 | 9.5±1.7 | |||
| Month 12 — % | 7.5±1.8 | 6.4±0.9 | 6.6±1.0 | <0.001 | 0.003 | 0.23 |
| Change from baseline — percentage points | −1.4±1.5 | −2.9±1.6 | −2.9±1.8 | <0.001 | <0.001 | 0.85 |
| Body weight — kg
| ||||||
| Baseline | 104.4±14.5 | 106.7±14.8 | 100.6±16.5 | |||
| Month 12 | 99.0±16.4 | 77.3±13.0 | 75.5±12.9 | <0.001 | <0.001 | 0.50 |
| Change from baseline | −5.4±8.0 | −29.4±8.9 | −25.1±8.5 | <0.001 | <0.001 | 0.02 |
| High-density lipoprotein cholesterol
| ||||||
| Percent change from baseline | 11.3±25.7 | 28.5±22.7 | 28.4±21.9 | 0.001 | 0.001 | 0.98 |
| Triglycerides
| ||||||
| Median percent change from baseline (interquartile range) | −14 (−40 to 3) | −44 (−65 to −16) | −42 (−56 to 0) | 0.002 | 0.08 | 0.17 |
| High-sensitivity C-reactive protein
| ||||||
| Median percent change from baseline (interquartile range) | −33.2 (−71 to 0) | −84 (−91 to −59) | −80 (−90 to −63) | <0.001 | <0.001 | 0.59 |
Plus–minus values are means ±SD. Post-randomization data were not available for nine patients in the medical-therapy group and one patient in the sleeve-gastrectomy group. P<0.05 for the comparisons with baseline values in all listed categories. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129.
Figure 1. Changes in Measures of Diabetes Control from Baseline.
Values for change in glycated hemoglobin (Panel A), change in fasting plasma glucose (Panel B), the average number of diabetes medications (Panel C), and change in body-mass index (BMI) (Panel D) were plotted at 3, 6, 9, and 12 months. Least-square means and standard errors from a repeated measures model are plotted for glycated hemoglobin, average number of medications, and BMI; medians and interquartile ranges are plotted for fasting plasma glucose. P values are for the comparison between each surgical group and the medical-therapy group and were calculated from a repeated-measures model that considers data over time.
DIABETES MEDICATIONS
During the trial, the average number of diabetes agents per patient per day tended to increase in the medical-therapy group but decreased significantly in the gastric-bypass group and the sleeve-gastrectomy group (P<0.001 for both comparisons) (Fig. 1C). There was a significant reduction in the majority of medication classes used for glycemic control for the two surgical groups at 12 months (Table 3). Insulin use remained high at 12 months (38%) in the medical-therapy group and was reduced to 4% in the gastric-bypass group and to 8% in the sleeve-gastrectomy group (P<0.001 for both comparisons).
Table 3.
Medication Use at Baseline and Month 12.*
| Medication | Baseline | Month 12 | ||||
|---|---|---|---|---|---|---|
| Medical Therapy (N = 41) | Gastric Bypass (N = 50) | Sleeve Gastrectomy (N = 49) | Medical Therapy (N = 39) | Gastric Bypass (N = 49) | Sleeve Gastrectomy (N = 49) | |
| number of patients (percent) | ||||||
| Diabetes medication
| ||||||
| Biguanide | 38 (93) | 42 (84) | 41 (84) | 38 (97) | 10 (20)† | 19 (39)† |
| Thiazolidinedione | 18 (44) | 25 (50) | 17 (35) | 20 (51) | 0† | 5 (10)† |
| Incretin mimetic | 20 (49) | 20 (40) | 21 (43) | 34 (87) | 1 (2)† | 10 (20)† |
| Secretagogue | 15 (37) | 17 (34) | 18 (37) | 10 (26) | 1 (2)† | 5 (10) |
| Insulin | 21 (51) | 23 (46) | 22 (45) | 15 (38) | 2 (4)† | 4 (8)† |
| Injectable agent‡ | 27 (66) | 30 (60) | 30 (61) | 31 (79) | 2 (4)† | 4 (8)† |
| No. of diabetes medications
| ||||||
| 0 | 1 (2) | 1 (2) | 1 (2) | 0 | 38 (78)§ | 25 (51)§ |
| 1 | 5 (12) | 10 (20) | 11 (22) | 2 (5) | 8 (16) | 10 (20) |
| 2 | 10 (24) | 13 (26) | 14 (29) | 9 (23) | 3 (6) | 9 (18) |
| ≥3 | 25 (61) | 26 (52) | 23 (47) | 28 (72) | 0 | 5 (10) |
| Cardiovascular medication
| ||||||
| Lipid-lowering agent | 34 (83) | 43 (86) | 38 (78) | 36 (92) | 13 (27)† | 19 (39)† |
| Antihypertensive agent | 31 (76) | 39 (78) | 33 (67) | 30 (77) | 16 (33)† | 13 (27)† |
| Beta-blocker | 6 (15) | 9 (18) | 6 (12) | 5 (13) | 9 (18.4) | 3 (6) |
| Calcium-channel blocker | 4 (10) | 4 (8) | 2 (4) | 3 (8) | 1 (2.0) | 1 (2) |
| ACE inhibitor or ARB | 25 (61) | 37 (74) | 30 (61) | 26 (67) | 9 (18)† | 11 (22)† |
| Diuretic | 11 (27) | 18 (36) | 14 (29) | 14 (36) | 5 (10)¶ | 9 (18) |
| Antithrombotic agent | 22 (54) | 21 (42) | 16 (33) | 24 (62) | 1 (2)† | 8 (16)† |
All P values in the footnotes were calculated on the basis of the 12-month data with the medical-therapy group as the comparator. Data for the 12-month analysis were missing for two patients in the medical-therapy group and for one patient in the gastric-bypass group. ACE denotes angiotensin-converting enzyme, and ARB angiotensin-receptor blocker.
P<0.001.
Injectable agents include insulin.
P<0.05 for the categorical comparison of the number of medications.
P<0.01.
WEIGHT LOSS
At 12 months, changes in body weight, BMI, waist circumference, and waist-to-hip ratio were greater after gastric bypass and sleeve gastrectomy than after medical therapy (Table 2, and Tables S2 and S3 in the Supplementary Appendix). The mean percentage of weight loss among patients undergoing either gastric bypass or sleeve gastrectomy was greater (−27.5 ± 7.3% and −24.7 ± 6.6%, respectively) than among those receiving medical therapy alone (−5.2 ± 7.7%) (P<0.001 for both comparisons). Changes in weight and in BMI were greater after gastric bypass than after sleeve gastrectomy (P = 0.02 and 0.03, respectively). The percent of excess weight loss for gastric bypass (88%) and sleeve gastrectomy (81%) was superior to that of medical therapy (13%) (P<0.001 for both comparisons). Both surgical groups had a significantly greater decrease in BMI over time than did the medical-therapy group (P<0.001 for both comparisons) (Fig. 1D).
OTHER HEALTH OUTCOMES
Table 2 shows changes in clinical and laboratory outcomes at 12 months. The reduction in prevalence of the metabolic syndrome was significantly greater in the two surgical groups than in the medical-therapy group (Table S3 in the Supplementary Appendix). Rates of hyper insulinemia and the HOMA-IR index improved markedly after each of the two surgical procedures, as compared with medical therapy alone. A significant decrease in triglycerides occurred at 12 months after gastric bypass, but not after sleeve gastrectomy, as compared with medical therapy. There was a marked increase in high-density lipoprotein (HDL) cholesterol and a significant decrease in the high-sensitivity CRP level after the two surgical procedures, as compared with medical therapy alone.
USE OF CARDIOVASCULAR MEDICATIONS
Although levels of total and LDL cholesterol did not differ significantly among groups after 12 months, there was a significant reduction in the number of medications needed to treat hyperlipidemia in the two surgical groups (Table 3). For example, lipid-lowering drugs were required at baseline in 86% and 78% of patients assigned to undergo gastric bypass and sleeve gastrectomy, respectively, but use declined to 27% and 39% after 12 months, as compared with 92% for medical therapy (P<0.001 for both comparisons). Likewise, there was no significant difference in values for systolic and diastolic blood pressure among the three groups at 12 months, but there was a significant reduction in the number of hypertension medications after the two bariatric procedures.
ADVERSE EVENTS
Table 4 shows significant adverse events that occurred up to 1 year after surgery or the initiation of medical therapy. Additional surgical interventions were required in four patients, including laparoscopic procedures for blood-clot evacuation, assessment of nausea and vomiting, and cholecystectomy after gastric bypass and jejunostomy for feeding access to treat a gastric leak after sleeve gastrectomy. There were no deaths, episodes of serious hypoglycemia requiring intervention, malnutrition, or excessive weight loss among the three groups.
Table 4.
Adverse Events at 12 Months.*
| Adverse Event | Medical Therapy (N = 43) | Gastric Bypass (N = 50) | Sleeve Gastrectomy (N = 49) |
|---|---|---|---|
| no. of patients (%) | |||
|
Serious adverse event | |||
| Requiring hospitalization | 4 (9) | 11 (22) | 4 (8) |
| Intravenous treatment for dehydration | 0 | 4 (8) | 2 (4) |
| Reoperation | 0 | 3 (6) | 1 (2) |
| Transfusion | 0 | 1 (2) | 1 (2) |
| Hemoglobin decrease ≥5 g/dl | 0 | 1 (2) | 0 |
| Gastrointestinal leak | 0 | 0 | 1 (2) |
| Transient renal insufficiency | 0 | 1 (2) | 0 |
| Cholelithiasis | 0 | 1 (2) | 0 |
| Arrhythmia or palpitations | 2 (5) | 0 | 1 (2) |
| Pleural effusion | 0 | 0 | 1 (2) |
| Ketoacidosis | 0 | 1 (2) | 0 |
| Wound infection | 0 | 1 (2) | 0 |
| Cellulitis | 1 (2) | 0 | 0 |
| Pneumonia | 0 | 2 (4) | 0 |
| Kidney stone | 1 (2) | 0 | 0 |
| Hernia | 0 | 1 (2) | 0 |
|
Other adverse event | |||
| Hypoglycemic episode† | 35 (81) | 28 (56) | 39 (80) |
| Anemia‡ | 3 (7) | 6 (12) | 6 (12) |
| Hypokalemia | 1 (2) | 2 (4) | 2 (4) |
| Anastomotic ulcer | 0 | 4 (8) | 0 |
| Excessive weight gain§ | 3 (7) | 0 | 0 |
Patients may have had more than one event. Seven patients in the medical-therapy group withdrew immediately after randomization. One patient in the sleeve-gastrectomy group had anemia before withdrawing from the study before surgery.
Hypoglycemic episodes were self-reported. Patients were classified according to whether they reported at least one episode of hypoglycemia during the follow-up period.
Anemia was defined as a hemoglobin level of less than 11.5 g per deciliter for women and less than 13.0 g per deciliter for men.
Excessive weight gain was defined as an increase of more than 5% over the baseline value.
DISCUSSION
In our study, obese patients with poorly controlled diabetes who underwent either gastric bypass or sleeve gastrectomy combined with medical therapy were significantly more likely to achieve a glycated hemoglobin level of 6.0% or less 12 months after randomization than were patients receiving medical therapy alone. Notably, many patients in the surgical groups, particularly those in the gastric-bypass group, achieved glycemic control without the use of diabetes medications (Table 3). The study population had relatively advanced disease, including many patients with major diabetes-related coexisting illnesses or evidence of end-organ damage, including retinopathy in 14 to 22% and nephropathy (microalbuminuria) in 14 to 29% (Table S1 in the Supplementary Appendix). The majority of patients had the metabolic syndrome and increased measures of systemic inflammation (median high-sensitivity CRP level, >4 mg per liter) (Table 2, and Tables S1 and S2 in the Supplementary Appendix). More than 60% of the surgical patients had moderate-to-severe fatty liver disease on the basis of biopsy samples obtained during surgery (Table S1 in the Supplementary Appendix). Accordingly, a significant improvement in type 2 diabetes (a reduction in glycated hemoglobin levels of 2.9 percentage points) can occur after bariatric surgery in obese patients with advanced diabetes, although modest improvement is feasible with the use of intensive medical therapy alone (a reduction of 1.4 percentage points).
Observational studies of bariatric procedures have shown rates of remission of type 2 diabetes of 55 to 95%, although resolution was often determined without biochemical evidence (levels of glycated hemoglobin or fasting plasma glucose) or with the use of more liberal definitions of remission (e.g., fasting plasma glucose, ≤125 mg per deciliter [6.9 mmol per liter]).5 A nonrandomized, prospective trial comparing bariatric surgery with conventional treatment of obesity also showed higher diabetes remission rates for surgery after 2 and 10 years but with gradual recurrence over time.8 A single previous randomized, controlled trial compared medical therapy with gastric banding in patients with moderate-to-severe obesity (BMI, 30 to 40) but involved patients with early diabetes (<2 years) of mild severity (glycated hemoglobin, <7.5%). In that study, gastric banding was superior to medical therapy in achieving glycemic control (glycated hemoglobin, ≤6.2%) and weight loss.10 In contrast, in our trial, patients had more advanced type 2 diabetes, with an average disease duration of more than 8 years and a mean baseline glycated hemoglobin level of 8.9 to 9.5% while undergoing treatment with an average of nearly three diabetes agents, including a relatively high use of insulin (44% of patients) or other injectable therapies (14%). The inclusion of patients with more advanced type 2 diabetes in the STAMPEDE trial probably explains the lower observed rate of diabetes remission; other differences from previous trials included less severe obesity, a greater proportion of men and black patients, and an older age.
In our study, results were generally similar in the two surgical groups although somewhat more favorable in the gastric-bypass group. Most differences between the gastric-bypass group and the sleeve-gastrectomy group were not significant, although it should be noted that the study was not adequately powered to detect modest differences between these two surgical procedures. Secondary end points, including BMI, body weight, waist circumference, and the HOMA-IR index, also showed more favorable results in the surgical groups than in the medical-therapy group (Table 2, and Tables S2 and S3 in the Supplementary Appendix). Maximal improvements after bariatric surgery occurred quickly, often within 3 months, and were maintained throughout the 12-month follow-up period. Reductions in the use of diabetes medications occurred before achievement of maximal weight loss, which supports the concept that the mechanisms of improvement in diabetes involve physiologic effects in addition to weight loss, probably related to alterations in gut hormones.17–20 As noted in observational studies, some adverse effects of surgical treatment were observed in this study but were modest in severity.6,7,9,21 Self-reported symptoms of hypoglycemia occurred with a similar frequency in the surgical and medical groups.
The mechanism of improved glycemic control appears to involve improvement in insulin sensitivity, with a marked reduction in insulin levels and improvement in the HOMA-IR index, which may be linked to the attenuation of chronic inflammation, as suggested by the greater reduction in high-sensitivity CRP in the surgery groups (−84% for gastric bypass and −80% for sleeve gastrectomy) than in the medical-therapy group (−33%). All patients received intensive medical therapy, including lifestyle counseling, home glucose monitoring, and the most effective pharmacotherapy currently available. Using these strategies, the patients receiving medical therapy alone did well, achieving a substantial reduction in glycated hemoglobin levels (−1.4 ± 1.5 percentage points, P<0.001) and body weight (−5.4 ± 8.0 kg, P<0.001) over 12 months. Although the study was not powered to assess the effects of improved glycemic control on clinical outcomes, improvements in cardiovascular risk factors were observed (Table 2, and Tables S2 and S3 in the Supplementary Appendix). Although lipoprotein and blood-pressure levels were similar in all three study groups at 12 months, improvements in the surgical groups allowed reduction or elimination of concomitant medications in many patients.
Important limitations of our study include the relatively short duration of follow-up (12 months) and the single-center, open-label nature of the study. Some adverse events occurred in the bariatric-surgery group, including in four patients who required reoperation. The durability and long-term safety profile of these results remain uncertain, but the protocol specifies further 4-year follow-up of all patients, which should allow additional assessment of long-term efficacy and safety results to guide patient counseling regarding specific bariatric procedures for the treatment of type 2 diabetes.
Despite these limitations, we conclude that bariatric surgery represents a potentially useful strategy for management of uncontrolled diabetes, since it has been shown to eliminate the need for diabetes medications in some patients and to markedly reduce the need for drug treatment in others. In addition, among patients undergoing surgery, cardiovascular risk factors improved, allowing reductions in lipid-lowering and antihypertensive therapies. Theoretically, such improvements have the potential to reduce cardiovascular morbidity and mortality, as shown in nonrandomized studies, although such benefits will need to be balanced with surgical risk and safety as shown in larger, multicenter clinical-outcome trials.8,22
Supplementary Material
Acknowledgments
Supported by a grant (EES IIS 19900) from Ethicon Endo-Surgery, a grant (R01-DK089547) from the National Institutes of Health, and LifeScan.
Dr. Schauer reports receiving payment for board membership from Ethicon Endo-Surgery, Surgiquest, Barosense, Remedy MD, and Stryker, consulting fees from Ethicon Endo-Surgery, Stryker, Gore, and Carefusion, payment for expert testimony from Physicians Review of Surgery, and lecture fees from Ethicon Endo-Surgery, Allergan, Cinemed, and Quadrant Health-care, holding a patent for a medical device to enhance weight loss in codevelopment with the Cleveland Clinic, royalties from Springer, having an equity interest in Intuitive Surgical, Barosense, Surgiquest, and RemedyMD, and receiving institutional grant support (to the Cleveland Clinic) from Ethicon Endo-Surgery and Bard Davol; Dr. Kashyap, receiving consulting fees from Ethicon; Dr. Brethauer, receiving consulting fees, lecture fees, and payment for board membership from Ethicon Endo-Surgery and lecture fees from Covidien; Dr. Kirwan, receiving grant support from Nestle and ScottCare; Dr. Nissen, receiving institutional grant support (to the Cleveland Clinic) from Orexigen and Vivus; and Dr. Bhatt, receiving institutional grant support (to Brigham and Women’s Hospital) from Amarin, Astra-Zeneca, Bristol-Myers Squibb, Eisai, and Sanofi Aventis and from Medtronic and the Medicines Company (to Boston VA Research Institute) and serving as the international principal investigator for the CRESCENDO cardiovascular outcome trial of the weight-loss drug rimonabant versus placebo. The Cleveland Clinic had received a grant from Sanofi Aventis for the CRESCENDO trial. No other potential conf lict of interest relevant to this article was reported.
We thank Chytaine Hall for recruitment support; Craig Balog, Debbie Gladish, Betty Moore, Karen Myers, Tammy Scebbi, Diane Smith, Maura Schnauffer, Randy Scott, and Janice Bell for statistics and data-management support; Matthew Kroh, M.D., Tomasz Rogula, M.D., Bipan Chand, M.D., Derrick Cetin, M.D., Betul Hatipoglu, M.D., Mario Skugor, M.D., Adi Mehta, M.D., Leslie Heinberg, Ellen Calogeras, Wendy Kirby, and Lauren Sullivan for medical-site support; Rishi Singh, M.D., and Lisa Yerian, M.D., for technical support; and Suzanne Turner and Mary Ann Citraro for graphical support.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2. 7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–42. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 5.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:3, 248.e5–256.e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 6.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–84. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scopinaro N, Marinari GM, Camerini GB, Papadia FS, Adami GF. Specific effects of biliopancreatic diversion on the major components of metabolic syndrome: a long-term follow-up study. Diabetes Care. 2005;28:2406–11. doi: 10.2337/diacare.28.10.2406. [DOI] [PubMed] [Google Scholar]
- 8.Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 9.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–50. doi: 10.1097/00000658-199509000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–23. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 11.Kashyap SR, Bhatt DL, Schauer PR. Bariatric surgery vs. advanced practice medical management in the treatment of type 2 diabetes mellitus: rationale and design of the Surgical Therapy And Medications Potentially Eradicate Diabetes Efficiently trial (STAMPEDE) Diabetes Obes Metab. 2010;12:452–4. doi: 10.1111/j.1463-1326.2009.01172.x. [Erratum, Diabetes Obes Metab 2010;12:833.] [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association. Standards of medical care in diabetes — 2011. Diabetes Care. 2011;34(Suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schauer PR, Ikramuddin S, Hamad G, et al. Laparoscopic gastric bypass surgery: current technique. J Laparoendosc Adv Surg Tech A. 2003;13:229–39. doi: 10.1089/109264203322333557. [DOI] [PubMed] [Google Scholar]
- 14.Eldar S, Heneghan HM, Brethauer S, Schauer PR. A focus on surgical preoperative evaluation of the bariatric patient — the Cleveland Clinic protocol and review of the literature. Surgeon. 2011;9:273–7. doi: 10.1016/j.surge.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Gill RS, Birch DW, Shi X, Sharma AM, Karmali S. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review. Surg Obes Relat Dis. 2010;6:707–13. doi: 10.1016/j.soard.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs. sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143–8. doi: 10.1001/archsurg.2010.326. [DOI] [PubMed] [Google Scholar]
- 17.Cummings DE, Overduin J, Shannon MH, Foster-Schubert KE. Hormonal mechanisms of weight loss and diabetes resolution after bariatric surgery. Surg Obes Relat Dis. 2005;1:358–68. doi: 10.1016/j.soard.2005.03.208. [DOI] [PubMed] [Google Scholar]
- 18.Kashyap SR, Daud S, Kelly KR, et al. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond) 2010;34:462–71. doi: 10.1038/ijo.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laferrère B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–16. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Peri-operative safety in the Longitudinal Assessment of Bariatric Surgery. N Engl J Med. 2009;361:445–54. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.