Chronic obstructive pulmonary disease (COPD) is characterized by an abnormal lung inflammatory response to inhaled noxious particles or gas including those present in cigarette smoke. Patients with COPD can have destructive processes (airspace enlargement) in their lungs alongside seemingly disparate chronic remodeling processes in their airways and pulmonary vasculature (1, 2). However, it has not been clear whether similar or distinct signaling pathways drive the development of the destructive versus remodeling lung pathologies in COPD lungs. A common feature of the pulmonary lesions associated with COPD is inflammation, and exaggerated T helper type 1 (Th1), Th2 cytokine, and Th17 responses have been linked to COPD pathogenesis (3–7). However, it has not been clear to what extent Th1, Th2, and Th17 cytokine responses contribute to individual pathologies in COPD lungs, or whether they interact synergistically or otherwise to promote the development of COPD. The article by Kang and colleagues in this issue of the Journal (pp. 1205–1217) addresses these knowledge gaps by showing for the first time that interleukin-18 (IL-18) is an upstream, master regulator that promotes a complex pattern of Th1 and Th17/Th2 cytokine responses in the lungs of mice (8). The IL-18–driven increases in Th1 cytokine responses are responsible for the airspace enlargement developing in the IL-18 transgenic mice, whereas the IL-18–driven increases in Th17/Th2 cytokine responses cause chronic remodeling in the airways and pulmonary vessels in the IL-18 transgenic mice. The authors also identify novel reciprocal regulation of the Th1 versus Th17/Th2 cytokine responses in the lungs and the development of the distinct lung pathologies associated with each cytokine response.
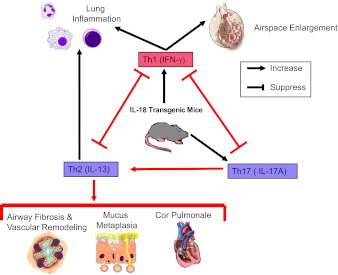
IL-18 is produced by myeloid leukocytes and lung epithelial cells (9) and binds to its receptor (IL-18R), which is expressed at low levels on naive T cells. In the presence of IL-12, IL-18 acts as a Th1 cytokine because IL-12 increases IL-18R expression by Th1 cells, which enables IL-18 to promote Th1-cell polarization and proliferation, secretion of interferon-γ (IFN-γ), and macrophage and polymorphonuclear neutrophil accumulation in tissues (9). However, in the absence of IL-12, IL-18 induces the release of Th2 and Th17 cytokines from activated Th1 cells and other leukocytes (9). Prior studies have linked IL-18 to COPD pathogenesis because IL-18 levels are increased in blood and lung samples from patients with COPD and correlate negatively with lung function (10–12), and IL-18R-α−/− mice are protected from cigarette smoke–induced airspace enlargement (10). However, until now, it has not been clear whether IL-18 contributes to other COPD lung pathologies. To address this issue, Kang and coworkers generated and evaluated dual construct transgenic mice that overexpress IL-18 in a doxycycline-inducible manner in the adult murine lung using the CC10 promoter. After 4 months of IL-18 overexpression in the lung, the mice developed robust lung inflammation and airspace enlargement along with impressive small fibrosis, airway mucus metaplasia, and vascular remodeling associated with pulmonary hypertension and right ventricular hypertrophy. The authors genetically deleted IFN-γ, IL-13, and IL-17A in IL-18 transgenic mice and thereby identified activities for IFN-γ in promoting cytotoxic lymphocyte responses in the lung, alveolar septal cell apoptosis, and airspace enlargement (Figure 1). Additionally, they found that: (1) IL-17A is required for IL-18-induced increases in lung IL-13 levels; and (2) IL-17A and IL-13 together promote lung inflammation, airway mucus metaplasia, and airway and vascular remodeling in IL-18 transgenic mice (Figure 1). Kang and colleagues also found that IFN-γ reduces lung levels of IL-17A and IL-13 to thereby abrogate IL-17A/IL-13–driven lung remodeling processes, and IL-17A and IL-13 both lower lung levels of IFN-γ to reduce airspace enlargement in IL-18 transgenic mice (Figure 1).
Figure 1.
IL-18 drives both the destructive and remodeling processes in the lungs of IL-18 transgenic mice. Novel pathways identified by Kang and coworkers in IL-18 transgenic mice are highlighted with red arrows and lines. Inducible overexpression of IL-18 in the adult murine airway epithelium induces Th1 cytokine expression, leading to increased cytotoxic lymphocytes responses, alveolar septal cell apoptosis, and airspace enlargement. In addition, IL-18 drives Th2 cytokines responses in a Th17-dependent manner, leading to mucus metaplasia, small airway fibrosis, and vascular remodeling with pulmonary hypertension and right ventricular hypertrophy. There is also reciprocal regulation of Th1 and Th17/Th2 cytokine responses and downstream lung pathologies in the lungs of IL-18 transgenic mice.
The article by Kang and colleagues is noteworthy for several reasons. First, the investigators identify a novel master cytokine regulator that can drive all of the key pathologies found in COPD lungs. Second, the authors demonstrate for the first time that Th1 and Th17/Th2 cytokine responses counterregulate both each other and the lung pathologies associated with each response in IL-18 transgenic mice. Third, IL-18 transgenic mice represent a new murine model of COPD characterized by robust airspace enlargement as well as impressive COPD-like remodeling processes in their airways and vasculature, unlike C57BL/6 wild-type mice exposed to smoke, which is the mostly commonly used murine model of COPD (13). Other strengths of the article are the use of the inducible transgenic system to avoid potential effects of IL-18 overexpression on lung development and the targeting overexpression of IL-18 to lung epithelial cells, which endogenously produce IL-18 in the COPD lung (11).
Future studies could address whether the increased lung levels of IL-18 in IL-18 transgenic mice are of similar magnitude to those occurring in the lungs of patients with COPD to assess whether the results of this study are relevant to human COPD. It would be of interest to determine whether IL-18 overexpression in macrophages would produce similar results given that macrophages are an important source of IL-18 in COPD lungs (11). Although this study highlights the activities of CD4+ T cells in COPD-like lung pathologies in IL-18 transgenic mice, the mice were housed under specific pathogen-free conditions and thus not stimulated by pathogen-derived antigens. It would be interesting to determine whether the lung pathologies in IL-18 transgenic mice would be exacerbated by infection of their respiratory tracts with bacteria or viruses that have been linked to exacerbations in human patients with COPD. For reasons that are not clear, human patients with COPD vary considerably in the extent to which they develop different lung lesions. Future studies could address whether the latter is related to genetically or environmentally determined differences in levels of IL-18 expression or signaling in different cells in different compartments of the lung.
The results of the study of Kang and coworkers could have therapeutic implications for COPD. First, they provide evidence that monotherapy targeting either a Th1 or a Th2 cytokine might have both deleterious and beneficial effects. Monotherapy targeting IFN-γ might limit emphysema progression but worsen airway and vascular remodeling, whereas monotherapy directed against IL-13 might limit the progression of chronic remodeling processes in the lungs but accelerate the progression of emphysema. Second, the studies identify IL-18 as a potential upstream target for future COPD therapeutics to limit both the destructive and remodeling processes occurring in COPD lungs. In this respect, it is noteworthy that neutralizing antibodies to IL-18 have efficacy in preclinical models of inflammation and tissue injury in other organ systems (14, 15). However, IL-18 has crucial host defense and antitumor activities (16), and gene therapy to increase IL-18 levels in tissues protects experimental animals from infection and tumor growth and metastasis (17, 18). Given that patients with COPD can have infective disease exacerbations and are at increased risk from developing lung cancer (19), it would be important to determine the safety as well as the efficacy of novel therapeutics targeting IL-18 in the lungs of patients with COPD.
Supplementary Material
Footnotes
Supported by PHS grants #HL084816, HL835111, and HL105339, and The Brigham and Women’s Hospital-Lovelace Respiratory Research Institute Consortium.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653 [DOI] [PubMed] [Google Scholar]
- 2.Voelkel NF, Cool CD. Pulmonary vascular involvement in chronic obstructive pulmonary disease. Eur Respir J Suppl 2003;46:28s–32s [DOI] [PubMed] [Google Scholar]
- 3.Barczyk A, Pierzchala W, Kon OM, Cosio B, Adcock IM, Barnes PJ. Cytokine production by bronchoalveolar lavage T lymphocytes in chronic obstructive pulmonary disease. J Allergy Clin Immunol 2006;117:1484–1492 [DOI] [PubMed] [Google Scholar]
- 4.Zhu X, Gadgil AS, Givelber R, George MP, Stoner MW, Sciurba FC, Duncan SR. Peripheral T cell functions correlate with the severity of chronic obstructive pulmonary disease. J Immunol 2009;182:3270–3277 [DOI] [PubMed] [Google Scholar]
- 5.Di SA, Caramori G, Gnemmi I, Contoli M, Vicari C, Capelli A, Magno F, D'Anna SE, Zanini A, Brun P, et al. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol 2009;157:316–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eustace A, Smyth LJ, Mitchell L, Williamson K, Plumb J, Singh D. Identification of cells expressing IL-17A and IL-17F in the lungs of patients with COPD. Chest 2011;139:1089–1100 [DOI] [PubMed] [Google Scholar]
- 7.Chen K, Pociask DA, McAleer JP, Chan YR, Alcorn JF, Kreindler JL, Keyser MR, Shapiro SD, Houghton AM, Kolls JK, et al. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS ONE 2011;6:e20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang M-J, Choi J-M, Kim BH, Lee C-M, Cho W-K, Choe G, Kim D-H, Lee CG, Elias JA. IL-18 induces emphysema and airway and vascular remodeling via IFN-γ, IL-17A, and IL-13. Am J Respir Crit Care Med 2012;185:1205–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith DE. The biological paths of IL-1 family members IL-18 and IL-33. J Leukoc Biol 2011;89:383–392 [DOI] [PubMed] [Google Scholar]
- 10.Kang MJ, Homer RJ, Gallo A, Lee CG, Crothers KA, Cho SJ, Rochester C, Cain H, Chupp G, Yoon HJ, et al. IL-18 is induced and IL-18 receptor alpha plays a critical role in the pathogenesis of cigarette smoke-induced pulmonary emphysema and inflammation. J Immunol 2007;178:1948–1959 [DOI] [PubMed] [Google Scholar]
- 11.Imaoka H, Hoshino T, Takei S, Kinoshita T, Okamoto M, Kawayama T, Kato S, Iwasaki H, Watanabe K, Aizawa H. Interleukin-18 production and pulmonary function in COPD. Eur Respir J 2008;31:287–297 [DOI] [PubMed] [Google Scholar]
- 12.Rovina N, Dima E, Gerassimou C, Kollintza A, Gratziou C, Roussos C. Interleukin-18 in induced sputum: association with lung function in chronic obstructive pulmonary disease. Respir Med 2009;103:1056–1062 [DOI] [PubMed] [Google Scholar]
- 13.Churg A, Cosio M, Wright JL. Mechanisms of cigarette smoke-induced COPD: insights from animal models. Am J Physiol Lung Cell Mol Physiol 2008;294:L612–L631 [DOI] [PubMed] [Google Scholar]
- 14.Yu S, Chen Z, Mix E, Zhu SW, Winblad B, Ljunggren HG, Zhu J. Neutralizing antibodies to IL-18 ameliorate experimental autoimmune neuritis by counter-regulation of autoreactive Th1 responses to peripheral myelin antigen. J Neuropathol Exp Neurol 2002;61:614–622 [DOI] [PubMed] [Google Scholar]
- 15.Plater-Zyberk C, Joosten LA, Helsen MM, Sattonnet-Roche P, Siegfried C, Alouani S, van de Loo FA, Graber P, Aloni S, Cirillo R, et al. Therapeutic effect of neutralizing endogenous IL-18 activity in the collagen-induced model of arthritis. J Clin Invest 2001;108:1825–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauw FN, Branger J, Florquin S, Speelman P, van Deventer SJ, Akira S, van der Poll T. IL-18 improves the early antimicrobial host response to pneumococcal pneumonia. J Immunol 2002;168:372–378 [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Ishii K, Hisaeda H, Hamano S, Zhang M, Nakanishi K, Yoshimoto T, Hemmi H, Takeda K, Akira S, et al. IL-18 gene therapy develops Th1-type immune responses in Leishmania major-infected BALB/c mice: is the effect mediated by the CpG signaling TLR9? Gene Ther 2004;11:941–948 [DOI] [PubMed] [Google Scholar]
- 18.Oshikawa K, Shi F, Rakhmilevich AL, Sondel PM, Mahvi DM, Yang NS. Synergistic inhibition of tumor growth in a murine mammary adenocarcinoma model by combinational gene therapy using IL-12, pro-IL-18, and IL-1beta converting enzyme cDNA. Proc Natl Acad Sci USA 1999;96:13351–13356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koshiol J, Rotunno M, Consonni D, Pesatori AC, De MS, Goldstein AM, Chaturvedi AK, Wacholder S, Landi MT, Lubin JH, et al. Chronic obstructive pulmonary disease and altered risk of lung cancer in a population-based case-control study. PLoS ONE 2009;4:e7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.