Abstract
Adaptive responding to threatening stressors is of fundamental importance for survival. Dysfunctional hyperactivation of corticotropin releasing factor type-1 (CRF1) receptors in stress response system pathways is linked to stress-related psychopathology and CRF1 receptor antagonists (CRAs) have been proposed as novel therapeutic agents. CRA effects in diverse animal models of stress that detect anxiolytics and/or antidepressants are reviewed, with the goal of evaluating their potential therapeutic utility in depression, anxiety, and other stress-related disorders. CRAs have a distinct phenotype in animals that has similarities to, and differences from, those of classic antidepressants and anxiolytics. CRAs are generally behaviorally silent, indicating that CRF1 receptors are normally in a state of low basal activation. CRAs reduce stressor-induced HPA axis activation by blocking pituitary and possibly brain CRF1 receptors which may ameliorate chronic stress-induced pathology. In animal models sensitive to anxiolytics and/or antidepressants, CRAs are generally more active in those with high stress levels, conditions which may maximize CRF1 receptor hyperactivation. Clinically, CRAs have demonstrated good tolerability and safety, but have thus far lacked compelling efficacy in major depressive disorder, generalized anxiety disorder, or irritable bowel syndrome. CRAs may be best suited for disorders in which stressors clearly contribute to the underlying pathology (e.g. posttraumatic stress disorder, early life trauma, withdrawal/abstinence from addictive substances), though much work is needed to explore these possibilities. An evolving literature exploring the genetic, developmental and environmental factors linking CRF1 receptor dysfunction to stress-related psychopathology is discussed in the context of improving the translational value of current animal models.
Keywords: Animal models, corticotropin releasing factor, CRF1 receptor, anxiety, depression, stress
1. Introduction
Small molecule, orally active, brain-penetrating corticotropin releasing factor type-1 antagonists (CRAs; see Table 1) represent a mechanistically novel class of agents for potential therapeutic use in the treatment of anxiety, depression, and other stress-related disorders (for reviews, see (Grigoriadis, 2005; Hauger, Risbrough, Brauns, & Dautzenberg, 2006; Kehne, 2007; Kehne & De Lombaert, 2002; Kehne & Maynard, 2008; Li, et al., 2005; Steckler & Dautzenberg, 2006). The purpose of this manuscript is to review a broad scientific literature that has examined the effects of CRAs in a variety of animal models, with a particular focus on those commonly used to detect antidepressant and anxiolytic activity. This section will be preceded by background on CRF1 pathways and CRAs and followed by a discussion of how these preclinical findings fit with currently available clinical data. Finally, findings from a rapidly evolving literature that is further refining our understanding of the genetic, developmental and environmental factors linking CRF1 receptor dysfunction to stress-related psychopathology will be discussed both in the context of how they impact our thinking about the therapeutic utility of CRAs and how they might help us further improve the translational value of current animal models.
Table 1.
CRF1 receptor antagonists. This table summarizes current publically-available information about compounds reported to be (or presumed to be, if data are not available) non-peptidic, potent, selective, orally active antagonists of the CRF1 receptor. Numerous agents listed are not in development but are used as pharmacological probes in animal studies. Some agents have reached clinical testing in various disorders, as described by publications and/or company press releases, and some are reported to be discontinued. “CRF1 Aff.” refers to reported or estimated affinity in CRF1 receptor binding assays (IC50 or Ki, in nM). (n.a. = not available)
| Name | Company/ Stakeholder |
Chemical Name | CRF1 Aff. |
Status (estimated farthest advanced) |
References |
|---|---|---|---|---|---|
| Antalarmin | N-butyl-N-ethyl-2,5,6-trimethyl-7-(2,4,6- trimethylphenyl)pyrrolo[3,2-e]pyrimidin-4-amine |
<10 | Preclinical | (Seymour, Schmidt, & Schulz, 2003) | |
| CP-154,526 | Pfizer | (2,5-dimethyl-3-(6-dimethyl-4-methylpyridin-3-yl)- 7-dipropylamino-pyrazolo [1,5-a]pyrimidine) |
<10 | Preclinical | (Schulz, et al., 1996; Seymour, et al., 2003) |
| CP-316,311 | Pfizer | 3,6-dimethyl-4-(pentan-3-yloxy)-2-(2,4,6- trimethylphenoxy)pyridine |
<10 | Phase II, depression; double blind, placebo controlled;no difference vs placebo |
(Binneman, et al., 2008); www.bindingdb.org |
| CRA1000 | Taisho | 2-[N-(2-methylthio-4-isopropylphenyl)-N- ethylamino]-4-[4-(3-fluorophenyl)-1,2,3,6- tetrahydropyridin-1-yl)-6-methylpyrimidine |
20–40 | Preclinical | (Chaki, et al., 1999; Okuyama, et al., 1999) |
| CRA1001 | Taisho | 2-[N-(2-bromo-4-isopropylphenyl)-N-ethylamino]- 4-[4-(3-fluoropheny l)-1,2,3,6-tetrahydropyridin-1- yl)-6-methylpyrimidine |
20–40 | Preclinical | (Chaki, et al., 1999; Okuyama, et al., 1999) |
| CRA0450 | Taisho | 1-[8-(2,4-dichlorophenyl)-2-methylquinolin-4-yl]- 1,2,3,6-tetrahydropyridin e-4-carboxamide benzenesulfonate |
40–60 | Preclinical | (Chaki, et al., 2004; Dawe, et al., 2001) |
| DMP696 | BMS | 4-(1,3-Dimethoxyprop-2-ylamino)-2,7-dimethyl-8- (2, 4-dichlorophenyl)pyrazolo[1,5-a]-1,3,5-triazine |
<10 | Preclinical | (Li, et al., 2005) |
| DMP904 | BMS | [4-(3-pentylamino)-2,7-dimethyl-8-(2-methyl-4- methoxyphenyl)-pyrazolo-[1,5 -a]-pyrimidine] |
n.a. | Preclinical | (Li, et al., 2005) |
| GSK561679 | GSK/ Neurocrine |
n.a. | n.a. | Phase II, depression; double blind, placebo controlled; registered 08/08; completed but results not published |
www.clinicaltrials.gov |
| Phase I, social anxiety disorder; registered 11/07; double blind; completed but results not published |
www.clinicaltrials.gov | ||||
| Phase II, PTSD, females;double blind, placebo controlled; registered 11/09 |
www.clinicaltrials.gov | ||||
| Experimental, human startle, females; registered |
www.clinicaltrials.gov | ||||
| Experimental, emotional processing (fMRI); double blind; registered 8/07; completed but results not published |
www.clinicaltrials.gov | ||||
| GSK586529 | GSK/ Neurocrine |
n.a. | n.a. | Phase I, depression | www.clinicaltrials.gov |
| GW876008 | GSK/ Neurocrine |
n.a. | n.a. | Phase II, social anxiety disorder; double blind, placebo controlled; no difference vs placebo |
www.clinicaltrials.gov |
| Phase II, IBS; double blind, placebo controlled; completed; results not published |
www.clinicaltrials.gov ; www.neurocrine.com | ||||
| Phase I, emotional processing (fMRI); registered 1/07; completed but results not published |
www.clinicaltrials.gov | ||||
| MJL-1-109-2 | pyrazolo[1,5-a]-1,3,5-triazin-4-amine,8-[4- (bromo)-2-chlorophenyl]-N, N-bis(2- methoxyethyl)-2,7-dimethyl-(9Cl) |
<10 | Preclinical | (Zhao, et al., 2007) | |
| MPZP | Salk Institute | N,N-bis(2-methoxyethyl)-3-(4-methoxy-2- methylphenyl)-2,5-dimethyl-pyrazolo [1,5-a] pyrimidin-7-amine |
<10 | Preclinical | (Richardson, et al., 2008) |
| MTIP | NIAAA/Eli Lilly |
3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1- ethylpropyl)-2,6-dimethyl-imidazo[1,2- b]pyridazine |
<10 | Preclinical | (Gehlert, et al., 2007) |
| NBI3b1996 | Neurocrine | (N-Cyclopropylmethyl-2,5-dimethyl-N-propyl-N'- (2,4,6-trichloro-phenyl)-pyr imidine-4,6-diamine) |
<10 | Preclinical | (Gehlert, et al., 2005) |
| NBI-34041 | GSK/Neuroc rine |
2-(2,4-dichlorophenyl)-4-methyl-6-(1-propylbutyl)- 7,8-dihydro-6H-1,3,6,8a-tetraazaacenaphthylene |
<10 | Reduced stress-induced ACTH release and corticosterone in Trier Social Stress Test |
(Ising, et al., 2007) |
| ONO-2333Ms | Ono | n.a. | n.a. | Program discontinued due to lack of efficacy (07/08) |
www.clinicaltrials.gov |
| Pexacerfont (BMS-562086) | BMS | 8-(6-methoxy-2-methylpyridin-3-yl)-2,7-dimethyl- N-[(1R)-1-methylpropyl]pyrazolo[1,5-a]-1,3,5- triazin-4-amine |
n.a. | Phase II, depression; listed as completed 10/07; results conveyed as personal communication from V. Coric, M.D. |
www.clinicaltrials.gov; |
| Phase II, GAD | (Coric, et al., 2010) | ||||
| Phase II, IBS; listed as completed 01/08 but results not published |
www.clinicaltrials.gov | ||||
| PF-572778 | Pfizer | n.a. | n.a. | ||
| R121919 (NBI27914) | GSK/Neuroc rine |
(2,5-dimethyl-3-(6-dimethyl-4-methylpyridin-3-yl)- 7-dipropylamino-pyrazolo [1,5-a]pyrimidine) |
<10 | Phase II (open-label; depression); active; discontinued due to abnormal liver function tests |
(Chen, et al., 1996; Saunders & Williams, 2001) |
| R27899 (CRA0450) | Taisho/Johns on & Johson |
1-[8-(2,4-dichlorophenyl)-2-methylquinolin-4-yl]- 1,2,3,6-tetrahydropyridin e-4-carboxamide benzenesulfonate |
50–60 | Preclinical | (Chaki, et al., 2004) |
| R317573 | Taisho/Johns on & Johnson |
46 | Phase IIa; double-blind, placebo controlled; regional cerebral glucose metabolism PET study |
(Schmidt, et al., 2010) | |
| Phase IIa; double-blind, randomized, placebo- controlled |
(Dawson, et al., 2009) | ||||
| SSR125543 | Sanofi- Aventis |
4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)- 2-cyclopropyl-1-(3-fluoro-4-methylphenyl)ethyl]5- methyl-N-(2-propynyl)-1,3-thiazol-2-amine hydrochloride |
<10 | Preclinical | (Gully, et al., 2002) |
1a. Stress Response System, CRF1 Receptors, and CRAs
The ability to respond in an adaptive manner to threatening stressors is of fundamental importance for survival of the species. Evolution has crafted a complex neurobiological stress response system (SRS) which mediates responses to external or internal stressors thereby serving an essential survival function. Many neurochemicals comprise the SRS, including norepinephrine (NE), serotonin (5HT), and GABA, neurotransmitters that are targets for currently marketed antidepressants and/or anxiolytics. Such neurochemical multiplicity likely reflects, at least in part, a redundancy in the SRS that ensures the robustness of this highly important survival mechanism.
In the last two decades, a neurochemical system that has been of great interest with regard to the functioning of the SRS is the peptide CRF acting upon postsynaptic CRF1 receptors (“CRF1 pathways”). CRF (also known as corticotropin-releasing hormone, or CRH) is a 41 amino-acid peptide identified by Wylie Vale and colleagues three decades ago (Vale, Spiess, Rivier, & Rivier, 1981) that has been implicated in mediating an organism’s behavioral, endocrine and autonomic responses to stress. The molecular pharmacology of CRF systems has been extensively studied and is the subject of a number of excellent reviews (Aguilera, Nikodemova, Wynn, & Catt, 2004; Bale & Vale, 2004; Hauger, et al., 2006; Hillhouse & Grammatopoulos, 2006; Perrin & Vale, 1999; Steckler & Dautzenberg, 2006), and while a detailed discussion is beyond the scope of the current review, Figure 1 summarizes some key points which are elaborated below.
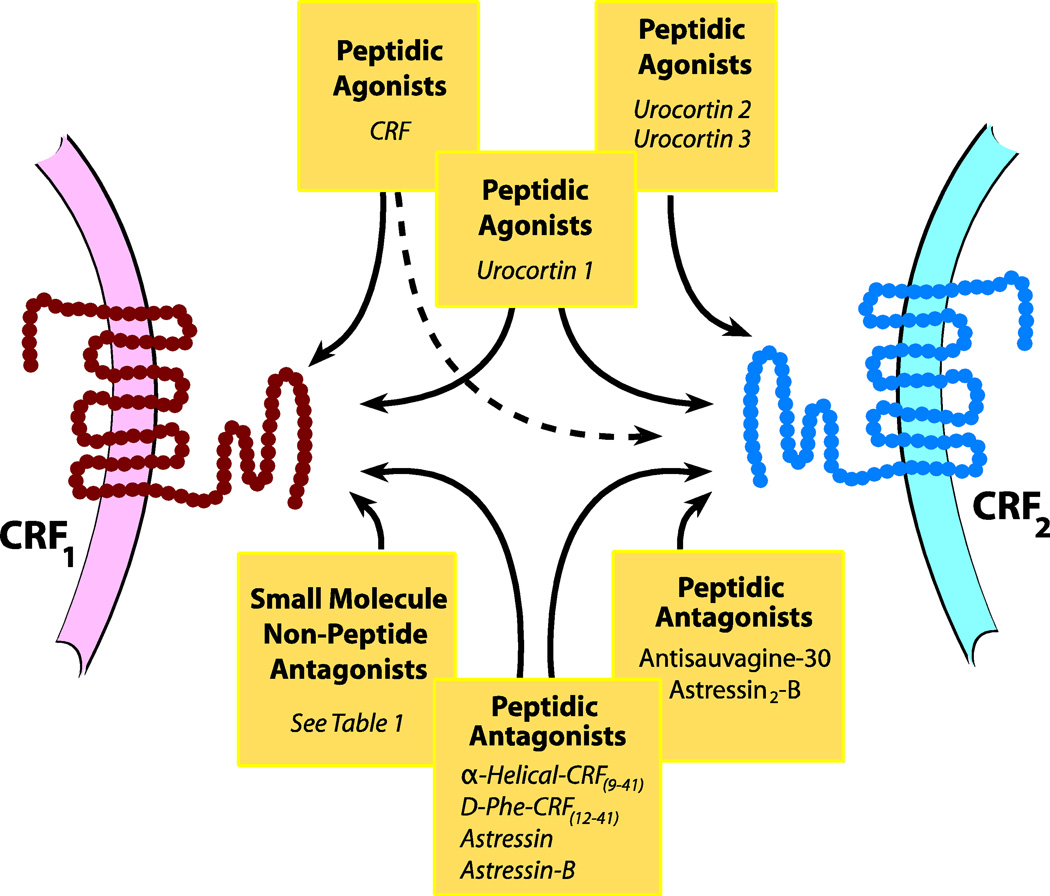
Figure 1. CRF Ligands and Receptors.
CRF and structurally related peptides urocortin 1, urocortin 2, and urocortin 3 (top of diagram) act as agonists with varying relative affinities for the CRF1 receptor (left) and CRF2 receptor (right). CRF shows the greatest selectivity for the CRF1 receptor whereas urocortin 2 and urocortin 3 show greatest selectivity for the CRF2 receptor. Not shown are the variants CRF1a (for the CRF1 receptor) and CRF2a, CRF2b, and CRF2c (for the CRF2 receptor). Peptidic antagonists (bottom of diagram) are either non-selective for the two subtypes or are selective for the CRF2 receptor. Small molecule, non-peptidic antagonists show a high degree of selectivity for the CRF1 receptor.
CRF belongs to a family of structurally-related peptides, with members including urocortin 1, urocortin 2 (“stresscopin-related peptide”), and urocortin 3 (“stresscopin). CRF acts upon two types of G-protein coupled receptors, CRF1 and CRF2, which are encoded by two distinct genes. There is one main structural variant of CRF1, termed CRF1a, whereas there are three main functional variants of the CRF2 receptor, designated CRF2a, CRF2b, and CRF2c. CRF1 and CRF2 receptors are present in the brain and periphery though with different neuroanatomical localizations (Hillhouse & Grammatopoulos, 2006). CRF1 receptors are primarily located in the anterior pituitary, amygdala, hippocampus, cortex, and cerebellum whereas CRF2 receptors are primarily located in choroid plexus, ventromedial hypothalamus and lateral septum (for a detailed description of the anatomical distribution of the isoforms, see (Hauger, et al., 2006; Hillhouse & Grammatopoulos, 2006). Adenylate cyclase/cyclic AMP is well-established as the dominant signaling pathway for both receptors, though the contributions of additional signaling pathways have been described (Hauger, et al., 2006).
CRF and the urocortins act as ligands with differing affinities for CRF1 and CRF2 receptors. Thus, whereas CRF shows preferential affinity for the CRF1 receptor subtype, urocortin 1 shares affinity for both subtypes, and urocortin 2 and 3 show preferential affinity for CRF2. Likewise, there are a number of peptidic agents which act with differing relative affinities as competitive antagonists at CRF1 and CRF2 receptors, with α-helical-CRF(9–41), D-Phe-CRF(12–41), astressin, and astressin-B non-selective and antisauvagine-30 and astressin-2B selective for CRF2. While there are no peptidic agents that are highly selective for CRF1, there are a number of small molecule, non-peptidic agents which have been identified as selective CRAs (see Table 1 for a list of the key compounds). CRAs are thought to bind to a site that is only part of the extracellular domain acted upon by peptidic agonists and antagonists, and in this regard, CRAs are allosteric inhibitors rather than competitive CRF1 receptor antagonists (Hauger, et al., 2006). Small molecule, non-peptidic antagonists that are selective for the CRF2 receptor have not yet been identified.
One approach to studying the functional roles of CRF1 and CRF2 receptors has been to evaluate the behavioral effects of direct intracerebral administration of CRF and related ligands and their interactions with CRF receptor antagonists (Campbell, Morrison, Walker, & Merchant, 2004; Heinrichs, De Souza, Schulteis, Lapsansky, & Grigoriadis, 2002; Howard, Carr, Hill, Valentino, & Lucki, 2008; Koob, 1999; Pelleymounter, et al., 2000; Pelleymounter, Joppa, Ling, & Foster, 2002, 2004; Zorrilla, et al., 2004). Activation of the CRF1 receptor has been primarily associated with anxiety- or depressive like behaviors, which can be reversed by peptidic CRF receptor antagonists. The role of CRF2 receptors is more complex: CRF2 receptor activation has been associated with both enhancement as well as inhibition of stress responsivity, and other lines of evidence suggest a primary role in the suppression of feeding behavior. While the CRF2 receptor also has potential relevance as a therapeutic target for the treatment of CNS disorders, the CRF1 receptor has been the primary target for pharmaceutical development and is the focus of the current review.
CRF uniquely participates in several key components of the SRS. CRF1 pathways modulate behavioral circuits important for defensive responding and stress coping including the prefrontal, cingulate and insular cortices; amygdala, hippocampus, bed nucleus of the stria terminalis (BNST), periaqueductal gray, and the monoamine pathways. The endocrine component of the SRS is comprised of the hypothalamic-pituitary-adrenal (HPA) axis. Following exposure to a stressor, CRF released from periventricular cells of the hypothalamus diffuses via the portal blood system to the anterior pituitary where it binds to CRF1 receptors and mediates adrenocorticotropin hormone (ACTH) release into the bloodstream. CRF is also contained within brainstem autonomic circuits, and in concert, these three different limbs, when transiently activated by stressors, produce a coordinated hyperarousal and subsequent coping response (Figure 2; for review, see (Kehne, 2007). In this manner, CRF1 pathways play an important and coordinating role in the normal functioning of the SRS.
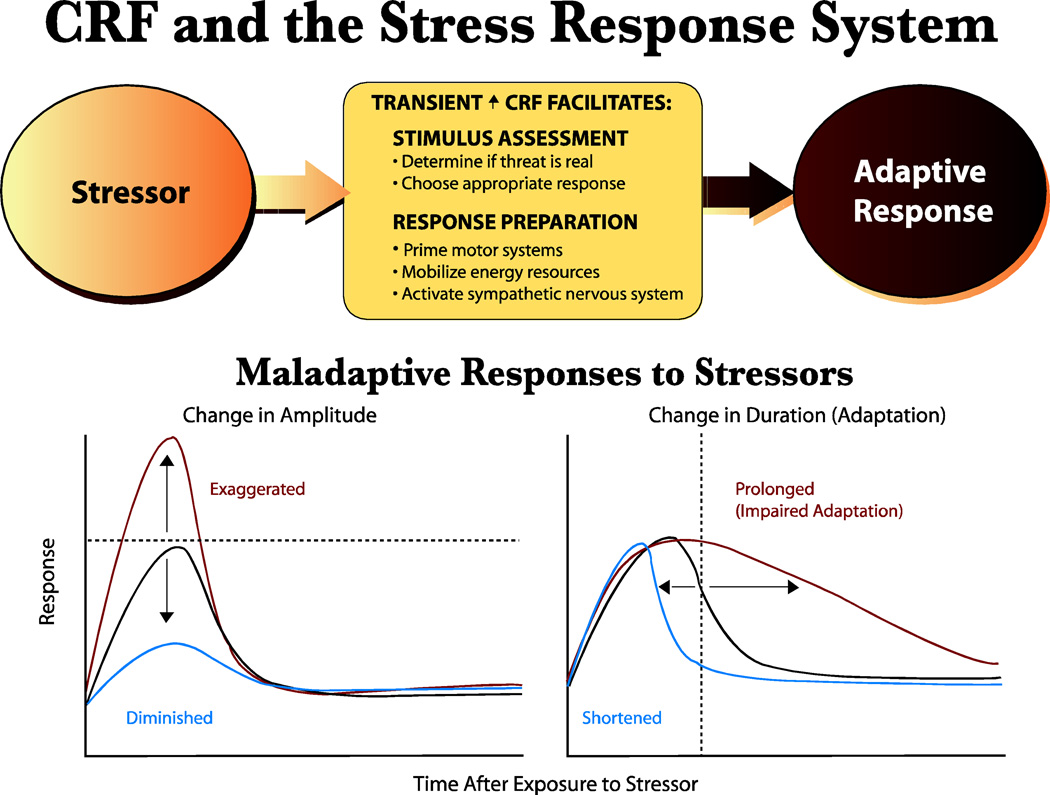
Figure 2. CRF and the Stress Response System (SRS).
TOP: The SRS is composed of behavioral, endocrine, and autonomic components which act in concert to generate an appropriate, adaptive response to a stressor. A key mediator in the SRS is CRF, acting through the CRF1 receptor, which is normally in a low state of basal activity. Stressor-induced CRF1 receptor activation facilitates physiological processes which allow the organism to evaluate the stressor and choose an adaptive response, while in parallel activating effector systems. Execution of a successful response will minimize the impact of the stressor and in parallel, feedback inhibitory systems will ensure that the stress response system will return to normal, pre-stress levels. BOTTOM: Abnormal hyperactivation of CRF pathways and CRF1 receptors can result in a dysfunctional SRS in which normal alarm reactions may be maladaptive. A complex interplay of genetic risk factors, vulnerability factors (prior history), stressor factors (intensity, duration, chronicity), may be expressed neuronally as imbalances in different CRF1 receptor pathways in the brain and functionally, as different alterations in the alarm reaction. Thus, CRF1 receptor hyperactivity seen in different disorders may be manifested as alarm reactions with exaggerated or diminished amplitude and/or prolonged or shortened duration. Different functional effects may reflect sensitization versus desensitization of CRF1 receptors in the same or different CRF pathways in the stress response system. Transient acute or repeated exposures to intense stressors may sensitize certain components giving rise to heightened or prolonged alarm reactions, whereas more chronic stressors may desensitize CRF1 receptors and give rise to blunted responses and decreased reactivity. Tonic elevations in stress hormones and anxiety levels may be expressions of extensive failure of adaptation of the SRS. CRF1 receptor antagonists may act to restore normal balance and reactivity in the stress response across a number of stress-related disorders. Figure from (Kehne, 2007), printed with permission from Bentham Publishers.
It is important to emphasize that stressor-induced responsiveness is a highly activated state that is not sustainable for long periods of time. The SRS is designed to be activated for a relatively short time frame during which rapid and extensive mobilization of energy resources and recruitment of neuronal coping pathways enables the performance of a coordinated adaptive response that optimizes chances for survival given the specific environmental conditions. This adaptive response could be a flight response, achieved by activation of brainstem mesencephalic locomotor centers which in turn drive spinal cord generators mediating alternating flexions and extensions of limb muscles that comprise locomotion. Alternatively, if flight is not an option, an organism will generally fight (though “playing dead”responses, comprised of inhibition of skeletal muscle activity akin to a freezing response, are an alternative adaptive behavior demonstrated by some species), and a specific stereotyped sequence of muscle activations will be engaged to help defend the safety of the organism. Fight and flight responses are situated at the high end of the defensive SRS behavioral spectrum, but it should be noted that there are numerous defensive behaviors available to an organism depending on the threat degree and resultant activation level of the SRS (Fanselow and Lester, 1988). If the stress response is successful, and the threat is evaded, then the eliciting stressor is removed and, through a natural decay process, the SRS is restored to its pre-stress, low basal state of activity. As it is critical to ensure that the state of SRS activation not be sustained, multiple feedback systems have evolved that, in parallel, serve to deactivate the SRS and rapidly bring it back to its low state of basal activity. An extremely important and robust regulatory mechanism is feedback inhibition of CRF pathways that occurs at the level of the brain and pituitary mediated by ACTH-induced release of corticosterone (or cortisol in humans). Thus, systems are in place to both actively engage the SRS to effectively deal with a threatening stressor and to subsequently shut it off. In the wild, the consequences of life-threatening encounters are often clear cut: survival (successful escape or dominance) or failure (death or severe injury). In the case of success, the SRS returns to a low state of activity, recovers, and the organism is prepared for future encounters with threatening stressors.
As illustrated in Figure 2, there are a number of ways in which this highly critical SRS could in theory become dysfunctional. In the hypothetical graphs at the bottom of the figure, an optimal physiological response to a stressor is indicated by a curve with an activational (ascending) component followed by deactivation decay. Dysfunctional overactivation of the stress response could be expressed as increased magnitude of a response (or alternatively a lowered threshold for response elicitation), by decreased decay (decreased adaptation, or habituation) of the response, or a combination of both. Functionally, one can conceptualize these types of outcomes as exaggerations of naturally-occurring stress responses. Conversely, it is also possible that dysfunctional stress responses could be expressed as a reduction in the normal responsiveness of the system, through a decreased magnitude of the response, an abnormally fast decay (indicative of hyperadaptation), or a combination of both. One can speculate that these opposite ends of the spectrum of abnormal stress responsiveness are expressed functionally as unique types of symptomotologies in different stress-related clinical disorders. As an example which will be further discussed below, opposite states of HPA axis reactivity have been measured in depression (evidence for reduced feedback inhibition of HPA) and PTSD (evidence for excessively high feedback inhibition of the HPA axis). Behaviorally, aberrant stress responses may be expressed as hyperresponsiveness (i.e. exagerrated startle responses in PTSD) or hyporesponsiveness (blunted emotional responses in depression) to external stimuli.
These scenarios describe different ways in which dysfunctions of the SRS may be expressed, but they do not describe how they come about. As will be apparent from further discussion below, there are likely many factors, genetic, developmental, and/or environmental, which can give rise to dysfunctions of the SRS. Extreme exposure to traumatic events may be sufficient to produce dysfunctions of the SRS in even the most resilient individuals with no underlying vulnerability to developing mental disorders, and this may be the result of normally-occurring changes in brain plasticity (i.e. sensitization and/or fear-conditioning processes). Factors related to the traumatic stressors, such as stressor type, intensity, duration, and repetition, may be important for determining the type of dysfunction of the SRS that occurs. Developmental factors likely play an important role as well, and in this regard, accumulating evidence is pointing toward the profound potential immediate and delayed adverse impact of traumatic experiences in childhood on the SRS and on the subsequent ability to cope with stress in adulthood. Furthermore, recent work is identifying potential genetic factors which might contribute in subtle ways to the development of a dysfunctional SRS, by rendering individuals more susceptible to developing long-term adverse stress responsiveness as a result of exposure to early life trauma.
Given their critical localization in the HPA axis and in multiple neural circuitries involved in both the generation and regulation of emotional behaviors and stress coping responses, CRF1 pathways have attracted considerable interest as a possible site of dysfunction in a number of different central nervous system (CNS)-based psychopathologies and as a target for novel pharmacological therapies.
1b. Classification of Mood & Anxiety Disorders
Hyperactivation of CRF1 receptors in dysfunctional SRS pathways has been linked to mood and anxiety disorders. As background for a discussion of the potential therapeutic utility of CRAs, it is helpful to understand the diversity of conditions that currently fall under the rubric of “mood”or “anxiety”disorders, as defined by DSM-IV.
Mood disorders are subclassified as depressive disorders (major depressive disorder (MDD) and dysthymia) and bipolar disorders (bipolar I and II). CRAs have primarily been targeted towards the treatment of MDD. It is important to note that specific depressive episodes may have additional features that can indicate a greater degree of severity, including the presence of severe despair (melancholia), schizophrenia-like psychotic episodes, or states of catatonia.
Anxiety disorders are subdivided into a number of different categories. Generalized anxiety disorder (GAD) is characterized by persistent, excessive worry occurring for at least 6 months. Specific phobias refer to anxiety provoked by specific feared situations or objects whereas social anxiety disorder (SAD, also called social phobia) is provoked by exposure to certain types of social or performance situations and is often accompanied by avoidance of those situations. Panic disorder is characterized by unexpected panic attacks (with or without agoraphobia) or anxiety about being in places or situations lacking escape. Posttraumatic stress disorder (PTSD) is characterized by re-experiencing of highly traumatic events, hyperarousal, and avoidance of stimuli associated with trauma. PTSD is distinguished from acute stress disorder which refers to PTSD symptoms that occur shortly after the trauma but which resolve within the first several months after exposure. Finally, obsessive compulsive disorder (OCD) is an anxiety disorder characterized by anxiety-provoking obsessions and anxiety-relieving compulsions.
It is acknowledged that the animal models that have been utilized for identifying potential anxiolytic and antidepressant agents lack the level of differentiation seen with the DSM-IV classifications, particularly in the case of anxiety disorders. This is clearly an area where improvements in animal models are needed, and will be revisited later in this manuscript. However, most animal models relevant to anxiety and depression are useful because they mimic key aspects of the disorders (e.g., heightened startle responses) rather than recreate all the diverse symptoms of the human conditions.
1c. Evidence for Hyperactivation of CRF1 Pathways in Depression and Anxiety
As reviewed in greater detail elsewhere (Kehne, 2007; Nemeroff, 2004a), chronic hyperactivation of CRF1 pathways has been implicated in human MDD. One line of evidence indicates that there is an elevated level of CRF1 receptor mediated drive of the pituitary limb of the HPA axis. Thus, chronically elevated plasma cortisol levels are reported at least in a subset (approximately half) of patients with MDD (Nemeroff, 1989, 1992). In tandem, there is a blunted suppression of HPA axis activity in response to dexamethasone, indicative of diminished feedback inhibition in some patients with MDD (Hatzinger, 2000; Nemeroff, 1989). Evidence for downregulated pituitary CRF1 receptors is provided by reports of blunted stimulation of ACTH release from the anterior pituitary following intravenous (IV) administration of CRF (Newport, et al., 2003).
Two primary sets of findings have been cited as evidence for hyperactivated brain CRF1 pathways in MDD. First, CRF levels in the cerebrospinal fluid (CSF) are reported to be elevated in at least some depressed patients (Nemeroff, 1992; Nemeroff, et al., 1984) and a correlation to a blunted ACTH response to IV-administered CRF is cited as evidence suggestive of a coordinated hyperactivation of HPA and non-HPA axis CRF pathways (Newport, et al., 2003). Second, biochemical studies have reported downregulated CRF1 receptor expression in multiple regions of postmortem brains of depressed suicide victims, including frontal cortex (Merali, et al., 2004; Merali, et al., 2006). Thus, sustained stimulation by chronically-elevated release of CRF could lead to desensitization of brain CRF1 receptors. Functionally, this could be expressed in multiple ways, for example, as a decreased responsiveness of primary SRS pathways to stress (hyporesponsiveness). Alternatively, desensitization could reduce a “restraining” or inhibitory effect of the frontal cortex on subcortical structures such as the amygdala and the periventricular nucleus of the hypothalamus, contributing to depressive symptomotology through a loss of regulation of the SRS.
Findings from postmortem studies of the brains of depressed suicide victims have also demonstrated elevated CRF immunoreactivity in specific brainstem monoaminergic nuclei, including NE cells of the locus coeruleus (Bissette, Klimek, Pan, Stockmeier, & Ordway, 2003; Merali, et al., 2006) and 5HT cells of the dorsal raphe (Austin, Janosky, & Murphy, 2003; Nemeroff, 2002). These pathways are targets for classic reuptake inhibitor antidepressants and interactions with CRF may be of functional relevance. In support of this conclusion, elevated NE activity produced by administration of the α2-adrenergic antagonist yohimbine (Vythilingam, et al., 2000) or decreased 5HT activity resulting from administration of a “5HT-depleting”diet (Tyrka, et al., 2004) is reported to result in an elevation of CRF levels in the CSF in humans.
An important observation is that hyperactivation of CRF1 pathways may be particularly evident in certain subpopulations of depressed patients (for reviews, (Kasckow, Baker, & Geracioti, 2001; Kehne, 2007). Thus, profound exaggerations of HPA axis activity and elevated levels of plasma cortisol are seen in depressed patients who also present with psychotic features (Belanoff, Kalehzan, Sund, Fleming Ficek, & Schatzberg, 2001; Schatzberg, 2003). High levels of CSF CRF and NE, and evidence for HPA axis hyperactivity, have been reported in severe melancholic depression (P. W. Gold & Chrousos, 1999, 2002; P. W. Gold, Gabry, Yasuda, & Chrousos, 2002). Finally, adult depressives with a history of exposure to early life trauma demonstrate evidence for excessive HPA axis dysfunction (for reviews, see (Heim, Plotsky, & Nemeroff, 2004; Nemeroff, 2004b). Further insights are provided by a recent genetic study which reported that certain CRF1 gene polymorphisms were associated with depression in adults who had a history of exposure to early life trauma (Bradley, et al., 2008). Taken together, these data suggest the possibility that CRAs might be particularly suitable for certain subpopulations of depressed patients in which CRF1 pathways show the greatest dysfunction and/or in which there may be a specific genetic signature and history of stressor exposure.
The evidence for hyperactivation of CRF1 pathways in anxiety disorders is more heterogeneous, showing differences across the various sub-disorders. In PTSD, evidence again includes the demonstration of increases in CSF levels of CRF (for reviews, see (Kasckow, et al., 2001; Kehne, 2007; Nemeroff, et al., 2006) and a dysfunctional HPA axis (e.g. low plasma cortisol but exaggerated stress-induced release; (Bremner, 2006). Evidence for elevated NE levels has also been reported in PTSD (for review, see (Kasckow, et al., 2001) and pharmacological activation with yohimbine was reported to evoke PTSD symptoms (Bremner, 2006; Southwick, Morgan, Charney, & High, 1999), HPA axis activation, and increases in CRF and NE in the CSF (Vythilingam, et al., 2000). As in melancholic depression, these data in PTSD are consistent with a hyperactivation of both NE and CRF pathways. Also analogous to the findings in depressed patients, the additional presence of psychosis in PTSD is associated with heightened CSF CRF levels (Sautter, et al., 2003), suggesting particularly severe CRF imbalances in this subpopulation. Thus, of the different anxiety disorders, the evidence for hyperactivation of CRF1 pathways is perhaps greatest for PTSD which may not be surprising, given that exposure to a traumatic stressor is the sine qua non of the disorder.
“Behavioral inhibition to the unfamiliar”, a heritable phenotype in children involving fearful or avoidant behavior in novel situations, has been identified as a risk factor for developing panic and phobic disorders (Smoller, et al., 2003). Genetic studies have demonstrated an association with the CRF gene (Smoller, et al., 2003; Smoller, et al., 2005) and imaging studies indicate abnormal activity in the SRS (Fox, Henderson, Marshall, Nichols, & Ghera, 2005; Schwartz, Wright, Shin, Kagan, & Rauch, 2003; Stein, 1998). Hyperactivation of CRF1 pathways have been implicated in panic disorder (for review, see (Strohle & Holsboer, 2003) though evidence is mixed that the HPA axis is dysfunctional in patients with panic disorder (e.g. (Kellner, et al., 2004). Recent genetic studies have shown an association between the presence of certain CRF1 receptor gene polymorphisms and panic disorder (Keck, et al., 2008). In GAD patients, however, CSF CRF levels were not elevated (Fossey, et al., 1996).
Interestingly, anxiety is comorbid in approximately 30% of patients with depression. In this population, heightened resistance to drug treatment (Bakish, 1999) and greater HPA axis activation in response to social stress (Young, Abelson, & Cameron, 2004) is reported, though no measurements of CSF levels of CRF have been reported.
1d. CRF1 Pathway Dysregulation and Other Stress-Related Disorders
A disorder that is frequently comorbid with anxiety (Lydiard, 2001, 2005) is irritable bowel syndrome (IBS), a stress-related gastrointestinal disorder characterized by disturbed bowel habits (diarrhea and/or constipation) and visceral abdominal pain (Lydiard, 2005). CRF is prominent in Barrington’s nucleus in the pons, which regulates bowel motility and can impact on other pelvic visceral functions. CRAs have been proposed as a novel pharmacological treatment for IBS, through blockade of both central and peripheral CRF1 receptors (for reviews, see (Martinez & Tache, 2006; Tache, 2004; Tache, Martinez, Wang, & Million, 2004; Tache, Million, Nelson, Lamy, & Wang, 2005). In IBS patients, functional imaging studies demonstrated heightened responsiveness of the brain’s “emotional motor system” to painful peripheral gut stimulation (Mayer, et al., 2005) and IV infusion of a non-selective peptidic CRF1/2 receptor antagonist, α-helical-CRF, produced improvements in gut stimulation-induced changes in gastrointestinal motility, visceral pain perception, and negative mood (Sagami, et al., 2004).
Work in recent years has linked hyperactivation of CRF1 receptors with drug addiction disorders and CRAs have been proposed as potential treatments (for reviews, see (Koob, 2008a, 2008b). A major risk factor for relapse to drug abuse is the occurrence of withdrawal symptoms, including anxiety. In animals, anxiety-like symptoms seen during withdrawal from drugs of abuse, such as cocaine, amphetamine, and morphine have been associated with heightened CRF release (Sarnyai, 1998; Sarnyai, et al., 1995; Sarnyai, Shaham, & Heinrichs, 2001), providing a rationale for the use of agents which block CRF1 pathways to treat addiction. Dependence on alcohol has also been linked to hyperactivation of CRF1 receptors (for review, see (Heilig & Koob, 2007). In alcohol-dependent adults, an association between the CRF1 receptor gene and excessive drinking has been reported (Treutlein, et al., 2006). Enhanced sensitivity to stress-induced drinking, heightened anxiety, and CRF1 receptor upregulation in the basolateral and medial amygdala have been reported in alcohol-dependent rats during chronic withdrawal (Sommer, Rimondini, Hansson, & Heileg, 2006). Rats genetically bred for high alcohol preference also show a dysfunctional upregulation of CRF1 receptors (Hansson, et al., 2006). Together, these results provide a rationale for further investigating CRAs for their potential utility in treating chronic drug and alcohol abuse.
1e. CRF1 Receptor Antagonists
As summarized in Table 1, numerous non-peptidic CRAs have been discovered, some of which have reached the stage of clinical evaluation. Many of these agents have been employed in animal studies as pharmacological tools or probes for evaluating the functional roles of CRF1 receptors. In the following section, two key assumptions are made in evaluating such studies: first, at the dose range used, the agent of interest achieves sufficient occupation of the relevant CRF1 receptor population to adequately test for a functional effect; and second, that it demonstrates selectivity as a CRF1 antagonist. It is beyond the scope of the present review to address in detail the data supporting these assumptions, however, a few points are worth emphasizing. First, the majority of the compounds described have been exhaustively evaluated in numerous in vitro assays for potency, efficacy, and selectivity as CRAs. That being said, one must always be cognizant of alternative mechanistic explanations, particularly when testing a compound in vivo. Second, many of the compounds described have been evaluated in ex vivo binding experiments which have demonstrated occupation of central CRF1 receptors following various modes of systemic administration (Chaki, et al., 2004; Gehlert, et al., 2007; Gehlert, et al., 2005; Gutman, Owens, Skelton, Thrivikraman, & Nemeroff, 2003; Heinrichs, et al., 2002; Keck, et al., 2001; Lelas, et al., 2004; Li, et al., 2003). Notably, several of the published receptor occupancy studies have been used in conjunction with efficacy determinations in behavioral assays to provide an estimate of the degree of receptor occupation needed to achieve efficacy. The results of these studies have generally been consistent in indicating that fairly high (50–85%) occupation of CRF1 receptors is needed to achieve efficacy. These data are important to bear in mind when ultimately evaluating clinical results obtained with CRAs.
2. CRAs in Animal Models
This section reviews preclinical studies characterizing the effects of CRAs in diverse animal models of stress, many of which are sensitive to anxiolytic and/or antidepressant agents, with the goal of evaluating their potential therapeutic utility in treating depression, anxiety, and other stress-related disorders. Although the primary focus is on anxiety and depression, additional studies exploring potential therapeutic utility in IBS and drug addictions, disorders in which anxiety symptoms may play a major role, are also reviewed as exciting future directions of this area.
Table 2 summarizes the effects of non-peptidic CRAs in a range of animal models. Several points should be noted regarding the organization of this table: First, peptidic CRF receptor antagonists are not covered, as these agents, among other shortcomings, do not readily penetrate into the brain. An assumption of the present review is that action on brain CRF1 receptors is a key requisite for a CRA that will be used to treat CNS-mediated psychopathology. Second, the table lists on separate rows assays which use the same dependent measure (i.e. performance on the elevated plus maze) but which have used some relevant procedural variation (i.e. presence or absence of prior stress). The reason for this is that, given the role of CRF in mediating the effects of stressors, standard models have often been modified to provide higher levels of stress and therefore greater sensitivity for detecting potential efficacy of CRAs. This tabular organization is intended to provide the reader with an appreciation of how efficacy in certain models may depend on differing levels of stress. Two primary types of information which are very relevant to evaluating CRAs are specified in the table, i.e. any specific environmental conditions or specific breeding/strain considerations which may alter the stress level of the animal being tested. Third, for the sake of simplicity, certain details (route of administration, pretreatment times used, etc.) are not included. As noted above, these agents all tend to be CNS-penetrating by multiple routes and generally CRF1 receptor selective, and an assumption is made that dosing conditions cover a range of levels of receptor occupancy. Specific commentaries will be made regarding these issues as appropriate. Finally, separate columns list ‘ACTIVE’ (i.e. demonstrating efficacy) and ‘INACTIVE’ CRAs, where this information is available. From an overall inspection of Table 2, it is apparent that CRAs have been extensively tested in some paradigms, and less so in others. This is important as more comprehensive evaluation is desirable to determine the generality and reproducibility of any individual finding, particularly when discrepant findings are seen. For the most part, discussion will focus on those paradigms in which multiple CRAs have been tested.
Table 2.
Summary of effects of CRF1 receptor antagonists (CRAs) in various animal models. This table describes compounds that are efficacious (ACTIVE) or which lack efficacy (INACTIVE) in numerous animal models. Column 1 summarizes the therapeutic application or other attribute (side effect potential, physiological system, etc.) associated with activity in the model. In Column 2, models are listed by behavioral endpoint or common name, and, if applicable, any special test conditions or parameters, and/or species/strain used. Species is summarized in Column 3. As is evident from this table, the same behavioral endpoint can be incorporated into different paradigms which have different therapeutic applications or interpretations.
| Therapeutic Application/System |
Assay (endpoint, special test conditions, strains, etc.) |
Species | Active CRAs | Inactive CRAs |
|---|---|---|---|---|
| Anxiety | Burying, defensive | Rat | R121919 (Heinrichs, et al., 2002); MPZP (Richardson, et al., 2008); MJL-1-109-2 (Zhao, et al., 2007) | |
| Anxiety | Burying, marble | Mouse | CP-154,526 (Hodgson, et al., 2007) | |
| Anxiety | Defensive Withdrawal | Rat | R121919 (Gutman, et al., 2003; Heinrichs, et al., 2002); DMP696 (Lelas, et al., 2004; McElroy, et al., 2002); DMP 696 (chronic) (Lelas, et al., 2004); DMP 904 (Lelas, et al., 2004); DMP 904 (chronic) (Lelas, et al., 2004); antalarmin (Zorrilla, et al., 2002); CP-154,526 (chronic) (Arborelius, et al., 2000) | |
| Anxiety | Elevated Plus Maze | Mouse | CP-154,526 (Hodgson, et al., 2007) | |
| Anxiety | Elevated Plus Maze | Rat | DMP 904 (7); CP-154,526 (chronic) (Mallo, et al., 2004) | R121919 (Heinrichs, et al., 2002); CRA0450 (Chaki, et al., 2004); antalarmin (Zorrilla, et al., 2002); CP 154,526 (Millan, et al., 2001); DMP695 (Millan, et al., 2001); MTIP (Gehlert, et al., 2007) |
| Anxiety | Elevated Plus Maze (exogenous CRF icv) | Mice | CRA1000 (Okuyama, et al., 1999); CRA1001 (Okuyama, et al., 1999); CP-154,526 (Okuyama, et al., 1999) | |
| Anxiety | Elevated Plus Maze (with prior maternal separation stress) | Rat | DMP696 (Maciag, et al., 2002) | |
| Anxiety | Elevated Plus Maze (with prior stress) | Rat | R121919 (Heinrichs, et al., 2002); CRA0450 (Chaki, et al., 2004) | |
| Anxiety | Elevated Plus Maze | Rat | CP-154,526 (chronic) (Mallo, et al., 2004) | |
| Anxiety | Elevated Plus Maze (“Low Anxiety Behavior, LAB, strain) | Rat | R121919 (Keck, et al., 2001) | |
| Anxiety | Elevated Plus Maze (“High Anxiety Behavior, HAB, strain) | Rat | R121919 (Keck, et al., 2001) | |
| Anxiety | Elevated Plus Maze, (“Swim High-Active” inbred strain) | Rat | R121919 (Gutman, et al., 2008) | |
| Anxiety | Food Intake (footshock stressor-suppressed) | Rat | CRA1000 (Sekino, Ohata, Mano-Otagiri, Arai, & Shibasaki, 2004) | |
| Anxiety | Food Intake (novel environment-suppressed; unpredictable mild chronic stress model) | Mouse | SSR125543 (Surget, et al., 2008) | |
| Anxiety | Freezing, conditioned | Mouse | CP-154,526 (Blank, et al., 2003) | |
| Anxiety | Freezing, conditioned (acquisition) | Rat | CP-154,526 (Hikichi, et al., 2000); CP-154,526 (Rau & Fanselow, 2007) | DMP696 (Hubbard, et al., 2007); |
| Anxiety | Freezing, conditioned (consolidation) | Rat | CP-154,526 (Rau & Fanselow, 2007) | |
| Anxiety | Freezing, conditioned (retention) | Rat | CP-154,526 (Hikichi, et al., 2000); DMP904 (Ho, et al., 2001) | |
| Anxiety | Freezing, conditioned (acquisition, retention) | Rat | Antalarmin (Deak, et al., 1999) | |
| Anxiety | Freezing, conditioned (acquisition, retention), prior stress-enhanced | Rat | Antalarmin (Deak, et al., 1999) | |
| Anxiety | Freezing, conditioned (prior stress-induced deficit) | Mouse | CP-154,526 (Blank, et al., 2003) | |
| Anxiety | Lick Suppression (Conditioned) | Mouse | CP-154,526 (Hodgson, et al., 2007) | |
| Anxiety | Lick Suppression (Unconditioned) | Rat | CP-154,526 (Millan, et al., 2001); DMP695 (Millan, et al., 2001) | CRA450 (Chaki, et al., 2004) |
| Anxiety | Light-Dark Box | Mouse | CRA1000 (Okuyama, et al., 1999); CRA1001 (Okuyama, et al., 1999); CP-154,526 (Okuyama, et al., 1999) | |
| Anxiety | Light-Dark Box (with prior swim stress) | Mouse | CRA1000 (Okuyama, et al., 1999); CRA1001 (Okuyama, et al., 1999); CP-154,526 (Okuyama, et al., 1999) | |
| Anxiety | Locomotion (reduction induced by diurnal phase of dark cycle) | Rat | CRA1000 (Ohata, Arai, & Shibasaki, 2002) | |
| Anxiety | Social Interaction | Rat | CP 154,526 (Millan, et al., 2001); DMP 695 (Millan, et al., 2001) | NBI3b1996 (Gehlert, et al., 2005) |
| Anxiety | Social Interaction, Flinders Sensitive Line | Rat | CP-154,526 (chronic, 14 d) (Overstreet, Keeney, et al., 2004) SSR 125543 (chronic, 14 d) (Overstreet & Griebel, 2004) | |
| Anxiety | Social Interaction (with prior stress) | Rat | NBI3b1996(Gehlert, et al., 2005) | |
| Anxiety | Social Interaction (anxiety induced with multiple infusions of urocortin) | Rat | NBI3b1996 (Gehlert, et al., 2005) | |
| Anxiety | Splash Test (with prior stress -- unpredictable mild chronic stress model) | Mouse | SSR125543 (Surget, et al., 2008) | |
| Anxiety | Startle, fear conditioning-enhanced (contextual cue) | Mouse | R121919 (Risbrough, et al., 2008) | |
| Anxiety | Startle, fear conditioning-enhanced (contextual cue) | Rat | ||
| Anxiety | Startle, fear conditioning-enhanced (discrete cue) | Mouse | R121919 (Risbrough, et al., 2008) | |
| Anxiety | Startle, fear conditioning-enhanced, (discrete cue) | Rat | CP-154,526 (Schulz, et al., 1996); | GSK876008(D. Walker, et al., 2008) |
| Anxiety | Startle, footshock stressor-enhanced | Rat | GSK876008(D. Walker, et al., 2008) | |
| Anxiety | Startle, light stressor-enhanced | Rat | GSK876008(D. Walker, et al., 2008) | |
| Anxiety | Startle, predator stressor-enhanced (initiation or acquisition) | Mouse | CRA0450(Adamec, et al., 2010) | |
| Anxiety | Vocalization, audible – maternal separation induced in pup | Guinea pig | CP-154,526 (Hodgson, et al., 2007) | |
| Anxiety | Vocalization, ultrasonic – maternal separation stressor induced | Rat | CP-154,526 (Hodgson, et al., 2007; Iijima & Chaki, 2005; Kehne, et al., 2000); NBI27914 (Ise, et al., 2008) | |
| Anxiety | Vocalization, ultrasonic – systemic CRF induced in rat pup (37° C) | Rat | NBI27914 (Ise, et al., 2008) | |
| Anxiety | Vocalizations, ultrasonic (conditioned footshock stressor-elicited) | Rat | CP 154,526 (Millan, et al., 2001); DMP 695 (Millan, et al., 2001) | |
| Depression | Chronic Mild Stress Induced Debilitation (weight gain,, physical state)(BALB/c) | Mouse | Antalarmin (Ducottet, et al., 2003) | |
| Depression | Differential Rate of Low Reinforcement 72 sec (DRL72) | Rat | SSR125543 (Louis, et al., 2006) | CRA0450 (Chaki, et al., 2004) |
| Depression | Forced Swim Test | Mouse | CP-154,526 (Hodgson, et al., 2007) | |
| Depression | Forced Swim Test | Rat | CP-154,526 (Hodgson, et al., 2007); LWH234 (Jutkiewicz, et al., 2005) | Antalarmin (Jutkiewicz, et al., 2005); CRA0450 (Chaki, et al., 2004); CP-154,526 (Jutkiewicz, et al., 2005); R121919 (Jutkiewicz, et al., 2005) |
| Depression | Forced Swim Test, “Swim High-Active” Strain | Rat | R121919 (Gutman, et al., 2008) | |
| Depression | Forced Swim Test, Flinders Sensitive Line | Rat | CP-154,526 (chronic, 14 d) (Overstreet, Keeney, et al., 2004) SSR 125543 (chronic, 14 d) (Overstreet & Griebel, 2004) | |
| Depression | Olfactory Bulbectomy | Rat | CRA0450 (Chaki, et al., 2004); CRA1000 (Okuyama, et al., 1999); CRA1001 (Okuyama, et al., 1999) | |
| Depression | Olfactory Bulbectomy | Rat | CRA0450 (chronic, 10 d) (Chaki, et al., 2004); CRA1000 (chronic, 7d)(Okuyama, et al., 1999); CRA1001 (chronic, 7d) (Okuyama, et al., 1999) | |
| Depression | Operant avoidance, inescapable shock (expression) | Rat | CP-154,526 (Mansbach, et al., 1997) | |
| Depression | Shuttle box avoidance, inescapable shock (acquisition) | Rat | CRA0450 (Chaki, et al., 2004) | |
| Depression | Shuttle box avoidance, inescapable shock (acquisition, expression) | Rat | Antalarmin (Deak, et al., 1999) | |
| Depression | Shuttle box avoidance, inescapable shock (consolidation) | Rat | CRA1000 (Takamori, et al., 2001); CP-154,526 (Takamori, et al., 2001) | |
| Depression | Shuttle box avoidance, inescapable shock (expression) | Rat | CRA1000 (Takamori, et al., 2001); CP-154,526 (Takamori, et al., 2001) | |
| Depression | Shuttle box avoidance, inescapable shock avoidance (acquisition) | Rat | CRA0450 (chronic, 8d)(Chaki, et al., 2004); CRA1000 (chronic, 8d) (Takamori, et al., 2001); CP-154,526 (chronic, 8d) (Takamori, et al., 2001) | |
| Depression | Tail suspension | Mouse | CRA0450 (Chaki, et al., 2004); CP-154,526 (Hodgson, et al., 2007); | |
| Depression | Tail suspension | Mouse | R121919 (subchronic) (Nielsen, Carey, & Gold, 2004); DMP 696 (subchronic) (Nielsen, et al., 2004) | CRA0450 (Chaki, et al., 2004); CP-154,526 (Hodgson, et al., 2007); |
| Drug Abuse (Alcohol) | Alcohol Intake (non-dependent) | Rat | MPZP (Richardson, et al., 2008); MTIP (Gehlert, et al., 2007) | |
| Drug Abuse (Alcohol) | Alcohol Intake (dependent/withdrawal) | Rat | MPZP (Richardson, et al., 2008); MTIP (Gehlert, et al., 2007) | |
| Drug Abuse (Alcohol) | Alcohol Intake (withdrawal-induced/dependent), mSP alcohol preferring strain | Rat | MTIP (Gehlert, et al., 2007) | |
| Drug Abuse (Alcohol) | Alcohol Intake (reinstatement of stress induced alcohol seeking), post-dependent | Rat | MTIP (Gehlert, et al., 2007) | |
| Drug Abuse (Alcohol) | Alcohol Intake (reinstatement of stress induced alcohol seeking), mSP alcohol preferring strain | Rat | MTIP (Gehlert, et al., 2007) | |
| Drug Abuse (Alcohol) | Elevate Plus Maze (withdrawal) | Rat | MTIP (Gehlert, et al., 2007) | |
| Drug Abuse (Alcohol) | Lick Suppression (Unconditioned; chronic withdrawal) | Rat | MTIP (Sommer, et al., 2007) | |
| Drug Abuse (Alcohol) | Social Interaction (Deficits Induced by Single or Repeated Withdrawals from Alcohol) | Rat | CP-154,526 (Overstreet, Keeney, et al., 2004); CRA1000 (Knapp, Overstreet, Moy, & Breese, 2004; Overstreet, Knapp, & Breese, 2004) | |
| Drug Abuse (Cocaine) | Cocaine Seeking (Reinstatement by a Conditioned Reinforcer) | Rat | CP-154,526 (Goeders & Clampitt, 2002) | |
| Drug Abuse (Cocaine) | Locomotion (Cocaine-Stimulated) | Rat | CP-154,526 (Lu, Liu, Huang, & Zhang, 2003) | |
| Drug Abuse (Cocaine) | Place Preference (Cocaine-Induced) | Rat | CP-154,526 (Lu, et al., 2003) | |
| Exogenous CRF (functional) | Freezing (icv CRF-induced) | Mouse | NBI 27914 (Pelleymounter, et al., 2000) | |
| Exogenous CRF (functional) | Grooming (icv CRF-induced) | Rat | Antalarmin (Howard, et al., 2008) | |
| Exogenous CRF (functional) | Burying (icv CRF-stimulated) | Rat | Antalarmin (Howard, et al., 2008) | |
| Exogenous CRF (functional) | Elevated Plus Maze (icv CRF-induced reduction) | Rat | Antalarmin (Zorrilla, et al., 2002) | |
| Exogenous CRF (functional) | Food Intake (icv CRF-suppressed) | Mouse | NBI 27914 (Pelleymounter, et al., 2000) | |
| Exogenous CRF (functional) | Forepaw Treading (icv CRF-induced) | Gerbil | CRA0450 (Chaki, et al., 2004) | |
| Exogenous CRF (functional) | Locomotion (icv CRF-reduced) | Rat | DMP696 (Campbell, et al., 2004) | NBI 27914 (Pelleymounter, et al., 2000) |
| Exogenous CRF (functional) | Locomotion (icv CRF-stimulated) | Rat | R121919 (Heinrichs, et al., 2002); Antalarmin (Zorrilla, et al., 2002) | |
| Exogenous CRF (functional) | Startle, CRF-enhanced (icv), C57Bl/6J strain | Mouse | R121919 (Risbrough, Hauger, Pelleymounter, & Geyer, 2003) | |
| Exogenous CRF (functional) | Startle, CRF-enhanced (icv) | Rat | CP-154,526 (Schulz, et al., 1996); GSK876008 (D. Walker, et al., 2008) | |
| HPA Axis | ACTH, plasma (basal) | Rat | R121919 (Heinrichs, et al., 2002) | |
| HPA Axis | ACTH, plasma (icv CRF-stimulated) | Rat | CP-154,526 (Schulz, et al., 1996) | |
| HPA Axis | ACTH, plasma (iv CRF-stimulated) | Rat | CRA1000 (Okuyama, et al., 1999); CRA1001 (Okuyama, et al., 1999); CP-154,526 (Okuyama, et al., 1999) | |
| HPA Axis | ACTH, plasma (basal) | Mouse | ||
| HPA Axis | ACTH, plasma (stressor-stimulated) | Rat | R121919 (Gutman, et al., 2003; Heinrichs, et al., 2002) | CRA1000 (Okuyama, et al., 1999); CRA1001 (Okuyama, et al., 1999); CP-154,526 (Okuyama, et al., 1999) |
| HPA Axis | ACTH, plasma (iv CRF-stimulated), rhesus | Monkey | Antalarmin (Broadbear, Winger, Rivier, Rice, & Woods, 2004) | |
| HPA Axis | ACTH, plasma (stressor-stimulated; high anxiety behavior, HAB, strain) | Rat | R121919 (Keck, et al., 2001) | |
| HPA Axis | Corticosterone, plasma (acute stress-induced) | Rat | DMP 904 (Lelas, et al., 2004); CP-154,526 (chronic) (Arborelius, et al., 2000) | CRA1000 (Okuyama, et al., 1999); CRA1001 (Okuyama, et al., 1999); CP-154,526 (Okuyama, et al., 1999) |
| HPA Axis | Corticosterone, plasma (basal) | |||
| HPA Axis | Corticosterone, plasma (icv CRF stimulated) | Rat | DMP696 (Campbell, et al., 2004) | |
| HPA Axis | Corticosterone, plasma (iv CRF-stimulated) | Rat | CRA1000 (Okuyama, et al., 1999); CRA1001 (Okuyama, et al., 1999); CP-154,526 (Okuyama, et al., 1999) | |
| HPA Axis | Corticosterone, plasma (stressor-stimulated); high-anxiety behavior, HAB, strain | Rat | R121919 in high anxiety rats (Keck, et al., 2001) | |
| Physiology | Cardiovascular Parameters (stressor-stimulated) | Rabbit | CP-154,526 (Nalivaiko & Blessing, 2003, 2004) | |
| Physiology | CRF mRNA (decreased; periventricular nucleus) | Rat | CP-154,526 (chronic) (Arborelius, et al., 2000) | |
| Physiology | CRF mRNA decrease; Barrington’s nucleus) | Rat | CP-154,526 (chronic) (Arborelius, et al., 2000) | |
| Physiology | CRF1 receptor mRNA (basolateral amygdala) | Rat | CP-154,526 (chronic) (Arborelius, et al., 2000) | |
| Physiology | CRF1 receptor mRNA (cerebellum) | Rat | CP-154,526 (chronic) (Arborelius, et al., 2000) | |
| Physiology | CRF1 receptor mRNA (parietal cortex) | Rat | CP-154,526 (chronic) (Arborelius, et al., 2000) | |
| Physiology | CRF1 receptor occupancy | Rat | R121919 (Heinrichs, et al., 2002); DMP696 (Li, et al., 2003); (Lelas, et al., 2004); | |
| Physiology | Dopamine Release (prefrontal cortex) | Rat | CP-154,526 (Millan, et al., 2001); DMP 695 (Millan, et al., 2001) | |
| Physiology | Neurogenesis (chronic mild stressor-reduced; hippocampus dentate gyrus) | Mouse | SSR125543 (Alonso, et al., 2004) | |
| Physiology | Norepinephrine firing, locus coeruleus (icv CRF induced) | Rat | CRA1000 (Okuyama, et al., 1999); CRA1001 (Okuyama, et al., 1999); CP-154,526 (Okuyama, et al., 1999; Schulz, et al., 1996) | |
| Physiology | Norepinephrine release (frontal cortex) | Rat | CP-154,526 (Millan, et al., 2001); DMP 695 (Millan, et al., 2001) | |
| Physiology | Serotonin release (frontal cortex) | Rat | CP-154,526 (Millan, et al., 2001); DMP 695 (Millan, et al., 2001) | |
| Physiology | cFOS (icv CRF stimulated; central n. amygdala) | Rat | DMP 696 (Campbell, et al., 2004) | |
| Physiology | cFOS (icv CRF stimulated; periventricular nucleus of hypothalamus) | Rat | DMP 696 (Campbell, et al., 2004) | |
| Schizophrenia | Locomotion (amphetamine-stimulated) | Rat | Antalarmin (Zorrilla, et al., 2002) | |
| Side Effects | Hexobarbital anesthesia potentiation | Mouse | CRA0450 (Chaki, et al., 2004) | |
| Side Effects | Hexobarbital anesthesia potentiation | Rat | CRA0450 (Chaki, et al., 2004) | |
| Side Effects | Inclined Plane, 10 day old pup | Rat | CP-154,526 (Kehne, et al., 2000) | |
| Side Effects | Locomotion | Mouse | DMP 904 (Lelas, et al., 2004); CRA0450 (Chaki, et al., 2004) | |
| Side Effects | Locomotion (spontaneous; habituated or non-habituated) | Rat | CRA0450 (Chaki, et al., 2004); antalarmin (Zorrilla, et al., 2002) | |
| Side Effects | Locomotion (unpredictable chronic mild stress) | Mouse | SSR125543 (Surget, et al., 2008) | |
| Side Effects | Locomotion (“High Anxiety Behavior”, HAB, strain) | Rat | R121919 (Keck, et al., 2001) | |
| Side Effects | Negative Geotaxis, pup | Rat | CP-154,526 (Hodgson, et al., 2007) | |
| Side Effects | Passive avoidance | Rat | CRA1000 (Okuyama, et al., 1999); CRA1001 (Okuyama, et al., 1999)CP-154,526 (Okuyama, et al., 1999) | |
| Side Effects | Rotarod (ataxia) | Mouse | CRA0450 (Chaki, et al., 2004) | |
| Side Effects | Rotarod (ataxia) | Rat | DMP 904(Lelas, et al., 2004) | |
| Side Effects | Staircase Test | Mouse | CRA0450 (Chaki, et al., 2004) | |
| Side Effects | Startle | Mouse | R121919 (Risbrough et al., 2003) | |
| Side Effects | Startle | Rat | GSK876008 (D. Walker et al., 2008) | |
| Side Effects (abuse potential) | Drug Discriminative Cue | Rat | DMP 696 (Lelas, Zeller, Ward, & McElroy, 2003) | |
| Side Effects (abuse potential) | Drug Substitution, chlordiazepoxide | Rat | DMP 696 (Lelas, et al., 2003); DMP 904 (Lelas, et al., 2004) |
2a. Anxiolytic Activity
Prior to discussing experimental data, it is important to address issues of definition. The terms stress, fear and anxiety are used interchangeably but are also used to denote different reactions to aversive stimulation (R. J. Blanchard, Yudko, Rodgers, & Blanchard, 1993; Charney & Deutch, 1996; Davis, Walker, & Lee, 1997b; de Jongh, Groenink, van der Gugten, & Olivier, 2003; Todorovic, et al., 2007). ‘Stress’ is perhaps the most broadly defined category and can refer to any bodily response to threatening demand (Selye, 1975) including endocrine responses and fearful/anxious behavioral responses (Weninger, et al., 1999). ‘Fear’ is usually used to denote defensive responding to clearly defined environmental cues, either innately aversive or learned. The fear state functions to cope with immediate threats, and is more intense than anxiety but also shorter-lived. For example, rodents will freeze when presented with cue that was previously paired with a pain-eliciting stimulus, or when presented with a natural predator, but freezing subsides quickly when the threatening stimulus is removed (R. J. Blanchard, Mast, & Blanchard, 1975; Cain, Blouin, & Barad, 2003). ‘Anxiety’ is often used to describe low level defensive responding elicited by more diffuse cues. The anxiety state can be much longer lasting than fear and functions to cope with more distant threats (Barlow, Chorpita, & Turovsky, 1996). Although the terms ‘stress’, ‘fear’ and ‘anxiety’ refer to different aspects of defensive responding, sometimes mediated by different brain regions (Davis, Walker, & Lee, 1997a), they are not always clearly dissociable in natural situations or laboratory experiments. Furthermore, therapeutic agents are routinely categorized as “anxiolytic” or “antianxiety”agents with no category for “antifear”drugs. However, as discussed above, anxiety disorders encompass a range of emotional states and it is not unreasonable to think that new agents might be more suitable as “antifear”agents than “antianxiety”drugs.
In the context of the present review, placing anxiety and fear at different ends of a spectrum of emotionality is deemed useful, and furthermore the term “high stress”will be used to denote a situation which is more “fear-evoking”relative to a “low stress”situation which is more “anxiety-provoking”. These distinctions will be clarified in the context of the following behavioral characterization of CRAs.
Although many different individual models have been used to evaluate compounds for anxiolytic potential, they can be broadly subdivided into two general classes, those measuring drug effects on spontaneous (or “unconditioned”) behaviors that have no explicit learning component, and those which measure effects on learned behaviors.
2a (i) Unconditioned Fear Models
The majority of CRA studies have focused on unconditioned fear models that do not explicitly involve emotional learning. Unconditioned fear has been defined as defensive responding resulting from the test situation itself, rather than cues associated with prior aversive experience (Takahashi, 2001). Such tests include elevated plus maze, defensive withdrawal, light-dark exploration, and social interaction tests.
Unconditioned fear models utilize ethologically-relevant behavioral endpoints which are thought to be sensitive indices of an animal’s natural fear. Given that rodents are small prey animals that are nocturnally active, these tests capitalize on a rodent’s natural tendency to avoid entry into brightly lit, open spaces and rather demonstrate a preference for enclosed, dark places. Classic benzodiazepines such as diazepam or chlordiazepoxide which potentiate transmission at GABAA receptors and are anxiolytic in humans, are generally active in these models, overcoming the animal’s natural anxiety/fear to increase exploration in the open and light. Notably, benzodiazepines have marked sedative, muscle relaxant, and ataxia-producing properties, but their anxiolytic actions in these (and other) animal models can often be demonstrated at doses below those that produce impairments as measured by generalized reductions, for example, in activity. One of the key incentives for identifying new anxiolytic agents is developing agents which have a wider therapeutic margin than benzodiazepines, in addition to lacking other undesirable properties, such as the abuse potential associated with this class of agents.
2a (i)(a) Elevated Plus Maze and Defensive Withdrawal
As seen in Table 1, CRAs have been extensively tested in models of unconditioned fear run under standardized testing conditions, though, at first inspection, it would appear that the pattern of outcomes has not been consistent across the different models used. For example, in the defensive withdrawal model, published reports indicate that CRAs are generally active, decreasing the latency before a rodent emerges from a dark, small, enclosed space into a brightly lit arena. In marked contrast, with a few exceptions, CRAs have generally been reported to lack anxiolytic-like activity in the elevated plus maze in mice or rats when the assay is run under a standardized set of test conditions. One exception is a study with CP-154,526, which was reported to be active following chronic administration (Mallo, et al., 2004). This is a single report which would benefit from replication and/or evaluation with other agents for confirmation.
It is not immediately clear why these two test paradigms would yield such contrasting outcomes for acutely-administered CRAs. One possibility is that the two test situations produce different levels of stress in the test animals. If the defensive withdrawal test conditions are inherently more stressful, then endogenous CRF1 pathways might be activated to a greater extent than during exposure to the elevated plus maze. Under these differential conditions, a CRA which, by definition, requires activated CRF1 pathways to exert efficacy, would be differentially effective in the more stressful defensive withdrawal test. It is not intuitively obvious that this would be the case, as it has been demonstrated that exposure to either test situation can produce elevations in plasma levels of ACTH and/or corticosterone, suggesting that both test situations are inherently stressful. However, it is not known whether specific brain CRF1 pathways (e.g., those involving limbic system structures such as the BNST or central nucleus of the amygdala) that likely mediate anxiolytic actions are differentially activated by these two test situations. Experimental approaches such as using microdialysis to measure CRF release would clearly be helpful in evaluating this hypothesis.
Other experiments in which specific manipulations were used to increase the animal’s level of stress indicate that testing in the elevated plus maze under standard conditions may not be sufficiently stressful to detect activity of CRAs. There are three independent lines of evidence in support of this conclusion. First, intracerbroventricular (ICV) administration of CRF results in an increased level of anxiety-like behavior as measured on the elevated plus maze. Rats spend less time exploring the open arms after ICV CRF, and this “anxiogenic” effect is reduced by the CRA antalarmin (Zorrilla, Valdez, Nozulak, Koob, & Markou, 2002). (Note that hereafter any compound cited in the text is a CRA, unless otherwise indicated). Second, exposure of rats to a swim stressor prior to testing also results in an anxiogenic-like effect on the elevated plus maze that is reversed by R121919 (Heinrichs, et al., 2002) or CRA0450 (Chaki, et al., 2004). Third, CRAs are active in the standard elevated plus maze when tested in rodent strains specifically bred for high endogenous stress levels. Thus, R121919 is anxiolytic when tested on the elevated plus maze in rats from a “High Anxiety Behavior”strain (selectively bred high levels of anxiety-like traits), but is inactive in the corresponding “low Anxiety Behavior”strain (Keck, et al., 2001). Again, it would be of interest to explore the dynamics of the CRF1 pathways of these two strains to determine if the CRA-sensitive strain demonstrated hyperactivation of CRF1 pathways relative to the insensitive strain.
2a (i)(b) Light Dark Test
Notably, in the light/dark test, a closely related paradigm, a pattern of results reminiscent of the elevated plus maze is seen. That is, CRAs tend to be inactive under standard, apparently “low stress” conditions, whereas anxiolytic activity is demonstrated in animals that have been pre-exposed to prior stressors (Okuyama et al, 1999). A subtle procedural variation may have relevance to the activity of CRAs in defensive withdrawal but not the Elevated Plus Maze or Light/Dark tests, even though they all seemingly measure a similar phenomenon: the natural aversion for open, brightly lit spaces in an unfamiliar environment. In the latter two, subjects begin trials in the presumably ‘more dangerous’ location and then distribute their time. In defensive withdrawal, subjects begin in the ‘safer’ location and then must emerge into a bright open space where they have no prior knowledge of safety. Thus, defensive withdrawal could be inherently more stressful because animals are less likely to enter bright open space with no history of safety in that space.
In summary, the majority of the data cited thus far are consistent with the conclusion that CRAs require sufficiently activated CRF1 pathways to demonstrate efficacy in unconditioned fear models.
2a (i)(c) Social Interaction
The social interaction test is a fourth unconditioned fear test sensitive to anxiolytics which measures the tendency of one animal to investigate and interact with another novel animal when the two are placed in close proximity in a test chamber. As with the previous tests, animals placed in this test paradigm show some inhibition of behavior as measured in this case by limited social interaction, presumably because of a naturalistic tendency to be wary or fearful of a novel interaction. There is some data published with CRAs demonstrating anxiolytic activity in the social interaction test in rats with the CP-154,526 and DMP695 (Millan, et al., 2001). In other studies, chronically administered CP-154,526 and SSR 125543 were anxiolytic in rats from the “Flinders Sensitive Line”(FSL) which have been selectively bred for the presence of depressive/anxious like traits (Overstreet, Keeney, & Hogg, 2004). Interestingly, in this model, CP-154,526 and SSR 125543 were not active in rats of the “Flinders Resistant Line”which were selectively bred for a low level of depressive-like traits, an outcome reminiscent of that seen previously with the “High” and “Low- Anxiety Behavior”rats. It would again be informative to understand the possible neurobiological differences between FSL and FRL rats with regard to measures of activity of CRF1 pathways in the brain and HPA axis and how they correlate with behavioral outcomes. A pattern of hyperactivation of CRF1 pathways in the FSL rat would be consistent with the reported efficacy of CRAs in this model.
The social interaction test has been utilized in an interesting paradigm which has been proposed as a rodent model of panic disorder. In this paradigm, direct injection of CRF or the peptide agonist urocortin into the basolateral nucleus of the amygdala produces an anxiogenic response relative to vehicle injection as measured with the social interaction test (Gehlert, et al., 2005). This anxiogenic effect was blocked by NBI3b1996. Two additional findings are of interest: First, repeated daily urocortin administration produced an apparent sensitization of CRF1 receptors such that the rat eventually developed a panic-prone state (Sajdyk, Schober, Gehlert, & Shekhar, 1999). This type of behavioral plasticity was proposed to be potentially relevant to the changes that underlie the development of panic disorder. However, it is also possible that this paradigm is relevant to PTSD which can develop as the result of repeated exposure to stressful situations (reminders). A second important observation was that NBI3b1996 also reversed the anxiogenic effect seen in normal rats exposed to a prior stressor, whereas the CRA was without effect in non-stressed, normal rats. An inference from this study is that the site of action of the CRA is on CRF1 receptors in the basolateral amygdala, a hypothesis that could be tested by direct administration studies of the antagonist into this area. To summarize, although there was some heterogeneity in the findings with CRAs using the social interaction test in rodents, converging lines of evidence again support the conclusion that CRAs are anxiolytic under conditions that produce higher levels of stress.
2a (i)(d) Light-Enhanced Startle
Walker and Davis have described an unconditioned fear test using the startle response as a behavioral endpoint which appears to be sensitive to CRAs (D. L. Walker & Davis, 2002a, 2008; D. L. Walker, Toufexis, & Davis, 2003). Startle is a brief, whole-body contraction that results from presentation of a sudden, intense stimulus, such as a loud sound or a puff of air to the body. Startle amplitude is dependent upon the intensity of the eliciting stimulus, such that, by using low intensity eliciting stimuli, a low (but non-zero) level of responding can be achieved. In the light-enhanced startle paradigm, rats exposed to intense, sustained bright light (which is naturally aversive to nocturnal rodents) show an elevated startle response in the presence of that light, relative to startle that is elicited in the dark. In this paradigm, GSK876008 reduced light-enhanced startle. The authors have performed previous work which has demonstrated that startle enhanced by light is mediated by the BNST, which is part of the “extended amygdala”. The BNST was also shown to mediate, at least in part, the potentiation of startle that is produced by exogenous CRF infused into the lateral ventricle. The authors have argued that the BNST mediates more prolonged, sustained states of anxiety. These findings will be revisited later in a discussion of contrasting results obtained with CRAs using the fear conditioned startle model. In the context of previously discussed results, one might conclude that prolonged exposure to light in a startle-testing environment was sufficiently stressful to activate endogenous CRF1 pathways, thereby allowing detection of CRA efficacy.
2a (i)(e) Distress Vocalizations
One final anxiolytic model of unconditioned fear in which CRAs have been extensively evaluated is in the separation-induced ultrasonic vocalization model in rat pups. In this model, rat pups removed from the litter and their mother elicit a series of repetitive “distress cries”which, from an ethological perspective, signals the mother that the pup has strayed from the litter. These distress cries are in the ultrasonic range and, when detected by the mother, cause her to leave the litter and retrieve the pup. Ultrasonic vocalizations can be viewed as a stimulus-dependent activation of the primitive stress response system in the rat pup, which serves a highly important adaptive purpose in enhancing the probability of survival of the pup.
Following up on initial studies (Kehne, et al., 1995; Kehne, et al., 1991; Winslow & Insel, 1990, 1991; Winslow, Insel, Trullas, & Skolnick, 1990; Winslow, Newman, & Insel, 1989) which pharmacologically characterized the ultrasonic vocalization test as a paradigm for evaluating potential anxiolytics, Kehne and colleagues were the first to report that CP-154,526 dose-dependently reduced maternal separation-induced vocalizations (Kehne, Coverdale, McCloskey, Hoffman, & Cassella, 2000). This anxiolytic-like effect occurred at doses widely separated from those that produced side effects, in contrast to benzodiazepines, which were anxiolytic with a considerably narrower therapeutic index. Subsequently, similar findings have been reported for CP-154,526 in two independent labs (Hodgson, et al., 2007; Iijima & Chaki, 2005) and recently extended with R121919 (Ise, Nagano, Okuda, & Ohta, 2008). In addition, it was recently demonstrated that exogenous CRF administered systemically to the rat (which can enter the brain because of the incomplete development of the blood-brain barrier at this early age of testing) elicited ultrasonic vocalizations and this effect was blocked by R121919 (Ise, et al., 2008), data that supported the notion that the separation stress activates CRF1 pathways, and is important for producing this well-defined, adaptive stress response.
The striking contribution of CRF1 receptors in the rat pup vocalization paradigm is interesting in relation to a body of animal evidence suggesting that overactivation of CRF1 pathways in early life can have long term detrimental effects lasting into adulthood, contributing to dysfunctional stress-responsiveness and psychopathology suggestive of anxiety and depression. In the animal models performed to study this phenomenon, the same stressor that effectively activates CRF1 pathways and elicits ultrasonic vocalizations in rat pups, maternal separation or isolation, is repeatedly used on successive days beginning at postnatal day 2 and up to about day 10 to deliver stress subchronically to the infant pup. When raised to adulthood and subsequently tested, rats exposed to this early life stress demonstrate evidence for hyperactivated CRF1 pathways, elevated reactivity of the HPA axis to stressors, and heightened anxiety as measured on the elevated plus maze (Maciag, et al., 2002). Importantly, the heightened anxiety demonstrated by these rats was blocked by DMP696, which had no clear anxiolytic activity in the non-separated controls (Maciag, et al., 2002). As mentioned earlier, CRAs generally lack anxiolytic profiles on the elevated plus maze when the test is run under “low stress” conditions. The finding that DMP696 was active but only in adult rats rendered anxious by prior exposure to early life stress is fully consistent with this conclusion. The potential clinical significance of these animal findings to the development of adult psychopathology in humans exposed to early life trauma is further discussed later in this review.
Finally, it should be mentioned that the basic “distress cry”phenomenon seen with rat pups is also seen with guinea pigs, though in this case, the vocalizations are in the audible range (Hodgson, et al., 2007). In addition, these vocalizations were reduced by CP-154,526, thereby extending the generality of the CRF1 receptor mediation of this phenomenon from rats to another species. Distress vocalizations can also be measured in non-human primates, though, to date, the effects of CRAs have not been reported in this species.
In an series of elegant studies, Sullivan and colleagues have demonstrated that exposure to early life stress in infant rats can result in aberrant attachment learning later in life, and have implicated CRF pathways that are part of a locus coeruleus-amygdala circuit (Moriceau, Raineki, Holman, Holman, & Sullivan, 2009; Moriceau, Shionoya, Jakubs, & Sullivan, 2009; Sullivan & Holman, 2009). Other recent work has demonstrated long-lasting effects of early life stress on CRF1 receptor expression to acute stressors in adulthood (Maniam & Morris, 2010; O'Malley, Dinan, & Cryan, 2010a, 2010b; Swinny, et al., 2010). It would be of interest to determine if CRAs could prevent the deleterious effects of early life stress in these models.
2a (ii) Conditioned Fear Models
The discussion thus far has focused on the effects of CRAs in models of unconditioned fear or anxiety. A second general class of tests in which CRAs have been evaluated measure conditioned fear and the two procedures which have been primarily used are conditioned freezing and fear potentiated startle. It should be noted at the onset of this discussion that, relative to unconditioned fear, far fewer studies have explored the effects of CRAs on the multiple aspects of fear conditioning. Thus, additional work is needed to fill these gaps to enable a better understanding of the clinical potential of CRAs in treating anxiety disorders, especially those with an aberrant or excessive conditioned fear component.
Fear conditioning (FC) is an experimental procedure in which a neutral conditioned stimulus (CS), often a light or tone, is paired with an aversive unconditioned stimulus (US), typically a footshock. After very few pairings, lasting memories are established, such that the CS comes to elicit responses that are characteristically expressed in the presence of danger. The responses are usually hard-wired, species-typical reactions that are themselves not learned (R. J. Blanchard & Blanchard, 1971; Bolles, 1970). Although the bulk of FC studies use auditory CSs and footshock USs, it should be noted that discrete CSs of any sensory modality are effective and more complex, configural cues such as the experimental context also work. Similarly, a number of US types are also effective at supporting FC, such as loud noise bursts, air puffs, and virtually any form of pain-eliciting stimulus. Recent reviews summarize major findings about fear conditioning (M. S. Fanselow & Poulos, 2005; J. E. LeDoux, 2000; Maren, 2001; Rosen, 2004; D. L. Walker & Davis, 2002b)(see also Figure 3 for a discussion of key neural substrates of fear conditioning).
Figure 3. Pavlovian fear conditioning circuitry.
In cue fear conditioning, animals learn to fear an innocuous stimulus, such as a tone. By pairing tone (conditioned stimulus: CS) and shock (unconditioned stimulus: US), the tone acquires the capacity to elicit defensive reactions, such as freezing (arrow pointing up). Tone and shock stimuli converge in the lateral amygdala (LA), resulting in associative plasticity in the tone→LA pathway. Subsequent presentations of the tone can now activate LA neurons. The LA then communicates with the central nucleus (CE), which controls the expression of fear by way of connections to specific circuits that mediate fear reactions (e.g. freezing, potentiated startle, autonomic responses and hormonal responses). The LA connects with CE directly and by way of connections to other amygdala areas, including the intercalated cell masses (ICM), which gate the output, and the basal nucleus (B), which processes contextual information from the hippocampus. As with discrete CSs, more complex configural cues can also enter into associations with the US via hippocampal connections to the basolateral amygdala. CRF and CRF1 receptors are present throughout fear conditioning circuits, including the basolateral and central amygdala, hippocampus as well as downstream effector regions such as the periacqueductal gray (freezing), nucleus reticularis pontis caudalis (potentiated startle), and the lateral (autonomic responses) and paraventricular (hormonal responses) nuclei of the hypothalamus. Thus, CRF1 pathways are well-positioned to modulate the learning, storage and expression of fear conditioning Figure from (Sotres-Bayon et al., 2006). Reprinted with permission from Elsevier.
A major advantage of studying FC is that anxiety disorders in humans seem to involve alterations of fear processing circuits in the brain (Marks, 1987; Ohman & Mineka, 2001; Seligman, 1971; Charney, 2003; Lang, Davis, & Ohman, 2000; J. LeDoux, 1996; Pitman & Delahanty, 2005). FC is thus a means of studying the circuits relevant to anxiety disorders, but may also be involved in the genesis or maintenance of these disorders. Studies of FC may have special relevance to understanding anxious disorders that are triggered by explicit environmental cues, such as panic, specific phobia, obsessive-compulsive disorder and post-traumatic stress disorder (Altemus, et al., 1992; Bremner & Brett, 1997).
Importantly, relative to other aversive behavior models, there is a good understanding of the neurocircuitry, physiology, molecular biology and structural underpinnings of FC learning, memory and performance. The knowledge of FC mechanisms has been advanced by studies that precisely time manipulations with respect to different phases of the fear conditioning procedure. For instance, drugs administered before training can be evaluated for their effects on learning (acquisition) mechanisms whereas application of the same drug after training may implicate memory consolidation mechanisms. Further, giving the drug before retention testing (at a time well-separated from the acquisition and consolidation phases) can assess possible effects on memory retrieval/expression mechanisms. Precisely timed manipulations, not discussed in detail here, can also implicate extinction, reconsolidation and recovery mechanisms relevant to fear coping. Importantly, detailed assessments of novel agents like CRAs on these different aspects of fear conditioning may provide important insights into how these agents might ultimately be best utilized in clinical practice as agents for treating anxiety disorders.
2a (ii)(a) Conditioned Fear and CRF Activation
There is some data to suggest that activation of CRF1 pathways occurs during the presentation of either fear-evoking unconditioned stimuli or conditioned fear stimuli. Microdialysis techniques have shown that exposure to natural predator threats results in CRF release in the central nucleus of the amygdala, a region critical for FC expression (Cook, 2004). Painful stimuli produce evidence for activation of CRF1 receptors in the central nucleus (Ji, Fu, Ruppert, & Neugebauer, 2007; Ji & Neugebauer, 2007). Furthermore, immunohistochemical studies provide evidence for elevated CRF levels in multiple brain regions known to be involved in FC, including the prefrontal cortex, basolateral amygdala, central nucleus of the amygdala, and hippocampal formation, following exposure to a fear-eliciting CS (Lehner, et al., 2008). In situ hybridization studies also demonstrate that acute footshocks induce brain CRF mRNA expression especially in the central nucleus of the amygdala and BNST (Funk, Li, & Le, 2006). Thus, the available evidence suggests that CRF is induced in FC circuits by pain evoking stimuli, by natural threats and by conditioned threats and therefore is available to modulate learning, memory and performance processes dependent on these regions.
FC can result in a wide array of autonomic, endocrine and behavioral responses to the CS, though the present analysis will focus on behavioral responses to a fearful CS. CRAs have been primarily characterized in FC models which utilize two particular behavioral responses: conditioned freezing and fear-potentiated startle.
2a (ii)(b) Conditioned Freezing
Rodent freezing is an immobile posture that is defined as the absence of all movements except those related to respiration (R. J. Blanchard & Blanchard, 1969; M. S. Fanselow, 1980). Freezing is not simply an absence of activity but rather represents an effortful cessation of movement including even vibrissae movements that are otherwise active during all awake behaviors. Conditioned freezing depends on projections within the amygdala (lateral to central nucleus) and from the amygdala to the ventral periaqueductal gray (Cain & LeDoux, 2008; M. S. Fanselow, 1991). Freezing has been described as the primary rodent reaction to fear-eliciting stimuli. Functionally, freezing may protect small rodents from predators by aiding in avoiding detection.
A number of studies have examined the effects of CRAs on ‘shock-elicited freezing’ behavior, with the underlying assumption that CRF manipulations affect the unconditioned stress response to aversive stimulation. However, this seemingly straightforward conclusion is complicated by two lines of evidence indicating that post-shock freezing is a result of context fear conditioning (learning), not an unconditioned response to shocks. First, rats shocked in one context and then immediately moved to a neutral second context do not exhibit freezing (M. S. Fanselow, 1980). Second, ‘shock-elicited freezing’ does not even occur in the shock context unless the rats have sufficient time before the shocks to compile a configural representation of the context CS (e.g. immediate shock deficit; Blanchard et al, 1976; Fanselow, 1986, 1990). Thus, post-shock freezing appears to reflect short-term context fear memory. CRF manipulations in this assay provide information about fear learning or expression, but cannot distinguish between the two.
As might be expected, CRF infusions prior to shocks lead to greater post-shock context freezing (Sherman and Kalin, 1988) though CRAs have not been evaluated for their activity in reversing this anxiogenic-like effect. However, several studies have evaluated the effects of CRAs on post-shock context freezing. Systemic antalarmin was reported to decrease post-shock freezing (Deak, et al., 1999) and direct administration of R121919 (NBI27914) into the central nucleus of the amygdala decreased both total freezing and latency to begin freezing following footshock (Bakshi, Smith-Roe, Newman, Grigoriadis, & Kalin, 2002). On the other hand, DMP696 given systemically, or directly into the central nucleus of the amygdala, prior to shocks was reported to have no effect on post-shock freezing (Hubbard, Nakashima, Lee, & Takahashi, 2007). There are at least two possible reasons for the lack of effects in this paper. First, context conditioning may have been stronger compared to the positive reports above because the experimenters used five footshocks instead of three. Indeed, rats were freezing above 90% in the post-shock interval, suggesting a possible ceiling effect. Second, rats were observed for only two minutes following the last footshock compared to 10–15 minutes in the above studies suggesting that longer post-shock intervals may be necessary to detect CRA antagonist effects on post-shock freezing. Further work is needed to evaluate these possible explanations.
Thus, the present data provide some support for the conclusion that CRAs can decrease short-term memory for context fear as measured by post-shock freezing. This effect can be a result of either decreased learning or decreased expression. Based on the data discussed thus far, it seems reasonable to hypothesize a role for CRF1 receptor activity in fear expression, given the effects of intra-CE manipulations, a region more closely associated with fear expression than fear learning/memory. (However, note a recent study indicating that the CE may be important for memory consolidation rather than expression: (Pitts, Todorovic, Blank, & Takahashi, 2009). However, manipulations aimed at the basolateral amygdala (BLA), that may implicate CRF1 receptors in FC learning, have not been thoroughly examined.
In a second set of studies, CRAs were administered prior to acquisition, but long-term memory was assessed, with testing occurring at least 24 hours post-acquisition. These studies can implicate learning or consolidation processes, but cannot distinguish between the two. However, they are usually not complicated by potential fear expression effects, since most drug effects have subsided by the time the long term memory test is performed. These studies include assessment of cue fear (tone or light CS), context fear and sometimes background context fear. Background context conditioning refers to fear acquired to the context during a cue fear conditioning session. Background context fear depends on similar neural mechanisms as foreground context fear (unsignaled shocks in the conditioning context) but has some subtle differences (Majchrzak, et al., 2006; Phillips & LeDoux, 1994).
Exogenous administration of CRF during FC acquisition, or shortly afterwards, tends to facilitate long term memory assessed at least 24 hours later. Rats infused with CRF, either ICV or intra-hippocampus (dorsal), before tone-shock pairings showed enhanced freezing to the tone and shock context one day later (Radulovic, Ruhmann, Liepold, & Spiess, 1999). CP 154,526, antalarmin, or DMP 696 administered before context fear conditioning result in reduced freezing at long term memory tests (Deak, et al., 1999; Hikichi, et al., 2000; Rau & Fanselow, 2007). Interestingly, Hubbard et al (2007) found that pre-training DMP 696 decreased long-term context fear when it was infused directly into the BLA but not when it was infused into the CE. This supports the notion that CRF1 receptor activity in BLA affects learning/consolidation mechanisms whereas CRF1 receptor activity in CE affects fear expression mechanisms, as mentioned above.
The data surveyed in the preceding sections point to a facilitatory role of CRF1 receptor activity in the learning, consolidation and/or expression of FC. It is possible to disentangle the contribution of CRF to these processes. Learning mechanisms are implicated with pre-training manipulations that affect short-term memory (STM) for FC. STM tests should be separate US-free sessions usually conducted 3–6 hours post-acquisition, before consolidation is complete. Of course, manipulations that are still present at the STM test, such as drugs with long half-lives, may affect fear expression. But expression effects can be studied in isolation by using pre-long-term memory (LTM) test manipulations (see below). According to our searches, STM has never been assessed with acute CRF or CRF1 manipulations in a separate US-free test session.
If CRF1 receptors are important for fear conditioning consolidation, then post-acquisition manipulations should affect LTM (McGaugh, 2000). Three studies to date have cleanly investigated the role of CRF and CRF1 receptors in FC consolidation. Radulovic et al (1999) infused CRF directly into the dorsal hippocampus immediately or 60-minutes after tone-shock pairings. They found that context-elicited freezing was increased 24-hours later relative to vehicle controls, but only with the immediately post-training infusion. Recall that context conditioning involves at least two distinct forms of learning: compilation of the context CS and then formation of the context-US association. Since context representations depend on the hippocampus and Pavlovian FC associations depend on the amygdala, this post-FC CRF effect likely reflects enhanced consolidation of the context representation memory rather than Pavlovian conditioning, although a modulatory role of FC consolidation cannot be ruled out. CRF also appears to modulate consolidation of the Pavlovian FC memory. Rau & Fanselow (2007) treated rats with CP 154,526 immediately following context FC and found reduced context-elicited freezing at a LTM test. Hubbard et al (2007) infused DMP 696 into the BLA 5-minutes, 3-hours or 9-hours after context FC. They found that context-elicited freezing 48 hours later was significantly impaired in the 5-minute and 3-hour groups, but not the 9-hour group, implicating BLA CRF1 receptors in consolidation of the context-US association. Note that in the two cases where timecourse was examined, early manipulations post-learning affected LTM but later manipulations did not. This pattern is consistent with a large body of memory consolidation work indicating a time window following aversive learning for affecting memory storage mechanisms (McGaugh, 2000).
A final set of studies examined the effects of CRAs on memory expression. Manipulations in these studies occur after learning and consolidation are complete, usually at least 24-hours post-training. CRF infused (ICV) prior to fear expression tests result in enhanced context freezing and conditioned suppression by a light CS (Cole & Koob, 1988; Swiergiel, Zhou, & Dunn, 2007). CRF1 receptor antagonists have an opposite effect. Systemic injections CP 154,526, antalarmin or DPC 904 before LTM tests impair context-elicited freezing (Deak, et al., 1999; Hikichi, et al., 2000; Ho, et al., 2001).
To summarize, the available evidence using the conditioned freezing model suggests that CRAs can reduce both the consolidation and expression of conditioned fear, effects which are likely mediated by actions in the basolateral amygdala and central amygdala, respectively. CRAs may also participate in fear acquisition (learning) however this possibility is difficult to assess given the substantial effects on fear expression. Further studies with short-lived CRF/ CRF1 receptor manipulations and US-free STM tests may clarify this issue.
Thus far, all of the studies described have been performed under “normal” fear conditioning conditions. Additional studies have begun to explore the effects of exposure to prior stress on subsequent fear conditioning. Such studies are likely of relevance to the etiology of certain anxiety disorders in which exposure to prior stress may result in a vulnerability to the development of dysfunctional emotional memories during subsequent fear conditioning associated with trauma.
Stress effects on learning and memory processes are complex, as studies suggest a critical time dependency in that stress immediately prior to fear conditioning produces an impairment, whereas stress separated from fear conditioning by at least 3-hours can enhance fear conditioning.
The most commonly reported effect of acute stress manipulations is an enhancement of context fear conditioning (Cordero, Venero, Kruyt, & Sandi, 2003). Mice subjected to 1 hour of restraint and then given context conditioning 3 hours later showed enhanced within-session freezing (Sananbenesi, Fischer, Schrick, Spiess, & Radulovic, 2003). Exposure to immobilization stress 1h prior to fear conditioning impaired the learning of context freezing, and this impairment was prevented by administration of CP-154526 prior to the immobilization period (Blank, Nijholt, Vollstaedt, & Spiess, 2003). In this experiment, CP-154,526 did not interfere with the acquisition of conditioned freezing in normal (non-stressed) mice.
Robust enhancing effects of acute stress (24 hours pre-FC) have been reported when the stress stimuli match the US used in conditioning. Rau and colleagues (Rau, DeCola, & Fanselow, 2005) conducted an extensive analysis of the interaction between a single footshock-stress session and fear conditioning. They compared 1-trial FC in control rats and rats that had been stressed 24-hours earlier with 15 strong footshocks in a separate context. When LTM was assessed for 1-trial FC, stressed rats froze more than twice as much as non-stressed rats. This effect was demonstrated for 1-trial FC using either a cue CS (tone) or a context CS. The footshock-stress session also did not appear to change shock sensitivity. Taken together, these data suggest that the footshock-stress session sensitized FC pathways to subsequent conditioning, either by enhancing learning or consolidation mechanisms. Finally, in a follow-up study, this same group demonstrated that systemic injections of CP 154,526 attenuated the stress-induced enhancement of 1-trial FC when given before, but not after, the stress session (Rau & Fanselow, 2007).
In related studies, Steven Maier and colleagues have extensively examined the effects of acute escapable (ES) versus inescapable (IS) electrical shocks on subsequent shock-reinforced FC and other behaviors (for review see (Maier & Watkins, 2005). Note that these studies employ intense shock-stress sessions [100 tailshocks from 1.0–1.6 mA, variable duration (up to 30s in some studies), 60s inter-shock interval] in order to induce learned helplessness in IS-treated rats. IS-treated rats show more within-session context freezing (‘shock-elicited freezing’), and more freezing at LTM tests, compared to ES-treated rats and homecage controls. This effect occurs when rats receive IS either 1-week or 1-day prior to context FC (Amat, et al., 2005; Baratta, et al., 2007). Interestingly, the critical variable in these studies appears to be control, as ES rats actually show suppressed conditioned freezing compared to homecage controls (Baratta, et al., 2007; Baratta, Lucero, Amat, Watkins, & Maier, 2008). These bi-directional effects of ES and IS on conditioned freezing are observed with either pre-FC or post-FC stress and thus appear to reflect changes in fear expression processes rather than (or in addition to) learning or consolidation processes. In an elegant series of experiments, this group has shown that the benefit of controllability with ES depends on the mPFC (Amat, et al., 2005) whereas the exacerbation of freezing with IS depends on CRF2 receptor activity in the dorsal raphe nucleus and neuronal activity in the BNST (Hammack, Richey, Watkins, & Maier, 2004). Interestingly, systemic CP-154,526 blocks the IS-induced potentiation of context freezing (Deak, et al., 1999) but intra-DRN antagonism with NBI27914 does not (Hammack, Pepin, DesMarteau, Watkins, & Maier, 2003). Thus, as with the Rau et al study, CRF1 receptor activity appears to be important for shock-stress facilitation of shock-reinforced FC but the site of action remains unknown.
Situations in which stress is separated from fear conditioning by a significant block of time may be relevant to situations in which a history of prior stress establishes a “vulnerability” for enhanced fear conditioning. Thus, additional studies with CRAs studying the effects of both proximal and distal prior stress on fear conditioning are warranted. Chronic stress has also been shown to enhance FC (Conrad, LeDoux, Magarinos, & McEwen, 1999; Cordero, et al., 2003; Sandi, Merino, Cordero, Touyarot, & Venero, 2001; Wood, Norris, Waters, Stoldt, & McEwen, 2008; Zurita, Martijena, Cuadra, Brandão, & Molina, 2000), especially context FC. Recent studies by Rau and colleagues reported that prior exposure to footshock stressors (more than one exposure was required) enhanced subsequent contextual fear conditioning of freezing (Rau & Fanselow, 2008). Strikingly, this vulnerability for enhanced conditioning was demonstrated to last for at least three months post exposure to the sensitizing shocks, and these authors proposed this as an animal model that may be relevant to PTSD (Rau & Fanselow, 2008). It would clearly be of interest to evaluate the effects of CRAs in these assays.
2a (ii)(c) Fear-Potentiated Startle
A second conditioned fear model which has been used to evaluate CRAs is the fear potentiated startle model. When rodents are classically conditioned by pairing a neutral stimulus (e.g. a light) with an aversive footshock stimulus, startle amplitude is augmented or potentiated when it is elicited in the presence of the conditioned stimulus relative to when it is elicited in its absence. This “fear-potentiated” or “fear-conditioned” startle can be reduced by benzodiazepines as well as by non-benzodiazepine anxiolytics such as the 5HT1A partial agonist buspirone. Mike Davis and colleagues have done an extensive amount of elegant work delineating the neural circuitry underlying startle and the modulatory pathways that mediate fear-potentiated startle.
Review of the literature reveals that, of several studies which have evaluated the effects of CRAs on fear-conditioned startle, only one using CP-154,526 reported an anxiolytic action. The remaining studies which were performed with R121919 in mice (Risbrough, et al., 2008) and GW876008 in rats (D. Walker, et al., 2008) reported no effects on cue-signaled, fear-conditioned startle. Further work by the Risbrough and Davis labs has provided some clarity regarding the effects of CRAs. For example, R121919 was reported to reduce startle that was potentiated in the presence of contextual cues, whereas it lacked efficacy when a discrete conditioned stimulus cue was used. As noted previously, in context conditioning, an association develops between the environmental cues in which an animal is exposed to the aversive stimulus, such that, when placed back in that environment, the animal becomes fearful. This general, “diffuse” conditioning contrasts to the more specific conditioning that occurs when a discrete cue (such as a light or sound) is used. Importantly, a considerable amount of data has indicated that different neural substrates mediate discrete versus contextual fear conditioning. Whereas the amygdala complex has been shown to mediate the cue-specific conditioning, the hippocampus, with its well-known role in spatial memory, has been shown to play an important role in context conditioning. Thus, the ability of CRAs to reduce contextual but not cue-enhanced fear conditioned startle suggests the involvement of CRF1 receptors in the hippocampus as opposed to the amygdala.
As discussed above, Walker and Davis have described an unconditioned startle paradigm (light-enhanced startle) which is sensitive to blockade by a CRA (D. Walker, et al., 2008) and which is mediated by the BNST, whereas fear potentiation with a cue is mediated by the central nucleus of the amygdala. The BNST was also shown to mediate, at least in part, the potentiation of startle that is produced by exogenous CRF infused into the lateral ventricle. It was argued that the BNST mediates more prolonged, sustained states of anxiety whereas the central nucleus of the amygdala mediates shorter duration, fast-onset fear responses mediated by discrete cues. This is supported by very recent data collected by the Davis lab. Fear-potentiated startle using long duration CSs (8 minutes) was blocked by both systemic and intra-BNST CRA application (GW876008). The same treatments had no effect on fear-potentiated startle with short duration CSs (3.7s)(Miles, Walker, & Davis, 2008).
The outcome of the various studies performed in mice and rats using startle, while complex, do imply that CRAs have some potential anxiolytic-like activity which might be seen in humans. One suggestion is that the potentiation to diffuse stimuli might be more relevant to anxiety disorders such as GAD, in which anxiety responses are not clearly linked to specific environmental cues, as in phobias. On the other hand, generalized anxiety might not be linked to environment stimuli at all, but rather produced by an endogenous neurochemical imbalance that may not involve hyperactivation of CRF1 pathways (a conclusion supported by the lack of evidence for elevated CRF levels in the CSF of patients with GAD). Disorders like PTSD, however, where symptoms can be elicited in presence of environmental cues similar to those in which exposure to a traumatic event occurred, may involve dysfunctional context conditioning and hyperactivation of CRF1 pathways, and, therefore, CRAs may be more likely to be useful in their treatment. Future clinical evaluation of CRAs in PTSD and other anxiety disorders is needed to evaluate these possibilities.
A recent study has explored the potential utility of CRAs in a predator stress animal model of PTSD (Adamec, Fougere, & Risbrough, 2010). In this model, exposure of C57BL6 mice to an intense, threatening stimulus (a cat) produced several enduring increases in behavioral reactivity. Thus, a week after predator exposure, mice exhibit elevated startle responses and delayed startle habituation, as well anxiogenic-like behaviors as measured in other paradigms (light/dark box, elevated plus maze). In stressed (but not unstressed) mice, CRA0450 administered prior to, or immediately after, stress exposure attenuated effects on startle measured a week later. The authors concluded that these results suggest that CRF1 receptor blockade can interfere with the acquisition (initiation) and consolidation of stressor effects, and indicate that CRAs may be useful as potential prophylactic treatments for PTSD.
Unlike the fear potentiated startle paradigm described previously, the predatory stress model involves only a single exposure to the stressor, so strictly speaking, it is not a classical conditioning paradigm. However, it is possible that there is conditioning to the contextual stimuli of the environment in which the stressor exposure occurred, a possibility that warrants further investigation. Furthermore, one can speculate that a potential mechanism underlying the long-term change in behavioral reactivity may be long term potentiation (LTP) in the central nucleus of the amygdala that is dependent on CRF1 receptor stimulation (Gallagher, Orozco-Cabal, Liu, & Shinnick-Gallagher, 2008).
2a (iii) Summary
To summarize, CRAs have been evaluated in a variety of unconditioned and conditioned animal models sensitive to anxiolytics and appear to have a unique “fingerprint” relative to classic anxiolytics, especially the benzodiazepines. The apparent mixed effects of CRAs in different animal models of unconditioned fear may result from different endogenous levels of stress produced by the test conditions. CRAs appear to significantly affect fear conditioning to contextual stimuli. Robust acute effects of CRAs have been measured in the maternal separation model suggesting that CRF1 pathways overactivated by stress in early infancy may be inhibited by CRAs thereby preventing SRS dysfunction and psychopathology that lasts into adulthood. Finally, it generally appears to be the case that CRAs demonstrate efficacy at doses widely separated from those that produce side effects, suggesting that they may be anxiolytic with an improved side effect profile relative to classic benzodiazepines. Importantly, as benzodiazepines have been reported to be active in all of the unconditioned tests run under standard conditions (in which no manipulations are used to enhance stress level), an important question is: what are the implications with regard to anxiolytic potential of CRAs in humans? This question will be revisited after discussion of profiling of CRAs in other animal models.
2b. Antidepressant Activity
Standard models for evaluating antidepressant potential of compounds are generally based on an animal’s behavioral response to an inescapable and/or unpredictable stressor. Stressors vary in type (swim stress, tail suspension, foot shock, etc) and chronicity (acute, subchronic, chronic). Typically, depressant-like behavior is indicated by a cessation of active coping behaviors related to attempts to escape or avoid the unpleasant situation.
2b (i) Forced Swim and Tail Suspension Tests
The Porsolt forced swim test, performed in mice and rats, measures swimming behavior as a behavioral endpoint, and the time that the animal eventually spends immobile as an index of a depressant-like state. Compounds are administered acutely or subchronically in this paradigm and antidepressant activity is measured as a decrease in immobility. In the tail suspension test, a mouse is suspended in air by its tail and the force generated by movements made to escape are measured, with immobility time again being the measure of the depressant-like state produced. Marketed antidepressants, serotonin-selective reuptake inhibitors (SSRIs); dual serotonin-norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors (MAOIs), and other agents generally decrease immobility time when administered acutely and/or subchronically in these paradigms.
As summarized in Table 2, CRAs have a mixed profile when run in either the forced swim test or tail suspension test run under standard conditions. An explanation for these mixed findings is that, as with the standard anxiolytic paradigms, the levels of stress induced by these paradigms may not be sufficiently high to produce a sustained activation of CRF1 pathways in the brain and HPA axis that may be necessary to see efficacy of CRAs (Overstreet, Keeney, et al., 2004; Takahashi, 2001). Consistent with this hypothesis, CRAs are reported to be effective in the forced swim test in rats that putatively demonstrate higher levels of endogenous stress. Thus, CP-154,526 decreased immobility time in FSL rats bred for depressive-like traits. Similarly, CP-154,526 (Overstreet, Keeney, et al., 2004) and R121919 were antidepressant in the forced swim test in a “Swim High, Active” strain rat (Gutman, et al., 2008).
2b (ii) Learned Helplessness
“Learned helplessness, a phenomenon first described in dogs by Martin Seligman in the 1970s, refers to the behavioral state produced in an animal following chronic exposure to inescapable stressors such as footshock. Coping behavior has classically been measured using a shuttle box apparatus, which requires the animal to perform movements to avoid or escape shock. Repeated exposure to inescapable shocks can result in avoidance/escape deficits (“learned helplessness”) which can be ameliorated by chronically administered antidepressants.
Antidepressant effects of either acute or chronic (8 days) dosing with CRA0450 decreased escaped failures in the rat learned helplessness test (Takamori, Kawashima, Chaki, Nakazato, & Kameo, 2001). CP-154,526 was also effective in this test (Takamori, et al., 2001). It should be noted that one study reported that antalarmin administered both prior to training and testing was ineffective in reversing the shuttlebox avoidance deficits resulting from exposure 24h earlier to inescapable tail shock (Deak, et al., 1999). This reason for this discrepancy is not clear.
These positive CRA results are interesting for several reasons. First, like classic antidepressants, efficacy was seen following chronic (8 d) daily administration, but unlike classic antidepressants, which are not active acutely, the CRAs worked after a single administration. This rapid-onset efficacy is consistent with a finding reported by Mansbach and colleagues with CP-154,526 in a learned helplessness paradigm that used an operant lever pressing task as a behavioral endpoint (Mansbach, Brooks, & Chen, 1997), and is cited as evidence that CRAs may offer a substantial benefit to depression treatment over classical agents that only work after chronic administration. A second finding of note was that CRAs showed efficacy when administered before inescapable shocks during training, but not when administered immediately after shocks, or before the subsequent testing session. Thus, CRAs appeared to prevent the acquisition of learned helplessness, but not its consolidation or retention. The simplest interpretation of blockade of acquisition is that the CRA might be producing analgesic effects which would diminish the aversiveness of the footshocks used to produce learned helplessness. CRAs are reported to lack robust analgesic properties using classic analgesia tests (Deak, et al., 1999). On the other hand, recent data suggest that there is a link between CRF1 receptors in the amygdala and pain-related anxiety behaviors (Ji, et al., 2007) and pain-related sensitization (Ji & Neugebauer, 2007). However, as noted above, CRAs appear to show activity in stressful assays that do not rely on pain, such as the forced swim test.
It is important to consider the implications of these results with regard to the expected effects in humans. That is, do these results imply that CRAs given to a patient with established depression (or even shortly after exposure to highly traumatic events) would not be effective? Would administering CRAs during exposure to trauma be of utility? Does “prophylactic” treatment with CRAs (i.e. administration prior to traumatic events) have practical utility? It should also be noted that results using inescapable shock paradigms were discussed in the previous section on anxiolytic actions of CRAs. Determining the relative importance of data gathered from inescapable shock experiments to anxiety and depression is clearly important for predicting clinical utility of CRAs. These are complex questions which should be considered as studies further evaluate the potential antidepressant utility of CRAs.
2b (iii) Olfactory Bulbectomy and DRL-72
Limited testing of CRAs has been performed in other tests which have been often used for evaluating potential antidepressant activity. Rats in which the olfactory bulb has been surgically removed demonstrate a syndrome of behavioral changes including increased emotionality that can be reversed by chronic administration of antidepressants. Reversal of emotionality was seen with either acutely or chronically administered CRA0450 (Chaki, et al., 2004), CRA1000 (Okuyama, et al., 1999), and CRA1001 (Okuyama, et al., 1999), providing additional data suggestive of rapid antidepressant activity. On the other hand, mixed results have been reported in differential rate of low reinforcement − 72s (DRL 72) test, with efficacy reported for SSR125543 (Louis, Cohen, Depoortere, & Griebel, 2006) but not for CRA0450 (Chaki, et al., 2004).
2b (iv) Chronic Mild Stress
Chronic stress paradigms have been elaborated to include different endpoints from those typically measured. In one paradigm, mice exposed to chronic mild stress demonstrated physical signs of debilitation such as loss of weight and decreased self-grooming which were ameliorated by SSR125543 given chronically (Ducottet, Griebel, & Belzung, 2003). Like known antidepressants, SSR125543 was also reported to decrease chronic stress-induced decreases in hippocampal neurogenesis (Alonso, et al., 2004). Interestingly, chronic mild stress has been shown to cause anhedonia in rats, a hallmark of human depression, and also increase CRF activity in the BNST (Stout et al, 2000). However, CRAs have not yet been evaluated in this assay.
A sizeable literature has indicated that chronic stress can have long term deleterious effects on the CNS which are mediated through excessive activation of the HPA axis (McEwen, 2005, 2007, 2008; Sapolsky, 1999, 2000, 2001, 2003). Excessive stimulation of glucocorticoid receptors in the hippocampus in particular may result in aberrant long terms alterations in cellular function, including cell death, thereby giving rise to impairments in hippocampal-related functions such as learning and memory. Such stress-related pathology might contribute to the pathology of depression as well as other CNS disorders in which impairments in cognitive function can be seen. More extensive evaluation of the effects of CRAs in animal models of stress-induced hippocampal pathology and associated impairments of cognitive function are clearly needed to help guide potential clinical work in this area.
2c. Other Activities
CRAs have been evaluated in two additional classes of animal models of disorders in which stress plays a significant role: irritable bowel syndrome and addictions to alcohol and other drugs of abuse. While not the focus of the present review, these will be briefly summarized below.
2c (i) Irritable Bowel Syndrome
As noted previously, IBS is a stress-related gastrointestinal disorder frequently comorbid with anxiety and characterized by disturbed bowel habits (diarrhea and/or constipation) and visceral abdominal pain (Lydiard, 2005). CRAs have been proposed as a novel pharmacological treatment for IBS, through blockade of both central and peripheral CRF1 receptors (for reviews, see (Martinez & Tache, 2006). As summarized in Table 2, CRAs ameliorate the stimulatory effects of exogenous CRF on colonic motility and colonic bowel function, and reduce stress-induced increases in visceral sensitivity (for reviews, see (Tache, et al., 2005). Visceral hyperalgesia and colonic motor function caused by ICV CRF or water avoidance stress were reduced by NBI35965 in rats. CP154526 or NBI35965 also attenuated the heightened visceral hypersensitivity in adult rats exposed to early life stress (maternal separation).
2c (ii) Drug and Alcohol Addictions
Hyperactivation of CRF1 receptors has been associated with drug addiction disorders and CRAs have been proposed as potential treatments (Koob, 2008a, 2008b). A major risk factor for relapse to drug abuse is the occurrence of withdrawal symptoms, including anxiety. Anxiety-like symptoms during withdrawal from drugs of abuse, such as cocaine, amphetamine, and morphine is accompanied by elevated CRF release, effects that can be attenuated by CRAs. Antalarmin was also shown to block the conditioned place aversion produced by naloxone-precipitated opiate withdrawal in rats (Stinus, Cador, Zorrilla, & Koob, 2005). By decreasing vulnerability to relapse during withdrawal and possibly prolonged abstinence from drugs of abuse, CRAs may be useful as treatments for drug addictions.
Alcohol dependence has also been associated with hyperactivation of CRF1 receptors (for review, see (Heilig & Koob, 2007). R121919 and MJL-1-109-2 decreased the elevated ethanol self-administration during acute withdrawal in ethanol-dependent rats. MTIP blocked withdrawal anxiety in alcohol-dependent rats and in rats genetically bred for high alcohol intake (Gehlert, et al., 2007) whereas MTIP was without effect on anxiety or on ethanol intake in normal (non-dependent) rats. These findings are further evidence supporting the conclusion that CRAs do not alter behavior in a basal, unstressed state. Rats genetically bred for high alcohol preference show a dysfunctional upregulation of CRF1 receptors and the lowered threshold for stress-induced reinstatement of alcohol seeking was reversed by antalarmin (Hansson, et al., 2006). These and other findings from preclinical studies indicate that CRAs may have utility in the treatment of alcohol addiction.
3. Clinical Status of CRF1 Receptor Antagonists
This section will review the status of CRAs that are in development for anxiety, depression, and other stress related disorders. The reader is referred to Table 1 for currently available information and references. Note that individual clinical studies on compounds that are being tested in humans are generally registered on the website “clinicaltrials.gov” prior to study initiation and are identified as such in Table 1.
3a. Anxiety Disorders
As summarized in Table 1, there is limited information, particularly actual clinical results, available to adequately evaluate the hypothesis that CRAs are anxiolytic in humans. However, as noted in Table 1, several agents are either being tested in, or have completed, Phase II clinical efficacy trials in different anxiety disorders.
GlaxoSmithKline, in collaboration with Neurocrine Biosciences, reported two compounds in clinical testing for anxiety disorders. GW876008 was evaluated in a Phase II double-blind, placebo controlled trial for the treatment of Social Anxiety Disorder, and was reported by Neurocrine Biosciences on its website to show no difference relative to placebo, though no further details are currently available.
Pexacerfont (BMS-562086) was tested in an 8 week, randomized, double-blind, placebo-controlled and active comparator (escitalopram, 20 mg/day) trial in GAD (Coric, et al., 2010). Pexacerfont (100 mg/day) was not significantly different from placebo on the primary outcome measure, the mean change from baseline to end point in the Hamilton Anxiety Scale total score. In this trial, the comparator escitalopram demonstrated significant efficacy relative to placebo. Pharmacokinetic assessment in 88 patients in the pexacerfont group demonstrated that 93% exceeded the projected human efficacious concentration (500 nM) at the end of week 1, and this was sustained until the end of the study.
A notable finding was that pexacerfont did not decrease the basal levels of salivary cortisol. Although this was interpreted as a positive attribute from the perspective of the drug’s safety profile, it was also acknowledged that it could represent an inadequate CRA dose, since pituitary CRF1 receptor blockade might be expected to diminish cortisol output. The authors acknowledged that there was a dose-limitation based on toxicological findings, precluding evaluation of a higher pexacerfont dose in a future clinical trial.
3b. Depressive Disorders
There are a number of CRAs being explored in clinical trials for potential utility in treating Major Depressive Disorder, but, as with the anxiety indication, there are currently very limited results and data available to evaluate the hypothesis. Neurocrine Biosciences, in collaboration with Janssen Pharmaceuticals, published the results of an open-label trial demonstrating potential antidepressant activity of R121919 in a small group of patients with Major Depressive Disorder. In this study, R121919 did not negatively impact the HPA axis, findings which were cited as important for support for the safety profile of the compound. Interestingly, in this study, R121919 did not reverse the stimulatory effect of intravenously administered CRF on ACTH release, at several times during this chronic study. Based on preclinical studies, a reduction might have been expected. However, an attenuating effect cannot be ruled out, in that there is no positive control (i.e. effect assessed in a placebo-treated group) needed for such a comparison. Unfortunately, this compound was discontinued because of abnormal liver function testing so further clinical data were not gathered.
Pfizer recently published the results of a Phase II, multi-center trial using the CRA CP-316,311 in MDD. A total of 123 patients were treated with 400 mg of CP-316,311 twice daily, or 100 mg of sertraline daily, or placebo in a 6-week fixed-dose, double-blind, placebo and sertraline-controlled trial. In an interim analysis, CP-316,311 was deemed safe and well tolerated in this study population, but it failed to demonstrate efficacy. In contrast, the positive control, the SSRI sertraline, was active and therefore the trial was discontinued. Based on preclinical studies utilizing receptor occupancy assessments and behavioral endpoints (efficacy in fear potentiated startle), the authors argued that an adequate dose was used to test for efficacy. However, no data in an antidepressant model were cited in this publication. Previous work has demonstrated that a CRA can have an anxiolytic-like action but, in the same animal and at the same dose, lack an apparent antidepressant-like effect, a finding which emphasizes the need to identify animal models that have appropriate translational value for the disorder being considered. The lack of an adequate PET imaging ligand for determining CRF1 receptor occupancy in human studies is another hurdle facing future clinical trials of CRAs.
The CRA pexacerfont (Bristol Myers Squibb) was tested in a randomized, double-blind placebo-controlled Phase II trial in women with MDD (n=260). Trial results were reported in a poster presentation at the December 2008 meeting of the American College of Neuropsychopharmacology (Vladamir Coric,, M.D., personal communication). In this study, subjects were treated for 8 weeks with pexacerfont (200 mg/day for week 1, then 100 mg/day through weeks 2–8, a dose regimen predicted to produce >80% receptor occupancy), placebo or the SSRI escitalopram (10 mg/day for 1 week; 20 mg/day for weeks 2–8). By the end of week 1, pexacerfont attained targeted plasma concentrations of > 500 nM (targeted plasma trough concentrations based on animal studies in anxiety models) which were sustained throughout the study. From a safety perspective, pexacerfont was well tolerated and no serious adverse events were reported. Efficacy assessment revealed that pexacerfont was inferior to placebo in change in the Hamilton Depression (HAMD)-17 scale from baseline to endpoint, however the comparator escitalopram (an SSRI) was not significantly active. Furthermore, neither pexacerfont nor escitalopram produced significant remission rates. In summary, pexacerfont was not effective in the treatment of MDD and was actually worse than placebo on some measures. The authors did note that the results of this trial should be tempered by several findings: first, this trial technically qualified as a “failed” trial in that the comparator escitalopram was not active. Second, the inability to assess human receptor occupancy did not allow for an assessment of the degree of actual CRF1 receptor occupation, and, given that only a single dose was used, it is possible that a higher dose may have been needed for efficacy. The targeted plasma concentration of 500 nM was based on the trough concentrations of pexacerfont accompanying efficacy in two rodent anxiolytic models, with simulations indicating that the dose regimen used would produce steady-state concentrations that would result in > 80% receptor occupancy in most patients within 1 week. Again, it is clear that the lack of robust, predictive animal model of antidepressant activity and the lack of a suitable PET ligand for assessing human receptor occupancy are major impediments to the assessment of novel antidepressant compounds.
Other CRAs were reported to be in clinical studies for depression. GlaxoSmithKline in collaboration with Neurocrine Biosciences reported Phase I (GSK586529) and Phase II (GSK561679; registered August 2008) trials in Major Depressive Disorder. Ono Pharmaceuticals listed ONO-2333Ms as a clinical compound and reported in July of 2008 that the program was discontinued because of “lack of efficacy” though no further details are currently available. SSR-125543 (Sanofi-Aventis) was reported by the company to be in Phase I evaluation.
3c. Other Stress-Related Disorders
Although not a primary focus of the current review, it is notable that several of the compounds described above are in clinical trials for Irritable Bowel Syndrome (IBS). There is an important contribution of stress to IBS, and anxiety is comorbid in a large percentage of these patients (Lydiard, 2001, 2005, 2007). Therefore, the outcomes of these studies will be of interest to compare with the data from the anxiety and depression trials.
In a Phase II study, oral pexacerfont (25 mg and 100 mg qd) was evaluated in a randomized, double-blind, placebo-controlled two-week study for its effects on colonic transit and bowel function in female patients with diarrhea-predominant irritable bowel syndrome (D-IBS)(Sweetser, et al., 2009). The 100-mg dose was comparable to a dose that inhibited colonic motility in stressed rats. No safety issues were identified but the compound did not significantly alter colonic or other regional transit or bowel function. The authors remarked that the role of central and peripheral CRF1 receptors in bowel function in D-IBS requires further study.
GW876008 is reported as a completed Phase II trial but results are not yet published. Finally, data on clinical validation of treating alcohol or drug addictions are not yet available.
3d. Biomarkers and Experimental Medicine Models
It is apparent from the previous section that one of the challenges faced in the clinical development of CRAs is overcoming the uncertainty involved in dose selection. At the level of animal models, ex vivo receptor occupancy studies can be performed to determine the plasma levels of compound that are associated with a given level of receptor occupancy in the targeted brain areas (e.g. cortex), and the level of receptor occupancy is derived from the active doses in relevant therapeutic models. A major disconnect to human testing is the inability to directly evaluate receptor occupancy in the human brain because of the lack of an appropriate positron emission tomography (PET) ligand. Discovering such a ligand will be extremely beneficial, but until that point is reached, other approaches may also be useful in helping to choose the clinical dose.
One such approach is to use brain imaging technology to measure the effects of a CRA on regional brain activity. This approach was demonstrated in a recent study in which 30- and 200-mg doses of the CRA R317573 were evaluated for their acute effects on regional cerebral glucose metabolism (rCMglu) using [18F] fluoro-2-deoxy-D:-glucose (FDG) PET in 12 healthy male volunteers rCMglu (Schmidt, et al., 2010). In this study, R317573 produced dose-related, brain region-dependent changes in rCMglu. Relative to placebo, both doses increased rCMglu in frontal cortical regions whereas decreases occurred in the putamen and right amygdala, regions that may be behaviorally relevant to depression and anxiety. One shortcoming of this technique is that it is not known if the observed effects are attributable to CRF1 receptor occupation in the specific regions in which changes occurred. Another concern is with regard to interpretation of the pattern of regional changes seen. Ideally, one would like to use specific patterns of changes in regional activity as a surrogate for antidepressant or anxiolytic action, but this level of analysis is not currently possible. Nevertheless this approach may have some utility for defining pharmacologically active doses of CRAs.
A second approach for determining dose selection is to measure the effects of a CRA on a specific hormone biomarker. This approach is illustrated by a study in which NBI-34041, a CRA reported to be in Phase I testing, was shown to reduce social stress-induced HPA axis activation induced by a psychological stressor, as measured using the Trier Social Stress Test (Ising, et al., 2007). As it is well-documented that stress-induced release of ACTH (from the anterior pituitary) and corticosterone (from the adrenal cortex) is a downstream consequence of activation of pituitary CRF1 receptors, these data provide key evidence that the compound had in vivo activity consistent with blockade of CRF1 receptors. Note, however, that there are several complications for the interpretation of these findings. First, as the reduction in stress-induced hormone release may be primarily due to CRA action on the CRF1 receptors in the anterior pituitary, which are outside of the blood-brain barrier, it may not tell us anything about the level of receptor occupancy in the brain (which presumably is primarily responsible for therapeutic action). Second, it has been shown in animal studies that central blockade of CRF1 receptors can reduce stress-induced HPA axis activation and therefore it is difficult to determine the relative contributions of central and peripheral sites of action. In addition, it has been demonstrated that there is redundancy in the HPA axis such that the peptide hormone vasopressin may potentially compensate for deficiencies in CRF signaling. Thus, particularly in a chronic dosing study, the effects of a CRA on ACTH or cortisol levels may be masked by compensatory changes mediated by vasopressin. Acknowledging these criticisms and caveats, this hormonal biomarker approach may nevertheless provide useful (though perhaps limited) functional information in humans regarding the CRF1 receptor blockade.
A third approach that can be useful for dose selection is to evaluate the CRA in a preliminary experimental medicine model of the target disorder. The CRA’s activity in such a model will then theoretically translate to a full-scale clinical trial in a patient population. A recent example of this approach is the evaluation of the CRA R317573 in healthy human subjects using the 7.5% CO2 model of anxiety (Colin Dourish, Gerry Dawson, personal communication). In this model, a subject breathes (through a face mask) a mixture comprised of 7.5% CO2, which produces an anxiety response which can be reduced by anxiolytic agents. Activity in this model is thought to predict potential utility in treating anxiety disorders, particularly GAD (Bailey, Argyropoulos, Kendrick, & Nutt, 2005; Bailey, Argyropoulos, Lightman, & Nutt, 2003; Bailey, Kendrick, Diaper, Potokar, & Nutt, 2007; Bailey & Nutt, 2008). In a poster presentation at the 2009 Society for Neuroscience Meeting by the British company P1Vital (Dawson, et al., 2009) it was reported that R317573 (40 mg, daily for 7 days) administered to 32 healthy volunteers was safe, well-tolerated, and reduced anxiety induced by 7.5% CO2. The plasma levels corresponding to the anxiolytic effect could be determined and used for subsequent clinical trials. As there are no additional clinical data on R317573 in treating GAD, it is not known how activity in the CO2 model translates to the target population for this compound in particular and the CRF1 receptor antagonist mechanism in general. Furthermore, it would be of interest to know how other CRAs tested in GAD (e.g. pexacerfont) would perform in the CO2 model, using the doses tested in the clinic. Finally, it is noteworthy that a more intense anxiety state can be induced by breathing a higher 35% concentration of CO2 (Bailey, et al., 2003) and reversal of this effect may be related to utility in treating panic disorder. It would be of interest to evaluate R317573 at this higher CO2 concentration to determine potential utility in treating panic disorder.
3e. Summary
Despite intensive pharmaceutical efforts in this area, there are limited clinical data currently available regarding the therapeutic utility of CRAs in treating depression, anxiety and other stress-related disorders. It is well-accepted that clinical evaluation in this area, particularly for antidepressants, is challenging given a high failure rate of clinical trials due to issues such as high placebo rate and difficulty in predicting clinical doses from animal models. Furthermore, the trials announced thus far have been performed under highly prescribed conditions, i.e. using a limited number of compounds, doses, subjects, target populations, etc. Taken together, these considerations and the relative paucity of published clinical data preclude making strong conclusions at this time regarding the efficacy of CRAs in treating depression, anxiety, and other stress-related disorders.
Nevertheless, given the high level of expectation generated that agents with this novel mechanism of action might be clinically robust, the present data set indicating lack of efficacy in treating psychiatric disorders is, overall, not encouraging. For depression, initial positive findings in a small, exploratory open-label trial in MDD must be tempered by negative findings in two subsequent larger placebo-controlled trials with active comparators, though in one of those trials the comparator did not produced the expected antidepressant effect. For the published GAD trial, the active comparator did produce a statistically-significant effect, and therefore the lack of even a trend for efficacy is discouraging for utility in this anxiety disorder. Given the lack of a suitable PET ligand for assessing brain CRF1 receptor occupancy, a major challenge encountered in these trials is the difficulty in precisely defining the predicted efficacious dose range. In this regard, it is encouraging that new research is exploring the utility of biomarkers and experimental medicine models which may help in defining clinical doses as well as predicting efficacy in treating the targeted disorder.
4. Translational Assessment of Animal Models & Future Directions
Although there are limited clinical data available at this time, the recent reports of lack of efficacy of CRAs in placebo-controlled Phase II/III clinical trials in patients with MDD (CP-316,311, pexacerfont) and GAD (pexacerfont) are not encouraging for the CRF1 receptor mechanism in treating these specific psychiatric disorders. Negative results in treating IBS are also not encouraging for this stress-related disorder. Although evidence for statistically significant efficacy of the CRA R121919 in treating MDD was noted in a Phase II clinical trial, the fact that the trial was open-label, lacked a placebo group and lacked a positive comparator make these results less compelling relative to those obtained from double-blind, placebo controlled trials with an active comparator. Acknowledging the limited availability of recent data with pexacerfont for in-depth analysis, it is nevertheless useful at this time to perform an interim assessment, reviewing some “what if” scenarios, and some potential implications for evaluations of CRAs in animal models.
As noted earlier, one of the striking findings from preclinical work was the general trend for lack of activity of CRAs in certain animal models of anxiety and depression that were run under fairly standardized conditions that may not have been sufficiently stressful to detect their efficacy. Elevating endogenous stress levels by various means (administration of exogenous CRF, exposure to prior stressors, use of genetic strains that are bred for high stress traits or excessive CRF tone) subsequently revealed efficacy of these agents in a number of situations.1 A fundamental question, therefore, is what relevance (if any) do these findings have to the clinical utility of CRAs in treating human stress disorders? There are a number of possible answers and some key ones will be proposed below along with potential implications for further refinement of animal models.
One scenario is that animal models that were adapted to detect activity of CRAs were indeed sufficient to predict their clinical efficacy, but the clinical trials performed thus far have simply not been adequate to demonstrate proof of concept. The advanced depression and anxiety trials reported above had SSRI comparators which served to validate two of the three trials, but the CRAs used may not have been optimized to fully test the hypothesis. The lack of an adequate biomarker for central action, including a PET ligand to allow an estimation of receptor occupancy following oral dosing, is a substantial impediment to the clinical evaluation of CRAs. In addition, given that CRAs don’t profile like standard antidepressants and anxiolytics in animal assays, there is the obvious difficulty in establishing which sensitive assay to use and therefore what predicted plasma (and brain) concentrations should be targeted in the clinical trials to achieve efficacy. It is entirely possible that adequate levels of receptor occupation have not been achieved in these clinical studies to achieve the desired efficacy. Clearly, future work is needed to identify better preclinical and clinical translational models, including predictive biomarkers, to improve our ability to predict efficacious plasma concentrations. Encouraging progress is being made in the use of experimental medicine models in early clinical development to both boost confidence that a CRA will have efficacy in the target population in advanced clinical trials and to establish efficacious plasma concentrations.
Another scenario is that the standard animal models which don’t require special conditions of stress and which are consistently sensitive to marketed anxiolytics and antidepressants but not CRAs, are correct in telling us that CRAs will not be effective in broadly treating anxiety and/or depression in humans. One undeniable challenge of discovering and developing new therapeutic agents with novel, clinically-untested mechanisms of action is that such agents may truly provide a “reality check” on the translational value (i.e. clinical predictability) of the animal models used to detect their efficacy. Do the models have limited predictive validity, only identifying compounds with certain mechanisms of action? Or are the models more robust, more “agnostic”, identifying therapeutically active agents that are mechanistically diverse?2 These are important questions that need to be continually reevaluated if progress is to be made in identifying new therapeutic treatments. It is inevitable that there will be casualties as a result of this process, either in the form of models which prove to be inadequate, and/or novel compounds which prove to lack efficacy, but these outcomes are to be expected and should be viewed in their proper perspective, to provide data for continued scientific progress.
As noted in the Introduction, there is a considerable degree of redundancy wired into the stress response system (SRS), and therefore, one explanation for the lack of activity of CRAs in standard antidepressant and anxiolytic models is that CRF1 receptor blockade alone is not sufficient to produce efficacy because of compensatory contributions of these other players. An implication is that CRF1 receptor antagonism in combination with other mechanisms, such as blockade of vasopressin receptors, may be required to fully express therapeutic efficacy in anxiety and depression.
It was beyond the scope of the current manuscript to comprehensively review the extensive literature on the behavioral effects of peptidic agonists and antagonists, but it is important to acknowledge many studies have elucidated potential contributions of CRF2 receptors to the anti-stress actions peptide antagonists. The precise roles of CRF2 receptors is complex and controversial, but some lines of evidence suggest that a CRF2 receptor antagonist and/or a dual-acting CRF1/CRF2 receptor antagonist might be a desirable therapeutic approach to treating psychiatric disorders and even be superior to targeting the CRF1 alone. Further exploration of this and other possibilities involved CRF2 receptors is clearly warranted.
If lack of activity in standard antidepressant and anxiolytic models is predictive of lack of efficacy in treating MDD and GAD, what, if anything is the significance of CRA efficacy in models in which high levels of stress presumably induce an activation of CRF pathways? While it is possible that activity of CRAs in “high stress” models is clinically irrelevant, an alternative explanation is that CRAs may prove to have clinical utility in alternative indications and/or certain subpopulations or subgroups of patients with mood and/or anxiety disorders, in which dysfunctionally overactivated CRF pathways are uniquely present. A growing body of preclinical literature briefly reviewed herein points to the potential utility of CRAs in treating drug and alcohol addictions, though big questions remain: How robust are the profiles of CRAs in these models? Do the models tell us that these agents could potentially be highly efficacious in these indications? Further preclinical effort in this area along with controlled clinical trials is needed to provide answers to these questions.
As noted earlier, there are numerous subclassifications of anxiety disorders, and currently, animal models are not able to reliably differentiate agents that uniquely target these different disorders. Increasing attention is being directed toward modeling, and understanding the relative contributions of CRF pathways to, diffuse, non-specific anxiety versus intense, cue-specific fear. Progress is being made in further understanding the complex neural mechanisms underlying both the genesis and the control of fear conditioning and the role of CRF1 receptors. Data from animal studies indicate that CRAs have effects in certain fear conditioning processes, though much work needs to be done to elaborate the role of CRF1 receptors and understand how this might relate to anxiety disorders such as PTSD. Exposure to a traumatic stressor is the sine qua non of PTSD, and therefore it is reasonable to predict that CRAs, which are effective in reducing the effects of excessive stress in animal models, might have clinical utility in the treatment of this disorder. There are potentially multiple ways in which CRAs may be used to treat PTSD. As PTSD is a chronic condition, CRAs may be useful in ameliorating the enduring symptoms if hyperactivation of CRF1 pathways is present. If CRF1 hyperactivation is important for the acquisition and/or consolidation of dysfunctional long-term aversive memories, then it is also possible that CRAs administered beginning shortly after the occurrence of the stressor might be beneficial in preventing the development of chronic PTSD. Indeed, some recent work with a predator stress animal model supports the idea that CRAs may have potential as prophylactic treatments in disorders like PTSD in which there is an exposure to a defined, intense stressor (Adamec, et al., 2010). It is of interest to note that the CRA GSK561679 is reported on clinicaltrials.gov to be in clinical testing for the treatment of PTSD in women though no data are currently available from this trial. In addition, new breakthroughs in pharmacological approaches to overcoming phobias by enhancing extinction processes (Davis, Barad, Otto, & Southwick, 2006; Davis, Myers, Chhatwal, & Ressler, 2007) or ameliorating intrusive negative memories by interfering with memory reconsolidation (Debiec, Doyere, Nader, & Ledoux, 2006; Diergaarde, Schoffelmeer, & De Vries, 2006; P. E. Gold, 2006; Nader, Schafe, & Le Doux, 2000; Pitman & Delahanty, 2005) provide new avenues for exploring agents with novel mechanisms of action like CRAs.
As also previously mentioned in this review, elevations in central levels of CRF and/or changes in reactivity of the HPA axis are measured in a diverse number of disorders, including PTSD, borderline personality disorder, some depressed patients, but the changes are not generally seen in all subjects, and may be particularly prominent in patients with certain characteristics. For example, elevated CRF may be particularly prominent in those patients with severe psychopathology, such as melancholic depression or depression with psychotic features and therefore one possibility is the CRAs will be particularly useful in treating adult psychopathology in these situations of severe illness. As these extreme conditions are challenging to model experimentally, an emphasis should be placed on explicitly demonstrating the presence of hyperactive CRF1 pathways in relevant animal models.
Another factor that may possibly contribute to hyperactivation of CRF1 pathways is a prior history of childhood trauma. As seen in preclinical studies, exposure to maternal separation is a potent stressor in animals that produces a dramatic acute stress response that is clearly mediated by CRF1 receptors. Furthemore, evidence indicates that repeated, subchronic activation of this otherwise normal, adaptive stress response produces evidence for marked, long-lasting dysfunctions in brain and HPA axis CRF1 pathways, and evidence for psychopathology in the form of heightened anxiety and depressive like symptoms in adulthood. In this context, it is important to note the rapidly expanding clinical literature emphasizing the profound, long lasting impact of exposure to early life trauma on adult psychopathology. For example, a review by Anda and colleagues reported a “dose-relationship” between exposure to childhood traumatic events and the presence of a wide variety of psychopathology in adulthood, including addictive behaviors, aggression, depression, and anxiety-like behaviors.
These results may help simplify some otherwise apparent unconnected observations. A history of childhood trauma may give rise to a vulnerability to develop a variety of different psychopathologies in adulthood. The precise type of pathology which can develop may depend on when in development the trauma occurs, on exposure to other types of environmental stressors, and/or genetic factors. Childhood traumatic stressors may cause excessive activation of stress responses mediated by CRF1 pathways that can have a lasting impact into adulthood, increasing the vulnerability of individuals to develop a range of adult psychopathologies. Thus, an implication is that CRAs may be useful for treating this subpopulation of individuals exposed to childhood trauma.
One of the fundamental questions in the field of stress-related psychopathology is: Why does one individual exposed to a specific set of defined traumatic environmental events go on to develop psychopathology (e.g. PTSD or depression) whereas another individual similarly exposed does not? Conversely, why do some individuals exposed to severe, extreme degrees of trauma avoid developing psychopathology? These questions have lead to considerable research into what factors contribute to vulnerability to develop psychopathology, as well as those factors that may lead to resilience (Charney, 2004; Yehuda & Flory, 2007). In the field of stress-related disorders, emerging data suggest that both genetic and environmental factors are important.
A report of an ongoing large clinical study in Atlanta called the “Grady Trauma Project” may shed some light on the factors influencing vulnerability and resilience, particularly with regard to role of CRF1 receptors (Binder & Nemeroff, 2009; Bradley, et al., 2008). This study reported evidence for a gene × environment interaction such that individuals who possess certain polymorphisms for the CRF1 receptor gene and have a history of early life trauma have a greater probability for have depression as adults. Conversely, presence of a different set of polymorphisms appear to actually “protect” the individual exposed to early life trauma against the development of depression as an adult. Further work in this area is clearly needed to understand this potential relationship between genes and environment, and this should include work with genetically-based animal models. Recent studies have begun to study the association of variants in genes that regulate the CRF system and treatment response to the SSRI antidepressant citalopram (Binder, et al., 2010). One could speculate that CRF1 polymorphisms present in certain psychiatric populations could have an impact on the efficacy of CRAs, though there currently are no data to support this hypothesis. In summary, there is a growing body of evidence supporting an important role of CRF1 pathways in childhood in the etiology of adult psychopathology and a potential influence of CRF1 receptor polymorphisms which may lead to new insights into novel treatment paradigms involving CRAs.
A clear challenge for both preclinical and clinical researchers exploring the neurobiology of mood and anxiety disorders is to better define the conditions under which CRF1 pathways are aberrantly hyperactivated and how CRAs may be effective in their normalization. Promising preclinical approaches include modeling early life stress to explore the long term developmental impact of trauma, and fear conditioning models in adult animals in which the contributions of different phases of aversive memory formation can be elucidated. This information, along with an understanding of the impact on normal brain function, will be important for obtaining a clearer understanding of how CRAs may be useful in the treatment of stress-related CNS disorders.
5. Summary
CRF1 pathways play a key role in the stress response system that is vital for an organism’s survival, and antagonism of CRF1 receptors provides a rationale approach for treating CNS disorders that may result from dysfunctional CRF hyperactivation. A substantial amount of data has been generated with a variety of CRAs in diverse animal models designed to detect antidepressant, anxiolytic, and anti-stress activity. In general, CRAs have a unique phenotype in animals that has both similarities to, and differences from, those of classic antidepressants and anxiolytics. From a safety perspective, CRAs tend to have benign behavioral profiles, consistent with the notion that CRF1 receptors are normally in a state of low basal activation. CRAs have been well-tolerated in humans, based on available clinical trial results with several agents. In animals, CRAs reduce stressor-induced activation of the HPA axis, effects presumably mediated by actions at pituitary (and possibly brain) CRF1 receptors, results that are also demonstrated in human studies in normal healthy volunteers. Reductions in hyperactive HPA axis activity may be important for reducing chronic stress-induced pathology. In animal models sensitive to anxiolytics and/or antidepressants, CRAs tend to be more effective in normalizing behavioral changes when high stress levels are a factor; conditions which presumably give rise to sustained, excessive activation of brain CRF1 receptors. In animal models of conditioned fear, CRAs may be useful in procedures involving context conditioning. Limited Phase II/III clinical trial results with two compounds, CP-316,311 and pexacerfont, suggest lack of efficacy in treating MDD and/or GAD, though a complete analysis (including an assessment the predictive validity of animal models) awaits full availability of these data.
The general lack of efficacy of CRAs in standard animal models of depression and anxiety may reflect the contribution of compensatory mechanisms attributable to redundancy in the SRS, and as such, therapeutic activity of CRF1 receptor antagonism may require combination with other mechanisms such as blockade of vasopressin receptors or CRF2 receptors. However, given their demonstrated activity in “high stress” animal models, CRAs may prove to have therapeutic utility in disorders in which defined traumatic stressors play a clear etiological role (e.g. posttraumatic stress disorder, early life trauma, withdrawal/abstinence from addictive substances), though much work is need to understand how these agents would best be utilized in their treatment. For example, being able to define with varying degrees of precision specific traumatic events may allow early treatment interventions with CRAs that could prevent or greatly inhibit the development of dysfunctional and disabling aversive memories seen in PTSD and panic disorder and possibly interfere with progressive pathology and deterioration produced by chronic stress. New findings regarding the genetic, developmental and environmental factors that contribute to stress-related psychopathology are providing insights into subpopulations that might particularly benefit from CRAs, and new avenues for improving the translational value of animal models.
Acknowledgments
The authors would like to thank our many colleagues for helpful discussions, and Betsy Kehne for her expert graphics assistance. This research was supported by the National Institute of Mental Health (NIMH) Grant No. F32MH077458 to Christopher K. Cain.
LIST OF ABBREVIATIONS
- CRA
CRF1 receptor antagonist
- CRF
corticotropin releasing factor
- HPA
hypothalamic-pituitary-adrenal
- SRS
stress response system
- ACTH
adrenocorticotropic Hormone
- CSF
cerebrospinal fluid
- ICV
intracerebroventricular
- IV
intravenous
- FC
fear conditioning
- BNST
bed nucleus of the stria terminalis
- GAD
generalized anxiety disorder
- SAD
social anxiety disorder
- PTSD
post-traumatic stress disorder
- OCD
obsessive compulsive disorder
- CNS
central nervous system
- NE
norepinephrine
- 5HT
serotonin
- GABA
gammaaminobutyric acid
- IBS
irritable bowel syndrome
- STM
short-term memory
- LTM
long-term memory
- SSRI
selective serotonin reuptake inhibitor
- SNRI
serotonin/norepinephrine reuptake inhibitor
- MAOI
monoamine oxidase inhibitor
- FSL
Flinders sensitive line
- FRL
flinders resistant line
- MDD
major depressive disorder
- BLA
basolateral amgydala
- CE
central amygdala
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Although trends were identified for CRAs as a class (e.g. “CRAs worked in Model A but not Model B”) there were examples of compounds which were exceptions to these generalizations which need to be accounted for. To this end, one needs to understand the CRA’s pharmacokinetics to determine if dosing conditions were optimized to detect efficacy. Furthermore, CRAs are allosteric inhibitors at CRF1 receptors, and there may be subtle differences in pharmacodynamics between compounds that could contribute to different in vivo profiles.
Evaluating the predictive validity of animal models used to identify potential antidepressants and anxiolytics is complicated by the concern that many models do not accurately reflect the onset of clinical effect. All currently marketed antidepressants require chronic administration before their therapeutic effects can be fully realized. As some antidepressant models are sensitive to agents administered acutely, these models may be better tuned to identifying acute pharmacological actions from a specific class of related mechanisms (e.g. potentiation of monoamines, by uptake blockade or by monoamine oxidase inhibition). Such models may be insensitive to novel mechanisms such as CRF1 receptor antagonism. Benzodiazepines such as triazolam or chlordiazepoxide, which are immediately active in treating anxiety disorders, have been traditionally used to validate anxiolytic models such as elevated plus maze or conflict testing. However, monoamine reuptake inhibitors (SSRIs, SNRIs), some of which are considered first-line treatments for anxiety disorders such as GAD, SAD, and/or PTSD, also require chronic administration for clinical efficacy. These agents have not always been adequately compared to benzodiazepines in animal models of anxiety, and hence the sensitivity of the animal models for detecting novel (e.g. nonbenzodiazepine) mechanisms of action may be unclear. Regardless, the argument has been set forth that the standard animal models have been adapted by introducing greater levels of stress and therefore have now become sensitive to a novel mechanism of action, CRF1 receptor antagonism.
Statement of Potential Conflict of Interest
JHK was previously employed at Neurogen Corporation and during part of that time was a project leader in a CRA program.
Note Added in Proof: In a press release dated 9/14/10, Neurocrine Biosciences, Inc. (www.neurocrine.com) announced top-line efficacy and safety results from a Phase II, double blind, placebo-controlled clinical trial with the CRA GSK561679 in patients with major depressive disorder in which 150 patients received 350 mg of GSK561679 daily and placebo. Statistical analyses of the primary endpoint, change from baseline in the Bech Melancholia scale at Week 6, and a key secondary endpoint, change from baseline in the HAMD-17 scale at Week 6, revealed no benefit of GSK561679 compared with placebo in the intent to treat population of 145 patients Safety assessment revealed that GSK561679 had no significant adverse events and was generally well tolerated. It was also stated that three separate academic collaborative clinical trials are ongoing to evaluate the effects of GSK561679 in post traumatic stress disorder, anxiety and alcoholism.
References
- Adamec R, Fougere D, Risbrough V. CRF receptor blockade prevents initiation and consolidation of stress effects on affect in the predator stress model of PTSD. Int J Neuropsychopharmacol. 2010;13:747–757. doi: 10.1017/S1461145709990496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera G, Nikodemova M, Wynn PC, Catt KJ. Corticotropin releasing hormone receptors: two decades later. Peptides. 2004;25:319–329. doi: 10.1016/j.peptides.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Alonso R, Griebel G, Pavone G, Stemmelin J, Le Fur G, Soubrie P. Blockade of CRF(1) or V(1b) receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol Psychiatry. 2004;9:278–286. 224. doi: 10.1038/sj.mp.4001464. [DOI] [PubMed] [Google Scholar]
- Altemus M, Pigott T, Kalogeras KT, Demitrack M, Dubbert B, Murphy DL, Gold PW. Abnormalities in the regulation of vasopressin and corticotropin releasing factor secretion in obsessive-compulsive disorder. Arch Gen Psychiatry. 1992;49:9–20. doi: 10.1001/archpsyc.1992.01820010009002. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Skelton KH, Thrivikraman KV, Plotsky PM, Schulz DW, Owens MJ. Chronic administration of the selective corticotropin-releasing factor 1 receptor antagonist CP-154,526: behavioral, endocrine and neurochemical effects in the rat. J Pharmacol Exp Ther. 2000;294:588–597. [PubMed] [Google Scholar]
- Austin MC, Janosky JE, Murphy HA. Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol Psychiatry. 2003;8:324–332. doi: 10.1038/sj.mp.4001250. [DOI] [PubMed] [Google Scholar]
- Bailey JE, Argyropoulos SV, Kendrick AH, Nutt DJ. Behavioral and cardiovascular effects of 7.5% CO2 in human volunteers. Depress Anxiety. 2005;21:18–25. doi: 10.1002/da.20048. [DOI] [PubMed] [Google Scholar]
- Bailey JE, Argyropoulos SV, Lightman SL, Nutt DJ. Does the brain noradrenaline network mediate the effects of the CO2 challenge? J Psychopharmacol. 2003;17:252–259. doi: 10.1177/02698811030173002. [DOI] [PubMed] [Google Scholar]
- Bailey JE, Kendrick A, Diaper A, Potokar JP, Nutt DJ. A validation of the 7.5% CO2 model of GAD using paroxetine and lorazepam in healthy volunteers. J Psychopharmacol. 2007;21:42–49. doi: 10.1177/0269881106063889. [DOI] [PubMed] [Google Scholar]
- Bailey JE, Nutt DJ. GABA-A receptors and the response to CO(2) inhalation - A translational trans-species model of anxiety? Pharmacol Biochem Behav. 2008;90:51–57. doi: 10.1016/j.pbb.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Bakish D. The patient with comorbid depression and anxiety: the unmet need. J Clin Psychiatry. 1999;60:20–24. [PubMed] [Google Scholar]
- Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J Neurosci. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, Watkins LR, Maier SF. Controllable versus uncontrollable stressors bi-di rectionally modulate conditioned but not innate fear. Neuroscience. 2007;146:1495–1503. doi: 10.1016/j.neuroscience.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Lucero TR, Amat J, Watkins LR, Maier SF. Role of the ventral medial prefrontal cortex in mediating behavioral control-induced reduction of later conditioned fear. Learn Mem. 2008;15:84–87. doi: 10.1101/lm.800308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH, Chorpita BF, Turovsky J. Fear, panic, anxiety, and disorders of emotion. Nebr Symp Motiv. 1996;43:251–328. [PubMed] [Google Scholar]
- Belanoff JK, Kalehzan M, Sund B, Fleming Ficek SK, Schatzberg AF. Cortisol activity and cognitive changes in psychotic major depression. Am J Psychiatry. 2001;158:1612–1616. doi: 10.1176/appi.ajp.158.10.1612. [DOI] [PubMed] [Google Scholar]
- Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol Psychiatry. 2010;15:574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Owens MJ, Liu W, Deveau TC, Rush AJ, Trivedi MH, Fava M, Bradley B, Ressler KJ, Nemeroff CB. Association of polymorphisms in genes regulating the corticotropin-releasing factor system with antidepressant treatment response. Arch Gen Psychiatry. 2010;67:369–379. doi: 10.1001/archgenpsychiatry.2010.18. [DOI] [PubMed] [Google Scholar]
- Binneman B, Feltner D, Kolluri S, Shi Y, Qiu R, Stiger T. A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. Am J Psychiatry. 2008;165:617–620. doi: 10.1176/appi.ajp.2008.07071199. [DOI] [PubMed] [Google Scholar]
- Bissette G, Klimek V, Pan J, Stockmeier C, Ordway G. Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology. 2003;28:1328–1335. doi: 10.1038/sj.npp.1300191. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. Journal of Comparative Physiological Psychology. 1969:67. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Defensive reactions in the albino rat. Learning and Motivation. 1971;2:351–362. [Google Scholar]
- Blanchard RJ, Fukunaga KK, Blanchard DC. Environmental control of defensive reaction to footshock. Bulletin of the Psychonomic Society. 1976;8:129–130. [Google Scholar]
- Blanchard RJ, Mast M, Blanchard DC. Stimulus control of defensive reactions in the albino rat. J Comp Physiol Psychol. 1975;88:81–88. doi: 10.1037/h0076213. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Yudko EB, Rodgers RJ, Blanchard DC. Defense system psychopharmacology: an ethological approach to the pharmacology of fear and anxiety. Behavioural Brain Research. 1993;58:155–166. doi: 10.1016/0166-4328(93)90100-5. [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Vollstaedt S, Spiess J. The corticotropin-releasing factor receptor 1 antagonist CP-154,526 reverses stress-induced learning deficits in mice. Behav Brain Res. 2003;138:207–213. doi: 10.1016/s0166-4328(02)00244-9. [DOI] [PubMed] [Google Scholar]
- Bolles RC. Species-specific defense reactions and avoidance learning. Psychol. Rev. 1970;77:32–48. [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD. Stress and brain atrophy. CNS Neurol Disord Drug Targets. 2006;5:503–512. doi: 10.2174/187152706778559309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Brett E. Trauma-related dissociative states and long-term psychopathology in posttraumatic stress disorder. J Trauma Stress. 1997;10:37–49. doi: 10.1023/a:1024804312978. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Rivier JE, Rice KC, Woods JH. Corticotropin-releasing hormone antagonists, astressin B and antalarmin: differing profiles of activity in rhesus monkeys. Neuropsychopharmacology. 2004;29:1112–1121. doi: 10.1038/sj.npp.1300410. [DOI] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. Temporally massed CS presentations generate more fear extinction than spaced presentations. J Exp Psychol Anim Behav Process. 2003;29:323–333. doi: 10.1037/0097-7403.29.4.323. [DOI] [PubMed] [Google Scholar]
- Cain CK, LeDoux JE. Brain mechanisms of Pavlovian and instrumental aversive conditioning. In: Nutt DJ, Blanchard RJ, Blanchard DC, Griebel G, editors. Handbook of Anxiety and Fear. Amsterdam: Elsevier Academic Press; 2008. pp. 103–125. Cain CK, LeDoux JE (2008), In D. J. Nutt, R. J. Blanchard, D. C. Blanchard & G. Griebel (Eds.), Handbook of Anxiety and Fear (pp. 103–125). Amsterdam: Elsevier Academic Press. [Google Scholar]
- Campbell BM, Morrison JL, Walker EL, Merchant KM. Differential regulation of behavioral, genomic, and neuroendocrine responses by CRF infusions in rats. Pharmacol Biochem Behav. 2004;77:447–455. doi: 10.1016/j.pbb.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Chaki S, Nakazato A, Kennis L, Nakamura M, Mackie C, Sugiura M, Vinken P, Ashton D, Langlois X, Steckler T. Anxiolytic- and antidepressant-like profile of a new CRF1 receptor antagonist, R278995/CRA0450. Eur J Pharmacol. 2004;485:145–158. doi: 10.1016/j.ejphar.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Chaki S, Okuyama S, Nakazato A, Kumagai T, Okubo T, Ikeda Y, Oshida Y, Hamajima Y, Tomisawa K. In vitro pharmacological profile of nonpeptide CRF1 receptor antagonists, CRA1000 and CRA1001. Eur J Pharmacol. 1999;371:205–211. doi: 10.1016/s0014-2999(99)00120-x. [DOI] [PubMed] [Google Scholar]
- Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr Scand Suppl. 2003:38–50. doi: 10.1034/j.1600-0447.108.s417.3.x. [DOI] [PubMed] [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Charney DS, Deutch A. A functional neuroanatomy of anxiety and fear: implications for the pathophysiology and treatment of anxiety disorders. Crit Rev Neurobiol. 1996;10:419–446. doi: 10.1615/critrevneurobiol.v10.i3-4.70. [DOI] [PubMed] [Google Scholar]
- Chen C, Dagnino R, Jr, De Souza EB, Grigoriadis DE, Huang CQ, Kim K-I, Liu Z, Moran T, Webb TR, et al. Design and Synthesis of a Series of Non-Peptide High-Affinity Human Corticotropin-Releasing Factor1 Receptor Antagonists. J Med Chem. 1996;39:4358–4360. doi: 10.1021/jm960149e. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Koob GF. Propranolol antagonizes the enhanced conditioned fear produced by corticotropin releasing factor. J Pharmacol Exp Ther. 1988;247:902–910. [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Cook CJ. Stress induces CRF release in the paraventricular nucleus, and both CRF and GABA release in the amygdala. Physiol Behav. 2004;82:751–762. doi: 10.1016/j.physbeh.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Venero C, Kruyt ND, Sandi C. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats. Evidence for a role of corticosterone. 2003;44:338–345. doi: 10.1016/s0018-506x(03)00160-0. [DOI] [PubMed] [Google Scholar]
- Coric V, Feldman HH, Oren DA, Shekhar A, Pultz J, Dockens RC, Wu X, Gentile KA, Huang SP, Emison E, Delmonte T, D'Souza BB, Zimbroff DL, Grebb JA, Goddard AW, Stock EG. Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress Anxiety. 2010;27:417–425. doi: 10.1002/da.20695. [DOI] [PubMed] [Google Scholar]
- Davis M, Barad M, Otto M, Southwick S. Combining pharmacotherapy with cognitive behavioral therapy: traditional and new approaches. J Trauma Stress. 2006;19:571–581. doi: 10.1002/jts.20149. [DOI] [PubMed] [Google Scholar]
- Davis M, Myers KM, Chhatwal J, Ressler KJ. Pharmacological treatments that facilitate extinction of fear: relevance to psychotherapy. NeuroRx. 2007;3:82–96. doi: 10.1016/j.nurx.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos Trans R Soc Lond B Biol Sci. 1997a;352:1675–1687. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. 1997b;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- Dawe GS, Huff KD, Vandergriff JL, Sharp T, O'Neill MJ, Rasmussen K. Olanzapine activates the rat locus coeruleus: in vivo electrophysiology and c-Fos immunoreactivity. Biol Psychiatry. 2001;50:510–520. doi: 10.1016/s0006-3223(01)01171-4. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Bailey JE, Papadopoulos A, Diaper A, Phillips SM, van der Ark P, Dourish CT, Nutt DJ. Society for Neuroscience. Chicago IL: Society for Neuroscience; 2009. Preliminary evidence of efficacy of a CRF1 receptor antagonist in the 7.5% CO2 model of anxiety. [Google Scholar]
- de Jongh R, Groenink L, van der Gugten J, Olivier B. Light-enhanced and fear-potentiated startle: temporal characteristics and effects of alpha-helical corticotropin-releasing hormone. Biol Psychiatry. 2003;54:1041–1048. doi: 10.1016/s0006-3223(03)00468-2. [DOI] [PubMed] [Google Scholar]
- Deak T, Nguyen KT, Ehrlich AL, Watkins LR, Spencer RL, Maier SF, Licinio J, Wong ML, Chrousos GP, Webster E, Gold PW. The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology. 1999;140:79–86. doi: 10.1210/endo.140.1.6415. [DOI] [PubMed] [Google Scholar]
- Debiec J, Doyere V, Nader K, Ledoux JE. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proc Natl Acad Sci U S A. 2006;103:3428–3433. doi: 10.1073/pnas.0507168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Schoffelmeer AN, De Vries TJ. Beta-adrenoceptor mediated inhibition of long-term reward-related memory reconsolidation. Behav Brain Res. 2006;170:333–336. doi: 10.1016/j.bbr.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Ducottet C, Griebel G, Belzung C. Effects of the selective nonpeptide corticotropin-releasing factor receptor 1 antagonist antalarmin in the chronic mild stress model of depression in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:625–631. doi: 10.1016/S0278-5846(03)00051-4. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditional and unconditional components of postshock freezing. Pavlovian Journal of Biological Science. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Associative vs topographical accounts of the immediate shock-freezing deficit in rats: Implication for the response selection rules governing species-specific defensive reactions. Learning and Motivation. 1986;17:16–39. [Google Scholar]
- Fanselow MS. Factors governing one-trial contextual conditioning. Animal Learning & Behavior. 1990;18:264–270. [Google Scholar]
- Fanselow MS. The midbrain periaqueductal gray as a coordinator of action in response to fear and anxiety. In: Depaulis A, Bandler R, editors. The midbrain periaqueductal grey matter: Functional,anatomical and immunohistochemical organization. New York: Plenum Publishing Corp.; 1991. [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Fossey MD, Lydiard RB, Ballenger JC, Laraia MT, Bissette G, Nemeroff CB. Cerebrospinal fluid corticotropin-releasing factor concentrations in patients with anxiety disorders and normal comparison subjects. Biol Psychiatry. 1996;39:703–707. doi: 10.1016/0006-3223(95)00197-2. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Gallagher JP, Orozco-Cabal LF, Liu J, Shinnick-Gallagher P. Synaptic physiology of central CRH system. Eur J Pharmacol. 2008;583:215–225. doi: 10.1016/j.ejphar.2007.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl- imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Shekhar A, Morin SM, Hipskind PA, Zink C, Gackenheimer SL, Shaw J, Fitz SD, Sajdyk TJ. Stress and central Urocortin increase anxiety-like behavior in the social interaction test via the CRF1 receptor. Eur J Pharmacol. 2005;509:145–153. doi: 10.1016/j.ejphar.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Clampitt DM. Potential role for the hypothalamo-pituitary-adrenal axis in the conditioned reinforcer-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology (Berl) 2002;161:222–232. doi: 10.1007/s00213-002-1007-4. [DOI] [PubMed] [Google Scholar]
- Gold PE. The many faces of amnesia. Learn Mem. 2006;13:506–514. doi: 10.1101/lm.277406. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. The endocrinology of melancholic and atypical depression: relation to neurocircuitry and somatic consequences. Proc Assoc Am Physicians. 1999;111:22–34. doi: 10.1046/j.1525-1381.1999.09423.x. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Gold PW, Gabry KE, Yasuda MR, Chrousos GP. Divergent endocrine abnormalities in melancholic and atypical depression: clinical and pathophysiologic implications. Endocrinol Metab Clin North Am. 2002;31:37–62. vi. doi: 10.1016/s0889-8529(01)00022-6. [DOI] [PubMed] [Google Scholar]
- Grigoriadis DE. The corticotropin-releasing factor receptor: a novel target for the treatment of depression and anxiety-related disorders. Expert Opin Ther Targets. 2005;9:651–684. doi: 10.1517/14728222.9.4.651. [DOI] [PubMed] [Google Scholar]
- Gully D, Geslin M, Serva L, Fontaine E, Roger P, Lair C, Darre V, Marcy C, Rouby PE, Simiand J, Guitard J, Gout G, Steinberg R, Rodier D, Griebel G, Soubrie P, Pascal M, Pruss R, Scatton B, Maffrand JP, Le Fur G. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4- methylphenyl)ethyl]5-methyl-N-(2-propynyl)-1,3-thiazol-2-amine hydrochloride (SSR125543A): a potent and selective corticotrophin-releasing factor(1) receptor antagonist. I. Biochemical and pharmacological characterization. J Pharmacol Exp Ther. 2002;301:322–332. doi: 10.1124/jpet.301.1.322. [DOI] [PubMed] [Google Scholar]
- Gutman DA, Coyer MJ, Boss-Williams KA, Owens MJ, Nemeroff CB, Weiss JM. Behavioral effects of the CRF1 receptor antagonist R121919 in rats selectively bred for high and low activity in the swim test. Psychoneuroendocrinology. 2008;33:1093–1101. doi: 10.1016/j.psyneuen.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Gutman DA, Owens MJ, Skelton KH, Thrivikraman KV, Nemeroff CB. The corticotropin-releasing factor1 receptor antagonist R121919 attenuates the behavioral and endocrine responses to stress. J Pharmacol Exp Ther. 2003;304:874–880. doi: 10.1124/jpet.102.042788. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Pepin JL, DesMarteau JS, Watkins LR, Maier SF. Low doses of corticotropin-releasing hormone injected into the dorsal raphe nucleus block the behavioral consequences of uncontrollable stress. Behav Brain Res. 2003;147:55–64. doi: 10.1016/s0166-4328(03)00133-5. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci. 2004;118:443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzinger M. Neuropeptides and the hypothalamic-pituitary-adrenocortical (HPA) system: review of recent research strategies in depression. World J Biol Psychiatry. 2000;1:105–111. doi: 10.3109/15622970009150573. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29:641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, De Souza EB, Schulteis G, Lapsansky JL, Grigoriadis DE. Brain penetrance, receptor occupancy and antistress in vivo efficacy of a small molecule corticotropin releasing factor type I receptor selective antagonist. Neuropsychopharmacology. 2002;27:194–202. doi: 10.1016/S0893-133X(02)00299-3. [DOI] [PubMed] [Google Scholar]
- Hikichi T, Akiyoshi J, Yamamoto Y, Tsutsumi T, Isogawa K, Nagayama H. Suppression of conditioned fear by administration of CRF receptor antagonist CP-154,526. Pharmacopsychiatry. 2000;33:189–193. doi: 10.1055/s-2000-7587. [DOI] [PubMed] [Google Scholar]
- Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- Ho SP, Takahashi LK, Livanov V, Spencer K, Lesher T, Maciag C, Smith MA, Rohrbach KW, Hartig PR, Arneric SP. Attenuation of fear conditioning by antisense inhibition of brain corticotropin releasing factor-2 receptor. Brain Res Mol Brain Res. 2001;89:29–40. doi: 10.1016/s0169-328x(01)00050-x. [DOI] [PubMed] [Google Scholar]
- Hodgson RA, Higgins GA, Guthrie DH, Lu SX, Pond AJ, Mullins DE, Guzzi MF, Parker EM, Varty GB. Comparison of the V1b antagonist, SSR149415, and the CRF1 antagonist, CP-154,526, in rodent models of anxiety and depression. Pharmacol Biochem Behav. 2007;86:431–440. doi: 10.1016/j.pbb.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Howard O, Carr GV, Hill TE, Valentino RJ, Lucki I. Differential blockade of CRF-evoked behaviors by depletion of norepinephrine and serotonin in rats. Psychopharmacology (Berl) 2008;199:569–582. doi: 10.1007/s00213-008-1179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard DT, Nakashima BR, Lee I, Takahashi LK. Activation of basolateral amygdala corticotropin-releasing factor 1 receptors modulates the consolidation of contextual fear. Neuroscience. 2007;150:818–828. doi: 10.1016/j.neuroscience.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M, Chaki S. Separation-induced ultrasonic vocalization in rat pups: further pharmacological characterization. Pharmacol Biochem Behav. 2005;82:652–657. doi: 10.1016/j.pbb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Ise S, Nagano N, Okuda S, Ohta H. Corticotropin-releasing factor modulates maternal separation-induced ultrasonic vocalization in rat pups via activation of CRF1 receptor. Brain Res. 2008;1234:59–65. doi: 10.1016/j.brainres.2008.07.079. [DOI] [PubMed] [Google Scholar]
- Ising M, Zimmermann US, Kunzel HE, Uhr M, Foster AC, Learned-Coughlin SM, Holsboer F, Grigoriadis DE. High-affinity CRF1 receptor antagonist NBI-34041: preclinical and clinical data suggest safety and efficacy in attenuating elevated stress response. Neuropsychopharmacology. 2007;32:1941–1949. doi: 10.1038/sj.npp.1301328. [DOI] [PubMed] [Google Scholar]
- Ji G, Fu Y, Ruppert KA, Neugebauer V. Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Mol Pain. 2007;3:13. doi: 10.1186/1744-8069-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Differential effects of CRF1 and CRF2 receptor antagonists on pain-related sensitization of neurons in the central nucleus of the amygdala. J Neurophysiol. 2007 doi: 10.1152/jn.00135.2007. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Wood SK, Houshyar H, Hsin LW, Rice KC, Woods JH. The effects of CRF antagonists, antalarmin, CP154,526, LWH234, and R121919, in the forced swim test and on swim-induced increases in adrenocorticotropin in rats. Psychopharmacology (Berl) 2005;180:215–223. doi: 10.1007/s00213-005-2164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasckow JW, Baker D, Geracioti TD., Jr Corticotropin-releasing hormone in depression and post-traumatic stress disorder. Peptides. 2001;22:845–851. doi: 10.1016/s0196-9781(01)00399-0. [DOI] [PubMed] [Google Scholar]
- Keck ME, Kern N, Erhardt A, Unschuld PG, Ising M, Salyakina D, Muller MB, Knorr CC, Lieb R, Hohoff C, Krakowitzky P, Maier W, Bandelow B, Fritze J, Deckert J, Holsboer F, Muller-Myhsok B, Binder EB. Combined effects of exonic polymorphisms in CRHR1 and AVPR1B genes in a case/control study for panic disorder. Am J Med Genet B Neuropsychiatr Genet. 2008 doi: 10.1002/ajmg.b.30750. [DOI] [PubMed] [Google Scholar]
- Keck ME, Welt T, Wigger A, Renner U, Engelmann M, Holsboer F, Landgraf R. The anxiolytic effect of the CRH(1) receptor antagonist R121919 depends on innate emotionality in rats. Eur J Neurosci. 2001;13:373–380. doi: 10.1046/j.0953-816x.2000.01383.x. [DOI] [PubMed] [Google Scholar]
- Kehne JH. The CRF1 receptor, a novel target for the treatment of depression, anxiety, and stress-related disorders. CNS Neurol Disord Drug Targets. 2007;6:163–182. doi: 10.2174/187152707780619344. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Baron BM, Harrison BL, McCloskey TC, Palfreyman MG, Poirot M, Salituro FG, Siegel BW, Slone AL, Van Giersbergen PL, et al. MDL 100,458 and MDL 102,288: two potent and selective glycine receptor antagonists with different functional profiles. Eur J Pharmacol. 1995;284:109–118. doi: 10.1016/0014-2999(95)00375-u. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Coverdale S, McCloskey TC, Hoffman DC, Cassella JV. Effects of the CRF(1) receptor antagonist, CP 154,526, in the separation-induced vocalization anxiolytic test in rat pups. Neuropharmacology. 2000;39:1357–1367. doi: 10.1016/s0028-3908(00)00043-5. [DOI] [PubMed] [Google Scholar]
- Kehne JH, De Lombaert S. Non-peptidic CRF1 receptor antagonists for the treatment of anxiety, depression and stress disorders. Curr Drug Targets CNS Neurol Disord. 2002;1:467–493. doi: 10.2174/1568007023339049. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Maynard GD. CRF1 receptor antagonists: treatment of stress-related disorders. Drug Discovery Today: Therapeutic Strategies, online publication. 2008 [Google Scholar]
- Kehne JH, McCloskey TC, Baron BM, Chi EM, Harrison BL, Whitten JP, Palfreyman MG. NMDA receptor complex antagonists have potential anxiolytic effects as measured with separation-induced ultrasonic vocalizations. Eur J Pharmacol. 1991;193:283–292. doi: 10.1016/0014-2999(91)90141-c. [DOI] [PubMed] [Google Scholar]
- Kellner M, Schick M, Yassouridis A, Struttmann T, Wiedemann K, Alm B. Metyrapone tests in patients with panic disorder. Biol Psychiatry. 2004;56:898–900. doi: 10.1016/j.biopsych.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism, corticotropin-releasing factor, and molecular genetic allostasis. Biol Psychiatry. 2008a;63:137–138. doi: 10.1016/j.biopsych.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, neuroplasticity (sensitization), and alcoholism. Proc Natl Acad Sci U S A. 2008b;105:8809–8810. doi: 10.1073/pnas.0804354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Emotional networks and motor control: a fearful view. Prog Brain Res. 1996;107:437–446. doi: 10.1016/s0079-6123(08)61880-4. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lehner M, Taracha E, Skorzewska A, Turzynska D, Sobolewska A, Maciejak P, Szyndler J, Hamed A, Bidzinski A, Wislowska-Stanek A, Plaznik A. Expression of c-Fos and CRF in the brains of rats differing in the strength of a fear response. Behav Brain Res. 2008;188:154–167. doi: 10.1016/j.bbr.2007.10.033. [DOI] [PubMed] [Google Scholar]
- Lelas S, Wong H, Li YW, Heman KL, Ward KA, Zeller KL, Sieracki KK, Polino JL, Godonis HE, Ren SX, Yan XX, Arneric SP, Robertson DW, Hartig PR, Grossman S, Trainor GL, Taub RA, Zaczek R, Gilligan PJ, McElroy JF. Anxiolytic-like effects of the corticotropinreleasing factor1 (CRF1) antagonist DMP904 [4-(3-pentylamino)-2,7-dimethyl-8-(2-methyl-4-methoxyphenyl)-pyrazolo-[1,5 -a]-pyrimidine] administered acutely or chronically at doses occupying central CRF1 receptors in rats. J Pharmacol Exp Ther. 2004;309:293–302. doi: 10.1124/jpet.103.058784. [DOI] [PubMed] [Google Scholar]
- Lelas S, Zeller KL, Ward KA, McElroy JF. The anxiolytic CRF(1) antagonist DMP696 fails to function as a discriminative stimulus and does not substitute for chlordiazepoxide in rats. Psychopharmacology (Berl) 2003;166:408–415. doi: 10.1007/s00213-002-1331-8. [DOI] [PubMed] [Google Scholar]
- Li YW, Fitzgerald L, Wong H, Lelas S, Zhang G, Lindner MD, Wallace T, McElroy J, Lodge NJ, Gilligan P, Zaczek R. The pharmacology of DMP696 and DMP904, non-peptidergic CRF1 receptor antagonists. CNS Drug Rev. 2005;11:21–52. doi: 10.1111/j.1527-3458.2005.tb00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YW, Hill G, Wong H, Kelly N, Ward K, Pierdomenico M, Ren S, Gilligan P, Grossman S, Trainor G, Taub R, McElroy J, Zazcek R. Receptor occupancy of nonpeptide corticotropin-releasing factor 1 antagonist DMP696: correlation with drug exposure and anxiolytic efficacy. J Pharmacol Exp Ther. 2003;305:86–96. doi: 10.1124/jpet.102.045914. [DOI] [PubMed] [Google Scholar]
- Louis C, Cohen C, Depoortere R, Griebel G. Antidepressant-like effects of the corticotropin-releasing factor 1 receptor antagonist, SSR125543, and the vasopressin 1b receptor antagonist, SSR149415, in a DRL-72 s schedule in the rat. Neuropsychopharmacology. 2006;31:2180–2187. doi: 10.1038/sj.npp.1301036. [DOI] [PubMed] [Google Scholar]
- Lu L, Liu Z, Huang M, Zhang Z. Dopamine-dependent responses to cocaine depend on corticotropin-releasing factor receptor subtypes. J Neurochem. 2003;84:1378–1386. doi: 10.1046/j.1471-4159.2003.01635.x. [DOI] [PubMed] [Google Scholar]
- Lydiard RB. Irritable bowel syndrome, anxiety, and depression: what are the links? J Clin Psychiatry. 2001;62(Suppl 8):38–45. discussion 46–37. [PubMed] [Google Scholar]
- Lydiard RB. Increased prevalence of functional gastrointestinal disorders in panic disorder: clinical and theoretical implications. CNS Spectr. 2005;10:899–908. doi: 10.1017/s1092852900019878. [DOI] [PubMed] [Google Scholar]
- Lydiard RB. Worried sick: antidepressants, stress, and inflammation. J Clin Psychiatry. 2007;68:1613–1614. doi: 10.4088/jcp.v68n1021. [DOI] [PubMed] [Google Scholar]
- Maciag CM, Dent G, Gilligan P, He L, Dowling K, Ko T, Levine S, Smith MA. Effects of a non-peptide CRF antagonist (DMP696) on the behavioral and endocrine sequelae of maternal separation. Neuropsychopharmacology. 2002;26:574–582. doi: 10.1016/S0893-133X(01)00398-0. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Majchrzak M, Ferry B, Marchand AR, Herbeaux K, Seillier A, Barbelivien A. Entorhinal cortex lesions disrupt fear conditioning to background context but spare fear conditioning to a tone in the rat. Hippocampus. 2006;16:114–124. doi: 10.1002/hipo.20138. [DOI] [PubMed] [Google Scholar]
- Mallo T, Berggard C, Eller M, Damberg M, Oreland L, Harro J. Effect of long-term blockade of CRF(1) receptors on exploratory behaviour, monoamines and transcription factor AP-2. Pharmacol Biochem Behav. 2004;77:855–865. doi: 10.1016/j.pbb.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Maniam J, Morris MJ. Long-term postpartum anxiety and depression-like behavior in mother rats subjected to maternal separation are ameliorated by palatable high fat diet. Behav Brain Res. 2010;208:72–79. doi: 10.1016/j.bbr.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Brooks EN, Chen YL. Antidepressant-like effects of CP-154,526, a selective CRF1 receptor antagonist. Eur J Pharmacol. 1997;323:21–26. doi: 10.1016/s0014-2999(97)00025-3. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Marks I. Panic, Anxiety and Their Disorders. New York: Oxford University Press; 1987. Fears, Phobias, and Rituals. [Google Scholar]
- Martinez V, Tache Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr Pharm Des. 2006;12:4071–4088. doi: 10.2174/138161206778743637. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, Chang L. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- McElroy JF, Ward KA, Zeller KL, Jones KW, Gilligan PJ, He L, Lelas S. The CRF(1) receptor antagonist DMP696 produces anxiolytic effects and inhibits the stress-induced hypothalamic-pituitary-adrenal axis activation without sedation or ataxia in rats. Psychopharmacology (Berl) 2002;165:86–92. doi: 10.1007/s00213-002-1239-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Anisman H. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci. 2004;24:1478–1485. doi: 10.1523/JNEUROSCI.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Kent P, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Bedard T, Anisman H. Corticotropin-releasing hormone, arginine vasopressin, gastrin-releasing Peptide, and neuromedin B alterations in stress-relevant brain regions of suicides and control subjects. Biol Psychiatry. 2006;59:594–602. doi: 10.1016/j.biopsych.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Miles LA, Walker D, Davis M. Society for Neuroscience Annual Meeting. Washington, D. C.: 2008. Corticotropin-releasing factor (CRF) type 1 receptors in the bed nucleus of the stria terminalis (BNST) mediate long-(minutes) but not short- (seconds) duration startle increases to shock-predicting cues. [Google Scholar]
- Millan MJ, Brocco M, Gobert A, Dorey G, Casara P, Dekeyne A. Anxiolytic properties of the selective, non-peptidergic CRF(1) antagonists, CP154,526 and DMP695: a comparison to other classes of anxiolytic agent. Neuropsychopharmacology. 2001;25:585–600. doi: 10.1016/S0893-133X(01)00244-5. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Raineki C, Holman JD, Holman JG, Sullivan RM. Enduring neurobehavioral effects of early life trauma mediated through learning and corticosterone suppression. Front Behav Neurosci. 2009;3:22. doi: 10.3389/neuro.08.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Shionoya K, Jakubs K, Sullivan RM. Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. J Neurosci. 2009;29:15745–15755. doi: 10.1523/JNEUROSCI.4106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E, Blessing WW. CRF1-receptor antagonist CP-154526 reduces alerting-related cutaneous vasoconstriction in conscious rabbits. Neuroscience. 2003;117:129–138. doi: 10.1016/s0306-4522(02)00818-7. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E, Blessing WW. CRF1 receptor antagonist CP-154,526 reduces cardiovascular responses during acute psychological stress in rabbits. Brain Res. 2004;1017:234–237. doi: 10.1016/j.brainres.2004.05.062. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Clinical significance of psychoneuroendocrinology in psychiatry: focus on the thyroid and adrenal. J Clin Psychiatry. 1989;50(Suppl):13–20. discussion 21-12. [PubMed] [Google Scholar]
- Nemeroff CB. New vistas in neuropeptide research in neuropsychiatry: focus on corticotropin-releasing factor. Neuropsychopharmacology. 1992;6:69–75. [PubMed] [Google Scholar]
- Nemeroff CB. New directions in the development of antidepressants: the interface of neurobiology and psychiatry. Hum Psychopharmacol. 2002;17(Suppl 1):S13–S16. doi: 10.1002/hup.396. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Early-Life Adversity, CRF Dysregulation, and Vulnerability to Mood and Anxiety Disorders. Psychopharmacol Bull. 2004a;38(Suppl 1):14–20. [PubMed] [Google Scholar]
- Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004b;65(Suppl 1):18–28. [PubMed] [Google Scholar]
- Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res. 2006;40:1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Heim C, Owens MJ, Ritchie JC, Ramsey CH, Bonsall R, Miller AH, Nemeroff CB. Cerebrospinal fluid corticotropin-releasing factor (CRF) and vasopressin concentrations predict pituitary response in the CRF stimulation test: a multiple regression analysis. Neuropsychopharmacology. 2003;28:569–576. doi: 10.1038/sj.npp.1300071. [DOI] [PubMed] [Google Scholar]
- Nielsen DM, Carey GJ, Gold LH. Antidepressant-like activity of corticotropin-releasing factor type-1 receptor antagonists in mice. Eur J Pharmacol. 2004;499:135–146. doi: 10.1016/j.ejphar.2004.07.091. [DOI] [PubMed] [Google Scholar]
- O'Malley D, Dinan TG, Cryan JF. Alterations in colonic corticotropin-releasing factor receptors in the maternally separated rat model of irritable bowel syndrome: differential effects of acute psychological and physical stressors. Peptides. 2010a;31:662–670. doi: 10.1016/j.peptides.2010.01.004. [DOI] [PubMed] [Google Scholar]
- O'Malley D, Dinan TG, Cryan JF. Neonatal maternal separation in the rat impacts on the stress responsivity of central corticotropin-releasing factor receptors in adulthood. Psychopharmacology (Berl) 2010b doi: 10.1007/s00213-010-1885-9. [DOI] [PubMed] [Google Scholar]
- Ohata H, Arai K, Shibasaki T. Effect of chronic administration of a CRF(1) receptor antagonist, CRA1000, on locomotor activity and endocrine responses to stress. Eur J Pharmacol. 2002;457:201–206. doi: 10.1016/s0014-2999(02)02663-8. [DOI] [PubMed] [Google Scholar]
- Ohman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychol Rev. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Okuyama S, Chaki S, Kawashima N, Suzuki Y, Ogawa S, Nakazato A, Kumagai T, Okubo T, Tomisawa K. Receptor binding, behavioral, and electrophysiological profiles of nonpeptide corticotropin-releasing factor subtype 1 receptor antagonists CRA1000 and CRA1001. J Pharmacol Exp Ther. 1999;289:926–935. [PubMed] [Google Scholar]
- Overstreet DH, Griebel G. Antidepressant-like effects of CRF1 receptor antagonist SSR125543 in an animal model of depression. Eur J Pharmacol. 2004;497:49–53. doi: 10.1016/j.ejphar.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Keeney A, Hogg S. Antidepressant effects of citalopram and CRF receptor antagonist CP-154,526 in a rat model of depression. Eur J Pharmacol. 2004;492:195–201. doi: 10.1016/j.ejphar.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelleymounter MA, Joppa M, Carmouche M, Cullen MJ, Brown B, Murphy B, Grigoriadis DE, Ling N, Foster AC. Role of corticotropin-releasing factor (CRF) receptors in the anorexic syndrome induced by CRF. J Pharmacol Exp Ther. 2000;293:799–806. [PubMed] [Google Scholar]
- Pelleymounter MA, Joppa M, Ling N, Foster AC. Pharmacological evidence supporting a role for central corticotropin-releasing factor(2) receptors in behavioral, but not endocrine, response to environmental stress. J Pharmacol Exp Ther. 2002;302:145–152. doi: 10.1124/jpet.302.1.145. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Joppa M, Ling N, Foster AC. Behavioral and neuroendocrine effects of the selective CRF2 receptor agonists urocortin II and urocortin III. Peptides. 2004;25:659–666. doi: 10.1016/j.peptides.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann. N. Y. Acad. Sci. 1999;885:312–328. doi: 10.1111/j.1749-6632.1999.tb08687.x. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn Mem. 1994;1:34–44. [PubMed] [Google Scholar]
- Pitman RK, Delahanty DL. Conceptually driven pharmacologic approaches to acute trauma. CNS Spectr. 2005;10:99–106. doi: 10.1017/s109285290001943x. [DOI] [PubMed] [Google Scholar]
- Pitts MW, Todorovic C, Blank T, Takahashi LK. The central nucleus of the amygdala and corticotropin-releasing factor: insights into contextual fear memory. J Neurosci. 2009;29:7379–7388. doi: 10.1523/JNEUROSCI.0740-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J. Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Rau V, Fanselow MS. Neurobiological and Neuroethological Perspectives on Fear and Anxiety. In: Kirmayer LJ, Lemelson R, Barad M, editors. Understanding Trauma: Integrating Biological, Clinical, and Cultural Perspectives. New York: Cambridge University Press; 2007. pp. 27–40. [Google Scholar]
- Rau V, Fanselow MS. Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress. 2008:1. doi: 10.1080/10253890802137320. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD, Zorrilla EP, Koob GF. MPZP: A novel small molecule corticotropin-releasing factor type 1 receptor (CRF(1)) antagonist. Pharmacol Biochem Behav. 2008;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Geyer MA, Hauger RL, Coste S, Stenzel-Poore M, Wurst W, Holsboer F. CRF(1) and CRF(2) Receptors are Required for Potentiated Startle to Contextual but not Discrete Cues. Neuropsychopharmacology. 2009;34:1494–1503. doi: 10.1038/npp.2008.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Pelleymounter MA, Geyer MA. Role of corticotropin releasing factor (CRF) receptors 1 and 2 in CRF-potentiated acoustic startle in mice. Psychopharmacology (Berl) 2003;170:178–187. doi: 10.1007/s00213-003-1535-6. [DOI] [PubMed] [Google Scholar]
- Rosen JB. The neurobiology of conditioned and unconditioned fear: a neurobehavioral system analysis of the amygdala. Behav Cogn Neurosci Rev. 2004;3:23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- Sagami Y, Shimada Y, Tayama J, Nomura T, Satake M, Endo Y, Shoji T, Karahashi K, Hongo M, Fukudo S. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958–964. doi: 10.1136/gut.2003.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Sananbenesi F, Fischer A, Schrick C, Spiess J, Radulovic J. Mitogen-activated protein kinase signaling in the hippocampus and its modulation by corticotropin-releasing factor receptor 2: a possible link between stress and fear memory. J Neurosci. 2003;23:11436–11443. doi: 10.1523/JNEUROSCI.23-36-11436.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Merino JJ, Cordero MI, Touyarot K, Venero C. Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience. 2001;102:329–339. doi: 10.1016/s0306-4522(00)00484-x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol. 1999;34:721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Atrophy of the hippocampus in posttraumatic stress disorder: how and when? Hippocampus. 2001;11:90–91. doi: 10.1002/hipo.1026. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress and plasticity in the limbic system. Neurochem Res. 2003;28:1735–1742. doi: 10.1023/a:1026021307833. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z. Neurobiology of stress and cocaine addiction. Studies on corticotropin-releasing factor in rats, monkeys, and humans. Ann N Y Acad Sci. 1998;851:371–387. doi: 10.1111/j.1749-6632.1998.tb09011.x. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Brain corticotropin-releasing factor mediates 'anxiety-like' behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- Saunders J, Williams JP. New developments in the study of corticotropin releasing factor. Annu Rep Med Chem. 2001;36:21–30. [Google Scholar]
- Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, Johnson JE, Cerbone A, Malaspina D. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry. 2003;54:1382–1388. doi: 10.1016/s0006-3223(03)00571-7. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF. New approaches to managing psychotic depression. J Clin Psychiatry. 2003;64(Suppl 1):19–23. [PubMed] [Google Scholar]
- Schmidt ME, Andrews RD, van der Ark P, Brown T, Mannaert E, Steckler T, de Hoon J, Van Laere K. Dose-dependent effects of the CRF(1) receptor antagonist R317573 on regional brain activity in healthy male subjects. Psychopharmacology (Berl) 2010;208:109–119. doi: 10.1007/s00213-009-1714-1. [DOI] [PubMed] [Google Scholar]
- Schulz DW, Mansbach RS, Sprouse J, Braselton JP, Collins J, Corman M, Dunaiskis A, Faraci S, Schmidt AW, et al. CP-154,526: a potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proc Natl Acad Sci USA. 1996;93:10477–10482. doi: 10.1073/pnas.93.19.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants "grown up": adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Sekino A, Ohata H, Mano-Otagiri A, Arai K, Shibasaki T. Both corticotropin-releasing factor receptor type 1 and type 2 are involved in stress-induced inhibition of food intake in rats. Psychopharmacology (Berl) 2004;176:30–38. doi: 10.1007/s00213-004-1863-1. [DOI] [PubMed] [Google Scholar]
- Seligman MEP. Phobias and Preparedness. Behavior therapy. 1971;2:307–320. [Google Scholar]
- Selye H. Implications of stress concept. N Y State J Med. 1975;75:2139–2145. [PubMed] [Google Scholar]
- Seymour PA, Schmidt AW, Schulz DW. The pharmacology of CP-154,526, a non-peptide antagonist of the CRH1 receptor: a review. CNS Drug Rev. 2003;9:57–96. doi: 10.1111/j.1527-3458.2003.tb00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman JE, Kalin NH. ICV-CRH alters stress-induced freezing behavior without affecting pain sensitivity. Pharmacol Biochem Behav. 1988;30:801–807. doi: 10.1016/0091-3057(88)90103-7. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Rosenbaum JF, Biederman J, Kennedy J, Dai D, Racette SR, Laird NM, Kagan J, Snidman N, Hirshfeld-Becker D, Tsuang MT, Sklar PB, Slaugenhaupt SA. Association of a genetic marker at the corticotropin-releasing hormone locus with behavioral inhibition. Biol Psychiatry. 2003;54:1376–1381. doi: 10.1016/s0006-3223(03)00598-5. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Yamaki LH, Fagerness JA, Biederman J, Racette S, Laird NM, Kagan J, Snidman N, Faraone SV, Hirshfeld-Becker D, Tsuang MT, Slaugenhaupt SA, Rosenbaum JF, Sklar PB. The corticotropin-releasing hormone gene and behavioral inhibition in children at risk for panic disorder. Biol Psychiatry. 2005;57:1485–1492. doi: 10.1016/j.biopsych.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Heileg M. CRH signaling and the dark side of addiction: long-lasting hyper-reactivity to stress in animals with a history of ethanol dependence. Neuropsychopharmacology. 2006;31:S208. [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of Voluntary Alcohol Intake, Behavioral Sensitivity to Stress, and Amygdala Crhr1 Expression Following a History of Dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction:historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry. 2006 Aug 15;60(4):329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Morgan CA, Charney DS, High JR. Yohimbine use in a natural setting: effects on posttraumatic stress disorder. Biol Psychiatry. 1999;46:442–444. doi: 10.1016/s0006-3223(99)00107-9. [DOI] [PubMed] [Google Scholar]
- Steckler T, Dautzenberg FM. Corticotropin-releasing factor receptor antagonists in affective disorders and drug dependence - an update. CNS Neurol Disord Drug Targets. 2006;5:147–165. doi: 10.2174/187152706776359619. [DOI] [PubMed] [Google Scholar]
- Stein MB. Neurobiological perspectives on social phobia: from affiliation to zoology. Biol Psychiatry. 1998;44:1277–1285. doi: 10.1016/s0006-3223(98)00265-0. [DOI] [PubMed] [Google Scholar]
- Stinus L, Cador M, Zorrilla EP, Koob GF. Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place aversion in rats. Neuropsychopharmacology. 2005;30:90–98. doi: 10.1038/sj.npp.1300487. [DOI] [PubMed] [Google Scholar]
- Stout SC, Mortas P, Owens MJ, Nemeroff CB, Moreau J. Increased corticotropin-releasing factor concentrations in the bed nucleus of the stria terminalis of anhedonic rats. Eur J Pharmacol. 2000;401:39–46. doi: 10.1016/s0014-2999(00)00412-x. [DOI] [PubMed] [Google Scholar]
- Strohle A, Holsboer F. Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry. 2003;36(Suppl 3):S207–S214. doi: 10.1055/s-2003-45132. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Holman PJ. Transitions in sensitive period attachment learning in infancy: The role of corticosterone. Neurosci Biobehav Rev. 2010;34:835–844. doi: 10.1016/j.neubiorev.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64:293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Sweetser S, Camilleri M, Linker Nord SJ, Burton DD, Castenada L, Croop R, Tong G, Dockens R, Zinsmeister AR. Do corticotropin releasing factor-1 receptors influence colonic transit and bowel function in women with irritable bowel syndrome? Am J Physiol Gastrointest Liver Physiol. 2009;296:G1299–G1306. doi: 10.1152/ajpgi.00011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiergiel AH, Zhou Y, Dunn AJ. Effects of chronic footshock, restraint and corticotropin-releasing factor on freezing, ultrasonic vocalization and forced swim behavior in rats. Behav Brain Res. 2007 doi: 10.1016/j.bbr.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Swinny JD, O'Farrell E, Bingham BC, Piel DA, Valentino RJ, Beck SG. Neonatal rearing conditions distinctly shape locus coeruleus neuronal activity, dendritic arborization, and sensitivity to corticotrophin-releasing factor. Int J Neuropsychopharmacol. 2010;13:515–525. doi: 10.1017/S146114570999037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tache Y. Corticotropin releasing factor receptor antagonists: potential future therapy in gastroenterology? Gut. 2004;53:919–921. doi: 10.1136/gut.2003.036400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tache Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol. 2004;141:1321–1330. doi: 10.1038/sj.bjp.0705760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tache Y, Million M, Nelson AG, Lamy C, Wang L. Role of corticotropin-releasing factor pathways in stress-related alterations of colonic motor function and viscerosensibility in female rodents. Gend Med. 2005;2:146–154. doi: 10.1016/s1550-8579(05)80043-9. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Role of CRF(1) and CRF(2) receptors in fear and anxiety. Neurosci Biobehav Rev. 2001;25:627–636. doi: 10.1016/s0149-7634(01)00046-x. [DOI] [PubMed] [Google Scholar]
- Takamori K, Kawashima N, Chaki S, Nakazato A, Kameo K. Involvement of corticotropin-releasing factor subtype 1 receptor in the acquisition phase of learned helplessness in rats. Life Sci. 2001;69:1241–1248. doi: 10.1016/s0024-3205(01)01217-6. [DOI] [PubMed] [Google Scholar]
- Todorovic C, Radulovic J, Jahn O, Radulovic M, Sherrin T, Hippel C, Spiess J. Differential activation of CRF receptor subtypes removes stress-induced memory deficit and anxiety. Eur J Neurosci. 2007;25:3385–3397. doi: 10.1111/j.1460-9568.2007.05592.x. [DOI] [PubMed] [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Saam C, Lascorz J, Soyka M, Preuss UW, Rujescu D, Skowronek MH, Rietschel M, Spanagel R, Heinz A, Laucht M, Mann K, Schumann G. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Carpenter LL, McDougle CJ, Kirwin PD, Owens MJ, Nemeroff CB, Strong DR, Price LH. Increased cerebrospinal fluid corticotropin-releasing factor concentrations during tryptophan depletion in healthy adults. Biol Psychiatry. 2004;56:531–534. doi: 10.1016/j.biopsych.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Anderson GM, Owens MJ, Halaszynski TM, Bremner JD, Carpenter LL, Heninger GR, Nemeroff CB, Charney DS. Cerebrospinal fluid corticotropin-releasing hormone in healthy humans: effects of yohimbine and naloxone. J Clin Endocrinol Metab. 2000;85:4138–4145. doi: 10.1210/jcem.85.11.6968. [DOI] [PubMed] [Google Scholar]
- Walker D, Yang Y, Ratti E, Corsi M, Trist D, Davis M. Differential Effects of the CRF-R1 Antagonist GSK876008 on Fear-Potentiated, Light- and CRF-Enhanced Startle Suggest Preferential Involvement in Sustained vs Phasic Threat Responses. Neuropsychopharmacology. 2008;213:29–42. doi: 10.1038/npp.2008.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Light-enhanced startle: further pharmacological and behavioral characterization. Psychopharmacology (Berl) 2002a;159:304–310. doi: 10.1007/s002130100913. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol Biochem Behav. 2002b;71:379–392. doi: 10.1016/s0091-3057(01)00698-0. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2009;34:1533–1542. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Weninger SC, Dunn AJ, Muglia LJ, Dikkes P, Miczek KA, Swiergiel AH, Berridge CW, Majzoub JA. Stress-induced behaviors require the corticotropin-releasing hormone (CRH) receptor, but not CRH. Proc Natl Acad Sci U S A. 1999;96:8283–8288. doi: 10.1073/pnas.96.14.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Serotonergic and catecholaminergic reuptake inhibitors have opposite effects on the ultrasonic isolation calls of rat pups. Neuropsychopharmacology. 1990;3:51–59. [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Serotonergic modulation of the rat pup ultrasonic isolation call: studies with 5HT1 and 5HT2 subtype-selective agonists and antagonists. Psychopharmacology. 1991;105:513–520. doi: 10.1007/BF02244372. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR, Trullas R, Skolnick P. Rat pup isolation calls are reduced by functional antagonists of the NMDA receptor complex. Eur J Pharmacol. 1990;190:11–21. doi: 10.1016/0014-2999(90)94107-9. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Newman JT, Insel TR. CRH and alpha-helical-CRH modulate behavioral measures of arousal in monkeys. Pharmacol Biochem Behavior. 1989;32:919–926. doi: 10.1016/0091-3057(89)90059-2. [DOI] [PubMed] [Google Scholar]
- Wood GE, Norris EH, Waters E, Stoldt JT, McEwen BS. Chronic immobilization stress alters aspects of emotionality and associative learning in the rat. Behav Neurosci. 2008;122:282–292. doi: 10.1037/0735-7044.122.2.282. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Flory JD. Differentiating biological correlates of risk, PTSD, and resilience following trauma exposure. J Trauma Stress. 2007;20:435–447. doi: 10.1002/jts.20260. [DOI] [PubMed] [Google Scholar]
- Young EA, Abelson JL, Cameron OG. Effect of comorbid anxiety disorders on the hypothalamic-pituitary-adrenal axis response to a social stressor in major depression. Biol Psychiatry. 2004;56:113–120. doi: 10.1016/j.biopsych.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Valdez GR, Fekete EM, Rivier JE, Vale WW, Rice KC, Weiss F, Zorrilla EP. Subtype-selective corticotropin-releasing factor receptor agonists exert contrasting, but not opposite, effects on anxiety-related behavior in rats. J Pharmacol Exp Ther. 2007;323:846–854. doi: 10.1124/jpet.107.123208. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Reinhardt LE, Valdez GR, Inoue K, Rivier JE, Vale WW, Koob GF. Human urocortin 2, a corticotropin-releasing factor (CRF)2 agonist, and ovine CRF, a CRF1 agonist, differentially alter feeding and motor activity. J Pharmacol Exp Ther. 2004;310:1027–1034. doi: 10.1124/jpet.104.068676. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002;952:188–199. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]
- Zurita A, Martijena I, Cuadra G, Brandão ML, Molina V. Early exposure to chronic variable stress facilitates the occurrence of anhedonia and enhanced emotional reactions to novel stressors: reversal by naltrexone pretreatment. Behav Brain Res. 2000;117:163–171. doi: 10.1016/s0166-4328(00)00302-8. [DOI] [PubMed] [Google Scholar]