Abstract
Rationale: Chronic obstructive pulmonary disease (COPD) is characterized by chronic inflammation, alveolar destruction, and airway and vascular remodeling. However, the mechanisms that lead to these diverse alterations have not been defined.
Objectives: We hypothesized that IL-18 plays a central role in the pathogenesis of these lesions.
Methods: We generated and characterized lung-specific, inducible IL-18 transgenic mice.
Measurements and Main Results: Here we demonstrate that the expression of IL-18 in the mature murine lung induces inflammation that is associated with the accumulation of CD4+, CD8+, CD19+, and NK1.1+ cells; emphysema; mucus metaplasia; airway fibrosis; vascular remodeling; and right ventricle cardiac hypertrophy. We also demonstrate that IL-18 induces type 1, type 2, and type 17 cytokines with IFN-γ–inhibiting macrophage, lymphocyte, and eosinophil accumulation while stimulating alveolar destruction and genes associated with cell cytotoxicity and IL-13 and IL-17A inducing mucus metaplasia, airway fibrosis, and vascular remodeling. We also highlight interactions between these responses with IL-18 inducing IL-13 via an IL-17A–dependent mechanism and the type 1 and type17/type 2 responses counterregulating each another.
Conclusions: These studies define the spectrum of inflammatory, parenchymal, airway, and vascular alterations that are induced by pulmonary IL-18; highlight the similarities between these responses and the lesions in COPD; and define the selective roles that type 1, type 2, and type 17 responses play in the generation of IL-18–induced pathologies.
Keywords: IL-18, chronic obstructive pulmonary disease, airway fibrosis, mucus metaplasia, vascular remodeling
At a Glance Commentary
Scientific Knowledge on the Subject
These studies suggest that IL-18 induces complex alterations in the lung including emphysema, airway and vascular remodeling, and cytotoxic responses. They also suggest that the emphysema and cytotoxic response are mediated by IFN-γ–dependent mechanisms and the airway and vascular remodeling are mediated by IL-17A– and IL-13–dependent mechanisms.
What This Study Adds to the Field
Type 1, 2, and 17 cytokines play distinct roles in the development of IL-18–induced chronic obstructive pulmonary disease–like parenchymal, airway, and vascular remodeling.
Chronic obstructive pulmonary disease (COPD) encompasses several clinical syndromes, most notably emphysema and chronic bronchitis (1, 2). It is a major unmet medical need in the United States and worldwide where it is the fourth and fifth leading cause of morbidity and mortality, respectively (3, 4). This is caused partly by our limited ability to treat people with COPD and a distinct lack of disease-modifying therapies (4, 5). Tissues from patients with COPD are characterized pathologically by chronic inflammation and varying degrees of emphysematous alveolar destruction, airway remodeling with tissue fibrosis and mucus metaplasia (6, 7), vascular remodeling with intimal hyperplasia, smooth muscle proliferation, and collagen deposition (8, 9). Importantly, a mechanistic construct that adequately accounts for the simultaneous existence of these varied tissue pathologic responses has not been put forth and animal models that simultaneously elicit these varied responses have not been commonly used. In particular, a mechanism that allows tissue destruction (emphysema) to coexist millimeters away from airway and vascular fibrotic responses has not been described (7).
Inflammation with infiltrating macrophages, neutrophils, lymphocytes, and occasionally eosinophils is seen throughout the bronchial tree and parenchyma of lungs from patients with COPD (10–13). It has long been assumed that this inflammation drives the varied pathologic alterations in this disease (14–16). A variety of lines of evidence have suggested that exaggerated type 1 inflammation plays an important role in the pathogenesis of emphysema (17, 18). Recent reports have also highlighted type 2 and type 17 cytokine production in the disorder (19–21). However, a pathologic schema that can account for these varied inflammatory and cytokine responses has not been put forth and the contributions that each of these responses make to the varied pathologies in COPD have not been adequately defined.
IL-18, a member of the IL-1 cytokine superfamily (22, 23), has an impressive ability to induce Th1-Tc1 differentiation and immune responses (22, 24). It can also contribute to the generation of type 2 immune responses (22) and plays a key role in the generation of Th17 responses in diseases characterized by autoimmunity (25, 26). Previous studies from our laboratory demonstrated that IL-18 is induced and activated after cigarette smoke (CS) exposure and that IL-18 receptor (R) 1 signaling plays a critical role in the pathogenesis of the pulmonary inflammation and emphysema in CS-exposed mice (27). In addition, increased levels of IL-18 have been noted in the serum and induced sputum from patients with COPD, which correlate with abnormal lung function (28, 29). Surprisingly, the ability of IL-18 to generate the varied pathologies characteristic of COPD has not been adequately defined. In addition, the ability of IL-18 to induce type 1, type 2, and type 17 responses in vivo and the ways that these different immune responses contribute to IL-18–induced tissue alterations and the varied pathologic lesions of COPD have not been adequately defined.
We hypothesized that IL-18 induces a broad spectrum of COPD-like inflammatory and remodeling responses in the murine lung. We also hypothesized that IL-18 induces a mixed type 1, type 2, and type 17 cytokine response and that each of these makes a distinct contribution to the pathogenesis of these varied pathologies. To test these hypotheses we generated transgenic (Tg) mice in which IL-18 was inducibly targeted to the lung and characterized the inflammatory and remodeling responses that were induced when the transgene was activated in adult animals. We also defined the contributions that a signature type 1 cytokine (IFN-γ), type 2 cytokine (IL-13), and type 17 cytokine (IL-17A) make in the pathogenesis of these alterations. These studies demonstrate that IL-18 induces tissue inflammation, emphysema, mucus metaplasia, airway fibrosis, and vascular remodeling with intimal hyperplasia, fibrosis, and cardiac right ventricle hypertrophy. They also highlight the exaggerated production of IFN-γ, IL-13, and IL-17A and exaggerated expression of moieties associated with cell cytotoxicity in these mice and demonstrate that IL-18 induces emphysema and the cytotoxic response via an IFN-γ–dependent mechanism and fibrotic airway remodeling, mucus metaplasia, and vascular remodeling via an IL-17A– and IL-13–dependent pathway. Lastly, they highlight important interactions between these pathways with IL-18 inducing IL-13 via an IL-17A–dependent mechanism and the IFN-γ and the IL-17A/IL-13 responses counterregulating one another. Aspects of these studies were presented at the Annual Meeting of the American Thoracic Society in 2010 (30).
Methods
Generation of IL-18–Expressing Tg Mice
We used an externally regulatable, dual construct overexpression Tg system that uses the Clara Cell 10-kD protein promoter as described by our laboratory (31–33). The required constructs were prepared, purified, and simultaneously microinjected (see Figure E1 in the online supplement). Tail biopsies were obtained from potential founder animals, DNA was isolated, and the presence or absence of the murine IL-18 and rtTA Tg sequences were evaluated via polymerase chain reaction and Western blot analysis. Six dual transgene-positive founder animals were obtained and backcrossed for more than 10 generations onto a C57BL/6J background to generate transgene-negative wild-type (WT, Tg−) and transgene-positive (Tg+) progeny. WT and Tg+ mice were kept on normal water until 6 weeks of age and then randomized to normal water or water with 1 g/L of doxycycline (Dox). In keeping with the known characteristics of the Clara cell 10-kD promoter (34, 35), immunohistochemistry demonstrated that IL-18 was produced in airway and type 2 alveolar epithelial cells after Dox administration (see Figure E2). Bronchoalveolar lavage (BAL) IL-18 levels were minimal in the absence of Dox. In contrast, after Dox, steady-state levels of BAL IL-18 between about 350 pg/ml and 4,500 pg/ml were appreciated (see Figures E3 and E4). The tissue and inflammatory responses in these mice were dose dependent. Thus, unless otherwise noted, findings from lungs from strain #8 mice are illustrated. All animal experimentation was conducted with the approval of the Institutional Animal Care and Use Committee of Yale School of Medicine.
Mice and Reagents
C57BL/6J WT and IFN-γ null mice (Jackson Laboratories, Bar Harbor, ME), IL-13 null mice (from Dr. A. McKenzie), and IL-17A null mice (from Dr. Y. Iwakura) were bred at Yale University. The reagents used for the experiments are described in detail in the online supplement (see Figure E5).
Preparation of BAL and Whole-Lung Single-Cell Suspensions
When BAL cell populations were being assessed, BAL was undertaken and cell differentials were evaluated after Diff Quick staining. A representative preparation can be seen in Figure E6. When whole-lung single-cell suspensions were being prepared, BAL was not undertaken, and the lungs were minced into small 1- to 2-mm2 pieces, mechanically disintegrated, filtered through a 70-μm strainer, and subjected to Ficoll-Paque density centrifugation as described previously by our laboratory and others (32, 36–38). Detailed methodology is provided in Figure E7.
Quantification of Cytokines
The levels of IFN-γ, IL-13, IL-17A (R&D, Minneapolis, MN), and IL-18 (MBL, Nagoya, Japan) were determined using commercial ELISA kits as per the manufacturer's instruction.
Histology, Morphometry, and Lung Histologic Mucus Index
Hematoxylin and eosin, periodic acid–Schiff, and trichrome staining were performed in the Research Histology Laboratory at Yale University School of Medicine. Lung morphometry was used to measure the alveolar chord length (a measure of the distance between alveolar walls and thus alveolar size) (27, 39) and the histologic mucus index (HMI, the number of periodic acid–Schiff–positive epithelial cells per unit airway basement membrane) as described by our laboratory (27, 39, 40).
Lung Collagen Content
The collagen in the entire right lung was quantitated using the Sircol Collagen Assay Kit (Biocolor Ltd., Carrickfergus, UK) according to the manufacturer's instructions.
Flow Cytometric Analysis
Single-cell suspensions were prepared as described previously, evaluated on a LSRII flow cytometer (Becton Dickinson, Franklin Lakes, NJ), and analyzed with FlowJo software (Tree Star, Ashland, OR). Intracellular cytokine staining was undertaken after stimulation with ionomycin (1 μg/ml) and phorbol myristate acetate (50 ng/ml) for 4 hours. The gating used excluded dead cells and can be seen in Figure E8. The isotype control staining can be seen in Figure E9.
mRNA Evaluations
Total lung RNA was obtained using trizol reagent (Invitrogen, Grand Island, NY) according to the manufacturer's instructions. Real-time reverse transcriptase polymerase chain reaction analysis was used as previously described by our laboratory (27) using the primers detailed in Figure E10.
Immunoblot Analysis
Whole-lung lysates were prepared and equal amounts of sample proteins were fractionated on 4–15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing conditions. Immunoreactive signal was detected using a chemiluminescent procedure (ECL Western blotting detection system; Amersham Life Science, Piscataway, NJ) according to the manufacturer's instructions.
Evaluation of Right Ventricular Hypertrophy
The whole heart was excised and the right ventricular (RV) free wall was dissected. The ratio of weights of the RV free wall and the septum plus left ventricular free wall was calculated and used as an index of RV hypertrophy (Fulton index) (41, 42).
TUNEL Analysis
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analysis was undertaken as previously described by our laboratory (27, 39).
Statistical Analyses
Evaluations were undertaken with SPSS (IBM, Armonk, NY) software. As appropriate, groups were compared with Student t test or with nonparametric Mann-Whitney U test. Values are expressed as mean ± SEM. Statistical significance was defined at a level of P less than 0.05.
Results
Characterization of the Inflammatory Responses Induced by IL-18
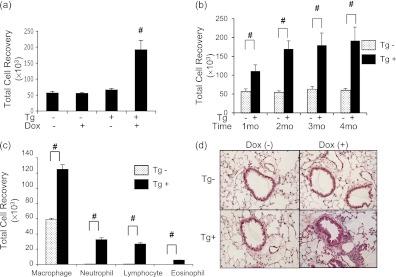
Six-week-old WT and Tg+ mice were placed on normal water or Dox water, maintained on these regimens for 1–4 months, and BAL and histologic changes were evaluated. In all cases Dox did not cause significant alterations in the BAL or tissue responses in WT mice. In addition, BAL and tissues from IL-18 Tg+ mice on normal water were not significantly different than those from WT animals. In contrast, Tg+ mice on Dox water manifest a significant increase in BAL total cell recovery. This response was most prominent after 4 months but could be appreciated after as little as 1 month of Dox administration (Figures 1a and 1b). Increases in BAL macrophage, neutrophil, lymphocyte, and to a lesser extent eosinophil recovery were also appreciated (Figure 1c, see Figure E6). This BAL response was associated with neutrophil-, macrophage-, and lymphocyte-rich tissue inflammation that was most prominent in peribronchial and perivascular locations (Figure 1d, see Figure E11).
Figure 1.
Inflammation in IL-18 transgenic (Tg) mice. Wild-type (Tg−) and IL-18 Tg (Tg+) mice were randomized to normal water (Dox−) or water with doxycycline (Dox+) for 1–4 months. All data represent evaluations that were done after 4 months of doxycycline (Dox+) water administration unless otherwise specified. The effects of IL-18 on bronchoalveolar lavage (BAL) total cell recovery (a), the kinetics of transgene-induced increases in BAL total cell recovery (b), BAL differential cell recovery (c), and peribronchial and perivascular inflammation (hematoxylin and eosin, ×20) (d) are illustrated. The values in a–c represent the mean ± SEM of evaluations in a minimum of eight mice. # P < 0.001. d is representative of a minimum of five similar evaluations.
Characterization of the Remodeling Responses Induced by IL-18
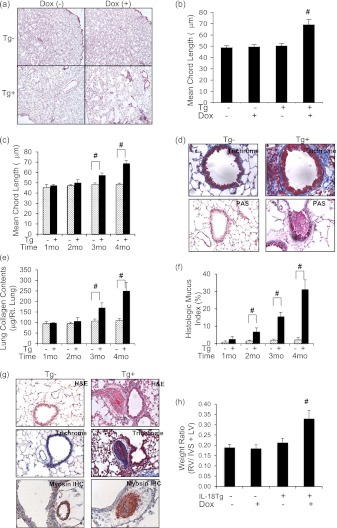
The protocols noted previously were also used to define the IL-18–induced remodeling responses. In all cases Dox did not regulate tissue remodeling in WT mice. In contrast, impressive alterations in parenchymal, airway, and vascular structures were noted in Tg+ mice on Dox water but not Tg+ mice on normal water. Light microscopy revealed alveolar remodeling with emphysematous septal destruction and significant increases in morphometrically determined alveolar chord length (Figures 2a–2c). Fibrotic airway remodeling and airway mucus metaplasia with goblet cell hyperplasia were also readily appreciated (Figure 2d and data not shown). The enhanced collagen accumulation and mucus metaplasia were noted in Tg mice treated with Dox for as little as 3 and 2 months, respectively (Figures 2e and 2f). Pulmonary vascular remodeling with the accumulation of collagen and myosin-positive cells were also observed in Tg+ mice on Dox water (Figure 2g). These vascular alterations were associated with hypertrophy of the right cardiac ventricle (Figure 2h). Collectively, these results demonstrate that lung-targeted IL-18 induces alveolar destruction, mucus metaplasia, fibrotic airway remodeling, pulmonary vascular remodeling, and cor pulmonale responses, which resemble the alterations in human COPD.
Figure 2.
Remodeling in IL-18 transgenic (Tg) mice. Wild-type (Tg−) and IL-18 Tg (Tg+) mice were randomized to normal water (Dox−) or water with doxycycline (Dox+) for 1–4 months. All data represent evaluations that were done after 4 months of doxycycline (Dox+) water administration unless otherwise specified. The effects of IL-18 on emphysematous alveolar destruction (hematoxylin and eosin [H&E], ×4) (a), alveolar chord length (b), the kinetics of alveolar enlargement (c), fibrotic airway remodeling (trichrome, ×40), mucus metaplasia (periodic acid–Schiff [PAS], ×20) (d), kinetics of collagen accumulation (e), kinetics of the mucus response (f), and vascular remodeling and myosin immunohistochemistry (IHC) (g) are illustrated. Right ventricular (RV) hypertrophy was assessed by calculating the ratio of the weights of the RV free wall and the interventricular septum (IVS) plus left ventricular (LV) free wall in h. In the PAS stains in d mucus stains purple. In the trichrome stains in d and g collagen fibers stain blue. In the IHC evaluation in g, myosin-positive cells stain brown. The values in b, c, e, f, and h represent the mean ± SEM of evaluations in a minimum of eight mice. # P < 0.001. d and g are representative of a minimum of five similar evaluations.
IL-18 Regulation of Th1, Th2, and Th17 Cells in the Lung
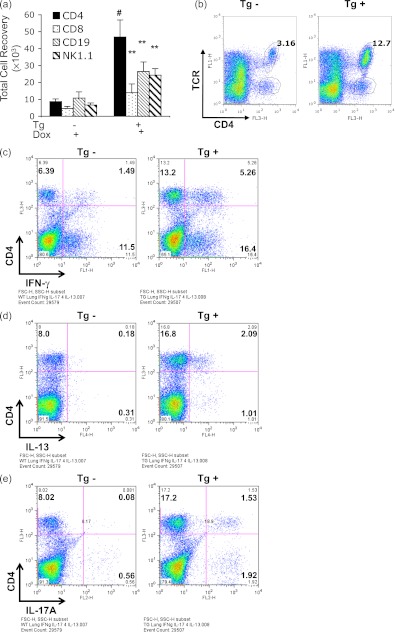
We next compared the numbers of CD4+, CD8+, CD19+, and NK1.1+ cells in lung parenchyma from WT and Tg+ mice on normal and Dox water. WT mice on normal and Dox water and Tg+ mice on normal water had similar numbers of cells that expressed these markers (data not shown). In contrast, lungs from Tg+ mice on Dox water contained significantly increased numbers of pulmonary CD4+, CD8+, CD19+, and NK1.1+ cells compared with lungs from WT mice on Dox water or normal water and Tg+ mice on normal water (Figures 3a and 3b and data not shown). Of these alterations, the increase in CD4+ cells was most prominent (Figure 3b). Intracellular cytokine staining and fluorescence-activated cell sorter analysis highlighted significantly elevated levels of intracellular IFN-γ, IL-13, and IL-17A, signature cytokines of type 1, 2, and 17 responses, respectively, in these lung cell populations (Figures 3c–3e, see Figure E12). In accord with these observations, whole-lung mRNA evaluations also revealed significantly increased levels of IFN-γ, IL-13, and IL-17A mRNA in lungs from Tg+ mice on Dox water compared with lungs from WT mice on normal or Dox water and Tg+ mice on normal water (see Figures E13a–E13c). The enhanced accumulation of these mRNA moieties was seen after as little as 2 months of Dox administration (see Figures E14a–E14c). These responses were not type 1, 2, or 17 cytokine-specific because Tg IL-18 also stimulated the expression of IL-6, CCL2, and CCL3 (see Figures E15a–E15c). Thus, IL-18 enhanced the accumulation of CD4+, CD8+, CD19+, and NK1.1+ cells and induced the expression of cytokines associated with type 1, 2, and 17 responses in the murine lung.
Figure 3.
Immunologic characterization of IL-18 transgenic (Tg) mice. Single-cell suspensions were prepared from whole lungs from wild-type (Tg−) and IL-18 Tg (Tg+) mice on doxycycline water (Dox+) for 4 months. Whole-lung cell gates were undertaken excluding dead cell populations and fluorescence-activated cell sorter analysis was used to evaluate the numbers of CD4+, CD8+, CD19+, and NK1.1+ cells (a). The gating for T-cell receptor (TCR) and CD4 double-positive (+) cells is in b. Fluorescence-activated cell sorter was also used to evaluate CD4+ cells that produced IFN-γ (c), IL-13 (d), and IL-17A (e). The values in a represent the mean ± SEM of evaluations in a minimum of eight mice. ** P < 0.01, # P < 0.001. b–e are representative of a minimum of three similar experiments.
Interactions among IFN-γ, IL-13, and IL-17A
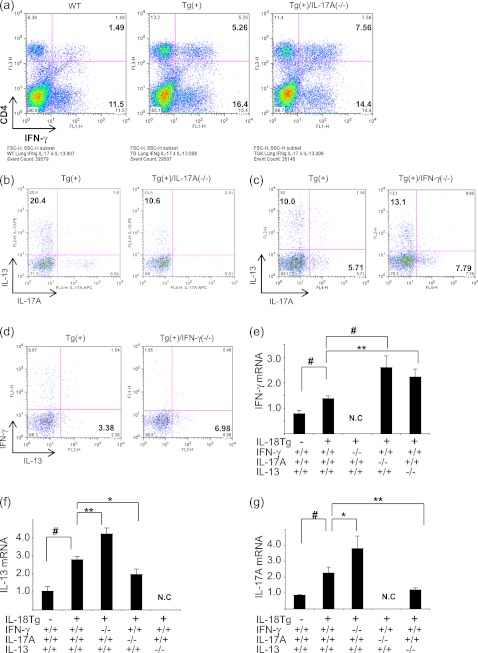
We next compared the cytokine responses in IL-18Tg+ mice and Tg+ mice with null IFN-γ (IL-18Tg+/IFN-γ−/−), IL-17A (IL-18Tg+/IL-17A−/−), and IL-13 (IL-18Tg+/IL-13−/−) loci. Interestingly, in the absence of IL-17A, the ability of IL-18 to induce the production of IFN-γ in CD4+ cells and the levels of pulmonary IFN-γ mRNA were significantly enhanced compared with the responses in IL-18Tg+ mice (Figures 4a and 4e). In addition, in the absence of IL-17A, the ability of IL-18 to induce the production of IL-13 in CD4+ cells and pulmonary IL-13 mRNA was significantly decreased compared with the responses in IL-18Tg+ mice (Figures 4b and 4f). In the absence of IFN-γ, the ability of IL-18 to induce the production of IL-17A and IL-13 in CD4+ cells and pulmonary IL-17A and IL-13 mRNA were significantly enhanced compared with the responses in IL-18Tg+ mice (Figures 4c, 4d, 4f, and 4g). Lastly, in the absence of IL-13, the ability of IL-18 to induce the production of IFN-γ in CD4+ cells and pulmonary IFN-γ mRNA was significantly enhanced compared with the responses in IL-18Tg+ mice (Figure 4e and data not shown). These studies highlight complex cytokine–cytokine interactions in which IL-18 stimulates IL-13 via an IL-17A–dependent mechanism and IL-18–induced IFN-γ and the IL-17A/IL-13 axis counterregulate one another.
Figure 4.
Interactions between IFN-γ, IL-17A, and IL-13 in IL-18 transgenic (Tg) mice. Wild-type (WT) (Tg−) and IL-18 Tg (Tg+) mice with WT (+/+) and null (−/−) genetic loci were on doxycycline (Dox) water for 4 months. Lung parenchymal cells were isolated and accumulation of CD4+ cells that produce IFN-γ (a) and IL-13 (b) in the presence (+/+) or absence (−/−) of IL-17A was evaluated. The accumulation of CD4+ IL-17A–producing cells (c) and IL-13–producing cells (d) was evaluated in the presence (+/+) or absence (−/−) of IFN-γ. The levels of mRNA encoding IFN-γ (e), IL-13 (f), and IL-17A (g) mRNAs were also evaluated. b–d were evaluated after the CD4+ lymphocyte population was gated. The values in e–g represent the mean ± SEM of evaluations in a minimum of eight mice. * P < 0.05, ** P < 0.01, # P < 0.001. N.C = not checked. a–d are representative of a minimum of three similar experiments.
IL-18 Induction and Cytokine Regulation of Molecules Associated with Cell Cytotoxicity
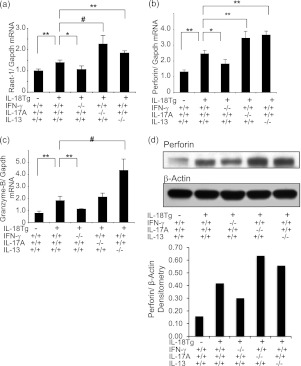
In keeping with the increased numbers of NK1.1+ and CD8+ cells in IL-18Tg+ mice noted previously, studies were undertaken to determine if cytotoxic responses were augmented in lungs from these animals. In keeping with recent studies that demonstrate that cytotoxic lymphocytes can play a role in alveolar destruction (43), the levels of mRNA encoding retinoic acid early transcript 1 (Raet-1), perforin, and granzyme B were significantly increased in lungs from IL-18Tg+ mice compared with those from WT controls (Figures 5a–5c). Protein levels were also increased (Figure 5d and data not shown). Interestingly, the levels of mRNA encoding these genes and their proteins were significantly decreased in IL-18Tg+/IFN-γ−/− mice and significantly enhanced in IL-18Tg+/IL-17A−/− and IL-18Tg+/IL-13−/− mice (Figures 5a–5d). Taken together, these studies demonstrate that IL-18 regulates pulmonary cytotoxic mediators via a stimulatory IFN-γ–dependent pathway and an IL-17A/IL-13 pathway that inhibits this activation.
Figure 5.
IL-18 induction and cytokine regulation of molecules associated with cell cytotoxicity. Wild-type (transgenic [Tg]−) and IL-18 Tg (Tg+) mice with the noted wild-type and null genetic loci were treated with doxycycline (Dox) water for 4 months. The levels of whole-lung mRNA encoding Raet-1 (a), perforin (b), and granzyme B (c) were evaluated. (d) Western blot analysis of perforin protein was also undertaken and analyzed using densitomoetry. The values in a–c represent the mean ± SEM of evaluations in a minimum of eight mice. * P < 0.05, ** P < 0.01, # P < 0.001. d is representative of a minimum of three similar evaluations.
Distinct Roles of IFN-γ, IL-17A, and IL-13 in IL-18–induced Inflammation
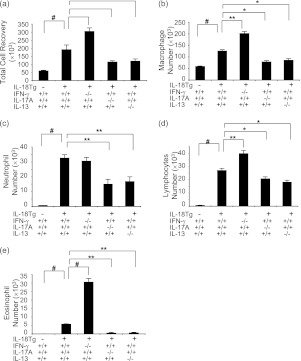
BAL total cell recovery and peribronchial and perivascular inflammation were increased in IL-18Tg+ mice on Dox water. These responses were significantly exaggerated in lungs from IL-18Tg+/IFN-γ−/− mice and ameliorated in lungs from IL-18Tg+/IL-17A−/− or IL-18Tg+/IL-13−/− mice (Figure 6a and data not shown). Interestingly, differential cellular recoveries were also altered with Tg+ mice that lacked IL-13 or IL-17A manifesting a marked decrease in macrophage, neutrophil, and lymphocyte recovery and an almost complete absence of eosinophils (Figures 6b–6e). In contrast, Tg+ mice that lacked IFN-γ manifest a significant increase in macrophage, lymphocyte, and eosinophil recovery (Figures 6b–6e). These studies demonstrate that IFN-γ, IL-17A, and IL-13 play distinct roles in IL-18–induced pulmonary inflammation.
Figure 6.
Roles of IFN-γ, IL-17A, and IL-13 in IL-18–induced pulmonary inflammation. Wild-type (transgenic [Tg]−) and IL-18 Tg (Tg+) mice with the noted wild-type and null genetic loci were placed on doxycycline (Dox) water for 4 months. Bronchoalveolar lavage (BAL) total cell recovery (a), BAL macrophage number (b), BAL neutrophil number (c), BAL lymphocyte number (d), and BAL eosinophil number (e) were evaluated. The noted values represent the mean ± SEM of evaluations in a minimum of eight mice. * P < 0.05, ** P < 0.01, # P < 0.001.
Cytokines in IL-18–induced Alveolar Destruction
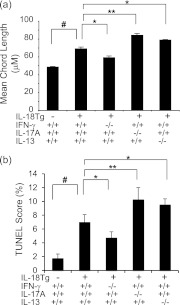
We next compared the alveolar responses in IL-18Tg+ mice that contained and had null mutations of IFN-γ, IL-13, or IL-17A. IL-18–induced alveolar remodeling was markedly decreased in mice that lacked IFN-γ (Figure 7a). In contrast, a significant increase in alveolar size and alveolar chord length was seen in mice that lacked IL-17A or IL-13 (Figure 7a). Interestingly, IL-18–induced alveolar destruction was associated with increased levels of epithelial cell apoptosis (as assessed with TUNEL evaluations), which was decreased in the absence of IFN-γ and increased in the absence of IL-13 or IL-17A (Figure 7b). These studies demonstrate that IFN-γ plays a critical role in the pathogenesis of IL-18–induced alveolar destruction, whereas the IL-17A–IL-13 axis feeds back to inhibit these destructive events.
Figure 7.
Roles of IFN-γ, IL-17A, and IL-13 in IL-18–induced alveolar remodeling. Wild-type (transgenic [Tg]−) and IL-18 Tg (Tg+) mice with the noted wild-type and null genetic loci were placed on doxycycline (Dox) water for 4 months. Alveolar chord length measurements (a) and TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) evaluations (b) are illustrated. The noted values represent the mean ± SEM of evaluations in a minimum of eight mice. * P < 0.05, ** P < 0.01, #P < 0.001.
Cytokines in IL-18–induced Airway and Vascular Responses
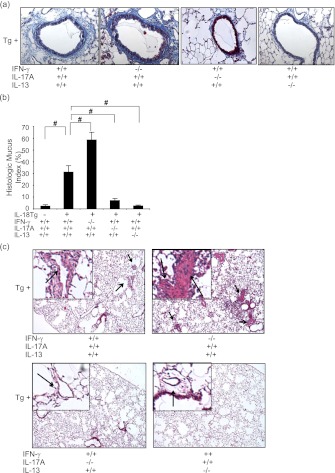
To define the pathogenesis of IL-18–induced airway and vascular responses, we compared these responses in IL-18Tg+ mice that contained and had null mutations of IFN-γ, IL-13, or IL-17A. IL-18–induced airway fibrosis and mucus metaplasia (assessed histologically and via the HMI) were significantly increased in mice that lacked IFN-γ (Figures 8a and 8b). In contrast, a significant decrease in airway fibrosis and mucus metaplasia was seen in mice that lacked IL-17A or IL-13 (Figures 8a and 8b). Similar events were seen in comparisons of the vascular remodeling responses with Tg+/IFN-γ null mice manifesting increased remodeling and Tg+/IL-13 null and Tg+/IL-17A null mice manifesting decreased vascular responses (Figure 8c). Collectively, these studies demonstrate that the IL-17A/IL-13 axis plays a critical role in the pathogenesis of IL-18–induced airway and vascular remodeling, whereas IFN-γ feeds back to inhibit these events.
Figure 8.
Roles of IFN-γ, IL-17A, and IL-13 in IL-18–induced airway and vascular remodeling. Wild-type (transgenic [Tg]−) and IL-18 Tg (Tg+) mice with the noted wild-type and null genetic loci were placed on doxycycline (Dox) water for 4 months. Fibrotic airway remodeling (trichrome, ×20) (a), histologic mucus index (b), and vascular remodeling responses (c) were evaluated. In c, arrows indicate remodeled blood vessels at lower (×4) and higher (×40) magnification. a and c are representative figures from a minimum of eight mice. The values in b represent the mean ± SEM of evaluations in a minimum of eight mice. # P < 0.001.
Cytokine Regulation of Matrix Molecule Accumulation and Gene Expression
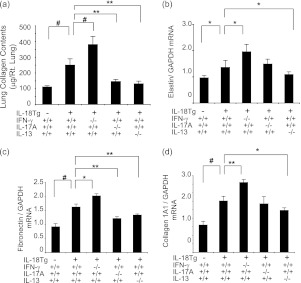
In accord with the fibrotic responses noted previously, total lung collagen accumulation and the levels of mRNA encoding elastin, fibronectin, and type I collagen α1 (col1a1) were significantly increased in lungs from Dox-treated IL-18Tg+ mice compared with appropriate controls (Figures 9a–9d). Interestingly, collagen accumulation and matrix molecule gene expression were significantly increased in IL-18Tg+ mice with null mutations of IFN-γ and significantly decreased in IL-18Tg+ mice with null mutations of IL-17A or IL-13 (Figures 9a–9d). These studies demonstrate that IL-17A/IL-13–mediated responses play critical roles in IL-18–induced collagen accumulation and matrix molecule gene expression, whereas IFN-γ suppresses these responses.
Figure 9.
Roles of IFN-γ, IL-17A, and IL-13 in IL-18–induced pulmonary collagen accumulation and matrix molecule gene expression. Wild-type (transgenic [Tg]−) and IL-18 Tg (Tg+) mice with the noted wild-type and null genetic loci were placed on doxycycline (Dox) water for 4 months. (a) Lung collagen content was evaluated using Sircol assays. The levels of mRNA encoding elastin (b), fibronectin (c), and collagen 1A1 (d) were evaluated with real-time reverse transcriptase polymerase chain reaction. The values in a–d represent the mean ± SEM of evaluations in a minimum of eight mice. * P < 0.05, ** P < 0.01, # P < 0.001. GAPDH = glyceraldehyde phosphate dehydrogenase.
Discussion
To define the chronic effector functions of IL-18 in the mature lung and elucidate mechanisms that could contribute to the pathogenesis of these responses we generated lung-specific inducible IL-18 Tg mice and characterized the inflammatory and remodeling responses that were induced by this transgene and the pathways that contribute to their generation. These studies demonstrate that IL-18 induces remarkably varied responses that mimic, in many ways, the pathologies in human COPD including chronic inflammation, emphysema, mucus metaplasia, airway fibrosis, vascular remodeling with intimal hyperplasia, and collagen deposition and RV hypertrophy. They also demonstrate that IL-18 simultaneously induces the signature cytokines associated with type 1, type 2, and type 17 responses and that each of these plays a specific role in the pathogenesis of the IL-18 effector repertoire. Specifically, IFN-γ–dependent pathways inhibited macrophage, lymphocyte, and eosinophil accumulation while stimulating alveolar destruction and moieties associated with cell cytotoxicity. In contrast, IL-13 and IL-17A were intimately associated with IL-18 optimally inducing IL-13 via an IL-17A–dependent mechanism and the IL-17/IL-13 axis stimulating mucus metaplasia and vascular and airway fibrosis. They also highlight important reciprocal interactions between these responses with the type 1 and type 17/type 2 cytokine responses feeding back to antagonize one another. It is important to point out that these studies do not define which in vivo IL-18–induced responses are caused by direct effects of IL-18 on specific cells or tissues. It is likely that complex gene–gene and cell–cell interactions are required to engender these complex responses. These are, however, the first studies to define the spectrum of parenchymal, airway, and vascular alterations that are induced by pulmonary IL-18 and the first to highlight the similarities between these responses and the lesions in COPD. They are also the first to demonstrate that IL-18 is able to simultaneously induce a mixed inflammatory response that contains exaggerated levels of IFN-γ, IL-13, and IL-17A; the first to define the selective roles that these cytokines play in the generation of IL-18–induced pathologies; and the first to define the reciprocal interactions between these pathways.
One of the most perplexing issues in COPD relates to the finding that two seemingly opposed biologic responses, the tissue rarification in emphysema and the tissue fibrosis in airways and blood vessels, coexist in close proximity to one another in the setting of ongoing inflammation (7, 44). Previous studies from our laboratory demonstrated that CS exposure induces and activates IL-18; that CS and viral pathogen-associated molecular patterns synergize to induce and activate IL-18; and that IL-18R signaling plays a critical role in the pathogenesis of CS and CS-viral pathogen-associated molecular pattern–induced inflammation, epithelial apoptosis, emphysema, and airway remodeling (27, 39). This prompted us to hypothesize that IL-18 might somehow explain this apparent paradox. In accord with findings in COPD (17–21), we noted that IL-18 induces a mixed mediator response characterized by signature type 1, type 2, and type 17 cytokine. Our finding that IL-18 induces Th1 and Th2 responses is in accord with findings in the literature (22, 24). These are, however, the first studies to demonstrate that IL-18 induces IL-13 via an IL-17A–dependent mechanism. They are also the first to provide a mechanism that can explain the emphysema-fibrosis paradox by demonstrating that the former is mediated by an IL-18–induced, IFN-γ–dependent pathway, whereas the latter is induced by an IL-17A/IL-13 response and that the IFN-γ and the IL-17A/IL-13 responses counterregulate each another. When the present and previous studies are viewed in combination they suggest that IL-18 might be a “central” mediator in COPD. In accord with this contention, studies from our laboratory and others have demonstrated that the levels of IL-18 in the circulation and sputum from patients with COPD are increased compared with controls (27, 28) where they correlate negatively with pulmonary function (28, 29). The studies also suggest that COPD is mediated by a complex inflammatory response and that optimal modeling of COPD needs to take this complexity into account.
Alterations in vascular structure are highly prevalent in COPD (45) and 6% of patients with COPD develop cor pulmonale annually (46). Studies of necropsy and surgical specimens have highlighted the importance of intimal hypertrophy with fibroelastic thickening, increased muscle mass, and elastin and collagen deposition in these lesions (46). Traditional concepts have suggested that these vascular alterations are the result of emphysema-associated blood vessel destruction or resulting hypoxia (46). However, published studies have failed to demonstrate direct correlations between emphysema and pulmonary artery pressures or right heart weight (46). In addition, in COPD, the extent of pulmonary vascular remodeling correlates with the severity of inflammatory cell vascular infiltration (47, 48). As a result, it has recently been proposed that the vascular alterations in COPD are the direct consequences of CS or the inflammatory mediators that are induced in this setting (46). Our studies support this contention and provide insight into mechanisms that might be operative by demonstrating that IL-18 is a potent stimulator of vascular remodeling that mediates these responses via its ability to induce IL-17A and subsequently IL-13 and inhibits this response via IFN-γ. They also demonstrate that these alterations are associated with right heart changes compatible with cor pulmonale. The demonstration that IL-13 is a mediator of pulmonary vascular remodeling is in accord with recent studies in pulmonary hypertension (41) and the well-documented ability of IL-13 to stimulate tissue fibrosis (49). They raise the possibility that similar mechanisms are involved in the vascular alterations in COPD and primary pulmonary hypertension.
Macrophage alterations are well documented in COPD and believed to contribute to disease pathogenesis (50, 51). In accord with this concept our studies demonstrate that IL-18 induces COPD-like tissue alterations while simultaneously increasing macrophage accumulation and epithelial apoptosis. Interestingly, these studies also demonstrated that these IL-18 effects were mediated via an IFN-γ–dependent pathway. These findings build on prior studies from our laboratory that demonstrated that Tg IFN-γ also induces emphysema and epithelial apoptosis (52) and that the IL-18R plays a critical role in CS-induced emphysema and epithelial apoptosis (27, 53). It is surprising, however, that IFN-γ–dependent pathways also inhibited IL-18–induced macrophage accumulation. On superficial analysis this dissociation between macrophage accumulation and emphysema might seem to be confusing. However, it is important to point out that IL-13 induces M2 (alternative) macrophage differentiation and that IFN-γ inhibits this and other IL-13–induced responses (54, 55). Thus, it is tempting to speculate that the IFN-γ–dependent decrease in macrophage accumulation in the IL-18 Tg mice is caused, at least in part, by the ability of these pathways to suppress M2 macrophage differentiation and accumulation. Additional investigation is required to test this speculation.
CS exposure causes epithelial cell stress and immune activation (43). The cytotoxic lymphocyte activation receptor NK cell group 2D (NKG2D) on circulating and tissue T cells links these responses by inducing cell lysis and enhancing innate and adaptive immunity after binding to stress-induced ligands, such as Raet-1 (43). Our studies demonstrate that IL-18 augments pulmonary expression of cells and molecules that are involved in cell cytotoxicity with IL-18 Tg mice manifesting increased levels of CD8+ and NK1.1+ cells and enhanced expression of Raet-1, perforin, and granzyme B. They also demonstrate that this response is induced via an IFN-γ–dependent mechanism and inhibited by IL-18–induced IL-17A and IL-13 responses. Recent studies have demonstrated that CS induces Raet-1 on lung epithelial cells, sustained NKG2D activation causes murine emphysema, and NKG2D ligand expression can be seen in COPD tissues where it correlates with morphologic and physiologic evidence of disease (43). This allows for the exciting hypothesis that IL-18–induced IFN-γ–dependent responses generate emphysema, at least in part, by augmenting cell cytotoxicity.
Enlarged alveoli in adult mice or humans can be caused by processes that alter alveolar development or processes that destroy normal septae (1, 31, 56–58). The emphysema in COPD is caused by the latter (1). Overexpression Tg modeling in the lung uses either the Clara cell 10-kD or surfactant apoprotein C promoters most frequently. Both promoters are activated in utero (31, 59, 60). Thus, to avoid confounding developmental effects, we used an externally regulatable Tg system developed in our laboratory that allows transgene expression to be induced after lung development has occurred. As a result, the findings in this submission are relevant to the adult lung. Hoshino and coworkers (61) also used Tg approaches to investigate the effects of IL-18 in the lung. In contrast to our studies, however, they used the SP-C promoter and, in most of their experiments, a constitutive Tg approach. When their transgene was induced for a short period of time in the adult lung they noted tissue inflammation that was similar to what we observed. However, they did not detect alveolar, airway, or vascular remodeling responses and did not comment on downstream moieties, such as IL-13 or IFN-γ. The basis for the difference in their findings in adult lungs and ours are not clear but likely relate to technical items, such as the promoter that was used or duration of transgene activation. Interestingly, when their transgene was activated during development (a situation that is relevant to such diseases as bronchopulmonary dysplasia [62]), they noted type 1 and 2 cytokines, alveolar enlargement, pulmonary hypertension, and cor pulmonale that was similar to the inflammatory and vascular responses in our animals. Importantly, IL-18 did not induce identical responses in early life and adult lungs because, in contrast to our findings, airway remodeling was not induced by developmentally activated IL-18, and null mutations of IFN-γ did not alter the developmentally induced increase in alveolar size, whereas null mutations of IL-13 did do so (61). In combination, the studies by Hoshino and coworkers (61) and our findings highlight the similarities and differences in the effects of IL-18 in the developing and adult lung.
Although COPD is referred to as a “disease,” it can be more accurately thought of as a constellation of heterogeneous syndromes (63–66). As a result, the clinical manifestations of the disease and the degree to which emphysema versus airway (chronic bronchitis) and vascular pathologies predominate can differ from individual to individual. This complexity is so profound that the National Institutes of Health is sponsoring ongoing studies of subpopulations and intermediate outcome measures in the disorder (65). To account for this heterogeneity, some have proposed that these differences are the result of disparate disease causes (67). Only time and study will determine if this is true. However, our data allow for another nonmutually exclusive hypothesis. Specifically, our studies demonstrate that a single abnormality, the exaggerated expression of IL-18, can induce many of the classic abnormalities that are seen in COPD and that these lesions are mediated by different mutually inhibiting mechanisms. Environmental or genetic influences that alter an individual's propensity to mount IL-18–stimulated type 1, type 2, or type 17 responses could regulate the balance of emphysema and airway and vascular remodeling thereby altering disease expression. These findings also have important implications regarding therapeutic approaches that are used in this disorder. If the inhibitory interactions of the type 1 and type 17/2 pathways described in this modeling system are operative in humans, one can see how an anti–IFN-γ–based monotherapy could decrease emphysema while increasing inflammation and airway and vascular remodeling. Similarly, one can see how an anti–IL-13 monotherapy (as has been proposed [68]) could decrease mucus metaplasia and airway and vascular fibrosis while increasing emphysema and cell cytotoxic responses. This suggests that optimal treatment of COPD needs to be directed at “central” mediators, such as IL-18, or use combination therapy to simultaneously control the multiple pathways that are operative in a given individual. Further investigation of the regulation and roles of IL-18 and type 1, 2, and 17 responses and the therapeutic use of interventions that control these pathways is warranted.
Supplementary Material
Acknowledgments
The authors thank Kathleen Bertier for excellent administrative assistance.
Footnotes
Supported by NIH Grants HL-079328 (J.A.E.) and HL-084225 (C.G.L.), and Flight Attendance Medical Research Institute Grant 82571 (J.A.E.). J.-M.C. received support from the Basic Science Research Program through the National Research Foundation of Korea (Grant 2010-0025389 and 2011-0012859).
Author Contributions: conceived the idea, M.-J.K., J.-M.C., and J.A.E.; performed experiments, M.-J.K., J.-M.C., B.H.K., C.-M.L., W.-K.C., G.C., and D.-H.K.; provided important reagent and tools, J.-M.C. and C.G.L.; analyzed data, M.-J.K., J.-M.C., C.G.L., and J.A.E.; and drafted the manuscript, M.-J.K. and J.A.E. All the authors reviewed the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201108-1545OC on March 1, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Senior RM, Shapiro SD. Chronic obstructive pulmonary disease: epidemiology, pathophysiology, and pathogenesis. : Fishman AP, Elias JA, Fishman JA, Grippi MA, Kaiser LR, Senior RM, Fishman's pulmonary diseases and disorders. New York: McGraw-Hill; 1998; pp. 659–681 [Google Scholar]
- 2.Wang Z, Zheng T, Zhu Z, Homer RJ, Riese RJ, Chapman HA, Jr, Shapiro SD, Elias JA. Interferon gamma induction of pulmonary emphysema in the adult murine lung. J Exp Med 2000;192:1587–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–555 [DOI] [PubMed] [Google Scholar]
- 4.Calverley PM. COPD: what is the unmet need? Br J Pharmacol 2008;155:487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsson K. Aspects on pathophysiological mechanisms in COPD. J Intern Med 2007;262:311–340 [DOI] [PubMed] [Google Scholar]
- 6.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004;364:709–721 [DOI] [PubMed] [Google Scholar]
- 7.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol 2009;4:435–459 [DOI] [PubMed] [Google Scholar]
- 8.Barbera JA, Blanco I. Pulmonary hypertension in patients with chronic obstructive pulmonary disease: advances in pathophysiology and management. Drugs 2009;69:1153–1171 [DOI] [PubMed] [Google Scholar]
- 9.Magee F, Wright JL, Wiggs BR, Pare PD, Hogg JC. Pulmonary vascular structure and function in chronic obstructive pulmonary disease. Thorax 1988;43:183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Shaughnessy TC, Ansari TW, Barnes NC, Jeffery PK. Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med 1997;155:852–857 [DOI] [PubMed] [Google Scholar]
- 11.Cosio MG, Guerassimov A. Chronic obstructive pulmonary disease. Inflammation of small airways and lung parenchyma. Am J Respir Crit Care Med 1999;160:S21–S25 [DOI] [PubMed] [Google Scholar]
- 12.Saetta M, Baraldo S, Corbino L, Turato G, Braccioni F, Rea F, Cavallesco G, Tropeano G, Mapp CE, Maestrelli P, et al. CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:711–717 [DOI] [PubMed] [Google Scholar]
- 13.Saetta M, Turato G, Maestrelli P, Mapp CE, Fabbri LM. Cellular and structural bases of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:1304–1309 [DOI] [PubMed] [Google Scholar]
- 14.Sutherland ER, Cherniack RM. Management of chronic obstructive pulmonary disease. N Engl J Med 2004;350:2689–2697 [DOI] [PubMed] [Google Scholar]
- 15.Yao H, Rahman I. Current concepts on the role of inflammation in COPD and lung cancer. Curr Opin Pharmacol 2009;9:375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001;163:1256–1276 [DOI] [PubMed] [Google Scholar]
- 17.Saetta M, Mariani M, Panina-Bordignon P, Turato G, Buonsanti C, Baraldo S, Bellettato CM, Papi A, Corbetta L, Zuin R, et al. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;165:1404–1409 [DOI] [PubMed] [Google Scholar]
- 18.Grumelli S, Corry DB, Song LZ, Song L, Green L, Huh J, Hacken J, Espada R, Bag R, Lewis DE, et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med 2004;1:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barczyk A, Pierzchala W, Kon OM, Cosio B, Adcock IM, Barnes PJ. Cytokine production by bronchoalveolar lavage T lymphocytes in chronic obstructive pulmonary disease. J Allergy Clin Immunol 2006;117:1484–1492 [DOI] [PubMed] [Google Scholar]
- 20.Zhu X, Gadgil AS, Givelber R, George MP, Stoner MW, Sciurba FC, Duncan SR. Peripheral T cell functions correlate with the severity of chronic obstructive pulmonary disease. J Immunol 2009;182:3270–3277 [DOI] [PubMed] [Google Scholar]
- 21.Di Stefano A, Caramori G, Gnemmi I, Contoli M, Vicari C, Capelli A, Magno F, D'Anna SE, Zanini A, Brun P, et al. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol 2009;157:316–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol 2001;19:423–474 [DOI] [PubMed] [Google Scholar]
- 23.Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol 2003;73:213–224 [DOI] [PubMed] [Google Scholar]
- 24.Dinarello CA. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin Nephrol 2007;27:98–114 [DOI] [PubMed] [Google Scholar]
- 25.Sakai A, Sugawara Y, Kuroishi T, Sasano T, Sugawara S. Identification of IL-18 and Th17 cells in salivary glands of patients with Sjogren's syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J Immunol 2008;181:2898–2906 [DOI] [PubMed] [Google Scholar]
- 26.Boraschi D, Dinarello CA. IL-18 in autoimmunity [review]. Eur Cytokine Netw 2006;17:224–252 [PubMed] [Google Scholar]
- 27.Kang MJ, Homer RJ, Gallo A, Lee CG, Crothers KA, Cho SJ, Rochester C, Cain H, Chupp G, Yoon HJ, et al. IL-18 is induced and IL-18 receptor alpha plays a critical role in the pathogenesis of cigarette smoke-induced pulmonary emphysema and inflammation. J Immunol 2007;178:1948–1959 [DOI] [PubMed] [Google Scholar]
- 28.Imaoka H, Hoshino T, Takei S, Kinoshita T, Okamoto M, Kawayama T, Kato S, Iwasaki H, Watanabe K, Aizawa H. Interleukin-18 production and pulmonary function in COPD. Eur Respir J 2008;31:287–297 [DOI] [PubMed] [Google Scholar]
- 29.Rovina N, Dima E, Gerassimou C, Kollintza A, Gratziou C, Roussos C. Interleukin-18 in induced sputum: association with lung function in chronic obstructive pulmonary disease. Respir Med 2009;103:1056–1062 [DOI] [PubMed] [Google Scholar]
- 30.Kang MJ, Choi JM, Lee CG, Elias JA. Interleukin-18 induces pulmonary inflammation and remodeling via an interleukin-17-dependent pathway. Presented at the American Thoracic Society International Conference. May 14–18, 2010, New Orleans, LA. Abstract 3994. [Google Scholar]
- 31.Ray P, Tang W, Wang P, Homer R, Kuhn C, III, Flavell RA, Elias JA. Regulated overexpression of interleukin 11 in the lung: use to dissociate development-dependent and -independent phenotypes. J Clin Invest 1997;100:2501–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med 2004;10:1095–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, Sohn MH, Cohn L, Homer RJ, Kozhich AA, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med 2009;206:1149–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Londhe VA, Maisonet TM, Lopez B, Jeng JM, Li C, Minoo P. A subset of epithelial cells with CCSP promoter activity participates in alveolar development. Am J Respir Cell Mol Biol 2011;44:804–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hackett BP, Shimizu N, Gitlin JD. Clara cell secretory protein gene expression in bronchiolar epithelium. Am J Physiol 1992;262:L399–L404 [DOI] [PubMed] [Google Scholar]
- 36.Cunningham RE. Tissue disaggregation. Methods Mol Biol 1994;34:225–228 [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Dong Z, Zhou R, Luo D, Wei H, Tian Z. Isolation of lymphocytes and their innate immune characterizations from liver, intestine, lung and uterus. Cell Mol Immunol 2005;2:271–280 [PubMed] [Google Scholar]
- 38.Jaatinen T, Laine J. Isolation of mononuclear cells from human cord blood by Ficoll-Paque density gradient. Curr Protoc Stem Cell Biol 2007;Chapter 2:Unit 2A.1 [DOI] [PubMed] [Google Scholar]
- 39.Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, Enelow R, Elias JA. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest 2008;118:2771–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee CG, Homer RJ, Cohn L, Link H, Jung S, Craft JE, Graham BS, Johnson TR, Elias JA. Transgenic overexpression of interleukin (IL)-10 in the lung causes mucus metaplasia, tissue inflammation, and airway remodeling via IL-13-dependent and -independent pathways. J Biol Chem 2002;277:35466–35474 [DOI] [PubMed] [Google Scholar]
- 41.Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, Hogaboam C, Taraseviciene-Stewart L, Voelkel NF, Rabinovitch M, et al. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med 2008;205:361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azoulay E, Eddahibi S, Marcos E, Levame M, Harf A, Schlemmer B, Adnot S, Delclaux C. Granulocyte colony-stimulating factor enhances alpha-naphthylthiourea-induced pulmonary hypertension. J Appl Physiol 2003;94:2027–2033 [DOI] [PubMed] [Google Scholar]
- 43.Borchers MT, Wesselkamper SC, Curull V, Ramirez-Sarmiento A, Sanchez-Font A, Garcia-Aymerich J, Coronell C, Lloreta J, Agusti AG, Gea J, et al. Sustained CTL activation by murine pulmonary epithelial cells promotes the development of COPD-like disease. J Clin Invest 2009;119:636–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653 [DOI] [PubMed] [Google Scholar]
- 45.Peinado VI, Pizarro S, Barbera JA. Pulmonary vascular involvement in COPD. Chest 2008;134:808–814 [DOI] [PubMed] [Google Scholar]
- 46.Wright JL, Levy RD, Churg A. Pulmonary hypertension in chronic obstructive pulmonary disease: current theories of pathogenesis and their implications for treatment. Thorax 2005;60:605–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barbera JA, Riverola A, Roca J, Ramirez J, Wagner PD, Ros D, Wiggs BR, Rodriguez-Roisin R. Pulmonary vascular abnormalities and ventilation-perfusion relationships in mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1994;149:423–429 [DOI] [PubMed] [Google Scholar]
- 48.Hale KA, Ewing SL, Gosnell BA, Niewoehner DE. Lung disease in long-term cigarette smokers with and without chronic air-flow obstruction. Am Rev Respir Dis 1984;130:716–721 [DOI] [PubMed] [Google Scholar]
- 49.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med 2001;194:809–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet 2011;378:1015–1026 [DOI] [PubMed] [Google Scholar]
- 51.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med 2009;360:2445–2454 [DOI] [PubMed] [Google Scholar]
- 52.Zheng T, Kang MJ, Crothers K, Zhu Z, Liu W, Lee CG, Rabach LA, Chapman HA, Homer RJ, Aldous D, et al. Role of cathepsin S-dependent epithelial cell apoptosis in IFN-gamma-induced alveolar remodeling and pulmonary emphysema. J Immunol 2005;174:8106–8115 [DOI] [PubMed] [Google Scholar]
- 53.Kang MJ, Lee CG, Cho SJ, Homer RJ, Elias JA. IFN-gamma-dependent DNA injury and/or apoptosis are critical in cigarette smoke-induced murine emphysema. Proc Am Thorac Soc 2006;3:517–518 [DOI] [PubMed] [Google Scholar]
- 54.Villalta SA, Deng B, Rinaldi C, Wehling-Henricks M, Tidball JG. IFN-gamma promotes muscle damage in the mdx mouse model of Duchenne muscular dystrophy by suppressing M2 macrophage activation and inhibiting muscle cell proliferation. J Immunol 2011;187:5419–5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 2011;11:750–761 [DOI] [PubMed] [Google Scholar]
- 56.Snider GL, Lucey EC, Stone PJ. Pitfalls in antiprotease therapy of emphysema. Am J Respir Crit Care Med 1994;150:S131–S137 [DOI] [PubMed] [Google Scholar]
- 57.Amy RW, Bowes D, Burri PH, Haines J, Thurlbeck WM. Postnatal growth of the mouse lung. J Anat 1977;124:131–151 [PMC free article] [PubMed] [Google Scholar]
- 58.Burri PH. Structural aspects of postnatal lung development: alveolar formation and growth. Biol Neonate 2006;89:313–322 [DOI] [PubMed] [Google Scholar]
- 59.Tang W, Geba GP, Zheng T, Ray P, Homer RJ, Kuhn C, III, Flavell RA, Elias JA. Targeted expression of IL-11 in the murine airway causes lymphocytic inflammation, bronchial remodeling, and airways obstruction. J Clin Invest 1996;98:2845–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou L, Dey CR, Wert SE, Whitsett JA. Arrested lung morphogenesis in transgenic mice bearing an SP-C-TGF-beta 1 chimeric gene. Dev Biol 1996;175:227–238 [DOI] [PubMed] [Google Scholar]
- 61.Hoshino T, Kato S, Oka N, Imaoka H, Kinoshita T, Takei S, Kitasato Y, Kawayama T, Imaizumi T, Yamada K, et al. Pulmonary inflammation and emphysema: role of the cytokines IL-18 and IL-13. Am J Respir Crit Care Med 2007;176:49–62 [DOI] [PubMed] [Google Scholar]
- 62.Vicencio AG, Lee CG, Cho SJ, Eickelberg O, Chuu Y, Haddad GG, Elias JA. Conditional overexpression of bioactive transforming growth factor-beta1 in neonatal mouse lung: a new model for bronchopulmonary dysplasia? Am J Respir Cell Mol Biol 2004;31:650–656 [DOI] [PubMed] [Google Scholar]
- 63.Celli BR. Roger S. Mitchell lecture. Chronic obstructive pulmonary disease phenotypes and their clinical relevance. Proc Am Thorac Soc 2006;3:461–465 [DOI] [PubMed] [Google Scholar]
- 64.Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, Fabbri LM, Goldin JG, Jones PW, Macnee W, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 2010;182:598–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.SubPopulations and InteRmediate Outcome Measures In COPD Study (SPIROMICS) [Internet; accessed 2011 Jul 23]. Available from: http://www.fnih.org/work/programs-development/spiromics.
- 66.Lee JH, Lee YK, Kim EK, Kim TH, Huh JW, Kim WJ, Lee SM, Lee S, Lim SY, Shin TR, et al. Responses to inhaled long-acting beta-agonist and corticosteroid according to COPD subtype. Respir Med 2010;104:542–549 [DOI] [PubMed] [Google Scholar]
- 67.Rennard SI. COPD heterogeneity: what this will mean in practice. Respir Care 2011;56:1181–1187 [DOI] [PubMed] [Google Scholar]
- 68.Walsh GM. Tralokinumab, an anti-IL-13 mAb for the potential treatment of asthma and COPD. Curr Opin Investig Drugs 2010;11:1305–1312 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.