SUMMARY
Thrombospondin (Thbs) proteins are induced in sites of tissue damage or active remodeling. The endoplasmic reticulum (ER) stress response is also prominently induced with disease where it regulates protein production and resolution of misfolded proteins. Here we describe a novel function for Thbs’ as ER resident effectors of an adaptive ER stress response. Thbs4 cardiac-specific transgenic mice were protected from myocardial injury while Thbs4−/− mice were sensitized to cardiac maladaptation. Thbs induction produced a unique profile of adaptive ER stress response factors and expansion of the ER and downstream vesicles. The type-3 repeat domain in Thbs’ bind the ER luminal domain of activating transcription factor 6α (Atf6α) to promote its nuclear shuttling. Thbs4−/−mice failed to show activation of Atf6α and other ER stress response factors with injury, and Thbs4-mediated protection was lost when Atf6α was deleted. Hence, Thbs’ can function inside the cell during disease/remodeling to augment ER function and protect through a mechanism involving regulation of Atf6α.
INTRODUCTION
The endoplasmic-reticulum (ER) stress response / unfolded protein response (UPR) has been studied for nearly 2 decades, revealing a role in many forms of human disease (Glembotski, 2007; Zhang and Kaufman, 2008). Engagement of the ER stress response acutely reduces protein synthesis in the ER, enhances protein degradation of damaged or misfolded proteins, and selectively induces expression of protective proteins (Glembotski, 2007; Zhang and Kaufman, 2008). While acute activation of the ER stress response is protective, prolongation of the response typically leads to cell death in the heart and other tissues (Zhang and Kaufman, 2008; Hamada et al., 2004; Cook et al., 2009; Xu et al., 2009). The ER stress response is initiated within the ER lumen and membrane through the chaperone protein BiP (Grp78) that directly binds Ca2+ and senses unfolded or damaged proteins. BiP then aids activation of three primary pathways; PKR-like ER kinase (PERK), inositol-requiring enzyme 1α (IRE1α) and activating transcription factor 6 (Atf6). PERK activation at the ER membrane results in phosphorylation and inactivation of eukaryotic translation initiation factor 2α (eIF2α) to reduce protein synthesis but also to selectively induce expression of the transcription factor Atf4. The second branch involves IRE1α, which is a kinase and RNAase that mediates selective splicing of the message encoding the transcription factor Xbp1. Finally, the third branch involves Atf6, an ER membrane bound transcription factor that shuttles to the Golgi with ER stress stimulation where it is cleaved into an active form that translocates to the nucleus.
Five thrombospondins (Thbs) genes are present in mammals that encode secreted matricellular Ca2+-binding glycoproteins involved in diverse biologic processes given their ability to bind numerous proteins and serve as interaction platforms in the extracellular matrix (ECM) (Stenina et al., 2007; Kazerounian et al., 2008). Thbs1 and 2 comprise a subfamily that form homo-and hetero-trimers. They contain a procollagen binding domain, a type 1 repeat domain that binds transforming growth factor β (TGFβ), and domains that bind β1 integrins, calreticulin, and the CD36 and CD47 receptors (Kazerounian et al., 2008). In contrast, Thbs3/4/5 constitute a different structural subfamily that forms homo- and hetero-pentamers. This subfamily lacks the procollagen domain and type 1 repeats, and they are less than 50% homologous to Thbs1 and 2 with presumably divergent functions (Stenina et al., 2007; Kazerounian et al., 2008). All 5 Thbs gene family members appear capable of low-level ubiquitous expression in diverse tissues, although each is dramatically induced by injury, stress, or acute remodeling events (Kazerounian et al., 2008). For example, Thbs1, Thbs2, Thbs3 and Thbs4 are each induced in the heart in response to hypertrophy or infarction injury (Schroen et al., 2004; Stenina et al., 2007), and loss of Thbs1 or Thbs2 in gene-targeted mice promoted greater cardiac disease with aging or stress stimulation (Swinnen et al., 2009; Schroen et al., 2004; Frangogiannis et al., 2005). Thbs1 and Thbs4 reside within the ER, either indefinitely or for a period of time before being secreted outside the cell, depending on Ca2+ levels or the cell type that was examined (Veliceasa et al., 2007).
RESULTS
Thbs4 is an inducible ER/vesicular protein in the heart that is protective
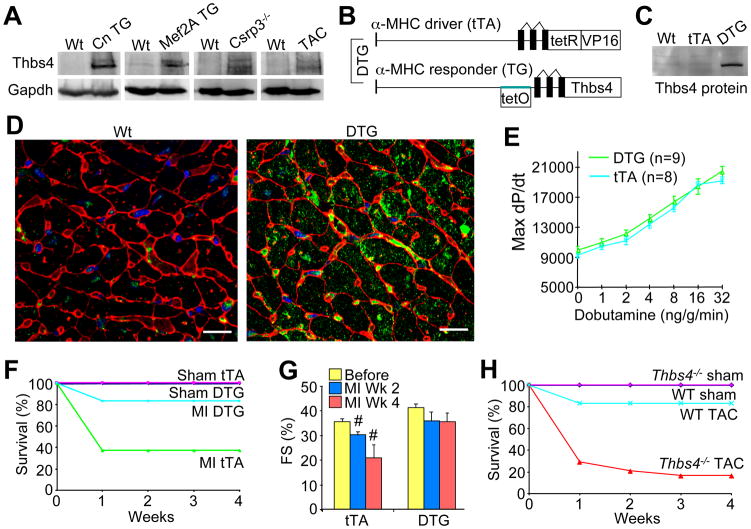
Thbs4 was identified as a cardiac induced gene in transgenic mouse models of heart disease, consistent with previous reports in the literature (Gabrielsen et al., 2007; Mustonen et al., 2008). Western blotting confirmed induction of Thbs4 protein in the hearts of transgenic mice expressing activated calcineurin (hypertrophic model), myocyte enhancer factor 2A (Mef2A, dilated cardiomyopathy), Csrp3−/− mice (muscle LIM protein gene, dilated cardiomyopathy) and after 2 weeks of cardiac pressure overload stimulation by transverse aortic constriction (TAC, Figure 1A). To model this induction of Thbs4 and examine its functional effect we generated 2 lines of Thbs4 overexpressing transgenic mice using a cardiac-specific binary tetracycline/doxycycline (Dox) regulated system in which double transgenic mice (DTG) express Thbs4 when Dox is removed from the diet (Sanbe et al., 2003) (Figure 1B). DTG mice (line 17.3) were removed from Dox at weaning to produce moderate expression of Thbs4 protein several weeks later in young adult hearts that approximated the level of endogenous Thbs4 induced in hearts of our disease models (Figure 1C).
Figure 1.
Thbs4 expression is up-regulated in diseased hearts and is protective. (A) Western blots for Thbs4 protein expression in different mouse models exhibiting cardiac disease. (B) Schematic diagram representing the binary transgenic system regulated by doxycycline to inducibly overexpress Thbs4 in the heart. (C) Representative western blot showing Thbs4 protein expression level in heart extracts from 4-month old DTG mice versus Wt and tTA only transgenic controls. (D) Immunohistochemistry for Thbs4 (green) in 4-month old mouse hearts. Red staining shows membranes and blue shows nuclei (scale bars = 10 μm). (E) Hemodynamic assessment of cardiac performance as maximal dP/dt in anesthetized, closed-chested mice in response to increasing doses of the β-adrenergic agonist dobutamine (measured in mmHg/s; +/− SD, N=6 mice each). (F) Survival plot for tTA and DTG mice following myocardial infarction (MI) injury (n = 10 male mice per group). (G) Cardiac ventricular fractional shortening (FS%) determined by echocardiography. At least 6 mice were analyzed in each group at 2 and 4 weeks after MI injury in the tTA control and DTG groups (#P<0.05 versus tTA before MI). (H) Survival plot in Thbs4−/− and Wt mice after pressure-overload by TAC (n = 10 male mice per group). Also See Figure S1 and S2
Immunohistochemical analysis of both endogenous or overexpressed Thbs4 from cardiac histological sections showed prominent localization within intracellular vesicles and the ER/SR compartment of cardiac myocytes, with some secretion into the extracellular space (Figure 1D, S1A and S1B). Importantly, Thbs4 was evenly distributed within the ER/SR compartment, and aggregation was not apparent by microscopy (Figure S1A and S1B). Induction of endogenous Thbs4 in the hearts of pressure-overloaded Wt mice also showed intracellular localization with minor accumulation in the extracellular space (Figure S1C). We also generated heart-specific Thbs1 overexpressing transgenic mice, which similarly showed intracellular ER localization of Thbs1 protein, without significant ECM association (Figure S1D). Overexpression of Thbs1 and 4 in skeletal muscle by transgenesis also showed primarily intracellular localization consistent with the ER/SR compartment (data not shown).
Adult Thbs4 DTG mice showed no cardiac functional or structural abnormalities associated with persistent overexpression during aging, or even with 12 weeks of pressure overload stimulation by TAC (Figure S2A–C). Assessment of cardiac ventricular performance by invasive hemodynamics also showed normal cardiac function and responsiveness to a β-adrenergic receptor agonist in Thbs4 DTG mice compared with single transgenic controls expressing the tetracycline transactivator protein (tTA, Figure 1E). Moreover, Thbs4 DTG mice were protected from lethality and maintained better cardiac function following MI injury compared with tTA controls (Figure 1F and 1G), and Thbs4 DTG mice showed no detriment after 12 weeks of TAC stimulation (Figure S2C). This effect was specific to the heart because Thbs4 skeletal muscle-specific overexpressing transgenic mice were not protected from MI injury (data not shown). Antithetically, Thbs4−/− mice were more susceptible to lethality and decompensation following TAC stimulation over 4 weeks (Figure 1H), as well as following MI injury (Figure S2D and S2E), further suggesting that Thbs4 is a beneficial or protective factor in the heart once induced. More recently, and in support of our observations, Thbs4−/− mice demonstrated greater cardiac decompensation with pressure overload stimulation (Cingolani et al., 2011).
Thbs4 induces a unique ER stress response signature in the heart
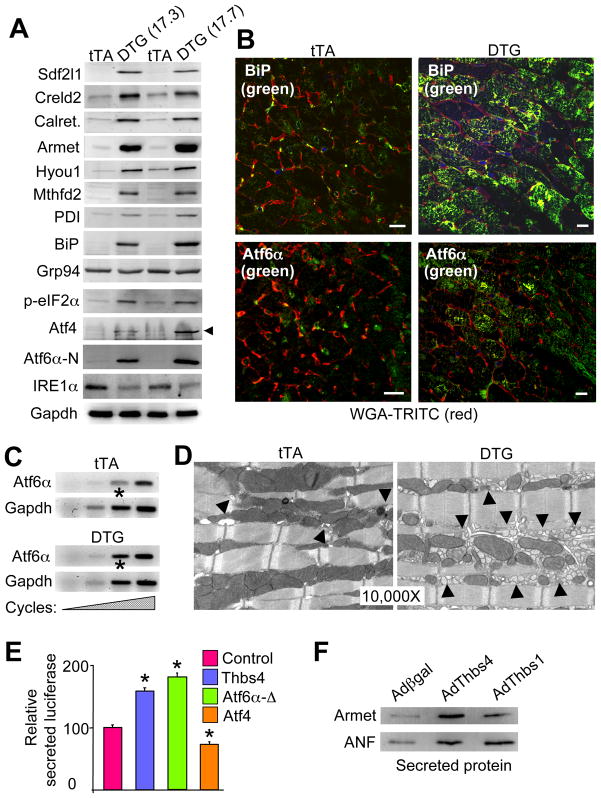
We performed a gene expression array analysis to examine why Thbs4 was cardioprotective, revealing a remarkable profile of induced ER chaperones and ER stress response genes (data not shown). Western blotting confirmed induction of BiP (Grp78), Sdf2l1, Creld2, calreticulin, Armet, Hyou1, Mthfd2, and PDI (Figure 2A). We also observed activation of the PERK pathway with phosphorylation of eIF2α and induction of downstream Atf4, robust induction/activation of Atf6α (cleaved nuclear form, N-terminus), and repression of IRE1α (Figure 2A). Downstream of IRE1α, we failed to observe activation or splicing of XBP-1 in Thbs4 DTG hearts or in cultured cardiomyocytes with various stressors (data not shown). Immunohistochemistry from cardiac tissue sections confirmed induction of BiP, Atf6α, calreticulin, Creld2 and Hyou1 proteins in the hearts of Thbs4 DTG mice compared with tTA controls (Figure 2B, and S3A), as well as induction of Atf6α mRNA in hearts or AdThbs4 infected neonatal cardiomyocytes in culture (Figure 2C, and data not shown). Interestingly, the induction and processing of Atf6α was unique amongst the membrane resident-cleaved bZIP transcription factor subfamily members, as we observed no induction or cleavage of Atf6β, Creb3L1, Crebl3L2, Creb3L3, Creb3L4 or Creb in Thbs4 hearts (Figure S3B). Thbs4 DTG hearts also showed no changes in the site 1 and site 2 proteases (S1P and S2P), Srebp1 and Srebp2, or insulin induced gene 1 and 2 (Insig1 and 2) (Figure S3B).
Figure 2.
Unique ER stress induction profile associated with Thbs4 expression. (A) Western blots for expression of ER stress response proteins from the hearts of two lines of Thbs4 DTG mice versus tTA control hearts. (B) Immunohistochemistry for Atf6α and BiP (green) in heart sections from tTA controls or Thbs4 DTG mice. Membranes of the cardiomyocytes are shown in (scale bars = 10 μm). (C) Increasing RT-PCR cycles for Atf6α mRNA in hearts of tTA control or Thbs4 DTG mice. Gapdh mRNA was used as a loading/normalization control. The asterisk shows increased Aft6α mRNA in the DTG hearts at the most linear cycle number in the RT-PCR reaction. (D) Transmission electron microscopy (EM) of heart sections from tTA control and Thbs4 DTG mice shows ER/SR and vesicles (arrowheads) in control and Thbs4 DTG hearts. (E) Luciferase activity in the media of NIH 3T3 cells transfected with a plasmid encoding a secreted version of luciferase that was co-transfected with Thbs4, Atf6α-Δ (constitutively nuclear) or Atf4 (all internally normalized to co-transfected β-gal plasmid). *P<0.05 versus the luciferase plasmid only. (F) Western blotting for the ER processed and secreted proteins Armet and ANF from concentrated media of neonatal rat cardiomyocytes previously infected with adenoviruses encoding β-galactosidase (control), Thbs4 or Thbs1. Also see Figure S3 and S4.
Ultrastructural analysis by transmission electron microscopy (EM) revealed a large expansion of the ER and post-ER vesicles in Thbs4 DTG hearts (Figure 2D). Consistent with this observation, Thbs4 overexpression by transfection in NIH 3T3 cells significantly increased secretory capacity, similar to increases observed with a constitutively nuclear mutant of Aft6α, while Atf4 overexpression suppressed secretion (Figure 2E). Indeed, adenoviral mediated overexpression of Thbs4 or Thbs1 in neonatal cardiomyocyte cultures increased Armet and atrial natriuretic factor (ANF) levels in the media (Figure 2F). Thus, Thbs4 induces Atf6α expression and processing to the activated form, it leads to mild PERK pathway activation, and it results in expansion of the ER compartment with greater vesicle content and secretory activity in the heart.
Thbs proteins are likely universal inducers of protective ER stress signaling
To determine if the ER effects observed with Thbs4 are a general feature of the Thbs family we also generated cardiac-specific (inducible) Thbs1 transgenic mice (Figure S4A and S4B). Remarkably, Thbs1 DTG mice showed a similar, albeit weaker pattern of ER stress response protein induction as observed in Thbs4 hearts (Figure S4C). Thbs1 overexpression also resulted in downregulation of its receptor CD47, but not CD36 (Figure S4C). To extend this paradigm outside the heart we also generated skeletal muscle-specific transgenic mice that overexpressed either Thbs4 or Thbs1 under control of the human skeletal α-actin promoter (Figure S4D and S4E). Remarkably, two separate lines each of Thbs1 and Thbs4 transgenic mice showed induction of the same ER stress response factors in skeletal muscle as observed in the heart (Figure S4D and S4E). These results suggest that induction of either the Thbs1/2 or Thbs3/4/5 subfamily members can mediate upregulation of a unique profile of protective ER stress response factors.
Thbs4 overexpression protects from protein aggregation-dependent cardiomyopathy
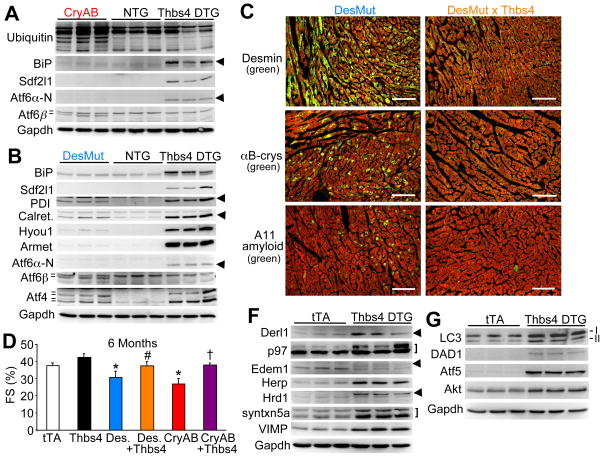
The heart is an ideal organ system for investigation of the ER stress response and the UPR given its susceptibility to protein aggregation-dependent cardiomyopathy (Wang and Robbins, 2006). For example, point mutations in the intermediate filament protein desmin or in αB-crystallin can lead to protein aggregation and disease in the heart, for which mouse models have been generated (Wang et al., 2001; Wang et al., 2001a). Separate transgenic mice expressing either a mutant αB-crystallin (CryAB) or desmin (DesMut) protein in the heart showed accumulation of ubiquitinated proteins and aggregation and deposition of endogenous desmin, αB-crystallin, and even amyloid formation in the heart (Figure 3A and 3C). Despite widespread protein aggregates and accumulation of ubiquitinated proteins, hearts from CryAB and DesMut transgenic mice showed mild or no upregulation of ER stress response genes compared with Thbs4 hearts, although CryAB and DesMut hearts did produce a unique signature of Atf6β protein processing (Figure 3A and 3B). By comparison, Thbs4 hearts showed no change in Atf6β processing, but strong Atf6α induction/activation (the cleaved nuclear form is shown, N-terminus), along with large induction of BiP, Armet and Sdf2l1 (Figure 3A and 3B). Atf4 protein was also differentially processed between Thbs4 hearts and DesMut hearts (different isoforms). Thus, Thbs4 produced a specific profile of ER stress response factor induction in the heart that was distinct from the profile observed in 2 models of protein misfolding and aggregation-based cardiomyopathy.
Figure 3.
Thbs4 induces a unique ER stress response signature that antagonizes protein aggregation and disease in the heart. (A) Western blots showing ER protein expression in αB-crystallin (CryAB) mutant or (B) desmin (DesMut) mutant hearts in comparison to non-transgenic (NTG) and Thbs4 DTG hearts. The hash marks or arrowheads show the different isoforms that were detected. (C) Immunohistochemistry of frozen heart sections from 6-month old mice of the indicated genotypes. Protein aggregation (green color) is dramatically reduced when Thbs4 is over-expressed. Red staining is for actin and shows the outline of cardiomyocytes (scale bars = 100 μm). (D) FS% as determined by echocardiography suggests that cardiac function is improved in both aggregation-prone cardiomyopathic transgenic mouse models when Thbs4 is overexpressed (N=6 or more mice in each group, *P<0.05 versus tTA; #P<0.05 versus DesMut; †P<0.05 versus CryAB). (F) Western blotting for proteins involved in ERAD from the hearts of Thbs4 DTG mice versus tTA control hearts. The arrowheads show the position of the relevant proteins, while the bracket shows 2 relevant bands. (G) Western blotting for proteins involved in autophagy (LC3, both I and II isoforms) or cellular protection. Also see Figure S5.
We next crossed Thbs4 DTG mice with both CryAB and DesMut mice, which strongly suggests that Thbs4 overexpression itself does not have an unspecific effect or predispose to protein aggregation. Indeed, Thbs4 overexpression rescued the appearance of protein aggregates in the hearts of CryAB and DesMut mice, as well as corrected their functional defect in ventricular performance from the ensuing cardiomyopathy at 6 months of age (Figure 3C and 3D).
In addition to simply enhancing ER functionality and induction of protective chaperones, the Thbs-dependent adaptive ER stress response was also associated with upregulated expression of key proteins underling the ER-associated protein degradation (ERAD) pathway in the heart (Figure 3F). For example, increased activity/expression of p97, Edem1, Hrd1 and related ERAD factors was observed, which are known to augment degradation and turnover of cytosolic proteins or aggregates, suggesting another mechanism of protection in CryAB and DesMut mice (Beskow et al., 2009; Kaneko et al., 2010). Thbs4 hearts also showed enhanced expression of other protective pathways, such as autophagy (LC3), and protection from cell death through upregulation of defender against apoptotic death (DAD1), Atf5, and Akt (Figure 3G).
Finally, we also instituted a separate cell culture-based model in which protein aggregation is induced by overexpression of a mutant cystic fibrosis transmembrane regulator (CFTR) protein (Kerbiriou et al., 2007). Wildtype CFTR protein is normally produced in the ER after which it is processed and trafficked to the membrane, while the mutant form of the protein forms ER aggregates and an ER stress response. Thbs4 co-overexpression reduced the appearance of intracellular aggregates due to CFTR508mut expression in primary MEFs (Figure S5).
Thbs4 protects from within the cell by shuttling through the ER
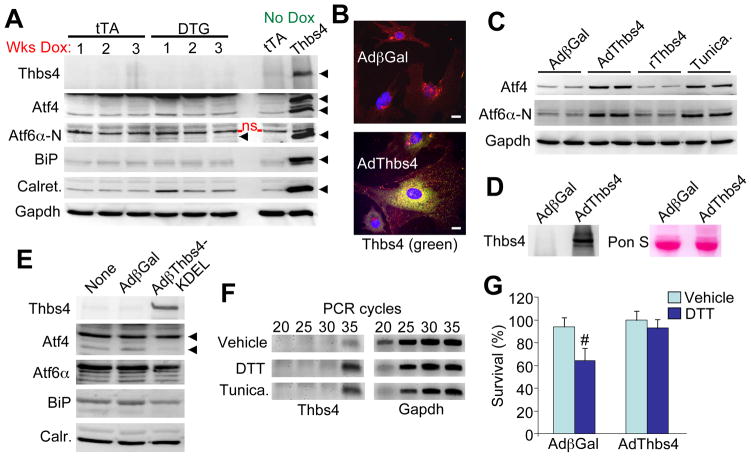
To further investigate Thbs4 signaling/function inside versus outside the cell we first examined Thbs4 DTG adult hearts in which expression was extinguished by feeding the mice Dox (Figure 4A). The rationale here is that cessation of Thbs4 expression would first extinguish intracellular regulatory effects, while any potential accumulation outside the cell should provide a more stable signal if it integrated into the ECM. However, within 1 week of Thbs4 protein shut-down, induction of BiP, Atf4 and Aft6α was lost, although a residual increase in calreticulin was observed that likely reflects initial ER expansion due to prior Thbs4 overexpression (Figure 4A). These results suggest that Thbs4 has its protective effect by events initiated from inside the cell and not through a stable reservoir in the ECM or some other aggregated source.
Figure 4.
Thbs4 functions from within the cell to promote adaptive ER stress response signaling. (A) Western blots of ER stress response protein expression in heart extracts from 3-month old tTA and DTG mice fed chow containing doxycycline for the indicated periods of time to extinguish Thbs4 expression. “No dox” controls are also shown and were generated from extracts of 4-month old DTG mice. An N-terminal Atf6α antibody from ProSci was used. The arrowheads so the specific band that was detected; ns = non-specific. (B) Immunocytochemistry for Thbs4 in adenovirus infected neonatal cardiomyocytes showing that Thbs4 (green) has an ER and Golgi staining pattern. Red staining is for membranes and blue stains the nuclei (scale bars = 10 μm). (C) Western blotting for Atf4 and Atf6α protein expression in neonatal rat cardiomyocytes infected for 24 hrs with recombinant adenovirus or treated with recombinant Thbs4 (2 μg/μL) or tunicamycin (1μg/μL). (D) Western blotting from media for Thbs4 after AdThbs4 infection versus Adβgal control in neonatal cardiomyocytes. Ponceau staining is a control for protein loading between the samples. (E) Western blotting for ER stress response proteins from neonatal cardiomyocytes following infection with a control (Adβgal) adenovirus or an adenovirus expressing Thbs4 fused with a KDEL ER retention/relocalization signal. (F) RT-PCR for Thbs4 or Gapdh in neonatal cardiomyocytes treated for 5 hours with ER stress inducers DTT (5μg/μL) or tunicamycin (10μg/μL). (G) AlamarBlue survival assay in DTT treated (8-hrs, 4μg/μL) neonatal cardiomyocytes infected with AdThbs4 or Adβgal (assay was run in duplicate in three separate experiments; #P<0.05 versus vehicle).
To further investigate the site of Thbs4 action we used a recombinant adenovirus to overexpress Thbs4 in cultured neonatal cardiomyocytes, which again demonstrated ER/Golgi staining, although secretion in the media was observed (Figure 4B and 4D). Induction of Aft4 and Atf6α (nuclear processed form) was only observed when cardiomyocytes were infected with AdThbs4, but not with recombinant Thbs4 supplied to the media or with conditioned media from prior AdThbs4 infected cardiomyocytes (Figure 4C, and data not shown). Tunicamycin, a known ER stress-inducing agent is shown as a control (Figure 4C).
We also engineered a recombinant version of Thbs4 that is retained in the ER using a retention/recycling signal in the primary sequence (KDEL). Remarkably, the ER resident version of Thbs4 was unable to induce Atf6α and the known profile of ER stress factor responsiveness observed with Wt Thbs4 (Figure 4E). This is consistent with Thbs4 functioning more directly on Atf6α as it translocates from the ER to the Golgi, then the nucleus once cleaved. The inability of the Thbs4-KDEL mutant to induce an ER stress response further suggests that the effects we observe with standard Thbs4 overexpression are not due to unspecific conditioning within the ER, or overloading/aggregation of Thbs4 protein in the ER.
The ER stress response generated by DTT or tunicamycin each induced endogenous Thbs4 expression in neonatal cardiomyocytes by RT-PCR analysis (Figure 4F). Importantly, overexpression of Thbs4 in neonatal cardiomyocytes was protective, similar to data obtained in the DTG mice, as less ER stress-dependent cell death was observed following DTT application (Figure 4G). Collectively, these results suggest that Thbs4 functions from within the cell as vesicles shuttle between the ER to Golgi and onwards, to induce an adaptive stress response that protects from cell death.
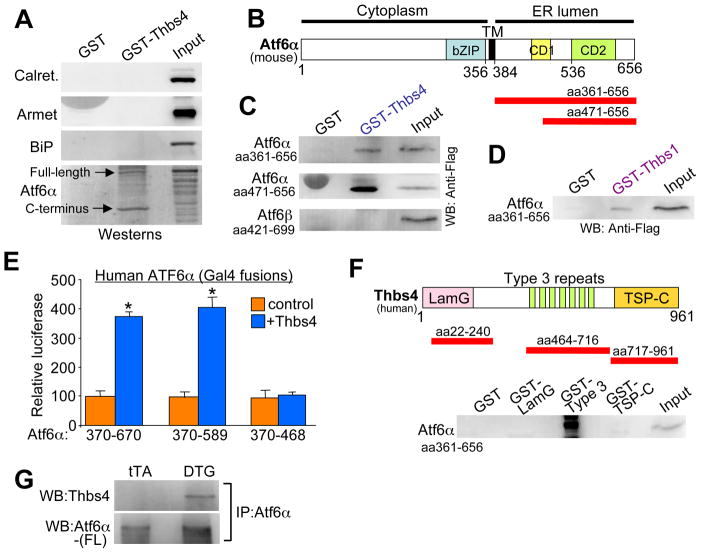
Thbs4 directly binds ATF6α to mediate its processing and activation
A GST-Thbs4 fusion protein was next generated to permit protein pull-downs followed by western blotting of suspected ER regulatory candidates from Thbs4 heart protein extracts. Thbs4 did not interact with calreticulin, Armet or BiP, but it did prominently interact with Atf6α, both the full-length form, and more preferentially, the C-terminus that corresponds to the ER luminal domain (Figure 5A and 5B). In vitro transcription-translation products of Atf6α corresponding to the luminal domain (aa #361-656) and a smaller more C-terminal region (aa #471-656) each bound GST-Thbs4 but not GST alone (Figure 5B and 5C). Importantly, the luminal domain of Atf6β did not bind GST-Thbs4, but GST-Thbs1 that similarly contains type 3 repeats also bound the Atf6α luminal domain (Figure 5C and 5D). Indeed, overexpression of Thbs1 in neonatal rat cardiomyocytes with a recombinant adenovirus also induced a similar profile of adaptive ER stress response factor expression as observed with Thbs4 (data not shown), similar to Thbs1 and Thbs4 DTG hearts.
Figure 5.
Thbs proteins directly interact with Atf6α. (A) Western blot following GST-Thbs4 pull-down of the indicated proteins from cardiac protein extracts from Thbs4 DTG hearts. A C-terminal Atf6α antibody from ProSci was used. (B) Mouse Atf6α schematic diagram showing relative size of the luminal domains used for GST pull-down experiments (red bars). (C) Western blot for tagged truncations of two different Atf6α luminal domains following GST-Thbs4 pull-down. The ER luminal domain of Atf6β did not interact with GST-Thbs4. There is a non-specific band in the GST (Atf6α 471–656) only lane due to extreme loading of purified GST. (D) Western blot for a tagged Atf6α luminal domain (amino acids 361–656) following GST-Thbs1 pull-down. (E) Gal4-dependent luciferase reporter activity with the luminal fragments of Atf6α (amino acid numbers shown) as fusions to full-length Gal4 transiently transfected into COS cells with or without Thbs4. The basal activity of each construct was set to 100, from which Thbs co-expression was compared. *P<0.05 versus no Thbs4 control. Results are averaged from 3 separate experiments. (F) Schematic diagram of human Thbs4 with the relative size of the GST fragments indicated (red bars). A western blot for Atf6α luminal domain following GST pull-down with the different Thbs4 domains is shown below the schematic. (G) Western blot for Thbs4 and Atf6α (FL, full-length is shown detected with Abcam N-terminal antibody) after Atf6α immunoprecipitation (with C-terminal ProSci antibody) from cardiac extracts of control tTA or Thbs4 DTG hearts.
We further refined the binding domain within human Atf6α using the Gal4 transcription factor fused to three different regions of the Atf6α luminal region. These Atf6α-Gal4 fusion constructs were transfected into HEK293 cells with a Gal4-dependent luciferase reporter, with or without a Thbs4 expression plasmid. Thbs4 co-expression enhanced Gal4 reporter activity of Atf6α fusions containing amino acids 370-670 and 370-589, but not 370-468, suggesting a minimal region of 468-589 as the interacting domain in Atf6α (Figure 5E). This region is not conserved within Atf6β. We also analyzed the domains in Thbs4 that might interact with the luminal domain of Atf6α using GST fusion fragments, which identified the calcium-binding type 3 repeat region (Figure 5F). Immunoprecipitation of full-length Aft6α from Thbs4 hearts, but not control tTA hearts, showed interaction with Thbs4 in vivo (Figure 5G). In conclusion, these results suggest a mechanism whereby Thbs conditions an adaptive ER stress response, in part, by interacting with Atf6α.
Thbs4−/− mice show defective induction of the ER stress response in the heart
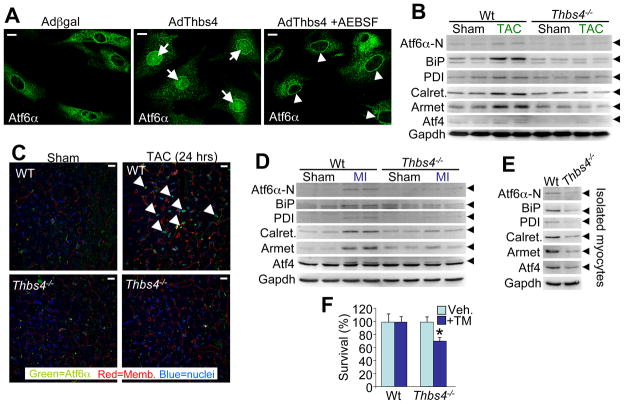
Overexpression of Thbs4 in neonatal rat fibroblasts also caused translocation and nuclear accumulation of endogenous Atf6α protein compared with very little nuclear localization of Atf6α in Adβgal infected controls (Figure 6A). This dramatic increase in nuclear content of Atf6α due to Thbs4 overexpression was prevented with AEBSF (Okada et al., 2003), a S1P specific protease inhibitor, showing that Thbs4-dependent activation of Atf6α occurs before nuclear translocation, likely in the Golgi (Figure 6A).
Figure 6.
Thbs4 is necessary for Atf6α activation in the heart. (A) Immunocytochemistry for Atf6α in neonatal rat fibroblasts with Adβgal (control) or AdThbs4 infection, with our without the S1P inhibitor AEBSF. The arrows show prominent nuclear localization of Atf6α, while the arrowheads show Atf6α retention in the Golgi and ER. (scale bars = 10 μm). (B) Western blots showing ER stress response protein expression in hearts of Wt and Thbs4−/− mice at baseline (sham) or after 48-hrs of TAC stress stimulation. Arrowheads show the indicated protein. (C) Immunohistochemistry for Atf6α following 24-hrs TAC or a sham surgical procedure. Nuclear localized Atf6α (arrowheads) was much more prominent in Wt hearts after TAC relative to Thbs4−/− hearts with TAC. (scale bars = 10 μm). (D) Western blots showing ER stress response protein expression in hearts of Wt and Thbs4−/− mice at baseline (sham) or 3-days after MI injury. (E) Western blot for ER stress response protein expression from isolated adult myocytes in temporary culture from Wt or Thbs4−/− hearts. (F) AlamarBlue survival assay in tunicamycin treated adult cardiomyocytes isolated from Wt or Thbs4−/−hearts (assay was run in duplicate; *P<0.05 versus vehicle). Also see Figure S6.
To gain further insight into the necessity of Thbs4 as a mediator of the ER stress response in the heart we further analyzed Thbs4−/− mice. Importantly, while four Thbs genes are expressed in the heart (1/2/3/4), Thbs4 is the most highly expressed compared with other tissues, and it appears to be of greatest inducibility in collective gene arrays from diseased hearts (Lawler et al., 1993; Lawler et al., 1995; and data not shown). As expected, hearts from surviving Thbs4−/− mice after 48-hrs of TAC stimulation showed defective Atf6α induction (nuclear form, N-terminus), with no or less induction of BiP, PDI, calreticulin, Armet, and Atf4 (Figure 6B). Indeed, the observed immediate translocation of Atf6α protein to the nucleus of hearts from Wt mice after 24 hrs of TAC was lost in Thbs4−/− mice (Figure 6C). These same ER stress factors were also induced 3 days after MI injury in Wt hearts, but again this induction was lost or blunted in Thbs4−/− mice (Figure 6D). We also enzymatically disassociated and cultured adult cardiomyocytes (4–8 hrs) from Wt and Thbs4−/−hearts, a process that spontaneously induced an ER stress response signature in the Wt cells, but much less so in the nulls (Figure 6E). Consistent with these observations, isolated myocytes from Thbs4−/− hearts in culture were more susceptible tunicamycin-induced killing than were isolated adult cardiac myocytes from Wt control mice (Figure 6F). Collectively, these results suggest that induction of Thbs4 protein in the mouse heart is required to fully induce the adaptive ER stress response.
Mechanism of Thbs4-mediated adaptive ER stress signaling
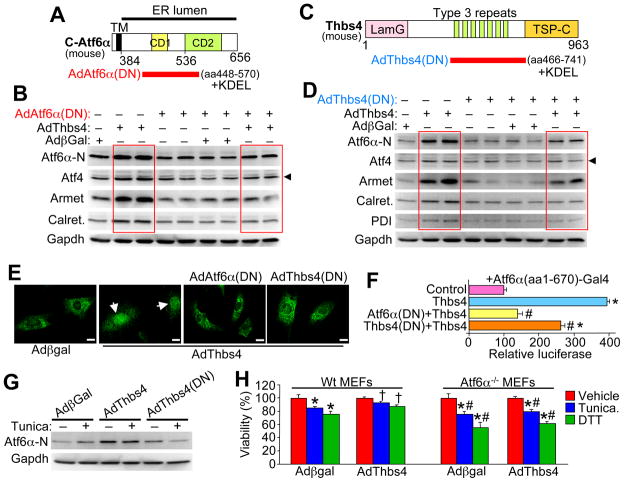
To probe more deeply into the mechanism whereby Thbs might activate Atf6α we generated a recombinant adenovirus expressing amino acids 448–570 of mouse Atf6α that contained the minimal Thbs4 binding domain to act as a dominant negative (Figure 7A). A signal and KDEL sequence were included to ensure proper residence within the ER compartment. Infection with AdAtf6α-dominant negative (DN) blocked the ability of co-infected AdThbs4 to fully induce nuclear Atf6α, as well as protein induction of Atf4, Armet, and calreticulin in cultured neonatal cardiomyocytes (Figure 7B). We also generated a dominant negative, ER-retained, Thbs4 mutant encoding only the type 3 repeat region that binds Atf6α (Figure 7C). Similar to the Atf6α(DN) construct, AdThbs4(DN) attenuated the ability of AdThbs4 co-infection to fully upregulate expression of Atf6α, Aft4, Armet, calreticulin, and PDI in cultured neonatal cardiomyocytes (Figure 7D). Consistent with these results, both AdAtf6α(DN) and AdThbs4(DN) blocked Thbs4-induced nuclear translocation and nuclear accumulation of endogenous nuclear/cleaved Atf6α (N-terminus) in primary fibroblast cultures (Figure 7E). Atf6α(DN) and Thbs4(DN) also blocked or significantly reduced that activity of Atf6α-Gal4 for a Gal4-dependent luciferase reporter as stimulated by Thbs4 co-transfection in HEK293 cells (Figure 7F). Finally, overexpression of Thbs4(DN) blocked tunicamycin stress-induced activation of endogenous Atf6α (nuclear form) in cultured neonatal cardiomyocytes (Figure 7G). Taken together, these results suggest that Thbs4 directly regulates Atf6α as a specific mechanism in mediating the adaptive ER stress response.
Figure 7.
Thbs4-Atf6α interaction is necessary for Atf6α function in ER stress induction in cardiomyocytes. (A) Schematic diagram of the region of mouse Atf6α that was used to make the dominant negative or decoy adenovirus (red bar). (B) Western blotting for the indicated proteins from neonatal cardiomyocytes infected with the indicated recombinant adenoviruses. The red-boxed areas show induction of ER stress response proteins by Thbs4 overexpression, which is attenuated with the Atf6α-dominant negative expressing adenovirus. (C) Schematic diagram of the region of Thbs4 that was used to make the dominant negative or decoy adenovirus (red bar). (D) Western blotting for the indicated proteins from neonatal cardiomyocytes infected with the indicated recombinant adenoviruses. The red-boxed areas show induction of ER stress response proteins by Thbs4 overexpression, which is attenuated with the Thbs4-dominant negative expressing adenovirus. (E) Immunocytochemistry for endogenous Atf6α in cultured neonatal rat fibroblasts 24 hrs after infection with the indicated adenoviruses. The arrows show prominent nuclear localization of endogenous Atf6α by AdThbs4 infection, which was blocked with either AdAtf6α(DN) or AdThbs4 (DN). (scale bars = 10 μm). (F) Gal4-dependent luciferase reporter assay with full-length human Atf6α-Gal4 that was co-transfected in HEK293 cells with combinations of plasmids encoding Thbs4 with either Atf6α(DN) or Thbs4(DN). Results were averaged from 3 independent experiments. *P<0.05 versus reporter alone (control); #P<0.05 versus Thbs4. (G) Western blotting for Atf6α (activated form) or Gapdh (control) in neonatal cardiomyocytes infected with the indicated adenoviruses with or without tunicamycin. The dominant negative Thbs4 construct prevents activation of endogenous Atf6α in response to tunicamycin treatment. (H) AlamarBlue survival assay in Wt or Atf6a−/− MEFs with or without AdThbs4 infection. Assay was performed in triplicate. *P<0.05 versus no drug; #P<0.05 versus Wt MEFs with AdThbs4. †P<0.05 versus Wt MEFs with Adβgal with same drug treatment. Also see Figure S6.
To extend the results obtained with dominant negative Thbs4 and Atf6α, we also utilized Aft6α null MEFs. If Atf6α is truly the primary mechanism for mediating the protective ER stress response profile downstream of Thbs4, its absence should reduce Thbs4-dependent protection. Indeed, Wt MEFs treated with DTT or tunicamycin showed enhanced cell death, which was significantly reduced by AdThbs4 infection (Figure 7H). However, Atf6α null MEFs, which showed even greater killing with DTT and tunicamycin compared with Wt MEFs, were not protected with AdThbs4 infection, suggesting that Atf6α is a primary mediator underlying Thbs4-dependent protection from ER stress-associated cell death (Figure 7H).
DISCUSSION
Once induced in select tissues, Thbs protein expression can be observed directly within cells or between cells in the extracellular space (Chen et al., 2000; Sodersten et al., 2007; Veliceasa et al., 2007; Greco et al., 2010). We observed prominent Thbs4 protein localization within the ER/SR compartment of adult cardiomyocytes of the heart, with some mild accumulation in the extracellular space or ECM. Indeed, while Thbs’ are generally secreted, examples of their intracellular retention in the ER or vesicles have been reported (Chen et al., 2000; Sodersten et al., 2007; Veliceasa et al., 2007; Hauser et al., 1995; Greco et al., 2010). Thbs’ are assembled into trimers (Thbs1 and 2) or pentamers (Thbs3, 4, and 5) in the ER, and Thbs1 was shown to be part of a complex with PDI, BiP, p72 and Grp94, and to reside in the ER compartment for up to 30 minutes before presumably shuttling to the Golgi for gylcosylation (Prabakaran et al., 1996; Kuznetsov, 1997; Hecht et al., 2001). More provocatively, Thbs1 is retained in the ER in response to low calcium levels in renal cell carcinoma tissue and cultured cells, while restoration of ER calcium permitted Thbs1 secretion (Veliceasa et al., 2007). Thus, Thbs’ could easily have a primary function in the ER or other associated intracellular vesicular compartments, either as they are being produced in complexes with known ER stress chaperones or ECM proteins, or if they maintain residence in the ER long-term, as regulators of the ER stress response in conjunction with calcium sensing.
Here we identified a novel role for Thbs in the ER stress response to presumably enhance the ability of a cell to secrete proteins, properly re-construct the ECM after injury, and deal with unfolded proteins during disease. We determined that the type 3 repeat domain in Thbs4 directly binds to Atf6α to facilitate its activation (Figure S6). All 5 Thbs contain this calcium-binding type 3 repeat domain, although its function remains essentially unknown (Kazerounian et al., 2008). Here we propose a working hypothesis that accounts for induction of all 5 Thbs genes during injury or tissue remodeling, incorporates the fact that these proteins are resident in the ER-vesicular network, and that builds from our new observations related to Atf6α activation by Thbs4 (Figure S6). We propose that the type 3 repeat domain in Thbs’ activates Atf6α to upregulate the protective ER stress response. Increased Atf6α activity is known to expand the ER compartment and upregulate other ER stress response effectors and chaperones (Adachi et al., 2008; Bommiasamy et al., 2009). In addition, a previously published mRNA expression analysis from mice containing a tamoxifen-inducible activated Atf6α transgene for heart-specific expression showed a very similar profile of enhanced ER stress response genes as our microarrays from Thbs4 DTG hearts, suggesting that Atf6α is the primary mediator of Thbs effects in the heart (Belmont et al., 2008; Martindale et al., 2006). It is also interesting to speculate that Thbs4 might be more evolutionarily divergent as a dedicated mediator of this adaptive ER stress/conditioning effect with injury compared with Thbs1 and 2. Indeed, loss of Thbs4−/− in the mouse compromised the ER stress response activation profile that is normally observed with acute injury to the heart, despite known induction and presence of Thbs1 and Thbs2 with cardiac injury (Schellings et al., 2009).
A critical observation that supports the importance and function of the type 3 repeat domain comes from known mutations in COMP (encodes Thbs5 protein) that causes genetic diseases resulting in skeletal dysplasia (Briggs et al., 1995; Hecht et al., 1995). Such mutations in COMP occur within the type 3 repeat domain that appears to affect its function and ability to traverse the ER compartment (Hecht et al., 2001; Hashimoto et al., 2003). Indeed mutations in this type 3 repeat region of COMP cause its retention in the ER, co-retention of type IX collagen and aggrecan, sequestration of several ER stress chaperones, and enlargement of the rER compartment (Hecht et al., 1998; Delot et al., 1999; Vranka et al., 2001; Chen et al., 2007). The inability of the mutant Thbs5 protein to leave the ER and be secreted causes a maladaptive ER stress response leading to cellular apoptosis (Hashimoto et al., 2003). These results suggest that the type 3 repeat domain is critical for proper ER conditioning and processing of Thbs proteins once generated, which also directly affects overall ER function and the ability to properly produce and secrete other ECM proteins. These results also suggest that Thbs proteins may have a chaperone-like function for processing large ECM-fated proteins for their construction outside the cell after injury. Interestingly, this type 3 repeat domain in Thbs’ binds calcium where is could also serve to sense the health of the ER environment. Indeed, reductions in ER calcium are known to cause Thbs1 ER retention and lack of secretion (Veliceasa et al., 2007).
While Thbs4 produced a protective ER stress response through Atf6α activation, we cannot exclude other protective functions of Thbs4 in the ER or associated vesicles that might be separate from Atf6α. Indeed, proteins that underlie ERAD were specifically upregulated in Thbs4-DTG hearts. Another possibility is that Thbs4 itself might function to enhance vesicular trafficking through the secretory pathway to help move more traditional ECM targeted proteins outside the cell. Indeed, while the ER was expanded in Thbs4 DTG hearts, we also observed a large increase in total vesicles throughout the cytosol and near the plasma membranes of cardiomyocytes, suggesting enhanced trafficking. We also observed increased markers of autophagy (LC3) and signs of greater autophagic vesicles by EM, especially in Thbs1 DTG hearts (Figure 3G, and data not shown). However, it is also possible that simply mobilizing Atf6α mediates all or much of the protective and adaptive ER functions observed in Thbs4 DTG hearts, especially given the results with the decoy pieces of Thbs4 (type 3 repeat domain) and the dominant negative luminal portion of Aft6α. While PERK and Atf4 were mildly activated in Thbs4 DTG hearts or by AdThbs4 infection in neonatal cardiomyocytes, this effect could also be due to a primary change in Atf6α, which secondarily changes BiP levels or other ER stress regulatory factors to possibly favor PERK activation. IRE1α was noticeably down-regulated by Thbs4 overexpression, which again could be due to adaptive secondary changes associated with Atf6α.
Our results show a unique paradigm whereby these Thbs proteins contain within their primary sequence all the necessary domains for ER adaptation and greater construction or turnover of the ECM with injury or disease. Thbs proteins contain a highly conserved domain that functions to condition the ER when expressed through a direct effect on Atf6α that results in expansion of the ER for greater capacity, along with more protective chaperones that aid in generation of complex ECM proteins. Thbs proteins also bind various ECM proteins where they likely facilitate their processing and eventual extrusion into the ECM, after which Thbs proteins are likely degraded. Such built in heterofunctionality allows inherent coordination of extracellular milieu content or dynamic extracellular replacement events with increased functioning of the ER and vesicular trafficking. Such conditions most likely arise after injury in adult tissues or during active developmental remodeling, or when ER calcium levels are altered during chronic disease.
EXPERIMENTAL PROCEDURES
Animals
Mice were produced by transgenesis to overexpress Thbs4 (and Thbs1) in the heart using a regulated binary system based on the tetracycline transactivator protein and the tet-operator in a modified α-myosin heavy chain promoter (cardiac-specific). All transgenic mice were in the FVB/N background while Thbs4−/− mice were obtained from Jackson laboratories and were in the C57BL/6 background.
Western blotting, immunohistochemistry and immunocytochemistry
Hearts from mice were processed according to routine protocols to permit immunohistochemistry and processing for protein extracts for western blotting. Cultured neonatal rat cardiomyocytes, primary fibroblasts, NIH 3T3, COS7, and HEK293 cells were also used throughout the study for mechanistic experiments with or without adenoviral infection to manipulate expression of Thbs1, Thbs4, Atf6α, or truncation mutants of Atf6α and Thbs4 that served as dominant negatives. Cells were fixed with cold methanol followed by 1-minute permeabilization in cold acetone, washed, processed and incubated with primary antibody at typical dilutions of 1:100. Dilutions for immunohistochemistry were 1:200 to 1:1000.
Cell culture, adenovirus infection, and RT-PCR
Primary neonatal rat cardiomyocytes were prepared from 1- to 2-day-old Sprague-Dawley rat pups and plated at 1.5 × 106 cells per 10 cm dish in 2% bovine growth serum. Cardiomyocytes or MEFs were infected with adenovirus for 2 hours and then fresh media applied. Cells were harvested 24 to 72 hours post-infection. For reverse transcriptase PCR (RT-PCR), RNA was isolated from either tissue or cells using the Qiagen fibrous tissue kit coupled with the Qiagen QIAShredder according to manufacturer instructions. Invitrogen SuperScript III One-Step RT-PCR system then used to make cDNA before PCR.
Transfections, Luciferase assays, GST pull down, and electron microscopy
FuGENE6 was utilized for transfection of COS7, NIH 3T3, and HEK293 cells according to manufacturer instructions. The 9xGAL4-luciferase reporter was co-transfected with pSV-β-galactosidase control vector to measure Gal4-Atf6α fusion domain function with Thbs4 overexpression. Forty-eight-hours post-transfection cells were harvested in lysis buffer and standard extracts generated to measure luciferase activity. GST pull-down assays were used to investigate Thbs1 and Thbs4 interaction with Atf6α. For these experiments, a GST-Thbs4 fusion plasmid was transformed into BL21 competent E. coli and the fusion protein was isolated for pull-downs with TNT lysate generated experimental proteins of interest. Electron microscopy was used to visualize ultrastructural features in the hearts of Thbs4 DTG mice using standard processing and imaging. A full detailed description of the experimental procedures can be found in the Supplemental Information.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (J.D.M., J.R., M.M., J.La. [HL049081], J.N.L., R.P.) and the Howard Hughes Medical Institute (to J.D.M.). We would like to thank Allen York, Mark Duquette and Zeenat Jamal for excellent technical assistance in conducting this study.
Footnotes
Supplementary information includes 6 figures and an Extended Experimental Methods sectio that can be found with this article online at:
Conflict of interest: None (no competing financial interests).
References
- Adachi Y, Yamamoto K, Okada T, Yoshida H, Harada A, Mori K. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struc Func. 2008;33:75–89. doi: 10.1247/csf.07044. [DOI] [PubMed] [Google Scholar]
- Belmont PJ, Tadimalla A, Chen WJ, Martindale JJ, Thuerauf DJ, Marcinko M, Gude N, Sussman MA, Glembotski CC. Coordination of growth and endoplasmic reticulum stress signaling by regulator of calcineurin 1 (RCAN1), a novel ATF6-inducible gene. J Biol Chem. 2008;283:14012–14021. doi: 10.1074/jbc.M709776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beskow A, Grimberg KB, Bott LC, Salomons FA, Dantuma NP, Young P. A conserved unfoldase activity for the p97 AAA-ATPase in proteasomal degradation. J Mol Biol. 2009;394:732–746. doi: 10.1016/j.jmb.2009.09.050. [DOI] [PubMed] [Google Scholar]
- Bommiasamy H, Back SH, Fagone P, Lee K, Meshinchi S, Vink E, Sriburi R, Frank M, Jackowski S, Kaufman RJ, Brewer JW. ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J Cell Sci. 2009;122:1626–1636. doi: 10.1242/jcs.045625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs MD, Hoffman SM, King LM, Olsen AS, Mohrenweiser H, Leroy JG, Mortier GR, Rimoin DL, Lachman RS, Gaines ES, Cekleniak JA, Knowlton RG, Cohn DH. Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat Genet. 1995;10:330–336. doi: 10.1038/ng0795-330. [DOI] [PubMed] [Google Scholar]
- Chen FH, Herndon ME, Patel N, Hecht JT, Tuan RS, Lawler J. Interaction of cartilage oligomeric matrix protein/thrombospondin 5 with aggrecan. J Biol Chem. 2007;282:24591–24598. doi: 10.1074/jbc.M611390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Zhao P, Borup R, Hoffman EP. Expression profiling in the muscular dystrophies: identification of novel aspects of molecular pathophysiology. J Cell Biol. 2000;151:1321–1336. doi: 10.1083/jcb.151.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani OH, Kirk JA, Seo K, Koitabashi N, Lee D, Ramirez-Correa G, Bedja D, Barth AS, Moens AL, Kass DA. Thromobospondin-4 is required for stretch-mediated contractility augmentation in cardiac muscle. Circ Res. 2011;109:1410–1414. doi: 10.1161/CIRCRESAHA.111.256743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook AR, Bardswell SC, Pretheshan S, Dighe K, Kanaganayagam GS, Jabr RI, Merkle S, Marber MS, Engelhardt S, Avkiran M. Paradoxical resistance to myocardial ischemia and age-related cardiomyopathy in NHE1 transgenic mice: a role for ER stress? J Mol Cell Cardiol. 2009;46:225–233. doi: 10.1016/j.yjmcc.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Délot E, King LM, Briggs MD, Wilcox WR, Cohn DH. Trinucleotide expansion mutations in the cartilage oligomeric matrix protein (COMP) gene. Hum Mol Genet. 1999;8:123–128. doi: 10.1093/hmg/8.1.123. [DOI] [PubMed] [Google Scholar]
- Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML. Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111:2935–2942. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- Gabrielsen A, Lawler PR, Yongzhong W, Steinbrüchel D, Blagoja D, Paulsson-Berne G, Kastrup J, Hansson GK. Gene expression signals involved in ischemic injury, Extracellular matrix composition and fibrosis defined by global mRNA profiling of the human left ventricular myocardium. J Mol Cell Cardiol. 2007;42:870–83. doi: 10.1016/j.yjmcc.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Glembotski CC. Endoplasmic reticulum stress in the heart. Circ Res. 2007;101:975–984. doi: 10.1161/CIRCRESAHA.107.161273. [DOI] [PubMed] [Google Scholar]
- Greco SA, Chia J, Inglis KJ, Cozzi SJ, Ramsnes I, Buttenshaw RL, Spring KJ, Boyle GM, Worthley DL, Leggett BA, Whitehall VL. Thrombospondin-4 is a putative tumour-suppressor gene in colorectal cancer that exhibits age-related methylation. BMC Cancer. 2010;10:494. doi: 10.1186/1471-2407-10-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Suzuki M, Yuasa S, Mimura N, Shinozuka N, Takada Y, Suzuki M, Nishino T, Nakaya H, Koseki H, Aoe T. Dilated cardiomyopathy caused by aberrant endoplasmic reticulum quality control in mutant KDEL receptor transgenic mice. Mol Cell Biol. 2004;24:8007–8017. doi: 10.1128/MCB.24.18.8007-8017.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Tomiyama T, Yamano Y, Mori H. Mutation (D472Y) in the type 3 repeat domain of cartilage oligomeric matrix protein affects its early vesicle trafficking in endoplasmic reticulum and induces apoptosis. Am J Pathol. 2003;163:101–110. doi: 10.1016/S0002-9440(10)63634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser N, Paulsson M, Kale AA, DiCesare PE. Tendon extracellular matrix contains pentameric thrombospondin-4 (TSP-4) FEBS Lett. 1995;17:307–310. doi: 10.1016/0014-5793(95)00675-y. [DOI] [PubMed] [Google Scholar]
- Hecht JT, Deere M, Putnam E, Cole W, Vertel B, Chen H, Lawler J. Characterization of cartilage oligomeric matrix protein (COMP) in human normal and pseudoachondroplasia musculoskeletal tissues. Matrix Biol. 1998;17:269–278. doi: 10.1016/s0945-053x(98)90080-4. [DOI] [PubMed] [Google Scholar]
- Hecht JT, Hayes E, Snuggs M, Decker G, Montufar-Solis D, Doege K, Mwalle F, Poole R, Stevens J, Duke PJ. Calreticulin, PDI, Grp94 and BiP chaperone proteins are associated with retained COMP in pseudoachondroplasia chondrocytes. Matrix Biol. 2001;20:251–262. doi: 10.1016/s0945-053x(01)00136-6. [DOI] [PubMed] [Google Scholar]
- Hecht JT, Nelson LD, Crowder E, Wang Y, Elder FF, Harrison WR, Francomano CA, Prange CK, Lennon GG, Deere M, Lawler J. Mutations in exon 17B of cartilage oligomeric matrix protein (COMP) cause pseudoachondroplasia. Nat Genet. 1995;10:325–329. doi: 10.1038/ng0795-325. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Koike H, Saito R, Kitamura Y, Okuma Y, Nomura Y. Loss of HRD1–mediated protein degradation causes amyloid precursor protein accumulation and amyloid-beta generation. J Neurosci. 2010;30:3924–3932. doi: 10.1523/JNEUROSCI.2422-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazerounian S, Yee KO, Lawler J. Thrombospondins in cancer. Cell Mol Life Sci. 2008;65:700–712. doi: 10.1007/s00018-007-7486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbiriou M, Le Drévo MA, Férec C, Trouvé P. Coupling cystic fibrosis to endoplasmic reticulum stress: Differential role of Grp78 and ATF6. Biochim Biophys Acta. 2007;1772:1236–1249. doi: 10.1016/j.bbadis.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Kuznetsov G, Chen LB, Nigam SK. Multiple molecular chaperones complex with misfolded large oligomeric glycoproteins in the endoplasmic reticulum. J Biol Chem. 1997;272:3057–3063. doi: 10.1074/jbc.272.5.3057. [DOI] [PubMed] [Google Scholar]
- Lawler J, Duquette M, Whittaker CA, Adams JC, McHenry K, DeSimone DW. Identification and characterization of thrombospondin-4, a new member of the thrombospondin gene family. J Cell Biol. 1993;120:1059–1067. doi: 10.1083/jcb.120.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J, McHenry K, Duquette M, Derick L. Characterization of human thrombospondin-4. J Biol Chem. 1995;270:2809–2814. doi: 10.1074/jbc.270.6.2809. [DOI] [PubMed] [Google Scholar]
- Martindale JJ, Fernandez R, Thuerauf D, Whittaker R, Gude N, Sussman MA, Glembotski CC. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res. 2006;98:1186–1193. doi: 10.1161/01.RES.0000220643.65941.8d. [DOI] [PubMed] [Google Scholar]
- Mustonen E, Aro J, Puhakka J, Ilves M, Soini Y, Leskinen H, Ruskoaho H, Rysä J. Thrombospondin-4 expression is rapidly upregulated by cardiac overload. Biochem Biophys Res Commun. 2008;373:186–191. doi: 10.1016/j.bbrc.2008.05.164. [DOI] [PubMed] [Google Scholar]
- Okada T, Haze K, Nadanaka S, Yoshida H, Seidah NG, Hirano Y, Sato R, Negishi M, Mori K. A serine protease inhibitor prevents endoplasmic reticulum stress-induced proteolysis but not transport of the membrane-bound transcription factor ATF6. J Biol Chem. 2003;278:31024–31032. doi: 10.1074/jbc.M300923200. [DOI] [PubMed] [Google Scholar]
- Prabakaran D, Kim PS, Dixit VM, Arvan P. Oligomeric assembly of thrombospondin in the endoplasmic reticulum of thyroid epithelial cells. Eur J Cell Biol. 1996;70:134–141. [PubMed] [Google Scholar]
- Sanbe A, Gulick J, Hanks MC, Liang Q, Osinska H, Robbins J. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ Res. 2003;92:609–616. doi: 10.1161/01.RES.0000065442.64694.9F. [DOI] [PubMed] [Google Scholar]
- Schellings MW, van Almen GC, Sage EH, Heymans S. Thrombospondins in the heart: potential functions in cardiac remodeling. J Cell Commun Signal. 2009;3:201–213. doi: 10.1007/s12079-009-0070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroen B, Heymans S, Sharma U, Blankesteijn WM, Pokharel S, Cleutjens JP, Porter JG, Evelo CT, Duisters R, van Leeuwen RE, Janssen BJ, Debets JJ, Smits JF, Daemen MJ, Crijns HJ, Bornstein P, Pinto YM. Thrombospondin-2 is essential for myocardial matrix integrity: increased expression identifies failure-prone cardiac hypertrophy. Circ Res. 2004;95:515–522. doi: 10.1161/01.RES.0000141019.20332.3e. [DOI] [PubMed] [Google Scholar]
- Södersten F, Ekman S, Niehoff A, Zaucke F, Heinegård D, Hultenby K. Ultrastructural immunolocalization of cartilage oligomeric matrix protein, thrombospondin-4, and collagen fibril size in rodent achilles tendon in relation to exercise. Connect Tissue Res. 2007;48:254–262. doi: 10.1080/03008200701587505. [DOI] [PubMed] [Google Scholar]
- Stenina OI, Topol EJ, Plow EF. Thrombospondins, their polymorphisms, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2007;27:1886–1894. doi: 10.1161/ATVBAHA.107.141713. [DOI] [PubMed] [Google Scholar]
- Swinnen M, Vanhoutte D, Van Almen GC, Hamdani N, Schellings MW, D’hooge J, Van der Velden J, Weaver MS, Sage EH, Bornstein P, Verheyen FK, VandenDriessche T, Chuah MK, Westermann D, Paulus WJ, Van de Werf F, Schroen B, Carmeliet P, Pinto YM, Heymans S. Absence of thrombospondin-2 causes age-related dilated cardiomyopathy. Circulation. 2009;120:1585–1597. doi: 10.1161/CIRCULATIONAHA.109.863266. [DOI] [PubMed] [Google Scholar]
- Veliceasa D, Ivanovic M, Hoepfner FT-S, Thumbikat P, Volpert OV, Smith ND. Transient receptor potential channel 4 controls thrombospondin-1 secretion and angiogenesis in renal cell carcinoma. FEBS let. 2007;274:6365–6377. doi: 10.1111/j.1742-4658.2007.06159.x. [DOI] [PubMed] [Google Scholar]
- Vranka J, Mokashi A, Keene DR, Tufa S, Corson G, Sussman M, Horton WA, Maddox K, Sakai L, Bächinger HP. Selective intracellular retention of extracellular matrix proteins and chaperones associated with pseudoachondroplasia. Matrix Biol. 2001;20:439–450. doi: 10.1016/s0945-053x(01)00148-2. [DOI] [PubMed] [Google Scholar]
- Wang X, Robbins J. Heart failure and protein quality control. Circ Res. 2006;99:1315–1328. doi: 10.1161/01.RES.0000252342.61447.a2. [DOI] [PubMed] [Google Scholar]
- Wang X, Osinska H, Dorn GW, 2nd, Nieman M, Lorenz JN, Gerdes AM, Witt S, Kimball T, Gulick J, Robbins J. Mouse model of desmin-related cardiomyopathy. Circulation. 2001a;103:2402–2407. doi: 10.1161/01.cir.103.19.2402. [DOI] [PubMed] [Google Scholar]
- Wang X, Osinska H, Klevitsky R, Gerdes AM, Nieman M, Lorenz J, Hewett T, Robbins J. Expression of R120G-alphaB-crystallin causes aberrant desmin and alphaB-crystallin aggregation and cardiomyopathy in mice. Circ Res. 2001;89:84–91. doi: 10.1161/hh1301.092688. [DOI] [PubMed] [Google Scholar]
- Xu J, Wang G, Wang Y, Liu Q, Xu W, Tan Y, Cai L. Diabetes- and angiotensin II-induced cardiac endoplasmic reticulum stress and cell death: metallothionein protection. J Cell Mol Med. 2009;13:1499–1512. doi: 10.1111/j.1582-4934.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.