Abstract
Gamma-hydroxybutyrate (GHB) is a drug with significant abuse potential. The present study aimed to assess the relative value of escalating doses of GHB to current GHB users via the Multiple Choice Procedure (MCP), and to validate that the dose rated highest with the MCP would be self-administered at a greater rate than placebo. Participants were 5 current GHB users who were not currently trying to stop using GHB. To examine the value of escalating doses of GHB, the following doses of GHB were used: 0 (placebo), 12.5, 25, 37.5, and 50 mg/kg. Participants typically assigned higher doses of GHB had higher crossover points on the MCP. During choice sessions, participants made repeated choices between administering GHB, placebo or nothing. All participants selected GHB exclusively (5 out of 5 instances) except for one participant who selected GHB on 4 out of 5 instances, thus 96% (i.e., 24/25) of choices were for active GHB. Based on these data, GHB appears likely to function as a dose-dependent reinforcer for humans based on our sample.
Keywords: Gamma-Hydroxybutyrate (GHB), Multiple Choice Procedure (MCP), reinforcer efficacy, substance abuse
Gamma-hydroxybutyrate (GHB) is a metabolite of gamma-amino-butyric acid (GABA) that was first synthesized in 1960, and was originally investigated as an anesthetic because of its ability to rapidly permeate the blood–brain barrier and induce a sleep-like state (Kam & Yoong, 1998; Leone, Vigna-Taglianti, Avanzi, Brambilla, & Faggiano, 2010). Subsequently, GHB has been investigated in the treatment of sleep disorders (e.g., Scrima, Hartman, Johnson, Thomas, & Hiller, 1990), depression (e.g., Mamelak, 2009), anxiety (e.g., Ferrara et al., 1999), and in the reduction of acute alcohol and opiate withdrawal symptoms (e.g., Gallimberti, Spella, Soncini, & Gessa, 2000). GHB is typically administered orally or intravenously.
The abuse potential of GHB became apparent during the 1980s and 1990s when the drug was unregulated and uncontrolled. GHB was routinely used by weightlifters to increase muscle mass based on unsubstantiated reports that it stimulated release of human growth hormones and enhanced effects of steroid use, and was later used by dieters for its purported weight control effects (Kam & Yoong, 1998). GHB has also been implicated as a date rape drug, or a drug administered to facilitate a sexual assault (Carter, Pardi, Gorsline, & Griffiths, 2009; Weiss & Colyer, 2010). GHB may be administered in order to produce symptoms of acute intoxication, amnesia, and diminished mental capacity (Wang, Swick, Carter, Thorpy, & Benowitz, 2009). Recognition of the potential for abuse and misuse of GHB led the United States Food and Drug Administration (FDA) to classify nonmedical GHB as a Schedule I controlled substance in 2000.
GHB is a central nervous system depressant exerting a peak effect 30–45 min postingestion (Abanades et al., 2006). Research has indicated that GHB is capable of producing initial stimulant effects (particularly in humans with no history of sedative abuse) and later sedative effects (e.g., Abanades et al., 2007; Oliveto et al., 2010). The adverse effects of GHB use include: dizziness, weakness, nausea, emesis, hypothermia, depressed respiration, apnea, difficulties with peripheral vision, agitation, confusion, delirium, hallucinations, loss of consciousness, and coma (Ingels, Rangan, Bellezzo, & Clark, 2000; Leone et al., 2010). There was a dramatic increase in hospital emergency department reports involving GHB from 1992–1996 (Greenblatt, 1997). GHB has also been a contributing factor in several deaths, typically in combination with alcohol or other drugs (Leone et al.). GHB withdrawal syndromes have been reported by a number of investigators, suggesting that some individuals have become dependent on GHB (e.g., Kim, Anderson, Dyer, Barker, & Blanc, 2007).
Preclinical studies of GHB have established GHB as a discriminative stimulus (Winter, 1981) and demonstrated that other substances (i.e., PCP, heroin) do not generalize to GHB in discrimination tests (e.g., Beardsley, Balster, & Harris, 1996). In a line of elegant behavioral pharmacology work, France and colleagues demonstrated that GHB exerts its effect primarily through the GABAb receptor, although other sites also contribute to GHB's effect (e.g., Carter, Koek & France, 2009; Koek, Mercer, Coop & France, 2009). Drug discrimination studies with ethanol and GHB have yielded mixed results, with cross generalization occurring between the two substances but only within a narrow range of doses (Colombo et al., 1995). In preclinical self-administration studies, rats bred to self-administer alcohol exhibited stronger preference for GHB self-administration over water than did alcohol-nonpreferring rats; however, even alcohol-preferring rats alternated between GHB and water (Colombo et al., 1998). Other self-administration studies using rhesus monkeys have demonstrated that GHB failed to produce reliable responding over placebo, serving as a weak positive reinforcer in only a few cases (Beardsley et al., 1996; Woolverton et al., 1999). Recent work has, however, demonstrated that baboons will self-administer GHB, demonstrating that it does function as a reinforcer in at least one nonhuman primate species (Goodwin, Kaminski, Griffiths, Ator & Weerts, 2011).
While research on drug discrimination, abuse potential, and the reinforcing efficacy of GHB in humans has been limited, several findings are noteworthy. For example, Carter, Griffiths, and Mintzer (2009) examined the cognitive effects of GHB compared to triazolam, and reported that while both substances produced increases in ratings of drug effects, GHB produced fewer cognitive and psychomotor impairments. Abanades et al. (2007) examined the abuse potential of GHB compared to other drugs (e.g., flunitrazepam, ethanol). Their results indicated that GHB produced more pleasurable effects (e.g., euphoria) than other substances, suggesting a high abuse potential among users seeking these types of effects (e.g., club drug users). Research has also demonstrated that GHB produces dose-related increases on subjective ratings of drug effects (e.g., sedative effects, stimulant effects, positive mood, dissociative effects) and drug liking (Oliveto et al., 2010).
An improved understanding of the reinforcing effects of GHB in current GHB users in humans is needed in order to better characterize its abuse potential. The present study used the Multiple Choice Procedure (MCP; Griffiths, Troisi, Silverman, & Mumford, 1993) to evaluate the relative value of GHB among current GHB users when compared to monetary compensation. The MCP was developed as a pragmatic approach for studying drug reinforcement (Griffiths et al., 1993), and has been validated for use with multiple substances of abuse (Griffiths, Rush, & Puhala, 1996; Kidorf, Stitzer, & Griffiths, 1995; Schmitz, Sayre, Hokanson, & Spiga, 2003). The MCP requires participants to make several choices between a substance and escalating amounts of monetary compensation. The monetary crossover point (the point at which the participant stops selecting the drug and begins selecting money) quantifies the putative reinforcing effects of the drug in reference to money.
Variants of this type of choice procedure have been used frequently to infer the reinforcing efficacy of drugs of abuse (de Wit, Pierri, & Johanson, 1989; Higgins, Bickel, & Hughes, 1993; Higgins, Roll, & Bickel, 1996; Johanson & de Wit, 1989; Johanson, Mattox, & Schuster, 1995; Woolverton & Johanson, 1984). Carter, Richards, Mintzer, and Griffiths (2006) used the MCP to determine the reinforcing efficacy of GHB, and compared the crossover values for GHB with those for other sedatives with known abuse potential (i.e., triazolam and pentobarbital). Their results indicated that the effects and overall abuse potential of GHB overlapped with those of the other sedative/hypnotic drugs. Additionally, they reported a dose-dependent increase in crossover point for GHB.
The aims of the present study were (1) to evaluate the relative value of escalating doses of GHB among current GHB users via the MCP, and (2) to examine the extent to which GHB would be self-administered relative to placebo. We hypothesized that monetary crossover points on the MCP would escalate as dose of GHB escalated (H1) and that GHB would be reliably chosen for self-administration over placebo (H2).
METHOD
Participants
The participants were 5 individuals who were currently using GHB but not seeking treatment for their GHB use. Participants were recruited from the greater Los Angeles area, and were Caucasian (n = 4) or Korean (n = 1). Two participants were female and the mean age was 35 (range 27–42).
Participants met the following inclusion criteria: (a) 21–45 years of age; (b) used GHB at least once a month for the past 2 years; (c) reported weekly GHB use during the past 6 months; and (d) were cleared for participation by the study physician after physical examination and laboratory tests were reviewed. Participants were excluded from participation if they met any of the following exclusion criteria: (a) history of or evidence suggestive of seizures or brain injury; (b) previous medically adverse reaction to GHB, including loss of consciousness, chest pain, or seizure; (c) history of major psychiatric illness other than drug dependence or disorders secondary to drug use; (d) evidence of untreated clinically significant heart disease or hypertension or untreated or unstable medical illness (including untreated thyroid disease, autoimmune disease, and tuberculosis); (e) pregnancy; (f) medication use during the study other than occasional use of acetaminophen or antacids; (g) significant family history of early cardiovascular morbidity or mortality; (h) current drug dependence (excepting nicotine); (i) currently on probation or parole; and (j) more than three uses of GHB per week over the past four weeks.
During the informed consent process, participants were instructed that they were taking part in a study designed to examine how sedative medications alter their behavior, mood and physiological functions. They were informed that GHB may be received and were instructed regarding the format of different experimental sessions. All study participants received counseling about substance dependence and were advised that treatment for substance abuse was indicated and available. Applicants not participating in the study received treatment referral information as appropriate. At completion of their participation, participants were again advised that treatment was indicated and available, and were given treatment referral information and assistance. Participants were evaluated weekly by a clinical psychologist to ascertain any changes in their desire to seek substance abuse counseling.
Apparatus
Physiological monitoring was conducted with a Space Labs medical system model #90367 (Redmond, WA).
Procedures
General procedures
Participants were housed as inpatients in the General Clinical Research Center (GCRC) of UCLA. They were instructed to refrain from illicit and prescription drug use and alcohol for the duration of the study. Additionally, participants were not allowed to have caffeine, nicotine or solid foods for 1 hr prior to their scheduled sessions. Participants who were cigarette smokers were required to smoke a cigarette of their preferred brand 1 hr before the beginning of experimental sessions in order to insure that the time since last cigarette was constant across participants. To monitor compliance with restrictions on alcohol, nicotine and other drug use, breath alcohol levels (BALs) and carbon monoxide (CO) levels were measured (via Intoximeter and Bedfont Smokerlyzer) and urine specimens were collected for drug screening prior to the start of each experimental session. Finally, a field sobriety test was conducted at the beginning of each experimental session to assess for acute intoxication.
Experimental sessions were conducted at approximately the same time of day for a given participant and were separated by 24 hr. Experimental sessions were conducted in a quiet room of the GCRC with research and medical staff present. Medical personnel monitored cardiac and respiratory function during drug administration periods and for 2 hr following drug administration. Participants remained comfortably seated during experimental sessions except for brief visits to the lavatory.
Putative reinforcing efficacy assessed via the MCP
The MCP (Griffiths et al., 1993) was used to assess relative value, compared to money, of escalating doses of GHB. The MCP required participants to make repeated choices between two potential reinforcers (drug vs. money). The MCP form consisted of 100 items assessing preference between today's dose of GHB and monetary compensation values ranging from $0.25 to $25.00. To complete the MCP, participants made a series of choices between today's dose of GHB (dose of drug that they sampled earlier in the day) and monetary compensation (see Figure 1 for a sample portion of the MCP form). Participants were instructed before each MCP form was administered that they would receive one of their choices on the last day of the phase. On Day 6, one of the choices that participants made on days 1–5 was randomly selected using a random number generator and the corresponding choice was given to participants (either money or drug).
Fig 1.
Sample Multiple Choice Procedure form. On each numbered line, the participant would circle their preferred item (GHB or money). In this example, the monetary crossover point is $1.50.
Phase 1
Phase 1 consisted of six sessions. At the beginning of the first five sessions, participants were seated and connected to physiological monitoring equipment and an indwelling catheter so that baseline physiological measures (heart rate, oxygen saturation, digit temperature and blood pressure), blood samples, and digital photographs of pupils could be taken prior to drug administration. After 30 min, participants received one of the following doses of orally delivered GHB or placebo, in an ascending order across sessions: 0 (placebo), 12.5, 25, 37.5, 50 mg/kg. The order of exposure to drug and placebo during these first five sessions was purposefully arranged so that we could ramp the dose up in an incremental fashion to assess tolerability in each participant and to facilitate the pharmacokinetic assay (described below).
Following placebo or drug administration, physiological measures, blood draws, and pupil photographs were taken over a 4-hr period. Physiological measures were recorded every 5 min and pupil photographs were taken every 15 min. Blood for pharmacokinetic analysis was drawn prior to and at 10, 20, 30, and 45 min and at 1, 1.5, 2, 2.5, 3, 4, 5, and 6 hr after dosing. A detailed description of the gas chromatography/mass spectrometry assay used in the present study is available in Frison, Tedeschi, Maietti, and Ferrara (2000). This assay is more convenient and simpler than other published assays, yet still produces valid and reliable measurements.
Four hr after placebo or drug ingestion, physiological measures were taken every 15 min for a 2-hr period. During this time, participants filled out the MCP form. Participants made repeated selections between the dose of drug they received during the session and an escalating amount of money. The point at which preference for money supersedes preference for the drug is referred to as the crossover point. The crossover point can be compared across days (e.g., across different doses of GHB) and the relative value of each dose can be compared. On Day 6, one of the participant's choices on the MCP was consequated as described above.
At the conclusion of the session, the indwelling catheter and physiological monitoring equipment were removed. The participant was then required to pass a field sobriety test and, being deemed free from any residual drug effects by the attending physician, was released from the session. Participants remained in the inpatient facility between sessions.
Phase 2
The second phase consisted of 6 days and was essentially a replication of Phase 1, with the following exceptions: (1) no indwelling catheter, as there was no pharmacokinetic assessment; and (2) doses used in Phase 2 were administered in a random fashion across participants as their tolerability and safety had been previously assessed. This procedure had the advantage of removing the order of dosing confound present in Phase 1 and provided a repeated assessment of each dose. After Phase 2, participants were eligible for discharge. Prior to their discharge, participants scheduled a time for their return for the completion of Phase 3 of the study.
Phase 3
Phase 3 consisted of nine sessions with approximately 24 hr separating sessions. Participants were re-admitted to the inpatient unit on GCRC for the 9-day duration of Phase 3. All sessions were 3 hr in length. When participants arrived for an experimental session, breath and urine samples were collected and screened (as in Phase 1) for the presence of any drug or metabolite. Baseline physiological measures and blood draws were collected and a field sobriety test was conducted.
Following collection of samples and assessment of vital signs and sobriety, participants were given a drug for immediate ingestion. During Sessions 1 and 3, the drug was labeled Drug A, and on Sessions 2 and 4 the drug was labeled Drug B. For some of the participants, Drug A was GHB and Drug B was placebo. The order was reversed for other the participants. The dose of GHB used was the dose that the participant rated as most valuable (i.e., had the highest crossover point) using the MCP in Phase 1. Participants were instructed to note the drug's letter (i.e., A or B) and to attempt to associate the letter with the effects of the drug. Blood pressure and heart rate were monitored every 20 min throughout the session. To conclude the session a field sobriety test was conducted and participants were dismissed.
The next five sessions were identical except that at the beginning of each session participants were given a choice between self-administering Drug A, Drug B, or neither. Preference for a “drug” was defined as selecting that drug a minimum of four times across the five choice sessions.
Compensation. Participants were paid $20 in vouchers to local restaurants and stores for completion of initial screening, and $30 in cash for completion of medical screening, including interviews, blood tests, and ECG, which was scheduled over several days. Participants were paid $1,400 in vouchers for the 3-week inpatient stay, with an additional $50 in gift certificates if the entire study was completed. The money that was earned during the MCP session was paid to participants in cash at the end of the study.
Drug dosing
Liquid GHB doses were diluted with water and administered to participants orally in a double-blind fashion.
Data collection
This was primarily a within-subject design and therefore no random assignment occurred; the exceptions being during the first five sessions of Phase 2 when GHB dose was randomized across participants and during the first four sessions of Phase 3 when order of placebo and GHB administration was randomized across participants.
Statistical analyses
Stata (StataCorp, 2009) was used to conduct all statistical analyses. We used repeated-measures ANOVA to analyze the MCP outcome of mean crossover point across GHB dose (placebo, 12.5, 25, 37.5, and 50 mg/kg) and Phase (1 and 2) of exposure. We followed the primary analysis with a trend analysis to examine specific patterns of MCP crossover points over time and across GHB dose. We used the binomial probability test to analyze participants' choices between GHB and placebo for each of the five Phase 3 sessions in which they were asked to make such a decision. Pharmacokinetic analyses were performed using WinNonlin Version 4.1 (Pharsight Corporation, 1998).
RESULTS
Physiological Measures for Safety
Several domains of physiological functioning were assessed during the present study to ensure participants' safety. There were no clinically significant deviations from baseline physiological measures that were taken for safety during the three phases of this experiment.
Pharmacokinetic Analysis
Across the four dosage levels, the mean half-life of GHB was 37.33 (+/− 5.84) min. The mean clearance was 30.27 min. The mean time that GHB was present at the maximum concentration (Tmax) was 30.31 min and the mean peak concentration (Cmax) was 2.06 mcg/ml.
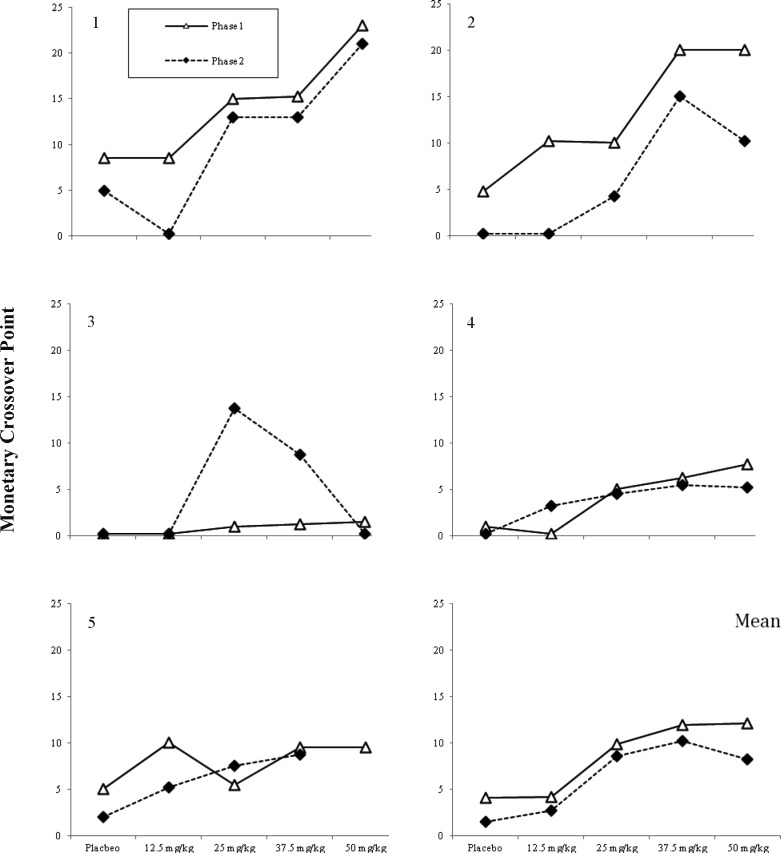
Choice as a Function of GHB Dose: MCP and Drug Choice
Figure 2 shows participant's monetary crossover points across phases and across GHB dose. Data are missing for the Phase 2 exposure to 50 mg/kg of GHB for Participant 5 because this participant had an adverse reaction (emesis) to the initial 50 mg/kg dose. In Phase 1, for 4 out of 5 participants, there appeared to be a linear increase in crossover values across GHB doses, Participant 5 being the exception. In Phase 2, a similar increase in crossover points was observed but there was a tendency to value the highest dose less than the 37.5 mg/kg dose, a pattern suggestive of the inverted u-shaped function clearly observed in Participant 3. A repeated- measures ANOVA applied to the data collapsed across Phases 1 and 2 revealed no significant impact of time on the crossover point, but there was a significant effect of dose; F(4, 36) Greenhouse-Geiser = 4.88, p < .05. A follow-up trend analysis revealed a significant (F = 11.77, p < .05) linear trend in the relationship between dose of GHB and mean monetary crossover point, and a significant (F = 9.75, p < .05) cubic trend in the relationship between dose of GHB and mean monetary crossover point. The cubic trend could likely be interpreted as a tolerance effect, even though the GHB was administered in a randomized fashion during Phase 2. The lower-right panel of Figure 2 displays the estimated mean crossover point for participants across GHB dose and time of exposure. Both the linear (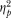 = 0.75) and cubic (
= 0.75) and cubic (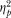 = 0.71) trends of the dose effect represent large effects on the MCP decisions across GHB dose and time of exposure.
= 0.71) trends of the dose effect represent large effects on the MCP decisions across GHB dose and time of exposure.
Fig 2.
Individual participant and mean monetary crossover points across GHB doses and phase of exposure.
During Phase 3, participants never declined to receive either Drug A or B. Four of five participants selected GHB over the placebo in five out of five sessions; the exception was Participant 5 who selected GHB in four out of five sessions. Thus 96% (i.e., 24/25) of choices were for active GHB; a pattern of choice significantly different (p < 0.05) than would be expected by chance alone.
Discussion
We evaluated the relative value of escalating doses of GHB to current GHB users via the MCP. The MCP crossover point generally increased in a stepwise manner as dose of GHB increased (during some trials, there was a tendency to value the highest dose less than the 37.5 mg/kg dose). This increase in crossover points serves as a proxy for the value of GHB to current GHB users, with higher crossover points suggesting higher reinforcing efficacy. Based on these results, GHB appears likely to function as a dose-dependent reinforcer for humans. To further substantiate this observation we assessed participant's proclivity to choose GHB relative to placebo in a choice paradigm. Participants overwhelmingly chose GHB over placebo when given the opportunity on five different occasions (24/25 choices were for GHB). These results provide further evidence that GHB can likely function as a reinforcer for humans engaged in recreational use of GHB, a finding that is consistent with the limited research in this area (e.g., Carter et al., 2006). The inclusion of two measures of drug taking (i.e., the MCP and a traditional choice task) in the same group of participants is a novel contribution to the existing literature on GHB in humans. The results from both assays suggest that GHB can serve as a reinforcer for regular users of GHB. This provides some additional validation of each procedure.
The results of the present study also have implications for current examinations of the utility of GHB in the treatment of an existing substance use disorder (i.e., to treat acute alcohol or opiate withdrawal symptoms). As mentioned previously, the potential therapeutic uses of GHB are often accompanied by concerns about the possible risk for abuse and misuse of the drug. Currently, researchers are examining the risks and benefits of applying GHB in the treatment of alcoholism (e.g., Leone et al., 2010; Sewell & Petrakis, 2011). Specifically, concerns exist about whether the use of GHB to treat a patient with a history of substance abuse will result in the abuse of GHB. When prescribed to treat alcohol withdrawal symptoms, GHB is typically prescribed in doses of 50–100 mg/kg divided over three to four doses per day (see Leggio, Kenna, & Swift (2008) for a review of GHB doses that have been effective in the treatment of alcohol withdrawal). The abuse potential of GHB does not necessarily preclude an examination of its utility as a treatment agent for reducing the symptoms of acute alcohol withdrawal. Given that our results showed a linear increase in crossover values across GHB doses (even at doses as low as 12.5 mg/kg and 25 mg/kg), additional research on the potential risks of prescribing GHB to a patient with a history of a substance use disorder is clearly warranted.
Several future directions for research are warranted based on our findings. During the second exposure to GHB on the MCP sessions, most participants' crossover point for the 50 mg dose was less than at the 37.5 mg dose. This finding may indicate a developing tolerance to GHB, or it may indicate that higher doses of GHB result in a level of intoxication or sedation that reduces the reinforcing value. Either way, this finding is interesting and warrants further evaluation. Additionally, future studies would benefit from collecting data on participants' typical GHB dose and route of administration in their home environments to determine whether this influences choices on the MCP or preference for lower versus higher doses. The extent to which these findings can be generalized to GHB-naïve participants is also an important area of future research, given the results of Oliveto et al. (2010) that indicated that stimulant-like effects of GHB are often reported by those without sedative abuse backgrounds.
Our results provide an enhanced understanding of the relative value, and potential reinforcing efficacy, of GHB that makes a significant scientific contribution to the very limited research currently available on the abuse potential and reinforcing value of GHB in humans. The sample size for the present study (N = 5) limits the external validity of the findings, and future replications of the present study using a larger participant sample may be warranted. While our results appear to support the hypothesis that GHB operates as a dose-dependent reinforcer in humans, further self-administration studies will be needed to substantiate this claim.
Acknowledgments
This work was supported by NIDA RO1DA14871. The authors wish to thank Arif Kareem, MD, Christina Harding, Ph.D., Desey Tzortzias, Todd Helmus, PhD, Christine Waimey, Ph.D., and James Anderson, Ph.D.
REFERENCES
- Abanades S, Farré M, Barral D, Torrens M, Closas N, Langohr K, Pastor A, et al. Relative abuse liability of gamma-hydroxybutyric acid, flunitrazepam, and ethanol in club drug users. Journal of Clinical Psychopharmacology. 2007;27(6):625–638. doi: 10.1097/jcp.0b013e31815a2542. [DOI] [PubMed] [Google Scholar]
- Abanades S, Farré M, Segura M, Pichini S, Barral D, Pacifici R, Pellegrini M, et al. Gamma-hydroxybutyrate (GHB) in humans: pharmacodynamics and pharmacokinetics. Annals of the New York Academy of Sciences. 2006;1074:559–576. doi: 10.1196/annals.1369.065. [DOI] [PubMed] [Google Scholar]
- Beardsley P.M, Balster R.L, Harris L.S. Evaluation of the discriminative stimulus and reinforcing effects of gammahydroxybutyrate (GHB) Psychopharmacology. 1996;127(4):315. doi: 10.1007/s002130050092. [DOI] [PubMed] [Google Scholar]
- Carter L.P, Griffiths R.R, Mintzer M.Z. Cognitive, psychomotor, and subjective effects of sodium oxybate and triazolam in healthy volunteers. Psychopharmacology. 2009;206(1):141–154. doi: 10.1007/s00213-009-1589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter L.P, Koek W, France C.P. Behavioral analyses of GHB: receptor mechanisms. Pharmacology & Therapeutics. 2009;121:100–114. doi: 10.1016/j.pharmthera.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter L.P, Pardi D, Gorsline J, Griffiths R.R. Illicit gamma-hydroxybutyrate (GHB) and pharmaceutical sodium oxybate (Xyrem®): Differences in characteristics and misuse. Drug and Alcohol Dependence. 2009;104((1–2)):1–10. doi: 10.1016/j.drugalcdep.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter L.P, Richards B.D, Mintzer M.Z, Griffiths R.R. Relative abuse liability of GHB in humans: A comparison of psychomotor, subjective, and cognitive effects of supratherapeutic doses of triazolam, pentobarbital, and GHB. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2006;31(11):2537–2551. doi: 10.1038/sj.npp.1301146. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Fà M, Lobina C, Reali R, Gessa G.L. Gamma-hydroxybutyric acid intake in ethanol-preferring sP and -nonpreferring sNP rats. Physiology & Behavior. 1998;64(2):197–202. doi: 10.1016/s0031-9384(98)00033-x. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Fadda F, Gessa G.L. Symmetrical generalization between the discriminative stimulus effects of gamma-hydroxybutyric acid and ethanol: occurrence within narrow dose ranges. Physiology & Behavior. 1995;57(1):105–111. doi: 10.1016/0031-9384(94)00215-q. [DOI] [PubMed] [Google Scholar]
- deWit H, Pierri J, Johanson C.E. Assessing individual differences in ethanol preference using a cumulative dosing procedure. Psychopharmacology. 1989;98(1):113–119. doi: 10.1007/BF00442016. [DOI] [PubMed] [Google Scholar]
- Ferrara S.D, Giorgetti R, Zancaner S, Orlando R, Tagliabracci A, Cavarzeran F, Palatini P. Effects of single dose of gamma-hydroxybutyric acid and lorazepam on psychomotor performance and subjective feelings in healthy volunteers. European Journal of Clinical Pharmacology. 1999;54(11):821–827. doi: 10.1007/s002280050560. [DOI] [PubMed] [Google Scholar]
- Frison G, Tedeschi L, Maietti S, Ferrara S.D. Determination of γ-hydroxybutyric acid (GHB) in plasma and urine by headspace solid-phase microextraction and gas chromatography/positive ion chemical ionization mass spectrometry. Rapid Communications in Mass Spectrometry. 2000;14(24):2401–2407. doi: 10.1002/1097-0231(20001230)14:24<2401::AID-RCM179>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gallimberti L, Spella M.R, Soncini C.A, Gessa G.L. Gamma-hydroxybutyric acid in the treatment of alcohol and heroin dependence. Alcohol (Fayetteville, N.Y.) 2000;20(3):257–262. doi: 10.1016/s0741-8329(99)00089-0. [DOI] [PubMed] [Google Scholar]
- Goodwin A.K, Kaminski B.J, Griffiths R.R, Ator N.A, Weerts E.M. Intravenous self-administration of gamma-hydroxybutyrate (GHB) in baboons. Drug and Alcohol Dependence. 2011;114:217–224. doi: 10.1016/j.drugalcdep.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J.C. Gamma hydroxy butyrate (GHB) abuse in the United States. Office of Applied Statistics: Substance Abuse and Mental Health Services Administration; 1997. [Google Scholar]
- Griffiths R.R, Rush C.R, Puhala K. Validation of the Multiple-Choice Procedure for investigating drug reinforcement in humans. Experimental and Clinical Psychopharmacology. 1996;4(1):97–106. [Google Scholar]
- Griffiths R.R, Troisi J, Silverman K, Mumford G. Multiple-choice procedure: an efficient approach for investigating drug reinforcement in humans. Behavioural Pharmacology. 1993;4(1):3–13. [PubMed] [Google Scholar]
- Higgins S.T, Bickel W.K, Hughes J.R. Methods in the human behavioral pharmacology of drug abuse. In: van Haaren F, editor. Methods in behavioral pharmacology. New York: Elsevier; 1993. pp. 475–498. (Ed.) [Google Scholar]
- Higgins S.T, Roll J.M, Bickel W.K. Alcohol pretreatment increases preference for cocaine over monetary reinforcement. Psychopharmacology. 1996;123:1–8. doi: 10.1007/BF02246274. [DOI] [PubMed] [Google Scholar]
- Ingels M, Rangan C, Bellezzo J, Clark R.F. Coma and respiratory depression following the ingestion of GHB and its precursors: Three cases. Journal of Emergency Medicine. 2000;19(1):47–50. doi: 10.1016/s0736-4679(00)00188-8. [DOI] [PubMed] [Google Scholar]
- Johanson C.E, de Wit H. The use of choice procedures for assessing the reinforcing properties of drugs in humans. NIDA Research Monograph. 1989;92:171–210. [PubMed] [Google Scholar]
- Johanson C.E, Mattox A, Schuster C.R. Conditioned reinforcing effects of capsules associated with high versus low monetary payoff. Psychopharmacology. 1995;120(1):42–48. doi: 10.1007/BF02246143. [DOI] [PubMed] [Google Scholar]
- Kam P.C.A, Yoong F.F.Y. Gamma-hydroxybutyric acid: an emerging recreational drug. Anaesthesia. 1998;53(12):1195–1198. doi: 10.1046/j.1365-2044.1998.00603.x. [DOI] [PubMed] [Google Scholar]
- Kidorf M, Stitzer M.L, Griffiths R.R. Evaluating the reinforcement value of clinic-based privileges through a multiple choice procedure. Drug and Alcohol Dependence. 1995;39(3):167–172. doi: 10.1016/0376-8716(95)01136-7. [DOI] [PubMed] [Google Scholar]
- Kim S.Y, Anderson I.B, Dyer J.E, Barker J.C, Blanc P.D. High-risk behaviors and hospitalizations among gamma-hydroxybutyrate (GHB) users. The American Journal of Drug and Alcohol Abuse. 2007;33(3):429–438. doi: 10.1080/00952990701312316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Mercer S.L, Coop A, France C.P. Behavioral effects of gamma-hydroxybutyrate, its precursor gamma-butyrolactone, and GABA(B) receptor agonists: time course, and differential antagonism by the GABA(B) receptor antagonist 3-aminopropyl (diethoxymethyl) phosphinic acid (CGP35348) Journal of Pharmacology and Experimental Therapeutics. 2009;330:876–883. doi: 10.1124/jpet.109.151845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Kenna G.A, Swift R.M. New developments for the pharmacological treatment of alcohol withdrawal syndrome. A focus on non-benzodiazepine GABAergic medications. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32:1106–1117. doi: 10.1016/j.pnpbp.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Leone M.A, Vigna-Taglianti F, Avanzi G, Brambilla R, Faggiano F. Gamma-hydroxybutyrate (GHB) for treatment of alcohol withdrawal and prevention of relapses. 2010. Retrieved from http://onlinelibrary.wiley.com/o/cochrane/clsysrev/articles/CD006266/abstract.html. [DOI] [PubMed]
- Mamelak M. Narcolepsy and depression and the neurobiology of gammahydroxybutyrate. Progress in Neurobiology. 2009;89(2):193–219. doi: 10.1016/j.pneurobio.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Oliveto A, Gentry W.B, Pruzinsky R, Gonsai K, Kosten T.R, Martell B, Poling J. Behavioral effects of gamma-hydroxybutyrate in humans. Behavioural Pharmacology. 2010;21(4):332–342. doi: 10.1097/FBP.0b013e32833b3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharsight Corporation. WinNonlin Version 4.1. Mountain View, CA: Pharsight Corporation; 1998. [Google Scholar]
- Schmitz J.M, Sayre S.L, Hokanson P.S, Spiga R. Assessment of the relative reinforcement value of smoking and drinking using a multiple-choice measurement strategy. Nicotine & Tobacco Research. 2003;5(5):729–734. doi: 10.1080/1462220031000158618. [DOI] [PubMed] [Google Scholar]
- Scrima L, Hartman P.G, Johnson F.H, Jr, Thomas E.E, Hiller F.C. The effects of gamma-hydroxybutyrate on the sleep of narcolepsy patients: a double-blind study. Sleep. 1990;13(6):479–490. doi: 10.1093/sleep/13.6.479. [DOI] [PubMed] [Google Scholar]
- Sewell R.A, Petrakis I.L. Does gamma-hydroxybutyrate (GHB) have a role in the treatment of alcoholism. Alcohol and Alcoholism. 2011;46(1):1–2. doi: 10.1093/alcalc/agq086. [DOI] [PubMed] [Google Scholar]
- StataCorp. (2009). Stata Statistical Software: Release 11. College Station, TX: StateCorp LP; 2009. [Google Scholar]
- Wang Y.G, Swick T.J, Carter L.P, Thorpy M.J, Benowitz N.L. Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion. Journal of Clinical Sleep Medicine. 2009;5(4):365–371. [PMC free article] [PubMed] [Google Scholar]
- Weiss K.G, Colyer C.J. Roofies, mickies and cautionary tales: Examining the persistence of the “Date-Rape Drug” crime narrative. Deviant Behavior. 2010;31(4):348. [Google Scholar]
- Winter J.C. The stimulus properties of gamma-hydroxybutyrate. Psychopharmacology. 1981;73(4):372–375. doi: 10.1007/BF00426468. [DOI] [PubMed] [Google Scholar]
- Woolverton W.L, Johanson C.E. Preference in rhesus monkeys, given a choice between cocaine and d,l-cathionine. Journal of the Experimental Analysis of Behavior. 1984;41:35–43. doi: 10.1901/jeab.1984.41-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton W.L, Rowlett J.K, Winger G, Woods J.H, Gerak L.R, France C.P. Evaluation of the reinforcing and discriminative stimulus effects of gamma-hydroxybutyrate in rhesus monkeys. Drug and Alcohol Dependence. 1999;54(2):137–143. doi: 10.1016/s0376-8716(98)00153-7. [DOI] [PubMed] [Google Scholar]