Abstract
Centrally acting Angiotensin II AT1 receptor blockers (ARBs) protect from stress-induced disorders and decrease anxiety in a model of inflammatory stress, the systemic injection of bacterial endotoxin lipopolysaccharide (LPS). In order to better understand the anxiolytic effect of ARBs, we treated rats with LPS (50 µg/kg) with or without three days of pretreatment with the ARB candesartan (1 mg/kg/day), and studied cortical benzodiazepine (BZ) and corticotrophin-releasing factor (CRF) receptors. We compared the cortical BZ and CRF receptors expression pattern induced by LPS with that produced in restraint stress. Inflammation stress produced a generalized increase in cortical BZ1 receptors and reduced mRNA expression of the GABAA receptor γ2 subunit in cingulate cortex; changes were prevented by candesartan pretreatment. Moreover, restraint stress produced similar increases in cortical BZ1 receptor binding, and candesartan prevented these changes. Treatment with candesartan alone increased cortical BZ1 binding, and decreased γ2 subunit mRNA expression in the cingulate cortex. Conversely, we did not find changes in CRF1 receptor expression in any of the cortical areas studied, either after inflammation or restraint stress. Cortical CRF2 receptor binding was undetectable, but CRF2 mRNA expression was decreased by inflammation stress, a change prevented by candesartan. We conclude that stress promotes rapid and widespread changes in cortical BZ1 receptor expression; and that the stress-induced BZ1 receptor expression is under the control of AT1 receptor activity. The results suggest that the anti-anxiety effect of ARBs may be associated with their capacity to regulate stress-induced alterations in cortical BZ1 receptors.
Keywords: Angiotensin II AT1 receptor blockers, Cortical benzodiazepine receptors, GABAA receptor subunits, CRF receptors, Anxiety, Stress
1. Introduction
Peripheral and brain Angiotensin II (Ang II) play active roles in the response to stress [1–2]. Through stimulation of AT1 receptors, brain Ang II participates in the regulation of hypothalamic-pituitary-adrenal (HPA) axis and the central and peripheral sympathetic activation during stress [1–3]. Brain and peripheral AT1 receptors are antagonized by Ang II AT1 receptor blockers (ARBs). Systemic ARB treatment inhibits the hormonal and central sympathetic stimulation during isolation [4–6], prevents the production of stress-induced gastric ulcers, decreases the central sympathetic response during cold-restraint [7–8], and limits the HPA axis stimulation during inflammation stress induced by systemic injection of the bacterial endotoxin lipopolysaccharide (LPS) [3,9].
ARBs effects have been proposed to be at least in part the result of AT1 receptor inhibition in cerebral cortex and other subcortical structures regulating both behavior and the hypothalamic reaction to stress [10–11]. In addition, systemic ARB administration reduces stress-driven anxiety in rodents [5,12] and ameliorates LPS-induced sickness behavior, a measure of inflammation-induced anxiety and depression in rodents [13].
The anti-anxiety effect of ARBs is associated with inhibition of isolation stress-induced alterations in cortical gamma amino butyric acid A (GABAA) and corticotrophin-releasing factor (CRF) systems [5], two major regulatory factors of the behavioral response to stress [14–17]. Sustained treatment with the ARB candesartan completely prevented the decrease in cortical CRF1 receptor and BZ1 binding produced by isolation stress [5]. These results suggested that a modulation of upstream neurotransmission processes regulating cortical CRF1 receptors and the GABAA complex were molecular mechanisms at least in part responsible for the anti-anxiety effect of centrally acting AT1 receptor antagonists.
While this is valuable information, it does not clarify whether the effects of ARBs on CRF1 receptors and the GABAA complex are limited to the stress of isolation or are part of a more general anti-stress effect. The initial hypothesis of a unitary “stress syndrome” has been challenged [18–20]. Each stressor is associated with different brain neurochemical “signatures” depending on the particular challenge to homeostasis, the age, previous experience (which influences adaptation mechanisms), and the nature, intensity and duration of the stress [18–20]. For this reason it is essential to determine if the effect of ARBs extends to other stressors, indicating a more general anti-stress effect. Isolation is a social and psychological stressor [5]. Two very different stressors were selected for this study [20–22]: restraint, a physical stressor with a strong psychological component; and LPS-induced inflammation, a particular type of physical stressor that represents an immediate threat and is associated with anxiety and depression, behavioral changes that are prevented by candesartan treatment [13]. The aim of this study was to clarify whether the effects of candesartan on cortical CRF1 receptors and the GABAA complex were limited to isolation stress or part of a more general anti-stress effect extending to very different stressors.
2. Material and Methods
2.1 Animals
Eight weeks old male Wistar Hannover rats initially weighing about 250 g were used (Taconic Farms; Germantown, NY, USA). Upon its arrival, animals were acclimatized to our animal facility for one week. Rats were kept in groups of three per cage under standard laboratory conditions with water and rat chow ad libitum, room temperature at 22°C, and 12 hours cycles of light and dark (lights on at 7:00 AM). A total of 48 rats were used for LPS treatment and 32 for restraint stress. The National Institute of Mental Health Animal Care and Use Committee (Bethesda, MD) approved all procedures. All efforts were made to minimize the number of animals used and their suffering according to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, NIH Publication No. 80-23, revised 1996.
2.2 Drugs
Candesartan (Astra-Zeneca, Mölndal, Sweden) was dissolved in 0.1 N Na2CO3 and then diluted in isotonic saline at a final pH of 7.5–8.0; this solution was protected from light and stored at 4°C. The ARB was administrated s.c. at 1 mg/kg/day. At this dose, candesartan effectively blocks peripheral and central AT1 receptors [3,9,13]. This AT1 blockade is associated with a small decrease in the blood pressure, that nevertheless continue within the normal range [9]. Enough drug was prepared to provide the three injections required per experiment. Fresh drug was prepared for each experiment.
LPS (Escherichia coli serotype 055:B5; Sigma Chemical Co., St Louis, MO, USA) was dissolved in sterile saline and administered i.p. at 50 µg/kg; we have found that at this dose LPS produces brain and peripheral inflammation as well as sickness behavior response [3,13].
2.2 Experimental protocol
2.2.1 Inflammation stress
Animals were divided into four groups: vehicle-saline (n=5), candesartan-saline (n=5), vehicle-LPS (n=7) and candesartan-LPS (n=7). For 3 consecutive days at 9:00 AM, rats received s.c. injections of vehicle or 1 mg/kg/day of candesartan. On day 3 starting at 10:00 AM, rats received an i.p. injection of sterile saline or LPS 50 µg/kg. Three hours after saline/LPS injection, animals were removed from their cages and taken to a different room to be killed by fast decapitation between 1:00 PM to 2:30 PM). Trunk blood was collected in polyethylene tubes with EDTA (1 mg/ml blood). Plasma was obtained by centrifugation at 1800 × g, 15 min at 4°C and it was stored at −80°C until used. Brains were frozen in cold isopentane and stored at −80°C until used. This strategy was repeated in another set of animals; the first set of brains was used for BZ binding autoradiography and the second for real-time PCR after selective dissection of cingulate cortex.
2.2.2 Restraint stress
Rats were divided into four groups of eight animals each: vehicle-control (not stressed), vehicle-restraint, candesartan-control and candesartan-restraint. Candesartan was injected (s.c. 1 mg/kg/day) at 9:00 AM, daily for three days. After treatment, half of vehicle- and half of candesartan-treated rats were randomly selected and individually housed in restrainers (model 82, IITC.INC, Woodland Hills, CA) for 2 hours starting at 9:30 AM. Not stressed rats were left undisturbed in their cages. Stressed and control rats were killed by decapitation between 11:30 AM to 1:30 PM. Plasma and brains obtained were stored at −80°C until used.
2.3 Hormones
Commercial radioimmunoassay kits were used to measure plasma corticosterone (MP Biomedicals, Orangeburg, NY, USA), and plasma ACTH (DiaSorin, Stillwater, MN, USA) following the manufactures’ protocols.
2.4 BZ receptors binding autoradiography
Fresh-frozen brains were sliced in a cryostat at −20°C. Sixteen µm thick coronal sections were thaw mounted on positively charged microslides and stored at −80°C until used (Daigger, Vernon Hills, IL). BZ binding sites were determined as previously described [23] with slight modifications. Brain sections were preincubated for 30 min at 4°C in freshly prepared 170mM Tris-HCl, pH 7.4 buffer, followed by incubation for 60 min at 4°C in assay buffer containing 3 nM of the BZ agonist [3H]-flunitrazepam (71.0 Ci/mmol; Perkin Elmer, Boston, MA), a drug that binds to GABAA α1, α2, α3 and α5 containing receptors. Non-specific binding was measured in consecutive sections co-incubated with the antagonist clonazepam 1 µM. A third set of slides was co-incubated with zolpidem 1 µM, an agonist that preferentially binds to GABAA α1-γ2 containing receptors (resembling the pharmacologically described BZ1 receptor) and with less affinity to GABAA α2 and α3 containing receptors and no affinity for GABAA α5-γ2 containing receptors (resembling the BZ2 receptor; [24–26]. After incubation, sections were washed twice for 2 min in incubation buffer at 4°C and dipped once in ice-cold distilled water. Slides were dried and exposed to Kodak Biomax MR films for 2 weeks together with 3H-labeled standards. The films were developed in GBX developer (Eastman Kodak Company, Rochester, NY) for 4 min, fixed in Kodak GBX fixer for 4 min, and rinsed in water for 5 min.
Optical densities of autoradiograms were quantified by comparison with 3H-labeled standards (American Radiolabeled Chemicals, St Louis, MO) using the Scion Image Program 4.0.2 (Scion Corporation, Frederick, MD) based on the NIH Image Program of the National Institutes of Health. Optical densities were then transformed to the corresponding values of fmol/mg.
2.5 CRF receptors binding autoradiography
Sixteen µm thick coronal sections were cut and thaw mounted on positively charged microslides (Daigger). CRF receptors were label with [125I]-sauvagine, a highly specific agonist for both CRF1 and CRF2 receptors. [125I]-sauvagine binding was peformed as earlier reported [27] with some modifications. Sections were pre-incubated twice for 10 min in 50 mM Tris-HCl pH 7.4 containing 10 mM MgCl2 and 2 mM EGTA, followed by incubation for 120 min at room temperature in the same buffer plus 0.1 % BSA, 0.05 % bacitracin, 0.04 TIU/ml aprotinin and 0.15 nM [125I]-sauvagine (Perkin-Elmer, Boston, MA, specific activity 2200 Ci/mmol). Two sets of adjacent section were used to quantify the receptor subtypes. Non-specific binding was defined by co-incubation with astressin 100 nM (Sigma). CRF1 receptors were defined as the [125I]-sauvagine binding displaced by 130 nM of the selective CRF1 receptor antagonist antalarmin. CRF2 receptors were defined as the [125I]-sauvagine binding not displaced by antalarmin but displaced by astressin. Following the incubation period, slides were washed twice, 5 min each, in 50 mM Tris-HCl pH 7.4 containing 0.01% Triton X-100 at 4°C. Slides were deep once in distilled water and immediately dried under cold air. The slides were exposed to Kodak Biomax MR films (Eastman Kodak Company) for 3 days together with [14C] standards, developed and quantified.
Cerebral areas for both BZ and CRF receptor quantification were defined according to Paxinos and Watson [28].
2.6 Measurement of mRNA expression by real-time PCR
Cingulate cortex areas 1 and 2 were microdissected from 350 µm coronal brain sections. Punch microdissection was performed under stereomicroscope control using a 1.25 mm Harris Uni-Core microdissection needle. Up to four punches per brain were performed in sections between −0.2 mm to −1.5 mm from bregma [28]. Obtained tissues were store at −80°C until used.
Total RNA was isolated individually from homogenized cingulate cortex samples using 400 µl TRIzol (Invitrogen, Carlsbad, CA, USA), followed by purification using an RNeasy Mini kit (Qiagen, Valencia, CA, USA). Synthesis of complementary DNA (cDNA) was performed using 0.2 µg of total RNA and Super-Script III first-Strand Synthesis SuperMix (Invitrogen). Real-time PCR was performed in a 20 µl reaction mixture consisting of 10 µl SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), 3 µl cDNA and 0.3 µM of each primer for a specific target on a DNA Engine Opticon™ (Bio-Rad, Hercules, CA, USA). Specific primers used are listed in Table 1. Amplification was performed at 95°C for 10 min, followed by 40 cycles at 95°C for 15 sec and 60°C for 60 sec. At the end of amplification, the specificity of the PCR was confirmed by determination of the melting temperature of the PCR product. Serial dilutions of cDNA were used to obtain a calibration curve. The individual targets for each sample were quantified by determining the cycle threshold and by using calibration curves. The relative amount of the target RNA was normalized with the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and expressed as arbitrary units relative to GAPDH. The expression of GAPDH was not affected by any of the treatments used (results not shown).
Table 1. Primers used for real-time PCR.
| Gene | Forward (5’ – 3’) |
| Reverse (5’ – 3’) | |
| CRF1 receptor | TGCATCCGTGGACCTCATTG |
| GGTAGCCATTGTTTGTCGTGTTGT | |
| CRF2 receptor | TGCCGCTGCGTCACCACCATA |
| AGCAGCCTTCCACAAACATCCAGA | |
| GABAA α1 subunit | ACCAGGCTTGGGAGAGCGTGT |
| CGTGGTCTGACACGGGTCCG | |
| GABAA α2 subunit | GCCAGAGAACAAGCCAGCCGA |
| ACCCCTAATACAGGCTCCCGGT | |
| GABAA β2 subunit | GACCCCAGGAGCACAATGCTTGC |
| CCACATGTCGTTCCAGGGCGT | |
| GABAA γ2 subunit | GCGGATGCTCACTGGATCACGA |
| TGCAACTGGCACTCGGCATCA | |
| GAPDH | ATGACTCTACCCACGGCAAG |
| TGGAAGATGGTGATGGGTTT |
2.7 Statistical analysis
All results are expressed as means ± SEM, for groups of animals measured individually. One-way ANOVA and post-hoc Newman-Keuls’ test were used to assess the significance of differences. P<0.05 was considered as statistically significant. All statistics were performed with the use of Prism 5.02 software (GraphPad Software for Science, San Diego, CA, USA)
3. Results
3.1 Plasma hormones
Three days candesartan treatment (1 mg/kg/day) did not affect basal adenocorticotropic hormone (ACTH) but significantly increased basal corticosterone plasma levels (Fig. 1). Plasma stress hormones were increased by three hours treatment with LPS (50 µg/kg i.p.): ACTH was more than 6-fold increased and corticosterone about 3-fold (Fig. 1A). Candesartan pre-treatment significantly reduced the LPS-induced ACTH plasma levels but it did not change that of corticosterone (Fig. 1A). Identical pattern of results was found for restraint stress (Fig. 1B).
Figure 1. Plasma stress hormones in the LPS and restraint stress models.
Animals pretreated with vehicle or ARB candesartan (1 mg/kg/day) were injected with LPS (50 µg/kg) for three hours (A) or subjected to restraint for two hours (B); control groups were either injected with sterile saline (A) or kept undisturbed in their home cages (B). Trunk blood was collected after decapitation. Values represent means ± SEM from groups of 7–9 rats measured individually. * p<0.05, ** p<0.01, *** p<0.001 as compared to vehicle-control; # p<0.05 as compared to vehicle-stress; one-way ANOVA and Newman-Keuls post-hoc test.
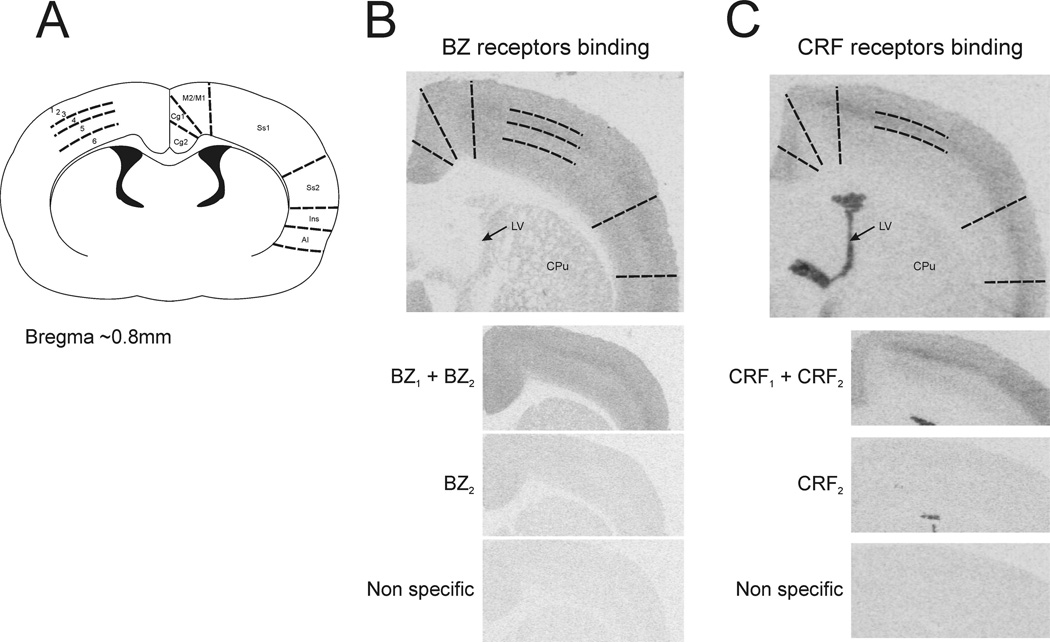
3.2 Cortical BZ receptors
BZ receptor binding was quantified in cortical subdivisions and layers as described by Paxinos and Watson [28] (Fig. 2A). Four tiers were clearly visible on cortical BZ binding corresponding to layers 1–3, layer 4, layer 5 and layer 6 (Fig. 2B). BZ1 receptor was the predominant subtype in the rat cortex with just a modest amount of BZ2 subtype that was not statistically different to the non-specific binding (Fig 2B).
Figure 2. Cortical BZ and CRF receptors binding summary.
A) Scheme of the rat brain coronal section showing the cortical areas where BZ and CRF receptors were measured. B) Representative pictures for BZ receptors binding; total binding (BZ1+BZ2), binding after zolpidem displacement (BZ2) and binding after clonazepam displacement (non specific). C) Representative pictures for CRF receptors binding; total binding (CRF1+CRF2), binding after antalarmin displacement (CRF2) and binding after astressin displacement (non specific). Cg1 and Cg2, cingulate cortex area 1 and 2; M2/M1, primary and secondary motor cortex; Ss1 and Ss2, somatosensory cortex 1 and 2; Ins, insular cortex; AI, agranular insular cortex; 1–6, cortical layers 1 to 6; LV, lateral ventricle; CPu, striatum.
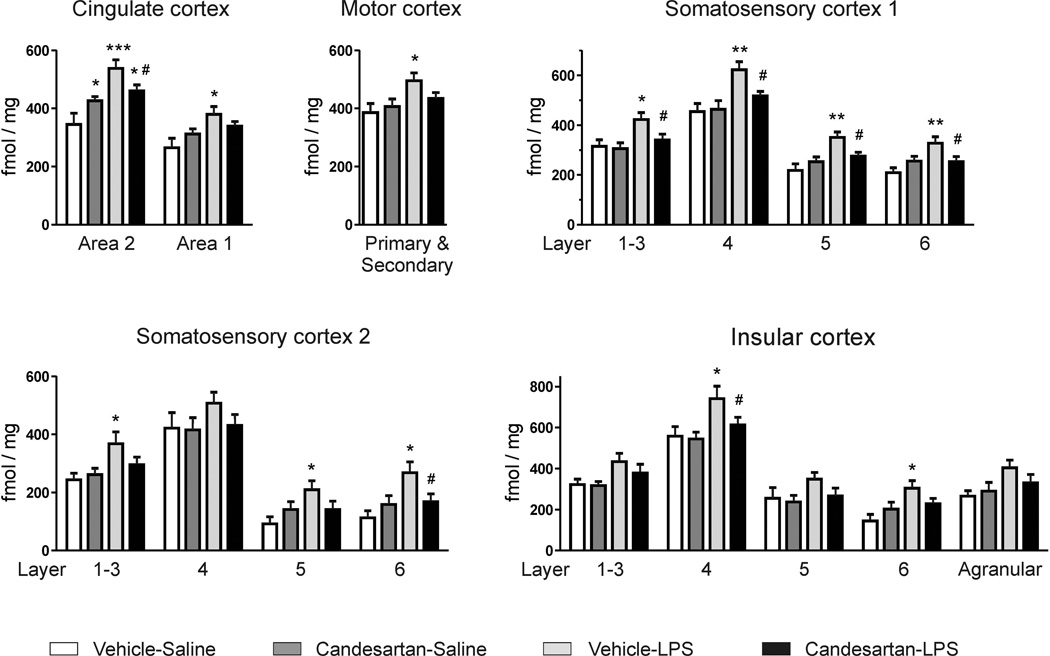
BZ1 receptor binding was increased by LPS administration in almost all cortical areas and layers measured with only few exceptions where the increase in binding did not reach statistical significance. These exceptions were somatosensory cortex 2 layer 4, insular cortex layers 1–3, insular cortex layer 5, and agranular insular cortex (Fig. 3). Candesartan alone increased basal BZ1 receptor binding in cingulate cortex area 2, and candesartan pre-treatment significantly prevented the generalized LPS-induced BZ1 receptor binding increment (Fig. 3).
Figure 3. Inflammation stress and BZ1 receptor binding in the rat brain cortex.
Animals pretreated with vehicle or ARB candesartan (1 mg/kg/day) were injected with sterile saline or LPS (50 µg/kg) for three hours. Values represent means ± SEM from groups of 7–9 rats measured individually. * p<0.05, ** p<0.01, *** p<0.001 as compared to vehicle-saline; # p<0.05 as compared to vehicle-LPS; one-way ANOVA and Newman-Keuls post-hoc test.
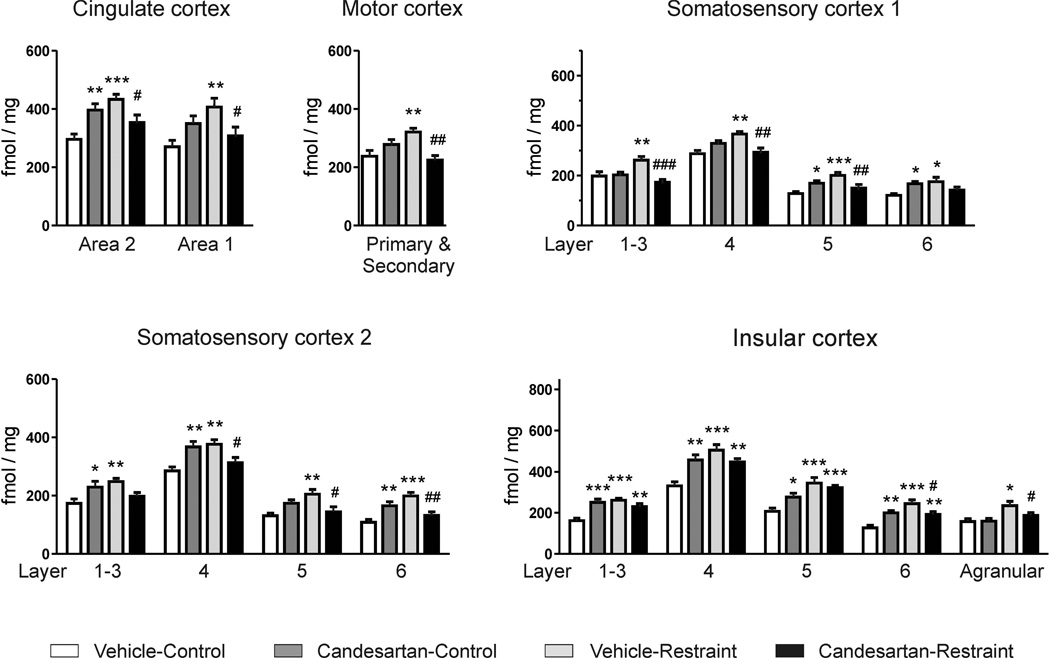
Restraint stress presented identical pattern of response. Restraint stress increased BZ1 receptor binding; candesartan alone increased BZ1 binding in cingulate cortex area 2, somatosensory cortex 1 layers 5 and 6, somatosensory cortex 2 layers 1–3, 4 and 6 and in all layers of insular cortex; and pre-treatment with candesartan prevented restraint-induced BZ1 increase (Fig. 4).
Figure 4. Restraint stress and BZ1 receptor binding in the rat brain cortex.
Animals pretreated with vehicle or ARB candesartan (1 mg/kg/day) were restraint for two hours or kept undisturbed in their home cages (control). Values represent means ± SEM from groups of 8 rats measured individually. * p<0.05, ** p<0.01, *** p<0.001 as compared to vehicle-control; # p<0.05, ## p<0.01, ### p<0.001 as compared to vehicle-restraint; one-way ANOVA and Newman-Keuls post-hoc test.
3.3 GABAA receptor subunits in cingulate cortex
Based in the binding results, cingulate cortex was selected as a representative area of the observed cortical changes and it was used to analyze gene expression in the LPS model.
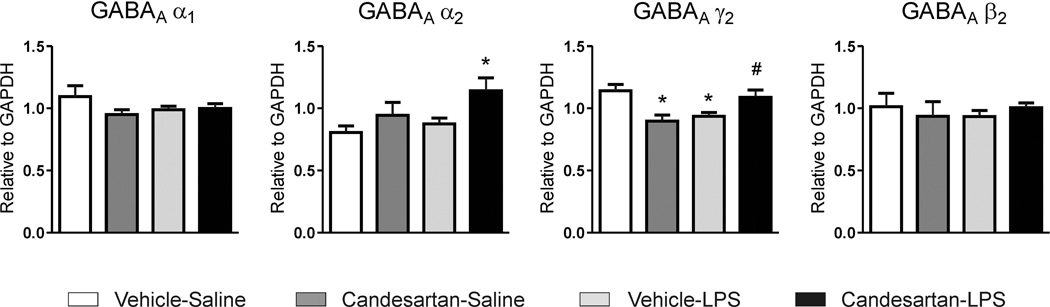
Neither LPS nor candesartan nor their combination produced significant changes on mRNA expression of GABAA α1 and GABAA β2 subunits in cingulate cortex (Fig. 5). The GABAA γ2 subunit was down-regulated by both LPS and candesartan when administered alone (Fig. 5). Pretreatment with candesartan prevented the LPS-induced decrease in GABAA γ2 mRNA expression, and up-regulated GABAA α2 subunit expression (Fig. 5).
Figure 5. mRNA expression of GABAA receptor subunits in cingulate cortex after inflammation stress.
Animals pretreated with vehicle or ARB candesartan (1 mg/kg/day) were injected with sterile saline or LPS (50 µg/kg) for three hours. Values represent means ± SEM from groups of 5–7 rats measured individually. * p<0.05 as compared to vehicle-saline; # p<0.05 as compared to vehicle-LPS; one-way ANOVA and Newman-Keuls post-hoc test.
3.4 Cortical CRF receptors
Three cortical tiers were obvious on cortical CRF receptors binding corresponding to layers 1–3, layer 4 and layer 5–6 [28] (Fig 2A and 2C). CRF1 receptors were the predominant subtype in the cerebral cortex with non-detectable binding for CRF2 receptors (Fig 2C). CRF1 binding was not significantly affected by any of the treatments either in immune (Table 2) or in restraint stress (not shown).
Table 2. CRF1 receptor binding after inflammation stress.
Animals pretreated with vehicle or ARB candesartan (1 mg/kg/day) were injected with LPS (50 µg/kg) for three hours. Values are expressed as fmol/mg protein and represent means ± SEM from groups of 5–7 rats measured individually.
| Cortical area | Vehicle- Saline |
Candesartan- Saline |
Vehicle- LPS |
Candesartan- LPS |
|---|---|---|---|---|
| Cingulate cortex Area 1 and 2 | 3.0 ± 0.3 | 3.4 ± 0.3 | 2.9 ± 0.3 | 3.0 ± 0.2 |
| Primary & Secondary Motor cortex | 1.6 ± 0.1 | 1.9 ± 0.2 | 1.7 ± 0.2 | 1.7 ± 0.2 |
| Somatosensory cortex Layer 1–3 | 3.3 ± 0.5 | 3.4 ± 0.3 | 2.7 ± 0.3 | 2.7 ± 0.1 |
| Somatosensory cortex Layer 4 | 4.2 ± 0.5 | 4.7 ± 0.6 | 4.2 ± 0.4 | 4.2 ± 0.2 |
| Somatosensory cortex Layer 5–6 | 1.5 ± 0.1 | 1.6 ± 0.2 | 1.3 ± 0.1 | 1.3 ± 0.1 |
| Insular cortex Layer 1–3 | 2.6 ± 0.2 | 2.8 ± 0.4 | 2.5 ± 0.3 | 2.6 ± 0.2 |
| Insular cortex Layer 4 | 4.7 ± 0.2 | 5.3 ± 0.7 | 4.7 ± 0.5 | 4.8 ± 0.2 |
| Insular cortex Layer 5–6 | 1.8 ± 0.1 | 1.9 ± 0.2 | 1.6 ± 0.2 | 1.8 ± 0.1 |
3.5 CRF receptor subtype mRNA in cingulate cortex
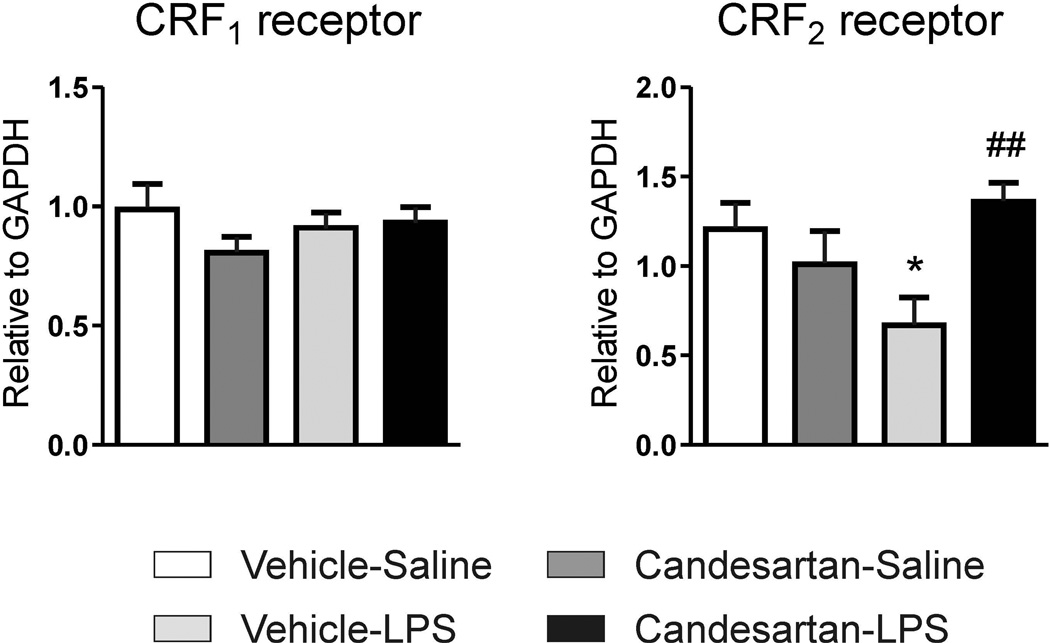
Expression of CRF1 receptor mRNA was not changed by any of the treatments in the inflammatory stress model. On the other hand, CRF2 mRNA expression was down-regulated by LPS administration (Fig. 6); and candesartan alone did not change CRF2 receptor expression but it prevented LPS-induced CRF2 down-regulation (Fig. 6).
Figure 6. mRNA expression of CRF receptors in cingulate cortex after inflammation stress.
Animals pretreated with vehicle or ARB candesartan (1 mg/kg/day) were injected with sterile saline or LPS (50 µg/kg) for three hours. Values represent means ± SEM from groups of 5–7 rats measured individually. * p<0.05 as compared to vehicle-saline; ## p<0.01 as compared to vehicle-LPS; one-way ANOVA and Newman-Keuls post-hoc test.
4. Discussion
The main findings of the present study are that brain AT1 receptor participates in the regulation of the cerebral cortical GABAA system in control, non-stressed rats; and that AT1 receptor blockade prevents the stress-induced alteration of cortical GABAA complex.
Candesartan administration produced significant hormonal effects in inflammatory and restraint stress, further proof of AT1 receptor regulation of the HPA axis response to stress [3,4,29]. While in isolation stress candesartan inhibits both the ACTH and corticosterone responses [4], in inflammation and restraint stress the ARB decreases ACTH release but does not affect corticosterone release (present results and [3,9]).
In addition, administration of candesartan alone enhanced basal corticosterone levels, an apparent paradoxical response, which is, however, in agreement with previous observations [3,6]. Candesartan decreases adrenal corticosterone content [6], and this may be interpreted as the result of increased corticosterone release. These observations indicate that enhanced corticosterone levels after candesartan administration may be mediated by direct effects on the adrenal gland, and that adrenal and/or circulating Ang II might inhibit basal corticosterone release. The report that Ang II decreases corticosterone production in adrenal cell cultures supports this hypothesis [30],
By contrast, Ang II infusion was reported to increase glucocorticoid release [31]. However, the amounts infused, 9 µg/hour, may result in concentrations many times higher than normal circulating Ang II levels [32], and the effect occurs only in the obese Zucker, but not in lean control rats [31].
On the other hand, other studies do not support an interaction of the Ang II with corticosterone. In a maternal separation stress model, candesartan potenciates ACTH release but does not affect corticosterone [33]. In Spontenously Hypertensive Rats, a model with over-stimulated Renin-Angiotensin System, ARBs do not affect basal corticosterone levels [34]. In A-ZIP/F-1 transgenic mice, ARBs do not reduce the elevated corticosterone levels characteristic of this model [35]. The conclusion is that the effect of ARBs and Ang II on corticosterone levels depends on the model studied and the conditions of the experiments, and the precise mechanism of physiological control has yet to be elucidated.,
It is well established that the GABAA complex is rapidly affected by acute stressors, indication of fast system plasticity. BZ receptors in the rat cortex are acutely modulated by cold swim, handling and isolation stress [5,36,37]. Their expression and stimulation is a major factor determining the GABAA complex activation and its participation in the behavioral response to stress and anxiety [14–16]. Acute stressors like restraint, infection, hypoxia or combined mild stressors influence the GABAA complex at two different levels: altering BZ1 binding sites and modulating the expression of selective GABAA receptor subunits [38–41]. Changes in GABAA receptor subunits expression induced by stress will then promote differential clustering of selective subunits conditioning the receptor activity and its participation in stress and anxiety [25,42–46]. While the GABAA γ2 subunit is essential for the synaptic clustering of the GABAA complex [39,41,47], anti-anxiety effects are commonly linked to receptors highly expressing the GABAA α2 subunits [42,43,48].
This study reveals fast, significant and widespread increases in cortical BZ1 binding after acute restraint or inflammatory stress. In addition, inflammatory stress significantly decreases the mRNA expression of the GABAA γ2 subunit in the cingulate cortex, without affecting the α1, α2 and β2 subunits. These results support the hypothesis that the cortical GABAA system regulates the response to stress [16,17,49]. While acute reduction of GABAA γ2 subunit may indicate decreased clustering of the GABAA receptor complex and decreased inhibitory activity leading to anxiety [50], increases in BZ1 binding should be interpreted as possible compensatory mechanisms to maintain the GABAA complex function during stress.
Reducing the normal level of brain AT1 receptor activation in non-stressed rats by administration of candesartan alone significantly enhanced cortical BZ1 binding, increased cortical GABAA α2 subunit mRNA expression and reduced the expression of γ2 subunit mRNA. Increased BZ1 binding and α2 subunit mRNA expression, which is associated with the anti-anxiety properties of the GABAA complex [48] may explain why candesartan administration reduces anxiety in control rats unchallenged by stress [5,13]. The apparently contradictory decrease of γ2 subunit mRNA as a result of candesartan treatment may possibly be interpreted as a compensatory mechanism to maintain the functional stability of the GABAA complex.
In this study, systemic candesartan administration is very effective in preventing the consequences of inflammatory and restraint stress on the cortical GABAA complex, abolishing the effects of both stress stimuli on cortical BZ1 binding, and reversing the LPS-induced decrease in γ2 subunit mRNA expression. Thus, candesartan eliminates the effect of inflammatory and restraint stress in the cortical GABAA complex, facilitating the return of normal BZ1 expression and restoring proper γ2 subunit mRNA expression to control levels during the response to stress. Overall, we interpret the effects of candesartan as stimulation of adaptive mechanisms aiming to restore the resting inhibitory GABA transmission [43,47,51].
It must be noted that both inflammatory and restraint stress produced significant increases in BZ1 binding, alterations in opposite direction from the significant decrease in BZ1 binding we earlier observed after isolation stress [5]. Our experience is in agreement with the literature, since it has been reported that alterations in both BZ receptor and GABAA subunit expression after stress are not consistent and are strongly influenced by gender, intensity and length of stress, kind of stressor and are dependent on the area studied [36,37,40,41,52].
Nevertheless, the inhibitory effect of ARB treatment on the stress-induced cortical alterations in the GABAA complex is shared across a variety of stress types: a) unpredictable restraint, a psychological stress perceived as a life threatening event where the body responds in anticipation to possible homeostatic needs [20]; b) systemic LPS administration, an immediate threat strongly activating the innate immune system and producing a robust inflammatory reaction associated with emotional responses characterized by sickness behavior [20–22]; c) isolation, a negative emotional stimulus representing a significant loss of normal social interaction [4–6]. In all cases, ARBs prevent stress-induced changes in the cortical GABAA system, and these effects occur whether BZ1 binding is decreased (isolation, [5]) or increased (inflammation and restraint, [present results]).
Common biological and molecular pathways may explain the similar effect of AT1 receptor blockade on cortical benzodiazepine receptors during acute inflammatory and restraint stress. There is a close and inverse interaction between AT1 receptor and GABAA activity in the brain. AT1 receptor activation directly inhibits GABAergic transmission and GABAA function controls the effects of AT1 receptor stimulation [53–56]. A similar direct control of GABAA activity may occur in the cerebral cortex, as a consequence of activation of local AT1 receptors [57].
In addition, regulation of GABAA function by AT1 receptor blockade may be indirect, the result of regulation of upstream molecular pathway(s) common to all the stress types studied. The hypothalamic paraventricular nucleus (PVN) is a major regulator of the hormonal and sympathetic response to stress [58]. In PVN, both inflammatory and restraint stress up-regulate AT1 receptors [3,59] and inducible pro-inflammatory enzymes such as COX-2 and iNOS [60,13]. Inflammation and enhanced AT1 receptor stimulation directly decrease GABAA transmission desinhibiting PVN sympathetic activity and outflow which ultimately promotes the stress-induced sympathetic response [53,56,60,61]. In turn, enhanced central sympathetic activation plays a major role in the regulation of cortical GABAA function and leads to anxiety [62,63]. Thus, ARBs may prevent stress-induced alterations in cortical GABAA function also as a consequence of decreased inflammation in the PVN, preserving GABAA inhibitory effects and reducing central sympathetic activation.
Conversely, none of the experimental conditions altered cortical CRF1 receptor binding, the principal CRF receptor expressed in rat cortex [20,64,65]. This contrasts with the clear decrease in cortical CRF1 receptor expression reported after isolation stress and with the prevention of such alterations by treatment with candesartan [5]. It is well established that activation of CRF1 receptors promotes anxiety-like behavior [20,66,67]. These results may be related to the intensity, length and type of stress studied, since alterations in CRF binding are dependent on the characteristics of the stress applied [68].
In agreement with previous observations, we have not detected significant levels of cortical CRF2 receptor binding [64,65], but we found clear expression of CRF2 mRNA in cingulate cortical samples [69]. Activation of CRF2 receptors has been proposed as antagonistic to CRF1 stimulation [20,66,67], and CRF2 receptor knock-out mice show increased anxiety behavior and higher sensitivity to acute stress [70–72]. In addition, central CRF2 receptors regulate the neuronal activation of limbic areas, including cingulate cortex, in an anxiety model [73,74]. We hypothesize that prevention of CRF2 receptor down-regulation by candesartan might be an additional mechanism explaining the anxiolytic and anti-stress effects observed with ARBs [75].
4.1 Conclusions
We conclude that cortical BZ1 receptor expression is under AT1 receptor control, and that very different stressors promote rapid and widespread changes in cortical BZ1 receptor expression. While the direction of the changes depends on the particular challenge to homeostasis, candesartan treatment prevents the stress-induced alterations in BZ1 receptor expression.
Conversely, the effects of candesartan in cortical CRF1 receptor expression appear to be stress-specific, and not essential for the anxiolytic effects of ARBs.
Overall, our results and earlier studies suggest that ARBs prevent the alterations in the cortical GABAA complex in very different stress models. The precise molecular mechanism for ARBs effect remains to be clarified; however, we propose that ARBs may affect cortical GABAA receptors by modulating AT1-GABAA interactions in cortex and/or by regulating hypothalamic PVN sympathetic system. Nevertheless, cortical GABAA regulation might be a common mechanism of ARBs associated with their anxiolytic effect. Since humans are often submitted to combinations of stressors, the present results provide additional and necessary information in support of the translational value of the studies.
Our results have important clinical implications. Abnormal responses to stress with alterations in the GABAA complex response are a vulnerability factor for mood disorders like anxiety and depression [20,43,49,50,76]. We demonstrated, in pre-clinical rodent models, that ARB administration prevents development of stress-induced gastric ulcers, decreases anxiety and significantly reduced inflammation-induced sickness behavior [5,7,13]. In diabetic patients, candesartan administration resets the HPA-axis and ameliorates their depressive symptoms [77]. Furthermore, the Renin-Angiotensin system is activated in subjects living alone with depressive symptomatology [78]. Based on our results and those of the literature, we suggest that treatment with ARBs may be effective on a wide range of brain conditions and mood disorders where abnormal responses to stress play a significant role.
Highlights.
Acute stress promptly increases benzodiazepine-1 receptors in cerebral cortex
Candesartan prevents GABAA receptor plasticity during stress
Candesartan prevents cortical CRF2 gene expression during stress
Angiotensin II AT1 receptors regulate stress and anxiety
Candesartan decreases anxiety
5. Acknowledgements
This research was supported by the Division of Intramural Research Programs, National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services, USA. We thank Astra-Zeneca, Mölndal, Sweden for the gift of candesartan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saavedra JM. Brain and pituitary angiotensin. Endocr Rev. 1992;13:329–380. doi: 10.1210/edrv-13-2-329. [DOI] [PubMed] [Google Scholar]
- 2.Saavedra JM, Benicky J. Brain and peripheral angiotensin II play a major role in stress. Stress. 2007;10:185–193. doi: 10.1080/10253890701350735. [DOI] [PubMed] [Google Scholar]
- 3.Sánchez-Lemus E, Benicky J, Pavel J, Saavedra JM. In vivo Angiotensin II AT1 receptor blockade selectively inhibits LPS-induced innate immune response and ACTH release in rat pituitary gland. Brain Behav Immun. 2009;23:945–957. doi: 10.1016/j.bbi.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armando I, Carranza A, Nishimura Y, Hoe KL, Barontini M, Terron JA, Falcon-Neri A, Ito T, Juorio AV, Saavedra JM. Peripheral administration of an Angiotensin II AT1 receptor antagonist decreases the hypothalamic-pituitary-adrenal response to isolation stress. Endocrinology. 2001;42:3880–3889. doi: 10.1210/endo.142.9.8366. [DOI] [PubMed] [Google Scholar]
- 5.Saavedra JM, Armando I, Bregonzio C, Juorio A, Macova M, Pavel J, Sanchez-Lemus E. A centrally acting, anxiolytic angiotensin II AT1 receptor antagonist prevents the isolation stress-induced decrease in cortical CRF1 receptor and benzodiazepine binding. Neuropsychopharmacology. 2006;31:1123–1134. doi: 10.1038/sj.npp.1300921. [DOI] [PubMed] [Google Scholar]
- 6.Armando I, Volpi S, Aguilera G, Saavedra JM. Angiotensin II AT1 receptor blockade prevents the hypothalamic corticotropin-releasing factor response to isolation stress. Brain Res. 2007;1142:92–99. doi: 10.1016/j.brainres.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bregonzio C, Armando I, Ando H, Jezova M, Baiardi G, Saavedra JM. Anti-inflammatory effects of angiotensin II AT1 receptor antagonism prevent stress-induced gastric injury. Am J Physiol Gastrointest Liver Physiol. 2003;285:G414–G423. doi: 10.1152/ajpgi.00058.2003. [DOI] [PubMed] [Google Scholar]
- 8.Bregonzio C, Seltzer A, Armando I, Pavel J, Saavedra JM. Angiotensin II AT(1) receptor blockade selectively enhances brain AT(2) receptor expression, and abolishes the cold-restraint stress-induced increase in tyrosine hydroxylase mRNA in the locus coeruleus of spontaneously hypertensive rats. Stress. 2008;11:457–466. doi: 10.1080/10253890801892040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sánchez-Lemus E, Murakami Y, Larrayoz-Roldan IM, Moughamian AJ, Pavel J, Nishioku T, Saavedra JM. Angiotensin II AT1 receptor blockade decreases lipopolysaccharide-induced inflammation in the rat adrenal gland. Endocrinology. 2008;149:5177–5188. doi: 10.1210/en.2008-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsutsumi K, Saavedra JM. Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am J Physiol. 1991;261:R209–R216. doi: 10.1152/ajpregu.1991.261.1.R209. [DOI] [PubMed] [Google Scholar]
- 11.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Distribution of angiotensin type-1 receptor messenger RNA expression in the adult rat brain. Neuroscience. 1998;82(3):827–841. doi: 10.1016/s0306-4522(97)00328-x. [DOI] [PubMed] [Google Scholar]
- 12.Barnes NM, Costall B, Kelly ME, Murphy DA, Naylor RJ. Anxiolytic-like action of DuP753, a non-peptide angiotensin II receptor antagonist. Neuroreport. 1990;1:20–21. doi: 10.1097/00001756-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Benicky J, Sánchez-Lemus E, Honda M, Pang T, Orecna M, Wang J, Leng Y, Chuang DM, Saavedra JM. Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology. 2011;36:857–870. doi: 10.1038/npp.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nutt DJ, Malizia AL. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. Br J Psychiatry. 2001;179:390–396. doi: 10.1192/bjp.179.5.390. [DOI] [PubMed] [Google Scholar]
- 15.Zavala F. Benzodiazepines, anxiety and immunity. Pharmacol Ther. 1997;75:199–216. doi: 10.1016/s0163-7258(97)00055-7. [DOI] [PubMed] [Google Scholar]
- 16.Biggio G, Concas A, Corda MG, Giorgi O, Sanna E, Serra M. GABAergic and dopaminergic transmission in the rat cerebral cortex: effect of stress, anxiolytic and anxiogenic drugs. Pharmacol Ther. 1990;48:121–142. doi: 10.1016/0163-7258(90)90077-f. [DOI] [PubMed] [Google Scholar]
- 17.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pacak K, Palkovits M, Yadid G, Kvetnansky R, Kopin IJ, Goldstein DS. Heterogeneous neurochemical responses to different stressors: test of Selye's doctrine of nonspecificity. Am J Physiol. 1998;275:R1247–R1255. doi: 10.1152/ajpregu.1998.275.4.R1247. [DOI] [PubMed] [Google Scholar]
- 19.Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic Systems in stress: structural and molecular genetic approaches. Physiol Rev. 2009 Apr;89(2):535–606. doi: 10.1152/physrev.00042.2006. [DOI] [PubMed] [Google Scholar]
- 20.Pêgo JM, Sousa JC, Almeida OF, Sousa N. Stress and the neuroendocrinology of anxiety disorders. Curr Top Behav Neurosci. 2010;2:97–117. doi: 10.1007/7854_2009_13. [DOI] [PubMed] [Google Scholar]
- 21.Maier SF. Bi-directional immune-brain communication: Implications for understanding stress, pain, and cognition. Brain Behav Immun. 2003;17:69–85. doi: 10.1016/s0889-1591(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 22.Besedovsky HO, del Rey A. Central and peripheral cytokines mediate immune-brain connectivity. Neurochem Res. 2011;36:1–6. doi: 10.1007/s11064-010-0252-x. [DOI] [PubMed] [Google Scholar]
- 23.Negro M, Fernández-López A, Calvo P. Autoradiographical study of types 1 and 2 of benzodiazepine receptors in rat brain after chronic ethanol treatment and its withdrawal. Neuropharmacology. 1995;34:1177–1182. doi: 10.1016/0028-3908(95)00064-d. [DOI] [PubMed] [Google Scholar]
- 24.Sanna E, Busonero F, Talani G, Carta M, Massa F, Peis M, Maciocco E, Biggio G. Comparison of the effects of zaleplon, zolpidem, and triazolam at various GABA(A) receptor subtypes. Eur J Pharmacol. 2002;451:103–110. doi: 10.1016/s0014-2999(02)02191-x. [DOI] [PubMed] [Google Scholar]
- 25.Benavides J, Peny B, Ruano D, Vitorica J, Scatton B. Comparative autoradiographic distribution of central omega (benzodiazepine) modulatory site subtypes with high, intermediate and low affinity for zolpidem and alpidem. Brain Res. 1993;604:240–250. doi: 10.1016/0006-8993(93)90375-w. [DOI] [PubMed] [Google Scholar]
- 26.Lüddens H, Wisden W. Function and pharmacology of multiple GABAA receptor subunits. Trends Pharmacol Sci. 1991;12:49–51. doi: 10.1016/0165-6147(91)90495-e. [DOI] [PubMed] [Google Scholar]
- 27.Sánchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Fourth Edition. New York: Academic Press; 1998. [Google Scholar]
- 29.Miesel A, Müller-Fielitz H, Jöhren O, Vogt FM, Raasch W. Double blockade of angiotensin II (AT(1))-receptors and ACE does not improve weight gain and glucose homeostasis better than single-drug treatments in obese rats. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spinedi E, Aguado L, Basilotta G, Carrizo D. Angiotensin II and glucocorticoid release: direct effect at the adrenal level and modulation of the adrenocorticotropin-induced glucocorticoid release. J Endocrinol Invest. 1989;12:321–327. doi: 10.1007/BF03349997. [DOI] [PubMed] [Google Scholar]
- 31.Müller H, Schweitzer N, Jöhren O, Dominiak P, Raasch W. Angiotensin II stimulates the reactivity of the pituitary-adrenal axis in leptin-resistant Zucker rats, thereby influencing the glucose utilization. Am J Physiol Endocrinol Metab. 2007;293:E802–E810. doi: 10.1152/ajpendo.00650.2006. [DOI] [PubMed] [Google Scholar]
- 32.Allan DR, Hui KY, Coletti C, Hollenberg NK. Renin vs. angiotensin-converting enzyme inhibition in the rat: consequences for plasma and renal tissue angiotensin. J Pharmacol Exp Ther. 1997;283:661–665. [PubMed] [Google Scholar]
- 33.Liebl C, Panhuysen M, Pütz B, Trümbach D, Wurst W, Deussing JM, Müller MB, Schmidt MV. Gene expression profiling following maternal deprivation: involvement of the brain Renin-Angiotensin system. Front Mol Neurosci. 2009;2:1–10. doi: 10.3389/neuro.02.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raasch W, Wittmershaus C, Dendorfer A, Voges I, Pahlke F, Dodt C, Dominiak P, Jöhren O. Angiotensin II inhibition reduces stress sensitivity of hypothalamo-pituitary-adrenal axis in spontaneously hypertensive rats. Endocrinology. 2006;147:3539–3546. doi: 10.1210/en.2006-0198. [DOI] [PubMed] [Google Scholar]
- 35.Rong X, Li Y, Ebihara K, Zhao M, Aini W, Kusakabe T, Hirata M, Miyamoto L, Murray M, Nakao K. An adipose tissue-independent insulin-sensitizing action of telmisartan: a study in lipodystrophic mice. J Pharmacol Exp Ther. 2009;331:1096–1103. doi: 10.1124/jpet.109.157099. [DOI] [PubMed] [Google Scholar]
- 36.Andrews N, Zharkovsky A, File SE. Acute handling stress downregulates benzodiazepine receptors: reversal by diazepam. Eur J Pharmacol. 1992;210:247–251. doi: 10.1016/0014-2999(92)90411-v. [DOI] [PubMed] [Google Scholar]
- 37.Motohashi N, Okamoto Y, Osada M, Yamawaki S. Acute swim stress increases benzodiazepine receptors, but not GABAA or GABAB receptors, in the rat cerebral cortex. Neurochem Int. 1993;23:327–330. doi: 10.1016/0197-0186(93)90076-h. [DOI] [PubMed] [Google Scholar]
- 38.Reyes-García MG, Hernández-Hernández F, Hernández-Téllez B, García-Tamayo F. GABA(A) receptor subunits RNA expression in mice peritoneal macrophages modulate their IL-6/IL-12 production. J Neuroimmunol. 2007;188:64–68. doi: 10.1016/j.jneuroim.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 39.Femenía T, Pérez-Rial S, Urigüen L, Manzanares J. Prodynorphin gene deletion increased anxiety-like behaviours, impaired the anxiolytic effect of bromazepam and altered GABAA receptor subunits gene expression in the amygdala. J Psychopharmacol. 2011;25:87–96. doi: 10.1177/0269881110367724. [DOI] [PubMed] [Google Scholar]
- 40.Feng Y, He X, Yang Y, Chen J, Yin K, Xia Y. Effect of delta-opioid receptor over-expression on cortical expression of GABAA receptor alpha1-subunit in hypoxia. Chin J Physiol. 2011;54:118–123. doi: 10.4077/cjp.2011.amm047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shlomit JP, Gal RL. Short and long term effects of juvenile stressor exposure on the expression of GABA(A) receptor subunits in rats. Stress. 2011 doi: 10.3109/10253890.2011.634036. [DOI] [PubMed] [Google Scholar]
- 42.Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, Fritschy JM, Rülicke T, Bluethmann H, Möhler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 43.Smith KS, Rudolph U. Anxiety and depression: mouse genetics and pharmacological approaches to the role of GABA(A) receptor subtypes. Neuropharmacology. 2012;62:54–62. doi: 10.1016/j.neuropharm.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soria C, Revilla V, Candelas MA, Calvo P, Fernández-López A. An autoradiographical saturation kinetic study of the different benzodiazepine binding sites in rat brain by using [3H] flunitrazepam as a radioligand. Biochem Pharmacol. 1995;50:1619–1625. doi: 10.1016/0006-2952(95)02044-6. [DOI] [PubMed] [Google Scholar]
- 45.Gupta A, Lind S, Eklund A, Lennmarken C. The effects of midazolam and flumazenil on psychomotor function. J Clin Anesth. 1997;9:21–25. doi: 10.1016/S0952-8180(96)00214-0. [DOI] [PubMed] [Google Scholar]
- 46.Iwata N, Cowley DS, Radel M, Roy-Byrne PP, Goldman D. Relationship between a GABAA alpha 6 Pro385Ser substitution and benzodiazepine sensitivity. Am J Psychiatry. 1999;156:1447–1449. doi: 10.1176/ajp.156.9.1447. [DOI] [PubMed] [Google Scholar]
- 47.Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, Belzung C, Fritschy JM, Lüscher B, Mohler H. Decreased GABAA-receptor clustering results in enhanced anxiety and bias for threat cues. Nat Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- 48.McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 49.Korpi ER, Sinkkonen ST. GABA(A) receptor subtypes as targets for neuropsychiatric drug development. Pharmacol Ther. 2006;109:12–32. doi: 10.1016/j.pharmthera.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uusi-Oukari M, Korpi ER. Regulation of GABA(A) receptor subunit expression by pharmacological agents. Pharmacol Rev. 2010;62:97–135. doi: 10.1124/pr.109.002063. [DOI] [PubMed] [Google Scholar]
- 52.Skilbeck KJ, Johnston GA, Hinton T. Stress and GABA receptors. J Neurochem. 2010;112:1115–1130. doi: 10.1111/j.1471-4159.2009.06539.x. [DOI] [PubMed] [Google Scholar]
- 53.Chen QH, Toney GM. Responses to GABA-A receptor blockade in the hypothalamic PVN are attenuated by local AT1 receptor antagonism. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1231–R1239. doi: 10.1152/ajpregu.00028.2003. [DOI] [PubMed] [Google Scholar]
- 54.Shekhar A, Johnson PL, Sajdyk TJ, Fitz SD, Keim SR, Kelley PE, Gehlert DR, DiMicco JA. Angiotensin-II is a putative neurotransmitter in lactate-induced panic-like responses in rats with disruption of GABAergic inhibition in the dorsomedial hypothalamus. J. Neurosci. 2006;26:9205–9215. doi: 10.1523/JNEUROSCI.2491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xing J, Lu J, Li J. Angiotensin II inhibits GABAergic synaptic transmission in dorsolateral periaqueductal gray neurons. Neurosci Lett. 2009;455:8–13. doi: 10.1016/j.neulet.2009.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pyner S. Neurochemistry of the paraventricular nucleus of the hypothalamus: implications for cardiovascular regulation. J Chem Neuroanat. 2009;38:197–208. doi: 10.1016/j.jchemneu.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 57.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortès C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol. 1997;18:383–439. doi: 10.1006/frne.1997.0155. [DOI] [PubMed] [Google Scholar]
- 58.Kenney MJ, Weiss ML, Haywood JR. The paraventricular nucleus: an important component of the central neurocircuitry regulating sympathetic nerve outflow. Acta Physiol Scand. 2003;177:7–15. doi: 10.1046/j.1365-201X.2003.01042.x. [DOI] [PubMed] [Google Scholar]
- 59.Leong DS, Terrón JA, Falcón-Neri A, Armando I, Ito T, Jöhren O, Tonelli LH, Hoe KL, Saavedra JM. Restraint stress modulates brain, pituitary and adrenal expression of angiotensin II AT(1A), AT(1B) and AT(2) receptors. Neuroendocrinology. 2002;75:227–240. doi: 10.1159/000054714. [DOI] [PubMed] [Google Scholar]
- 60.Yamaguchi N, Ogawa S, Okada S. Cyclooxygenase and nitric oxide synthase in the presympathetic neurons in the paraventricular hypothalamic nucleus are involved in restraint stress-induced sympathetic activation in rats. Neuroscience. 2010;170:773–781. doi: 10.1016/j.neuroscience.2010.07.051. [DOI] [PubMed] [Google Scholar]
- 61.Zhang ZH, Yu Y, Wei SG, Felder RB. Centrally administered lipopolysaccharide elicits sympathetic excitation via NAD(P)H oxidase-dependent mitogen-activated protein kinase signaling. J Hypertens. 2010;28:806–816. doi: 10.1097/HJH.0b013e3283358b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kellogg CK, Inglefield JR, Taylor MK, Pleger GL. Importance of hypothalamic function to stressor-induced responsiveness of the GABAA receptor in the cerebral cortex: a non-corticosterone influence. Brain Res. 1993;609:244–252. doi: 10.1016/0006-8993(93)90879-r. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka M, Yoshida M, Emoto H, Ishii H. Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur J Pharmacol. 2000;405:397–406. doi: 10.1016/s0014-2999(00)00569-0. [DOI] [PubMed] [Google Scholar]
- 64.Primus RJ, Yevich E, Baltazar C, Gallager DW. Autoradiographic localization of CRF1 and CRF2 binding sites in adult rat brain. Neuropsychopharmacology. 1997;17:308–316. doi: 10.1016/S0893-133X(97)00071-7. [DOI] [PubMed] [Google Scholar]
- 65.Rominger DH, Rominger CM, Fitzgerald LW, Grzanna R, Largent BL, Zaczek R. Characterization of [125I]sauvagine binding to CRH2 receptors: membrane homogenate and autoradiographic studies. J Pharmacol Exp Ther. 1998;286:459–468. [PubMed] [Google Scholar]
- 66.Saavedra JM, Pavel J. Angiotensin II AT1 receptor antagonists inhibit the Angiotensin-CRF-AVP axis and are potentially useful for the treatment of stress-related and mood disorders. Drug Dev Res. 2005;65:237–269. [Google Scholar]
- 67.Bravo JA, Dinan TG, Cryan JF. Alterations in the central CRF system of two different rat models of comorbid depression and functional gastrointestinal disorders. Int J Neuropsychopharmacol. 2011;14:666–683. doi: 10.1017/S1461145710000994. [DOI] [PubMed] [Google Scholar]
- 68.Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotrophin-releasing hormone receptor-2 displays anxiety-like behavior and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 69.Rybnikova EA, Pelto-Huikko M, Rakitskaya VV, Shalyapina VG. Localization of corticoliberin receptors in the rat brain. Neurosci Behav Physiol. 2003;33:399–404. doi: 10.1023/a:1022807926406. [DOI] [PubMed] [Google Scholar]
- 70.Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 71.Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptation to stress and impared cardiovascular function in mice lacking corticotrophin-releasing hormone receptor-2. Nat Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- 72.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 73.Skórzewska A, Bidziński A, Lehner M, Turzyńska D, Sobolewska A, Wisłowska-Stanek A, Maciejak P, Szyndler J, Płaźnik A. The localization of brain sites of anxiogenic-like effects of urocortin-2. Neuropeptides. 2011;45:83–92. doi: 10.1016/j.npep.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 74.Skórzewska A, Lehner M, Hamed A, Wisłowska-Stanek A, Turzyńska D, Sobolewska A, Płaźnik A. The effect of CRF2 receptor antagonists on rat conditioned fear responses and c-Fos and CRF expression in the brain limbic structures. Behav Brain Res. 2011;221:155–165. doi: 10.1016/j.bbr.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 75.Saavedra JM, Ando H, Armando I, Baiardi G, Bregonzio C, Juorio A, Macova M. Anti-stress and anti-anxiety effects of centrally acting angiotensin II AT1 receptor antagonists. Regul Pept. 2005;128:227–238. doi: 10.1016/j.regpep.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 76.Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov. 2011;10:685–697. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pavlatou MG, Mastorakos G, Lekakis I, Liatis S, Vamvakou G, Zoumakis E, Papassotiriou I, Rabavilas AD, Katsilambros N, Chrousos GP. Chronic administration of an angiotensin II receptor antagonist resets the hypothalamic-pituitary-adrenal (HPA) axis and improves the affect of patients with diabetes mellitus type 2: preliminary results. Stress. 2008;11:62–72. doi: 10.1080/10253890701476621. [DOI] [PubMed] [Google Scholar]
- 78.Häfner S, Baumert J, Emeny RT, Lacruz ME, Bidlingmaier M, Reincke M, Kuenzel H, Holle R, Rupprecht R, Ladwig KH for the MONICA/KORA Study Investigators. To live alone and to be depressed, an alarming combination for the renin-angiotensin-aldosterone-system (RAAS) Psychoneuroendocrinology. 2012;37:230–237. doi: 10.1016/j.psyneuen.2011.06.007. [DOI] [PubMed] [Google Scholar]