Abstract
Rationale: Hyperventilation of hot humid air induces transient bronchoconstriction in patients with asthma; the underlying mechanism is not known. Recent studies showed that an increase in temperature activates vagal bronchopulmonary C-fiber sensory nerves, which upon activation can elicit reflex bronchoconstriction.
Objectives: This study was designed to test the hypothesis that the bronchoconstriction induced by increasing airway temperature in patients with asthma is mediated through cholinergic reflex resulting from activation of these airway sensory nerves.
Methods: Specific airway resistance (SRaw) and pulmonary function were measured to determine the airway responses to isocapnic hyperventilation of humidified air at hot (49°C; HA) and room temperature (20–22°C; RA) for 4 minutes in six patients with mild asthma and six healthy subjects. A double-blind design was used to compare the effects between pretreatments with ipratropium bromide and placebo aerosols on the airway responses to HA challenge in these patients.
Measurements and Main Results: SRaw increased by 112% immediately after hyperventilation of HA and by only 38% after RA in patients with asthma. Breathing HA, but not RA, triggered coughs in these patients. In contrast, hyperventilation of HA did not cause cough and increased SRaw by only 22% in healthy subjects; there was no difference between their SRaw responses to HA and RA challenges. More importantly, pretreatment with ipratropium completely prevented the HA-induced bronchoconstriction in patients with asthma.
Conclusions: Bronchoconstriction induced by increasing airway temperature in patients with asthma is mediated through the cholinergic reflex pathway. The concomitant increase in cough response further indicates an involvement of airway sensory nerves, presumably the thermosensitive C-fiber afferents.
Keywords: asthma, cough, bronchoconstriction, TRPV1, ipratropium
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Recent studies suggest that increasing temperature within the physiological range can sensitize and stimulate C-fiber sensory nerves in the lung that express the thermosensitive transient receptor potential vanilloid type 1 channels (TRPV1). Activation of these sensory nerves is known to trigger various symptoms associated with airway inflammatory diseases, such as cough and bronchoconstriction.
What This Study Adds to the Field
This study suggests that hyperventilation of hot humid air evoked coughs and bronchoconstriction in patients with mild asthma but not in healthy subjects. The airway constriction is mediated through the cholinergic reflex pathway.
It is extensively documented that breathing cold dry air induces bronchoconstriction in patients with asthma, which results primarily from injury of airway mucosa and release of various bronchoactive autacoids, such as leukotrienes and histamine (1). In contrast, the effects of an increase in temperature on the airway functions in patients with asthma is generally overlooked despite the fact that hyperthermia occurs frequently under normal and pathophysiological conditions. The most common causes of hyperthermia are elevated metabolic rate (e.g., during exercise) and hindered heat dissipation (e.g., in a warm environment). Hyperthermia can also occur under disease conditions, such as in patients suffering from severe fever. Furthermore, tissue inflammation is known to lead to local hyperemia and an increase in tissue temperature in the inflamed area (2, 3). A recent study has reported that the average end-expiratory temperature plateau (as an indirect measurement of the lung tissue temperature) is 2.7°C higher in children with asthma than that in healthy control subjects (4).
An earlier study by Aitken and Marini (5) has shown that, after hyperventilation of the air with different combinations of temperature and humidity in patients with asthma, the most intense bronchoconstriction occurring immediately was generated by breathing hot humid air, which caused an almost 2-fold increase in airway constriction generated by cold dry air at the same time point. The bronchoconstriction caused by hyperventilation of cold dry air developed slowly and reached a peak after a delay of 5 to 10 minutes, whereas the bronchoconstrictive response to hot humid air developed much more rapidly in the same patients (5), suggesting a possible involvement of neural reflexes. However, this possibility was not tested in their study, and the underlying mechanism was not known.
A recent study in our lab has shown that vagal C-fiber sensory endings innervating the lungs were activated when the intrathoracic temperature was elevated to above a threshold of approximately 39.2°C (6). To avoid other indirect and complex effects of systemic hyperthermia, follow-up studies were performed in isolated vagal pulmonary sensory neurons; the results further demonstrated that a direct stimulatory effect of increasing temperature is mediated through activation of thermosensitive ion channels, namely the transient receptor potential vanilloid type (TRPV) receptors, expressed in these neurons (7–9). More importantly, stimulation of these TRPV-expressing bronchopulmonary C-fiber sensory nerves can elicit an array of pulmonary defense reflex responses, including cough and bronchoconstriction (10–12). Based upon the existing information, we hypothesized that hyperventilation of hot humid air increases airway temperature and evokes bronchoconstriction in patients with asthma by activating vagal bronchopulmonary C-fiber afferents. Furthermore, the airway constriction is mediated through cholinergic reflex pathways and therefore can be prevented by pretreatment with ipratropium bromide, a muscarinic receptor anatogonist, in these patients.
Some of the results of these studies have been previously reported in the form of an abstract (13).
Methods
Subjects
Patients with asthma and healthy subjects were recruited by public advertisement. A screening test was performed in each subject after informed consent was obtained. The diagnosis of asthma was confirmed according to the standard clinical guidelines in each patient (14). Due to the need to stop therapeutic medications for 2 weeks before beginning the study, patients who had severe asthma or poor asthma control were excluded. The study protocol was approved by the Institutional Review Board at the University of Kentucky and the Human Research Protection Office of the United States Department of Defense.
Hyperventilation Challenge
A device designed to deliver air of desired temperature and humidity was constructed by the University of Kentucky Center for Manufacturing. Briefly, a humidified gas mixture of 4.5% CO2 balance air at high temperature (HA; 49°C and 75–80% relative humidity measured by an Extech Hygro-Thermometer model RH101; Nashua, NH) or at room temperature (RA; 20–22°C and 65–75% relative humidity) was delivered at 300 L/min through a large-bore (3-in) stainless steel conduit. During the hyperventilation challenge, the subject breathed via a mouthpiece into this free stream of humidified gas mixture at approximately 40% of maximal voluntary ventilation for 4 minutes; CO2 was added to maintain an isocapnic condition during hyperventilation. Humidity was generated from sterile isotonic saline by an ultrasonic atomizer (Sonaer Ultrasonics, Farmingdale, NY). The amounts of water content delivered in RA and HA were 12 to 14 and 56 to 60 mg H2O/L of air, respectively. Levels of end-tidal temperature (time constant, 0.1 s) (Physitemp model IT-18; Clifton, NJ) and CO2 (Novametrix 1260; Wallingford, CT) were measured before and after 2 minutes of hyperventilation when these changes reached steady state.
Pulmonary Function Measurements
Airway resistance (Raw) and thoracic gas volume (Vtg) were measured by a whole-body constant-volume plethysmography (SensorMedics, Homestead, FL) for 6 minutes before and 16 minutes immediately after the hyperventilation challenge. During each measurement, the subject was asked to pant at a frequency of approximately 2 Hz for 8 s. Raw and Vtg (with shutter closed) were determined by computer using the center-fit method for the slope measurement within the flow range of ±0.5 L/s for Raw. Specific airway resistance (SRaw) was calculated as: Raw × Vtg. Spirometry test was also performed along with the measurements of other physiological variables (body temperature, heart rate, arterial blood pressure, and oxygen saturation) before and after the challenge.
Study Design
Two study series were performed. In study 1, the responses to HA and RA hyperventilation challenges were compared in patients with asthma and in healthy subjects. In study 2, aerosolized ipratropium bromide (500 μg; 2.5 ml of 0.02% solution) and placebo (2.5 ml of sterile isotonic saline) were administered in a double-blind fashion, and their effects on the response to HA hyperventilation challenge were determined in patients with asthma.
Statistical Analysis
Unless noted otherwise, a two-way ANOVA was used for the statistical evaluation of the results. When the ANOVA showed a significant interaction, pairwise comparisons were made with a post hoc analysis (Tukey’s test). Data are reported as means ± SEM. P values of < 0.05 were considered significant.
Results
Six patients with asthma (21–26 yr of age; SD, 23 ± 1 yr) and six healthy subjects (19–46 yr of age; SD, 26 ± 4 yr) were enrolled in this study. Subject characteristics are shown in Table 1.
TABLE 1.
SUBJECT CHARACTERISTICS*
| Patient Type, Subject No. | Age (yr) | Sex | Height (cm) | Weight (kg) | FEV1 (L) | FEV1 (% of predicted normal)† | FEV1/FVC (%) |
| Asthma | |||||||
| 1 | 23 | M | 180 | 95 | 4.54 | 96 | 73 |
| 2 | 26 | F | 165 | 64 | 2.66 | 80 | 76 |
| 3 | 25 | M | 180 | 108 | 4.18 | 89 | 84 |
| 4 | 22 | F | 160 | 61 | 3.05 | 95 | 86 |
| 5 | 23 | F | 175 | 73 | 2.91 | 77 | 78 |
| 6 | 21 | F | 170 | 66 | 3.04 | 85 | 74 |
| Healthy | |||||||
| 1 | 21 | M | 188 | 89 | 6.07 | 117 | 87 |
| 2 | 29 | F | 178 | 75 | 3.86 | 101 | 84 |
| 3 | 46 | F | 168 | 54 | 3.25 | 105 | 81 |
| 4 | 23 | M | 188 | 77 | 5.42 | 105 | 71 |
| 5 | 19 | M | 180 | 71 | 4.12 | 88 | 87 |
| 6 | 22 | F | 165 | 59 | 3.55 | 105 | 86 |
Exclusion criteria for patients with asthma included chronic systemic corticosteroid use, exacerbation of asthma symptoms within the last month, other chronic lung diseases, acute respiratory illnesses within the last 6 wk, history of smoking, and congenital or acquired heart disease. Healthy subjects were nonsmokers who had no sign or previous record of pulmonary or cardiovascular disease.
Predicted normal values obtained from Reference 40.
Study 1
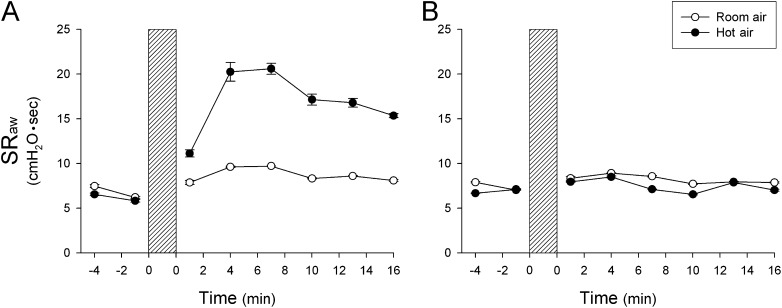
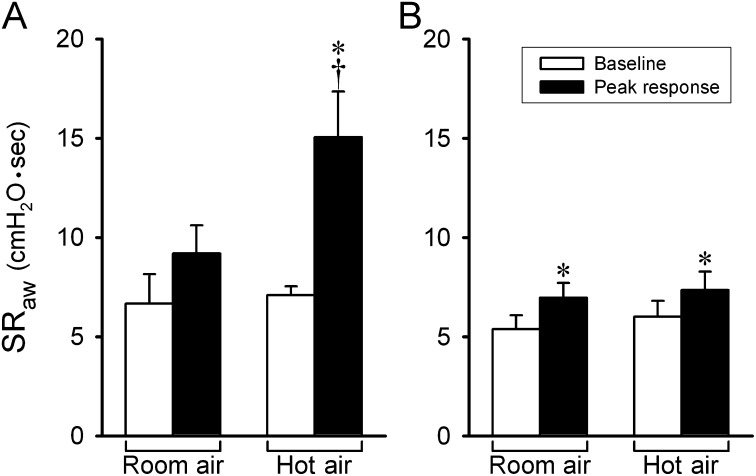
The responses to HA and RA challenges were tested in a randomized order among subjects. Only one experiment was performed on a given day in each subject. In patients with asthma, hyperventilation of humidified HA did not change the end-tidal CO2 concentration but generated a significant increase in end-tidal air temperature (Δ = 1.5 ± 0.1°C; P < 0.05) (Table 2). SRaw increased immediately after hyperventilation and declined slowly toward baseline after more than 8 minutes (Figure 1A); the average SRaw increased significantly from a baseline value of 7.09 ± 0.45 cm H2O·s to a peak of 15.06 ± 2.29 cm H2O·s after the HA challenge (P < 0.05) (Figure 2A). In the same patients, hyperventilation of humidified RA gas mixture did not cause a significant increase in SRaw (baseline, 6.66 ± 1.49 cm H2O·s; peak response, 9.19 ± 1.41 cm H2O·s; P > 0.05) (Figures 1A and 2A). Wheezing in the chest was detected by auscultation during hyperventilation of HA in five of the six patients but was not detected in any patient during hyperventilation of RA.
TABLE 2.
CHANGES IN END-TIDAL TEMPERATURE AND CO2 CONCENTRATION CAUSED BY HYPERVENTILATION OF HUMIDIFIED AIR AT ROOM AND HIGH TEMPERATURE*
| ET Temperature (°C) |
ET CO2 (%) |
|||
| Patient Type, Treatment | Before | During | Before | During |
| Asthma | ||||
| RA | 33.5 ± 0.3 | 33.1 ± 0.3 | 4.49 ± 0.27 | 4.53 ± 0.34 |
| HA | 33.2 ± 0.2 | 34.7 ± 0.1† | 4.52 ± 0.35 | 4.64 ± 0.28 |
| Healthy | ||||
| RA | 34.1 ± 0.6 | 33.0 ± 0.7 | 4.48 ± 0.36 | 5.11 ± 0.31 |
| HA | 32.7 ± 0.7 | 34.3 ± 0.6† | 5.02 ± 0.24 | 4.82 ± 0.40 |
Definition of abbreviations: ET = end tidal; HA = humidified air at high temperature; RA = humidified air at room temperature.
Measurements were made before and at 2 min after the beginning of the 4-min hyperventilation; the latter was measured immediately after the hyperventilation was interrupted for three to six breaths while the subject breathed room air during these measurements.
Significant difference (P < 0.05; n = 6; paired t test) between before and during the hyperventilation challenge.
Figure 1.
Representative responses of specific airway resistance (SRaw) to hyperventilation of humidified room air (open circles) and hot air (closed circles) in a patient with asthma (A) and a healthy subject (B). Each point represents the data averaged over four consecutive breaths. During hyperventilation (shaded bars), the subjects breathed a gas mixture of 4.5% CO2 balance air at 40% of maximal voluntary ventilation for 4 minutes. Only one experiment was performed in each subject on the same day. Data are means ± SEM of four breaths.
Figure 2.
Group data showing a comparison of the peak responses of specific airway resistance (SRaw) to hyperventilation of humidified room air and hot air in patients with asthma (n = 6) (A) and healthy subjects (n = 6) (B). Baseline and peak SRaw were averaged over eight and four consecutive breaths before and after hyperventilation challenge, respectively, in each subject. Data are means ± SEM. *Significantly different (P < 0.05) from the baseline. †Significant difference (P < 0.05) comparing the corresponding data between room air and hot air.
In healthy subjects, hyperventilation of humid HA and RA gas mixtures caused similar changes in the end-tidal temperature and CO2 concentration as those in patients with asthma (Table 2). However, in contrast to that in patients with asthma, the SRaw responses were distinctly smaller, and there was no difference between the responses to HA and RA challenges in healthy subjects (Figures 1B and 2B). Wheezing was not detected during or after either of these challenges.
When the forced expiratory test was performed at approximately 8 minutes after the HA challenge, the ratio between FEV1 and FVC was still significantly reduced (P < 0.05) from baseline (before HA challenge) in patients with asthma but not in healthy subjects (Table 3). The reduction was mainly generated by a reduced FEV1 without a significant change in FVC. There was no significant change in residual volume after the HA challenge in patients with asthma (before: 1.34 ± 0.21 L; after: 1.53 ± 0.27 L; P > 0.05) or healthy individuals (before: 1.55 ± 0.20 L; after: 1.63 ± 0.32 L; P > 0.05).
TABLE 3.
CHANGES IN FORCED EXPIRATORY VOLUMES CAUSED BY HYPERVENTILATION OF HUMIDIFIED AIR AT HIGH TEMPERATURE AND AT ROOM TEMPERATURE
| FEV1* (L) |
FVC (L) |
FEV1/FVC |
||||
| Patient Type, Treatment | Before | During | Before | During | Before | During |
| Asthma | ||||||
| RA | 3.52 ± 0.29 | 3.44 ± 0.30 | 4.35 ± 0.46 | 4.43 ± 0.46 | 82.33 ± 3.24 | 78.67 ± 3.42* |
| HA | 3.45 ± 0.32 | 3.04 ± 0.21* | 4.30 ± 0.46 | 4.29 ± 0.41 | 81.17 ± 2.94 | 71.67 ± 2.36* |
| Healthy | ||||||
| RA | 4.27 ± 0.32 | 4.44 ± 0.49 | 5.39 ± 0.65 | 5.31 ± 0.65 | 81.20 ± 4.41 | 81.60 ± 4.72 |
| HA | 4.43 ± 0.48 | 4.48 ± 0.55 | 5.43 ± 0.72 | 5.43 ± 0.71 | 83.40 ± 4.37 | 83.40 ± 3.83 |
Definition of abbreviations: HA = humidified air at high temperature; RA = humidified air at room temperature.
Forced expiratory tests were performed before and at ∼8 min after the hyperventilation challenge in patients with asthma (n = 6) and healthy subjects (n = 5; the test was not done after HA in one subject).
†Significant difference (P < 0.05; paired t test) between before and after the hyperventilation challenge.
In patients with asthma, the bronchoconstriction generated after the HA challenge was also clearly illustrated by a concave shape and a reduced peak flow in the expiratory flow-volume loops (e.g., Figure 3B), whereas RA challenge had no effect on the flow-volume loop in these patients (e.g., Figure 3A).
Figure 3.
Effect of hyperventilation of humidified room air (RA) and hot air (HA) on forced expiratory flow-volume loops in a patient with asthma: baseline (blue lines) and response after hyperventilation challenge (red lines).
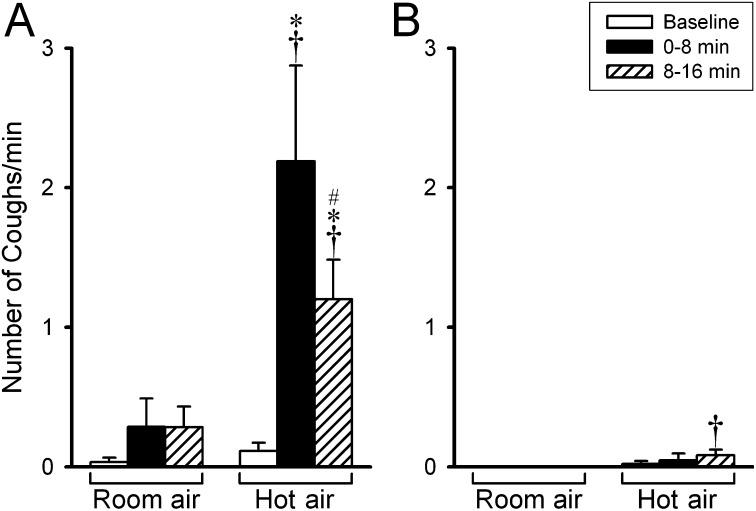
Hyperventilation of humidified HA also triggered coughs in five of the six patients with asthma, which began during the hyperventilation challenge and continued after the termination of HA challenge. Coughs were identified by the sound generated and verified by the short and sharp expiratory flow and pressure recorded at the mouthpiece in some of the experiments. The cough frequency increased from 0.11 ± 0.06 coughs per minute at the baseline to 2.19 ± 0.68 coughs per minute during the first 8 minutes after HA challenge (Figure 4A). In contrast, hyperventilation of RA rarely caused coughs in the same patients. In healthy individuals, neither HA nor RA hyperventilation generated cough (Figure 4B). The cough data during the hyperventilation challenge are not reported because they were not recorded in some of the subjects.
Figure 4.
Group data showing a comparison of the cough responses to hyperventilation of humidified room air and hot air in patients with asthma (n = 6) (A) and healthy subjects (n = 6) (B). Cough frequencies were averaged in 8-minute durations before and after hyperventilation challenge in each subject. Data are means ± SEM and were collected in the same experiments as performed in Figure 2. *Significantly different (P < 0.05) from the baseline. †Significant difference (P < 0.05) comparing the corresponding data between room air and hot air. #Significantly different (P < 0.05) from that in the first 8 minutes after hyperventilation.
We used isotonic saline to humidify the inspired gas mixture in this study because inhalation of aerosolized distilled water is known to induce airway constriction in patients with asthma (15). In two of these six patients with asthma, we also tested their responses to HA challenge when humidity was generated by distilled water, but peak SRaw and cough responses to HA hyperventilation were similar to those when humidity was generated by saline in these patients.
In patients with asthma, hyperventilation of HA caused small but significant increases in heart rate (baseline: 80 ± 4 beats/min; after HA challenge: 85 ± 5 beats/min; P < 0.05) and body temperature (baseline: 36.3 ± 0.2°C; after HA challenge: 36.6 ± 0.2°C; P < 0.05) but did not generate any significant change in blood pressure or oxygen saturation. Similar changes in heart rate and body temperature were found in healthy subjects after HA challenge.
Study 2
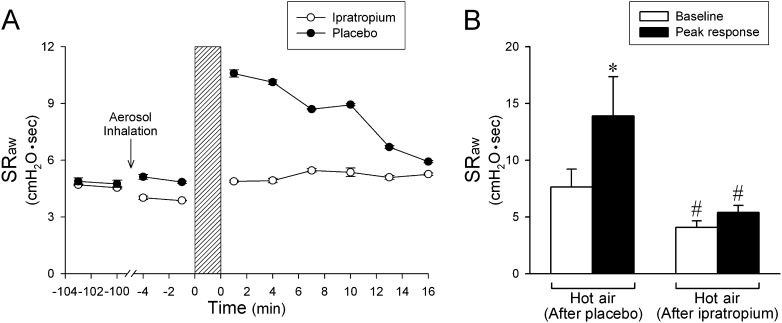
This study series was performed in the same patients with asthma tested in study 1. The HA challenge was studied 90 minutes after inhalation of ipratropium or placebo aerosol when ipratropium was expected to reach its full effect. Pretreatment with inhalation of ipratropium aerosol did not cause any change in heart rate (P > 0.05), blood pressure (P > 0.05), or any side effects (e.g., dizziness or blurred vision) in these patients but significantly reduced the baseline SRaw (6.12 ± 0.72 cm H2O·s before and 4.07 ± 0.58 cm H2O·s after ipratropium; P < 0.05) (Figure 5A). More importantly, ipratropium prevented the increase in SRaw generated by HA hyperventilation (peak SRaw after HA challenge: 5.41 ± 0.62 cm H2O·s; P > 0.05) (Figures 5A and 5B). Pretreatment with placebo in the same manner did not change the baseline SRaw (7.84 ± 1.59 cm H2O·s before and 7.63 ± 1.58 cm H2O·s after placebo; P > 0.05) and failed to prevent the HA-induced bronchoconstriction in the same patients (peak SRaw after HA challenge: 13.90 ± 3.45 cm H2O·s; P < 0.05) (Figures 5A and 5B). The blocking effect of ipratropium pretreatment on the HA-evoked bronchoconstriction was also evident in the flow-volume loops obtained in these patients (e.g., Figures 3C and 3D).
Figure 5.
Effect of pretreatment with ipratropium aerosol on the airway response to hot humid air challenge in six patients with asthma. (A) Representative specific airway resistance (SRaw) responses obtained in a patient with asthma. Ipratropium and placebo aerosols were administered in a double-blind fashion, and the response to hot air challenge was tested 90 minutes after the aerosol inhalation. The two tests were performed about 1 week apart. (B) Average data collected from all six patients with asthma comparing the peak SRaw responses to hot humid air challenge after ipratropium and placebo pretreatments. Baseline and peak SRaw were averaged over eight and four consecutive breaths before and after hyperventilation of hot air, respectively, in each subject. Data are means ± SEM. *Significantly different (P < 0.05) from the baseline. #Significant difference (P < 0.05) comparing the corresponding data between ipratropium and placebo pretreatments.
Pretreatment with ipratropium, however, did not prevent the cough response elicited by the hyperventilation of humidified HA in patients with asthma (Figure 6). There was no difference in the cough frequency during the first 8 minutes after HA challenge between ipratropium and placebo pretreatments in the same patients (Figure 6).
Figure 6.
Group data showing a comparison of the cough responses to hot humid air hyperventilation challenge after placebo (A) and ipratropium (B) pretreatments in six patients with asthma. Cough frequencies were averaged in 8-minute durations before and after hyperventilation challenge in each subject. Data are means ± SEM (n = 6) and were collected in the same experiments performed in Figure 5. For further details, see legend of Figure 5. *Significantly different (P < 0.05) from the baseline. #Significantly different (P < 0.05) from that in the first 8 minutes after hyperventilation. There was no significant difference (P > 0.05) in the corresponding data between ipratropium and placebo pretreatments.
The effect of ipratropium was not tested in healthy subjects because the HA hyperventilation challenge caused only a very small increase in SRaw and because there was no difference between their responses to HA and RA challenges.
Discussion
The results of this study showed that hyperventilation of HA triggered an immediate and reversible increase in airway resistance in patients with mild asthma but caused either only a very small or no response in healthy subjects. More importantly, the bronchoconstriction in these patients was completely prevented by pretreatment with ipratropium aerosol, indicating an involvement of cholinergic reflex. Breathing HA also triggered coughs consistently in these patients, suggesting an involvement of the airway sensory nerves that are responsible for eliciting the cough reflex. Furthermore, this reflex bronchoconstriction is likely generated by the increase in airway temperature because hyperventilation of RA did not generate any change in airway resistance in the same patients.
The mechanisms underlying these responses are not fully understood, but several factors possibly involved should be carefully considered. One of these factors is the activation of specific types of airway sensory nerves that are sensitive to an increase in temperature. The entire respiratory tract (from larynx to alveolar wall) is extensively innervated by vagal sensory nerves, of which the majority (∼75%) are nonmyelinated (C-) afferent fibers (16). It is well documented that these bronchopulmonary C-fibers can be activated by various inhaled irritants and certain endogenous chemical mediators and play an important role in protecting the lung under normal and disease conditions (10–12). Stimulation of these afferents triggers bronchoconstriction, cough, mucus secretion, and other cardiopulmonary reflex responses in various species, including humans (10, 11, 17, 18). One of the most prominent characteristics of these bronchopulmonary C-fiber afferents is the expression of TRPV type 1 (TRPV1) receptors at the sensory nerve terminals (19, 20).
TRPV1, a member of the superfamily of TRP ion channel proteins, is a tetrameric membrane protein that forms a nonselective, non–voltage-gated cation channel (21, 22). TRPV1 and three other subtypes of TRPV channels (TRPV2–4) are known as the primary sensors for detecting warm temperature in mammalian species (23–25). Indeed, a recent study in our laboratory has demonstrated that the afferent activity of these vagal pulmonary C-fiber endings was elevated when the temperature in the isolated perfused thoracic chamber was raised to above a threshold of approximately 39.2°C in anesthetized rats (6). More recent studies using the whole-cell perforated patch clamping technique have shown that an increase in temperature within the normal physiological range (35–41°C) evoked inward currents (voltage-clamp mode) and membrane depolarization and action potentials (current-clamp mode) in isolated pulmonary sensory neurons (7). These responses were reduced by more than 50% after pretreatment with AMG 9810, a selective antagonist of the TRPV1 channel, indicating that hyperthermia can exert a direct stimulatory effect on pulmonary sensory neurons and that this effect is mediated primarily through activation of the TRPV1 channel (7). More importantly, these bronchopulmonary sensory neurons were activated by increasing temperature to the level considerably lower than 43°C, which was initially reported as the temperature threshold for activating the heterologously expressed TRPV1 receptor (21).
A recent study reported that the average end-expiratory temperature plateau in children with asthma is 2.7°C higher than that in healthy individuals. Furthermore, the increased temperature is closely correlated with the number of eosinophils in induced sputum, indicating a coupling between the lung tissue temperature and the degree of inflammation in asthmatic airways (4). On the basis of results obtained in our previous studies described above (6, 7, 9), we suggest that the increase in airway tissue temperature due to inflammatory reaction can activate bronchopulmonary C-fiber sensory nerves during asthma exacerbation, which may play a part in the manifestation of various asthma symptoms, such as cough and bronchoconstriction. This possibility and the degree of its contribution remain to be tested.
In this study we used HA as a tool to raise the airway temperature, and an air temperature of 49°C was chosen to mimic that used by Aitken and Marini in their original study (5). Although this temperature is relatively high compared with the range of environmental temperatures, it is conceivable that the same elevation in airway tissue temperature as that in the present study can be generated by breathing HA at a lower temperature when the exposure time is lengthened. Surprisingly, it only generated a small increase of 1.5 to 1.6°C in the end-tidal temperature plateau in patients with asthma and in healthy subjects, probably due to the short duration (4 min) of exposure. We did not make direct measurement of the increase in local temperature in the airway tissue after HA hyperventilation, but we believe that it was probably underestimated by the measured increase in end-tidal temperature. Although we do not know if the increase in airway temperature induced by breathing HA in this study was sufficient to activate the TRPV1 in the airways, a recent report by Gavva and coworkers (26) has lent strong support to this possibility. Their clinical trial studies showed that treatment with the TRPV1 antagonist caused a significant increase in body temperature in healthy human volunteers, indicating that TRPV1 is active even at the normal resting body temperature and that the tonic activity of TRPV1 is involved in regulating normal core temperature (26). Hence, a slight increase in tissue temperature is expected to activate these channels. However, because the involvement of TRPV1 was not directly tested in this study, we cannot make a more definitive conclusion on its possible role in eliciting the responses observed in this study.
An important question remains as to why the same hot humid air challenge triggered cough and reflex bronchoconstriction in patients with asthma but not in healthy subjects. In addition, fever is not known to induce cough or bronchoconstriction in individuals without concurrent airway inflammatory diseases. Increasing evidence suggests an important role of bronchopulmonary C-fiber sensory nerves in the manifestation of various symptoms associated with airway inflammation, such as coughs and bronchoconstriction (27–29). Indeed, in addition to its role as a thermal sensor, TRPV1 can be activated by a number of endogenous chemical mediators, such as lipooxygenase metabolites and hydrogen ions, that are detected in the bronchoalveolar lavage fluid, sputum, or exhaled breath condensate of patients with inflammatory airway diseases (27, 30, 31). Moreover, cough sensitivity to the TRPV1 activators, capsaicin and citric acid aerosol, was markedly elevated in patients with asthma and chronic obstructive pulmonary disease (32). This is not surprising because certain endogenous inflammatory mediators (e.g., prostaglandin E2, bradykinin) can markedly enhance the sensitivity of TRPV1 and lower its threshold for activation (28). Recent studies further revealed that increased expression of the TRPV1 in bronchopulmonary sensory nerves may be responsible, at least in part, for the increased TRPV1-mediated responses in allergic airway inflammation (33, 34). Whether there is an enhanced sensitivity or an increased expression of TRPV1 in the airways during chronic inflammation, the same stimulation, such as an increase in airway temperature, is expected to evoke a greater afferent discharge of the bronchopulmonary C-fiber sensory nerves and consequently more intense reflex responses (e.g., cough and bronchoconstriction). The bronchoconstrictive response may be further amplified in asthmatic airways due to the hypertrophy and hyperplasia of airway smooth muscles known to develop during the process of chronic inflammation–induced airway remodeling (35).
Cough is a protective reflex function elicited by chemical and/or physical activation of the cough receptors that are specific types of sensory endings innervating the respiratory tract. Our study showed that ipratropium completely prevented the bronchoconstriction (Figure 5) but did not prevent the cough response triggered by the HA inhalation challenge in patients with asthma (Figure 6), suggesting that the cough reflex was elicited by a direct activation of the cough receptors and not by an indirect effect generated by bronchoconstriction. Several important new findings of the physiological properties and morphological characteristics of these cough receptors have been described in recent reviews (36). It is generally recognized that the vagal bronchopulmonary C-fiber sensory ending is one type of these cough receptors. Indeed, aerosolized capsaicin, a selective activator of TRPV1, is one of the most effective tussive agents commonly used for testing cough sensitivity in humans (17). Our observation that increasing airway temperature consistently triggered coughs in patients with asthma but not in healthy individuals supports the hypothesis that the sensitivity and/or expression of TRPV1 are up-regulated in the airways of patients with asthma.
In their study, Aitken and Marini (5) clearly described the important role of water content contained in HA for delivering the “heat load” to the airways. Sheppard and coworkers have reported a bronchoconstriction triggered by inhalation of distilled water or hypoosmolar saline aerosols in patients with asthma (15, 37). This response was attenuated by atropine, indicating the involvement of the cholinergic reflex (15). Indeed, it was later discovered that distilled water can stimulate C-fiber afferents and rapidly adapting receptors in the airways due to the low chloride ion concentration (38, 39). However, a possible involvement of the stimulatory effect of water can be ruled out in our study for the following reasons: (1) the humidity was generated from isotonic saline, which did not cause bronchoconstriction in the study by Sheppard and colleagues (15); (2) the amount of water content contained in the HA in our study was relatively low compared with that delivered by aerosol in their study (15); and (3) there was no detectable difference in the two patients with asthma tested in this study in their responses to hyperventilation of HA whether the humidity was generated from isotonic saline or distilled water.
In summary, this study showed that hyperventilation of HA triggered coughs and a reflex bronchoconstriction in patients with mild asthma but not in healthy individuals. The bronchoconstriction is mediated through the cholinergic reflex, suggesting that the airway sensory nerves, presumably the bronchopulmonary C-fibers, are activated by an increase in airway tissue temperature in these patients.
Supplementary Material
Acknowledgments
The authors thank Dr. Tom Henninger for designing and manufacturing the device for regulating temperature and humidity of gas mixture, Dr. Richard Kryscio for statistical consultation, Robert Morton for technical assistance, and the nursing staff at the University of Kentucky Clinical Research Development and Operations Center for assistance.
Footnotes
This study was supported in part by National Institutes of Health grant HL-96914 (L.Y.L.), a Department of Defense DMRDP/ARATD award administered by the US Army Medical Research & Materiel Command (USAMRMC) Telemedicine & Advanced Technology Research Center (TATRC) under Contract Number W81XWH-10-2-0189 (L.Y.L.), by a grant from the University of Kentucky Clinical Research Development & Operations Center (D.H.), and by a grant from the Kentucky Pediatric Research Institute (D.H.).
Originally Published in Press as DOI: 10.1164/rccm.201201-0088OC on April 13, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Anderson SD, Daviskas E. The mechanism of exercise-induced asthma is. J Allergy Clin Immunol 2000;106:453–459 [DOI] [PubMed] [Google Scholar]
- 2.Gourine AV, Rudolph K, Korsak AS, Kubatko J, Tesfaigzi J, Kozak W, Kluger MJ. Role of capsaicin-sensitive afferents in fever and cytokine responses during systemic and local inflammation in rats. Neuroimmunomodulation 2001;9:13–22 [DOI] [PubMed] [Google Scholar]
- 3.Planas ME, Rodriguez L, Sanchez S, Pol O, Puig MM. Pharmacological evidence for the involvement of the endogenous opioid system in the response to local inflammation in the rat paw. Pain 1995;60:67–71 [DOI] [PubMed] [Google Scholar]
- 4.Piacentini GL, Peroni D, Crestani E, Zardini F, Bodini A, Costella S, Boner AL. Exhaled air temperature in asthma: methods and relationship with markers of disease. Clin Exp Allergy 2007;37:415–419 [DOI] [PubMed] [Google Scholar]
- 5.Aitken ML, Marini JJ. Effect of heat delivery and extraction on airway conductance in normal and in asthmatic subjects. Am Rev Respir Dis 1985;131:357–361 [DOI] [PubMed] [Google Scholar]
- 6.Ruan T, Gu Q, Kou YR, Lee LY. Hyperthermia increases sensitivity of pulmonary c-fibre afferents in rats. J Physiol 2005;565:295–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ni D, Gu Q, Hu HZ, Gao N, Zhu MX, Lee LY. Thermal sensitivity of isolated vagal pulmonary sensory neurons: role of transient receptor potential vanilloid receptors. Am J Physiol Regul Integr Comp Physiol 2006;291:R541–R550 [DOI] [PubMed] [Google Scholar]
- 8.Ni D, Lee LY. Lack of potentiating effect of increasing temperature on responses to chemical activators in vagal sensory neurons isolated from trpv1-null mice. Am J Physiol Lung Cell Mol Physiol 2008;295:L897–L904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ni D, Lee LY. Effect of increasing temperature on trpv1-mediated responses in isolated rat pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 2008;294:L563–L571 [DOI] [PubMed] [Google Scholar]
- 10.Coleridge JC, Coleridge HM. Afferent vagal c fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 1984;99:1–110 [DOI] [PubMed] [Google Scholar]
- 11.Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary c-fibers. Respir Physiol 2001;125:47–65 [DOI] [PubMed] [Google Scholar]
- 12.Lee LY, Undem BJ. Brochopulmonary vagal sensory nerves. : Undem BJ, Weinreich D, Advances in vagal afferent neurobiology. Boca Raton, FL: CRC Press; 2005. pp. 279–313 [Google Scholar]
- 13.Hayes DJ, Collins PB, Lin RL, Lee LY. Cholinergic involvement of hyperthermia-induced bronchoconstriction in asthma: a translational study. Am J Respir Crit Care Med 2011;183:A5556 [Google Scholar]
- 14.National Asthma Education and Prevention Program Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol 2007;120:S94–S138 [DOI] [PubMed] [Google Scholar]
- 15.Sheppard D, Rizk NW, Boushey HA, Bethel RA. Mechanism of cough and bronchoconstriction induced by distilled water aerosol. Am Rev Respir Dis 1983;127:691–694 [DOI] [PubMed] [Google Scholar]
- 16.Jammes Y, Fornaris E, Mei N, Barrat E. Afferent and efferent components of the bronchial vagal branches in cats. J Auton Nerv Syst 1982;5:165–176 [DOI] [PubMed] [Google Scholar]
- 17.Dicpinigaitis PV. Experimentally induced cough. Pulm Pharmacol Ther 2007;20:319–324 [DOI] [PubMed] [Google Scholar]
- 18.Fuller RW, Dixon CM, Barnes PJ. Bronchoconstrictor response to inhaled capsaicin in humans. J Appl Physiol 1985;58:1080–1084 [DOI] [PubMed] [Google Scholar]
- 19.Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol 2001;127:113–124 [DOI] [PubMed] [Google Scholar]
- 20.Watanabe N, Horie S, Michael GJ, Keir S, Spina D, Page CP, Priestley JV. Immunohistochemical co-localization of transient receptor potential vanilloid (trpv)1 and sensory neuropeptides in the guinea-pig respiratory system. Neuroscience 2006;141:1533–1543 [DOI] [PubMed] [Google Scholar]
- 21.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997;389:816–824 [DOI] [PubMed] [Google Scholar]
- 22.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev 2007;87:165–217 [DOI] [PubMed] [Google Scholar]
- 23.Benham CD, Gunthorpe MJ, Davis JB. Trpv channels as temperature sensors. Cell Calcium 2003;33:479–487 [DOI] [PubMed] [Google Scholar]
- 24.Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annu Rev Neurosci 2006;29:135–161 [DOI] [PubMed] [Google Scholar]
- 25.Patapoutian A, Peier AM, Story GM, Viswanath V. Thermotrp channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci 2003;4:529–539 [DOI] [PubMed] [Google Scholar]
- 26.Gavva NR, Bannon AW, Surapaneni S, Hovland DN, Jr, Lehto SG, Gore A, Juan T, Deng H, Han B, Klionsky L, et al. The vanilloid receptor trpv1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci 2007;27:3366–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geppetti P, Materazzi S, Nicoletti P. The transient receptor potential vanilloid 1: role in airway inflammation and disease. Eur J Pharmacol 2006;533:207–214 [DOI] [PubMed] [Google Scholar]
- 28.Lee LY, Gu Q. Role of trpv1 in inflammation-induced airway hypersensitivity. Curr Opin Pharmacol 2009;9:243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takemura M, Quarcoo D, Niimi A, Dinh QT, Geppetti P, Fischer A, Chung KF, Groneberg DA. Is trpv1 a useful target in respiratory diseases? Pulm Pharmacol Ther 2008;21:833–839 [DOI] [PubMed] [Google Scholar]
- 30.Jia Y, Lee LY. Role of trpv receptors in respiratory diseases. Biochim Biophys Acta 2007;1772:915–927 [DOI] [PubMed] [Google Scholar]
- 31.Gu Q, Lee LY. Characterization of acid signaling in rat vagal pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 2006;291:L58–L65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doherty MJ, Mister R, Pearson MG, Calverley PM. Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease. Thorax 2000;55:643–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe N, Horie S, Spina D, Michael GJ, Page CP, Priestley JV. Immunohistochemical localization of transient receptor potential vanilloid subtype 1 in the trachea of ovalbumin-sensitized guinea pigs. Int Arch Allergy Immunol 2008;146:28–32 [DOI] [PubMed] [Google Scholar]
- 34.Zhang G, Lin RL, Wiggers M, Snow DM, Lee LY. Altered expression of trpv1 and sensitivity to capsaicin in pulmonary myelinated afferents following chronic airway inflammation in the rat. J Physiol 2008;586:5771–5786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiappara G, Gagliardo R, Siena A, Bonsignore MR, Bousquet J, Bonsignore G, Vignola AM. Airway remodelling in the pathogenesis of asthma. Curr Opin Allergy Clin Immunol 2001;1:85–93 [DOI] [PubMed] [Google Scholar]
- 36.Canning BJ. Functional implications of the multiple afferent pathways regulating cough. Pulm Pharmacol Ther 2011;24:295–299 [DOI] [PubMed] [Google Scholar]
- 37.Eschenbacher WL, Boushey HA, Sheppard D. Alteration in osmolarity of inhaled aerosols cause bronchoconstriction and cough, but absence of a permeant anion causes cough alone. Am Rev Respir Dis 1984;129:211–215 [PubMed] [Google Scholar]
- 38.Fox AJ, Barnes PJ, Dray A. Stimulation of guinea-pig tracheal afferent fibres by non-isosmotic and low-chloride stimuli and the effect of frusemide. J Physiol 1995;482:179–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pisarri TE, Jonzon A, Coleridge HM, Coleridge JC. Vagal afferent and reflex responses to changes in surface osmolarity in lower airways of dogs. J Appl Physiol 1992;73:2305–2313 [DOI] [PubMed] [Google Scholar]
- 40.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179–187 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.