Abstract
Though much of the research on atherosclerosis has focused on the intimal accumulation of lipids and inflammatory cells, there is an increasing amount of interest in the role of the adventitia in coordinating the immune response in atherosclerosis. In this review of the contributions of the adventitia and adventitial lymphocytes to the development of atherosclerosis, we discuss recent research on the formation and structural nature of adventitial immune aggregates, potential mechanisms of crosstalk between the intima, media, and adventitia, specific contributions of B lymphocytes and T lymphocytes, and the role of the vasa vasorum and surrounding perivascular adipose tissue (PVAT). Furthermore, we highlight techniques for the imaging of lymphocytes in the vasculature.
Keywords: Lymphocytes, Atherosclerosis, Adventitia, Imaging
Introduction
Atherosclerosis and its clinical manifestations of stroke and myocardial infarction are the leading cause of morbidity and mortality in the Western world1. Understanding the pathophysiology of atherosclerosis and potential means of preventing its progression are of critical importance since the initial manifestation of coronary artery disease is sudden cardiac death or myocardial infarction in over half of individuals2. Though much emphasis has been placed on investigating atherosclerosis through intimal accumulation of lipids and inflammatory cells, recent research suggests the adventitia may also play a critical role in coordinating the progression of the disease. It has long been known that damage to the adventitia in the setting of both percutaneous coronary angioplasty3,4 and the placement of a circumferential silastic collar5 can precede neointimal formation in porcine and rabbit models, and more recent studies have elucidated some of the potential mechanisms of this adventitial contribution to lesion development. The adventitia is a major site of lymphocyte accumulation and organization6–8, and histological studies in both humans and mice suggest that the adventitia may contribute to the adaptive and innate immune responses that regulate atherosclerosis6,7,9–11. These studies further underscore the current notion that immunomodulatory therapy may be an effective strategy to add to our clinical arsenal for the treatment of patients12. Understanding how the surrounding tissues such as the perivascular fat and the vasa vasorum influence lymphocyte recruitment and subsequent modulation of atherosclerosis progression may ultimately elucidate mechanisms of crosstalk between adventitial lymphocytes and the atherosclerotic plaque.
Molecular and cellular imaging can serve as a powerful tool to aid our understanding of the pathophysiology of atherosclerosis13. Given the close proximity of the different layers of the arterial wall, careful selection of the imaging target and imaging modality is critical. Targeted molecular imaging of intimal macrophage density could provide a means to not only determine the degree of inflammation in atherosclerotic plaque14–16, but also monitor response to medical therapy17. Advances in targeted cellular and molecular imaging make it possible not only to identify the presence of lymphocytes within the adventitia and atherosclerotic plaque but to also study the role lymphocytes play in the progression of atherosclerosis. However, differentiation between cells within the intima and adventitia using certain imaging modalities may prove very challenging. Thus, the use of multimodality imaging may provide adequate sensitivity and spatial resolution to reliably localize labeled cells or molecular targets within the arterial wall and provide functional information on their role in atherosclerosis progression.
Immune cells in the Adventitia of Diseased Arteries
The presence of lymphocytes in the adventitia surrounding atherosclerotic lesions has long been recognized8, yet our understanding of their organization and their role in the progression of atherosclerosis is just recently unfolding. The majority of murine work in this area uses the aorta as a model as the small size of murine coronary arteries make detailed study of their structure and function challenging. The aggregation of lymphocytes and formation of organized structures critically depend on surrounding adventitial cells that orchestrate this process. Murine studies have demonstrated the presence of leukocytes in the adventitia adjacent to atherosclerotic lesions that are comprised of collections of T lymphocytes, B lymphocytes, follicular dendritic cells and tingible body macrophages (predominantly found in germinal centers)6,7,10,18,19.
Structural organization of the adventitial infiltrates has been shown to range from loose T lymphocyte aggregates to lymphoid follicles with B lymphocyte and dendritic cell nodular centers surrounded by parafollicular T lymphocytes, termed aortic tertiary lymphoid organs (ATLOs)6,7,10,18–20. ATLOs can be distinguished from paraaortic lymph nodes as ATLOs border the external elastic lamina and lack capsules10. The presence of these ATLOs in the adventitia has been associated with advanced atherosclerotic plaques6–8,10, suggesting that they may act as centers of a local humoral response with B lymphocyte subset selection, maturation, and antibody production following antigen presentation by dendritic cells6,7.
Several structural aspects of these advanced adventitial lymphoid aggregates support this idea. Lymphoid aggregates in the adventitia of a patient with chronic periaortitis were found to react positively to peanut lectin, which binds B cells in germinal centers19. Consistent with this idea, recent important work by Grabner and colleagues, demonstrated that adventitial B cell follicles (B220+) contained follicular dendritic cell networks and T cell (CD3ε+) and plasma cell (CD138+) compartments, an organization that promotes antigen presentation, selection, and plasma cell antibody production typical of germinal centers10. These findings are further supported by the observation that the follicular B lymphocyte centers within the ATLOs show high concentrations of Ki-67 positive proliferating cells, suggesting B lymphocytes are undergoing affinity maturation7,10 Addtionally, The extensive networks of high endothelial venules, lymph vessels, and blood vessels observed in these ATLOs facilitate the recruitment of immune cells to these developing lymphoid organs10.
Several chemokines related to the recruitment of lymphocytes likely orchestrate the initiation and further organization of these adventitial lymphoid structures. Expression of CCL21 is known to be important in the development of lymphoid follicles with segregated T and B cell areas as well as extensive networks of lymphatic vessels and high endothelial venules in the thyroid21,22, and medial smooth muscle cells acting as lymphoid tissue organizers in aged apoE−/− mice produce CCL21 when activated through lymphotoxin β-receptor (LTβR) signalling10. Furthermore, CCL21 and CCL19 gradients help mediate the movement of follicular B cells to T cell compartments within lymphoid follicles, and expression of CCR7, their receptor, is upregulated on B cells upon exposure to antigen10,23. CXCL13 has likewise been shown to be produced by LTβR-activated medial smooth muscle cells in Apoe−/− mice10 and is known to be produced by follicular dendritic cells. Together with its receptor CXCR5, which is present on all mature B cells24, CXCL13 likely plays a crucial role in the recruitment of B cells from the vasculature into the developing adventitial lymphoid aggregate. Interestingly, Muniz and colleagues recently showed that depletion of CD11c+ dendritic cells inhibited LTβR signalling-mediated lymphangiogenesis in thyroid tertiary lymphoid structures, suggesting that lymphatic vascular development may depend on dendritic cell recruitment and activation25. It is likely that dendritic cells in developing ectopic adventitial lymphoid structures likewise mediate the development of follicular lymphatic vasculature, which raises the interesting possibility that chemokines responsible for dendritic cell recruitment, such as CCL2, CXCL10, and CXCL922 may be important in the progression of ATLO development25.
T lymphocytes in the Adventitia and in Atherosclerosis
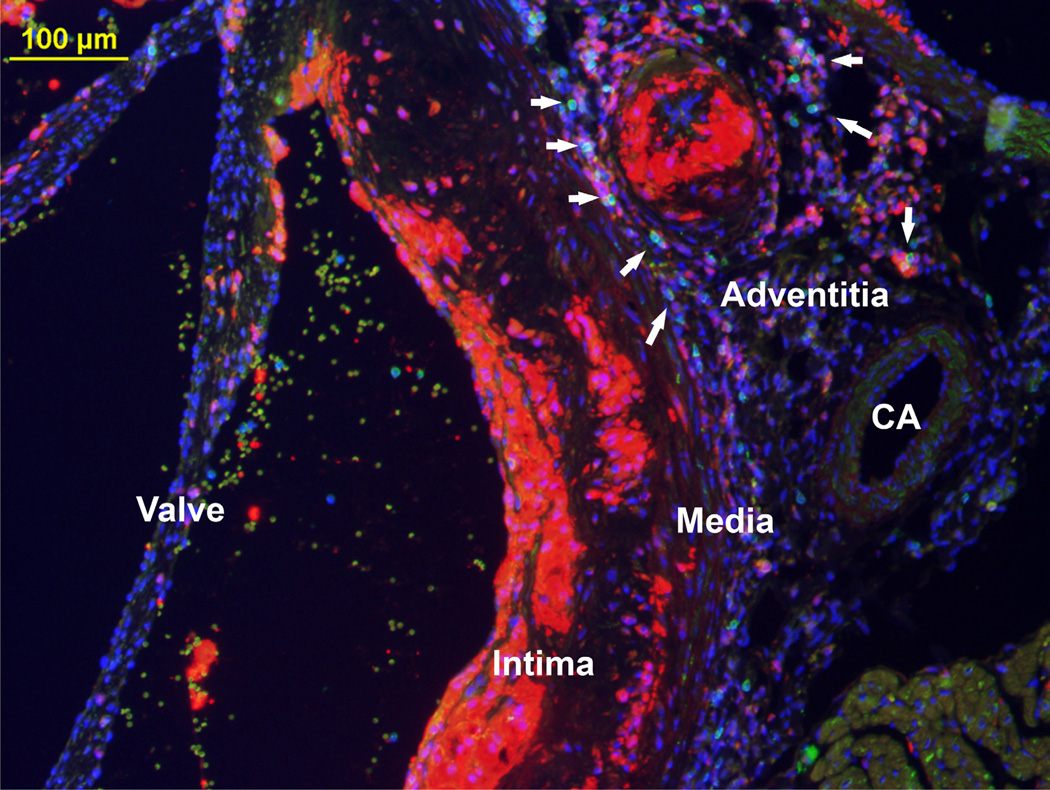
The role of T lymphocytes in the development and progression of atherosclerosis has been an area of active investigation26,27. T lymphocyte subsets and their interaction with other inflammatory cells within the adventitia, especially within ATLOs, is an area of great interest10,28. Lymphocytes populate the adventitia as previously shown via molecular imaging29. Figure 2 depicts an image of T lymphocytes aggregating in the aortic root adjacent to an atherosclerotic plaque in an Ldlr−/− mouse fed a Western diet for 16 weeks. This aggregation of T lymphocytes and adventitial macrophages may represent the beginning formation of an ATLO as it borders the external elastic lamina.
Figure 2. Fluorescent microscopy (10X) of a cross-section from the aortic root of a 24 week old Ldlr−/− mouse fed Western diet for 16 weeks.
Macrophages appear red (anti-Mac-2), T lymphocytes appear green (anti-CD3), and nuclei appear blue (DAPI). The overlay RGB image clearly demonstrates the intima, media, and adventitia with the presence of an atherosclerotic plaque containing macrophages and T lymphocytes. Adjacent to the coronary artery (CA) is an area with T lymphocytes as well as an area of adventitial macrophages.
Adventitial T lymphocytes are present early in the development of atherosclerosis, in loose aggregates6,18. Much is known about the role of various T lymphocyte subsets, including CD4+ T lymphocytes, CD8+ T lymphocytes, regulatory T lymphocytes, Th-17 cells, and natural killer T cells in atherosclerosis20,26,27,30,31, with a focus on their role in the developing intima, and this has recently been nicely reviewed26,27,32. Adoptive transfer of CD4+ T lymphocytes specifically reactive to oxidized low density lipoprotein (oxLDL) leads to a greater increase development in atherosclerosis than naïve CD4+ T lymphocytes or CD4+ T lymphocytes from mice immunized with keyhole limpet hemocyanin (KLH)33, suggesting that CD4+ T lymphocytes play a key role in mediating specific antigen-driven responses during atherosclerosis development33. Additional studies demonstrating T lymphocytes in the atherosclerotic plaque specific for not only oxLDL but also for heat shock protein supports the concept that multiple antigens may activate T lymphocytes and other immune cells in the artery wall.34,35 In a recent study, Hermansson and colleagues demonstrated that T cell hybridomas generated from human ApoB100 transgenic mice responded with production of IL-2 upon in vitro exposure of native LDL and purified ApoB10036. These T cells were found to be CD4+ and expressed a lone T cell receptor variable β chain, TRBV31. Interestingly, serological analysis of these mice revealed the presence of IgG antibodies against oxLDL, suggesting that these T cells reactive to native ApoB100 may induce B cells to produce antibodies against oxLDL36. Therefore, more work is needed to fully understand the important autoantigens stimulating responses that modulate atherosclerosis development and the specificity of the autoantibodies that they produce. However, clearly CD4+ T lymphocytes can undergo activation and clonal expansion in response to plaque antigens34 which could lead to local autoantibody production. Yet, the role of ATLOs in this process is poorly understood. Production of cytokines is another mechanism whereby T lymphocytes regulate atherosclerosis formation, and adventitial T lymphocytes could impact intimal plaque development by passage of these cytokines through the channels connecting the adventitia with the media.
IL-17A producing T cells are also present in the adventitia and have been implicated in atherogenesis. Blockade of IL-17A led to a significant reduction in aortic CXCL1 expression, aortic macrophage accumulation and atherosclerosis31. Follow on studies to further explore the role of these Th-17 cells in the adventitia are needed. ATLOs have also been shown to harbor a large number of Fox3P+ Treg that are predominantly found within the T lymphocyte region10. It appears there are significantly more Tregs present in ATLOs surrounding well established plaques than there are in early loose adventitial T lymphocyte aggregates6,10, yet Tregs have been shown to be atheroprotective37 through complex interactions with transcription growth factor-β and IL-1026. Further studies are needed to characterize the complex interactions of T lymphocyte subsets, cytokines, chemokines, and other inflammatory cells in the adventitia.
B lymphocytes in the Adventitia and in Atherosclerosis
Multiple autopsy studies have reported the presence of B lymphocytes in both the plaque and adventitia surrounding atherosclerotic lesions with a predominance of B lymphocytes found in the adventitia7,11,38. Though the contributions of T lymphocytes, macrophages and other proinflammatory cells have been well studied, the role of B lymphocytes in atherogenesis is less well understood. B lymphocyte aggregates have been observed within the nodular centers of adventitial ATLOs and may undergo selection, maturation, and antibody production following antigen presentation by dendritic cells7,10. Real-time PCR performed on directional artherectomy samples obtained from coronary arteries of six patients with coronary artery disease demonstrated evidence of antigen-driven clonal expansion and evolution of B lymphocytes in human atherosclerotic plaques39. Additionally, in their survey of inflammatory cell infiltration in atherosclerosis, Parums and Mitchinson noted that advanced plaques with evidence of medial attenuation showed significant adventitial plasma cell and B lymphocyte accumulation19,40. Plasma cells have been demonstrated in the adventitia around ATLOs10 and in the coronary arteries in the setting of transplant vasculopathy41, providing further evidence that B lymphocytes may differentiate into plasma cells within the adventitial environment. These studies establish the presence of activated B lymphocytes in diseased arteries, but fall short of identifying a functional role for B lymphocytes in atherogenesis.
Some of the first data to address a functional role for B lymphocytes in atherosclerosis was published in 2002 by two groups42,43. Caligiuri and colleagues42 demonstrated that splenectomy increased atherosclerosis development in response to 12 weeks of Western feeding in Apoe−/− mice. As splenectomy results in a significant decrease of both B and T lymphocytes, they performed adoptive transfer of purified splenic B lymphocytes into splenectomized Apoe−/− mice to determine a role specifically for B lymphocytes. Indeed, adoptive transfer of B lymphocytes into splenectomized Apoe−/− mice resulted in a 90% reduction in lesion area compared with controls. Additionally, they found that adoptive transfer of B lymphocytes to non-splenectomized mice also led to a 30% reduction in atherosclerosis, suggesting an atheroprotective role for B lymphocytes. That same year, Major et.al. also demonstrated the atheroprotective properties of B lymphocytes using a different approach. They performed bone marrow reconstitution of Ldlr−/− mice with bone marrow from either B lymphocyte-deficient µMT mice or C57Bl/6 mice43. The µMT mice lack peripheral B lymphocytes due to a deletion of genomic DNA sequences that encode the transmembrane domain of the B cell receptor µ heavy chain44. The µMT bone marrow recipients developed a significant 2-fold increase in atherosclerosis after only 4 weeks of Western feeding compared with C57BL/6 bone marrow recipient mice. This difference remained present even following 12 weeks of Western diet. Taken together, these studies suggested an atheroprotective role for B lymphocytes.
Two more recent studies further expanded our understanding of the role of B lymphocytes in atherosclerosis. Ait-Oufella and Kyaw demonstrated that B lymphocyte depletion with CD20-specific monoclonal antibody resulted in a reduction of atherosclerosis in both Apoe−/− and Ldlr−/− mice45,46. Further, consistent with these findings, injection of splenic B2 lymphocytes from C57BL/6 mice into Apoe−/− Rag-2−/− mice (both B and T lymphocyte deficient) and Apoe−/− µMT (B lymphocyte deficient) resulted in aggravation of atherosclerosis46. Interestingly, depletion of mature B lymphocytes with CD20-specific monoclonal antibody diminished pathogenic T lymphocyte activiation with reduced IFN-γ secretion and IL-17 production45. Taken together, these studies provide evidence for an atherogenic role for B lymphocytes. Notably, CD20 monoclonal antibody treatment depletes B2 lymphocytes more substantially than B1 lymphocytes45,47 and adoptive transfer of B1 lymphocytes to Apoe−/− Rag-2−/−, unlike B2 cells, did not aggravate atherosclerosis46. Moreover, follow-on studies by Kyaw and colleagues recently provided the first direct evidence that B1a lymphocytes are atheroprotective48. Consistent with previous reports49, they demonstrated that splenectomized Apoe−/− mice had a marked depletion of B1a lymphocytes in the peritoneal cavity and an increase in atherosclerosis42,48. Moreover, splenectomized Apoe−/− mice had reduced IgM in plasma and in atherosclerotic lesions48. Adoptive transfer of B1a but not B2 lymphocytes into splectomized mice restored plasma levels and lesion IgM deposits and reduced the size of atherosclerotic lesions and the prevalence of necrotic cores48. Intriguingly, B1a cells lacking the ability to secrete IgM were not able to attenuate the development of atherosclerosis upon adoptive transfer to splenectomized Apoe−/− mice, identifying IgM production as important to B1a lymphocyte-mediated atheroprotection48. In support of secreted IgM as important in B lymphocyte-mediated atheroprotection is the recent study by Lewis and colleagues demonstrating that sIgM−/− Ldlr−/− mice deficient in serum IgM developed larger and more complex atherosclerotic lesions with increased apoptosis in aortic root lesions in response to both low and high-fat diets50. Moreover, a wealth of data provided by the Witztum group and others demonstrated that IgM natural antibodies, such as IgM-E06, bind oxidized lipoproteins, block uptake of oxLDL by macrophage scavenger receptors, and attenuate atherosclerosis51–55. Taken together, results suggest that the effect of B lymphocytes on atherosclerosis is subset dependent with B2 lymphocytes promoting atherosclerosis and B1 lymphocytes attenuating atherosclerosis. The importance of B lymphocyte subsets and the potential for the therapeutic manipulation of B lymphocyte populations in atherosclerosis has recently been reviewed56.
Immune cell infiltrates in the adventitia in humans
Though the presence of lymphocytes in the adventitia in humans has been documented since the early 1960’s, the natural question of whether fully-developed ATLOs similar to those characterized in mice also develop in humans has not been thoroughly explored. In the setting of advanced atherosclerotic abdominal aortic aneurysms, adventitial lymphoid follicles with an abundance of B lymphocytes and T helper cells that stain positively for the proliferation marker, Ki-67, have been reported19,57. These follicles also react with peanut lectin, indicating that they were likely germinal centers of B cell origin. Although these characterizations fall short of demonstrating the intricate high endothelial venules, lymph vessels, and blood vessels, demonstrated by Grabner and colleagues, they do provide compelling evidence that at least some of the adventitial lymphoid organizational characteristics observed in mice may be applicable to humans. Additionally, since atherosclerosis of the coronary arteries is the major clinical problem linked to the mortality associated with atherosclerosis, it is important to consider whether findings of immune regulation from studies in the aorta are also applicable to the coronary arteries. Studies in human coronary arteries have shown adventitial lymphoid infiltrates similar to those present in the human aorta58. While the vasa vasorum of noninflammed human coronary arteries do not form a dense capillary plexus59, like the aorta, with intimal thickening and the progression of coronary artery lesions, the vasa vasorum grow to form a dense network with projections through the media into the intima59, allowing recruitment of immune cells. Further studies are needed to fully characterize the immune cell environment in the adventitia surrounding coronary arteries in humans.
Intimal/Adventitial Communication
Though much of the research on atherogenesis has focused on the intimal environment, increasing evidence supports the idea that the adventitia may influence the progression of intimal lesions3–5,60–63. Several studies suggest that an intact adventitia is necessary for normal intimal endothelial morphology. Surgical removal of the vasa vasorum and subsequent adventitial ischemia results in abnormal intimal endothelium61,62, and restriction of vasa vasorum blood volume through ligation of the intercostal arteries leads to intimal necrosis63. Furthermore, adventitial injury in both the setting of percutaneous coronary angioplasty3,4 and placement of a circumferential silastic collar5 leads to neointimal formation. Together, this evidence indicates that communication exists between the intima and adventitia and that the adventitia plays a role in plaque development.
Collagenous Conduits
A significant contribution to our understanding of potential mechanisms whereby cells in the intimal and medial compartments in the vessel wall may regulate and be regulated by the adventitia came when Grabner and colleagues demonstrated the presence of a dense reticular network of collagenous conduits connecting the medial layer of the vessel wall with the underlying adventitia10. In studies using intravenous injection of fluorescently labeled dextran particles, they elegantly demonstrated that, like lymph node conduits, these adventitial conduits could transport small (10kD), but not large (500 kD) particles into the medial layer of the vessel wall. These conduits could serve as a possible means for the transport of soluble chemokines or antigens between the activated medial smooth muscle cells or intimal plaque and adventitial cells. Notably, these conduit networks are similar to those known to facilitate lymphocyte organization in the white pulp of the spleen64 and to those that facilitate the filtering of soluble substances within lymph nodes65 The observation that ATLO development parallels the severity of the intimal plaque suggests that crosstalk between the intima and adventitia is likely. Lötzer and colleagues described how activation of the LTβR and tumor necrosis factor receptor subfamily, member 1A stimulates medial smooth muscle cells to transform into cells resembling lymphoid tissue organizers that interact with the adventitial inflammatory infiltrates and may play a role in generating the ATLO organizational structures already discussed66.
Vasa vasorum
Another way in which the adventitia communicates with the developing intima is through the vasa vasorum. Studies in dogs and humans have shown that in the noninflammed aorta, the vasa vasorum network acts to nourish the outer segments of the vessel wall and the outer two thirds of the media, while the intima and inner third of the media is fed via passive oxygen diffusion through the luminal endothelium67,68 Caution must be exercised when extrapolating data on immune cell recruitment to the vessel wall in mice to humans as the vessel wall thickness in mice is small enough that the adventitial vasculature may adequately perfuse a large portion of the normal vessel wall. However, with the progression of atherosclerosis in humans, oxygen diffusion is impaired and the vasa vasorum grows to become the primary source of nutrients for the entire vessel wall69,70. These intimal projections of the vasa vasorum have long been known to be important in immune cell recruitment to intimal plaques in humans71,72. Thus, the vasa vasorum may have similar functions in the migration of lymphocytes to the adventitia and subsequent modulation of the immune response in atherosclerotic plaques in humans and mice.
In addition to enabling cellular migration to the intima20, the vasa vasorum likely plays a key role in the migration of lymphocytes to the adventitia and subsequent modulation of the immune response in atherosclerotic plaques. T lymphocytes have been observed entering adventitial immune tissues via the vasa vasorum18,29, and this may be facilitated by increased expression of integrins and selectins critical for inflammatory cell recruitment in microvessels73. Furthermore, microvessels in the proximity of adventitial inflammatory cell clusters have been shown to be reactive to HECA-452 and peripheral lymph node addressin (PNAd), both markers of specialized high endothelial venules (HEVs) by which lymphocytes enter lymphoid tissue, These findings provide evidence that the structures needed for lymphocyte trafficking into functional lymphoid aggregates are present in the adventia7,10. HEVs were also shown to enable the migration of lymphocytes from the vasculature into lymphoid tissue in cases of chronic inflammation74, suggesting these microvessels may be necessary for the development of ATLOs and subsequent immune modulation of atherosclerosis development. It is possible that the conduits described by Grabner and colleagues allow for the transport of antigens, cytokines and chemokines between the intima and adventitia that subsequently promote neovascularization of the vasa vasorum and the coordination of cells recruited through the vasa vasorum into the tertiary lymphoid structures described earlier.
Perivascular adipose tissue
The complex interplay of inflammatory and anti-inflammatory cytokines and adipokines produced within perivascular adipose tissue (PVAT) and their impact on the atherosclerotic plaque has previously been reviewed75. Though many of the cardiovascular risk factors associated with atherosclerosis lead to an increase in the development of PVAT, a recent noninvasive computed tomographic study of patients demonstrated that doubling of a patients pericoronary fat volume resulted in a 2.5-fold increase in the presence of atherosclerotic plaque in the underlying coronary segments, and this remained significant following adjustment for traditional cardiovascular risk factors and overall pericardial fat volume76. PVAT surrounding these atherosclerotic plaques intriguingly demonstrates a marked increase in inflammatory cell density77. Additionally, it is important to recognize that PVAT surrounds the adventitia with no limitation on cellular trafficking between the two tissues. As PVAT increases in size with increasing development of atherosclerosis76, the increased inflammatory cell burden may easily traffic to the adventitia and play a role in developing inflammatory responses. Interestingly, PVAT-derived oxidative stress has been shown to play a major role in endothelial dysfunction of the vasa vasorum78,79 and may also provide a stimulus for formation of microvessels. Additionally, vasoactive factors produced by PVAT79,80 and other adipokines and inflammatory chemokines may not only modulate microvessel function but also lead to increased recruitment of lymphocytes81 Interestingly, in addition to a role in intimal plaque progression, PVAT lymphocytes have also been implicated in the regulation of blood pressure. Angiotensin II infusion increased the number of T cells in the PVAT, T cell production of TNFα, and the amount of both intercellular adhesion molecule-1 and RANTES in the aortic wall, while TNFα agonists hindered the hypertensive response to angiotensin II82. Thus, the complex interplay of PVAT, lymphocytes, and the adventitial vasa vasorum, may impact not only atherogenesis, but other common vascular disorders.
Disruption of the medial barrier
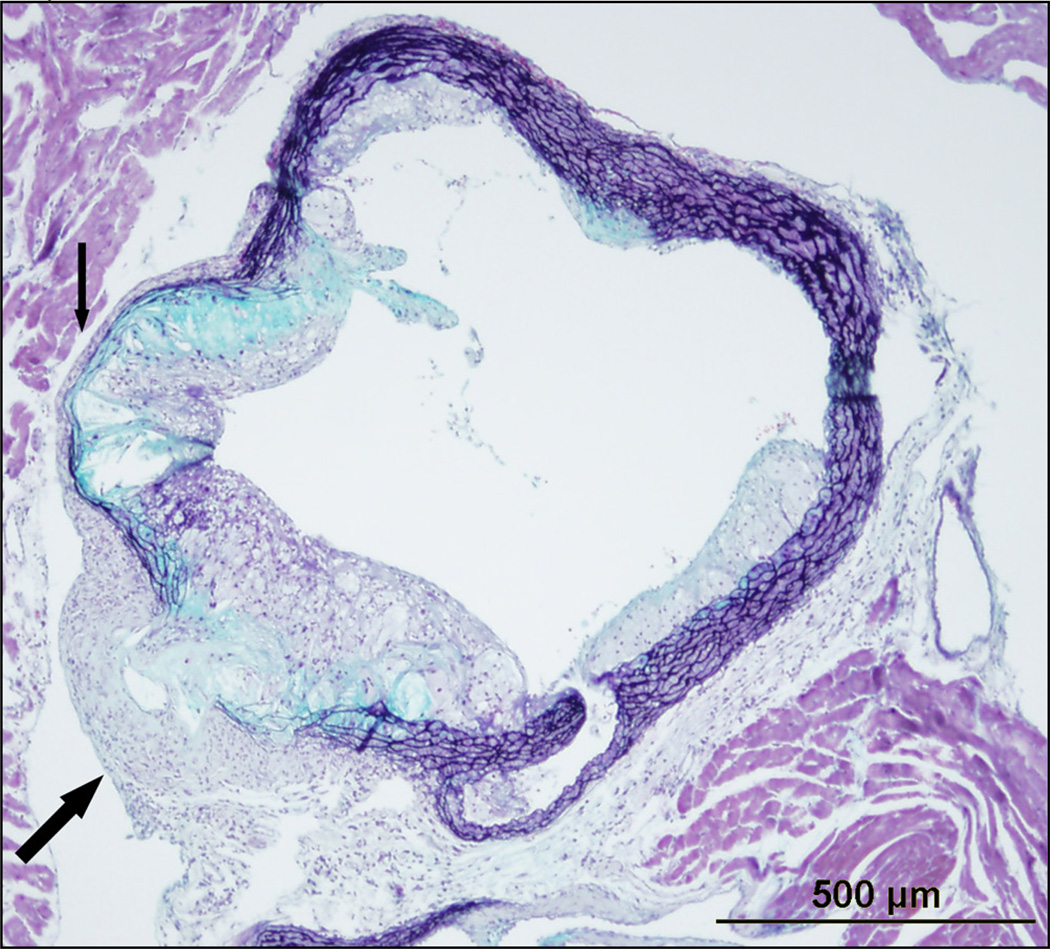
Immunohistochemical studies of atherosclerotic lesions demonstrating accumulations of lymphocytes and macrophages in the intima and the adventitia but not the media83–86 suggest that the media normally acts as a barrier to the trafficking of phagocytes and lymphocytes between intima and adventitia. It has been suggested that the elastic laminae bordering and within the medial layer may provide a physical barrier to lymphocyte trafficking through the media. In addition, interesting studies by Cuffy et.al. and Dal Canto et.al. suggest that the media of the vessel wall may be “immunoprivileged”, inhibiting immune cells from trafficking through it87,88. Initial insights into this phenomenon came with the study by Dal Canto and colleagues. They developed an animal model of chronic vasculitis by infecting IFN-γR−/− mice with γ-herpesvirus 68, noting that the chronicity of the infection was due to the inability of T cells and macrophages to infiltrate the infected media and clear the γ-herpesvirus 6888. Follow on studies demonstrated that medial VSMC produced indoleamine 2,3-dioxygenase (IDO), a factor that mediates immune privilege of the fetus cohabitating within the mother87. Inhibition of IDO by 1-methyl-tryptophan increased medial infiltration by allogeneic T cells suggesting that cytokines produced by VSMC inhibit immune cells from trafficking into the media87. Whether as a physical barrier or due to active anti-inflammatory mechanisms such as production of IDO, the healthy intact media appears to serve as barrier to immune cell trafficking between the intimal and advential compartments. The collagenous conduits and the vasa vasorum provide a method to bypass this barrier and allow communication between these compartments by allowing cytokines, chemokines, and cells a path through the media. In addition to these pathways, disruption of the media itself may allow for greater communication. As early as 1981, Parums and Mitchinson recognized that advanced plaques surrounded by an intact media typically elicit little adventitial inflammation, while medial thinning around advanced plaques was associated with more prevalent adventitial inflammatory infiltrates40. In this setting, dendritic cells and macrophages may migrate from the plaque to the adventitial inflammatory foci, where they subsequently could serve as antigen presenting cells89,90. It has previously been suggested that medial attenuation allows normally sequestered antigens within the intima to be recognized by lymphocytes in the adventitia19. Activation of adventitial immune cells due to compromise of the medial barrier could lead to further release of cytokines and chemokines that could travel to the intima via conduits and enhance inflammatory cell recruitment and intimal lesion formation. The breakdown of the media itself in disease states such as advanced atherosclerosis (Figure 1), arterial injury4,91 and arterial aneurysm provide a context in which the physical barrier of the media could be so compromised that it allows direct cellular trafficking. Studies in porcine models of vascular injury demonstrate that medial disruption results in migration of adventital cells into the intima4,91.
Figure 1. Movat’s stain of a cross section of a 24 week old Ldlr−/− mouse fed a Western diet for 16 weeks.
As indicated by the small arrow, there is thinning of the media and breakdown of the internal and external elastic lamina in the setting of advanced atherosclerotic plaque. The large arrow points to an advanced atherosclerotic lesion that has breached the internal elastic lamina, media, and external elastic lamina with evidence of necrotic core and cholesterol crystals within the adventitia. This breach enables emigration of intimal macrophages, dendritic cells, and lymphocytes to the adventitia and compromises the barrier status of the media. There is compensatory thickening of the adventitia in this region which likely serves to contain the breached media.
Abdominal aortic aneurysms (AAA) are perhaps the most well-known settings of medial breakdown. Though our understanding of the mechanisms leading to AAA continue to evolve, inflammation and matrix degradation are clear hallmarks of its development, and inflammation appears primarily in the adventitia and outer media of the vessel wall60. AAA has long been associated with atherosclerosis, but it is important to recognize that the severity of atherosclerosis does not correlate with AAA development92. Notably, not all patients with severe atherosclerosis develop AAA, and not all patients with AAA are afflicted with severe atherosclerosis, suggesting that the two can develop in a parallel response to common risk factors instead of in a causative manner. It is well known that macrophages in the setting of AAA produce several factors that contribute to the degradation of the extracellular matrix, including cathepsins93, and multiple matrix metalloproteinases94–96. A similar process occurs in atherogenesis, where macrophages produce matrix degrading enzymes, leading to medial barrier disruption and subsequent increased communication between the intima and adventitia97.
Immune cells in the Adventitia of Non-diseased Arteries
Adventitial immune infiltrates that develop in association with advanced intimal atherosclerotic plaques have been well characterized10,18. However, the existence and function of resident immune cells in the aorta is less well understood. Galkina and colleagues provided important insight into this question through studies utilizing flow cytometry which enabled quantitiative analysis of the number and percentage of leukocytes in the vessel wall. Through their carefully controlled studies, they demonstrated the presence of macrophages, dendritic cells, T lymphocytes and B lymphocytes in the normal, non-inflamed aortas in C57BL/6 mice, indicating that there is a resident population of leukocytes in the vessel wall regulated by constitutive trafficking18. Immunohistochemical data from the same study provided evidence that many of these leukocytes, including the lymphocytes, were present in the adventitia. Using en face immunoconfocal microscopy, Jongstra-Bilen and colleagues demonstrated that the adventitia of normocholesterolemic 3–6 month old C57BL/6 mice contain accumulations of T cells and macrophages, providing further evidence for a constituitive immune cell presence in the non-inflamed adventitia98. Mice on the atherogenic Apoe−/− background have greater numbers of baseline aortic immune cells than C57BL/6 mice even prior to Western diet feeding. These numbers are greater in the absence of visible intimal lesions, suggesting adventitial accumulation. Of note, while total leukocyte numbers in the aorta of Apoe−/− mice are greater than in C57BL/6 mice, the percentage of specific leukocyte cell types differs. Apoe−/− have an increase in the percentage of macrophages and dendritic cells compared to C57BL/6 mice, but no change in the percentage of T cells and reduced B cell percentages18, suggesting a link between the type of adventitial immune cells and propensity of the mice to develop atherosclerosis. It is intriguing to hypothesize that the immune milieu of the normal vessel may be present to respond to early atherogenic signals and perform innate anti-inflammatory functions which become altered in the setting of chronic hyperlipemia (Apoe−/− mice) and overwhelmed in the setting of marked hyperlipidemia (addition of Western diet) or other vascular insults. Of note, the greatest percentage of B lymphocytes is seen in the aorta of C57BL/6 mice. Moreover, studies in Apoe−/− mice with deletion of the helix-loop-helix factor Inhibitor of Differentiation 3 (Id3) which results in significantly reduced number of B cells in the aorta reveals significantly more atherosclerosis compared to Apoe−/− controls in response to Western diet feeding99. Atherosclerosis is present in Id3−/−Apoe−/− mice as early as 4 weeks after Western diet feeding, raising the intriquing hypothesis that B lymphocytes resident in aortic adventitia may limit early and exaggerated atherosclerosis. Notably, a functionally significant polymorphism in the human Id3 gene is associated with increased intima medial thickness in humans100. Indeed, analysis of human vessels also supports the existence of resident adventitial leukocytes. Immunohistochemical studies in aortas of children prior to the development of atherosclerosis have reported leukocytes surrounding the vasa vasorum in the adventitia20. However, more studies of the adventitia of normal human arteries is needed to fully characterize the immune cells present.
Imaging Lymphocytes and the Adventitia in Atherosclerosis
Molecular and cellular imaging can characterize lymphocyte biology in vivo101–104,105, and several of these, and other approaches can and have been applied to elucidating leukocyte biology in the adventitia. For example, intravital microscopy has enabled clear visualization of the process of leukocyte rolling and adhesion on the vascular endothelium106, serving as a potentially powerful technique to study lymphocyte interactions within small vessels. Intravital microscopy was used to demonstrate the importance of P-selectin in leukocyte adhesion to the endothelium, as P-selectin-deficient mice have significantly reduced leukocyte recruitment and attenuated inflammatory disease101. Two-photon intravital microscopy can even visualize the interactions of living cells deep within tissues without significant concern for phototoxicity or photobleaching107,108. Detection of adhesion molecule expression using synthetic optical probes can also be used to identify key molecules in the adventitia regulating leukocyte recruitment and adventitial growth. Fluorophore binding of an αvβ3 integrin targeted peptide identified marked expression of this adhesion molecule in the adventitia of hyperlipidemic rabbits that correlated with adventitial thickness109. In vivo imaging of this and other synthetic optical probes with advanced imaging modalities could provide key insights into factors regulating the expression of these molecules in the adventitia throughout the course of disease. Additionally, lymphocytes can be engineered to study their role in the immune system through production of bioluminescence activity or detection via positron emission tomography following specific gene activation102–104. Galkina et. al. utilized a mouse model where green fluorescent protein (GFP) was knocked in to the CXCR6 locus to determine that T lymphocyte recruitment to the vessel wall is mediated by CXCR6110. These T lymphocytes are predominantly found in the adventitial layer6,18. Multiphoton microscopy of the carotid artery of Apoe−/− mice indeed demonstrated that adoptively transferred labeled lymphocytes were mainly found in the adventitial layer29. Noninvasive optical imaging of luminescent proteins could be applied to help determine the site and timing of lymphocyte recruitment and activation during the course of atherogenesis. Similarly, multimodality contrast agents can be targeted to molecules on lymphocytes and determine not only functional characteristics but also identify and track certain lymphocyte subsets111,105,112.
Simple imaging techniques have recently led to the novel finding that B lymphocytes home to regions prone to and with existing atherosclerosis. Initial imaging, validated by flow cytometry data, confirmed that B lymphocytes were present in non-diseased aorta99. Quantitation of near-infrared fluorescence-mediated tomographic imaging of Cy5.5 conjugated to CD19 or B220-specific antibodies demonstrated B lymphocytes in the aorta of Apoe−/− mice fed a chow diet. The B lymphocyte-deficient µMT Apoe−/− mouse was used as a control for nonspecific signal. There was significantly greater fluorescence present in the aorta of the Apoe−/− mouse when compared with the aorta from the µMT Apoe−/− mouse. Interestingly, this technique also provided evidence that B lymphocytes were predominantly detected in regions of the aorta prone to the development of atherosclerosis (arch and abdominal aorta). Phosphor imaging of B lymphocytes radiolabeled with indium-111 oxyquinoline and adoptively transferred into µMT Apoe−/− mice demonstrated constituitive homing of B lymphocytes to these very same regions. Moreover, transfer of B lymphocytes radiolabeled with indium-111 oxyquinoline to µMT Apoe−/− mice with existing early atherosclerosis revealed that B lymphocytes trafficked predominantly to regions of lipid deposition99. Advantages of adapting this technique to perform imaging of radiolabeled immune cells are the ability to isolate and label specific cell types, the high sensitivity afforded by the modality, and the lack of impact on cellular function, While labeling specific cells via surface molecules may alter cellular function, non-specific radiolabeling with indium-111 oxine is unlikely to significantly impact cell surface receptor expression, cellular trafficking or function113,114. The technique could have been further improved through incorporation of radioautographic microscopy, which would have helped determine the location of the radiolabeled cell within the layers of the artery. To complement this approach, labeling lymphocytes with CFSE or other analogues have been used. Histology of aortic sections after transfer of labeled B cells demonstrate that they trafficked to the adventitia18,99.
Finally, the characterization of the adventitial vasa vasorum network has also provided significant insight into potential clinical imaging techniques. Contrast-enhanced ultrasound has demonstrated that increased contrast in the adventitia strongly correlates with adventitial vasa vasorum and the progression of atherosclerosis115–118. Contrast-enhanced magnetic resonance imaging provides another means of quantifying the presence of vasa vasorum in atherosclerotic lesions119. Furthermore, multimodality imaging with MRI and positron emission tomographic imaging enabled highly sensitive detection of neovessels within the plaque and the necessary spatial resolution to distinguish intimal neovessels from the adventitial vasa vasorum120. The ability to target adhesion molecules expressed on the endothelium may also enable early detection of neovascularization and characterization of atherosclerosis121. These imaging agents can also be readily modified to deliver therapeutic agents that can disrupt the formation of vasa vasorum and attenuate the progression of atherosclerosis122. Thus, the same imaging agents used to perform targeted imaging can also serve as a vehicle to provide targeted drug delivery. Molecular and cellular imaging can be a powerful tool to characterize and study atherosclerotic plaque in animals13, Many of these techniques also hold the potential to provide structural characterization of molecules and cell types present in all layers of the artery wall in patients throughout the course of atherosclerosic lesion development. The current knowledge on molecular imaging of atherosclerosis in clinical practice has been thoroughly reviewed123,124. These advanced imaging techniques may help in the early identification of patients with atherosclerosis who are at risk for rapid lesion progression or plaque rupture and subsequent acute atherothrombotic events.
Conclusion
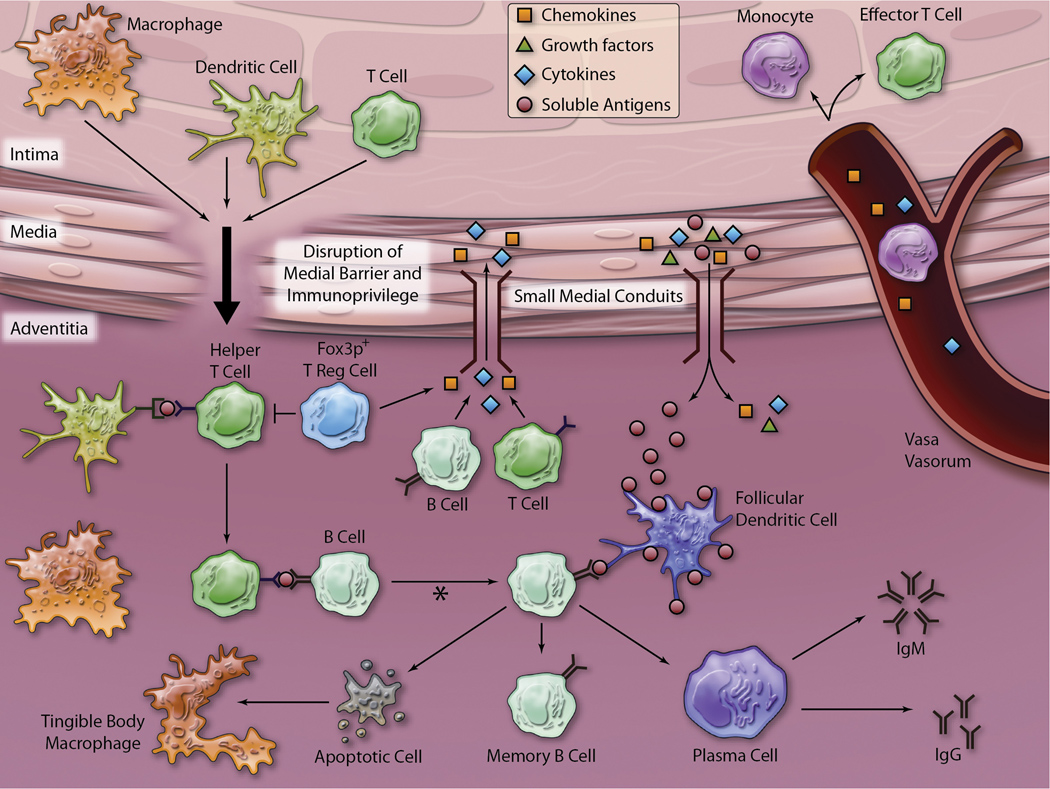
Evidence continues to mount highlighting the importance of lymphocytes and the adventitia in the coordination of the immune response in atherosclerosis. As illustrated in Figure 3, inflammatory chemokines, cytokines, and growth factors from the atherosclerotic plaque in the intima result in neovascularization arising from the adventitial vasa vasorum, accumulation of adventitial T and B lymphocytes which organize along with other inflammatory cells to form early immune tissues and eventually give rise to form ATLOs. ATLOs may serve as sites for immune responses to plaque antigens such as HSP60 or modified lipoproteins. Resident immune cell accumulation in the adventitia and PVAT may play a role in innate protection from atherogenic antigens or cytokines. Finally, PVAT may modulate inflammation through migration of immune cells in the PVAT into the adjacent adventitia and transfer of adipokines, chemokines, and cytokines from local adipocytes across the media via conduits and microvessels. While further studies are necessary to fully characterize the importance of the adventitia in regulating the immune response to atherosclerosis, it is clear that we can no longer view atherosclerosis as a disease of the intima but recognize it as an inflammatory disease of the entire arterial wall.
Figure 3. Proposed model of immune system activity in the adventitia.
Small medial conduits, as demonstrated by Gräbner and colleagues, may enable soluble antigens, cytokines, growth factors, and chemokines to traffic between the intima and the adventitia. Chemokines and cytokines passing through these conduits may aid in the recruitment of leukocytes to the growing atherosclerotic plaque via the vasa vasorum. Additionally, growth factors secreted by cells within the growing plaque may stimulate neovascularization from the adventitial vasa vasorum. As shown in Figure 1, the media underlying advanced atherosclerotic plaques may be breached, compromising the barrier status of the media. In this model, cells can then cross the media and present antigen to helper T lymphocytes. The helper T lymphocytes (negatively regulated by Fox3p+ T reg lymphocytes) then stimulate B lymphocytes to undergo clonal expansion, isotype switching, and affinity maturation (asterisk). B lymphocytes with low affinity for antigen presented on follicular dendritic cells may undergo apoptosis and be removed by tingible body macrophages via efferocytosis. The B lymphocytes selected through high affinity interaction with antigen presented on follicular dendritic cells may undergo differentiation into memory B lymphocytes and plasma cells that may produce IgM or IgG autoantibodies locally or traffick to lymph nodes.
Acknowledgments
Sources of Research Support: Figures in this review were generated from research supported in part by NIH training grant T32 HL007355-29 (MJL), AHA Mid-Atlantic Affiliate postdoctoral fellowship award 10POST3560000 (MJL), NIH grant R01 HL096447 and NIH P01 HL55798 (CAM).
Non-standard Abbreviations and Acronyms
- AAA
Abdominal Aortic Aneurysm
- APC
Antigen Presenting Cell
- ATLO
Aortic Tertiary Lymphoid Organ
- Ig
Immunoglobulin
- IFN-γ
Interferon gamma
- IL
Interleukin
- MIP-1α
Macrophage Inflammatory Protein-1 alpha
- MMP
Matrix Metalloproteinase
- Th
T Helper
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
References
- 1.Heart Disease and Stroke Statistics-2006 Update. Dallas, TX: The American Heart Association; 2006. [Google Scholar]
- 2.Kannel WB. Some lessons in cardiovascular epidemiology from Framingham. Am J Cardiol. 1976;37:269–282. doi: 10.1016/0002-9149(76)90323-4. [DOI] [PubMed] [Google Scholar]
- 3.Wilcox JN, Waksman R, King SB, Scott NA. The role of the adventitia in the arterial response to angioplasty: the effect of intravascular radiation. Int J Radiat Oncol Biol Phys. 1996;36:789–796. doi: 10.1016/s0360-3016(96)00299-4. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y, O'Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94:1655–1664. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- 5.Booth RF, Martin JF, Honey AC, Hassall DG, Beesley JE, Moncada S. Rapid development of atherosclerotic lesions in the rabbit carotid artery induced by perivascular manipulation. Atherosclerosis. 1989;76:257–268. doi: 10.1016/0021-9150(89)90109-3. [DOI] [PubMed] [Google Scholar]
- 6.Moos MP, John N, Grabner R, Nossmann S, Gunther B, Vollandt R, Funk CD, Kaiser B, Habenicht AJ. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:2386–2391. doi: 10.1161/01.ATV.0000187470.31662.fe. [DOI] [PubMed] [Google Scholar]
- 7.Houtkamp MA, de Boer OJ, van der Loos CM, van der Wal AC, Becker AE. Adventitial infiltrates associated with advanced atherosclerotic plaques: structural organization suggests generation of local humoral immune responses. J Pathol. 2001;193:263–269. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH774>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz CJ, Mitchell JR. Cellular infiltration of the human arterial adventitia associated with atheromatous plaques. Circulation. 1962;26:73–78. doi: 10.1161/01.cir.26.1.73. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe M, Sangawa A, Sasaki Y, Yamashita M, Tanaka-Shintani M, Shintaku M, Ishikawa Y. Distribution of inflammatory cells in adventitia changed with advancing atherosclerosis of human coronary artery. J Atheroscler Thromb. 2007;14:325–331. doi: 10.5551/jat.e489. [DOI] [PubMed] [Google Scholar]
- 10.Grabner R, Lotzer K, Dopping S, Hildner M, Radke D, Beer M, Spanbroek R, Lippert B, Reardon CA, Getz GS, Fu YX, Hehlgans T, Mebius RE, van der Wall M, Kruspe D, Englert C, Lovas A, Hu D, Randolph GJ, Weih F, Habenicht AJ. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J Exp Med. 2009;206:233–248. doi: 10.1084/jem.20080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higuchi ML, Gutierrez PS, Bezerra HG, Palomino SA, Aiello VD, Silvestre JM, Libby P, Ramires JA. Comparison between adventitial and intimal inflammation of ruptured and nonruptured atherosclerotic plaques in human coronary arteries. Arq Bras Cardiol. 2002;79:20–24. doi: 10.1590/s0066-782x2002001000003. [DOI] [PubMed] [Google Scholar]
- 12.Packard RR, Lichtman AH, Libby P. Innate and adaptive immunity in atherosclerosis. Semin Immunopathol. 2009;31:5–22. doi: 10.1007/s00281-009-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipinski MJ, Fuster V, Fisher EA, Fayad ZA. Technology Insight: targeting of biological molecules for evaluation of high-risk atherosclerotic plaques with magnetic resonance imaging. Nat Clin Pract Cardiovasc Med. 2004;1:48–55. doi: 10.1038/ncpcardio0013. [DOI] [PubMed] [Google Scholar]
- 14.Hyafil F, Cornily JC, Feig JE, Gordon R, Vucic E, Amirbekian V, Fisher EA, Fuster V, Feldman LJ, Fayad ZA. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med. 2007;13:636–641. doi: 10.1038/nm1571. [DOI] [PubMed] [Google Scholar]
- 15.Amirbekian V, Lipinski MJ, Briley-Saebo KC, Amirbekian S, Aguinaldo JG, Weinreb DB, Vucic E, Frias JC, Hyafil F, Mani V, Fisher EA, Fayad ZA. Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc Natl Acad Sci U S A. 2007;104:961–966. doi: 10.1073/pnas.0606281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipinski MJ, Frias JC, Amirbekian V, Briley-Saebo KC, Mani V, Samber D, Abbate A, Aguinaldo JG, Massey D, Fuster V, Vetrovec GW, Fayad ZA. Macrophage-specific lipid-based nanoparticles improve cardiac magnetic resonance detection and characterization of human atherosclerosis. JACC Cardiovasc Imaging. 2009;2:637–647. doi: 10.1016/j.jcmg.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudd JH, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, Fuster V, Fayad ZA. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50:892–896. doi: 10.1016/j.jacc.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parums DV, Dunn DC, Dixon AK, Mitchinson MJ. Characterization of inflammatory cells in a patient with chronic periaortitis. Am J Cardiovasc Pathol. 1990;3:121–129. [PubMed] [Google Scholar]
- 20.Wick G, Romen M, Amberger A, Metzler B, Mayr M, Falkensammer G, Xu Q. Atherosclerosis, autoimmunity, and vascular-associated lymphoid tissue. Faseb J. 1997;11:1199–1207. doi: 10.1096/fasebj.11.13.9367355. [DOI] [PubMed] [Google Scholar]
- 21.Furtado GC, Marinkovic T, Martin AP, Garin A, Hoch B, Hubner W, Chen BK, Genden E, Skobe M, Lira SA. Lymphotoxin beta receptor signaling is required for inflammatory lymphangiogenesis in the thyroid. Proc Natl Acad Sci U S A. 2007;104:5026–5031. doi: 10.1073/pnas.0606697104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marinkovic T, Garin A, Yokota Y, Fu YX, Ruddle NH, Furtado GC, Lira SA. Interaction of mature CD3+CD4+ T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid. J Clin Invest. 2006;116:2622–2632. doi: 10.1172/JCI28993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reif K, Ekland EH, Ohl L, Nakano H, Lipp M, Forster R, Cyster JG. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416:94–99. doi: 10.1038/416094a. [DOI] [PubMed] [Google Scholar]
- 24.Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 25.Muniz LR, Pacer ME, Lira SA, Furtado GC. A Critical Role for Dendritic Cells in the Formation of Lymphatic Vessels within Tertiary Lymphoid Structures. J Immunol. 2011 doi: 10.4049/jimmunol.1004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallat Z, Taleb S, Ait-Oufella H, Tedgui A. The role of adaptive T cell immunity in atherosclerosis. J Lipid Res. 2009;50(Suppl):S364–S369. doi: 10.1194/jlr.R800092-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tavora F, Kutys R, Li L, Ripple M, Fowler D, Burke A. Adventitial lymphocytic inflammation in human coronary arteries with intimal atherosclerosis. Cardiovasc Pathol. 2010;19:e61–e68. doi: 10.1016/j.carpath.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Maffia P, Zinselmeyer BH, Ialenti A, Kennedy S, Baker AH, McInnes IB, Brewer JM, Garside P. Images in cardiovascular medicine. Multiphoton microscopy for 3-dimensional imaging of lymphocyte recruitment into apolipoprotein-E-deficient mouse carotid artery. Circulation. 2007;115:e326–e328. doi: 10.1161/CIRCULATIONAHA.106.658492. [DOI] [PubMed] [Google Scholar]
- 30.Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135:169–175. [PMC free article] [PubMed] [Google Scholar]
- 31.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansson GK, Jonasson L. The discovery of cellular immunity in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2009;29:1714–1717. doi: 10.1161/ATVBAHA.108.179713. [DOI] [PubMed] [Google Scholar]
- 33.Zhou X, Robertson AK, Hjerpe C, Hansson GK. Adoptive transfer of CD4+ T cells reactive to modified low-density lipoprotein aggravates atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:864–870. doi: 10.1161/01.ATV.0000206122.61591.ff. [DOI] [PubMed] [Google Scholar]
- 34.Paulsson G, Zhou X, Tornquist E, Hansson GK. Oligoclonal T cell expansions in atherosclerotic lesions of apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:10–17. doi: 10.1161/01.atv.20.1.10. [DOI] [PubMed] [Google Scholar]
- 35.Rossmann A, Henderson B, Heidecker B, Seiler R, Fraedrich G, Singh M, Parson W, Keller M, Grubeck-Loebenstein B, Wick G. T-cells from advanced atherosclerotic lesions recognize hHSP60 and have a restricted T-cell receptor repertoire. Exp Gerontol. 2008;43:229–237. doi: 10.1016/j.exger.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Hermansson A, Ketelhuth DF, Strodthoff D, Wurm M, Hansson EM, Nicoletti A, Paulsson-Berne G, Hansson GK. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J Exp Med. 2010;207:1081–1093. doi: 10.1084/jem.20092243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 38.Aubry MC, Riehle DL, Edwards WD, Maradit-Kremers H, Roger VL, Sebo TJ, Gabriel SE. B-Lymphocytes in plaque and adventitia of coronary arteries in two patients with rheumatoid arthritis and coronary atherosclerosis: preliminary observations. Cardiovasc Pathol. 2004;13:233–236. doi: 10.1016/j.carpath.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Burioni R, Canducci F, Saita D, Perotti M, Mancini N, De Marco D, Clementi N, Chieffo A, Denaro M, Cianflone D, Manfredi AA, Colombo A, Maseri A, Clementi M. Antigen-driven evolution of B lymphocytes in coronary atherosclerotic plaques. J Immunol. 2009;183:2537–2544. doi: 10.4049/jimmunol.0901076. [DOI] [PubMed] [Google Scholar]
- 40.Parums D, Mitchinson MJ. Demonstration of immunoglobulin in the neighbourhood of advanced atherosclerotic plaques. Atherosclerosis. 1981;38:211–216. doi: 10.1016/0021-9150(81)90118-0. [DOI] [PubMed] [Google Scholar]
- 41.Wehner JR, Fox-Talbot K, Halushka MK, Ellis C, Zachary AA, Baldwin WM., 3rd B cells and plasma cells in coronaries of chronically rejected cardiac transplants. Transplantation. 2010;89:1141–1148. doi: 10.1097/TP.0b013e3181d3f271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109:745–753. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Major AS, Fazio S, Linton MF. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2002;22:1892–1898. doi: 10.1161/01.atv.0000039169.47943.ee. [DOI] [PubMed] [Google Scholar]
- 44.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 45.Ait-Oufella H, Herbin O, Bouaziz JD, Binder CJ, Uyttenhove C, Laurans L, Taleb S, Van Vre E, Esposito B, Vilar J, Sirvent J, Van Snick J, Tedgui A, Tedder TF, Mallat Z. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2010;207:1579–1587. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kyaw T, Tay C, Khan A, Dumouchel V, Cao A, To K, Kehry M, Dunn R, Agrotis A, Tipping P, Bobik A, Toh BH. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J Immunol. 2010;185:4410–4419. doi: 10.4049/jimmunol.1000033. [DOI] [PubMed] [Google Scholar]
- 47.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, Tedder TF. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med. 2004;199:1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kyaw T, Tay C, Krishnamurthi S, Kanellakis P, Agrotis A, Tipping P, Bobik A, Toh BH. B1a B Lymphocytes Are Atheroprotective by Secreting Natural IgM That Increases IgM Deposits and Reduces Necrotic Cores in Atherosclerotic Lesions. Circ Res. 2011 doi: 10.1161/CIRCRESAHA.111.248542. [DOI] [PubMed] [Google Scholar]
- 49.Wardemann H, Boehm T, Dear N, Carsetti R. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J Exp Med. 2002;195:771–780. doi: 10.1084/jem.20011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis MJ, Malik TH, Ehrenstein MR, Boyle JJ, Botto M, Haskard DO. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:417–426. doi: 10.1161/CIRCULATIONAHA.109.868158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wool GD, Cabana VG, Lukens J, Shaw PX, Binder CJ, Witztum JL, Reardon CA, Getz GS. 4F Peptide reduces nascent atherosclerosis and induces natural antibody production in apolipoprotein E-null mice. Faseb J. 2011;25:290–300. doi: 10.1096/fj.10-165670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horkko S, Bird DA, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner JA, Friedman P, Dennis EA, Curtiss LK, Palinski W, Witztum JL. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaw PX, Horkko S, Tsimikas S, Chang MK, Palinski W, Silverman GJ, Chen PP, Witztum JL. Human-derived anti-oxidized LDL autoantibody blocks uptake of oxidized LDL by macrophages and localizes to atherosclerotic lesions in vivo. Arterioscler Thromb Vasc Biol. 2001;21:1333–1339. doi: 10.1161/hq0801.093587. [DOI] [PubMed] [Google Scholar]
- 54.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Backhed F, Miller YI, Horkko S, Corr M, Witztum JL, Binder CJ. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Binder CJ, Shaw PX, Chang MK, Boullier A, Hartvigsen K, Horkko S, Miller YI, Woelkers DA, Corr M, Witztum JL. The role of natural antibodies in atherogenesis. J Lipid Res. 2005;46:1353–1363. doi: 10.1194/jlr.R500005-JLR200. [DOI] [PubMed] [Google Scholar]
- 56.Kyaw T, Tipping P, Toh BH, Bobik A. Current understanding of the role of B cell subsets and intimal and adventitial B cells in atherosclerosis. Curr Opin Lipidol. 2011 doi: 10.1097/MOL.0b013e32834adaf3. [DOI] [PubMed] [Google Scholar]
- 57.Ramshaw AL, Parums DV. Immunohistochemical characterization of inflammatory cells associated with advanced atherosclerosis. Histopathology. 1990;17:543–552. doi: 10.1111/j.1365-2559.1990.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 58.Mitchinson MJ. Chronic periaortitis and periarteritis. Histopathology. 1984;8:589–600. doi: 10.1111/j.1365-2559.1984.tb02371.x. [DOI] [PubMed] [Google Scholar]
- 59.Barger AC, Beeuwkes R, 3rd, Lainey LL, Silverman KJ. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med. 1984;310:175–177. doi: 10.1056/NEJM198401193100307. [DOI] [PubMed] [Google Scholar]
- 60.Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res. 2007;75:640–648. doi: 10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barker SG, Tilling LC, Miller GC, Beesley JE, Fleetwood G, Stavri GT, Baskerville PA, Martin JF. The adventitia and atherogenesis: removal initiates intimal proliferation in the rabbit which regresses on generation of a 'neoadventitia'. Atherosclerosis. 1994;105:131–144. doi: 10.1016/0021-9150(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 62.Chignier E, Eloy R. Adventitial resection of small artery provokes endothelial loss and intimal hyperplasia. Surg Gynecol Obstet. 1986;163:327–334. [PubMed] [Google Scholar]
- 63.Heistad DD, Marcus ML, Larsen GE, Armstrong ML. Role of vasa vasorum in nourishment of the aortic wall. Am J Physiol. 1981;240:H781–H787. doi: 10.1152/ajpheart.1981.240.5.H781. [DOI] [PubMed] [Google Scholar]
- 64.Nolte MA, Belien JA, Schadee-Eestermans I, Jansen W, Unger WW, van Rooijen N, Kraal G, Mebius RE. A conduit system distributes chemokines and small blood-borne molecules through the splenic white pulp. J Exp Med. 2003;198:505–512. doi: 10.1084/jem.20021801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 66.Lotzer K, Dopping S, Connert S, Grabner R, Spanbroek R, Lemser B, Beer M, Hildner M, Hehlgans T, van der Wall M, Mebius RE, Lovas A, Randolph GJ, Weih F, Habenicht AJ. Mouse aorta smooth muscle cells differentiate into lymphoid tissue organizer-like cells on combined tumor necrosis factor receptor-1/lymphotoxin beta-receptor NF-kappaB signaling. Arterioscler Thromb Vasc Biol. 2010;30:395–402. doi: 10.1161/ATVBAHA.109.191395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heistad DD, Marcus ML, Law EG, Armstrong ML, Ehrhardt JC, Abboud FM. Regulation of blood flow to the aortic media in dogs. J Clin Invest. 1978;62:133–140. doi: 10.1172/JCI109097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geiringer E. Intimal vascularization and atherosclerosis. J Pathol Bacteriol. 1951;63:201–211. doi: 10.1002/path.1700630204. [DOI] [PubMed] [Google Scholar]
- 69.Heistad DD, Armstrong ML. Blood flow through vasa vasorum of coronary arteries in atherosclerotic monkeys. Arteriosclerosis. 1986;6:326–331. doi: 10.1161/01.atv.6.3.326. [DOI] [PubMed] [Google Scholar]
- 70.Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V. Neovascularization in human atherosclerosis. Circulation. 2006;113:2245–2252. doi: 10.1161/CIRCULATIONAHA.105.578955. [DOI] [PubMed] [Google Scholar]
- 71.O'Brien KD, McDonald TO, Chait A, Allen MD, Alpers CE. Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation. 1996;93:672–682. doi: 10.1161/01.cir.93.4.672. [DOI] [PubMed] [Google Scholar]
- 72.O'Brien KD, Allen MD, McDonald TO, Chait A, Harlan JM, Fishbein D, McCarty J, Ferguson M, Hudkins K, Benjamin CD, et al. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993;92:945–951. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balakrishnan KR, Kuruvilla S. Images in cardiovascular medicine. Role of inflammation in atherosclerosis: immunohistochemical and electron microscopic images of a coronary endarterectomy specimen. Circulation. 2006;113:e41–e43. doi: 10.1161/CIRCULATIONAHA.105.537464. [DOI] [PubMed] [Google Scholar]
- 74.Freemont AJ. Functional and biosynthetic changes in endothelial cells of vessels in chronically inflamed tissues: evidence for endothelial control of lymphocyte entry into diseased tissues. J Pathol. 1988;155:225–230. doi: 10.1002/path.1711550308. [DOI] [PubMed] [Google Scholar]
- 75.Rajsheker S, Manka D, Blomkalns AL, Chatterjee TK, Stoll LL, Weintraub NL. Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol. 2010;10:191–196. doi: 10.1016/j.coph.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mahabadi AA, Reinsch N, Lehmann N, Altenbernd J, Kalsch H, Seibel RM, Erbel R, Mohlenkamp S. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: a segment analysis. Atherosclerosis. 2010;211:195–199. doi: 10.1016/j.atherosclerosis.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 77.Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, Meda P, Chizzolini C, Meier CA. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- 78.Ketonen J, Shi J, Martonen E, Mervaala E. Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57Bl/6 mice. Circ J. 74:1479–1487. doi: 10.1253/circj.cj-09-0661. [DOI] [PubMed] [Google Scholar]
- 79.Gao YJ, Takemori K, Su LY, An WS, Lu C, Sharma AM, Lee RM. Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res. 2006;71:363–373. doi: 10.1016/j.cardiores.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 80.Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. Faseb J. 2002;16:1057–1063. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 81.Zhang H, Zhang C. Regulation of Microvascular Function by Adipose Tissue in Obesity and Type 2 Diabetes: Evidence of an Adipose-Vascular Loop. Am J Biomed Sci. 2009;1:133–142. doi: 10.5099/aj090200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Billingham ME. Cardiac transplant atherosclerosis. Transplant Proc. 1987;19:19–25. [PubMed] [Google Scholar]
- 84.Emeson EE, Robertson AL., Jr T lymphocytes in aortic and coronary intimas. Their potential role in atherogenesis. Am J Pathol. 1988;130:369–376. [PMC free article] [PubMed] [Google Scholar]
- 85.van der Wal AC, Das PK, Bentz van de Berg D, van der Loos CM, Becker AE. Atherosclerotic lesions in humans. In situ immunophenotypic analysis suggesting an immune mediated response. Lab Invest. 1989;61:166–170. [PubMed] [Google Scholar]
- 86.Salomon RN, Hughes CC, Schoen FJ, Payne DD, Pober JS, Libby P. Human coronary transplantation-associated arteriosclerosis. Evidence for a chronic immune reaction to activated graft endothelial cells. Am J Pathol. 1991;138:791–798. [PMC free article] [PubMed] [Google Scholar]
- 87.Cuffy MC, Silverio AM, Qin L, Wang Y, Eid R, Brandacher G, Lakkis FG, Fuchs D, Pober JS, Tellides G. Induction of indoleamine 2,3-dioxygenase in vascular smooth muscle cells by interferon-gamma contributes to medial immunoprivilege. J Immunol. 2007;179:5246–5254. doi: 10.4049/jimmunol.179.8.5246. [DOI] [PubMed] [Google Scholar]
- 88.Dal Canto AJ, Swanson PE, O'Guin AK, Speck SH, Virgin HW. IFN-gamma action in the media of the great elastic arteries, a novel immunoprivileged site. J Clin Invest. 2001;107:R15–R22. doi: 10.1172/JCI11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bobryshev YV. Dendritic cells and their involvement in atherosclerosis. Curr Opin Lipidol. 2000;11:511–517. doi: 10.1097/00041433-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 90.Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med. 2008;5:91–102. doi: 10.1038/ncpcardio1086. [DOI] [PubMed] [Google Scholar]
- 91.Scott NA, Cipolla GD, Ross CE, Dunn B, Martin FH, Simonet L, Wilcox JN. Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation. 1996;93:2178–2187. doi: 10.1161/01.cir.93.12.2178. [DOI] [PubMed] [Google Scholar]
- 92.Johnsen SH, Forsdahl SH, Singh K, Jacobsen BK. Atherosclerosis in abdominal aortic aneurysms: a causal event or a process running in parallel? The Tromso study. Arterioscler Thromb Vasc Biol. 2010;30:1263–1268. doi: 10.1161/ATVBAHA.110.203588. [DOI] [PubMed] [Google Scholar]
- 93.Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998;102:576–583. doi: 10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nollendorfs A, Greiner TC, Nagase H, Baxter BT. The expression and localization of membrane type-1 matrix metalloproteinase in human abdominal aortic aneurysms. J Vasc Surg. 2001;34:316–322. doi: 10.1067/mva.2001.115962. [DOI] [PubMed] [Google Scholar]
- 95.Newman KM, Jean-Claude J, Li H, Scholes JV, Ogata Y, Nagase H, Tilson MD. Cellular localization of matrix metalloproteinases in the abdominal aortic aneurysm wall. J Vasc Surg. 1994;20:814–820. doi: 10.1016/s0741-5214(94)70169-5. [DOI] [PubMed] [Google Scholar]
- 96.Raffetto JD, Barros YV, Wells AK, Khalil RA. MMP-2 induced vein relaxation via inhibition of [Ca2+]e-dependent mechanisms of venous smooth muscle contraction. Role of RGD peptides. J Surg Res. 2008;159:755–764. doi: 10.1016/j.jss.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu P, Sun M, Sader S. Matrix metalloproteinases in cardiovascular disease. Can J Cardiol. 2006;22(Suppl B):25B–30B. doi: 10.1016/s0828-282x(06)70983-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med. 2006;203:2073–2083. doi: 10.1084/jem.20060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Doran AC, Lipinski MJ, Oldham SN, Garmey JC, Campbell KA, Skaflen MD, Cutchins A, Lee DJ, Glover DK, Kelly KA, Galkina EV, Ley K, Witztum JL, Tsimikas S, Bender TP, McNamara CA. B-Cell Aortic Homing and Atheroprotection Depend on Id3. Circ Res. 2011 doi: 10.1161/CIRCRESAHA.111.256438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Doran AC, Lehtinen AB, Meller N, Lipinski MJ, Slayton RP, Oldham SN, Skaflen MD, Yeboah J, Rich SS, Bowden DW, McNamara CA. Id3 is a novel atheroprotective factor containing a functionally significant single-nucleotide polymorphism associated with intima-media thickness in humans. Circ Res. 2010;106:1303–1311. doi: 10.1161/CIRCRESAHA.109.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rivera-Nieves J, Burcin TL, Olson TS, Morris MA, McDuffie M, Cominelli F, Ley K. Critical role of endothelial P-selectin glycoprotein ligand 1 in chronic murine ileitis. J Exp Med. 2006;203:907–917. doi: 10.1084/jem.20052530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Balagopalan L, Sherman E, Barr VA, Samelson LE. Imaging techniques for assaying lymphocyte activation in action. Nat Rev Immunol. 2011;11:21–33. doi: 10.1038/nri2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koya RC, Mok S, Comin-Anduix B, Chodon T, Radu CG, Nishimura MI, Witte ON, Ribas A. Kinetic phases of distribution and tumor targeting by T cell receptor engineered lymphocytes inducing robust antitumor responses. Proc Natl Acad Sci U S A. 2010;107:14286–14291. doi: 10.1073/pnas.1008300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Patel MR, Chang YF, Chen IY, Bachmann MH, Yan X, Contag CH, Gambhir SS. Longitudinal, noninvasive imaging of T-cell effector function and proliferation in living subjects. Cancer Res. 2010;70:10141–10149. doi: 10.1158/0008-5472.CAN-10-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Malviya G, Galli F, Sonni I, Pacilio M, Signore A. Targeting T and B lymphocytes with radiolabelled antibodies for diagnostic and therapeutic applications. Q J Nucl Med Mol Imaging. 2010;54:654–676. [PubMed] [Google Scholar]
- 106.Zarbock A, Ley K. New insights into leukocyte recruitment by intravital microscopy. Curr Top Microbiol Immunol. 2009;334:129–152. doi: 10.1007/978-3-540-93864-4_6. [DOI] [PubMed] [Google Scholar]
- 107.Ishii T, Ishii M. Intravital two-photon imaging: a versatile tool for dissecting the immune system. Ann Rheum Dis. 2011;70(Suppl 1):i113–i115. doi: 10.1136/ard.2010.138156. [DOI] [PubMed] [Google Scholar]
- 108.Okada T. Two-photon microscopy analysis of leukocyte trafficking and motility. Semin Immunopathol. 2010;32:215–225. doi: 10.1007/s00281-010-0210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heroux J, Gharib AM, Danthi NS, Cecchini S, Ohayon J, Pettigrew RI. High-affinity alphavbeta3 integrin targeted optical probe as a new imaging biomarker for early atherosclerosis: initial studies in Watanabe rabbits. Mol Imaging Biol. 2009;12:2–8. doi: 10.1007/s11307-009-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Galkina E, Harry BL, Ludwig A, Liehn EA, Sanders JM, Bruce A, Weber C, Ley K. CXCR6 promotes atherosclerosis by supporting T-cell homing, interferon-gamma production, and macrophage accumulation in the aortic wall. Circulation. 2007;116:1801–1811. doi: 10.1161/CIRCULATIONAHA.106.678474. [DOI] [PubMed] [Google Scholar]
- 111.van Tilborg GA, Vucic E, Strijkers GJ, Cormode DP, Mani V, Skajaa T, Reutelingsperger CP, Fayad ZA, Mulder WJ, Nicolay K. Annexin A5-functionalized bimodal nanoparticles for MRI and fluorescence imaging of atherosclerotic plaques. Bioconjug Chem. 2010;21:1794–1803. doi: 10.1021/bc100091q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang J, Fu Y, Li G, Zhao RY, Lakowicz JR. Direct observation of chemokine receptors 5 on T-lymphocyte cell surfaces using fluorescent metal nanoprobes 2: Approximation of CCR5 populations. Biochem Biophys Res Commun. 2011;407:63–67. doi: 10.1016/j.bbrc.2011.02.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pozzilli P, Signore A, Pozzilli C. Detrimental effect of indium-111 on human lymphocytes. J Nucl Med. 1984;25:830. [PubMed] [Google Scholar]
- 114.Signore A, Beales P, Sensi M, Zuccarini O, Pozzilli P. Labelling of lymphocytes with indium 111 oxine: effect on cell surface phenotype and antibody-dependent cellular cytotoxicity. Immunol Lett. 1983;6:151–154. doi: 10.1016/0165-2478(83)90097-4. [DOI] [PubMed] [Google Scholar]
- 115.Giannarelli C, Ibanez B, Cimmino G, Garcia Ruiz JM, Faita F, Bianchini E, Zafar MU, Fuster V, Garcia MJ, Badimon JJ. Contrast-enhanced ultrasound imaging detects intraplaque neovascularization in an experimental model of atherosclerosis. JACC Cardiovasc Imaging. 2010;3:1256–1264. doi: 10.1016/j.jcmg.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 116.Coli S, Magnoni M, Sangiorgi G, Marrocco-Trischitta MM, Melisurgo G, Mauriello A, Spagnoli L, Chiesa R, Cianflone D, Maseri A. Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries: correlation with histology and plaque echogenicity. J Am Coll Cardiol. 2008;52:223–230. doi: 10.1016/j.jacc.2008.02.082. [DOI] [PubMed] [Google Scholar]
- 117.Moguillansky D, Leng X, Carson A, Lavery L, Schwartz A, Chen X, Villanueva FS. Quantification of plaque neovascularization using contrast ultrasound: a histologic validation. Eur Heart J. 2011;32:646–653. doi: 10.1093/eurheartj/ehq197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee SC, Carr CL, Davidson BP, Ellegala D, Xie A, Ammi A, Belcik T, Lindner JR. Temporal characterization of the functional density of the vasa vasorum by contrast-enhanced ultrasonography maximum intensity projection imaging. JACC Cardiovasc Imaging. 2010;3:1265–1272. doi: 10.1016/j.jcmg.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Calcagno C, Mani V, Ramachandran S, Fayad ZA. Dynamic contrast enhanced (DCE) magnetic resonance imaging (MRI) of atherosclerotic plaque angiogenesis. Angiogenesis. 2010;13:87–99. doi: 10.1007/s10456-010-9172-2. [DOI] [PubMed] [Google Scholar]
- 120.Calcagno C, Cornily JC, Hyafil F, Rudd JH, Briley-Saebo KC, Mani V, Goldschlager G, Machac J, Fuster V, Fayad ZA. Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced MRI and 18F-FDG PET. Arterioscler Thromb Vasc Biol. 2008;28:1311–1317. doi: 10.1161/ATVBAHA.108.166173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kaufmann BA, Carr CL, Belcik JT, Xie A, Yue Q, Chadderdon S, Caplan ES, Khangura J, Bullens S, Bunting S, Lindner JR. Molecular imaging of the initial inflammatory response in atherosclerosis: implications for early detection of disease. Arterioscler Thromb Vasc Biol. 2010;30:54–59. doi: 10.1161/ATVBAHA.109.196386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Winter PM, Neubauer AM, Caruthers SD, Harris TD, Robertson JD, Williams TA, Schmieder AH, Hu G, Allen JS, Lacy EK, Zhang H, Wickline SA, Lanza GM. Endothelial alpha(v)beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2103–2109. doi: 10.1161/01.ATV.0000235724.11299.76. [DOI] [PubMed] [Google Scholar]
- 123.Tardif JC, Lesage F, Harel F, Romeo P, Pressacco J. Imaging biomarkers in atherosclerosis trials. Circ Cardiovasc Imaging. 2011;4:319–333. doi: 10.1161/CIRCIMAGING.110.962001. [DOI] [PubMed] [Google Scholar]
- 124.Noble S, Heinonen T, Tardif JC. Imaging biomarkers of atherosclerosis. Biomark Med. 2008;2:555–565. doi: 10.2217/17520363.2.6.555. [DOI] [PubMed] [Google Scholar]