Abstract
Rationale: Given the role of vascular endothelial growth factor (VEGF) in lung development, we hypothesized that polymorphisms in VEGF-A may be associated with lung function.
Objectives: The current study was designed to assess the role of genetic variants in VEGF-A as determinants of airway function from infancy through early adulthood.
Methods: Association between five single-nucleotide polymorphisms (SNPs) in VEGF-A and lung function were assessed longitudinally in two unselected birth cohorts and cross-sectionally among infants. Replication with two SNPs was conducted in adults and children with asthma. We investigated the functionality of the SNP most consistently associated with lung function (rs3025028) using Western blotting to measure the ratio of plasma VEGF-A165b/panVEGF-A165 among homozygotes.
Measurements and Main Results: In two populations in infancy, C-allele homozygotes of rs3025028 had significantly higher VmaxFRC, forced expiratory flow50, and forced expiratory flow25–75 compared with other genotype groups. Among preschool children (age 3 yr), C allele of rs3025028 was associated with significantly higher specific airway conductance, with similar findings observed for lung function in school-age children. For FEV1/FVC ratio similar findings were observed among adolescents and young adults (birth cohort), and then replicated in adults and schoolchildren with asthma (cross-sectional studies). For rs3025038, plasma VEGF-A165b/panVEGF-A165 was significantly higher among CC versus GG homozygotes (P ≤ 0.02) at birth, in school-age children, and in adults.
Conclusions: We report significant associations between VEGF-A SNP rs3025028 and parameters of airway function measured throughout childhood, with the effect persisting into adulthood. We propose that the mechanism may be mediated through the ratios of active and inhibitory isoforms of VEGF-A165, which may be determined by alternative splicing.
Keywords: lung function, vascular endothelial growth factor-A, genetics
At a Glance Commentary
Scientific Knowledge on the Subject
Poor lung function is associated with increased mortality, yet the genes underlying this heritable trait remain largely undiscovered.
What This Study Adds to the Field
We have identified variation in the VEGF-A gene that predicts lung function in five populations and speculate that the mechanism is through splice variation.
Lung function is highly heritable, with a recent twin study indicating that genetic factors account for 61% of the variability (1). Recent metaanalyses of genome-wide association studies (GWAS) for lung function in tens of thousands of subjects identified a number of novel genome-wide significant loci (2, 3), but the discovered loci accounted for a small proportion of the variance in lung function (∼3%), with most of the variance remaining unexplained (4, 5). Longitudinal cohort studies indicate that lung function tracks throughout childhood into adult life (6, 7) and poor lung function is associated with adverse outcomes, including increased mortality (8). It is plausible to surmise that individuals who initiate their adult years with lower levels of airway function may be at increased risk of obstructive lung diseases later in life.
There are many different ways in which lung function can be measured. Ideally, one would use the same test at all ages to facilitate longitudinal comparisons, but for technical reasons this is not possible. In children younger than 2 years, maximal expiratory flows can be measured while the child is sedated (9–11). In preschool children, forced expiratory maneuvers, such as FEV1 and FVC, are often inaccurate and measures taken during tidal breathing (e.g., specific airways conductance [sGaw]) are preferable (12). In young school-age children, although many can manage forced expiratory maneuvers, the FEV1 often approaches the FVC, making the FEV1/FVC ratio less useful than the FEV1 alone (12). In older children and adults, FEV1/FVC ratio is indicative of airway narrowing and shows little variation with size (13). Thus, the most informative test to measure lung function differs by age.
The structural development and maintenance of the human lung is dependent on numerous factors, including vascular endothelial growth factor (VEGF)-A. In mouse embryos, the epithelial cells of the leading edge of the developing airway produce VEGF-A, which stimulates endothelial cell differentiation, migration, and proliferation, resulting in a primitive vascular plexus around the developing airways (14). Studies in human vascular endothelial cells have identified that the VEGF-A gradient determines the signal transduction response of endothelial cells (15). VEGF-A has multiple isoforms, and the most abundant in human lung seems to be VEGF-A165 (16). A further level of complexity is added by the fact that VEGF-A165 has one active (VEGF-A165a) and one inhibitory (VEGF-A165b) isoform (17), with VEGF-A165b being formed by differential splicing between exon 7 and 3′ untranslated region (see Figure E1 in the online supplement) (18). Despite the critically important role of VEGF-A in the development and structural maintenance of the lung (16), only one study has reported on the association between VEGF-A variants and airflow obstruction (19), and single-nucleotide polymorphisms (SNPs) in this region were not associated with lung function in large GWA studies (5).
We hypothesized that polymorphisms in VEGF-A may contribute to the variance in lung function. In a candidate gene association study we assessed the role of genetic variants in VEGF-A as determinants of airway function using age-appropriate lung function measurements obtained in five populations from different geographic areas (United States, United Kingdom, and Croatia). We then investigated the function of the most consistently associated SNP located in intron 7 (rs3025038). Based on its location and our observations we hypothesize that rs3025038 may alter the ratio of active and inactive isoforms of VEGF-A165. Finally, we investigated the reasons why SNPs in this region were not associated with lung function in previous large GWA studies. Some of this work has previously been published as an abstract (20).
Methods
Participants
Lung function was assessed longitudinally in two unselected birth cohorts (Tucson Children’s Respiratory Study [TCRS] [7, 21] and Manchester Asthma and Allergy Study [MAAS] [22]), and at a single time point in a study of infants in Indianapolis (23). Replication studies were performed in cross-sectional studies of adults from Manchester (United Kingdom) and children with asthma from Croatia. A description of the population is presented in Table E1. All studies were approved by the Research Ethics Committees or Institutional Review boards. Informed consent was obtained from all parents or participants, and children gave their assent if appropriate. Only subjects of European ancestry were included. A detailed description of all methods is available in the online supplement.
Variables
Measures of lung function appropriate to the age and ability of subjects were made in each population: thoracic compression technique (TCT) in infants (11), whole-body plethysmography during tidal breathing in preschool children (11), and spirometry in older children and adults (24).
Data Sources and Measurement
Infancy.
In the TCRS group, maximal expiratory flow at functional residual capacity (VmaxFRC) was recorded from partial expiratory flow–volume curves by TCT (23, 25) at age 2.3 months (n = 57). In the Indiana group, healthy full-term infants (n = 30) underwent measurement of forced expiratory flow (FEF) using the raised-volume rapid TCT (11, 23) to measure FEF50 and FEF25–75 in the first 3 months of life.
Preschool children.
In the MAAS group, at age 3 years, sGaw was measured using whole-body plethysmography (n = 466) (26, 27).
Elementary school children.
In the MAAS group, at ages 5 (n = 660) and 8 years (n = 638) spirometry was used to measure FEV1. In the TCRS group, at age 6 years, voluntary partial flow–volume curves were obtained to calculate VmaxFRC (n = 266) (7).
Middle school children.
In the TCRS (n = 292) and MAAS (n = 656) groups, at age 11 years spirometry was used to assess lung function (FEV1/FVC ratio).
Adolescents and young adults.
In the TCRS group, at age 16 (n = 243) and 22 years (n = 230) spirometry was used to measure FEV1/FVC ratio.
Replication among adults and children with asthma.
FEV1/FVC ratio was measured using spirometry among 596 adults from Manchester (parents of children participating in MAAS; age 28–61 yr; 246 males), and in 403 Croatian children with physician-diagnosed asthma aged 6–18 years.
Genotyping
Five haplotype tagging SNPs were selected in three discovery populations (TCRS, MAAS, and Indiana): rs833068, rs833070, rs3025028, rs10434, and rs2146323) (see Table E2). For replication populations (adults from Manchester and children with asthma from Croatia), only SNPs of interest were typed (rs3025028 and rs10434). Finally, in MAAS we genotyped an additional 41 SNPs in the VEGF-A gene to give dense coverage of this region (see Table E3). Genotyping methods are described in the online supplement.
Investigation of the Function of SNP rs3025038
We used Western blotting to measure the ratio of plasma VEGF-A165b to panVEGF-A165 among homozygotes in rs3025038 from 45 cord blood samples in MAAS, 29 adults from Manchester, and 61 Croatian children with asthma (see Figures E2–E4).
Investigation of Coverage of VEGF-A Region in GWA Studies
Because the two associated SNPs are not included in most commercially available genome-wide genotyping arrays, we investigated the coverage of VEGF-A in the arrays to investigate why this region was not associated with lung function in recent GWAS. DNA samples (MAAS; n = 880) were genotyped using Illumina 610-quad chip (Illumina, Inc., San Diego, CA); standard quality control measures were used. Genotypes were imputed (to ∼6 million) with IMPUTE v2.1.2 (http://mathgen.stats.ox.ac.uk/impute/impute.html) with 1,000 Genomes and HapMap Phase 3 reference genotypes using the recommended parameters.
Statistical Methods
Measures of lung function were expressed as z scores to facilitate comparisons. In the regression analysis lung function was adjusted as appropriate (see the online supplement). Associations between genotype and lung function were first assessed by analysis of variance. The fit of the three genetic models (log-additive, recessive, and dominant) was evaluated based on the lowest value for Akaike information criteria (values are presented in the online supplement; see Tables E4–E5). Kruskal-Wallis rank test or t test was used to assess differences between groups. Where two time points were available using the same measure of lung function, longitudinal analysis of the data was performed using generalized estimating equation models. P values less than or equal to 0.05 were considered significant. In the protein analysis, we used the normalized values of VEGFA-165b and panVEGFA-165 to calculate VEGFA-165b/panVEGFA-165 ratio; geometric mean and 95% confidence intervals are presented. We analyzed data using STATA (version 10.0) (StataCorp, College Station, TX) and SPSS (version 19.0) (IBM, New York, NY) statistical software.
Results
Genotyping success rates were generally high (≥93%) (see Tables E2 and E3). Similar allele frequencies for all SNPs were observed in Tucson, Indianapolis, Manchester, and Croatia (see Table E6); no deviation from Hardy-Weinberg equilibrium could be detected and patterns of linkage disequilibrium were similar in the three populations (see Figure E5). Only subjects of European ancestry were included in the present study.
Association between Polymorphisms in VEGF-A and Lung Function in Different Age Groups
We tested the association between VEGF genetic variation and lung function in three discovery populations (TCRS, Indiana, and MAAS); no associations were seen for SNPs rs833068, rs833070, and rs2146323, but we observed significant associations between SNPs rs3025028 and rs10434 and lung function at different ages in all three populations.
Infancy.
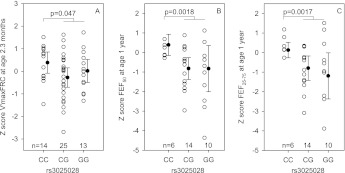
Associations between VEGF-A SNPs and lung function measures in infancy are summarized (by genotype) in Table E7. When specific genetic models were tested, a recessive model was the best fit for rs3025028 and rs10434. Although the number of infants with available data from TCRS was small, CC homozygotes of rs3025028 had significantly higher VmaxFRC compared with other genotype groups (P = 0.047) (Figure 1A). A similar trend was seen for AA homozygotes of SNP rs10434 (P = 0.087). Similarly, in Indiana, mean z scores for FEF50 and for FEF25–75 were significantly higher among CC homozygotes of rs3025028 (P < 0.002) (Figures 1B and 1C) and AA homozygotes of rs10434 (P < 0.01) than among carriers of the other two genotypes.
Figure 1.
Airway function data by vascular endothelial growth factor (VEGF) rs3025028 in the Tucson (A) and Indiana samples (B and C). Adjusted z score values and the mean (95% confidence interval) are plotted. P values shown on the graph represent t tests. FEF = forced expiratory flow.
Preschool children.
Associations between VEGF-A SNPs and lung function measures in TCRS and MAAS throughout childhood are summarized (by genotype) in Table 1. Among young preschool children at age 3 years in MAAS, carriers of the C allele of rs3025028 had significantly higher sGaw in multiple logistic regression analysis adjusted for sex, allergic sensitization, and allergen exposure (28) (P = 0.02) (Figure 2A). We observed similar association for carriers of the A allele of rs10434 (P = 0.03).
TABLE 1.
AIRWAY FUNCTION BY VEGF SNPs FOR YOUNG CHILDREN
|
Z Scores of Adjusted Airway Function* |
|||||||||||||||||
| MAAS Age 3 Years sGaw |
MAAS Age 5 Years FEV1 |
MAAS Age 8 Years FEV1 |
TCRS Age 6 Years Maximal Flow |
||||||||||||||
| VEGF SNPs | n | Mean | 95% CI | P | n | Mean | 95% CI | P | n | Mean | 95% CI | P | n | Mean | 95% CI | P | |
| rs833068 | AA | 38 | −0.28 | −0.61 to 0.04 | 67 | −0.08 | −0.28 to 0.13 | 62 | −0.13 | −0.32 to 0.07 | 27 | 0.36 | −0.14 to 0.85 | ||||
| AG | 183 | −0.07 | −0.23 to 0.09 | 274 | 0.02 | −0.08 to 0.12 | 255 | −0.03 | −0.13 to 0.07 | 105 | −0.12 | −0.29 to 0.06 | |||||
| GG | 187 | −0.11 | −0.27 to 0.05 | 0.58 | 268 | −0.06 | −0.16 to 0.04 | 0.74 | 273 | −0.04 | −0.14 to 0.04 | 0.68 | 128 | 0.02 | −0.15 to 0.19 | 0.09 | |
| rs833070 | CC | 109 | −0.11 | −0.31 to 0.09 | 171 | 0.01 | −0.12 to 0.17 | 151 | −0.02 | −0.14 to 0.09 | 61 | 0.22 | −0.07 to 0.51 | ||||
| CT | 198 | −0.02 | −0.18 to 0.14 | 299 | −0.09 | −0.18 to 0.01 | 286 | −0.04 | −0.13 to 0.04 | 127 | −0.10 | −0.25 to 0.06 | |||||
| TT | 87 | −0.17 | −0.39 to 0.05 | 0.78 | 140 | 0.12 | −0.11 to 0.17 | 0.94 | 151 | −0.09 | −0.20 to 0.03 | 0.48 | 73 | −0.02 | −0.27 to 0.23 | 0.13 | |
| rs2146323 | AA | 42 | −0.24 | −0.56 to 0.07 | 66 | −0.15 | −0.36 to 0.06 | 78 | −0.11 | −0.29 to 0.06 | 31 | 0.01 | −0.36 to 0.37 | ||||
| AC | 188 | −0.10 | −0.26 to 0.06 | 288 | 0.00 | −0.10 to 0.10 | 270 | −0.07 | −0.16 to 0.03 | 115 | −0.12 | −0.28 to 0.05 | |||||
| CC | 169 | −0.03 | −0.12 to 0.14 | 0.22 | 272 | −0.03 | −0.13 to 0.07 | 0.55 | 255 | 0.02 | −0.12 to 0.07 | 0.34 | 108 | 0.13 | −0.08 to 0.35 | 0.18 | |
| rs3025028 | CC | 89 | 0.03 | −0.19 to 0.24 | 127 | 0.12 | −0.03 to 0.27 | 112 | 0.14 | 0.00 to 0.29 | 53 | 0.22 | −0.11 to 0.56 | ||||
| CG | 201 | −0.05 | −0.21 to 0.11 | 288 | −0.04 | −0.14 to 0.06 | 288 | −0.04 | −0.14 to 0.04 | 137 | −0.04 | −0.20 to 0.12 | |||||
| GG | 102 | −0.29 | −0.50 to −0.09 | 0.02 | 176 | −0.10 | −0.23 to 0.03 | 0.04 | 161 | −0.12 | −0.23 to 0.01 | 0.009 | 65 | −0.21 | −0.42 to 0.001 | 0.065 | |
| rs10434 | AA | 103 | 0.01 | −0.20 to 0.21 | 144 | 0.10 | −0.04 to 0.24 | 134 | 0.07 | −0.06 to 0.20 | 57 | 0.19 | −0.14 to 0.54 | ||||
| AG | 206 | −0.07 | −0.23 to 0.08 | 306 | −0.04 | −0.14 to 0.06 | 299 | −0.06 | −0.14 to 0.03 | 131 | 0.02 | −0.14 to 0.18 | |||||
| GG | 99 | −0.30 | −0.51 to −0.09 | 0.03 | 166 | −0.06 | −0.19 to 0.07 | 0.12 | 162 | −0.09 | −0.21 to 0.03 | 0.08 | 60 | −0.25 | −0.47 to −0.04 | 0.048 | |
Definition of abbreviations: CI = confidence interval; MAAS = Manchester Asthma and Allergy Study; sGaw = specific airways conductance; SNP = single-nucleotide polymorphism; TCRS = Tucson Children’s Respiratory Study; VEGF = vascular endothelial growth factor.
Adjusted for MAAS: age 3 years sGaw was adjusted for sensitization and exposure, height, and sex; FEV1 (age 5 and 8 yr) was adjusted for height and sex. Adjusted for TCRS: VmaxFRC at age 6 years adjusted for height and sex. P value is for analysis of variance model.
Figure 2.
Lung function by rs3025028 genotype (A) specific airway conductance in Manchester Asthma and Allergy Study (MAAS) age 3 years, (B) FEV1 age 5 years in MAAS, (C) FEV1 age 8 years in MAAS, and (D) VMax FRC age 6 years in Tucson Children’s Respiratory Study (TCRS). Adjusted z score values and the mean (95% confidence interval) are plotted.
Elementary school children.
In MAAS at age 5 and 8 years, FEV1 (adjusted for height and sex) was significantly higher among CC homozygotes of rs3025028 compared with the CG/GG group (P ≤ 0.03) (Figures 2B and 2C). There was a trend for carriers of the A allele of rs10434 to have better lung function. In the longitudinal model, both SNPs were significantly associated with FEV1 (P < 0.03). Similar findings were observed among children in TCRS, in whom adjusted maximal flows at age 6 years were higher in CC homozygotes of rs3025028 (Figure 2D) compared with the CG-GG group.
Middle school children.
For lung function measures at age 11 years in MAAS and TCRS (FEV1, FEV1/FVC), there was no association with any SNP (see Table E8).
Adolescents and young adults.
The association between rs3025028 and rs10434 and lung function (FEV1/FVC ratio) among adolescents and young adults (age 16 and 22 years) in TCRS is summarized in Table 2. The A allele of rs10434 was associated with significantly higher FEV1/FVC ratio (P < 0.05); similar results were seen for the C allele of rs3025028 at age 22 years (P = 0.01), with a trend at age 16 years. In the longitudinal model, both SNPs were significantly associated with FEV1/FVC ratio (P < 0.020).
TABLE 2.
AIRWAY FUNCTION BY VEGF SNPs FOR YOUNG ADULTS
|
Z Scores of Adjusted FEV1/FVC Ratio* |
|||||||||
| TCRS Age 16 Years |
TCRS Age 22 Years |
||||||||
| VEGF-A SNPs | n | Mean | 95% CI | P | n | Mean | 95% CI | P | |
| rs3025028 | CC | 53 | 0.11 | −0.16 to 0.37 | 50 | 0.30 | 0.01 to 0.59 | ||
| CG | 122 | 0.05 | −0.12 to 0.22 | 112 | −0.03 | −0.20 to 0.14 | |||
| GG | 62 | −0.24 | −0.52 to 0.04 | 0.107 | 62 | −0.26 | −0.53 to −0.01 | 0.012 | |
| rs10434 | AA | 54 | 0.18 | −0.07 to 0.44 | 48 | 0.31 | 0.004 to 0.62 | ||
| AG | 115 | 0.09 | −0.10 to 0.27 | 107 | 0.02 | −0.16 to 0.20 | |||
| GG | 56 | −0.26 | −0.56 to 0.041 | 0.048 | 55 | −0.27 | −0.57 to −0.02 | 0.014 | |
Definition of abbreviations: CI = confidence interval; SNP = single-nucleotide polymorphism; TCRS = Tucson Children’s Respiratory Study; VEGF = vascular endothelial growth factor.
Adjusted for height and sex. P value is for analysis of variance model.
Replication among adults and children with asthma.
Next, we examined whether SNPs rs3025028 and rs10434 affect lung function in adults from Manchester and in Croatian children with asthma (Table 3). In Manchester adults the FEV1/FVC ratio was significantly higher in the CC (80.9%; 95% confidence interval, 79.9–81.9) than the CG-GG group (79.5%; 95% confidence interval, 79–80.1; P = 0.02) of rs3025028. Similar results were seen for rs10434.
TABLE 3.
AIRWAY FUNCTION BY VEGF SNPs FOR ADULTS AND CHILDREN WITH ASTHMA
|
Z Scores of Adjusted FEV1/FVC Ratio* |
|||||||||||||
| Manchester Adults |
Croatian Asthma Study (All Children) |
Croatian Asthma Study (Children >10 yr) |
|||||||||||
| VEGF-A SNPs | n | Mean | 95% CI | P | n | Mean | 95% CI | P | n | Mean | 95% CI | P | |
| rs3025028 | CC | 130 | 0.22 | 0.05 to 0.39 | 68 | 0.23 | 0.00 to 0.45 | 30 | 0.18 | −0.15 to 0.51 | |||
| CG | 275 | 0.02 | −0.09 to 0.14 | 202 | 0.01 | −0.12 to 0.14 | 123 | −0.22 | −0.38 to −0.06 | ||||
| GG | 162 | −0.05 | −0.20 to 0.10 | 0.02 | 130 | −0.07 | −0.24 to 0.10 | 0.05 | 68 | −0.34 | −0.56 to −0.12 | 0.018 | |
| rs10434 | AA | 139 | 0.23 | 0.06 to 0.39 | 65 | 0.24 | 0.01 to 0.48 | 29 | 0.16 | −0.18 to 0.49 | |||
| AG | 299 | −0.04 | −0.15 to 0.07 | 203 | 0.00 | −0.13 to 0.14 | 123 | −0.23 | −0.39 to −0.07 | ||||
| GG | 153 | −0.03 | −0.18 to 0.13 | 0.03 | 123 | −0.09 | −0.26 to 0.08 | 0.03 | 64 | −0.35 | −0.58 to −0.13 | 0.024 | |
Definition of abbreviations: CI = confidence interval; SNP = single-nucleotide polymorphism; VEGF = vascular endothelial growth factor.
Adjusted for Manchester adults: age, sex, height, and cigarette smoking pack-years. Adjusted for Croatia Asthma Study: height, age, and sex. P value is for analysis of variance model.
In a population of children with asthma in Croatia, the C allele of rs3025028 was associated with a higher FEV1/FVC ratio (P = 0.05). Similarly, the A allele of rs10434 was associated with a higher FEV1/FVC ratio (P = 0.03). This analysis was repeated excluding children aged less than 10 years (in whom FEV1/FVC ratio approximates 100%); for children older than 10 years (median age, 14 yr), C allele of rs3025028 and A allele of rs10434 were associated with a higher FEV1/FVC ratio (P = 0.02), with no such association in children less than 10 years.
Effect size.
To estimate the effect size of genetic variation in VEGF on lung function, we used eta squared from analysis of variance models. In MAAS, rs3025038 explained 1.19% of the variance at age 3 years, 0.58% at age 5 years, and 0.94% at age 8 years. In adults, rs3025038 explained 0.86% of the variance in FEV1/FVC ratio.
Functional Effect of rs3025028 on VEGF-A Splice Variants
Because SNP rs3025028 was most consistently associated with lung function, we next investigated its potential function. Because this SNP is located between exons 7 and 8, we hypothesized that it might alter splicing and affect the balance between the inhibitory (VEGF-A165b) and active (VEGF-A165a) isoforms (see Figure E1). We measured VEGF-A165b and total VEGF-A165 levels (Western blot) in serum and plasma samples from individuals selected based on genotype. Already at birth (cord plasma in MAAS), the VEGF-A165b/VEGF-A165 ratio was significantly higher among the CC group (n = 22) compared with the GG group (n = 23; P = 0.004) (see Table E9). Similar findings were observed in Croatian children with asthma (30 CC, 31 GG) and adults from Manchester (16 CC, 13 GG), in that the ratio of plasma VEGF-A165b to panVEGF-A165 was consistently significantly higher among C compared with G allele homozygotes (P ≤ 0.02) (Figure 3; see Table E9).
Figure 3.
The vascular endothelial growth factor (VEGF)-A165b/VEGF-A165 ratio in three populations. (A) Manchester adults. (B) Manchester Asthma and Allergy Study (MAAS) children. (C) Croatian children.
Comparison with Results of GWAS for Lung Function
Using GWAS data imputed to approximately 6 million SNPs, we measured linkage disequilibrium (LD) between rs3025028 (typed on Sequenom) and all other SNPs (typed on the GWAS array or imputed) in the 31-kb region containing VEGF-A (Figure 4). The highest LD with the genotyped SNPs was low (r2 ≤0.15). For imputed SNPs, the highest LD seen was with rs10434 (r2 = 0.78) (Figure 4). The imputation quality of this SNP (info score, 0.461) would nominally pass imputation QC (the recommended info score threshold is >0.4). However, the probabilistic genotypes were converted to called genotypes in only 9% of samples, which implies that the imputation of this SNP is suboptimal. Imputation of SNPs in a 31-kb region containing VEGF-A (see Figure E6A) is generally poorer than in broader regions of the genome (see Figure E6B).
Figure 4.
The genomic location (NCBI build 36) on chromosome 6 of single-nucleotide polymorphism (SNP) rs3025028 is indicated with a red cross. Red diamonds indicate SNPs genotyped on Illumina 610 quad. Blue diamonds indicate imputed SNPs. Linkage disequilibrium measure used was r2.
Additional Genotyping of VEGF-A
Finally, we genotyped a dense panel of 41 additional SNPs in MAAS (see Figure E7, Table E10). Significant associations are shown in Table E11. Although SNPs in the same LD block as rs3025028 are associated with lung function, none is more consistently associated than rs3025028.
Discussion
This is the first study to report associations between genetic variants in VEGF-A and lung function measured in the general population. SNPs rs10434 and rs3025028 were associated with lung function from infancy through to adulthood (with the exception of puberty), in four population-based cohorts and in one asthma cohort. Taken together, these findings strongly suggest that variants in VEGF play an important role in the genetic determination of airway function from birth to adulthood. We have also provided evidence that the SNP most consistently associated with lung function is also associated with the ratio of the active and inhibitory isoforms of VEGF-A165, suggesting that this SNP (or another SNP in the same haplotype block) may be functional.
Genetics of Lung Function
Studies of the genetics of lung function have generally used subjects with asthma and focused on lung function decline and remodeling (29–32). One previous study has focused on VEGF-A and lung function in subjects with asthma (19). Sharma and coworkers (19) identified SNP rs4711750 to be associated with FEV1/FVC in children with asthma and also with FEV1/FVC decline over time. Because this SNP was not in LD with the SNPs analyzed in the current study (r2 <0.05), we genotyped one population (MAAS) for rs4711750 and found no association with lung function (data not shown; P > 0.2). None of the SNPs typed in the study by Sharma and coworkers (19) were in LD with rs3025028 (r2 <0.24).
A metaanalysis of GWAS in the SpiroMeta consortium, comprising 20,288 individuals of European ancestry, tested the association between cross-sectional lung function measures and approximately 2.5 million genotyped or imputed SNPs (2). Several possible new candidate genes were identified, but the effect size of each variant was small accounting for approximately 0.14% of the variation in FEV1/FVC ratio. No SNPs in the VEGF-A region of chromosome 6 were associated with lung function, and this region (3 MB up or downstream) did not feature in the top 2,000 hits reported. Using data from an Illumina 610Quad array and the Sequenom genotyped rs3025028, we were able to establish that SNP rs3025028 is not tagged by the SNPs on this array (r2 <0.15). Using imputed data demonstrated that Sequenom typed rs3025028 nominally tagged an imputed SNP (rs10434; r2 ∼0.8); the imputation quality for rs10434 was poor (info score ∼ 0.4). Because the probabilistic genotype could be confidently called less than 10% of the time, this provides an explanation why this SNP was not identified as an associate of lung function in the GWA study (2). Although an important tool in genetics research, GWA studies have explained only a small proportion of the variance of complex disease to date, despite sample sizes approaching 100,000. Even increasing the number of participants in a GWAS modestly improves the ability to explain the variance of disease (33). It is likely that methods other than GWAS will be required to uncover the remaining variance of heritable complex traits. Among these methods we maintain that the candidate gene approach is still an important tool in the arsenal of researchers investigating the genetics of complex disease. Others have previously highlighted that current GWAS arrays offer inadequate coverage for many asthma candidate genes (34).
Strengths and Weaknesses of the Study
Measures of lung function used in the analysis were appropriate to the age of the subject; consequently, a series of different lung function measures were used and data were presented as z scores to facilitate comparisons across time. In infancy, the use of rapid thoracoabdominal compression techniques provides the means of assessing airway function during sedation (35). Measuring lung function between ages 2 and 6 years remains challenging because children are too old to sedate, but too young to perform forced expiratory maneuvers (12); measurement of specific airways conductance can be done during tidal breathing and has been shown to be a robust and reproducible measure (26, 36). With age, forced expiratory maneuvers, such as spirometry, become feasible, but in primary school age children the FEV1/FVC ratio is often greater than 95%, making the FEV1 a more useful measure than FEV1/FVC ratio in this age group (12). In older children and adults and in asthma, FEV1/FVC ratio is the most important parameter for detecting an obstructive impairment (13).
Associations with lung function were not seen at age 11 years in the TCRS and MAAS. This likely reflects the differential growth of lung and height during this phase, with lung growth seeming to lag behind (37, 38), further complicated by the timing of onset of puberty.
We included only subjects of European ancestry in this study, and the fact that we were able to replicate this finding in multiple populations makes population stratification an unlikely explanation. Additionally, the genomic inflation factor in MAAS GWAS data is 1.006, which is low.
VEGF-A as a Candidate Gene for Lung Function
VEGF-A is a 34- to 46-kD dimeric glycoprotein that acts as a mitogen, stimulating endothelial cell proliferation (39). The deletion of a single allelic copy of the VEGF gene leads to embryonic death caused by failure of blood vessel development (40). Reduction of the expression of VEGF isoforms during fetal life impairs lung microvascular, airway, and airspace maturation in mice (41). Interestingly, overexpression of VEGF in late fetal stages of gestation was lethal in mice, because pups were unable to establish respiration (42). Neovascularization of the bronchi and partial obstruction of the conducting airways was observed. The results of these studies suggest that tight regulation of VEGF expression is necessary for normal airway and lung parenchymal growth, making VEGF-A a biologically plausible candidate gene for lung function.
VEGF-A biology is complicated, with active (VEGF-A165a) and inhibitory (VEGF-A165b) isoforms. Functionally, VEGF-A165b binds to VEGF receptor 2 with the same affinity as VEGF-A165a but does not result in receptor activation and inhibits VEGF-A165a–mediated angiogenesis in vivo (43). The relative ratio of VEGF-A(xxx)b/VEGF-A(xxx)a is known to be important in human diseases including diabetic retinopathy (44) and preeclamptic placentae (45). VEGF-A165b is formed by differential splicing between exon 7 and 3′ untranslated region skipping exon 8 (which is translated in VEGF-A165a) so that the isoforms differ by 6-amino acids at the COOH terminal sequence (17). The variant most strongly associated with lung function in the current study (rs3025028) is located in intron 7. We propose that this variant in VEGF-A (or another SNP in the same haplotype block) might alter the relative ratio of VEGF-A165a and VEGF-A165b, resulting in differences in lung function. We acknowledge, however, that the association is statistical and further work is required to confirm causation.
In conclusion, to the best of our knowledge we report for the first time significant associations between VEGF-A SNP rs3025028 and parameters of airway function measured from birth throughout childhood with the effect persisting into adulthood, in five populations (only one of which was an asthma population). We propose that the mechanism of this effect may be mediated through the ratios of active and inhibitory isoforms of VEGF-A165, which may be determined by alternative splicing.
Supplementary Material
Acknowledgments
The authors thank the all study participants and acknowledge the dedication of the study teams.
Footnotes
Supported by Asthma UK Grant No 04/014, Moulton Charitable Foundation, NIH grant HL-56177, and SFundação para a Ciência e Tecnologia Portugal (S.M.).
Author Contributions: A.S., A.C., and F.D.M. contributed to study conception and design; A.S., A.C., F.D.M., and S.M. obtained funding; S.M., J.H., J.W., J.A.C., M.B., B.K.B., R.T., M.J., and P.G. contributed to acquisition of data; A.S., A.C., F.D.M., D.A.S., J.W., J.A.C., and D.B. analyzed and interpreted data; A.C., A.S., F.D.M., and D.A.S. drafted the report; and all authors contributed to revision of the report and approved the version submitted.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201112-2191OC on March 29, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ingebrigtsen T, Thomsen S, van der Sluis S, Miller M, Christensen K, Sigsgaard T, Backer V. Genetic influences on pulmonary function: a large sample twin study. Lung 2011;189:323–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Repapi E, Sayers I, Wain LV, Burton PR, Johnson T, Obeidat M, Zhao JH, Ramasamy A, Zhai G, Vitart V, et al. Genome-wide association study identifies five loci associated with lung function. Nat Genet 2010;42:36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hancock DB, Eijgelsheim M, Wilk JB, Gharib SA, Loehr LR, Marciante KD, Franceschini N, van Durme YM, Chen TH, Barr RG, et al. Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat Genet 2010;42:45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss ST. Lung function and airway diseases. Nat Genet 2010;42:14–16 [DOI] [PubMed] [Google Scholar]
- 5.Soler Artigas M, Loth DW, Wain LV, Gharib SA, Obeidat M, Tang W, Zhai G, Zhao JH, Smith AV, Huffman JE, et al. Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet 2011;43:1082–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med 2003;349:1414–1422 [DOI] [PubMed] [Google Scholar]
- 7.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet 2007;370:758–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorlie PD, Kannel WB, O'Connor G. Mortality associated with respiratory function and symptoms in advanced age. The Framingham Study. Am Rev Respir Dis 1989;140:379–384 [DOI] [PubMed] [Google Scholar]
- 9.Murray CS, Pipis SD, McArdle EC, Lowe LA, Custovic A, Woodcock A. Lung function at one month of age as a risk factor for infant respiratory symptoms in a high risk population. Thorax 2002;57:388–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez FD, Taussig LM, Morgan WJ. Infants with upper respiratory illnesses have significant reductions in maximal expiratory flow. Pediatr Pulmonol 1990;9:91–95 [DOI] [PubMed] [Google Scholar]
- 11.Anonymus. The raised volume rapid thoracoabdominal compression technique. The Joint American Thoracic Society/European Respiratory Society Working Group on Infant Lung Function. Am J Respir Crit Care Med 2000;161:1760–1762 [DOI] [PubMed] [Google Scholar]
- 12.Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, Bisgaard H, Davis GM, Ducharme FM, Eigen H, et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med 2007;175:1304–1345 [DOI] [PubMed] [Google Scholar]
- 13.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948–968 [DOI] [PubMed] [Google Scholar]
- 14.Healy AM, Morgenthau L, Zhu X, Farber HW, Cardoso WV. VEGF is deposited in the subepithelial matrix at the leading edge of branching airways and stimulates neovascularization in the murine embryonic lung. Dev Dyn 2000;219:341–352 [DOI] [PubMed] [Google Scholar]
- 15.Akeson A, Herman A, Wiginton D, Greenberg J. Endothelial cell activation in a VEGF-A gradient: relevance to cell fate decisions. Microvasc Res 2010;80:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol 2006;290:L209–L221 [DOI] [PubMed] [Google Scholar]
- 17.Bates DO, Cui T-G, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res 2002;62:4123–4131 [PubMed] [Google Scholar]
- 18.Nowak DG, Woolard J, Amin EM, Konopatskaya O, Saleem MA, Churchill AJ, Ladomery MR, Harper SJ, Bates DO. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci 2008;121:3487–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma S, Murphy AJ, Soto-Quiros ME, Avila L, Klanderman BJ, Sylvia JS, Celedon JC, Raby BA, Weiss ST. Association of VEGF polymorphisms with childhood asthma, lung function and airway responsiveness. Eur Respir J 2009;33:1287–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson A, Wu J, Marinho S, Hankinson J, Martinez FD, Custovic A. Functional variant in vascular endothelial growth factor (VEGFA) gene is a strong predictor of airway function in children and adults. Presented at the 28th Symposium of the Collegium Internationale Allergologicum. May, 2010, Ischia, Italy [Google Scholar]
- 21.Taussig LM, Wright AL, Morgan WJ, Harrison HR, Ray CG. The Tucson Children's Respiratory Study. I. Design and implementation of a prospective study of acute and chronic respiratory illness in children. Am J Epidemiol 1989;129:1219–1231 [DOI] [PubMed] [Google Scholar]
- 22.Custovic A, Simpson BM, Murray CS, Lowe L, Woodcock A. The National Asthma Campaign Manchester Asthma and Allergy Study. Pediatr Allergy Immunol 2002;13:32–37 [DOI] [PubMed] [Google Scholar]
- 23.Jones M, Castile R, Davis S, Kisling J, Filbrun D, Flucke R, Goldstein A, Emsley C, Ambrosius W, Tepper RS. Forced expiratory flows and volumes in infants. Normative data and lung growth. Am J Respir Crit Care Med 2000;161:353–359 [DOI] [PubMed] [Google Scholar]
- 24.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–338 [DOI] [PubMed] [Google Scholar]
- 25.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med 1995;332:133–138 [DOI] [PubMed] [Google Scholar]
- 26.Lowe L, Murray CS, Custovic A, Simpson BM, Kissen PM, Woodcock A. Specific airway resistance in 3-year-old children: a prospective cohort study. Lancet 2002;359:1904–1908 [DOI] [PubMed] [Google Scholar]
- 27.Lowe LA, Simpson A, Woodcock A, Morris J, Murray CS, Custovic A. Wheeze phenotypes and lung function in preschool children. Am J Respir Crit Care Med 2005;171:231–237 [DOI] [PubMed] [Google Scholar]
- 28.Lowe LA, Woodcock A, Murray CS, Morris J, Simpson A, Custovic A. Lung function at age 3 years: effect of pet ownership and exposure to indoor allergens. Arch Pediatr Adolesc Med 2004;158:996–1001 [DOI] [PubMed] [Google Scholar]
- 29.Barton SJ, Koppelman GH, Vonk JM, Browning CA, Nolte IM, Stewart CE, Bainbridge S, Mutch S, Rose-Zerilli MJ, Postma DS, et al. PLAUR polymorphisms are associated with asthma, PLAUR levels, and lung function decline. J Allergy Clin Immunol 2009;123:1391–400 [DOI] [PubMed] [Google Scholar]
- 30.Dijkstra A, Howard TD, Vonk JM, Ampleford EJ, Lange LA, Bleecker ER, Meyers DA, Postma DS. Estrogen receptor 1 polymorphisms are associated with airway hyperresponsiveness and lung function decline, particularly in female subjects with asthma. J Allergy Clin Immunol 2006;117:604–611 [DOI] [PubMed] [Google Scholar]
- 31.Jongepier H, Boezen HM, Dijkstra A, Howard TD, Vonk JM, Koppelman GH, Zheng SL, Meyers DA, Bleecker ER, Postma DS. Polymorphisms of the ADAM33 gene are associated with accelerated lung function decline in asthma. Clin Exp Allergy 2004;34:757–760 [DOI] [PubMed] [Google Scholar]
- 32.Koppelman GH, Sayers I. Evidence of a genetic contribution to lung function decline in asthma. J Allergy Clin Immunol 2011;128:479–484 [DOI] [PubMed] [Google Scholar]
- 33.Park JH, Wacholder S, Gail MH, Peters U, Jacobs KB, Chanock SJ, Chatterjee N. Estimation of effect size distribution from genome-wide association studies and implications for future discoveries. Nat Genet 2010;42:570–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michel S, Liang L, Depner M, Klopp N, Ruether A, Kumar A, Schedel M, Vogelberg C, von Mutius E, von Berg A, et al. Unifying candidate gene and GWAS approaches in asthma. PLoS ONE 2010;5:e13894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ATS/ERS statement: raised volume forced expirations in infants: guidelines for current practice. Am J Respir Crit Care Med 2005;172:1463–1471 [DOI] [PubMed] [Google Scholar]
- 36.Nielsen KG, Bisgaard H. Discriminative capacity of bronchodilator response measured with three different lung function techniques in asthmatic and healthy children aged 2 to 5 years. Am J Respir Crit Care Med 2001;164:554–559 [DOI] [PubMed] [Google Scholar]
- 37.Borsboom GJ, Van Pelt W, Quanjer PH. Pubertal growth curves of ventilatory function: relationship with childhood respiratory symptoms. Am Rev Respir Dis 1993;147:372–378 [DOI] [PubMed] [Google Scholar]
- 38.Degroodt EG, Quanjer PH, Wise ME, van Zomeren BC. Changing relationships between stature and lung volumes during puberty. Respir Physiol 1986;65:139–153 [DOI] [PubMed] [Google Scholar]
- 39.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 2004;25:581–611 [DOI] [PubMed] [Google Scholar]
- 40.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996;380:435–439 [DOI] [PubMed] [Google Scholar]
- 41.Galambos C, Ng YS, Ali A, Noguchi A, Lovejoy S, D'Amore PA, DeMello DE. Defective pulmonary development in the absence of heparin-binding vascular endothelial growth factor isoforms. Am J Respir Cell Mol Biol 2002;27:194–203 [DOI] [PubMed] [Google Scholar]
- 42.Akeson AL, Cameron JE, Le Cras TD, Whitsett JA, Greenberg JM. Vascular endothelial growth factor-A induces prenatal neovascularization and alters bronchial development in mice. Pediatr Res 2005;57:82–88 [DOI] [PubMed] [Google Scholar]
- 43.Woolard J, Wang W-Y, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, Cui T-G, Sugiono M, Waine E, Perrin R, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res 2004;64:7822–7835 [DOI] [PubMed] [Google Scholar]
- 44.Perrin RM, Konopatskaya O, Qiu Y, Harper S, Bates DO, Churchill AJ. Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia 2005;48:2422–2427 [DOI] [PubMed] [Google Scholar]
- 45.Bates DO, MacMillan PP, Manjaly JG, Qiu Y, Hudson SJ, Bevan HS, Hunter AJ, Soothill PW, Read M, Donaldson LF, et al. The endogenous anti-angiogenic family of splice variants of VEGF, VEGF(xxx)b, are down-regulated in pre-eclamptic placentae at term. Clin Sci 2006;110:575–585 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.