Abstract
The aim of this study is to present a new method for determining the root-derived extracellular acid phosphomonoesterase (EAPM) activity fraction within the total EAPM activity of soil. EAPM activity was determined for roots, organic and mineral soil. Samples were collected using paired PVC cylinders, inserted to a depth of 15 cm, within seven selected forest stands. Root-derived EAPM formed between 4 and18% of the total EAPM activity of soil from forests of differing maturity. A new approach, presented in this work, enables separation of root-derived EAPM activity from total soil EAPM. Separation of root-derived EAPM from soil provides a better understanding of its role in P-cycling in terrestrial ecosystems. The method presented in this work is a first step towards the separation of root- and microbe-derived EAPM in soils, which are thought to possess different kinetic properties and different sensitivity to environmental change.
1. Introduction
Extracellular acid phosphomonoesterase (EAPM) (orthophosphoric monoester phosphohydrolase, E.C. 3.1.3.2) plays an important role in the mineralization of soil organic phosphorus in a range of terrestrial ecosystems [1, 2]. This enzyme may be produced in soil by microorganisms including bacteria, protozoa, and mycorrhizal or saprophytic fungi and by plant roots [3–5]. Root-derived EAPM is bound onto root surfaces or released to external media as a part of the root exudates [6].
Different ecosystems are thought to have either plant- or microbe-derived EAPM prevailing in soil [7, 8]. Nevertheless, there were no available data indicating the significance of plant roots versus soil microorganisms in the production of EAPM and thus their relative importance for P-cycling. EAPM from roots is known to possess different kinetic properties and sensitivity to other factors of environment compared to that derived from microorganisms [9]. Consequently, these two fractions of total soil EAPM may respond differently to climate change and other environmental perturbations [6].
Separation of plant root- and microbe-derived EAPM in soil is difficult. Hence, we have developed a new approach, focusing on the activity of root-derived EAPM as a part of the total EAPM activity of soil in different forest ecosystems.
2. Material and Methods
2.1. Site and Soil Sampling
In total, seven forest stands were selected for this study. These included young (19 years) and old (207 years) beech (Fagus sylvatica L.) stands (480 m asl, N 49°16′54′′, E 16°37′52′′), young (33 years, oak 60%, hornbeam 30%, beech 10%) and old (133 years, oak 87%, Douglas fir 6%, beech 4%, and larch 3%) oak (Quercus robur L.) stands (460 m asl, N 49°32′16′′, E 16°79′75′′), and young (15 years, spruce 100%), middle-aged (51 years, spruce 68%, larch 32%), and old (94 years, spruce 92%, larch 6%, beech 2%) spruce (Picea abies L.) stands (500 m asl, N 49°32′19′′, E 16°78′54′′). Soils within the studied stands were Dystric Luvisol (young beech and old oak), Haplic Cambisol (old beech and young oak), Dystric Cambisol (old spruce), Gleyic Cambisol (young spruce), and Leptic Cambisol (middle aged spruce) [10].
Five pairs of PVC cylinders (15 cm long, 5.9 cm dia) were randomly inserted in every stand; cylinders within the same pair were always inserted side by side, to ensure similarity. After transportation to the laboratory in plastic bags, the litter layer in all cylinders was removed. One cylinder in each pair was used for separation of all the roots, which were washed in tap water and then in demineralized water. The soil from the second cylinder was separated into organic (F + H horizons) and mineral part to ensure consequent determination of EAPM activity of naturally developed soil layers without their artificial mixing together both parts were separately sieved through a 5 mm mesh, homogenised, and weighed.
2.2. Root Analysis
All roots from each of the cylinders were incubated, in succinate-borate buffer (pH 4.8) at 37°C for 30 min., with p-nitrophenyl phosphate (p-NPP) as a substrate [11]. The substrate dissolved in succinate-borate buffer was applied in ratio 12 mL per 0.5 g of fresh roots.
2.3. Soil Analysis
EAPM was measured separately in organic and mineral soil. Fresh soil (1 g) was incubated, in 12 mL of succinate-borate buffer (pH 4.8) at 37°C for 1 h, with p-NPP as a substrate [11]. EAPM activity was consequently calculated per the total amount of organic and mineral soil of every cylinder (data were pooled together), and, further, EAPM of roots of the same cylinders was added to obtain the total EAPM of the whole cylinder. Results were consequently calculated per 100 cm2 of soil surface.
2.4. Statistical Treatment
Values are given as means of five replicates with standard errors (SE). Significant differences were calculated using one-way ANOVA plus Fisher's LSD test.
3. Results and Discussion
The total soil activity of EAPM, including roots, was significantly (P < 0.05) higher in young oak, old oak, and young spruce than that in other forest stands (Figure 1). Significantly (P < 0.05), the lowest total EAPM activity was found in the soil from the old beech forest stand. From the total EAPM activity of soil, up to 18% was derived from roots (Figure 2). The proportion of root-derived EAPM was higher for all spruce stands (at average >12%) than for beech or oak stands, due to higher EAPM related to unit fresh root mass (Table 1).
Figure 1.
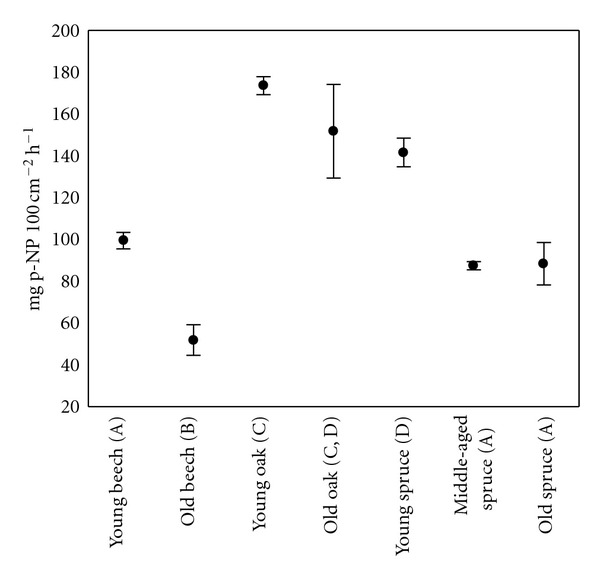
Total soil activity of EAPM, including roots (up to 15 cm depth), from seven forest stands (Mean ± SE). Different letters (in brackets) mark significant differences (P < 0.05).
Figure 2.
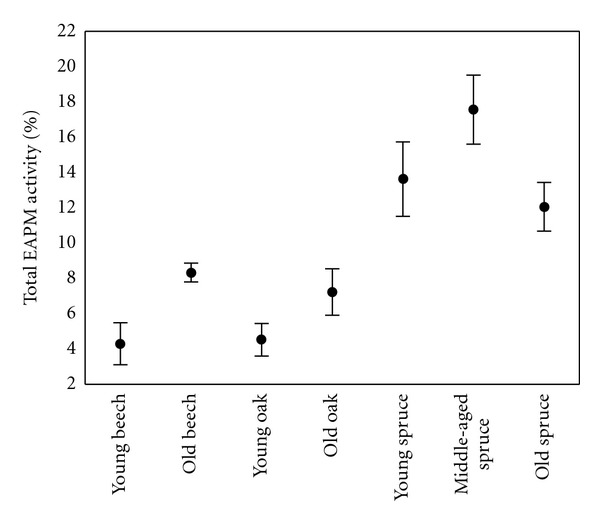
Proportion of root-derived EAPM within total soil EAPM up to 15 cm depth (Mean ± SE).
Table 1.
Total Root Mass and EAPM Activity of Roots in Seven Forest Stands (Mean ± SE). Different Letters Mark Significant Differences (P < 0.05).
| Forest | g fresh roots | mg p-NP g−1 fresh roots h−1 |
|---|---|---|
| Young beech | 3.98 ± 0.85a | 0.27 ± 0.03a |
| Old beech | 6.79 ± 1.10ab | 0.17 ± 0.03a |
| Young oak | 7.49 ± 1.27bc | 0.26 ± 0.02a |
| Old oak | 9.77 ± 1.00cd | 0.27 ± 0.02a |
| Young spruce | 4.18 ± 0.52ae | 1.18 ± 0.10b |
| Middle-aged spruce | 6.95 ± 0.86bde | 0.58 ± 0.08c |
| Old spruce | 4.02 ± 1.11a | 0.81 ± 0.13d |
Historically, different approaches have been tested to separate acid phosphomonoesterase activity in soils. These have included separation of the intra- and extracellular APM pool [12–15], assessment of APM in rhizosphere versus bulk soil [3, 16, 17] or within particle-size fractions [18–20], soluble versus immobilized soil APM fractions [21–24], or phosphatase bonded to humic substances [25]. In addition to these, fractions of APM derived from plant roots have been studied in intact roots, external-root solution (as a part of rhizodeposition), root apoplastic sap, total root, and root segment extracts. Anatomical-physiological studies of surface-bound phosphomonoesterase activity in cross sections of roots and mycorrhizal associations have also been carried out [3, 6, 26–29].
As APM from roots and microorganisms is known to possess different kinetic properties, separation of APM sources in soil components allows us to better understand the response of P-transformation in soil in different conditions. The new approach presented in this work does not enable us to distinguish between root- and microbe-derived EAPM in soil, nor can we determine if the studied forest ecosystems have either plant- or microbe-derived EAPM prevalent in the soil. Nevertheless, the presented approach enables separation of root-derived EAPM activity from EAPM of the soil which may originate from both microorganisms and roots. The results presented in this work showed up to 18% of EAPM in soil to be root-derived when mycorrhizal status of roots was not considered. This work represents a first step in research leading to separation of root- and microbe-derived EAPM in soils.
Origin of phosphomonoesterase was shown to affect its Michaelis-Menten characteristics (K m and V max values) and response to pollutants (e.g., Cu) and other compounds in soil [30, 31]. Also, Gould et al. [9] reported that properties of microbe- and root-derived EAPM were different including their kinetic parameters and temperature sensitivity. Further research is necessary to separate the importance of root- and microbe-derived sources of EAPM in the soils of different ecosystems in order to better understand their importance in P-cycling and to evaluate their sensitivity to climate change and other types of environmental perturbations.
In conclusion, root-derived EAPM forms a lesser part of the total EAPM activity of soils in forest ecosystems. These findings can be generalized for acid forest soils where EAPM is of microbial and root origin. Alkaline soils with dominance of alkaline phosphomonoesterase of microbial origin are hypothesised to have especially plant root-derived EAPM activity; however, it still remains to be experimentally determined.
Acknowledgments
This paper was created within the framework of the Grant TA02020867 and the IGA Projects 47/2010–2012.
References
- 1.Nannipieri P, Ceccanti B, Conti C, Bianchi D. Hydrolases extracted from soil: their properties and activities. Soil Biology and Biochemistry. 1982;14(3):257–263. [Google Scholar]
- 2.Sarapatka B. Phosphatase Activities (ACP, ALP) in Agroecosystem Soils. Uppsala, Sweden: Swedish University of Agricultural Sciences; 2003. [Google Scholar]
- 3.George TS, Gregory PJ, Wood M, Read D, Buresh RJ. Phosphatase activity and organic acids in the rhizosphere of potential agroforestry species and maize. Soil Biology and Biochemistry. 2002;34(10):1487–1494. [Google Scholar]
- 4.Martin SM, Byers TJ. Acid hydrolase activity during growth and encystment in Acanthamoeba castellanii. Journal of Protozoology. 1976;23(4):608–613. doi: 10.1111/j.1550-7408.1976.tb03851.x. [DOI] [PubMed] [Google Scholar]
- 5.Shaw G, Read DJ. The biology of mycorrhiza in the Ericaceae. XIV. Effects of iron and aluminium on the activity of acid phosphatase in the ericoid endophyte Hymenoscyphus ericae (Read) Korf and Kernan. New Phytologist. 1989;113:529–533. [Google Scholar]
- 6.Asmar F, Gissel-Nielsen G. Extracellular phosphomono- and phosphodiesterase associated with the released by the roots of barley genotypes: a non-destructive method for the measurement of the extracellular enzymes of roots. Biology and Fertility of Soils. 1997;25(2):117–122. [Google Scholar]
- 7.Colvan SR, Syers JK, O’Donnell AG. Effect of long-term fertiliser use on acid and alkaline phosphomonoesterase and phosphodiesterase activities in managed grassland. Biology and Fertility of Soils. 2001;34(4):258–263. [Google Scholar]
- 8.Rodríguez H, Fraga R, Gonzalez T, Bashan Y. Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant and Soil. 2006;287(1-2):15–21. [Google Scholar]
- 9.Gould WD, Coleman DC, Rubink AJ. Effect of bacteria and amoebae on rhizosphere phosphatase activity. Applied and Environmental Microbiology. 1979;37(5):943–946. doi: 10.1128/aem.37.5.943-946.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shek DTL, Ma HK, Merrick J, editors. Positive Youth Development: Development of A Pioneering Program in a Chinese Context. London, UK: Freund Publishing Company; 2002. [Google Scholar]
- 11.Rejšek K. Acid phosphomonoesterase activity of ectomycorrhizal roots in norway spruce pure stands exposed to pollution. Soil Biology and Biochemistry. 1991;23(7):667–671. [Google Scholar]
- 12.Renella G, Landi L, Nannipieri P. Hydrolase activities during and after the chloroform fumigation of soil as affected by protease activity. Soil Biology and Biochemistry. 2002;34(1):51–60. [Google Scholar]
- 13.Stromberger ME, Klose S, Ajwa H, Trout T, Fennimore S. Microbial populations and enzyme activities in soils fumigated with methyl bromide alternatives. Soil Science Society of America Journal. 2005;69(6):1987–1999. [Google Scholar]
- 14.Klose S, Acosta-Martínez V, Ajwa HA. Microbial community composition and enzyme activities in a sandy loam soil after fumigation with methyl bromide or alternative biocides. Soil Biology and Biochemistry. 2006;38(6):1243–1254. [Google Scholar]
- 15.Margon A, Fornasier F. Determining soil enzyme location and related kinetics using rapid fumigation and high-yield extraction. Soil Biology and Biochemistry. 2008;40(9):2178–2181. [Google Scholar]
- 16.Hernesmaa A, Björklöf K, Kiikkilä O, Fritze H, Haahtela K, Romantschuk M. Structure and function of microbial communities in the rhizosphere of Scots pine after tree-felling. Soil Biology and Biochemistry. 2005;37(4):777–785. [Google Scholar]
- 17.Zhao Q, Zeng DH, Lee DK, He XY, Fan ZP, Jin YH. Effects of Pinus sylvestris var. mongolica afforestation on soil phosphorus status of the Keerqin Sandy Lands in China. Journal of Arid Environments. 2007;69(4):569–582. [Google Scholar]
- 18.Rojo MJ, Carcedo SG, Mateos MP. Distribution and characterization of phosphatase and organic phosphorus in soil fractions. Soil Biology and Biochemistry. 1990;22(2):169–174. [Google Scholar]
- 19.Kandeler E, Palli S, Stemmer M, Gerzabek MH. Tillage changes microbial biomass and enzyme activities in particle-size fractions of a Haplic Chernozem. Soil Biology and Biochemistry. 1999;31(9):1253–1264. [Google Scholar]
- 20.Marx MC, Kandeler E, Wood M, Wermbter N, Jarvis SC. Exploring the enzymatic landscape: distribution and kinetics of hydrolytic enzymes in soil particle-size fractions. Soil Biology and Biochemistry. 2005;37(1):35–48. [Google Scholar]
- 21.Nannipieri P, Ceccanti B, Cervelli S, Matarese E. Extraction of phosphatase, urease, proteases, organic carbon and nitrogen from soil. Soil Science Society of America Journal. 1980;44:1011–1016. [Google Scholar]
- 22.Pascual JA, Moreno JL, Hernández T, García C. Persistence of immobilised and total urease and phosphatase activities in a soil amended with organic wastes. Bioresource Technology. 2002;82(1):73–78. doi: 10.1016/s0960-8524(01)00127-4. [DOI] [PubMed] [Google Scholar]
- 23.Criquet S, Ferre E, Farnet AM, Le Petit J. Annual dynamics of phosphatase activities in an evergreen oak litter: influence of biotic and abiotic factors. Soil Biology and Biochemistry. 2004;36(7):1111–1118. [Google Scholar]
- 24.Fornasier F, Margon A. Bovine serum albumin and Triton X-100 greatly increase phosphomonoesterases and arylsulphatase extraction yield from soil. Soil Biology and Biochemistry. 2007;39(10):2682–2684. [Google Scholar]
- 25.Gosewinkel U, Broadbent FE. Decomplexation of phosphatase from extracted soil humic substances with electron donating reagents. Soil Science. 1986;141:261–267. [Google Scholar]
- 26.Richardson AE, Hadobas PA, Hayes JE. Acid phosphomonoesterase and phytase activities of wheat (Triticum aestivum L.) roots and utilization of organic phosphorus substrates by seedlings grown in sterile culture. Plant, Cell and Environment. 2000;23(4):397–405. [Google Scholar]
- 27.Alvarez M, Godoy R, Heyser W, Härtel S. Anatomical-physiological determination of surface bound phosphatase activity in ectomycorrhizae of Nothofagus obliqua . Soil Biology and Biochemistry. 2005;37(1):125–132. [Google Scholar]
- 28.Tamas L, Dudikova J, Durceková K, Huttova J, Mistrik I, Zelinova V. The impact of heavy metals on the activity of some enzymes along the barley root. Environmental and Experimental Botany. 2008;62:86–91. [Google Scholar]
- 29.Priya P, Sahi SV. Influence of phosphorus nutrition on growth and metabolism of Duo grass (Duo festulolium) Plant Physiology and Biochemistry. 2009;47(1):31–36. doi: 10.1016/j.plaphy.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Gibson BR, Mitchell DT. Phosphatases of ericoid mycorrhizal fungi: kinetic properties and the effect of copper on activity. Mycological Research. 2005;109(4):478–486. doi: 10.1017/s095375620400214x. [DOI] [PubMed] [Google Scholar]
- 31.Goil MM, Harpur RP. A comparison of the non-specific acid phosphomonoesterase activity in the larva of Phocanema decipiens (nematoda) with that of the muscle of its host the codfish (Gadus morhua) Zeitschrift fur Parasitenkunde. 1979;60(2):177–183. doi: 10.1007/BF00927973. [DOI] [PubMed] [Google Scholar]


