Abstract
Introduction
Noninvasive methods are needed to detect distal sensory polyneuropathy in HIV-infected persons on antiretroviral therapy (ART).
Methods
Quantitative sudomotor axon reflex test (QSART) and Utah Early Neuropathy Scale (UENS), small-fiber sensitive measures, were assessed in subjects with and without clinical neuropathy. Pain was assessed by visual analog scale (VAS).
Results
Twenty-two subjects had symptoms and signs of neuropathy, 19 had neither, and all were receiving ART. Median sweat volume (μL) was lower at all testing sites in those with neuropathy compared to those without (p<0.01 for all). UENS and VAS (mm) were higher in neuropathy subjects (p<0.05 for each). Lower sweat volume at all sites correlated with higher pin UENS subscore, total UENS, and VAS (p<0.05 for all). In multivariable analyses adjusting for age, CD4+ T cells, sex, and use of “d-drug” ART, QSART and UENS remained associated (p=0.003).
Conclusion
QSART and UENS have not been previously studied in this patient population and may identify small-fiber neuropathy in HIV-infected, ART-treated persons.
Keywords: HIV, QSART, UENS, neuropathy, antiretroviral
INTRODUCTION
Antiretroviral therapy (ART) has dramatically improved morbidity and mortality of HIV infection and AIDS, and nucleoside reverse transcriptase inhibitors (NRTIs) remain a cornerstone of therapy for HIV infection. However, neurologic complications from these agents, particularly the “d-drugs” didanosine (ddI) and stavudine (d4T), are common and may be debilitating and irreversible(Cherry et al., 2006; Evans et al., 2011). Mitochondrial toxicity may explain the predilection of these agents to cause peripheral neuropathy(Cui et al., 1997). Although newer classes of ART are available, NRTIs including d4T remain important treatment options in resource-limited settings, causing peripheral neuropathy in many individuals(Maritz et al., 2010). Current therapies for neuropathy in HIV-infected persons on ART provide only symptomatic relief with limited efficacy(Phillips et al., 2010; Simpson et al., 2008; Simpson et al., 2010; Valcour et al., 2009). Antiretroviral therapy-associated neuropathy may present with non-specific symptoms and minimal objective clinical findings, leading to a delayed diagnosis. As a predominately small-fiber neuropathy, nerve conduction studies are often normal, especially early(Brew, 2003). Therefore, reliable and noninvasive methods are needed to detect and monitor distal sensory polyneuropathy in HIV-infected persons on ART.
Quantitative sudomotor axon reflex test (QSART) is sensitive(Low et al., 2006) and noninvasive. It has been studied in other small-fiber neuropathies, including diabetes, where it correlates with somatic C fiber loss of intraepidermal nerve fiber density (IENFD) in some studies(Novak et al., 2001; Singer et al., 2004; Tobin et al., 1999) but not in others(Periquet et al., 1999; Smith et al., 2006). Autonomic dysfunction has been described in previous HIV positive cohorts and is greater in patients with longer duration of infection(Becker et al., 1997; Freeman et al., 1990). Therefore, it is reasonable to hypothesize that sudomotor fibers innervating sweat glands, which are unmyelinated sympathetic C fibers, would be injured in parallel and would have abnormal function in HIV neuropathy.
Traditional neuropathy examination scales used to assess HIV patients with neuropathy (e.g. the Total Neuropathy Score and Brief Peripheral Neuropathy Screen) focus a large proportion of their score on large fiber functions, such as vibration sensation and absent deep tendon reflexes. These scales may be less sensitive to the small-fiber neuropathy characteristic of ART, which could lead to lower sensitivity in HIV-infected patients with earlier neuropathy (Cherry et al., 2005; Cornblath et al., 1999). The Utah Early Neuropathy Scale (UENS), while incorporating large-fiber measurements, preferentially weights small-fiber changes(Singleton et al., 2008) and may be useful in this population.
Neither QSART nor UENS have been evaluated in HIV-infected patients on ART. The purpose of our study was to characterize QSART and UENS in HIV-infected patients virologically suppressed on ART at risk for distal sensory polyneuropathy. We hypothesized that QSART and UENS would distinguish HIV-infected persons with distal sensory polyneuropathy on ART from HIV-infected patients without neuropathy.
MATERIALS AND METHODS
Study design
Data are from a prospective cohort of chronically HIV-infected individuals enrolled from June 2005 to September 2008. The cohort was established to assess the contribution of oxidant stress to the pathogenesis of ART-related toxicities, including metabolic and neurologic complications.
Study population
Inclusion criteria. Age ≥ 18 years, HIV diagnosis ≥ 12 months prior to enrollment, current ART including at least two NRTIs for ≥ 24 consecutive weeks, and plasma HIV-1 RNA < 10,000 copies/mL within 180 days of enrollment. At subsequent study follow-up visits, viral load could be higher as this was an observational cohort study, and providers were able to adjust antiretroviral therapy as necessary. All participants were receiving primary HIV care at the Comprehensive Care Center in Nashville, Tennessee. Exclusion criteria. Active opportunistic infection, documented lactic acidosis within 12 months of enrollment, diabetes mellitus not diet-controlled, recent systemic anti-neoplastic chemotherapy, and any history of exposure to pharmacologic agents associated with peripheral neuropathy other than ART. The study was approved by the Vanderbilt University Institutional Review Board, and all participants provided written informed consent. Individuals were categorized as neuropathy subjects if they had at least one of the following symmetrical signs of peripheral neuropathy - decreased vibratory sensation at the distal interphalangeal joint of the great toes, decreased or absent Achilles deep tendon reflexes (DTRs) with intact patellar reflexes, and/or decreased sharp sensation of the lower extremities measured by pin prick - and bilateral symptoms consistent with peripheral neuropathy. Initial exam was performed by the research coordinator prior to UENS to avoid bias. These criteria have been previously used in studies of distal sensory polyneuropathy in the setting of HIV infection(Robinson-Papp et al., 2009). Study participants were classified as non-neuropathy subjects if they did not have signs or symptoms of peripheral neuropathy.
Data collection
Current ART, body mass index (BMI), smoking status, and self-reported use of anti-inflammatory drugs were assessed. A targeted neurologic exam was performed using the UENS with a 128 Hz tuning fork and a number two safety pin, as previously described(Singleton et al., 2008). Current and recent history of neuropathic pain was assessed using a standard 100-mm visual analog scale (VAS)(Jensen et al., 2003; Price et al., 1994). QSART was performed using the Q-Sweat Quantitative Sweat Measurement System (WR Medical Electronics Corporation, Stillwater, MN) with iontophoresis of 10% acetylcholine at 2 mA over 5 minutes, recording the sweat response for an additional 5 minutes, for a total of 10 minutes. QSART was recorded at four standard sites (forearm at 75% of the distance from the ulnar epicondyle to the pisiform bone, proximal lateral leg 5 cm distal to the fibular head, distal leg medial aspect 5 cm proximal to the medial malleolus, and dorsal proximal foot), as previously described(Sletten et al., 2010). The most recent HIV-1 RNA and CD4+ T cell determinations (assayed at a commercial reference laboratory – Laboratory Corporation of America, Birmingham, AL) within 3 months of the above measures were obtained from the medical record.
Statistical analyses
Characteristics of study participants are presented using median and interquartile range (IQR) for continuous variables unless otherwise stated. Frequencies and proportions were used for categorical variables. Spearman's correlation was used to assess univariate relationships between QSART, UENS, VAS, age, CD4+ T cells, HIV-1 RNA, and BMI. Wilcoxon rank-sum test was used to compare the distribution of continuous variables between groups. Multivariable analyses of the associations between QSART, UENS, and VAS were performed using linear fixed-effects regression models adjusting for covariates CD4+ T cells (unit 100 cells/mm3), age (unit 1 year), sex, and current or prior use of d-drug NRTIs (d4T and/or ddI). For these multivariable models, total sweat volume, total UENS, and worst pain over the past week by VAS were chosen as those were felt to be of a priori clinical significance. Exponential of the regression coefficients were then computed, indicating fold change in the outcome variable of interest by one interquartile increase in the corresponding covariate. Adjustment was not made for multiple comparisons. Analyses were performed using Stata IC version 10.0 (Stata Corporation, College Station, TX).
RESULTS
Clinical characteristics
Clinical characteristics of the 41 study subjects (22 neuropathy; 19 non-neuropathy) are shown in Table 1. There were nine women and median age was 48 years. Forty-nine percent were of non-white race, and there was a trend toward more persons of non-white race in the neuropathy group (p=0.06). Subjects had median (IQR) CD4+ T cells 455 (333–717) cells/mm3. Fifty-six percent had undetectable HIV-1 RNA levels, defined as below the limit of assay detection (<50 copies/mL), and HIV-1 RNA ranged from 50 to 26750 copies/mL. Median BMI overall was 24.9 (22.6–27.8) kg/m2. BMI did not differ in neuropathy versus non-neuropathy subjects or in males versus females. Five subjects (12%) were receiving ddI and two (5%) d4T. One individual was previously taking ddI 238 days before neuropathy assessments were performed. Twenty-nine (71%) subjects were receiving protease inhibitor (PI)-containing ART and 15 (36%) a non-nucleoside reverse transcriptase inhibitor (NNRTI). The distribution of current ART did not differ according to gender or by cases versus controls. Clinical characteristics did not differ according to current or prior use of d-drug NRTIs or in those with detectable versus undetectable viral load.
Table 1.
Clinical characteristics of study subjects.
| Characteristica | Overall (N=41) | No Neuropathy (N=19) | Neuropathy (N=22) | pb | Males (N=32) | Females (N=9) | pc |
|---|---|---|---|---|---|---|---|
| Sex – n (%) | |||||||
| Male | 32 (78%) | 14 (24%) | 18 (82%) | 0.70 | -- | -- | -- |
| Female | 9 (22%) | 5 (26%) | 4 (18%) | ||||
| Race – n (%) | |||||||
| White | 21 (51%) | 13 (68%) | 8 (36%) | 0.06 | 17 (53%) | 4 (44%) | 0.71 |
| Non-white | 20 (49%) | 6 (32%) | 14 (64%) | 15 (47%) | 5 (56%) | ||
| Age, year | 48 (43–52) | 48 (40–55) | 48 (44–51) | 0.74 | 48 (43–52) | 49 (42–55) | 0.72 |
| CD4+ T cells/mm3 | 455 (333–717) | 430 (333–791) | 502 (281–717) | 0.89 | 472 (338–723) | 416 (286–509) | 0.46 |
| HIV-1 RNA (median, range) | 50 (50–26750) | 50 (50–419) | 53 (50–26750) | 0.24 | 50 (50–11605) | 60 (50–26750) | 0.17 |
| HIV-1 RNA < 50 – n (%) | 23 (56%) | 12 (63%) | 11 (50%) | 0.53 | 20 (63%) | 3 (33%) | 0.14 |
| BMI, kg/m2 | 24.9 (22.6–27.8) | 25.7 (22.6–28.8) | 24.3 (22.3–27.3) | 0.60 | 24.3 (22.5–26.9) | 28.8 (23.5–44.2) | 0.12 |
| NRTIs – n (%) | |||||||
| Abacavir | 9 (22%) | 6 (32%) | 3 (14%) | 0.26 | 8 (25%) | 1 (11%) | 0.65 |
| Tenofovir | 20 (49%) | 9 (47%) | 11 (50%) | 1.00 | 16 (50%) | 4 (44%) | 1.00 |
| Zidovudine | 22 (54%) | 10 (53%) | 12 (54%) | 1.00 | 16 (50%) | 6 (67%) | 0.46 |
| d4T | 2 (5%) | 1 (5%) | 1 (5%) | 1.00 | 1 (3%) | 1 (11%) | 0.39 |
| ddI | 5 (12%) | 1 (5%) | 4 (18%) | 0.35 | 4 (12%) | 1 (11%) | 1.00 |
| PI-based ART – n (%) | 29 (71%) | 13 (68%) | 16 (73%) | 1.00 | 24 (75%) | 5 (56%) | 0.40 |
| Atazanavir | 10 (24%) | 3 (16%) | 7 (32%) | 0.29 | 8 (25%) | 2 (22%) | 1.00 |
| Lopinavir | 14 (34%) | 8 (42%) | 6 (27%) | 0.34 | 12 (37%) | 2 (22%) | 0.69 |
| Nelfinavir | 3 (7%) | 1 (5%) | 2 (9%) | 1.00 | 2 (6%) | 1 (11%) | 0.53 |
| Fosamprenavir | 2 (5%) | 1 (5%) | 1 (5%) | 1.00 | 2 (6%) | 0 (0%) | 1.00 |
| NNRTI-based ART – n (%) | 15 (36%) | 7 (37%) | 8 (36%) | 1.00 | 11 (35%) | 4 (44%) | 0.70 |
| Efavirenz | 10 (24%) | 4 (21%) | 6 (27%) | 0.72 | 7 (22%) | 3 (33%) | 0.66 |
| Nevirapine | 5 (12%) | 3 (16%) | 2 (9%) | 0.64 | 4 (13%) | 1 (11%) | 1.00 |
Median (IQR) unless otherwise noted
For difference between individuals with and without neuropathy;
For difference according to sex
-- = reference
HIV=human immunodeficiency virus, BMI=body mass index, NSAID=nonsteroidal anti-inflammatory drug, NRTI=nucleoside reverse transcriptase inhibitor, ART=antiretroviral therapy, PI=protease inhibitor, NNRTI=non-nucleoside reverse transcriptase inhibitor
Quantitative sudomotor axon reflex test (QSART)
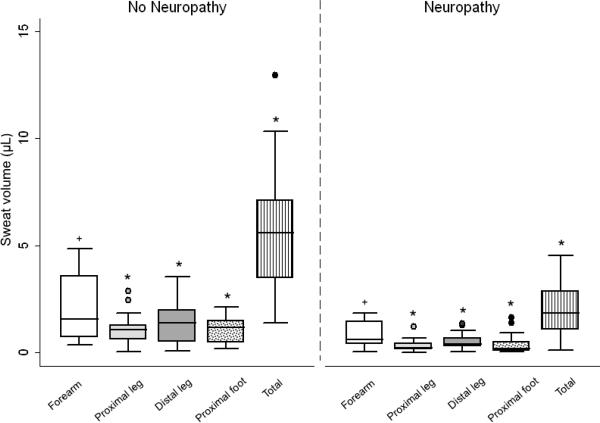
Figure 1A compares sweat volume in neuropathy and non-neuropathy subjects. A length-dependent decrease was seen in both study groups, as expected. Sweat volume did not differ in males compared to females at the forearm [1.22 (0.40–1.74) vs. 1.00 (0.40–1.40) μL, p=0.56], proximal leg [0.50 (0.17–1.08) vs. 0.42 (0.21–0.87) μL, p=0.85], distal leg [0.65 (0.32–1.49) vs. 0.48 (0.36–1.06) μL, p=0.63], or foot [0.48 (0.13–1.32) vs. 0.44 (0.24–1.17) μL, p=0.98], although the number of females was small (n=9). Neuropathy subjects had significantly lower median sweat volume at all testing sites compared to non-neuropathy subjects (p=0.006 at forearm, p<0.001 for other testing sites, and p<0.001 for total sweat volume). Sweat volume did not correlate with age, BMI, CD4+ T cells, or HIV-1 RNA. Response latency of sweat production was prolonged in neuropathy subjects compared to non-neuropathy subjects at the proximal leg [2.20 (1.52–3.00) vs. 1.07 (0.58–2.12) minutes, p=0.03)]. Response latency did not differ according to sex. Sweat volume at the distal leg was lower in those with current or prior exposure to ddI compared to those who did not have exposure to this agent [0.29 (0.06–0.52) vs. 0.67 (0.36–1.43) μL, p=0.02], but did not differ at any site in those with versus without current or prior exposure to d4T.
Figure 1.
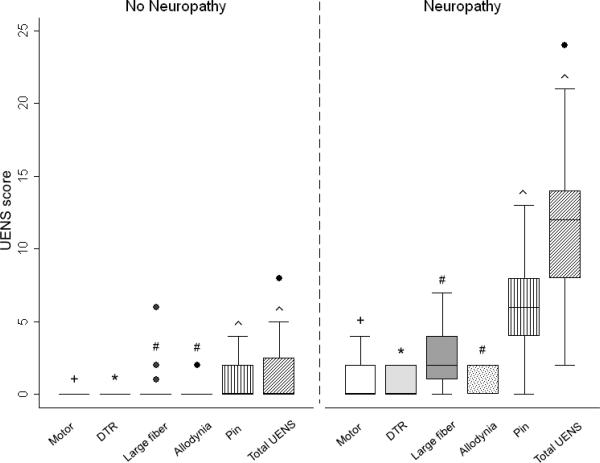
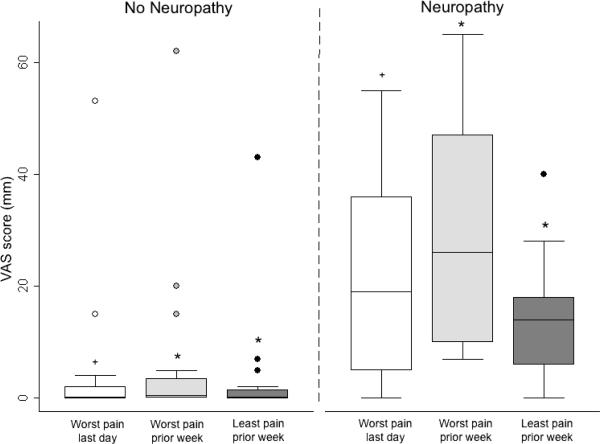
Neuropathy assessments according to the presence (neuropathy subjects) or absence (non-neuropathy subjects) of clinical neuropathy. (A) Sweat volume (μL) by quantitative sudomotor axon reflex test (QSART). Testing sites are as follows: white bar=forearm, light gray bar=proximal leg, dark gray bar=distal leg, dot filled bar=proximal foot, and vertical filled bar=total sweat volume. +p=0.006 for difference between study groups and *p<0.001 for difference between study groups. (B) Utah Early Neuropathy Scale (UENS). Elements of the UENS are as follows: white bar=motor subscore, light gray bar=deep tendon reflex (DTR) subscore, dark gray bar=large fiber subscore, dot filled bar=allodynia subscore, vertical filled bar=pin sensation subscore, and diagonal filled bar=total UENS score. +p=0.02 for difference between study groups, *p=0.009 for difference between study groups, #p=0.001 for difference between study groups, and ^p<0.001 for difference between study groups. (C) Visual analog scale (VAS, mm). Elements of the VAS are as follows: white bar=worst pain over the last day, light gray bar=worst pain during the prior week, and dark gray bar=least pain over the prior week. +p=0.001 for difference between study groups and *p<0.001 for difference between study groups.
Utah Early Neuropathy Scale (UENS) and visual analog scale (VAS)
UENS and VAS were incorporated into the protocol at a later time than QSART; thus, data were available in 33 individuals (16 without neuropathy, 17 with neuropathy). All elements of the UENS [median (range)] were higher in neuropathy subjects than non-neuropathy subjects, particularly the pin subscore [6 (4–8) vs. 0 (0–2)] and total UENS [12 (8–14) vs. 0 (0–2.5), p<0.001 for both], as shown in Figure 1B. There was no correlation between UENS and age, BMI, CD4+ T cells, or HIV-1 RNA. UENS did not differ according to sex, current or prior use of d-drugs or detectable versus undetectable viral load.
Compared to non-neuropathy subjects, neuropathy subjects had significantly higher VAS scores for worst pain over the past 24 hours (p=0.001) and also for worst pain over the past week and least pain over the past week (p<0.001 for both), as shown in Figure 1C. Compared to those with undetectable viral load, study participants with detectable viral load tended to have higher scores for worst pain over the past 24 hours (p=0.06).
Correlations between measures of neuropathy
Univariate analysis (Table 2). Proximal leg. Lower proximal leg sweat volume was associated with higher pin (p=0.003) and large fiber (p=0.008) UENS subscores and with total UENS score (p<0.001). It was associated with higher worst pain over the past week and least pain over the past week VAS scores (p=0.02 for both). Distal leg. Lower distal leg sweat volume was associated higher pin UENS subscore (p=0.03) and total UENS score (p=0.01). Lower distal leg sweat volume was associated with higher worst pain over the past 24 hours (p=0.01) and with higher worst pain over the past week and least pain over the past week (p=0.03 for both). Foot. Lower foot sweat volume was associated with higher pin (p=0.008), motor (trend, p=0.05) and DTR (p=0.02) UENS subscores and higher total UENS (p=0.001). Lower foot sweat volume was associated with higher least pain in the past week (p=0.01) and tended to be associated with higher worst pain in the past week (p=0.05). Total. Lower total sweat volume was associated with higher pin (p=0.006), allodynia (p=0.03), large fiber (p=0.02), and DTR (p=0.02) UENS subscores and total UENS (p<0.001). Higher total sweat volume was associated with higher scores on all elements of the VAS (p<0.05 for all). Multivariable analysis. Table 3 shows results of multivariable analysis with fixed-effect regression models of the association between total sweat volume by QSART, total UENS score, and worst pain over the past week by VAS, adjusting for the following covariates: age, CD4+ T cells, sex, and current or prior d-drug NRTI use. Lower total sweat volume was associated with higher total UENS (β=0.79; 95% CI: 0.68–0.92, p=0.003). Higher total UENS was associated with higher worst pain over the past week (β=1.25, 95% CI: 1.17–1.34, p<0.001).
Table 2.
Correlations between neuropathy assessments.
| QSART measurement site | Spearman's rho | p |
|---|---|---|
| Forearm | ||
| UENS | ||
| Motor | −0.26 | 0.13 |
| Pin | −0.38 | 0.02 |
| Allodynia | −0.30 | 0.08 |
| Large fiber | −0.36 | 0.03 |
| DTR | −0.27 | 0.11 |
| Total UENS score | −0.51 | 0.002 |
| VAS | ||
| Worst pain in the past 24 hours | −0.26 | 0.15 |
| Worst pain in the past week | −0.33 | 0.06 |
| Least pain in the past week | −0.37 | 0.04 |
| Proximal Leg | ||
| UENS | ||
| Motor | −0.29 | 0.09 |
| Pin | −0.49 | 0.003 |
| Allodynia | −0.19 | 0.27 |
| Large fiber | −0.45 | 0.008 |
| DTR | −0.29 | 0.09 |
| Total UENS score | −0.55 | <0.001 |
| VAS | ||
| Worst pain in the past 24 hours | −0.23 | 0.20 |
| Worst pain in the past week | −0.40 | 0.02 |
| Least pain in the past week | −0.41 | 0.02 |
| Distal Leg | ||
| UENS | ||
| Motor | −0.30 | 0.07 |
| Pin | −0.36 | 0.03 |
| Allodynia | −0.24 | 0.17 |
| Large fiber | −0.19 | 0.27 |
| DTR | −0.30 | 0.08 |
| Total UENS score | −0.41 | 0.01 |
| VAS | ||
| Worst pain in the past 24 hours | −0.43 | 0.01 |
| Worst pain in the past week | −0.38 | 0.03 |
| Least pain in the past week | −0.38 | 0.03 |
| Proximal Foot | ||
| UENS | ||
| Motor | −0.34 | 0.05 |
| Pin | −0.45 | 0.008 |
| Allodynia | −0.32 | 0.06 |
| Large fiber | −0.31 | 0.07 |
| DTR | −0.39 | 0.02 |
| Total UENS score | −0.53 | 0.001 |
| VAS | ||
| Worst pain in the past 24 hours | −0.30 | 0.09 |
| Worst pain in the past week | −0.35 | 0.05 |
| Least pain in the past week | −0.41 | 0.01 |
| Total Sweat Volume | ||
| UENS | ||
| Motor | −0.32 | 0.06 |
| Pin | −0.46 | 0.006 |
| Allodynia | −0.37 | 0.03 |
| Large fiber | −0.40 | 0.02 |
| DTR | −0.37 | 0.02 |
| Total UENS score | −0.58 | <0.001 |
| VAS | ||
| Worst pain in the past 24 hours | −0.35 | 0.04 |
| Worst pain in the past week | −0.42 | 0.01 |
| Least pain in the past week | −0.47 | 0.007 |
QSART=quantitative sudomotor axon reflex test, UENS=Utah Early Neuropathy Scale, DTR=deep tendon reflex, VAS=visual analog scale
Table 3.
Multivariable assessment of associations between neuropathy measures.
| Parameter | Regression coefficient (95% CI)a | p |
|---|---|---|
| Total sweat volume by QSART and total UENS score | ||
| Variable | ||
| UENS score | 0.79 (0.68–0.92) | 0.003 |
| Age (unit=1 year) | 0.98 (0.86–1.12) | 0.81 |
| CD4+ T cells (unit = 100 cells/mm3) | 1.00 (0.99–1.00) | 0.08 |
| Sex | 0.61 (0.06–5.96) | 0.66 |
| Current or prior d-drug NRTI use | 0.33 (0.03–3.72) | 0.36 |
| Total sweat volume by QSART and worst pain over past week by VAS | ||
| Variable | ||
| Worst pain over past week | 0.96 (0.91–1.02) | 0.20 |
| Age (unit=1 year) | 0.98 (0.84–1.13) | 0.79 |
| CD4+ T cells (unit = 100 cells/mm3) | 1.00 (0.99–1.00) | 0.08 |
| Sex | 0.84 (0.05–12.89) | 0.90 |
| Current or prior d-drug NRTI use | 0.34 (0.02–5.18) | 0.42 |
| Total UENS score and worst pain over past week by VAS | ||
| Variable | ||
| Worst pain over last week | 1.25 (1.17–1.34) | <0.001 |
| Age (unit=1 year) | 1.03 (0.87–1.23) | 0.66 |
| CD4+ T cells (unit = 100 cells/mm3) | 0.99 (0.99–1.00) | 0.62 |
| Sex | 0.10 (0.00–2.46) | 0.15 |
| Current or prior d-drug NRTI use | 0.46 (0.01–11.07) | 0.62 |
Data are exponential of regression coefficients (95% confidence interval), which indicates fold change in the outcome variable of interest by one interquartile increase in the corresponding covariate.
CI=confidence interval, QSART=quantitative sudomotor axon reflex test, UENS=Utah Early Neuropathy Scale, VAS=visual analog scale, NRTI=nucleoside reverse transcriptase inhibitor
DISCUSSION
The most important finding of this study is that QSART and UENS, noninvasive measures of small-fiber function, were significantly different in HIV-infected persons on ART with clinical neuropathy compared to those without clinical neuropathy and were highly correlated. We are, to our knowledge, the first to report the use of QSART and UENS for neuropathy assessment in the setting of HIV infection. Toxic neuropathy from ART is often irreversible, difficult to diagnose, and challenging to treat. Thus, early detection is paramount to allow interventions such as changing ART before further small-fiber damage occurs. Our data suggest that timely diagnosis of distal sensory polyneuropathy in HIV-infected individuals on ART may be possible with QSART and UENS.
In an HIV-negative population with clinically suspected small-fiber neuropathy, QSART had a sensitivity of 80%, compared to 75% for autonomic testing and 67% for quantitative sensory testing (QST)(Tobin et al., 1999). A recent report from the Polyneuropathy Task Force recommends QSART as a sensitive screening test for small-fiber neuropathy(England et al., 2009). QSART also has good reliability when skin temperature is controlled(Abou-Zeid et al., 2011; Sletten et al., 2010).
There are observed sex differences in sweat volume by QSART in HIV-negative populations, with females having lower sweat volumes compared to males(Low, 1997). Sweat volume was lower at all testing sites in females compared to males in our study, but the difference was not statistically significant. Although we did not find a difference in sweat volume analyzing data separately according to sex, this may be due to evenly matched controls and cases for sex and because there were fewer females than males in our study. A difference may be seen in a larger study.
Other HIV neuropathy studies have employed either the Total Neuropathy Score(Cornblath et al., 1999) or the Neuropathy Impairment Score of the lower limbs plus seven tests [NIS(LL) + 7 tests](Dyck et al., 1997). Both of these measures contain both nerve conduction study data and multiple measures of large fiber function utilizing vibration, deep tendon reflexes and strength, which dominate the scores. The Brief Peripheral Neuropathy Screen(Cherry et al., 2005) also has a majority of score dedicated to vibration and deep tendon reflexes. UENS is a novel neuropathy physical examination score predominately dedicated to an anatomical map of sensory loss in the lower extremities, detecting early subtle changes in sensory neuropathy. UENS has a sensitivity of 92% for early sensory loss, superior to scores that are equally devoted to large and small fibers, and an interrator reliability of 94%(Singleton et al., 2008). In our study, pin sensation subscore, the most sensitive UENS component for small-fiber change, was the predominant UENS subscore correlating with sweat volume by QSART, suggesting that the correlation with total UENS score may be driven by pin subscore. We extend observations published in HIV-negative persons showing correlations of sweat volume at the foot(Singleton et al., 2008) with UENS to include other testing sites by QSART and to HIV-infected individuals.
A limitation of this study is that IENFD, a gold standard for assessing small-fiber changes, was not available. Skin biopsy with quantitation of IENFD has a positive predictive value of 75% and a negative predictive value of 90%(McArthur et al., 1998). It has been studied in the setting of HIV infection, where it was reduced in persons with neuropathy(Polydefkis et al., 2002) and correlated with neuropathy severity and pain intensity(Zhou et al., 2007). QSART evaluates autonomic C fibers and IENFD assesses somatic C fibers; in small-fiber predominant polyneuropathy, both fiber populations are often affected simultaneously(Low et al., 2006). QSART and IENFD have not been compared in HIV-infected individuals, and there are conflicting data in HIV-negative populations: one study in persons with sensory neuropathy found good agreement between these tests (Novak et al., 2001), but they were not correlated in studies of small-fiber polyneuropathy due to impaired glucose tolerance(Smith et al., 2006) or painful sensory neuropathy(Periquet et al., 1999).
The purpose of our study was not to evaluate QSART as a replacement for IENFD but, rather, as a noninvasive adjunctive screening test for distal sensory polyneuropathy. In a broader sense, QSART may be a useful screening tool in the research setting for small-fiber polyneuropathy regardless of etiology. There is currently no gold standard for the diagnosis of distal small-fiber polyneuropathy. We chose to evaluate QSART rather than QST as a noninvasive test for small-fiber neuropathy, since the latter depends on patient cooperability and can be abnormal in patients without neuropathy (Backonja et al., 2009; Freeman et al., 2003). Future studies should compare QSART, QST, UENS and IENFD in HIV-infected persons on ART with distal sensory polyneuropathy. Because of the feasibility of UENS (and potentially QSART) in resource-limited settings where neurotoxic ART use remains common, future studies should also assess these measures in these settings and populations.
Our study has other limitations. The small sample size limits the ability to draw conclusions regarding the effect of specific classes of ART on our neuropathy measures. The smaller proportion of women than men in our study limits detection of gender-specific differences in outcomes. This is a substudy of a larger observational cohort that was not designed to exclude other potential causes of axonal neuropathy, including vitamin B12 deficiency, thyroid disease, and autoimmune disease. UENS and VAS were incorporated into the study protocol at varying times; therefore, not all study participants had these tests, limiting the data available for analyses. Because our study did not include an HIV-negative control group, we cannot exclude a contribution of HIV infection to neuropathy in some of the individuals, particularly those with detectable viral load at the time of neuropathy assessment. A larger study of HIV-infected individuals on and not on ART is needed. Another potential limitation of our study is that QSweat, when compared to the Mayo-QSART, may underestimate sweat volume for an individual patient, although there was a linear relationship between the volume estimates for the two devices in a recent study(Sletten et al., 2010). Although we did not attempt to correct for multiple comparisons in this exploratory analysis with a small sample size, several associations demonstrated low p-values that would likely remain statistically significant with correction.
In summary, in HIV-infected individuals, the majority of whom were virologically suppressed on ART, sweat volume by QSART was lower in those with clinical evidence of neuropathy on ART than in those without clinical neuropathy. QSART loss correlated with small-fiber changes on UENS, a targeted small-fiber neurological examination score. Larger studies including other measures of small-fiber neuropathy are needed to confirm and extend our observations. QSART and UENS, noninvasive measures, appear promising for early detection of distal sensory polyneuropathy in HIV-infected persons on ART.
Acknowledgments
Sources of support: Primary funding support was provided by NIH/NCCAM Career Development Award K23 AT002508 (TH), the Vanderbilt CTSA grant RR024975 from NCRR/NIH, 1K23 NS056009-01A1 (ACP), and the Vanderbilt Meharry Center for AIDS Research (AI54999).
ABBREVIATIONS
- ART
antiretroviral therapy
- BMI
body mass index
- CI
confidence interval
- d4T
stavudine
- ddI
didanosine
- DTR
deep tendon reflex
- IQR
interquartile range
- NIS(LL) + 7 tests
neuropathy impairment score of the lower limbs plus seven tests
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- NRTI
nucleoside reverse transcriptase inhibitor
- PI
protease inhibitor
- QSART
quantitative sudomotor axon reflex test
- QST
quantitative sensory testing
- UENS
Utah Early Neuropathy Scale
- VAS
visual analog scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meetings at which parts of data were presented: Part of the material in this manuscript was presented at the following national meetings: 1) 133rd American Neurological Association Annual Meeting, Salt Lake City, Utah, September 23, 2008; 2) 19th Annual Meeting of the American Autonomic Society, Kauai, Hawaii, October 31, 2008; 3) American Association of Neuromuscular and Electrodiagnostic Medicine 57th Annual Meeting, Québec City, Québec, Canada, October 8, 2010; and 4) 18th Conference on Retroviruses and Opportunistic Infections, Boston, MA, February 28, 2011.
REFERENCES
- 1.Abou-Zeid E, Artibee K, Shi Y, Wang L, Peltier AC. Reliability of the quantitative sudomotor axon reflex test (QSART) using QSWEAT. Neurology. 2011;76:A575. [Google Scholar]
- 2.Backonja MM, Walk D, Edwards RR, Sehgal N, Moeller-Bertram T, Wasan A, Irving G, Argoff C, Wallace M. Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain. 2009;25:641–647. doi: 10.1097/AJP.0b013e3181a68c7e. [DOI] [PubMed] [Google Scholar]
- 3.Becker K, Gorlach I, Frieling T, Haussinger D. Characterization and natural course of cardiac autonomic nervous dysfunction in HIV-infected patients. AIDS. 1997;11:751–757. doi: 10.1097/00002030-199706000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Brew BJ. The peripheral nerve complications of human immunodeficiency virus (HIV) infection. Muscle Nerve. 2003;28:542–552. doi: 10.1002/mus.10484. [DOI] [PubMed] [Google Scholar]
- 5.Cherry CL, Skolasky RL, Lal L, Creighton J, Hauer P, Raman SP, Moore R, Carter K, Thomas D, Ebenezer GJ, Wesselingh SL, McArthur JC. Antiretroviral use and other risks for HIV-associated neuropathies in an international cohort. Neurology. 2006;66:867–873. doi: 10.1212/01.wnl.0000203336.12114.09. [DOI] [PubMed] [Google Scholar]
- 6.Cherry CL, Wesselingh SL, Lal L, McArthur JC. Evaluation of a clinical screening tool for HIV-associated sensory neuropathies. Neurology. 2005;65:1778–1781. doi: 10.1212/01.wnl.0000187119.33075.41. [DOI] [PubMed] [Google Scholar]
- 7.Cornblath DR, Chaudhry V, Carter K, Lee D, Seysedadr M, Miernicki M, Joh T. Total neuropathy score: validation and reliability study. Neurology. 1999;53:1660–1664. doi: 10.1212/wnl.53.8.1660. [DOI] [PubMed] [Google Scholar]
- 8.Cui L, Locatelli L, Xie MY, Sommadossi JP. Effect of nucleoside analogs on neurite regeneration and mitochondrial DNA synthesis in PC-12 cells. J Pharmacol Exp Ther. 1997;280:1228–1234. [PubMed] [Google Scholar]
- 9.Dyck PJ, Davies JL, Litchy WJ, O'Brien PC. Longitudinal assessment of diabetic polyneuropathy using a composite score in the Rochester Diabetic Neuropathy Study cohort. Neurology. 1997;49:229–239. doi: 10.1212/wnl.49.1.229. [DOI] [PubMed] [Google Scholar]
- 10.England JD, Gronseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, Asbury AK, Szigeti K, Lupski JR, Latov N, Lewis RA, Low PA, Fisher MA, Herrmann DN, Howard JF, Jr., Lauria G, Miller RG, Polydefkis M, Sumner AJ. Practice Parameter: evaluation of distal symmetric polyneuropathy: role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review). Report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology. 2009;72:177–184. doi: 10.1212/01.wnl.0000336345.70511.0f. [DOI] [PubMed] [Google Scholar]
- 11.Evans SR, Ellis RJ, Chen H, Yeh TM, Lee AJ, Schifitto G, Wu K, Bosch RJ, McArthur JC, Simpson DM, Clifford DB. Peripheral neuropathy in HIV: prevalence and risk factors. AIDS. 2011;25:919–928. doi: 10.1097/QAD.0b013e328345889d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman R, Chase KP, Risk MR. Quantitative sensory testing cannot differentiate simulated sensory loss from sensory neuropathy. Neurology. 2003;60:465–470. doi: 10.1212/wnl.60.3.465. [DOI] [PubMed] [Google Scholar]
- 13.Freeman R, Roberts MS, Friedman LS, Broadbridge C. Autonomic function and human immunodeficiency virus infection. Neurology. 1990;40:575–580. doi: 10.1212/wnl.40.4.575. [DOI] [PubMed] [Google Scholar]
- 14.Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4:407–414. doi: 10.1016/s1526-5900(03)00716-8. [DOI] [PubMed] [Google Scholar]
- 15.Low PA. Laboratory Evaluation of Autonomic Neuropathy. In: Low PA, editor. Clinical Autonomic Disorders. Lippincott-Raven; Philadelphia: 1997. pp. 179–208. [Google Scholar]
- 16.Low VA, Sandroni P, Fealey RD, Low PA. Detection of small-fiber neuropathy by sudomotor testing. Muscle Nerve. 2006;34:57–61. doi: 10.1002/mus.20551. [DOI] [PubMed] [Google Scholar]
- 17.Maritz J, Benatar M, Dave JA, Harrison TB, Badri M, Levitt NS, Heckmann JM. HIV neuropathy in South Africans: frequency, characteristics, and risk factors. Muscle Nerve. 2010;41:599–606. doi: 10.1002/mus.21535. [DOI] [PubMed] [Google Scholar]
- 18.McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol. 1998;55:1513–1520. doi: 10.1001/archneur.55.12.1513. [DOI] [PubMed] [Google Scholar]
- 19.Novak V, Freimer ML, Kissel JT, Sahenk Z, Periquet IM, Nash SM, Collins MP, Mendell JR. Autonomic impairment in painful neuropathy. Neurology. 2001;56:861–868. doi: 10.1212/wnl.56.7.861. [DOI] [PubMed] [Google Scholar]
- 20.Periquet MI, Novak V, Collins MP, Nagaraja HN, Erdem S, Nash SM, Freimer ML, Sahenk Z, Kissel JT, Mendell JR. Painful sensory neuropathy: prospective evaluation using skin biopsy. Neurology. 1999;53:1641–1647. doi: 10.1212/wnl.53.8.1641. [DOI] [PubMed] [Google Scholar]
- 21.Phillips TJ, Cherry CL, Cox S, Marshall SJ, Rice AS. Pharmacological treatment of painful HIV-associated sensory neuropathy: a systematic review and meta-analysis of randomised controlled trials. PLoS One. 2010;5:e14433. doi: 10.1371/journal.pone.0014433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polydefkis M, Yiannoutsos CT, Cohen BA, Hollander H, Schifitto G, Clifford DB, Simpson DM, Katzenstein D, Shriver S, Hauer P, Brown A, Haidich AB, Moo L, McArthur JC. Reduced intraepidermal nerve fiber density in HIV-associated sensory neuropathy. Neurology. 2002;58:115–119. doi: 10.1212/wnl.58.1.115. [DOI] [PubMed] [Google Scholar]
- 23.Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56:217–226. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 24.Robinson-Papp J, Gonzalez-Duarte A, Simpson DM, Rivera-Mindt M, Morgello S. The roles of ethnicity and antiretrovirals in HIV-associated polyneuropathy: a pilot study. J Acquir Immune Defic Syndr. 2009;51:569–573. doi: 10.1097/QAI.0b013e3181adcefa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson DM, Brown S, Tobias J. Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology. 2008;70:2305–2313. doi: 10.1212/01.wnl.0000314647.35825.9c. [DOI] [PubMed] [Google Scholar]
- 26.Simpson DM, Schifitto G, Clifford DB, Murphy TK, Durso-De Cruz E, Glue P, Whalen E, Emir B, Scott GN, Freeman R. Pregabalin for painful HIV neuropathy: a randomized, double-blind, placebo-controlled trial. Neurology. 2010;74:413–420. doi: 10.1212/WNL.0b013e3181ccc6ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singer W, Spies JM, McArthur J, Low J, Griffin JW, Nickander KK, Gordon V, Low PA. Prospective evaluation of somatic and autonomic small fibers in selected autonomic neuropathies. Neurology. 2004;62:612–618. doi: 10.1212/01.wnl.0000110313.39239.82. [DOI] [PubMed] [Google Scholar]
- 28.Singleton JR, Bixby B, Russell JW, Feldman EL, Peltier A, Goldstein J, Howard J, Smith AG. The Utah Early Neuropathy Scale: a sensitive clinical scale for early sensory predominant neuropathy. J Peripher Nerv Syst. 2008;13:218–227. doi: 10.1111/j.1529-8027.2008.00180.x. [DOI] [PubMed] [Google Scholar]
- 29.Sletten DM, Weigand SD, Low PA. Relationship of Q-sweat to quantitative sudomotor axon reflex test (QSART) volumes. Muscle Nerve. 2010;41:240–246. doi: 10.1002/mus.21464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith AG, Russell J, Feldman EL, Goldstein J, Peltier A, Smith S, Hamwi J, Pollari D, Bixby B, Howard J, Singleton JR. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29:1294–1299. doi: 10.2337/dc06-0224. [DOI] [PubMed] [Google Scholar]
- 31.Tobin K, Giuliani MJ, Lacomis D. Comparison of different modalities for detection of small fiber neuropathy. Clin Neurophysiol. 1999;110:1909–1912. doi: 10.1016/s1388-2457(99)00164-9. [DOI] [PubMed] [Google Scholar]
- 32.Valcour V, Yeh TM, Bartt R, Clifford D, Gerschenson M, Evans SR, Cohen BA, Ebenezer GJ, Hauer P, Millar L, Gould M, Tran P, Shikuma C, Souza S, McArthur JC. Acetyl-l-carnitine and nucleoside reverse transcriptase inhibitor-associated neuropathy in HIV infection. HIV Med. 2009;10:103–110. doi: 10.1111/j.1468-1293.2008.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou L, Kitch DW, Evans SR, Hauer P, Raman S, Ebenezer GJ, Gerschenson M, Marra CM, Valcour V, Diaz-Arrastia R, Goodkin K, Millar L, Shriver S, Asmuth DM, Clifford DB, Simpson DM, McArthur JC. Correlates of epidermal nerve fiber densities in HIV-associated distal sensory polyneuropathy. Neurology. 2007;68:2113–2119. doi: 10.1212/01.wnl.0000264888.87918.a1. [DOI] [PubMed] [Google Scholar]