Abstract
The aim of this work was to evaluate the spectrophotometric methodology for determining the total flavonoid content (TFC) in herbal drug and derived products from Bauhinia monandra Kurz. Several analytical parameters from this method grounded on the complex formed between flavonoids and AlCl3 were evaluated such as herbal amount (0.25 to 1.25 g); solvent composition (ethanol 40 to 80%, v/v); as well as the reaction time and AlCl3 concentration (2 to 9%, w/v). The method was adjusted to aqueous extractives and its performance studied through precision, linearity and preliminary robustness. The results showed an important dependence of the method response from reaction time, AlCl3 concentration, sample amount, and solvent mixture. After choosing the optimized condition, the method was applied for the matrixes (herbal material and extractives), showing precision lower than 5% (for both parameters repeatability and intermediate precision), coefficient of determination higher than 0.99, and no important influence could be observed for slight variations from wavelength or AlCl3 concentration. Thus, it could be concluded that the evaluated analytical procedure was suitable to quantify the total flavonoid content in raw material and aqueous extractives from leaves of B. monandra.
1. Introduction
Bauhinia species, such as B. monandra Kurz, are popularly known in Brazil as “Pata-de-Vaca” and are widely used in traditional medicine as antidiabetic or antioxidant agent. Although studies showed such activities, they were not still elucidated which group(s) of compound(s) is/are responsible for such properties [1, 2]. In the literature the presence of flavonoids in the leaves of vegetable is related [1]. Additionally, the several pharmacologic properties and wide occurrence in the vegetable kingdom with restricted distribution inside of an order, family, or gender; these compounds have been widely used as chemical markers of first choice for several active herbal drugs [3–9].
The general technique most employed for quantification of flavonoids is based on the spectrophotometric determination of complex flavonoid-AlCl3, which provides a bathochromic displacement and the hyperchromic effect [10]. Although present in official codes, the method proposed initially by Christ and Müller [11] presents several analytic limitations that can be configured as significant source of error. Initially, the technique was developed for analysis of herbal materials containing O-glycoside flavonoids, the ones which, after acid hydrolise and extraction with organic solvent, are quantified starting from the compound formed between the flavonoid aglycones and AlCl3. However, the technique has been indiscriminately used for vegetable species containing C-glycosides flavonoids. How they do not undergo acid hydrolyze, they are discarded in the aqueous phase during the liquid-liquid extraction due to the polarity from glucosyl residue [5, 6, 12].
Additionally, several factors have been revealed as crucial during the formation of the flavonoid-AlCl3 complexes such as: the reaction time, the concentration of the reagent (AlCl3 and flavonoid content/plant material), and the chemical structure of the polyphenol. Likewise, the study of these parameters becomes fundamental during the development of the method [6, 12]. Thus, the use of multivariate approach is quite suitable for satisfactory evaluation of this kind of challenge. Experimental designs are widely used multivariate tools for the systematic and effective evaluation of simultaneously modifications of several variables in a specific system [13, 14]. Special attention has been direct to central composite design (CCD). This second-order design is the most often employed to study and optimize several systems such as: reactional conditions [12]; operational parameters [15]; formulation compositions [16]; method stability [17], and/or extractive performance [18, 19]. With this kind of second-order design, it is possible to create response surfaces that allow the ranking of each variable according to its significance on the studied responses [13, 14, 20]. Therefore, with reduced time and experimental effort, it may be possible to predict what experimental condition will produce a desired or optimum response.
Therefore, the objective of this work was the evaluation of a methodology for quantification of total flavonoids without acid hydrolyzes, in the herbal material and aqueous extract from leaves of B. monandra.
2. Material and Methods
2.1. Materials
The leaves of the Bauhinia monandra Kurz were collected in November of 2004, in Campina Grande, PB, and taxonomically identified. Voucher specimen was identified by Dra. Maria Iracema Bezerra Loyola and deposited at Department of Biology/UFRN under registration number 2611. The dried and ground material was used in all experiments. All reagents used were grade proanalysis.
2.2. Methods
2.2.1. Wavelength Selection
Herbal Material —
For the determination of the maximum of absorption, a sample of 0.5 g of the herbal material (d 50 = 0.615 mm) was submitted to a general procedure for flavonoids quantification using ethanol 80% (v/v) as liquid extractor. The UV-Vis spectra (200 to 500 nm) was obtained after 30 minutes of complex with AlCl3 5% (w/v) in hydroalcoholic solution 80% (v/v).
Extractives —
The spectrum from 200 to 500 nm was obtained in spectrophotometer (Cary 1E, Varian, Japan) 30 minutes after addition of 2.0 mL of AlCl3 2.5% (w/v) to the diluted aqueous extractives (dilution = 1/208.33). The same sample without AlCl3 was used as compensation solution.
2.2.2. Optimization of Reaction Parameters for Formation of Flavonoid-AlCl3 Complex
The influences of reaction time and concentration of AlCl3 were studied through an experimental design 22 (AlCl3 = 2.5 or 7.5%, w/v; time = 15 or 35 min), added of 3 central points (AlCl3 = 5%, w/v; time = 25 min) and 4 star points (AlCl3 = 1.46 or 8.54%, w/v; time = 10.86 or 39.14 min) [12]. Each point represents an average of three determinations. The results were adjusted by PLS to a mathematical model, which was evaluated statistically and used to generate response surface.
The independent variable factors for experimental design were the concentration of AlCl3 and time. The dependent variable (response) was the absorbance. To compare the effect of factors, their values were coded (Table 1). A second-order model was established for the responses by PLS (1). The validation of the mathematical model was performed through analysis of variance, multiple-correlation coefficients, and estimation of the lack of fit using STATISTICA 6.0 (Statsoft, USA):
Table 1.
Matrix of central composite design (CCD) applied to evaluate the influence of reaction time and AlCl3 concentration on the method responses (absorbance).
| Coded variables | Natural variables | Method response | ||||
|---|---|---|---|---|---|---|
| Experiment | Time (min) | AlCl3 (%) | Time (min) | AlCl3 (%) | Drug (A.U.) | Extractives (A.U.) |
| 1 | −1 | −1 | 15 | 2.5 | 0.255 | 0.400 |
| 2 | 1 | −1 | 35 | 2.5 | 0.258 | 0.398 |
| 3 | −1 | 1 | 15 | 7.5 | 0.258 | 0.409 |
| 4 | 1 | 1 | 35 | 7.5 | 0.264 | 0.400 |
| 5 | 0 | 0 | 25 | 5 | 0.258 | 0.409 |
| 6 | 0 | 0 | 25 | 5 | 0.258 | 0.410 |
| 7 | 0 | 0 | 25 | 5 | 0.256 | 0.409 |
| 8 | 0 | −1.414 | 25 | 1.46 | 0.262 | 0.397 |
| 9 | 0 | 1.414 | 25 | 8.54 | 0.259 | 0.413 |
| 10 | −1.414 | 0 | 10.86 | 5 | 0.260 | 0.405 |
| 11 | 1.414 | 0 | 39.14 | 5 | 0.260 | 0.414 |
A.U.: absorbance units.
| (1) |
2.2.3. General Procedure for Total Flavonoids Quantification in Herbal Material
The sample of raw material was transferred to a round-bottom flask of 250 mL and added of 30.0 mL from hydroalcoholic solution. The mixture was heated up in water bath, under reflux, by 30 min. After cooling at room temperature, the extractive was filtered to a volumetric flask of 100 mL through small amount of cotton. The residue of herbal drug and the cotton were resubmitted to reflux with more 30.0 mL from the same solvent by 30 min. The procedure was repeated once more. The volume was completed to 100 mL originating the stock solution (SS). 1.0 mL from SS was transferred to a 25 mL volumetric flask of, added of 2.0 mL from AlCl3 solution and the volume was completed with distilled water (probe solution, PS). The same procedure was repeated without the addition of AlCl3 for preparation of contrast solution (CS). The absorbance of PS against CS was determined in spectrophotometer at 410 nm.
The result express as the percentage of total flavonoid content (TFC), calculated as rutin, through (2), and it represents the average of three determinations
| (2) |
where A = absorbance (A.U.); DF = dilution factor; w = mass of plant material (g); ld = loss on drying of plant material (8%, w/w); A 1cm 1% = specific absorption for rutin-AlCl3 complex (259,4).
Evaluation of the Solvent Concentration —
0.5 g of raw material was extract with ethanol 40, 60, 80, or 100% (v/v), according to the general procedure for herbal material. The absorbance was determined by spectrophotometry at 410 nm after 25 min using AlCl3 5% (w/v). The total flavonoid content (TFC) was expressed by the average of three determinations.
Evaluation of Herbal Material Concentration —
Samples of the raw material ranged from 0.25 to 1.25 g were submitted to the general procedure of quantification using ethanol 80% (v/v) as solvent. The absorbance was determined by spectrophotometry at 410 nm after 25 min using AlCl3 5% (w/v). The results express the average of three determinations.
2.2.4. General Procedure for Total Flavonoids Quantification in the Aqueous Extractives
The sample was constituted by aqueous extractive prepared by decoction for 10 min at plant : solvent proportion of 15 : 100 (w/v). The solution was filtered and the volume completed to 100 mL with distilled water (extractive solution, ES). Aliquot of 3.0 mL from ES was diluted to 100 mL with water distilled in volumetric flask (stock solution, SS). Another aliquot of 4.0 mL of SS was transferred to a 25 mL volumetric flask and added of 2 solution mL from AlCl3 2.5% (w/v) (probe solution, PS). The same procedure was repeated without the addition of AlCl3 for preparation of the contrast solution (CS). The absorbance of PS against CS was determined in spectrophotometer in 410 nm after 15 minutes of reaction. The total flavonoid content was calculated according to (2).
2.2.5. Calibration Curve for Aqueous Extractives
The aqueous extractive from leaves of B. monandra was filtered through filter paper and diluted with distillated water to concentrations ranged from 0.3 to 1.44 mg/mL. 2.0 mL of AlCl3 2.5% (w/v) were added to each diluted solution and the solution was made up to 25.0 mL with distillated water. The absorbance was measured at 410 nm after 15 min, using the diluted solution without AlCl3 as contrast solution. The calibration curve was made by linear regression and the results represented the average of three determinations to each concentration.
2.2.6. Repeatability and Intermediary Precision
The repeatability was evaluated in triplicate on the same day for three samples, while the intermediary precision was assessed for two consecutive days. The dates were expressed for relative standard deviation (RSD%) [21].
2.2.7. Preliminary Robustness
The robustness of the method was evaluated through the determination of the absorbance of the solution extractive complexed with aluminum chloride in the concentrations of 5.0 ± 0.5% (w/v) and absorbance determinate at 410 ± 2 nm. Each determination represents average of three determinations [21].
3. Results and Discussion
3.1. Wavelength Selection for Herbal Drug and Extractives
The UV-spectrum for herbal drug, aqueous extractive, and rutin after reaction with AlCl3 are presented in Figure 1. A very similar UV-profile was observed for all sample tested and three maximums were observed. Due to the higher specificity of absorptions at wavelength corresponding to the band I derived from cynamoil moiety of flavonoids, the determinations were performed at 410 nm for both herbal material and respective aqueous extractives.
Figure 1.
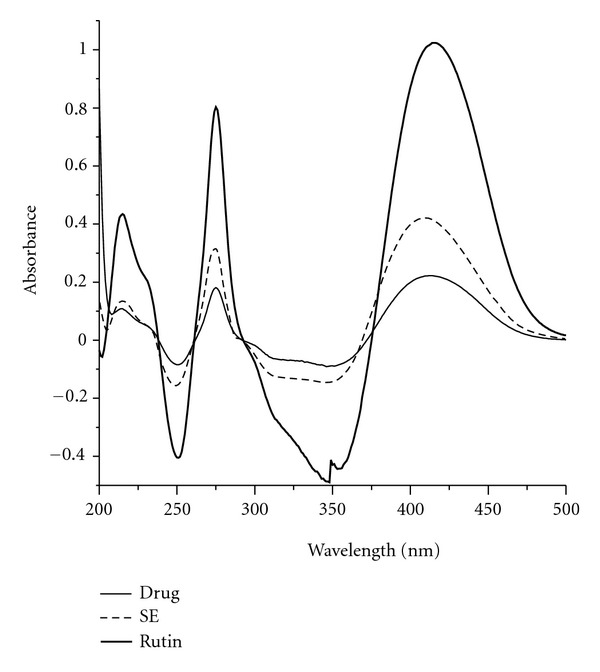
Spectrum for AlCl3-flavonoid complexes: herbal material (drug), aqueous extractive (SE) and standard (rutin).
3.2. Total Flavonoid Content in Herbal Material
3.2.1. Reactional Conditions for Formation of Flavonoid-AlCl3 Complex in Herbal Material
The results for method performance upon several reaction times and/or AlCl3 concentrations are shown in Table 1. The experimental data for herbal material were used to generate second-order models for each dependent variable. Due to the lack-of-fit test was not significant, the experimental variations could be attributed only to a randomized error. Thus, the fitted models provided an adequate approximation of the true values, and no violations of the model assumptions occurred.
The mathematical models that explain each surface are calculated by (1) and the data used to generate response surface (Figure 2(a)).
Figure 2.
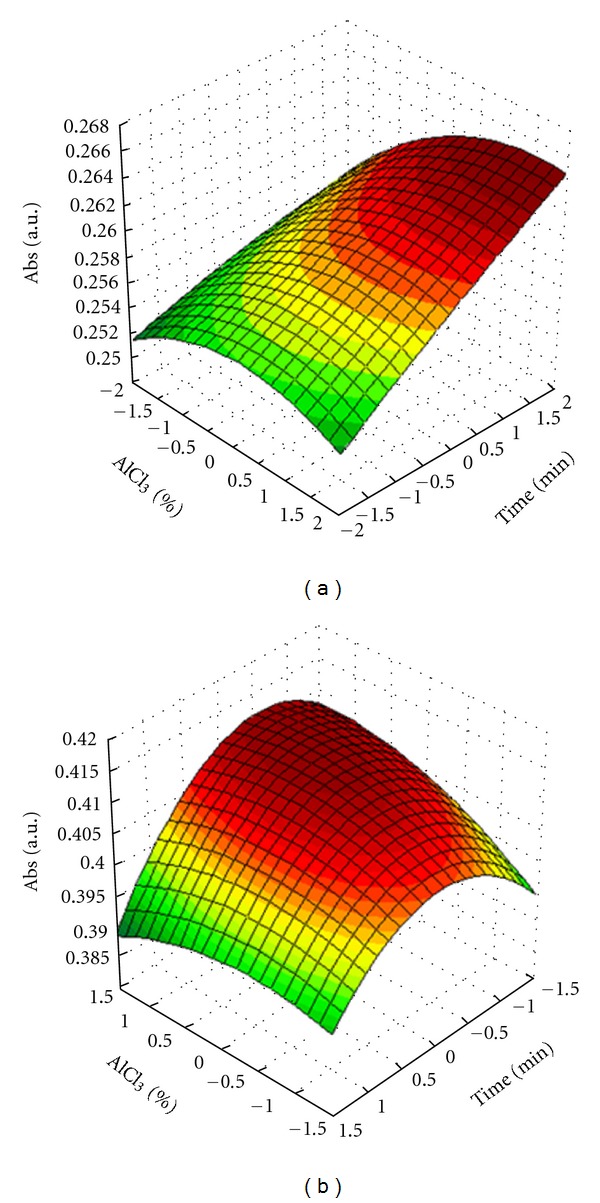
Effects of reaction time and AlCl3 concentration on the spectrophotometric responses for the analytical methodology applied to drug material (a) and aqueous extractives (b) from leaves of B. monandra.
The ANOVA was significant for both factors, concentration of AlCl3 and time, demonstrating that the variation of these significantly influence the response studied (Table 2).
Table 2.
ANOVA for CCD applied to evaluate the influence of reaction time and AlCl3 concentration on the method responses for both drug material and extractives from leaves of B. monandra.
| Source | F (Drug) | F (Extractives) |
|---|---|---|
| AlCl3 (linear) | 546.76* | 0.68 |
| AlCl3 (quadratic) | 131.85* | 0.63 |
| Time (linear) | 1654.53* | 3.59 |
| Time (quadratic) | 2.351 | 5.69 |
| Interaction (time × AlCl3) | 38.36* | 0.64 |
*Significant for α = 0.05.
In agreement with the Table 3, the t-test for the coefficients revealed statistical importance for lineal terms of variables concentration of AlCl3 and, mainly, time. The quadratic terms exercised negative effect on the response only being significant the factor AlCl3.
Table 3.
t-test for effects of factors on the method response for drug and extractive solution from leaves of B. monandra.
| Source | t (Drug) | t (Extractives) |
|---|---|---|
| Average | 3092.65* | 143.01 |
| AlCl3 (linear) | 23.38* | 0.83 |
| AlCl3 (quadratic) | −11.48* | −0.80 |
| Time (linear) | 40.68* | −1.90 |
| Time (quadratic) | −1.53 | −2.40 |
| Interaction (time × AlCl3) | 6.19 | −0.80 |
*Significant for α = 0.05.
In that way, the elected conditions for determining a higher response for total flavonoid content in plant material were 5% (m/v) of AlCl3 and 25 minutes of reaction.
3.2.2. Influence of Solvent and Plant Concentration on the Assay Performance for the Herbal Material
The total flavonoids content, calculated for drug material when 0.5 g was extracted with ethanolic solutions in different concentrations (40 to 100%), showed that alcohol 80% (v/v) determines a higher response for the method (Figure 3).
Figure 3.
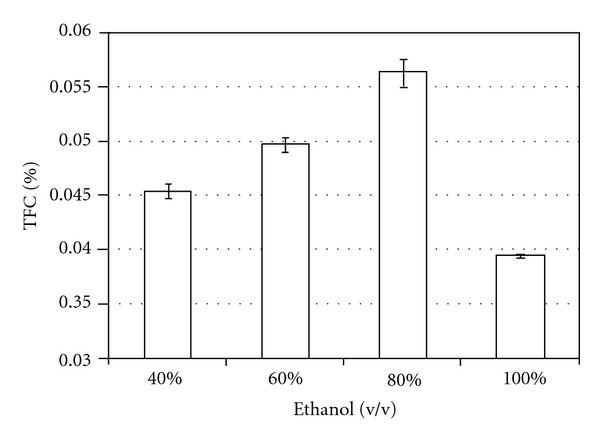
Influence of extractive solvent on the total flavonoid content (TFC) from drug material.
Selected ethanol 80% as better concentration of solvent extractor, the influence of proportion plant : solvent was evaluated using samples ranged from 0.25 to 1.25 g. Analyzing the results, it was verified that the best parameters, were 0.5 g of herbal material and 80% (v/v) of ethanol, where the higher response was obtained. The apparent decrease of flavonoid content with increase of plant proportion can be explained by the possible saturation of solvent or method failure (Figure 4) [22].
Figure 4.
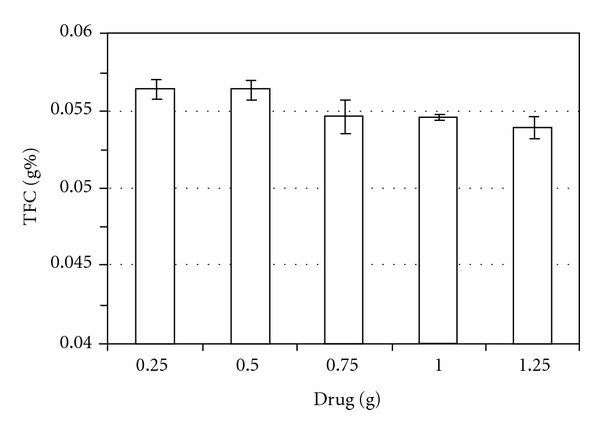
Influence of drug amount on the total flavonoid content (TFC) from drug material.
3.2.3. Repeatability and Intermediary Precision
The data for procedure precision by repeatability and intermediary precision showed that the method was precise due to the lower relative standard deviation (RSD%) for both parameters. The repeatability showed relative standard deviation of 2.5%, while no statistical difference could be observed for intermediary precision, although the method was appraised on different days. Thus the method could be considered in according with the legal requirements for analytical performance of procedures for evaluation of phytopharmaceuticals and phytomedicines (Table 4).
Table 4.
Repeatability and intermediate precision of the method for drug material and aqueous extractive from leaves of B. monandra (results in absorbance).
| Drug | Aqueous extractives | |||||
|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Repet. | Day 1 | Day 2 | Repet. | |
| Average | 0.266 | 0.276 | 0.271 | 0.412 | 0.417 | 0.414 |
| SD | 0.004 | 0.004 | 0.006 | 0.006 | 0.13 | 0.011 |
| RSD% | 1.701 | 1.739 | 2.50 | 1.53 | 3.19 | 2.68 |
3.3. Total Flavonoid Content in the Aqueous Extractives
3.3.1. Reactional Conditions for Formation of Flavonoid-AlCl3 Complex in Aqueous Extractives
The statistical analysis by ANOVA (Table 2) and t-test for coefficients of equation (Table 3), showed no significant variation for different terms of equation. Although the literature relates that the reaction time and concentration of AlCl3 are critical for flavonoids determination and should be optimized for each matrix vegetable, it was not the discovery for the aqueous extractive solution of B. monandra. Anyway, the surface generated by experimental data suggested a tendency to an optimal conditions at 2.5% (w/v) of AlCl3 after 15 minutes for reaction time, which allow to determine the flavonoids content without risk of method failure (Figure 2(b)).
3.3.2. Method Evaluation for Aqueous Extractives
The calibration curve was obtained by the absorbance concentrations (mg/mL) using eight dilutions. The regression analysis was performed and the resulting equation was “Abs = 0.0396 + 4.73x.” The coefficient of determination for standard curves were greater than 0.99 (R 2 = 0.998). Thus, the calculated straight line could explain more than 99% of the experimental data (Figure 5).
Figure 5.

Calibration curve for aqueous extractives for flavonoids-AlCl3 complex from leaves of B. monandra.
Table 4 shows the precision of method. This presented RSD of 2.68% for repeatability, with RSD intrasample varying from 1.53 to 3.19%. This improvement of the variation between days can be explained by the introduction of different extracts for each day of analysis [19].
Regarding the robustness of the method, the analysis by two-way ANOVA with repetition revealed that the response did not undergo any significant interference when small changes were inserted in the concentrations of AlCl3 and/or in the maximum wavelength (Table 5). This became, therefore, a robust method for determination of TFC in aqueous extractives of B. monandra.
Table 5.
ANOVA for the preliminary robustness of the procedure for TFC in aqueous extracts from leaves of B. monandra.
| Source of variation | F | F critical |
|---|---|---|
| AlCl3 (%) | 0.29 | 3.55 |
| λ (nm) | 1.56 | |
| Interation | 1.23 |
*Significant for α = 0.05.
4. Conclusions
This study showed that the method initially developed by Schmidt and Ortega [6] for aerial parts of Passiflora was also appropriate for the determination of the total flavonoids content (TFC) in the herbal material and aqueous extractive from B. monandra Kurz. However, before adoption of the proposed procedure, several tests were performed in order to obtain the optimized analytical conditions. After that, the results allowed to conclude that the proposed procedure is simple, fast and precise, and can be used to support the quality evaluation of raw material and f the aqueous extractives from B. monandra.
Acknowledgments
The authors are grateful to FAPERN (PPP 2007) and CNPq for financial support in the form of Grants (477131/2007-7, 470179/2009-0) and Fellowship Awards (312537/2009-3; 557224/2010-1; 501834/2010-9).
References
- 1.Argolo ACC, Sant’Ana AEG, Pletsch M, Coelho LCBB. Antioxidant activity of leaf extracts from Bauhinia monandra . Bioresource Technology. 2004;95(2):229–233. doi: 10.1016/j.biortech.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Da Silva KL, Cechinel Filho V. Plantas do gênero Bauhinia: composição química e potencial farmacológico. Quimica Nova. 2002;25(3):449–454. [Google Scholar]
- 3.Deutsches Arzneibuch. 10 edition. Stuttgart, Germany: Deutscher Apotheker Verlag; 1992. [Google Scholar]
- 4.Glasl H, Becker U. Flavonol-O-glyckoside: photometrische Gehaltsbestimmung. Deutsche Apotheker Zeitung. 1984;124(43):2147–2152. [Google Scholar]
- 5.Petry RD, Souza KCB, Bassani VL, Petrovick PR, González Ortega G. Doseamento do teor de flavonóides totais em extratos hidroalcoólicos de Passiflora alata Dryander (maracujá) Revista Brasileira de Farmácia. 1998;79(1-2):7–10. [Google Scholar]
- 6.Schmidt PC, Ortega GG. Passionsblumenkraut: Bestimmung des Gesamtflavonoidgehaltes von Passiflorae herba . Deutscher Apotheker Zeitung. 1993;47:17–26. [Google Scholar]
- 7.Soares LAL, Bassani VL, González Ortega G, Petrovick PR. Total flavonoid determination for the quality control of aqueous extractives from Phyllanthus niruri L. Acta Farmaceutica Bonaerense. 2003;22(3):203–207. [Google Scholar]
- 8.Silva IV, Ferreira MS, Warderley AG, Fernandes MJ, Soares LAL, De Souza TP. Influence of extractive parameters on the preparation of a solution from Psidium guajava L. Latin American Journal of Pharmacy. 2009;28(1):116–120. [Google Scholar]
- 9.Marques GS, Monteiro RPM, Leão WF, et al. Avaliação de procedimentos para quantificação espectrofotométrica de flavonóides totais em folhas de Bauhinia forficata Link. Quimica Nova. 2012;35(3):517–522. [Google Scholar]
- 10.Mabry TJ, Markhama KR, Thomas MB. The Systematic Identification of Flavonoids. Berlin, Germany: Springer; 1970. [Google Scholar]
- 11.Christ B, Müller KH. Zur serienmaessigen Bestimmung des Gehaltes an Flavonol-Derivaten in Drogen. Archiv der Pharmazie. 1960;293(65):1033–1042. [Google Scholar]
- 12.Petry RD, González Ortega G, Silva WB. Flavonoid content assay: influence of the reagent concentration and reaction time on the spectrophotometric behavior of the aluminium chloride—flavonoid complex. Pharmazie. 2001;56(6):465–470. [PubMed] [Google Scholar]
- 13.Box GE, Huntera WG, Hunter JS. Response surface methods. In: Box GE, Hunter WG, Hunter JS, editors. Statistics for Experimenters. New York, NY, USA: Wiley; 1978. pp. 510–539. [Google Scholar]
- 14.Myers RH, Montgomery DC. Response Surface Methodology: Process and Product Optimization Using Designed Experiments. New York, NY, USA: Wiley; 1995. [Google Scholar]
- 15.Soares LAL, Schmidt PC, González Ortega G, Petrovick PR. Efeito da Força e da Velocidade de Compressão sobre as Propriedades de Comprimidos Contendo Alta Concentração de Extrato Seco Vegetal. Acta Farmaceutica Bonaerense. 2003;22:147–154. [Google Scholar]
- 16.Soares LA, Ortega GG, Petrovick PR, Schmidt PC. Optimization of tablets containing a high dose of spray-dried plant extract: a technical note. AAPS PharmSciTech. 2005;6(3):367–371. doi: 10.1208/pt060346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soares LAL, De Souza TP, Gonzalez Ortega G, Petrovick PR. Use of central composite design to evaluate the robustness of a LC-method. Latin American Journal of Pharmacy. 2007;26(6):904–908. [Google Scholar]
- 18.da Cunha FP, da Costa LJL, Fernandes AJD, de Souza TP, Soares LA. Development and optimization of extractives from Astronium urundeuva (allemão) Engl. by factorial design. Brazilian Archives of Biology and Technology. 2009;52(3):647–652. [Google Scholar]
- 19.Silva KGH, Xavier FH, Farias IEG, et al. Stationary cuvette: a new approach to obtaining analytical curves by UV-VIS spectrophotometry. Phytochemical Analysis. 2009;20(4):265–271. doi: 10.1002/pca.1122. [DOI] [PubMed] [Google Scholar]
- 20.Davies L. Efficiency in Research, Development and Production: The Statistical Design and Analysis of Chemical Experiments. Cambrigde, UK: Royal Society of Chemistry; 1993. [Google Scholar]
- 21.Q2B: validation of analytical procedures: methodology. In: Proceedings of the International Conference on Harmonization (ICH '96); 1996. [Google Scholar]
- 22.List PM, Schmidt PC. Technologie Pflanzlicher Arzneizubereitungen. Stuttgart, Germany: Wissenschaftliche Verlagsgesellschaft; 1984. [Google Scholar]


