Abstract
Nuclear factor kappa B (NF-κB) is a key signaling molecule in the elaboration of the inflammatory response. Data indicate that curcumin, a natural ingredient of the curry spice turmeric, acts as a NF-κB inhibitor and exhibits both anti-inflammatory and anti-cancer properties. Curcumin analogues with enhanced activity on the NF-κB and other inflammatory signaling pathways have been developed including the synthetic monoketone compound termed 3,5-Bis(2-fluorobenzylidene)-4-piperidone (EF24). 3,5-Bis(2-pyridinylmethylidene)-4-piperidone (EF31) is a structurally-related curcumin analogue whose potency for NF-κB inhibition has yet to be determined. To examine the activity of EF31 compared to EF24 and curcumin, mouse RAW264.7 macrophages were treated with EF31, EF24, curcumin (1–100µM) or vehicle (DMSO 1%) for 1 hour. NF-κB pathway activity was assessed following treatment with lipopolysaccharide (LPS) (1µg/mL). EF31 (IC50 ~5µM) exhibited significantly more potent inhibition of LPS-induced NF-κB DNA binding compared to both EF24 (IC50~35µM) and curcumin (IC50 >50µM). In addition, EF31 exhibited significantly greater inhibition of NF-κB nuclear translocation as well as the induction of downstream inflammatory mediators including pro-inflammatory cytokine mRNA and protein (tumor necrosis factor-α, interleukin-1β, and interleukin-6). Regarding the mechanism of these effects on NF-κB activity, EF31 (IC50~1.92µM) exhibited significantly greater inhibition of IκB kinase β compared to EF24 (IC50~131µM). Finally, EF31 demonstrated potent toxicity in NF-κB-dependent cancer cell lines while having minimal and reversible toxicity in RAW264.7 macrophages. These data indicate that EF31 is a more potent inhibitor of NF-κB activity than either EF24 or curcumin while exhibiting both anti-inflammatory and anticancer activities. Thus, EF31 represents a promising curcumin analogue for further therapeutic development.
Keywords: Macrophages, Curcumin, NF-κB, Inflammation, Anti-cancer
1. Introduction
Mounting data suggest that inflammation may serve as a common mechanism of multiple diseases including cardiovascular disease, diabetes, cancer, neurodegenerative diseases, and certain neuropsychiatric disorders [1–7]. The nuclear factor kappa B (NF-κB) pathway has been identified as a key mediator of inflammation and therefore serves as an important target for drug development and discovery [8–10].
NF-κB is a family of transcription factors with five subunits (p52, p50, RelB, c-Rel, and RelA/p65) that form hetero- and homodimers which remain inactive in the cytoplasm when associated with IκB proteins [11]. Activation of IκB kinases (IκKα, IκKβ, and NF-κB essential modulator [NEMO]/IκKγ) results in the phosphorylation of the inhibitory IκB (IκBα, IκBβ, and IκBε) proteins bound to NF-κB. NF-κB is consequently released and translocates to the nucleus where it interacts with other transcription factors and transcriptional co-factors to regulate expression of an array of genes, many of which are involved in inflammatory signaling (e.g. cytokines, chemokines, adhesion molecules, and acute phase proteins) as well as proliferation and apoptosis [11]. The NF-κB pathway can be activated by multiple inflammatory stimuli including cytokines and pathogen-derived molecules such as lipopolysaccharide (LPS). LPS is a cell wall component of gram-negative bacteria, and with the aid of accessory proteins (LPS-binding protein and CD14) it is recognized by the toll-like receptor 4 (TLR-4) and myeloid differentiation factor (MD)-2 complex on the surface of mononuclear myeloid cells [12]. Activation and dimerization of the TLR4-MD2 monomer complex induces a cascade of signaling molecules that include myeloid differentiation factor 88, interleukin (IL)-1 receptor associated protein kinase, and tumor necrosis factor (TNF) receptor activated factor-6, leading to activation of NF-κB and mitogen-activated protein kinase (MAPK) pathways [13]. LPS is a common inflammatory stimulus in clinical and laboratory studies, and its effects on NF-κB and inflammatory mediators have been well characterized [14, 15].
The search for NF-κB blockers has identified several promising natural compounds, including curcumin. Curcumin is a component of the curry spice, turmeric, and is derived from the root of the plant Curcuma longa, a member of the ginger family. Curcumin has been used throughout Asia for culinary applications, and as a treatment for a variety of ailments ranging from acute infection to chronic disease (e.g. inflammatory bowel syndrome, diabetes, and asthma) [16–18]. Curcumin has also been shown to exhibit anti-inflammatory, anti-bacterial/ fungal/ viral, anti-cancer, and anti-oxidant activities in laboratory animals [18, 19]. Although curcumin targets many transcription factors (ATF3, AP-1, STAT-3), protein kinases (PKA, PKC), enzymes, growth factors, inflammatory mediators, and anti-apoptotic proteins [20], there has been considerable interest in curcumin’s ability to inhibit NF-κB pathway activity. However, due to its low bioavailability, poor absorption and rapid metabolism [21], clinical trials have involved doses of curcumin as high as 12 g/day orally. Although this dosage regimen may be unacceptable to some patients, no dose-limiting toxicities have been reported [22, 23]. Nevertheless, curcumin at high doses has shown potential efficacy in patients with advanced stage pancreatic cancer [24]. These data suggest that curcumin has therapeutic potential that may be limited by dosing considerations.
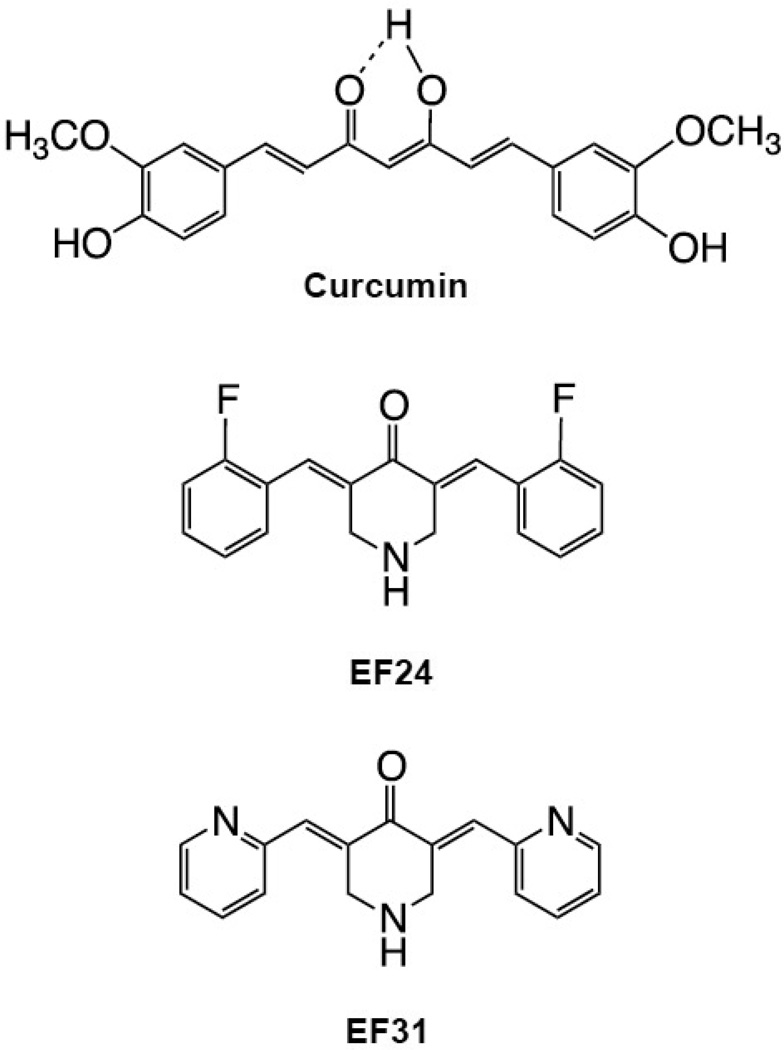
Structural curcumin analogs have been created to optimize the therapeutic effects of curcumin by increasing potency, slowing metabolism, and increasing absorption. Two promising monoketone curcumin analogs include 3,5-Bis(2-fluorobenzylidene)-4-piperidone (EF24), and the more recently developed 3,5-Bis(2-pyridinylmethylidene)-4-piperidone (EF31), which replaces the carbon-flourine bond at the 1-position of the terminal benzene rings with nitrogen to give the corresponding pyridine rings (Figure 1). The NF-κB inhibition activity of EF24 has been previously reported [25]. EF24 was found to block IκB kinase, phosphorylation of IκB, and the translocation of NF-κB to the nucleus. EF31 has shown a better physical profile with increased stability and solubility (Sun and Snyder, unpublished data), however its effect on NF-κB activity has yet to be determined. In the current study, the capacity of EF31 to inhibit NF-κB and its downstream inflammatory mediators was examined in mouse RAW264.7 macrophages as well as NF-κB-dependent cancer cell lines. EF31 was found to be a more potent inhibitor than either EF24 or curcumin of NF-κB as well as other inflammatory signaling pathways such as mitogen-activated protein kinases (MAPKs).
Figure 1. Chemical structures of curcumin and the monoketone analogs.
2. Methods
2.1. Materials
Dulbecco’s modified Eagle’s medium (DMEM) and RPMI 1640 were purchased from Cellgro (Manassas, VA). Weymouth’s medium and Leibovitz’s L-15 medium were purchased from ATCC (Manassas, VA). Curcumin was purchased from Sigma (St. Louis, MO) (C1386-5G), and the structurally related compounds EF24 and EF31 were prepared at Emory University as described previously by Adams et al., 2004. Dimethyl sulfoxide (DMSO) was used to dissolve all compounds, and all dilutions were made from a 10mM stock. LPS (Escherichia coli O55:B5) was obtained from Sigma and was suspended in saline to a final concentration of 1 µg/mL. Antibodies against NF-κB and the secondary goat anti-rabbit antibody conjugated to Fluor 488 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Primers for mouse TNF-α, IL-1β, IL-6, and GAPDH were obtained from Qiagen (Valencia, CA).
2.2. Cell cultures
Mouse RAW 264.7 macrophage cells (ATCC, Manassas, VA) were cultured in DMEM supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, and 10% fetal bovine serum (HyClone Labs, Logan, UT) at 37 °C with 5% CO2. A2780 cells, a human ovarian carcinoma cell line (Sigma), were cultured in RPMI 1640 supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, and 10% fetal bovine serum at 37 °C with 5% CO2. MDA-MB-231 cells, a human breast cancer cell line (ATCC), were cultured in Leibovitz’s L-15 supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, and 10% fetal bovine serum at 37 °C with 100% air. EMT6 cells, a mouse mammary carcinoma cell line (ATCC), were cultured in Weymouths’s MB 752/1 supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, and 15% fetal bovine serum at 37 °C with 5% CO2. MIA PaCa-2 cells, a pancreatic carcinoma cell line (ATCC), were cultured in DMEM supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, 10% fetal bovine serum, and 2.5% horse serum at 37 °C with 5% CO2.
2.3. Assessment of transcription factor-DNA binding in RAW cells
RAW 264.7 mouse macrophage cells were plated at 3.4 × 105 cells/well in 60 mm × 15 mm dishes, incubated overnight, and were then incubated in triplicate with either EF24, EF31, curcumin, or vehicle (DMSO 1%) for 1 hour prior to LPS (1 µg/mL) or saline treatment. Nuclear proteins were collected 15 minutes after LPS treatment using the extraction kit protocol from Active Motif (Carlsbad, CA). The 15 minute collection time-point after LPS treatment was derived from a time course study (see Figure 2A). Nuclear protein samples were analyzed in triplicate using the NF-κB p65 DNA binding ELISA kit (Active Motif) or the MAPK family DNA binding ELISA kit (Active Motif) according to manufacturer instructions.
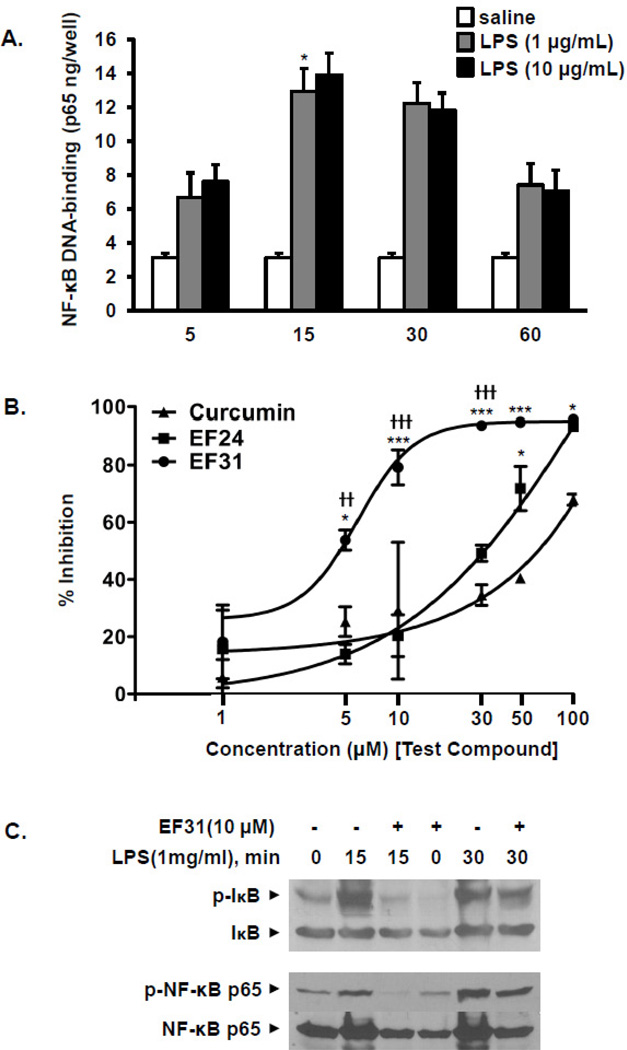
Figure 2. EF31 is a potent inhibitor of LPS-induced NF-κB DNA binding activity.
A) Mouse RAW264.7 macrophages were grown in 60 mm × 15 mm cell culture dishes and treated with LPS (1 or 10 µg/mL) or saline. Nuclear proteins were collected at different time-points (5 minutes– 60 minutes) as described in section 2.3. NF-κB DNA-binding activity was measured using a DNA-binding ELISA. Results shown are representative of three separate experiments. All conditions were run in triplicate, and values shown are means (±SEM). * p < 0.05 vs. 5 minutes- LPS (1 µg/mL) treated group. B) Mouse RAW264.7 macrophages were pre-treated with curcumin, EF24, EF31 (1, 5, 10, 30, 50, or 100 µM), or vehicle (DMSO 1%) for 1 hour prior to treatment with LPS (1 µg/mL) for 15 minutes. Nuclear proteins were collected and NF-κB DNA-binding was measured using a DNA-binding ELISA. Results shown are representative of three separate experiments. All conditions were run in triplicate, and values shown are means (±SEM) expressed as percent inhibition from vehicle. * p < 0.05, *** p < 0.001 vs. curcumin at same concentration; †† p < 0.01, ††† p < 0.001 vs. EF24 at same concentration. C) Mouse RAW264.7 macrophages were grown in 60 mm × 15 mm cell culture dishes and pre-treated with EF31 (10 µM), or vehicle (DMSO 1%) for 1 hour prior to treatment with LPS (1 µg/mL) or saline for 15 or 30 minutes. Whole cell extracts and western blot analyses were conducted for unphosphorylated and phosphorylated IkB and NFkB p65 as described in section 2.9. Results shown are representative of three separate experiments.
2.4. Assessment of NF-κB nuclear translocation in RAW cells
RAW 264.7 mouse macrophage cells were plated at 20,000 cells/400 µl/well in 8-well glass chamber slides (Nunc, USA) and incubated overnight. Cells were then treated with the test compounds or vehicle (DMSO 1%) for 1 hour prior to treatment with LPS (1 µg/mL) for 15 minutes. Cells were then processed for confocal microscopy as previously described (Kasinski et al., 2008). Briefly, cells were washed with ice-cold phosphate-buffered saline (PBS) and fixed using 2% paraformaldehyde for 30 minutes at room temperature. Triton-X 100 (0.1%) was used to permeabilize the cells for 20 minutes. Cells were then washed (3X) with PBS and blocked using bovine serum albumin (1%) for 1 hour followed by an overnight incubation at 4°C with rabbit anti-p65 NF-κB antibody (1:500). Cells were washed with PBS and incubated with goat anti-rabbit IgG conjugated with Alexa Fluor 488 (1:1000) along with Hoechst 33342 (1 µM) for 1 hour at room temperature. After washing the cells with PBS, the chambers were removed and the slides were imaged using an LSM510 confocal microscope set for fluorescein isothiocyanate (argon/2 laser- excitation at 488 nm, emission at 510 nm) and 4,6-diamidino-2-phenylindole (diode laser- excitation at 405 nm, emission at 420 nm). Images were quantified and analyzed using Metamorph for Olympus (Olympus America, Center Valley, PA). The nuclear region was defined using the Hoechst 33342 staining and was then used to calculate the average of the nuclear NF-κB fluorescence intensity. Samples were run in triplicate and 20 representative cells were chosen at random in each treatment well (total of 60 cells per treatment group) to assess NF-κB nuclear translocation.
2.5. Isolation of total RNA and cytokine RT-PCR
RAW 264.7 mouse macrophage cells were plated at 1.8 × 105 cells/well in 6-well plates and incubated overnight. Cells were then treated with the test compounds or vehicle (DMSO 1%) in triplicate for 1 hour prior to stimulation with LPS (1 µg/mL) or saline. Three hours after LPS treatment, cells were collected and total RNA was extracted using the RNeasy Mini kit (Qiagen, Hilden, Germany) followed by reverse transcription PCR and amplification of 1µL of cDNA by real-time PCR (Applied Biosystems, 7500 Fast, Carlsbad, CA).
2.6. Cytokine protein ELISA
RAW 264.7 mouse macrophage cells were plated at 1.8 × 105 cells/well in 6-well plates and incubated overnight in DMEM. Cells were then treated with the test compounds or vehicle (DMSO 1%) in triplicate for 1 hour prior to stimulation with LPS (1 µg/mL) or saline. Six hours after LPS stimulation, cell supernatants were collected and centrifuged. Cytokine protein concentrations were assayed using mouse IL-1β, TNF-α and IL-6 ELISA from R&D (Minneapolis, MN) according to manufacturer’s recommendations.
2.7. Assessment of IκKβ inhibition
Kinase recombinant protein of the catalytic domain, purchased from Invitrogen (Carlsbad, CA.), was incubated with the compounds or vehicle (DMSO 1%) in triplicate for 30 minutes before inhibition of IκKβ activity was assayed using the Z’-Lyte kinase assay kit from Invitrogen. In a 10 µL kinase reaction, the IκKβ transfers the gamma-phosphate of ATP to a single serine/ threonine residue in the synthetic peptide substrate (2 µM). The peptide is labeled with two fluorophores (coumarin and fluorescein), one at each end, to make up a fluorescence resonance energy transfer (FRET) pair. In the development reaction, 5 µL of a site-specific protease recognizes and cleaves non-phosphorylated peptides. Cleavage disrupts FRET between the coumarin and the fluorescein on the peptide. Five µl of stop reagent is added to halt the development reaction before the plate is read. Plates were read using an Envision 2102 plate reader from Perkin Elmer (Waltham, MA). During detection, a ratio-metric read-out of the donor emission over the acceptor emission quantitates reaction progress. Percent phosphorylation was calculated using controls. ATP concentrations were equal to Km values for the kinase.
2.8. Cell Viability/ proliferation assay
All cell lines were plated at 5000 cells/well in 96-well plates and incubated overnight. Cells were treated with test compounds or vehicle (DMSO 1%) in triplicate for either 1 or 48 hours before viability was assayed using the CellTiter 96 Aqueous non-radioactive cell proliferation assay (MTS) kit from Promega (Madison, WI).
2.9. Assessment of EF31 effects on phosphorylation of MAPKs, IκB, and NF-κB p65
For western blot analysis, cell extracts were prepared by directly dissolving cells in 1 × SDS sample buffer (Bio-Rad, Hercules, CA) before SDS-PAGE. Separated proteins were then electrophoretically transferred onto a nitrocellulose membrane. The membrane was blocked for 1 hr in a 5% milk/TBST solution, and then incubated overnight in the presence of the primary antibody (1:500 dilution) raised against phospho- NF-kB (p65), total NF-kB (p65), phospho IκB-α, total IκB-α, phospho P38, total P38, phospho ERK, total ERK, phospho JNK, total JNK (Cell Signaling, Danvers, MA). The washed membrane was subsequently incubated with anti-mouse and anti-rabbit second antibody (1:2000 dilution) conjugated with horseradish peroxidase for 1 h. The membrane was washed again and visualized using a chemoluminescence kit (Amersham Biosciences Corp, Piscataway, NJ) and autoradiography.
2.10. Data Analysis
Overall treatment effects were determined in each experiment by either a one- or two-way analysis of variance (ANOVA) using GraphPad Prism 5. Significant interactions were followed by post-hoc Bonferroni test to determine differences between specific groups of interest. An α level of p < 0.05 was used in all statistical tests. A t-test was used to determine a significant difference between control and LPS in Figure 3B.
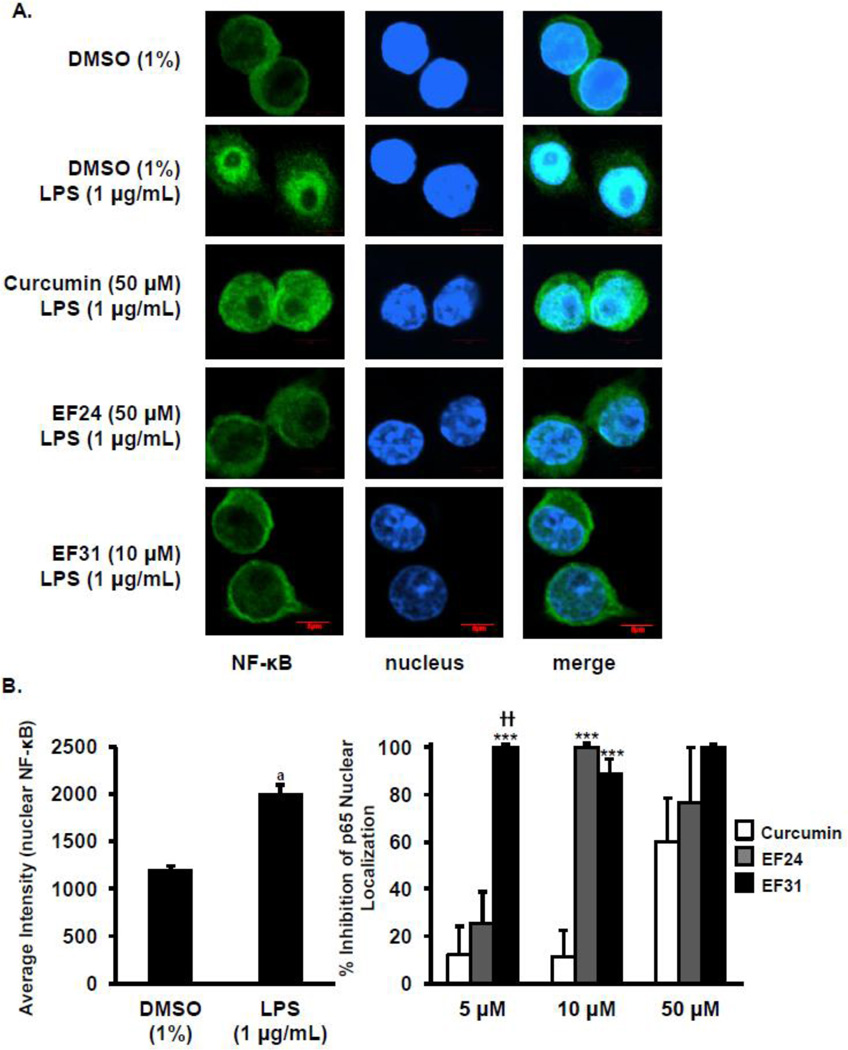
Figure 3. EF31 impairs LPS-induced NF- κB nuclear translocation.
A) Mouse RAW264.7 macrophages were grown in 8-well chamber slides and pre-treated with curcumin, EF24, EF31 (5, 10, or 50 µM), or vehicle (DMSO 1%) for 1 hour prior to treatment with LPS (1 µg/mL) for 15 minutes. Cells were then fixed and processed as described in section 2.4. Images were obtained using a LSM510 confocal microscope. Scale bar = 5µm. B) The induction of NF-κB nuclear translocation by LPS was quantified by measuring nuclear p65 fluorescence intensity as described in section 2.4. Results shown are representative of three separate experiments. All conditions were run in triplicate, and values shown are means (±SEM) expressed as percent inhibition from vehicle. *** p < 0.001 vs. curcumin at same concentration; †† p < 0.01 vs. EF24 at same concentration; a p < 0.001 vs. vehicle.
3. Results
3.1. EF31 is a potent inhibitor of NF-κB DNA-binding and IκKβ phosphorylation
To establish the best time-point to measure NF-κB DNA-binding activity, mouse RAW264.7 macrophages were treated with LPS (1 or 10 µg/mL) or saline for 5, 15, 30, or 60 minutes after which nuclear proteins were collected and analyzed using NF-κB DNA-binding ELISA. Treatment with LPS at both 1 and 10 µg elicited a peak in NF-κB DNA-binding by 15 minutes (Figure 2A). Statistical analysis revealed a significant time × treatment interaction (F [6, 24] = 4.209, p = 0.005). Post hoc analysis identified no significant differences between 1 and 10 µg LPS. Within cells treated with 1 µg, only the 15 minute time-point was significantly different from the 5 minute time-point (p < 0.05).
To examine the activity of EF31 compared to EF24 and curcumin, mouse RAW264.7 macrophages were treated with curcumin, EF24, EF31 (1, 5, 10, 30, 50, or 100 µM), or vehicle for 1 hour, and NF-κB DNA-binding activity was assessed 15 minutes following treatment with LPS (1 µg/mL). Potent inhibition of NF-κB DNA-binding was observed at concentrations of 5–10 µM for EF31, 30–50 µM for EF24, and 50–100 µM for curcumin (Figure 2B). Analysis of these results with a two-way ANOVA revealed a significant treatment × concentration interaction (F [10, 36] = 4.001, p=0.0010). The dose response indicated that the IC50 value of EF31 was in the range of 4.5–5.3 µM, 34.1–36.2 µM for EF24, and >50 µM for curcumin. Due to a wide concentration range of inhibition (50–100 µM), the IC50 for curcumin could not be determined. Of note, lower concentrations of EF31 were tested including 0.01 µM, and 0.10 µM with no effect on NF-κB DNA-binding (data not shown). Finally, whole cell p-IκB and NF-κB p-p65 levels were attenuated by 1 hour pretreatment with E31 after 15 or 30 min LPS treatment (Figure 2C).
NF-κB nuclear translocation was assessed using fluorescence microscopy to determine the nuclear localization of p65 following LPS. The treatment protocol used in the experiment shown in Figure 2B was employed, i.e. nuclear localization of NF-κB was determined after treating with EF31, EF24 and curcumin (5, 10, 50 µM) or vehicle for 1 hour and then for 15 minutes with LPS (1 µg/mL). In the absence of LPS, p65 was observed almost exclusively in the cytoplasm. However, the nuclear content of p65 increased dramatically following LPS as indicated by the overlapping of the p65-fluor 488 green fluorescence with the Hoesch blue staining (Figure 3A). A two-way ANOVA revealed a significant treatment × concentration interaction (F [6, 24] = 3.914, p = 0.0072). Nuclear p65 localization was significantly reduced by EF31 at all concentrations examined (p < 0.05 vs. vehicle + LPS) (Figure 3B). Similar results were found with EF24, but only at the 10 and 50 µM concentrations (p < 0.05 vs. vehicle + LPS). Finally, curcumin tended to decrease p65 nuclear localization at 50 µM (p > 0.05 vs. vehicle + LPS).
3.2. EF31 blocks LPS-induced cytokine mRNA expression in mouse RAW264.7 macrophages
To determine the best time-point at which to measure mRNA expression, mouse RAW264.7 macrophages were treated with LPS (1 or 10 µg/mL) or saline, and mRNA was extracted at different time-points (15 minutes, 1, 3, or 6 hours) as described in section 2.5. Treatment with LPS at both concentrations resulted in a time dependent increase in mRNA expression for TNF-α, IL-1β, and IL-6 (Supplementary Figure 1). The 3 hour time-point was chosen to examine the effects of the compound-dependent NF-κB inhibition on mRNA expression because it was the earliest time-point that showed a significant increase in mRNA expression compared to the saline treated group for the cytokines examined. Moreover, this time-point has been previously used in mouse RAW264.7 macrophages treated with LPS to examine pro-inflammatory cytokine mRNA expression [26, 27].
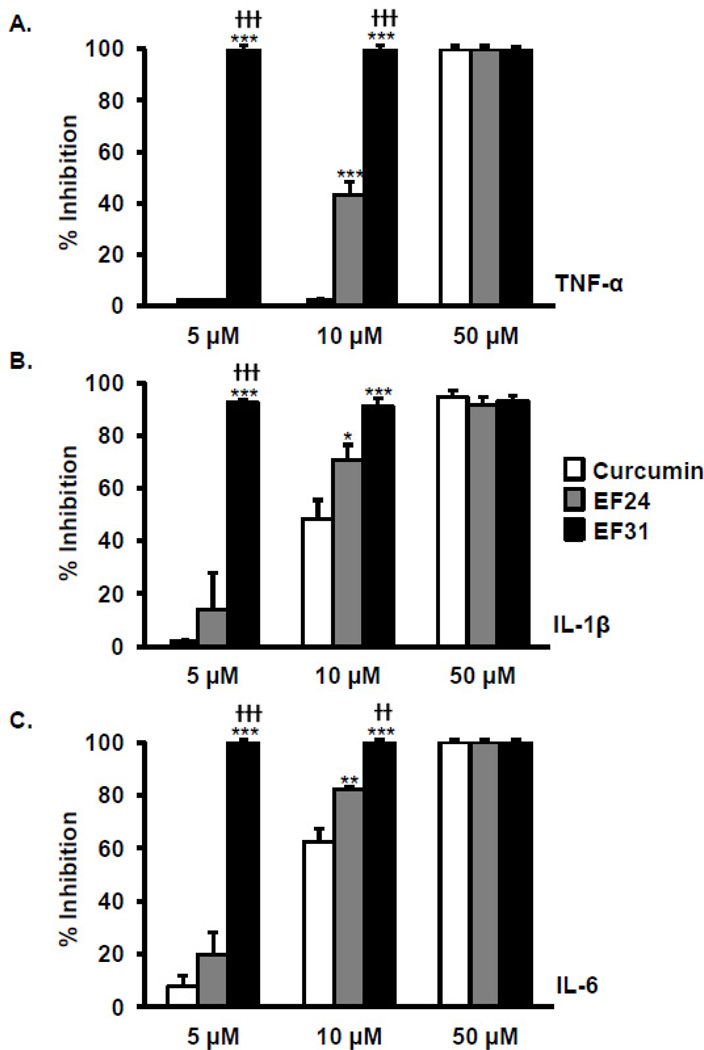
Mouse RAW264.7 macrophages were treated with curcumin, EF24, EF31 (5, 10, or 50 µM), or vehicle for 1 hour, and mRNA was extracted 3 hours following treatment with LPS (1 µg/mL) (Figure 4). The concentrations for the test compounds were chosen to cover the range of IC50 values revealed in the previous experiments. Expression of TNF-α, IL-1β, and IL-6 mRNA were inhibited in a dose–dependent manner with all compounds. Statistical analysis revealed a treatment × concentration interaction (TNF-α, F [4, 18] = 7.712, p = 0.0008; IL-1β, F [4, 18] = 40.71, p < 0.0001; IL-6, F [4, 18] = 31.11, p < 0.0001). Of note, no inhibition of LPS-induced TNF-α was found with curcumin.
Figure 4. EF31 inhibits LPS-induced pro-inflammatory cytokine mRNA expression.
Mouse RAW264.7 macrophages were grown in 6-well plates and pre-treated with curcumin, EF24, EF31 (5, 10, or 50 µM), or vehicle (DMSO 1%) for 1 hour prior to treatment with LPS (1 µg/mL) for 3 hours. Whole cell mRNA was then collected as described in section 2.5 to assess TNF-alpha (A), IL-1 beta (B), and IL-6 (C) gene expression using RT-PCR. Results shown are representative of three separate experiments. All conditions were run in triplicate, and values shown are means (±SEM) expressed as percent inhibition from vehicle. *** p < 0.001 vs. curcumin at same concentration; † p < 0.05, †† p < 0.01, ††† p < 0.001 vs. EF24 at same concentration.
3.3. EF31 is a potent inhibitor of cytokine protein release
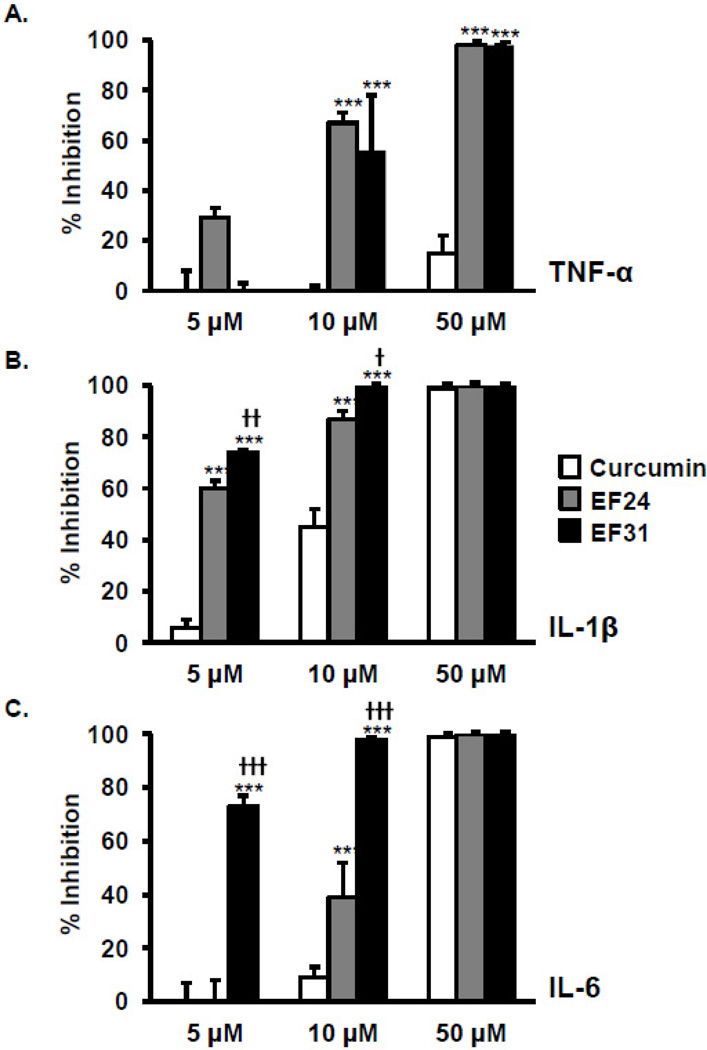
To assess the effects of EF31 on LPS-induced release of cytokine proteins, mouse RAW264.7 macrophages were treated with curcumin, EF24, EF31 (5, 10, or 50 µM), or vehicle for 1 hour and then with LPS (1 µg/mL). Cytokine protein released in medium was measured at 16 hours post LPS treatment. The 16 hours time-point was selected as it had been previously published using mouse RAW264.7 macrophages treated with LPS [28]. EF31 inhibited cytokine protein release at all concentrations tested (5–10 µM), while both EF24 (10–50 µM) and curcumin (10–50 µM) required higher concentrations to achieve similar results. Statistical analysis revealed a treatment × concentration interaction (TNF-α, F [4, 18] = 398.3, p < 0.0001; IL-1β, F [4, 18] = 19.00, p < 0.0001; IL-6, F [4, 18] = 50.42, p < 0.0001) (Figure 5). Of note, lower concentrations of EF31 (< 5 µM) did not block cytokine protein release (data not shown).
Figure 5. EF31 inhibits LPS-induced production of pro-inflammatory cytokine proteins.
Mouse RAW264.7 macrophages were grown in 6-well plates and pre-treated with curcumin, EF24, EF31 (5, 10, or 50 µM), or vehicle (DMSO 1%) for 1 hour prior to treatment with LPS (1 µg/mL) for 16 hours. Medium was collected and assessed for concentrations of TNF-alpha (A), IL-1 beta (B), and IL-6 (C) protein using ELISAs. Results shown are representative of three separate experiments. All conditions were run in triplicate, and values shown are means (±SEM) expressed as percent inhibition from vehicle. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. curcumin at same concentration; †† p < 0.01, ††† p < 0.001 vs. EF24 at same concentration. (Of note, LPS + DMSO treated samples yielded protein levels of approximately 1,400 pg/mL for TNF-α, 15 pg/mL for IL-1β, and 11,000 pg/mL for IL-6)
3.4. Mechanism for EF31-dependent inhibition of the NF-κB pathway
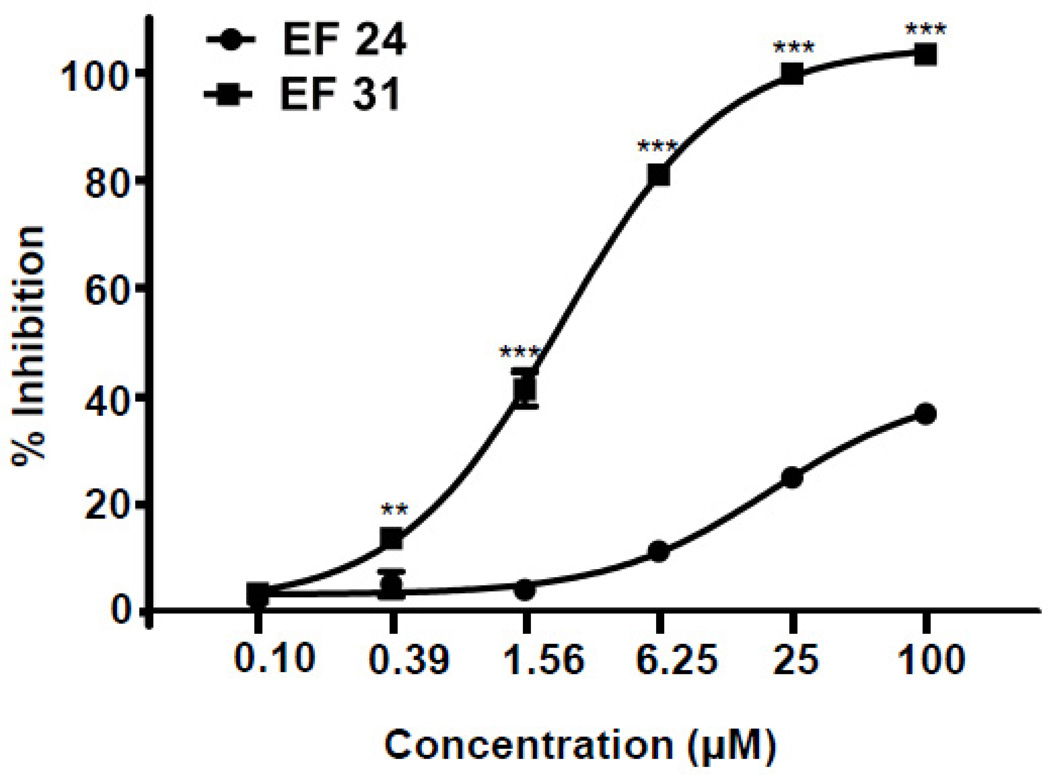
To examine whether EF31 directly inhibits IκKβ activity, recombinant IκKβ was pre-incubated with EF24, EF31 (0.0977, 0.391, 1.56, 6.25, 25, 100 µM), or vehicle for 30 minutes. The activity of IκKβ was measured using a Z’Lyte kinase assay kit. EF31 exhibited significantly greater inhibition of the recombinant kinase activity compared to EF24 (p < 0.01) for concentrations higher than 100 nM (Figure 6). The dose response indicated that the IC50 value of EF31 was ~1.92 µM and ~131 µM for EF24. Statistical analysis revealed a significant treatment × concentration interaction (F [5, 24] = 198.0, p < 0.0001).
Figure 6. Mechanism for EF31-dependent inhibition of the NF-κB pathway.
IκKβ recombinant protein was pre-incubated with EF24, EF31 (0.0977, 0.391, 1.56, 6.25, 25, 100 µM), or vehicle (DMSO 1%) for 30 minutes. Inhibition of IκKβ activity was measured using a Z’-Lyte kinase assay kit as described in section 2.7. Results shown are representative of three separate experiments. All conditions were run in triplicate, and values shown are means (±SEM) expressed as percent inhibition from vehicle. ** p < 0.01, *** p < 0.001 vs. EF24 at same concentration.
3.5. Inhibition of NF-κB DNA-binding activity by EF31 is reversible
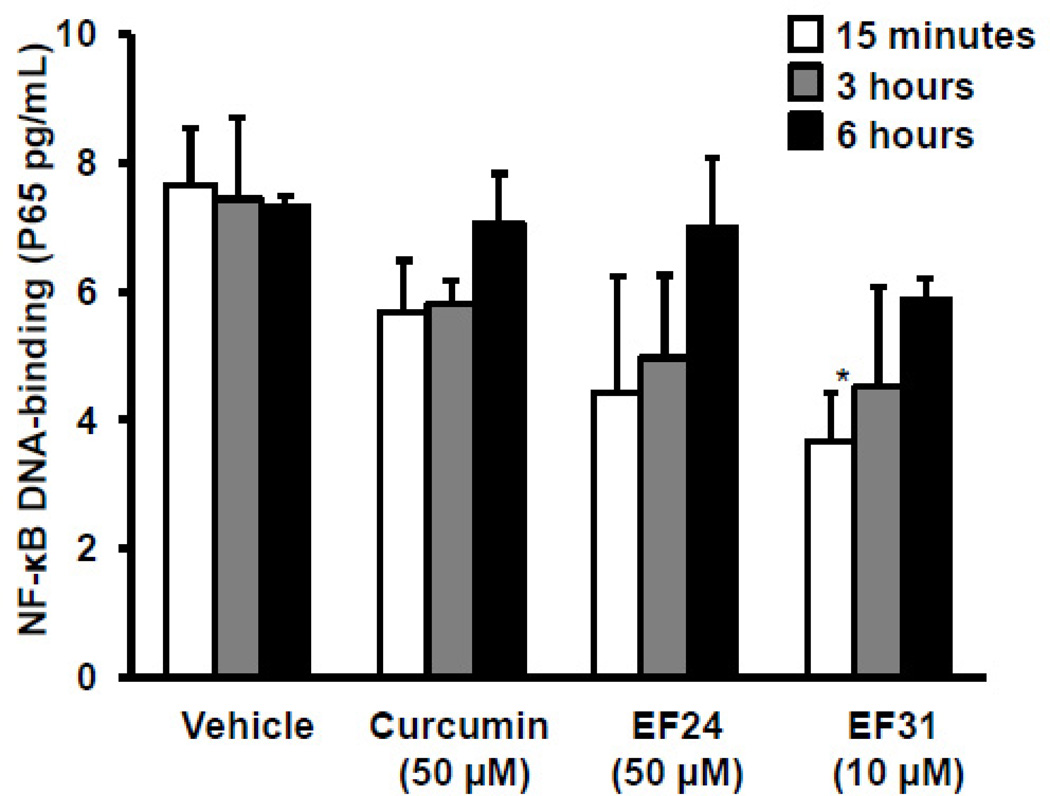
To examine whether EF31’s inhibition of NF-κB DNA-binding activity is reversible, mouse RAW264.7 macrophages were treated with curcumin (50 µM), EF24 (50 µM), or EF31 (10 µM), or vehicle for 1 hour. Cells were then washed and medium was replaced and treated with LPS (1 µg/mL). Nuclear proteins were collected at different time-points, and NF-κB DNA-binding activity was measured. NF-κB DNA-binding increased in a time-dependent manner (Figure 7). Statistical analysis revealed a significant effect of treatment (F [3, 24]= 3.883, p = 0.0215), but not time. Post-hoc analysis identified a significant difference between vehicle and EF31 at 15 minutes (p < 0.05), but not at 3 or 6 hours.
Figure 7. Inhibition of NF-κB DNA-binding by EF31 is reversible.
Mouse RAW264.7 macrophages were grown in 60 mm × 15 mm cell culture dishes and pre-treated with curcumin (50 µM), EF24(50 µM), EF31 (10 µM), or vehicle (DMSO 1%) for 1 hour. Cells were then washed and medium was replaced and treated with LPS (1 µg/mL) or saline. Nuclear proteins were collected at different time-points (15 minutes– 6 hours) as described in section 2.3. NF-κB DNA-binding activity was measured using a DNA-binding ELISA. Results shown are representative of three separate experiments. All conditions were run in triplicate, and values shown are means (±SEM). * p < 0.05 vs. vehicle + LPS at 15 minutes.
3.6. At concentrations that inhibit NF-κB, EF31 does not reduce cell viability/proliferation in mouse RAW264.7 macrophages
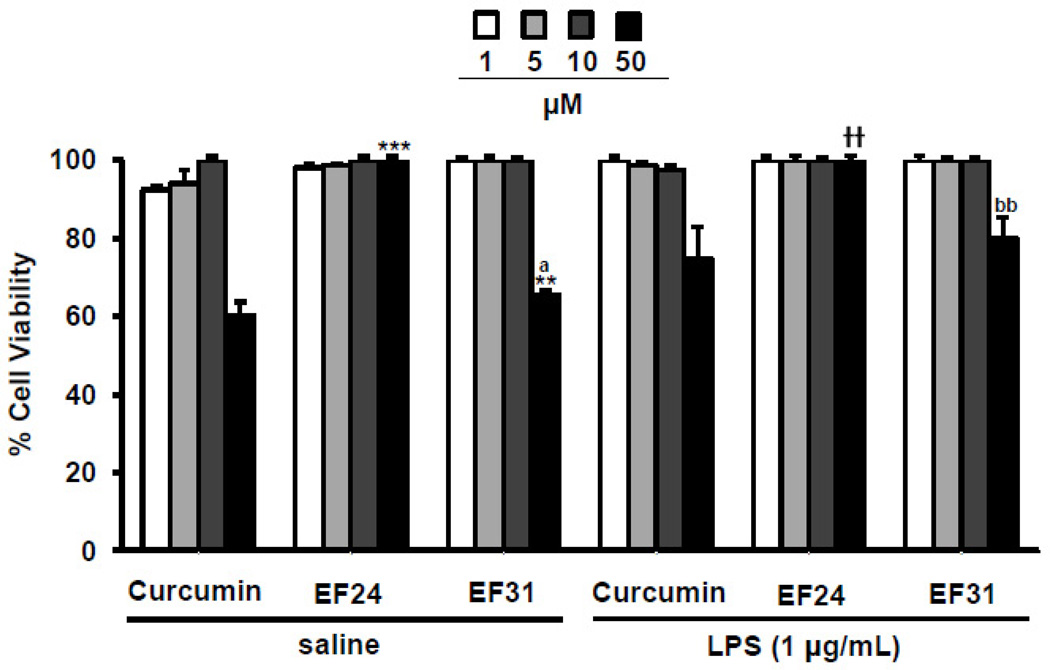
To assess the effects of EF31 on cell viability, mouse RAW264.7 macrophages were treated with curcumin, EF24, EF31 (1, 5, 10, or 50 µM), or vehicle for 1 hour, and then with LPS or saline for 15 minutes. Cell viability was assayed using a cell proliferation (MTS) kit. Statistical analysis showed a concentration effect in the LPS treated groups (F [3, 24]= 4.052, p = 0.0183) and a significant treatment × concentration interaction in the saline treated groups (F [6, 24]= 5.295, p= 0.0013). Post-hoc analysis showed a significant difference between curcumin and both analogs, EF24 and EF31 at the 50 µM concentration (p < .01) in the saline treated groups only. However, EF31 showed no reductions in cell viability as compared to vehicle treatment for concentrations that were previously found to inhibit NF-κB DNA binding (5–10 µM). Nevertheless, a higher concentration of 50 µM did reduce cell viability by approximately 50% (Figure 8). EF24 did not have an effect on cell viability for any of the concentrations tested, while curcumin showed significant reductions in cell viability at 50 µM (a concentration that inhibits NF-κB DNA-binding activity by about 50%). Of note, these data also suggest that the EF31-mediated reduction in NF-κB DNA-binding activity in mouse RAW264.7 macrophages was not due to a loss of viable cells.
Figure 8. EF31 shows no reduction in cell viability at concentrations that inhibit NF-κB.
Mouse RAW264.7 macrophages were grown in 96-well plates and pre-treated with curcumin, EF24, EF31 (1, 5, 10, or 50 µM), or vehicle (DMSO 1%) for 1 hour prior to treatment with LPS (1 µg/mL) or saline for 15 minutes. Cell viability/ proliferation was then measured as described in section 2.8. Results shown are representative of three separate experiments. All conditions were run in triplicate, and values shown are means (±SEM) expressed as percent of vehicle. ** p < 0.01, *** p < 0.001 vs. curcumin at same concentration in the saline treated groups; a p < 0.05 vs. EF24 at same concentration in the saline treated groups; †† p < 0.01 vs. curcumin at same concentration in the LPS treated groups; bb p < 0.01 vs. EF24 at same concentration in the LPS treated groups.
3.7. EF31 is cytotoxic in cancer cell lines
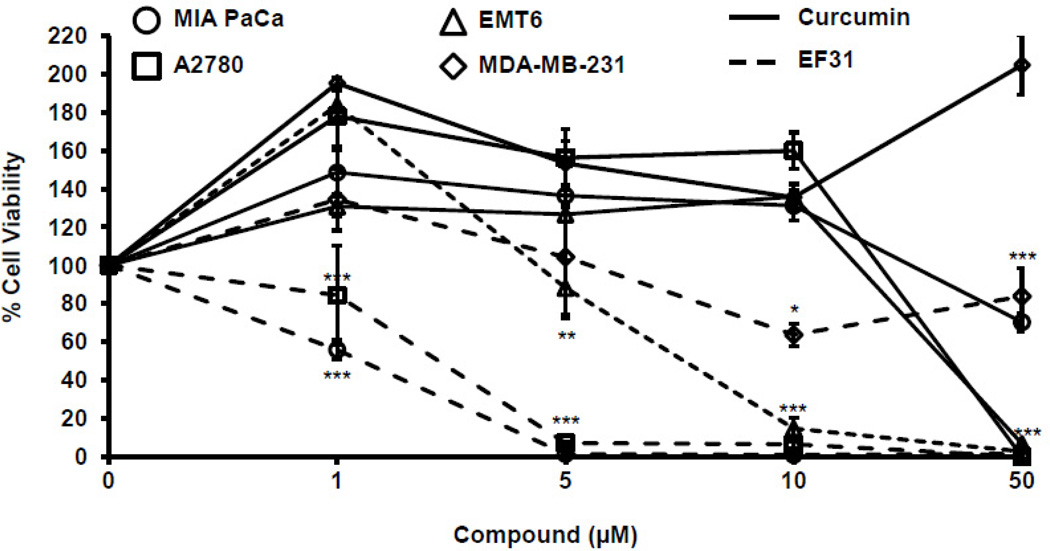
Human ovarian carcinoma cells (A2780), human breast cancer cells (MDA-MB-231), human pancreatic carcinoma cells (MIA PaCa-2), and mouse mammary carcinoma cells (EMT6), all of which depend heavily upon the NF-κB pathway for proliferation, were treated with curcumin, EF31 (1, 5, 10, or 50 µM), or vehicle for 48 hours. Cell viability was then assayed using a cell proliferation (MTS) kit. Statistical analysis showed a significant interaction between treatment × concentration in the MIA PaCa-2 cells (F [3, 16]= 15.76, p < 0.0001), A2780 cells (F [3, 16]= 16.28, p < 0.0001), and the EMT6 cells (F [3, 16]= 55.74, p < 0.0001), while the MDA-MB-231 cells showed a significant treatment effect (F [1, 16]= 46.44, p < 0.0001) and a significant concentration effect (F [3, 16]= 6.118, p = 0.0057). In all cancer cell lines, EF31 showed a dose response effect on cell viability using the same treatment paradigm as in the mouse RAW264.7 macrophages (Figure 8). Moreover, when compared to curcumin, EF31 was consistently more potent in reducing cell viability for all cancer cell lines tested (Figure 9).
Figure 9. EF31 shows potent toxicity on NF-κB dependent cancer cell lines.
Cancer cell lines were grown in 96-well plates and treated with curcumin, EF31 (1, 5, 10, or 50 µM), or vehicle (DMSO 1%) for 48 hours. Cell viability/ proliferation was measured as described in section 2.8. Results shown are representative of three separate experiments. All conditions were run in triplicate, and values shown are means (±SEM) expressed as percent of vehicle. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. curcumin at same concentration.
3.8. EF31 inhibits mitogen-activated protein kinase pathways
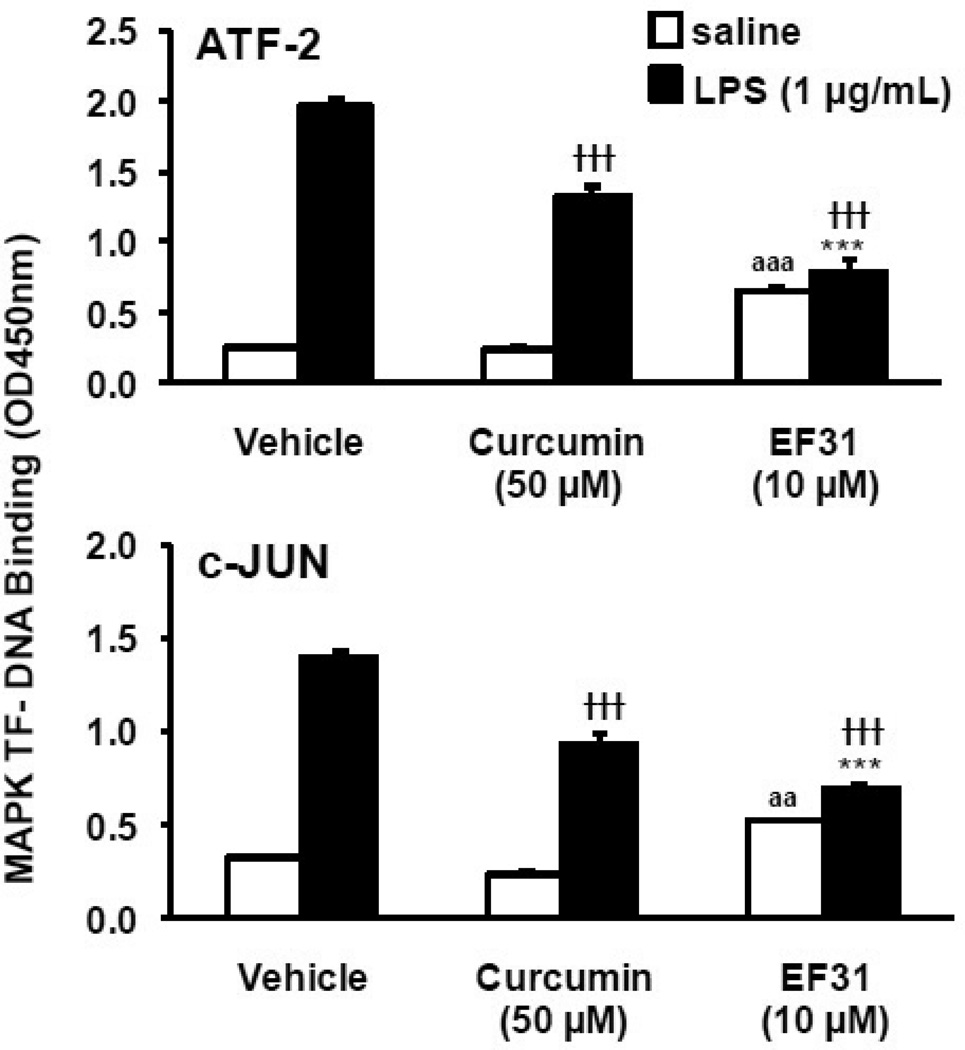
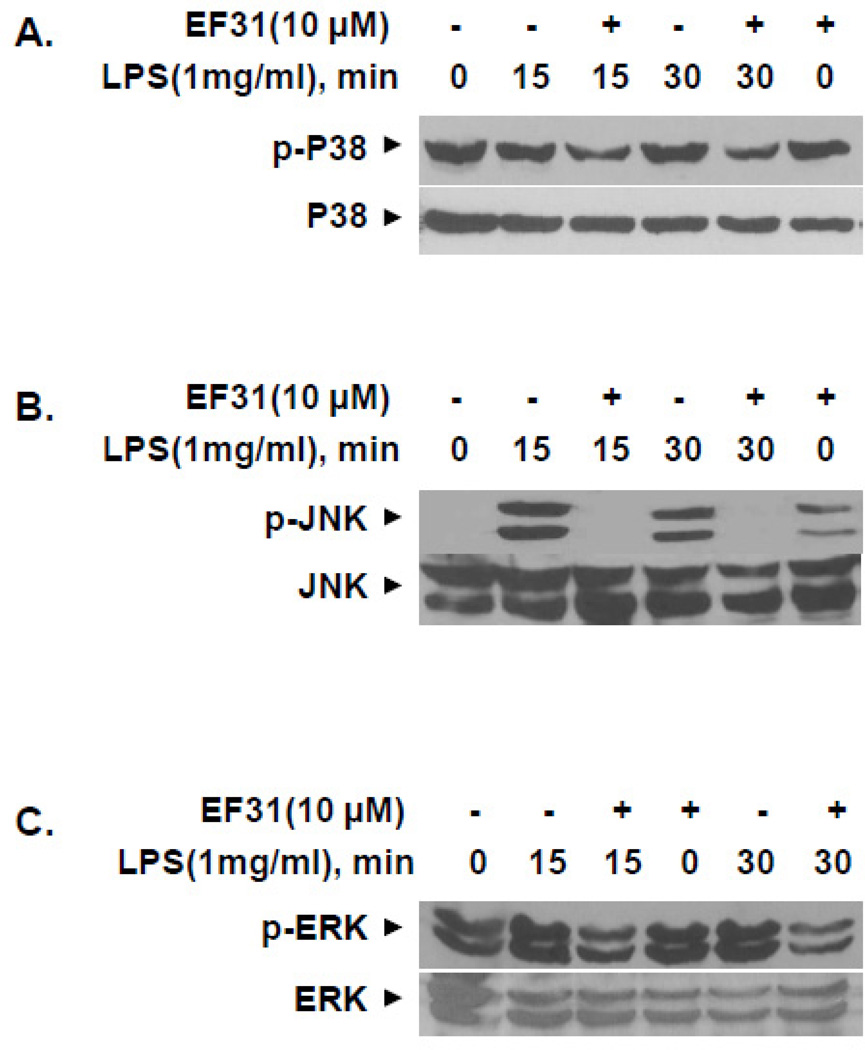
To explore whether the effects of EF31 on cytokine mRNA and protein extended to other kinase mediated pathways, activity in the MAPK pathway was assessed following treatment with EF31. Mouse RAW264.7 macrophages were treated with curcumin (50 µM), EF31 (10 µM), or vehicle for 1 hour prior to LPS (1 µg/mL) or saline treatment. Nuclear proteins were collected 15 minutes after LPS treatment and MAPK transcription factor (ATF-2 and c-JUN)-DNA binding activity was measured using a DNA-binding ELISA. This time-point was chosen based on a MAPK transcription factor time course (Supplementary Figure 2) showing that at 15 minutes the DNA-binding activity of both transcription factors (c-JUN and ATF-2) is significantly increased. EF31 significantly reduced the DNA-binding activity for both transcription factors in the LPS treated groups. A two-way ANOVA showed a significant compounds (EF31 or curcumin) × treatment (LPS or saline) interaction (ATF-2, F [2, 12]= 285.5, p < 0.0001; c-JUN, F [2, 12]= 83.08, p < 0.0001) (Figure 10). Similar results were found for curcumin; however, post-hoc analysis revealed that in the LPS treated groups, EF31-induced inhibition was significantly greater than curcumin for both transcription factors (p < 0.001). Finally, inhibition of LPS-induced MAPK pathway activity by EF31 was examined using western blot of whole cell p-ERK, p-JNK, and p-p38. As shown in Figure 11, treatment with EF31 for 1 hr blocked p-JNK after both 15 and 30 minutes of treatment with LPS. LPS-induced p-ERK and p-p38 were also attenuated at both time points, although to lesser degrees.
Figure 10. EF31 inhibits MAPK transcription factor DNA-binding activity.
Mouse RAW264.7 macrophages were grown in 60 mm × 15 mm cell culture dishes and pretreated with curcumin (50 µM), EF31 (10 µM), or vehicle (DMSO 1%) for 1 hour prior to treatment with LPS (1 µg/mL) or saline for 15 minutes. Nuclear proteins were collected as described in section 2.3 and transcription factor DNA-binding was measured using a DNA-binding ELISA for ATF-2 (top) and c-Jun (bottom). Results shown are representative of three separate experiments. All conditions were run in triplicate, and values shown are means (±SEM). *** p < 0.001 vs. curcumin + LPS; ††† p < 0.001 vs. vehicle + LPS; aa p < 0.01, aaa p < 0.001 vs. vehicle + saline.
Figure 11. EF31 inhibits lipopolysaccharide-induced phosphorylation of MAPKs p38, JNK, and ERK.
Mouse RAW264.7 macrophages were grown in 60 mm × 15 mm cell culture dishes and pre-treated with EF31 (10 µM), or vehicle (DMSO 1%) for 1 hour prior to treatment with LPS (1 µg/mL) or saline for 15 or 30 minutes. Whole cell extracts and western blot analyses were conducted for unphosphorylated and phosphorylated (p) p38 (A), JNK (B), and ERK (C) and as described in section 2.9. Results shown are representative of three separate experiments.
4. Discussion
The results presented here demonstrate that EF31 is a potent inhibitor of NF-κB DNA-binding and nuclear translocation. EF31 also attenuated induction of downstream pro-inflammatory cytokine mRNA and protein mediators, and directly inhibited IκKβ activity. Of note, these effects were observed at concentrations that showed no toxicity in mouse RAW264.7 macrophages, while maintaining potent toxicity in NF-κB-dependent cancer cell lines.
Regarding the mechanism of these effects, the data indicate that EF31, like EF24, directly interacts with IκKβ, thereby blocking the phosphorylation of IκB, a necessary step in the activation of NF-κB and its translocation to the nucleus [25, 29]. IκB binds to NF-κB subunits where it is thought to interact with the NF-κB DNA-binding/nuclear localization region, preventing NF-κB translocation to the nucleus. Upon activation, the IκB kinases (IκKα, IκKβ, and NEMO) phosphorylate the inhibitory IκB proteins bound to NF-κB. IκB is then polyubiquitinated and degraded by the 26S proteasome, and consequently, the p65 nuclear localization sequence is unmasked and other amino acids are in turn phosphorylated allowing translocation to the nucleus and activation of gene transcription. The mechanism by which EF31 inhibits IκKβ remains to be elucidated. One possibility is that EF31 may partially occupy the ATP binding site of the kinase as has been suggested for EF24 and curcumin [25]. By interacting with the ATP binding site on the IκKβ, EF31 would block the phosphorylation of the IκB protein and thereby block activation of NF-κB. Furthermore, curcumin has also been shown to indirectly inhibit IκKβ through interactions with other kinases such as Akt, protein kinase C (PKC), and MAPK, which have also been implicated in the activation of NF-κB [30]. Whether EF31 is active against these kinases remains to be determined.
EF31 was also found to block downstream cytokine mRNA expression and cytokine protein release. Indeed, in mouse RAW 264.7 macrophages, cytokine mRNA expression was significantly reduced by EF31 as well as EF24 and curcumin in a dose dependent manner at concentrations between 5–50 µM. Treatment with these compounds also blocked cytokine protein release at 16 hours post LPS. However, both EF24 and curcumin required significantly higher concentrations to achieve results similar to EF31. EF31’s increased potency may be related to its structure. Both EF31 and EF24 are shorter than curcumin, which could confer an advantage by allowing these compounds to be more adaptable to the globular binding pocket of IκKβ [25]. Moreover, the nitrogen-carbon bonds in the terminal rings of EF31 are less bulky than the fluorine-carbon bonds in EF24. The nitrogen allows for favorable steric effects in the kinase binding site, and because they are good proton acceptors, they also have an advantage in the formation of hydrogen bonds to the target proteins (Shi and Snyder, unpublished data). Nevertheless, further testing is required to determine the binding site of EF31 on IκKβ and the mechanism for increased potency. Although experiments presented here suggest that EF31 may be inhibit LPS-induced cytokines production via effects on the NF-κB pathway, further experiments are required to demonstrate that EF31 directly impacts expression of genes regulated by NF-κB. For example, a luciferase assays using an NF-κB-driven promoter would confirm that EF31 disrupts expression of genes that are regulated by NF-κB. This is especially the case given findings regarding the effects of EF31 on MAPK pathways (discussed below).
Similar to EF24, EF31 exhibited more potent toxicity on NF-κB-dependent cancer cell lines than curcumin. The NF-κB pathway plays an important role in the survival and proliferation of the cancer cell lines tested [3, 25, 31–33]. Sustained cell viability has been shown to depend in part on NF-κB-regulated gene expression of survival proteins such as Bcl-xL and other anti-apoptotic signaling molecules. By blocking the activation of the NF-κB pathway and thus the expression of survival signals, apoptotic signaling pathways may be activated and thereby reduce cell viability and proliferation. Another mechanism that may be involved in EF31’s effects on cancer cell viability is increased expression of the protein phosphatase and tensin homolog (PTEN). PTEN is a negative regulator of the kinase Akt, which is involved in the survival of some cancer cell lines [34]. Expression of PTEN results in cell cycle arrest and apoptosis, however, activation of the NF-κB subunit p65 has been shown to reduce expression of PTEN by interfering with transcription cofactors in the nucleus [35]. By blocking the disinhibition of the p65 subunit of NF-κB, EF31 in turn may increase the expression of PTEN. A similar mechanism of action has been reported for EF24 [31]. Moreover, there is evidence suggesting that EF24 and EF31 both use a redox-dependent mechanism to induce apoptosis in cancer cells [33, 36]. Although low concentrations of EF31 (1–30 µM) had cytotoxic effects on the cancer cell lines tested, it did not show significant reductions in cell viability/proliferation in mouse RAW264.7 macrophages, possibly related to the specific roles of NF-κB in these cancer cell lines. Related to this topic, curcumin tended to reduce RAW264.7 macrophage viability at the concentration employed in most experiments (50 µm), opening up the possibility that some portion of curcumin effects reported here may have been due to cell toxicity.
Another issue regarding the development of curcumin analogs for therapeutic use is whether or not the inhibition of NF-κB activity is reversible. EF31 is a Michael acceptor, with the potential to form carbon bonds that irreversibly bind to the ATP pocket of IκKβ and thereby could irreversibly block NF-κB activity. NF-κB is important for the survival of healthy cells as well as cancer cell lines, and if inhibition is irreversible, it could induce cellular apoptosis and destroy vulnerable cells/ tissues as well as compromise the immune response. Indeed, IκKα, IκKβ, and p65 knockout mice typically die as embryos or shortly after birth, while p50 KO mice are viable but show degenerative alterations including increased spontaneous cell death in the brain, abnormal capillaries, and fewer myelinated axons of the optic nerve [37]. In the current studies, mouse RAW26.7 macrophages treated with EF31 and LPS regained NF-κB DNA-binding activity in a time-dependent manner, suggesting that the inhibition is reversible. Nevertheless, recovery of NF-κB activity measured using a DNA-binding ELISA could have also been due in part to cell proliferation, and not necessarily to uncoupling of the compound from the IκKβ. Although, NF-κB DNA-binding activity in the current studies serves as an indirect indicator of upstream IκB kinase activity, Sun et al. (2009) showed that binding of EF24 and EF31 to thiol (SH) is reversible. This suggests that binding of the compounds to the nucleophile SH of the cysteine in the binding pocket of the IκB kinase is also reversible, which is consistent with the recovery of downstream NF-κB DNA-binding in the current studies.
In addition to effects on NF-κB, at a 10 µM concentration, EF31 significantly reduced DNA-binding activity of the activator protein (AP)-1 transcription factors activating transcription factor-2 (ATF-2) and c-Jun, as well as whole cell p-JNK, suggesting that EF31 may have broad effects on inflammatory signaling pathways including MAPKs. These results are consistent with data showing that curcumin inhibits the activity of p38 and c-Jun N-terminal kinase (JNK): kinases involved in the downstream activation of MAPK transcription factors [38, 39]. Of note, our results for MAPK transcription factor-DNA binding and p-JNK also showed a significant increase in the saline group treated with EF31. This may be related to interactions between the NF-κB and MAPK pathways. Nijboer et al., reported a significant increase in AP-1 activity when NF-κB was inhibited in a model of hypoxic-ischemic brain damage [40]. The mechanism by which NF-κB inhibition can up-regulate activity in the JNK/AP-1 pathway, may be via down-regulation of NF-κB-dependent expression of the proteins growth arrest and DNA-damage-inducible (Gadd45) β and x-linked inhibitor of apoptosis protein (XIAP). The proteins Gadd45β and XIAP have been shown to inhibit the activation of the JNK/AP-1 pathway by interacting with kinases MAPK kinase (MAP2K) 7 and transforming growth factor β-activated kinase (TAK) 1, respectively [40]. Thus, the EF31-related increase in AP-1 transcription factor-DNA binding activity in our results may be due to potent inhibition of the NF-κB pathway and down-regulation of the inhibitory proteins Gadd45β and or XIAP. However, this remains speculative and requires further testing.
In summary, EF31 is one in a series of curcumin analogs that show potent inhibition of NF-κB DNA-binding activity and nuclear translocation in conjunction with potent toxicity in NF-κB dependent cancer cell lines. Although preliminary studies suggest that EF31 may exhibit greater serum concentrations after oral administration that curcumin (see Supplementary Table 1), more extensive bioavailability studies are needed. Pending these and toxicity studies, EF31 may serve as a relevant endpoint or a further step in the development of curcumin analogues for therapeutic applications in inflammatory disorders and cancer.
Highlights.
EF31 is a potent inhibitor of Iκβ kinase activity, NF-κB translocation, and NF-κB DNA-binding activity in RAW264.7 macrophages
EF31 blocks the expression of pro-inflammatory cytokine mRNA and protein
EF31 inhibits MAPK transcription factors
EF31 exhibits potent anti-cancer activity in various cancer cell lines
Supplementary Material
Acknowledgments
We like to thank Songli Xu and Debbie Martinson for microscope training and assistance with cell imaging.
This work was supported by the National Institutes of Mental Health [Grants R21MH085210, F31MH085460 (to T.W.W.P. and A.O., respectively)], the National Institute of Health [Grants R21CA82995-01A1, 1P50CA128613, R01CA109366 (to M.S., J.D., and H.S., respectively)], the U.S. Department of Defense, Division of U.S. Army [Grants DAMD170010241, 1U54HG003918 (to M.S. and J.P.S., respectively), and the Emory Institute for Drug Discovery.
ABBREVIATIONS
- A2780
human ovarian carcinoma cells
- AP-1
activator protein 1
- ATF-2
activating transcription factor-2
- ANOVA
analysis of variance
- CD
cluster of differentiation
- DMSO
Dimethyl sulfoxide
- DMEM
Dulbecco’s modified Eagle’s medium
- EF24
3,5-Bis(2-fluorobenzylidene)-4-piperidone
- EF31
3,5-Bis(2-pyridinylmethylidene)-4-piperidone
- EMT6
mouse mammary carcinoma cells
- ERK
extracellular-signal-regulated kinase
- FLSD
Fisher's least significant difference
- FRET
fluorescence resonance energy transfer
- Gadd45β
growth arrest and DNA-damage-inducible
- IκKβ
IκB kinases β
- IL
interleukin
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MAP2K7
MAPK kinase 7
- NF-κB
Nuclear Factor kappa B
- MAPK
mitogen-activated protein kinase
- MDA-MB-231
human breast cancer cells
- MD-2
myeloid differentiation factor 2
- MIA PaCa-2
human pancreatic carcinoma cells
- PBS
phosphate-buffered saline
- PKC
protein kinase C
- PTEN
protein phosphatase and tensin homolog
- SH
thiol
- TAK1
transforming growth factor β-activated kinase
- TLR-4
toll-like receptor 4
- TNF
tumor necrosis factor
- XIAP
x-linked inhibitor of apoptosis protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare, financial or otherwise, regarding findings presented in this manuscript.
Parts of this work were previously presented: As part of Thesis at Emory University, Olivera A, Hu F, Brown AP, Shim H, Liotta DC, Miller AH, Pace TWW (2009) EF31, a novel curcumin analog, is a potent inhibitor of nuclear factor-kappa b activity in mouse RAW264.7 macrophages. Brain, Behavior, and Immunity. Volume 23, Supplement 2, July 2009, pp. S52–S53. PNIRS 2009, Psychoneuroimmunology Research Society Annual Meeting 2009, Keystone, CO., and Brown A, Yoon, Y., Sun, A., Liang, Z., Wang, S., Snyder, J.P., Liotta, D.C., and Shim, H. (2007) Development of curcumin analogs for anti-cancer therapy. Proceedings of the American Association for Cancer Research 48:5551.
Contributor Information
Anlys Olivera, Email: aolive3@emory.edu.
Terry W. Moore, Email: terry.moore@emory.edu.
Fang Hu, Email: fhu@emory.edu.
Andrew P. Brown, Email: andrew.brown@emory.edu.
Aiming Sun, Email: asun2@emory.edu.
Dennis C. Liotta, Email: dliotta@emory.edu.
James P. Snyder, Email: jsnyder@emory.edu.
Younghyoun Yoon, Email: yyoon2@emory.edu.
Hyunsuk Shim, Email: hshim@emory.edu.
Adam I. Marcus, Email: aimarcu@emory.edu.
Andrew H. Miller, Email: amill02@emory.edu.
Thaddeus W. W. Pace, Email: twpace@emory.edu.
References
- 1.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couzin-Frankel J. Inflammation bares a dark side. Science. 2010;330:1621. doi: 10.1126/science.330.6011.1621. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 4.Tracy RP. Emerging relationships of inflammation, cardiovascular disease and chronic diseases of aging. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S29–S34. doi: 10.1038/sj.ijo.0802497. [DOI] [PubMed] [Google Scholar]
- 5.Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 7.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giuliani C, Napolitano G, Bucci I, Montani V, Monaco F. Nf-kB transcription factor: role in the pathogenesis of inflammatory, autoimmune, and neoplastic diseases and therapy implications. Clin Ter. 2001;152:249–253. [PubMed] [Google Scholar]
- 9.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 12.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 13.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 15.Ulevitch RJ, Tobias PS. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr Opin Immunol. 1999;11:19–22. doi: 10.1016/s0952-7915(99)80004-1. [DOI] [PubMed] [Google Scholar]
- 16.Jagetia GC, Aggarwal BB. "Spicing up" of the immune system by curcumin. J Clin Immunol. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 17.Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Ann N Y Acad Sci. 2005;1056:206–217. doi: 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as "Curecumin": from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30:85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 22.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 24.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 25.Kasinski AL, Du Y, Thomas SL, Zhao J, Sun SY, Khuri FR, et al. Inhibition of IkappaB kinase-nuclear factor-kappaB signaling pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol Pharmacol. 2008;74:654–661. doi: 10.1124/mol.108.046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol. 2002;169:6408–6416. doi: 10.4049/jimmunol.169.11.6408. [DOI] [PubMed] [Google Scholar]
- 27.Chen YL, Huang YL, Lin NY, Chen HC, Chiu WC, Chang CJ. Differential regulation of ARE-mediated TNFalpha and IL-1beta mRNA stability by lipopolysaccharide in RAW264.7 cells. Biochem Biophys Res Commun. 2006;346:160–168. doi: 10.1016/j.bbrc.2006.05.093. [DOI] [PubMed] [Google Scholar]
- 28.Means TK, Pavlovich RP, Roca D, Vermeulen MW, Fenton MJ. Activation of TNF-alpha transcription utilizes distinct MAP kinase pathways in different macrophage populations. J Leukoc Biol. 2000;67:885–893. doi: 10.1002/jlb.67.6.885. [DOI] [PubMed] [Google Scholar]
- 29.Shu HG, Brown A, Yoon Y, Gao H, Purcell J, Snyder JP, Liotta DC, Shim H. Radiosensitization of glioma cells with the curcumin analogs EF24 and UBS109. AACR 99th Annual Meeting.2008. [Google Scholar]
- 30.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 31.Selvendiran K, Tong L, Vishwanath S, Bratasz A, Trigg NJ, Kutala VK, et al. EF24 induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by increasing PTEN expression. J Biol Chem. 2007;282:28609–28618. doi: 10.1074/jbc.M703796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holcomb B, Yip-Schneider M, Schmidt CM. The role of nuclear factor kappaB in pancreatic cancer and the clinical applications of targeted therapy. Pancreas. 2008;36:225–235. doi: 10.1097/MPA.0b013e31815b3207. [DOI] [PubMed] [Google Scholar]
- 33.Adams BK, Cai J, Armstrong J, Herold M, Lu YJ, Sun A, et al. EF24, a novel synthetic curcumin analog, induces apoptosis in cancer cells via a redox-dependent mechanism. Anticancer Drugs. 2005;16:263–275. doi: 10.1097/00001813-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 35.Vasudevan KM, Gurumurthy S, Rangnekar VM. Suppression of PTEN expression by NF-kappa B prevents apoptosis. Mol Cell Biol. 2004;24:1007–1021. doi: 10.1128/MCB.24.3.1007-1021.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun A, Lu YJ, Hu H, Shoji M, Liotta DC, Snyder JP. Curcumin analog cytotoxicity against breast cancer cells: exploitation of a redox-dependent mechanism. Bioorg Med Chem Lett. 2009;19:6627–6631. doi: 10.1016/j.bmcl.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Lu Z, Li WL, Yu SP, Wei L. Cell death and proliferation in NF-kappaB p50 knockout mouse after cerebral ischemia. Brain Res. 2008;1230:281–289. doi: 10.1016/j.brainres.2008.06.130. [DOI] [PubMed] [Google Scholar]
- 38.Camacho-Barquero L, Villegas I, Sanchez-Calvo JM, Talero E, Sanchez-Fidalgo S, Motilva V, et al. Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int Immunopharmacol. 2007;7:333–342. doi: 10.1016/j.intimp.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Chen YR, Tan TH. Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene. 1998;17:173–178. doi: 10.1038/sj.onc.1201941. [DOI] [PubMed] [Google Scholar]
- 40.Nijboer CH, Heijnen CJ, Groenendaal F, van Bel F, Kavelaars A. Alternate pathways preserve tumor necrosis factor-alpha production after nuclear factor-kappaB inhibition in neonatal cerebral hypoxia-ischemia. Stroke. 2009;40:3362–3368. doi: 10.1161/STROKEAHA.109.560250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.