Abstract
Reactive oxygen species (ROS) play important roles in the occurrence and development in diabetic cardiomyopathy (DC). Ferulic acid is one of the ubiquitous compounds in diet. Sodium ferulate (SF) is its sodium salt. SF has potent free radical scavenging activity and can effectively scavenge ROS. The study investigated the effect of SF on cardioprotection in diabetic rats. The diabetic rats induced by streptozotocin (STZ) were treated with SF (110mg/kg) by gavage per day for 12 weeks. Results showed that the levels of nitric oxide (NO) and superoxide dismutase (SOD) activity in plasma and myocardium in SF-treated group were significantly higher than those in diabetic control group. The levels of malondialdehyde (MDA) in plasma and myocardium in SF-treated group were significantly lower than those in diabetic control group. Expression of connective tissue growth factor (CTGF) in myocardium in SF-treated group was apparently lower than that in diabetic control group. Compared with normal control group, electron micrographs of myocardium in diabetic control group showed apparently abnormality, while that was significantly ameliorated in SF-treated group. The study demonstrated that SF has a cardioprotective effect via increasing SOD activity and NO levels in plasma and myocardium, inhibiting oxidative stress in plasma and myocardium, and inhibiting the expression of CTGF in myocardium in diabetes rats.
Keywords: diabetic cardiomyopathy, sodium ferulate, oxidative stress, nitric oxide, superoxide dismutase, connective tissue growth factor.
Introduction
Diabetic cardiomyopathy (DC) is the most common serious complication of diabetes mellitus.1 DC has become one of the leading causes of the increased mortality in both type 1 and type 2 diabetes. Clinically, DC can occur without major vascular lesions, suggesting a primary role for direct effects of diabetes on cardiomyocytes.1,2 Previous studies have demonstrated oxidative damage and myocardial fibrosis play important roles in the occurrence and development of DC.3,4
Oxidative stress occurs when there is a serious imbalance between generation of reactive oxygen species (ROS) and its clearance by body's endogenous antioxidative defenses.5 ROS include several sorts, such as superoxide anion, hydrogen peroxide, peroxynitrite, and hydroxyl.6 Reports have showed there are overproduction of ROS and impairment of antioxidant enzymes activities, such as superoxide dismutase (SOD), in the heart of diabetic animal model.7,8 Recent studies have shown that antioxidative medicine include probucol, N-acetylcysteine, SOD can protect the heart from diabetes-induced damage.4,7,9,10
Nitric oxide (NO) also plays important roles in the tissue damage induced by oxidative stress.5 NO normally is produced by endothelial nitric oxide synthase (eNOS), but is also produced by inducible nitric oxide synthase (iNOS) in the target tissues under inflammatory states.5 NO can interact with superoxide anion to form peroxynitrite.5 Peroxynitrite can cause nitrosative stress and has dramatic propathogenic effects in tissues and organs.4,5
Sodium ferulate (SF) is sodium salt of ferulic acid (Fig. 1). Ferulic acid, 4-hydroxy-3-methox-ycinnamic acid, is a sort of plant phenolic compound (Fig. 1). Ferulic acid is one of the ubiquitous compounds in diet, especially rich in grains, fruits, vegetables and beverage.11,12 Moreover, it is also the effective component of Chinese medicinal herbs Ligusticum walliichi (Chuanxiong) which has long been used for menoxenia, amenia, cardiovascular and cerebrovascular diseases in China.13 Ferulic acid, because of its phenolic nucleus and unsaturated side chain can readily form a resonance stabilized phenoxy radical, has potent free radical scavenging activity and can effectively scavenge ROS.12 Ferulic acid is unstable and can't be easily solved in water. Compared with ferulic acid, SF is more stable and more easily solved in water. So SF was used to treat diabetic rats in the present study. Our previous study showed that SF exhibits its protective effect on kidney in diabetic rats, which is attributed to its roles of increasing antioxidant enzymes activity, inhibiting oxidative stress.14
Figure 1.
Chemical structures of ferulic acid and sodium ferulate.
Although SF and ferulic acid exhibit beneficial effects in many diseases, whether SF or ferulic acid has beneficial effects in DC is still unclear. In the present study, diabetic rats induced by streptozotocin (STZ) were orally administered with SF, and the cardioprotective effect of SF was investigated.
Materials and methods
Animals
Male SD rats with the age of 8-10 weeks old (weighing 230-260g) were purchased from Animal Experimental Center of Zhejiang University.
Drugs and reagents
STZ were purchased from Sigma Company, USA. 3,3-diaminobenzidine tetrahydrochloride (DAB) was bought from Sigma Chemical Co., USA. Chloral hydrate was purchased from Fluka, USA. SOD assay kits (Intra-assay CV: 5.05%; Inter-assay CV: 3.32%), malondialdehyde (MDA) assay kits (Intra-assay CV: 3.5%; Inter-assay CV: 4.11%), and NO assay kits (Intra-assay CV: 2.0%; Inter-assay CV: 5.19%), protein quantification kits were purchased from Jiancheng Bio-engineering Company, Nanjing, China. Blood glucose assay kit was purchased from Changcheng Clinical Reagents Company, Baoding, Hebei, China. All assay kits were used according to the manufacturers' instructions strictly. SF was kindly provided from Limin pharmaceutical Company, Guangdong, China. Goat anti-rat connective tissue growth factor (CTGF) polyclonal antibody (sc-14939), rabbit anti-rat iNOS polyclonal antibody (sc-649), mouse anti-goat IgG (sc-2489) and mouse anti-rabbit IgG (sc-2491) were purchased from Santa Cruz Biotechnology, Inc., Santa Cruz, CA.
Study design
Rats were housed at 23-25°C in a 12-hour light/dark cycle with access to standard powdered rat chow and normal water. The scientific project, including animal care was supervised and approved by Animals Ethics Committee of the Second Affiliated Hospital, Zhejiang University. Diabetes was induced in SD rats by a single STZ injection (60 mg/kg i.p., dissolved in citrate buffer) after overnight fast. 72h after injection, the rats with blood glucose higher than 16.65mmol/L were considered as successfully diabetic model rats. All normal control group rats were treated with citrate buffer. Diabetic animals were randomized into diabetic control group and SF-treated group. Rats in the SF-treated group were treated with SF (110mg/kg) by gavage per day.14 Rats in the diabetic control group and the normal control group were treated with the same volume of normal saline by gavage per day. Every group included 10 rats. All rats in those three groups were not treated with insulin and other hypoglycemic agent, and could drink and eat freely in the experiment period. After 12 weeks, rats in the three groups were weighed. Rats were anesthetized by intraperitoneal (i.p.) injection of 350mg/kg chloral hydrate, the thoracic cavities were opened, and blood samples were directly obtained from the hearts.15 Plasma were separated and stored in freezer (-80°C) for analysis. Heart of every rat was lavaged with icecold normal saline, removed and weighed. Part of left ventricular tissue was fixed with 10% formalin for immunohistochemical examination. Four rats were randomly selected from every group. Part of left ventricular tissue of these rats was used for electron microscope assay. The remaining myocardial tissues were preserved in -80°C freezer for analysis.
Heart weight/Body weight (HW/BW) and blood glucose assay
HW/BW was calculated according to the formula: HW/BW=100×[Heart weight (g)/Body weight (g)]. Blood glucose was assayed by method of glucose oxidase.
Determination of SOD activity, MDA, and NO
SOD activity in plasma and myocardium were assayed by the method of xanthine oxidase according to the kit's instruction. The method is based on that SOD can inhibit superoxide anion reacting with hydroxylamine to generate nitrite. The content of SOD can be determined according to the formula provided by the kit. SOD results in plasma and myocardium were expressed as nitrite unit per milliliter (NU/ml) and nitrite unit per milligram protein (NU/mg protein) respectively.
MDA is the end product of lipid peroxidation. The content of MDA can indicate the extent of lipid peroxidation. MDA in plasma and myocardium were assayed by thiobarbituric acid method according to the kit's instruction. The method is based on that MDA can react with thiobarbituric acid to produce a red complex which has a peak absorbance at 532nm. The content of MDA can be determined by this method. MDA results in plasma and myocardium were expressed as nanomoles per milliliter (nmol/ml) and nanomoles per milligram protein (nmol/mg protein) respectively.
NO in plasma and myocardium were assayed by nitrate reductase method according to the kit's instruction. NO is unstable and easily oxidized to generate nitrite and nitrate. NO level is determined by measuring the total levels of nitrite/ nitrate. Samples were incubated with nitrate reductase to convert all nitrate to nitrite. Total nitrite/nitrate was assessed by spectrophotometer at 550 nm. NO results were in plasma and myocardium were expressed as nanomoles per milliliter (nmol/ml) and nanomoles per milligram protein (nmol/mg protein) respectively.
10% (w/v) myocardial tissue homogenate was made by homogenizer in ice bath. Sample protein content was quantified by the method of Coomassie brilliant blue with BSA as the standard.
Immunohistochemical assay for CTGF and iNOS
Paraffin-embedded left ventricular tissue blocks were sectioned at 4μm (8 rats per group), and we stained 8 sections per group for CTGF and iNOS respectively. The sections were deparaffinized, rehydrated, treated with target retrieval solution, and blocked with 3% hydrogen peroxide. After blocking with 10% normal goat serum, the slides were incubated with primary antibody (both in a 1:500 dilution). The slides were then incubated with mouse anti-goat IgG (in a 1:400 dilution for CTGF) and goat anti-rabbit IgG (in a 1:400 dilution for iNOS) labeled by biotin and further incubated with streptavidin peroxidase solution. The staining was visualized by reaction with DAB chromogen. Slides were counterstained with hematoxylin, washed in running water and mounted with resinous mounting medium. Positive staining was shown in yellow.
For quantification (8 sections per group respectively) of immunohistochemical staining for CTGF and iNOS, three random 100× microscopic fields per slide were photographed using a standard Olypus DP71 Light Microscope.16 Then a computer imaging analysis software Image-Pro Plus 6.0 (Media Cybernetics, America) was used for digital photographs analysis by calculation of integrated optical density (IOD) referencing the method introduced previously.16,17 IOD, the integral calculus of the stained area times the intensity of stain in each pixel in the area, indicates the total amount of staining material in that area.17
Ultrastructural examination by electron microscope
Left ventricular myocardial tissues were cut as the size of about 1mm3 (4 rats per group). Then myocardial tissues were fixed in 2.5% glutaraldehyde, followed in 1% osmic acid. After dehydration by ethanol and embedded by epoxy resins, the myocardial tissue were sectioned at 70 nm. The sections (4 sections per group) were stained with uranyl acetate and lead citrate, and examined in JEM-1230 (Japan) transmission electron microscope.
Statistical analysis
Data were expressed as means ± SD values. Data were analyzed using one-way analysis of variance (ANOVA) and secondary analysis for significance with the Turkey-Kramer post test. All analyses were performed using SPSS version 11.0 (SPSS Inc., USA). P <0.05 was considered statistically significant.
Results
Effect of SF on HW, BW, HW/BW, and blood glucose
In the ninth week of our experiment, we found that two rats in normal control group were dead; we neither dissected them nor searched for possible causes since we thought that two rats' death in normal control group would not affect our experiment accuracy at that time. Besides, we lost two rats in diabetic control group and two rats in SF-treated group respectively, which might be as the result of their hyperglycemic conditions. The BW and HW in the normal control group were significantly higher than those in the diabetic control and SF-treated group (P<0.01). No significant difference in BW and HW were found between the SF-treated group and the diabetic control group (P>0.05). HW/BW in the normal control group was significantly lower than that in the diabetic control and SF-treated group (P<0.05). No significant difference in HW/BW was found between the SF-treated group and the diabetic control group (P>0.05). Blood glucose in the diabetic control group and the SF-treated group were significantly higher than that in the normal control group (P<0.01), but no significant difference was found between the SF-treated group and the diabetic control group (P >0.05) (Table 1).
Table 1.
Effect of sodium ferulate on HW, BW, HW/BW, and blood glucose.
| Group | Normal control group | Diabetic control group | SF-treated group |
|---|---|---|---|
| HW (g) | 1.45±0.13 | 1.19±0.20** | 1.16±0.16** |
| BW (g) | 476.25±52.19 | 347.88±70.8** | 355.25±45.78** |
| HW/BW (×100) | 0.31±0.03 | 0.35±0.04* | 0.33±0.04 |
| Blood glucose (mmol/L) | 5.25±0.82 | 27.56±1.47** | 27.93±2.72** |
Values are expressed as means ± SD (n=8 rats/group)
HW, heart weight; BW, body weight; HW/BW, heart weight/body weight
* P <0.05, ** P <0.01 compared with normal control group
Effect of SF on SOD activity, MDA and NO levels in plasma and myocardium
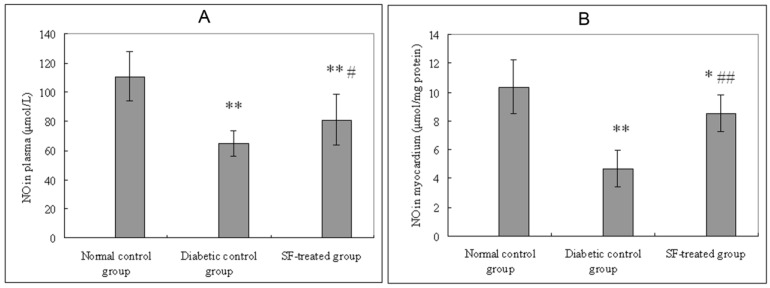
Plasma SOD activity in the diabetic control group were significantly lower than that in the normal control group (P<0.01). Plasma SOD activity in the SF-treated group were significantly higher than that in the diabetic control group (P<0.05). Myocardial SOD activity in the diabetic control group were significantly lower than that in the normal control group (P<0.01). Myocardial SOD activity in the SF-treated group were significantly higher than that in the diabetic control group (P<0.05) (Fig. 2).
Figure 2.
Effect of SF on SOD activity in plasma (A) and myocardium (B). Values are expressed as means ± SD (n=8 rats/group). SF, sodium ferulate; SOD, superoxide dismutase. **P<0.01 compared with normal control group; #P <0.05 compared with diabetic control group.
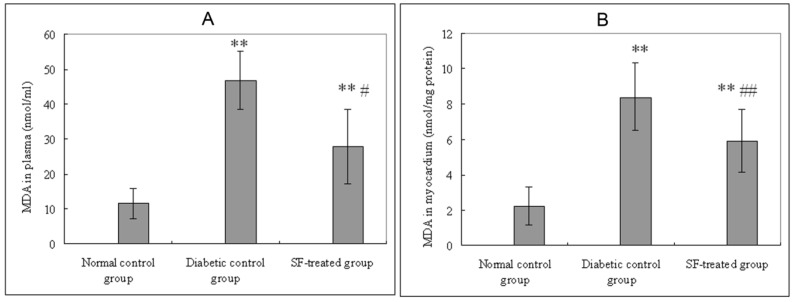
Plasma MDA levels in the diabetic control group were significantly higher than that in both the normal control group (P<0.01) and the SF-treated group (P<0.05). Myocardial MDA levels in the diabetic control group were significantly higher than that in both the normal control group (P<0.01) and the SF-treated group (P<0.01) (Fig. 3).
Figure 3.
Effect of SF on MDA levels in plasma (A) and myocardium (B). Values are expressed as means ± SD (n=8 rats/group). SF, sodium ferulate; MDA, malondialdehyde. **P<0.01 compared with normal control group; #P<0.05, ##P<0.01 compared with diabetic control group.
Plasma and myocardial NO levels in the diabetic control group were significantly lower than that in the normal control group (P<0.01). Plasma and myocardial NO in SF-treated group were significantly higher than that in the diabetic control group (P<0.05, P<0.01) (Fig. 4).
Figure 4.
Effect of SF on NO level in plasma (A) and myocardium (B). Values are expressed as means ± SD (n=8 rats/group). SF, sodium ferulate; NO, nitric oxide. *P<0.05, **P<0.01 compared with normal control group; #P<0.05, ##P<0.01 compared with diabetic control group.
Effect of SF on the expression of CTGF and iNOS in myocardium
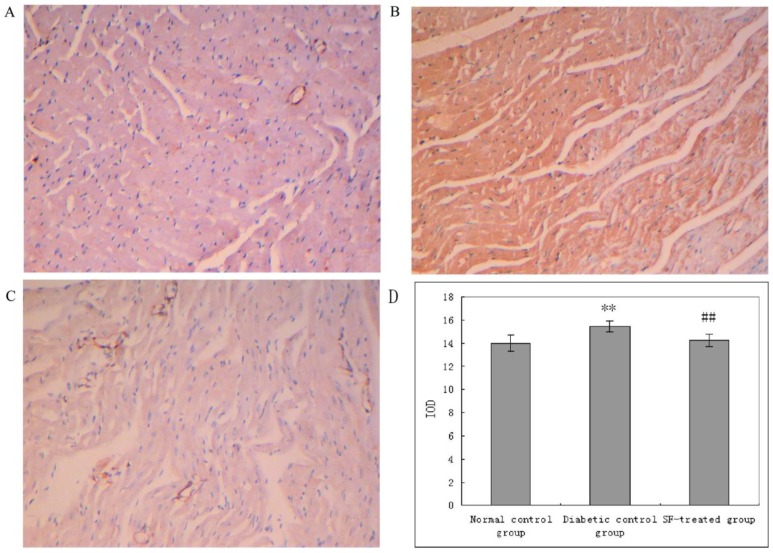
The yellow staining indicative of CTGF expression was prominent in myocardium in diabetic control group compared with that in the normal control group. The yellow staining indicative of CTGF expression in myocardium in the SF-treated group was apparently diminished compared with that in the diabetic control group. The analysis of IOD showed that CTGF expression was significantly higher in myocardium in the diabetic control group compared with that in the normal control group (P<0.01), and that its expression was notably reduced in myocardium in the SF-treated group compared with that in the diabetic control group (P<0.01). The results suggest that treatment with SF decreases diabetes-induced CTGF expression (Fig. 5).
Figure 5.
Immunohistochemical staining for CTGF in myocardium (A: normal control group; B: diabetic control group; C: SF-treated group; D: Quantitation of CTGF expressed in IOD). SF, sodium ferulate; CTGF, connective tissue growth factor; IOD, integrated optical density. Original magnification ×100. **P<0.01 compared with normal control group; ##P<0.01 compared with diabetic control group.
The yellow staining indicative of iNOS expression was prominent in myocardium in diabetic control group compared with that in the normal control group. The yellow staining indicative of iNOS expression in myocardium in the SF-treated group was not apparently diminished compared with that in the diabetic control group. The analysis of IOD showed that iNOS expression was significantly increased in myocardium in the diabetic control group compared with that in the normal control group (P<0.01), and that its expression was not notably reduced in myocardium in the SF-treated group compared with that in the diabetic control group (P>0.05). The results suggest treatment with SF did not inhibit diabetes-induced iNOS expression (Fig. 6).
Figure 6.
Immunohistochemical staining for iNOS in myocardium (A: normal control group; B: diabetic control group; C: SF-treated group; D: Quantitation of iNOS expressed in IOD). SF, sodium ferulate; iNOS, inducible nitric oxide synthase; IOD, integrated optical density. Original magnification ×100. **P<0.01 compared with normal control group.
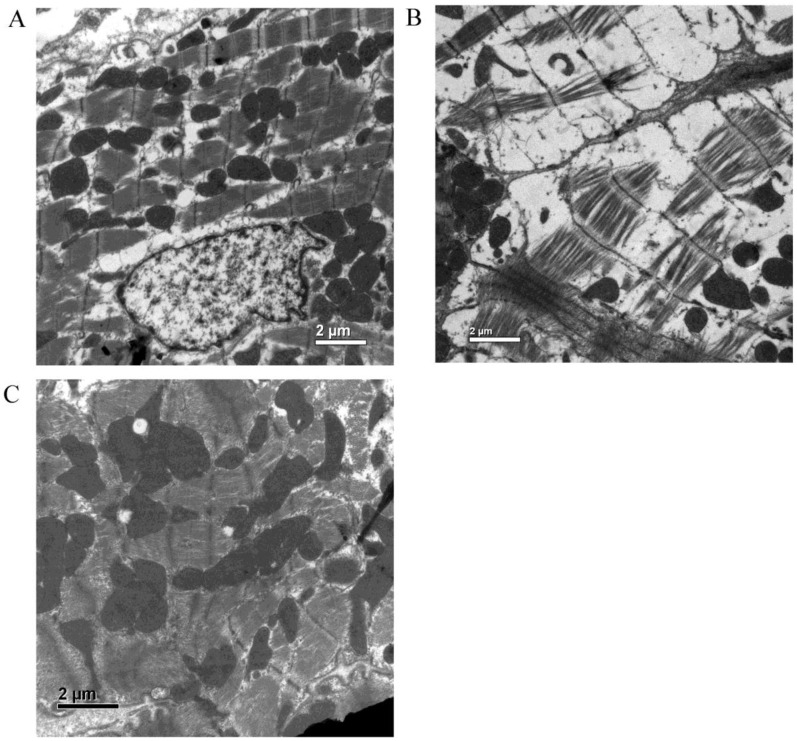
Ultrastructural examination by electron microscope
Electron micrographs of hearts in the normal control group showed normal mitochondria profiles and regular myofibrillar organization with evident M-lines and H-lines. In the hearts of the diabetic control group, sarcoplasm were prominent swelling, with disorganization and loss of myofibrils, destruction of M-lines and H-lines, inconformity of Z-bands thickness, and loss of mitochondria. In the hearts of the SF-treated group, the abnormalities were significantly improved, indicating the cardioprotective effect of SF in diabetic rats (Fig. 7).
Figure 7.
Electron micrographs of myocardium (A: normal control group; B: diabetic control group; C: SF-treated group). SF, sodium ferulate. Original magnification ×10000.
Discussion
There have been large numbers of studies reporting a decrease in body weight in diabetic rats compared with normal control rats.18-20 However, as for the effects of SF on body weight and organ weight in diabetic rats, a consensus has not been reached. An increase in body weight by ferulic acid (FA) in diabetic rats was reported by Balasubashini MS et al.18 Different from this increase effect, Fujita A et al reported that there were no significant differences in body weight and kidney weight between diabetic control groups and FA-treated groups, although an increase in kidney weight could be observed between diabetic groups and normal control groups.19 Thyagaraju BM et al pointed out a decrease in absolute testis weight and an increase in relative testis weight, but FA treatment did not significantly affect the body weight gain and testicular weight of diabetic rats.20 In view of the results above, and considering the different animal models and different experimental conditions, what we found is reasonable and could be understood.
As many studies have reported, SF has a hypoglycemic effect, and there has been a variety of mechanisms demonstrated to underlie this anti-hyperglycemic effect. Jung EH et al reported three main mechanisms of the hypoglycemic effect of FA: inhibiting the activity of α-glucosidase and thus reducing glucose absorption from the small intestine; increasing hepatic glucokinase activity and glycogen storage; elevating plasma insulin level.21 In a study by Balasubashini MS et al, they showed that the anti-oxidant properties of FA decrease oxidative stress and toxicity on the pancreas caused by STZ, which could help the beta cells to proliferate and secrete more insulin, and thus lead to increased utilization of glucose by the extra hepatic tissues and decreased blood glucose level.12,18 In our study, we did not notice significant decrease in glucose levels by SF, this might be because of the fluctuation of glucose test strips we used. Some glucose values were high and others were low, which led to an unchanged result: being no significantly differences of the average blood glucose levels between SF-treated group and diabetic control group.
As unwanted byproducts of aerobic metabolism, ROS are constantly generated in cells. Under normal physiological conditions, endogenous antioxidant defense system in our body can effectively scavenge ROS and protect our body from damage induced by ROS.5,8 In diabetic condition, many mechanisms such as glucose autoxidation, protein glycation, defective mitochondria, increasing activity of cytosolic xanthine oxidase, production of mediators of inflammation, etc, contribute to the overproduction of ROS.22,23 Moreover, studies have showed antioxidant enzymes activities are decreased in diabetes.7,8 The serious imbalance between ROS production and the decline of antioxidative ability leads to the enhancement of oxidative stress in diabetes. ROS can injury cell membrane, induce lipid peroxidation, and increase membrane permeability, which can significantly lead to cells functional and structural abnormalities, even death.4-8 Recently, study also showed that ROS can augment myocardial fibrosis in diabetes.24 In diabetes, these changes can cause myocardial remodeling, lead to the occurrence and development of DC. Antioxidant enzymes in our body include superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase. SOD, which can effectively scavenge superoxide radicals, is the most important antioxidant enzyme in our body. Shen's study showed overexpression of manganese superoxide dismutase (MnSOD) to the heart can protect heart from oxidative damage in diabetic mice.7 SF is an effective antioxidant. Previous studies have demonstrated SF can inhibit oxidative stress and lipid peroxidative reaction, increase antioxidant enzymes activity, and exhibit beneficial roles in many diseases.12,14 Our results in the study showed that SF can increase SOD activity in plasma and myocardium, and decrease the level of MDA in plasma and myocardium in diabetic rats.
The role of NO and NOS in DC are unclear at present. In the heart, the principal isoenzymes of NOS that produce NO are eNOS and iNOS, both of which are present in cardiac myocytes and vascular endothelium cells.5,25 Moderate levels of NO, which is a cardioprotective factor and can inhibit cell apoptosis, maintain the function of heart, is mainly produced by eNOS under normal physiological conditions.26,27 However, iNOS is implicated in diabetes-related heart dysfunctions.26,27 Several studies have shown that the expression of iNOS in myocardium in diabetes is upregulated, but the levels of NO are decreased or unaltered.28-30 NO is very reactive and excessive production of NO in the heart of diabetes may be quickly scavenged by free radicals such as superoxide anions, and at the same time, leads to the formation of peroxynitrite, which has dramatic propathogenic effects in the heart in diabetes (in addition to oxidizing proteins, peroxynitrite also causes lipid peroxidation which can lead to cellular damage).4,5 This is the reason why levels of NO are decreased or unaltered in the heart of diabetes. Diabetic rats administrated by iNOS inhibitor or diabetic mice targeted deletion of the gene for iNOS exhibit improved heart function, which suggested that NO, superoxide anions and their reaction product, peroxynitrite, play important roles in DC.26,27 Because of the molecular structure of NO, regardless produced by eNOS or iNOS, is the same. It is plausible that NO itself can't damage heart in diabetes. Peroxynitrite, the reaction product of NO and ROS, is the archcriminal and can damage the heart in diabetes. Our study showed that the expression of iNOS in myocardium was significantly higher in diabetic rats, but Plasma and myocardial NO in the diabetic control group were significantly lower than that in the normal control group. This is because that, although increased iNOS caused increased NO, elevated ROS at the same time could interact with NO and result in its ultimate decrease in diabetic rats. What is more, the present study suggests SF can not influence the expression of iNOS in the heart in diabetic rats. Also, SF can not increase NO levels directly. However, it seemed that the NO levels were indeed elevated in SF-treated group compared with that in diabetic control group. The truth is, SF can increase the activity of SOD and decrease the production of ROS. The quantity of NO scavenged by ROS is decreased, which leads to the increase of NO levels in myocardium in SF-treated group. In other words, SF increase NO levels indirectly by decreasing the production of ROS and thus decreasing the interaction of NO and ROS.
CTGF is increasingly being implicated in structural and functional abnormality of the heart in diabetes.31 CTGF is a potent profibrotic protein and play important roles in tissue and organ fibrosis.32 CTGF expression has been shown to correlate with the severity and progression of fibrosis in multiple fibrotic diseases.32 CTGF has diverse biological actions including the regulation of fibroblast proliferation, angiogenesis collagen synthesis, extracellular matrix deposition, and cellular apoptosis.32 The expression of CTGF is increased in the myocardium of rats with STZ-induced diabetes.31 Hyperglycemia, ROS, angiotensin II, vascular endothelial growth factor, protein kinase C, transforming growth factor-β (TGF-β), advanced glycation end products can up-regulate the expression of CTGF.31,33 Our study showed the expression of CTGF in myocardium in the diabetic control group was significantly increased compared with the normal control group. But the expression of CTGF in the SF-treated group was significantly lower than that in the diabetic control group. It may be one of the mechanisms of cardioprotective effect of SF in diabetes.
In summary, the present study demonstrated that SF has a cardioprotective effect via increasing SOD activity and NO levels in plasma and myocardium, inhibiting oxidative stress in plasma and myocardium, and inhibits the expression of CTGF in myocardium in diabetes rats. SF may be used for the treatment of DC.
Table 2.
Effect of sodium ferulate on SOD activity, MDA levels and NO levels in plasma and myocardium.
| Groups | SOD activity | MDA levels | NO levels | |||
|---|---|---|---|---|---|---|
| Plasma | Myocardium | Plasma | Myocardium | Plasma | Myocardium | |
| Normal control group | 133.27±7.29 | 368.51±44.33 | 11.56±4.49 | 2.22±1.06 | 110.83±16.88 | 10.35±1.89 |
| Diabetic control group | 101.16±7.2** | 238.69±24.42** | 46.85±8.24** | 8.41±1.91** | 64.91±8.5** | 4.66±1.28** |
| SF-treated group | 111.32±10.83** # | 277.88±29.63** # | 27.79±10.62** # | 5.92±1.77** ## | 80.97±17.43** # | 8.49±1.27* ## |
Values are expressed as means ± SD (n=8 rats/group).
SF, sodium ferulate; SOD, superoxide dismutase; MDA, malondialdehyde; NO, nitric oxide.
*P<0.05, **P<0.01 compared with normal control group; #P<0.05, ##P<0.01 compared with diabetic control group.
Acknowledgments
This work was supported by research grants from the National natural Science foundation of China (No: 30671007) and the grants from the Technology Department of Zhejiang Province, China (No: 2005C30063)
Abbreviations
- BW
body weight
- CTGF
connective tissue growth factor
- DC
diabetic cardiomyopathy
- FA
ferulic acid
- HW
heart weight
- iNOS
inducible nitric oxide synthase
- IOD
integrated optical density
- MDA
malondialdehyde
- NO
nitric oxide
- ROS
reactive oxygen species
- SF
sodium ferulate
- SOD
superoxide dismutase
- STZ
streptozotocin.
References
- 1.Trost S, LeWinter M. Diabetic cardiomyopathy. Curr Treatm Opt Cardiovasc Med. 2001;3:481–492. doi: 10.1007/s11936-001-0022-9. [DOI] [PubMed] [Google Scholar]
- 2.Dyntar D, Sergeev P, Klisic J. et al. High glucose alters cardiomyocyte contacts and inhibits myofibrillar formation. J Clin Endocrinol Metab. 2006;91:1961–1967. doi: 10.1210/jc.2005-1904. [DOI] [PubMed] [Google Scholar]
- 3.Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;47:693–700. doi: 10.1016/j.jacc.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 4.Kumar S, Sitasawad SL. N-acetylcysteine prevents glucose/glucose oxidase- induced oxidative stress, mitochondrial damage and apoptosis in H9c2 cells. Life Sci. 2009;84:328–336. doi: 10.1016/j.lfs.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Cai L. Suppression of nitrative damage by metallothionein in diabetic heart contributes to the prevention of cardiomyopathy. Free Radic Biol Med. 2006;41:851–861. doi: 10.1016/j.freeradbiomed.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Ritchie RH. Evidence for a causal role of oxidative stress in the myocardial complications of insulin resistance. Heart Lung Circ. 2009;18:11–18. doi: 10.1016/j.hlc.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Shen X, Zheng S, Metreveli NS. et al. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes. 2006;55:798–805. doi: 10.2337/diabetes.55.03.06.db05-1039. [DOI] [PubMed] [Google Scholar]
- 8.Cai L, Kang YJ. Oxidative stress and diabetic cardiomyopathy: a brief review. Cardiovasc Toxicol. 2001;1:181–193. doi: 10.1385/ct:1:3:181. [DOI] [PubMed] [Google Scholar]
- 9.Singal PK, Bello-Klein A, Farahmand F. et al. Oxidative stress and functional deficit in diabetic cardiomyopathy. Adv Exp Med Biol. 2001;498:213–220. doi: 10.1007/978-1-4615-1321-6_27. [DOI] [PubMed] [Google Scholar]
- 10.Kaul N, Siveski-Iliskovic N, Hill M. et al. Probucol treatment reverses antioxidant and functional deficit in diabetic cardiomyopathy. Mol Cell Biochem. 1996;160-161:283–288. doi: 10.1007/BF00240060. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Z, Moghadasian MH. Chemistry, natural sources, dietary intake and pharmaco-kinetic properties of ferulic acid: a review. Food Chem. 2008;109:1–12. doi: 10.1016/j.foodchem.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 12.Graf E. Antioxidant potential of ferulic acid. Free Radical Biol Med. 1992;13:435– 448. doi: 10.1016/0891-5849(92)90184-i. [DOI] [PubMed] [Google Scholar]
- 13.Barone E, Calabrese V, Mancuso C. Ferulic acid and its therapeutic potential as a hormetin for age-related diseases. Biogerontology. 2009;10:97–108. doi: 10.1007/s10522-008-9160-8. [DOI] [PubMed] [Google Scholar]
- 14.Zhao TF, Zhang L, Deng HC. Renal protective effect and its mechanism of sodium ferulate in diabetic rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24:445–449. [PubMed] [Google Scholar]
- 15.Chung WY, Zhang HQ, Zhang SP. Peripheral muscarinic receptors mediate the anti-inflammatory effects of auricular acupuncture. Chin Med. 2011;6:3. doi: 10.1186/1749-8546-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan JF, Yu SJ, Zhao ZW. et al. The expression of Fas/FasL and apoptosis in yak placentomes. Anim Reprod Sci. 2011;128:107–116. doi: 10.1016/j.anireprosci.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Wang CJ, Zhou ZG, Holmqvist A. et al. Survivin Expression Quantified by Image Pro-plus Compared With Visual Assessment. Appl Immunohistochem Mol Morphol. 2009;17:530–535. doi: 10.1097/PAI.0b013e3181a13bf2. [DOI] [PubMed] [Google Scholar]
- 18.Balasubashini MS, Rukkumani R, Viswanathan P. et al. Ferulic Acid Alleviates Lipid Peroxidation in Diabetic Rats. Phytother Res. 2004;18:310–314. doi: 10.1002/ptr.1440. [DOI] [PubMed] [Google Scholar]
- 19.Fujita A, Sasaki H, Doi A. et al. Ferulic acid prevents pathological and functional abnormalities of the kidney in Otsuka Long-Evans Tokushima Fatty diabetic rats. Diabetes Res Clin Pract. 2008;79:11–17. doi: 10.1016/j.diabres.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Thyagaraju BM, Muralidhara. Ferulic Acid Supplements Abrogate Oxidative Impairments in Liver and Testis in the Streptozotocin-Diabetic Rat. Zoolog Sci. 2008;25:854–860. doi: 10.2108/zsj.25.854. [DOI] [PubMed] [Google Scholar]
- 21.Jung EH, Kim SR, Hwang IK. et al. Hypoglycemic Effects of a Phenolic Acid Fraction of Rice Bran and Ferulic Acid in C57BL/KsJ-db/db Mice. J Agric Food Chem. 2007;55:9800–9804. doi: 10.1021/jf0714463. [DOI] [PubMed] [Google Scholar]
- 22.Wolff SP, Dean RT. Glucose autoxidation and protein modification. The potential role of 'autoxidative glycosylation' in diabetes. Biochemical Journal. 1987;245:243–250. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonnefont-Rousselot D. Glucose and reactive oxygen species. Current Opinion in Clinical Nutrition Metabolic Care. 2002;5:561–568. doi: 10.1097/00075197-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Aragno M, Mastrocola R, Alloatti G. et al. Oxidative stress triggers cardiac fibrosis in the heart of diabetic rats. Endocrinology. 2008;149:380–8. doi: 10.1210/en.2007-0877. [DOI] [PubMed] [Google Scholar]
- 25.Balligand JL, Ungureanu D, Kelly RA. et al. Abnormal contractile function due to induction of nitric oxide synthesis in rat cardiac myocytes follows exposure to activated macrophage-conditioned medium. J Clin Invest. 1993;91:2314–2319. doi: 10.1172/JCI116461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marfella R, Di Filippo C, Esposito K. et al. Absence of inducible nitric oxide synthase reduces myocardial damage during ischemia reperfusion in streptozotocin-induced hyperglycemic mice. Diabetes. 2004;53:454–462. doi: 10.2337/diabetes.53.2.454. [DOI] [PubMed] [Google Scholar]
- 27.Cheng X, Cheng XS, Kuo KH. et al. Inhibition of iNOS augments cardiovas- cular action of noradrenaline in streptozotocin-induced diabetes. Cardiovasc Res. 2004;64:298–307. doi: 10.1016/j.cardiores.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 28.Nagareddy PR, Xia Z, McNeill JH. et al. Increased expression of iNOS is associated with endothelial dysfunction and impaired pressor responsiveness in streptozotocin-induced diabetes. Am J Physiol Heart Circ Physiol. 2005;289:H2144–H2152. doi: 10.1152/ajpheart.00591.2005. [DOI] [PubMed] [Google Scholar]
- 29.Farhangkhoee H, Khan ZA, Chen S. et al. Differential effects of curcumin on vasoactive factors in the diabetic rat heart. Nutr Metab (Lond) 2006;3:27–35. doi: 10.1186/1743-7075-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farhangkhoee H, Khan ZA, Mukherjee S. et al. Heme oxygenase in diabetes-induced oxidative stress in the heart. J Mol Cell Cardiol. 2003;12:1439–1448. doi: 10.1016/j.yjmcc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Way KJ, Isshiki K, Suzuma K. et al. Expression of connective tissue growth factor is increased in injured myocardium associated with protein kinase C beta2 activation and diabetes. Diabetes. 2002;51:2709–2718. doi: 10.2337/diabetes.51.9.2709. [DOI] [PubMed] [Google Scholar]
- 32.Leask A, Holmes A, Abraham DJ. Connective tissue growth factor: a new and important player in the pathogenesis of fibrosis. Curr Reumatol Rep. 2002;4:136–142. doi: 10.1007/s11926-002-0009-x. [DOI] [PubMed] [Google Scholar]
- 33.Ban CR, Twigg SM. Fibrosis in diabetes complications: pathogenic mechanisms and circulating and urinary markers. Vasc Health Risk Manag. 2008;3:575–596. doi: 10.2147/vhrm.s1991. [DOI] [PMC free article] [PubMed] [Google Scholar]